Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent responsible for the Coronavirus Disease-2019 (COVID-19) pandemic, has infected over 185 million individuals across 200 countries since December 2019 resulting in 4.0 million deaths. While COVID-19 is primarily associated with respiratory illnesses, an increasing number of clinical reports indicate that severely ill patients often develop thrombotic complications that are associated with increased mortality. As a consequence, treatment strategies that target COVID-associated thrombosis are of utmost clinical importance. An array of pharmacologically active compounds from natural products exhibit effects on blood coagulation pathways, and have generated interest for their potential therapeutic applications towards thrombotic diseases. In particular, a number of snake venom compounds exhibit high specificity on different blood coagulation factors and represent excellent tools that could be utilized to treat thrombosis. The aim of this review is to provide a brief summary of the current understanding of COVID-19 associated thrombosis, and highlight several snake venom compounds that could be utilized as antithrombotic agents to target this disease.
Keywords: COVID-19, Antithrombotic, Snake venom, Anticoagulant, Antiplatelet, Thrombosis
1. COVID-19 pandemic: a public health crisis of the globe
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) now represents a major global health threat responsible for the respiratory illness known as Coronavirus Disease-2019 (COVID-19) [1]. Early cases of COVID-19 emerged in the city of Wuhan in Hubei province, China, in December 2019 [2] and were considered to have started through zoonotic transmission associated with Huanan Seafood Wholesale Market [3]. However, it is now believed that COVID-19 diagnoses were being documented as early as November 2019 [4], and low levels of SARS-CoV-2 may have been spreading throughout the province as early as mid-October to mid-November 2019 [5]. COVID-19 rapidly disseminated, and by January 2020, cases were confirmed in nine countries, including China, Japan, Nepal, Taiwan, Thailand, Singapore, South Korea, the United States, and Vietnam [6]. On the 3rd of March 2020, COVID-19 cases expanded to 72 countries [7], and on the 11th of March 2020, the World Health Organization declared the outbreak a global pandemic [8], [9]. As of the 17th of August 2021, the current outbreak has affected the lives of approximately 207 million people and resulted in over 4.3 million deaths across 200 countries [10].
As shown in Table 1 , COVID-19 has a range of clinical manifestations with mild symptomatic presentations, often characterized by fatigue, fever, dry cough, anorexia, myalgia, and dyspnea [11], [12]. Severe complications can include pneumonia, acute respiratory distress syndrome (ARDS), renal failure, heart failure, and multiple organ failure, and are fatal for some individuals [12], [13]. Clinical data has also shown that many patients with severe COVID-19 exhibit coagulation abnormalities such as microvascular thrombosis and venous or arterial thrombosis, often associated with increased mortality [14], [15], [16]. While several therapeutic agents have been proposed to target COVID-19-associated thrombosis, there are currently no effective treatments for this issue [16]. Novel pharmacologically active compounds from natural products have been an important source of numerous clinically useful agents [17], [18]. In particular, snake venoms are rich sources of bioactive molecules, many of which interfere with the blood coagulation cascade and platelet aggregation [19], [20]. The utilization of isolated venom compounds as potential therapeutics has received increasing attention, and several drugs marketed for clinical use have been successfully designed from animal venoms [21], [22], [23], [24], [25]. This paper aims to briefly review the thrombotic complications observed in severe COVID-19 patients and highlights several venom antithrombotic compounds that could be utilized as potential therapeutic agents to target this issue. A thorough understanding of COVID-19-associated thrombosis can have major implications for drug targeting and discovery. The studies discussed herein were identified in public databases (MEDLINE, Scopus) in the life and biomedical sciences covering the antithrombotic potential of snake venoms and their purified components from 2010 to the present. The public search engines ScienceDirect (https://www.sciencedirect.com/), PubMed (pubmed.ncbi.nlm.nih.gov), and Google Scholar (https://scholar.google.com/) were used for searching the public databases with combinations of keywords such as snake venom, antithrombotic, thrombolytic, anticoagulant and antiplatelet.
Table 1.
Some common symptoms of COVID-19 complications and baseline severity categorization as outlined by the US Food and Drug Administration.
| Severity Category | Clinical parameters/symptoms |
|---|---|
| SARS-CoV-2 infection without symptoms | Positive virologic test; asymptomatic |
| Mild COVID-19 | Positive virologic test; symptoms - fever, cough, sore throat, headache, muscle pain, nausea, vomiting, diarrhea, and loss of taste or smell; No shortness of breath or dyspnea |
| Moderate COVID-19 | Positive virologic test, respiratory rate ≥ 20 breaths per minute, heart rate ≥ 90 beats per minute, saturation of oxygen (SpO2) > 93% on room air at sea level; symptoms - those from mild illness and shortness of breath with exertion |
| Severe COVID-19 | Positive virologic test, respiratory rate ≥ 30 breaths per minute, heart rate ≥ 125 beats per minute, SpO2 ≤ 93% on room air at sea level, PaO2/FiO2 < 300; symptoms - those from moderate illness and shortness of breath at rest, or respiratory distress |
| Critical COVID-19 | Positive virologic test, Evidence of critical illness, defined by at least one of the following: Respiratory failure (requiring at least one of the following: Endotracheal intubation and mechanical ventilation, oxygen delivered by high flow nasal cannula (heated, humidified), oxygen delivered via reinforced nasal cannula at flow rates >20 L/min with fraction of delivered oxygen ≥0.5), non-invasive positive pressure ventilation, ECMO, or clinical diagnosis of respiratory failure), Shock, or Multi-organ dysfunction. Critical complications may also include pulmonary embolism (PE), stroke, microvascular thrombosis, venous or arterial thrombosis |
2. The etiology of hematological complications and coagulopathy in COVID-19 patients
Micro and macro-vascular coagulation are not uncommon in COVID-19 patients. Local direct vascular and endothelial injury in the lung and other organs leads to microvascular clot formation and angiopathy [26], [27]. COVID-19 patients frequently develop hypercoagulability with hyperfibrinogenemia in the systemic circulation and present with extensive vessel thrombosis and significant thromboembolic sequelae such as thrombosis in deep veins (DVT), pulmonary arteries (PE), coronary arteries (myocardial infarction) or arteries of the brain (stroke) [16], [28], [29], [30], [31], [32], [33]. Coagulation profiles have revealed increased D-dimer and fibrin degradation products; abnormal prothrombin or activated partial thromboplastin time, altered fibrinogen levels; platelet activation; and increased clot strength in thromboelastometry [34], [35], [36]. In severe COVID-19 patients, PE and stroke have been reported in 20-30% and 3-5%, respectively, while post-mortem reports of lung and other organs have demonstrated thickened and stenosed vascular lumen in association with infiltration of polymorphonuclear and mononuclear cells and endothelial or mononuclear cell apoptosis [16], [26], [30], [31], [32], [37], [38], [39].
Several mechanisms leading to the thromboinflammatory response are actively investigated. These include cytokine-mediated upregulation of tissue factor resulting in thrombin generation; increased fibrin generation coupled with reduced fibrinolysis due to imbalances between PAI-1, activated protein C, and tissue factor pathway inhibitor, and hypoxic vaso-occlusion-induced microvascular and macrovascular thrombosis [40], [41], [42], [43]. In addition, neutrophil extracellular traps (NETs) have also been implicated in propagating microvascular thrombi [44], [45]. NETs are tangles of neutrophil-released DNA decorated with antimicrobial and nuclear proteins [46], [47] that propagate intravascular thrombosis by augmenting activation of factor XII, tissue factor, and both trapping and activating platelets. As a consequence, both extrinsic and contact pathways of coagulation are initiated [48], [49], [50], [51]. Indeed, severe COVID-19 patients are found to have increased markers of neutrophil activation and NET formation in their serum [52], and are correlated or preceded the development of venous thromboembolism [53]. Although critical for host defense, the dysregulated inflammatory response can result in endothelial disruption, tissue damage, and free activation of coagulation [42].
The SARS-CoV-2 spike glycoprotein (S-glycoprotein) mediates viral entry into cells through angiotensin-converting enzyme 2 (ACE2) receptors, and the same has been identified in cells expressing ACE2 from autopsy specimens of SARS pneumonia [54], [55], [56], [57], [58], [59]. The S protein-ACE2 interaction leads to increased expression of NFκB (nuclear factor kappa-light-chain-enhancer of activated B cells), leading to the secretion of various proinflammatory cytokines, including interleukin (IL)-1β, IL-6, tumor necrosis factor-alpha, transforming growth factor-beta 1, and monocyte chemoattractant protein 1. These all have been implicated in dysregulated inflammation and thrombogenesis [60].
Mc Gonagle et al. [61] described the lung-restricted vascular immunopathology associated with COVID-19 and termed it diffuse pulmonary intravascular coagulopathy (PIC). In its early stage of development, PIC is distinct from disseminated intravascular coagulation (DIC) and presents with elevated cardiac enzyme (reflecting emergent ventricular stress induced by pulmonary hypertension) and D-dimer (reflecting pulmonary vascular bed thrombosis with fibrinolysis) levels in the background of normal fibrinogen and platelet levels [61]. The authors detailed that a macrophage activation syndrome (MAS)-like phenotype causes alveolar and interstitial inflammation, triggering extensive immunothrombosis distinct from the usual MAS and DIC.
Up to 95% of COVID-19-induced acute respiratory distress syndrome (ARDS) is associated with blood clots in the pulmonary micro- and macrovasculature with or without DIC, and both blood and alveolar fluid studies have been consistent with a prothrombotic state [62], [63], [64], [65], [66]. Mechanistically, there is increased thrombin production (elevated tissue factor) accompanied by the compromised fibrinolytic response (elevated Plasminogen activator inhibitor-1). The bidirectional relationship between coagulation and the innate immune system causes the initial hemostatic dysregulation to be primarily localized to the lungs. This cross-talk is facilitated by platelet degranulation and coordinated interactions with activated T cells, NETs, tissue factor-bearing microparticles, neutrophils, monocytes, dendritic cells, as well as coagulation proteases [42], [66], [67], [68]. These protective immunothrombi create a sterile barrier promoting pathogen recognition and prevent pathogen invasion. However, the mechanism can become maladaptive to interfere with organ perfusion [46], [69], [70]. In addition, studies on ARDS have shown both fibrin deposition (intra- and extra-vascular) and unregulated fibrinolysis during the process [64], [71].
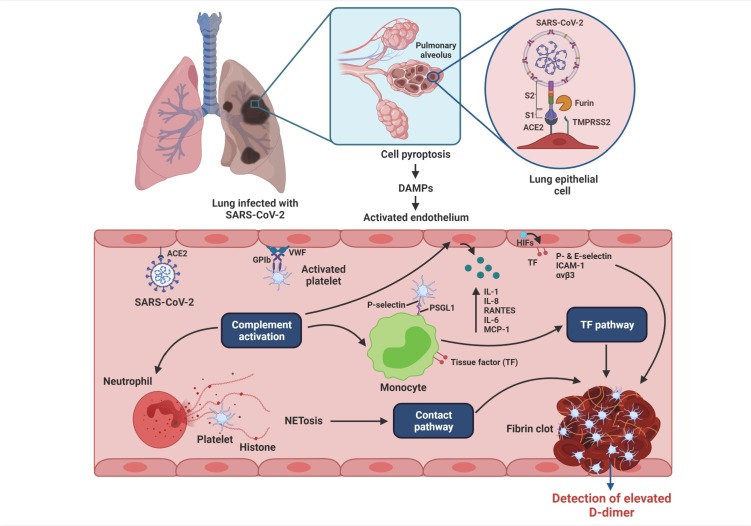
A mechanism for SARS-CoV-2-associated thrombus formation was proposed by McFadyen et al. [16]. Cytokine-activated endothelium upregulates von Willebrand factor, P-selectin, E-selectin, integrin αvβ3, and intercellular adhesion molecule leading to the recruitment of platelets and leukocytes and compliment activation. The inflamed endothelium releases inflammatory cytokines, including IL-8, IL-1, IL-6, RANTES, and monocyte chemoattractant protein-1. Neutrophil-derived NETs cause direct activation of the contact pathway, while the activated complement upregulates endothelial and monocyte tissue factor levels leading to activation of platelets. The hypoxic environment further upregulates endothelial tissue factors through the production of hypoxia-inducible factors. These mechanisms orchestrate an unchecked generation of thrombin, leading to thrombus formation (Fig. 1 ) [16].
Fig. 1.
Proposed mechanisms of COVID-19-associated thrombosis [Redrawn from McFadyen et al. [16], Circulation Research, 127(4), pp.571-587]. SARS-CoV-2 gains entry to host lung epithelial cells by the binding of the transmembrane spike (S) glycoprotein to ACE-2 (angiotensin-converting enzyme 2). The S1 subunit of the S protein binds to ACE-2 and mediates viral attachment. Proteolytic cleavage of the S protein at the S1/2 junction by the proteases, furin, and TMPRSS-2 (transmembrane protease serine 2), facilitates viral entry. SARS-CoV-2 can also directly invade the endothelial cells by binding to ACE-2. Infected cells undergo pyroptosis leading to the release of danger-associated molecular patterns (DAMPs) and triggering the release of proinflammatory cytokines and chemokines. The activated endothelium upregulates the expression of VWF (von Willebrand factor) and adhesion molecules including ICAM (intercellular adhesion molecule)-1, αvβ3, P-selectin and E-selectin leading to recruitment of platelets and leukocytes and complement activation. Neutrophils release neutrophil extracellular traps (NETS), causing direct activation of the contact pathway. Complement activation potentiates these mechanisms by increasing endothelial and monocyte tissue factor (TF), further platelet activation and amplifies endothelial inflammation, which increases production of proinflammatory cytokines from the endothelium including IL (interleukin)-1, IL-8, RANTES (regulated on activation, normal T-cell expressed and secreted), IL-6, and MCP (monocyte chemoattractant protein)-1. The hypoxic environment can induce HIFs (hypoxia-inducible factors) which upregulates endothelial TF expression. These mechanisms ultimately lead to the unchecked generation of thrombin, resulting in thrombus formation. The fibrin degradation product, D-dimer, which is a marker of coagulation activation, appears to be a strong prognostic marker associated with high mortality in patients with COVID-19.
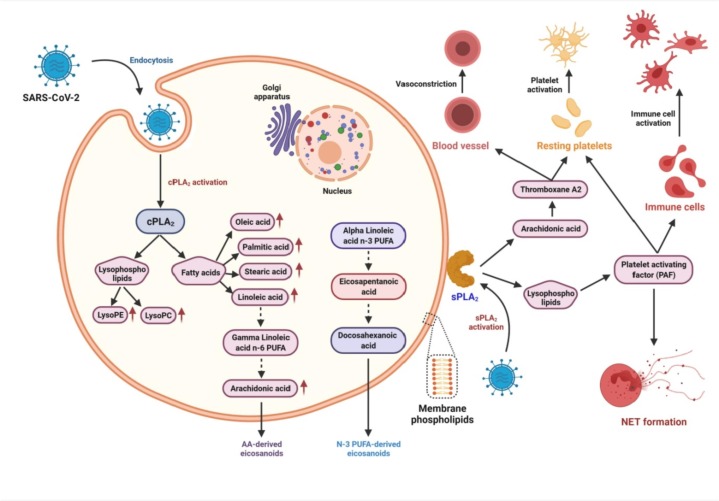
Further, phospholipase A2 (PLA2) has been implicated in SARS-CoV-2 entry and pathogenesis (Fig. 2 ). PLA2 is involved in the early steps of the arachidonic acid (AA) pathway, and platelet-activating factor (PAF) and eicosanoids generated through AA pathway play a crucial role in the context of coagulopathy and thrombosis in COVID-19 [72]. PAF causes platelet activation and aggregation and further releases NET from neutrophils, which can activate platelets and initiate the coagulation cascade that results in thrombosis [73], [74], [75], [76], [77], [78]. Similarly, AA and the eicosanoid thromboxane A2 have platelet-activating effects [72], [79]. Indeed, studies have demonstrated NET markers, hypercoagulability, and increased platelet activity in COVID-19 patients presenting with thrombotic complications [80], [81], [82], [83].
Fig. 2.
SARS-CoV-2 and activation of phospholipase A2s events [Redrawn from Casari et al. [72], Progress in Lipid Research, 82, p.101092]. Pathways activated by cytosolic phospholipase A2 (cPLA2) and secretory phospholipase A2 (sPLA2) potentially involved in virus entry and pathogenesis including COVID-19-associated thrombosis.
Overall, SARS-CoV-2 infects epithelial and endothelial cells that lead to the secretion of proinflammatory cytokines, and the subsequent dysregulated immune system and thromboinflammatory changes damage the vascular and organ systems, including cardiovascular and nervous, renal, liver, gastrointestinal, ocular, and dermatological systems [26].
3. Antithrombotic therapy in COVID-19 patients: Current strategy and key issues
Because of the clinicopathologic reports showing that severe coagulopathy is associated with SARS-CoV-2 infection, the International Society on Thrombosis and Hemostasis (ISTH) has released recent interim guidance about hospital management of coagulopathy in COVID-19 patients [84]. Similarly, quite a few groups of clinicians in close unanimity have developed and prepared guidelines for the diagnosis, treatment and/or prevention of venous thromboembolism (VTE) as well as risk assessment in clinically diagnosed COVID-19 patients [85], [86]. Noteworthy, several other countries may be expected to have similar thrombosis treatment guidelines soon. Table 2 shows the recommended and non-recommended antithrombotic drugs for the treatment of thrombotic complications in COVID-19 patients.
Table 2.
A list of some antithrombotic drugs which are either recommended or not-recommended for treatment and/or prevention of coagulopathy in COVID-19 patients admitted to hospital. The common adverse effects of these drugs are also mentioned.
| Category of drug | Mechanism of action and commercial name | Common adverse effects | Recommendation for the treatment of COVID-19 patient | References |
|---|---|---|---|---|
| 1. Anticoagulants (blood thinner) | A. Direct inhibitor of thrombin | |||
| Argatroban | Life-threatening bleeding complications in patients who receive direct oral anticoagulants (DOACs) [170], liver injury and gastrointestinal disorders [171], universal unobtainability of specific reversal agents [85]. | Recommended for the treatment of circuit thrombosis post LMWH treatment. | [172] | |
| Should be considered in a patient specific manner. | [173] | |||
| Not recommended because of possible adverse drug-to-drug interaction. | [174] | |||
| Low risk of interaction with other drugs. | [87] | |||
| Bivalirudin | -do- | No data available on drug-drug interaction. | [87] | |
| Betrixaban | -do- | -do- | [174] | |
| Dabigatran | -do- | Moderate risk of interaction with other drugs. | [87] | |
| B. Direct inhibitor of factor Xa | ||||
| Apixaban | -do- | Not recommended; high risk of interaction with some drugs. | [87], [174] | |
| Edoxaban | -do- | -do- | [87], [174] | |
| Fondaparinux | -do-, long life-related toxicity [85]. | Not recommended; low to high risk of interaction with some other drugs. However, it is preferred over UHF due to no need to monitor the aPTT which requires patient contact. | [85], [87], [174] | |
| Heparin, unfractionated (UFH) | Non-bleeding complications including heparin-induced thrombocytopenia, skin lesions [175]. | Low risk of interaction with other drugs. Recommended for use in COVID-19 patient with COVID-19 with renal insufficiency. | [85], [87] | |
| Heparin, low molecular weight (LMWH) (enoxaparin, dalteparin) | Same as above but the magnitude of side effect is significantly less. | Recommended for the prevention of thrombotic events and organ damage. Low risk of interaction with other drugs. | [87], [174] | |
| Rivaroxaban | Life-threatening bleeding complications in patients who are receiving direct oral anticoagulants (DOACs) [170], liver injury and gastrointestinal disorders [171]. | Not recommended; high risk of interaction with some drugs. | [87], [174] | |
| C. Vitamin K antagonist | ||||
| Warfarin (dicoumarol) | Narrow therapeutic index, drug-drug and drug-food interactions, requirement of a varied dosing range for maintaining therapeutic international normalized ratio (INR) [85]. | Not recommended for low therapeutic index and interaction with some other drugs and foods; however, recommended for use in COVID-19 patient with renal insufficiency. | [85], [174] | |
| 2. Antiplatelet drugs (inhibitors of platelet aggregation) | Abciximab (inhibitor of platelet αIIbβ3 receptor) | Bleeding complications, thrombocytopenia, and cost of medicine [176]. | Interaction with anticoagulant should be studied. Should be considered in a patient-specific manner. | [87], [173] |
| Aspirin (irreversible inhibitor of ADP receptor of platelet) | Increased risk of bleeding [177]. | Very less study. Does not seem to provide additional benefit to moribund patents. | [90] | |
| Clopidogrel (irreversible inhibitor of ADP P2Y12 receptor of platelet) | Increased risk of bleeding [177]. | Moderate risk of interaction with other drugs, may be used in combination with anticoagulants. However, there is no advantage or disadvantage of use in COVID-19 patient. | [87], [90] | |
| Eptifibatide (Inhibitor of platelet αIIbβ3 receptor) | Bleeding complications, thrombocytopenia, and cost of medicine [176]. | No such direct evidence of its use in COVID-19. However, coronary thrombosis in COVID-19 patients may be unresponsive to optimal pharmacological αIIbβ3 infusion. | [178] | |
| Ticagrelor (inhibitor of ADP P2Y12 receptor) | Increased risk of bleeding [177]. | No current evidence of its therapeutic use in COVID-19 patient; however, high risk of interaction with some other drugs. | [87] | |
| Tirofiban (inhibitor of platelet αIIbβ3 receptor) | Bleeding complications, thrombocytopenia, and cost of medicine [176]. | Same as Eptifibatide. | [178] | |
| Vorapaxar (inhibitor of thrombin-induced platelet aggregation) | Increased bleeding [179]. | No current evidence of its use in treating COVID-19 patient. | ||
| 3. Phosphodiesterase inhibitor | Dipyridamole (inhibitor of nucleoside transport and PDE3) | In-depth studies are warranted to determine its mechanism of action, adverse effects and doses requirements [180]. | Recommended as an adjunct therapy. | [181] |
| 4. Thrombolytic drugs (clot bursting) | Alteplase (tissue plasminogen activator, tPA) | Increased risk of bleeding sometime which is sever or even fatal. Risk of gastrointestinal angioedema is also reported. | Less commonly used. However, recommended in a low dose to critically ill patients even after the treatment with anticoagulants. However, further clinical phase-II studies to determine the safety, efficacy, and optimal dosing of t-PA to treat moderate/severe COVID-19–induced acute respiratory distress syndrome (ARDS) is necessary. | [182], [183], [184] |
| Lumbrokinase (direct fibrinolytic enzyme) | Studies are warranted to know the safety of this drug. | No current evidence of its use in treating COVID-19 patient. | ||
| Nattokinase (fibrinolytic enzyme of bacterial origin) | -do- | -do- | ||
| Reteplase (recombinant non-glycosylated human tPA) | Bleeding complications, stomach pain, vomiting. | Recommended for vascular interventional management of superior mesenteric vein thrombosis. However, more case studies are necessary. | [184], [185] | |
| Streptokinase (plasminogen activator) | Drug allergy, higher rate of hemorrhagic stroke. | Low risk of interaction with other drugs. In a limited trial recommended its use in thrombolysis in pulmonary thrombosis. However, the effectiveness of thrombolysis by this drug should be confirmed in randomized clinical trials. | [87], [186] | |
| Tenecteplase (recombinant tPA | Bleeding at venepuncture sites. Further studies on safety, efficacy, and optimal dosing of this drug are warranted. | Due to acute shortages of Alteplase, use of Tenecteplase as replacement is recommended. However, clinical trials are required. | [184], [187] | |
| Urokinase (directly cleaves plasminogen to produce plasmin) | Bleeding complications, but further clinical studies are necessary. | No such evidence of its therapeutic use. | ||
However, as mentioned below, several vital concerns are raised against the current anticoagulant treatment of COVID-19 patients with thrombotic complications.
-
1.
There is no uniformity in treatment regime or explicit unanimity concerning the timing, dosage, and duration of anticoagulation treatment for the in-patient management of coagulopathy in COVID-19 patients in addition to the need for post-discharge prophylaxis [85].
-
2.
COVID-19 patients receiving antiplatelet agents and anticoagulants risk drug-drug interactions [87].
-
3.
Should COVID-19 patients with disseminated intravascular coagulation (DIC) syndrome (a pathological condition of blood clot formation throughout the body) who do not have bleeding and are devoid of immobility be administered a prophylactic or therapeutic dose of anticoagulation [87].
-
4.
Occurrence of thrombosis is frequently reported in seriously ill COVID-19 patients regardless of the prophylactic usage of low molecular weight heparin (LMWH). Consequently, there is a crucial need for randomized clinical trials, which may be used for selecting the most suitable antithrombotic drugs [88].
-
5.
Some randomized clinical studies have explicitly demonstrated detrimental consequences in COVID-19 patients after treatment with oral anticoagulants [89].
-
6.
Clinical studies have shown that COVID-19 patients treated with therapeutic doses of anticoagulants were devoid of mortality benefits [89].
-
7.
Antiplatelet therapy with ADP receptor antagonists does not provide additional benefit on mortality of COVID-19 patients; however, an enthralling risk of harm is unlikely to be associated with this therapy [90].
Therefore, except for the use of LMWH, there is a lack of practical approaches regarding anticoagulants’ therapeutic use and choice in the clinical management of COVID-19. The use of certain antithrombotic drugs to treat coagulopathy in COVID-19 patients has produced contradictory results (Table 2). Consequently, an extensive drug discovery programme has been suggested to develop efficacious but safe antithrombotic drugs for the treatment and/or prophylaxis of hypercoagulability disorders in COVID-19 patients. In this regard, snake venoms, which contain an arsenal of relatively non-toxic but highly efficacious proteins and polypeptides, may be proposed as a choice for the treatment of thrombosis-associated complications in SARS-CoV-2 infected patients.
4. A brief account on antithrombotic drug prototypes from snake venoms
Snake venom toxins often act synergistically as complexes that aid the snakes in immobilizing, killing, and digesting their prey [91], [92], [93]; however, several of these compounds are non-toxic in their isolated forms [94], [95]. More importantly, they exhibit a wide array of pharmacological effects that can be exploited to develop life-saving drug prototypes for diverse diseases, including thrombotic disorders [96], [97], [98]. It has been observed that toxins present in the venoms of Viperid and Crotalide, and a few snakes of the Elapid family target different components of the hemostatic system, including blood coagulation factors and platelet function, that results in coagulopathy in victims upon envenomation [99], [100]. Nevertheless, it is essential to appreciate that the clinical manifestation of coagulopathy is a concerted consequence of the action of several venom toxins rather than a single protein [91], [92], [93]. Therefore, relatively non-toxic venom proteins and peptides from these families of venomous snakes have been extensively characterized for their potential use as drug prototypes for the treatment and/or prevention of thrombotic disorders. Notably, the primary advantage of these toxins over other cardiovascular drugs is the high binding affinity towards their targets. Since these toxins have naturally evolved to target the components of the hemostatic system, their specificity and selectivity are incomparable [99], [101]. Furthermore, most of these toxins are very potent and can exhibit their pharmacological traits at picomolar to nanomolar concentrations [94], [95].
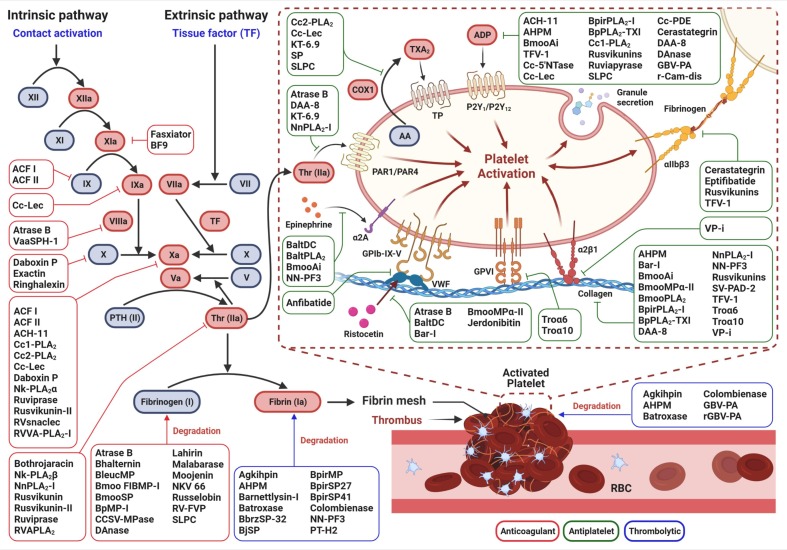
The antithrombotic action of venom toxins can be classified into three major categories – (i) anticoagulant (blood thinner), (ii) antiplatelet, and (iii) thrombolytic (clot bursting), and several proteins and peptides have been characterized from snake venoms in the last decade that target the pathways involved in each category (Table 3 , Fig. 3 ). In the following sections, we discuss some candidate antithrombotic drug prototypes purified from snake venoms for which the mechanism of antithrombotic action has been established.
Table 3.
List of snake venom toxins demonstrating antithrombotic potential.
| Category of drug | Name of the toxin | Snake species | Molecular mass and nature | Mechanism of action | Type of study | References |
|---|---|---|---|---|---|---|
| 1. Anticoagulant (blood thinner) | ACF I | Agkistrodon acutus | 29.6 kDa, homodimeric snaclec | Inhibition of factor Xa and factor IX | In vitro and in vivo | [115] |
| ACF II | Agkistrodon acutus | 29.4 kDa, homodimeric snaclec | Inhibition of factor Xa and factor IX | In vitro and in vivo | [114] | |
| ACH-11 | Agkistrodon acutus | 1.3 kDa, peptide | Inhibition of factor Xa | In vitro and in vivo | [116] | |
| Atrase B | Naja atra | 49.4 kDa, P-III SVMP | Fibrinogenolytic activity, inhibition of coagulation factor VIII | In vitro and in vivo | [105] | |
| Ba SpII RP4 | Bothrops alternatus | 14.2 kDa, PLA2 | Phospholipid hydrolysis | In vitro | [188] | |
| BF9 | Bungarus fasciatus | 6.2 kDa, KSPI | Inhibition of factor XIa | In vitro | [189] | |
| Bhalternin | Bothrops alternatus | 31.5 kDa, SVSP | Degradation of Aα and Bβ chains of fibrinogen | In vitro and in vivo | [125] | |
| BleucMP | Bothrops leucurus | 23.8 kDa, SVMP | Degradation of Aα and Bβ chains of fibrinogen | In vitro and in vivo | [190] | |
| Bmoo FIBMP-I | Bothrops moojeni | 22.8 kDa, SVMP | Degradation of Aα and Bβ chains of fibrinogen | In vitro and in vivo | [191] | |
| BmooSP | Bothrops moojeni | 30 kDa, SVSP | Degradation of Aα and Bβ chains of fibrinogen | In vitro and in vivo | [192] | |
| Bothrojaracin | Bothrops jararaca | 27 kDa, snaclec | Inhibition of thrombin | In vitro and in vivo | [119] | |
| BpMP-I | Bothropoides pauloensis | 23 kDa, SVMP | Degradation of Aα and Bβ chains of fibrinogen | In vitro and in vivo | [193] | |
| Cc1-PLA2 and Cc2-PLA2 | Cerastes cerastes | 13.5 and 13.4 kDa, PLA2 | Inhibition of factor Xa | In vitro | [110] | |
| Cc-Lec | Cerastes cerastes | 34.2 kDa, snaclec | Inhibition of factor Xa and factor IXa | In vitro and in vivo | [104] | |
| CCSV-MPase | Cerastes cerastes | 70 kDa, SVMP | Degradation of Bβ chain of fibrinogen | In vitro and in vivo | [194] | |
| Daboxin P | Daboia russelii | 13.5 kDa, PLA2 | Inhibition of factor X and factor Xa | In vitro and in vivo | [103] | |
| DAnase | Deinagkistrodon acutus | 25 kDa, homodimeric SVSP | Degradation of Aα and Bβ chains of fibrinogen | In vitro and in vivo | [127] | |
| Exactin | Hemachatus haemachatus | 6.6 kDa, 3FTx | Inhibition of factor X | In vitro | [107] | |
| Fasxiator | Bungarus fasciatus | 6.9 kDa, KSPI | Inhibition of factor XIa | In vitro and in vivo | [161] | |
| Lahirin | Naja kaouthia | 6.5 kDa, fibrinogenolytic peptide | Preferential degradation of Aα chain of fibrinogen, followed by Bβ and γ chains | In vitro | [195] | |
| LmrTX | Lachesis muta rhombeata | 14.2 kDa, PLA2 | Phospholipid hydrolysis | In vitro and in vivo | [196] | |
| Malabarase | Trimeresurus malabaricus | 23.4 kDa, SVSP | Degradation of Aα and Bβ chains of fibrinogen | In vitro | [197] | |
| Metalloproteinase SP | Agkistrodon acutus | 22.9 kDa, SVMP | Degradation of Aα, Bβ, and γ chains of fibrinogen | In vitro and in vivo | [198] | |
| Moojenin | Bothrops moojeni | 45 kDa, SVMP | Degradation of Aα and Bβ chains of fibrinogen | In vitro and in vivo | [124] | |
| NEUPHOLIPASE | Daboia russelii | 13.0 kDa, PLA2 | Phospholipid hydrolysis | In vitro and in vivo | [122] | |
| Nk-PLA2α and Nk-PLA2β | Naja kaouthia | 13.4 and 13.2 kDa, PLA2 | Inhibition of factor Xa and thrombin, respectively, and phospholipid hydrolysis | In vitro | [111] | |
| NKV 66 | Naja kaouthia | 66 kDa, SVMP | Degradation of Aα chain of fibrinogen | In vitro | [199] | |
| NnPLA2-I | Naja naja | 15.2 kDa, PLA2 | Phospholipid hydrolysis and inhibition of thrombin | In vitro | [118] | |
| PA11 | Pseudechis australis | 14 kDa, PLA2 | Phospholipid hydrolysis | In vitro and in vivo | [200] | |
| Ringhalexin | Hemachatus haemachatus | 7.4 kDa, 3FTx | Inhibition of factor X | In vitro | [108] | |
| Russelobin | Daboia russelii | 51.3 kDa, SVSP | Degradation of Aα and Bβ chains of fibrinogen | In vitro and in vivo | [126] | |
| Rusvikunin | Daboia russelii | 6.9 kDa, KSPI | Inhibition of fibrinogen clotting and plasma clotting activity of thrombin | In vitro and in vivo | [95] | |
| Rusvikunin-II | Daboia russelii | 7.1 kDa, KSPI | Inhibition of factor Xa and fibrinogen clotting activity of thrombin | In vitro and in vivo | [94] | |
| Ruviprase | Daboia russelii | 4.4 kDa, peptide | Inhibition of thrombin and factor Xa | In vitro and in vivo | [117] | |
| Rv(i) PLA2 | Daboia russelii | 13.6 kDa, PLA2 | Phospholipid hydrolysis | In vitro | [201] | |
| RVAPLA2 | Daboia russelii | 13.8 kDa, PLA2 | Hydrolysis of plasma phospholipids and by non-enzymatic inhibition of thrombin | In vitro and in vivo | [121] | |
| RV-FVP isoforms | Daboia russelii | 32.9-34. kDa, SVSP | Degradation of Aα and Bβ chains of fibrinogen | In vitro and in vivo | [202] | |
| RVsnaclec | Daboia russelii | 66.3 kDa, heterodimeric snaclec | Inhibition of factor Xa | In vitro and in vivo | [113] | |
| RVVA-PLA2-I | Daboia russelii | 58.0 kDa, homodimeric PLA2 | Hydrolysis of plasma phospholipids and by non-enzymatic inhibition of factor Xa | In vitro | [112] | |
| SLPC | Deinagkistrodon acutus | 14 kDa, snaclec-like | Degradation of α, β, and γ chains of fibrinogen | In vitro and in vivo | [203] | |
| VaaSPH-1 | Vipera ammodytes ammodytes | 35 kDa, SVSP | Inhibition of factor VIIIa | In vitro | [106] | |
| 2. Antiplatelet drug candidates (inhibitors of platelet aggregation) | ACH-11 | Agkistrodon acutus | 1.3 kDa, peptide | Inhibition of ADP-induced platelet aggregation | In vitro and in vivo | [116] |
| Agkisacucetin (trade name Anfibatide) | Agkistrodon acutus | 30 kDa, heterodimeric snaclec | Inhibition of GPIb-VWF interaction | In vitro and in vivo | [131] | |
| AHPM | Agkistrodon halys Pallas | 110 kDa, dimeric SVMP | Inhibition of collagen- and ADP-induced platelet aggregation | In vitro and in vivo | [204] | |
| Atrase B | Naja atra | 49.4 kDa, P-III SVMP | Inhibition of ristocetin- and thrombin-induced platelet aggregation, cleavage of integrin GPIb | In vitro and in vivo | [105] | |
| BaltDC | Bothrops alternatus | 32 kDa, SVMP | Inhibition of ristocetin- and epinephrine-induced platelet aggregation | In vitro | [205] | |
| BaltPLA2 | Bothrops alternatus | 14 kDa, PLA2 | Inhibition of ADP- and epinephrine-induced platelet aggregation | In vitro | [206] | |
| Barnettlysin-I | Bothrops barnetti | 23.38 kDa, SVMP | Inhibition of ristocetin-, collagen- and VWF-induced platelet aggregation | In vitro and in vivo | [143] | |
| BmooAi | Bothrops moojeni | 15.2 kDa, homodimeric toxin (unknown class) | Inhibition of epinephrin-, collagen- and ADP-induced platelet aggregation | In vitro | [207] | |
| BmooMPα-II | Bothrops moojeni | 22.5 kDa, SVMP | Inhibition of ristocetin-, collagen- and ADP-induced platelet aggregation | In vitro | [208] | |
| BmooPLA2 | Bothrops moojeni | 13.6 kDa, PLA2 | Inhibition of collagen- and ADP-induced platelet aggregation | In vitro | [209] | |
| BpirPLA2-I | Bothrops pirajai | 13.7 kDa, PLA2 | Inhibition of collagen- and ADP-induced platelet aggregation | In vitro | [210] | |
| BpPLA2-TXI | Bothrops pauloensis | 13.6 kDa, PLA2 | Inhibition of collagen- and ADP-induced platelet aggregation | In vitro | [211] | |
| Cc1-PLA2 and Cc2-PLA2 | Cerastes cerastes | 13.5 and 13.4 kDa, PLA2 | Inhibition of ADP- and arachidonic acid-induced platelet aggregation via targeting P2Y12 and TPα receptors | In vitro | [110] | |
| Cc-5′NTase | Cerastes cerastes | 70 kDa, 5′-NT | Inhibition of ADP-induced platelet aggregation | In vitro and in vivo | [212] | |
| Cc-Lec | Cerastes cerastes | 35.2 kDa, snaclec | Inhibition of ADP-, arachidonic acid- and fibrinogen-induced platelet aggregation | In vitro and in vivo | [104] | |
| Cc-PDE | Cerastes cerastes | 73.5 kDa, PDE | Inhibition of ADP- and ATP-induced platelet aggregation | In vitro and in vivo | [213] | |
| Cerastategrin | Cerastes cerastes | 13.8 kDa, disintegrin | Inhibition of ADP-induced platelet aggregation, integrin αIIbβ3 antagonist | In vitro and in vivo | [134] | |
| DAA-8 | Deinagkistrodon acutus | 1 kDa, peptide | Inhibition of collagen-, thrombin- and ADP-induced platelet aggregation | In vitro and in vivo | [214] | |
| DAnase | Deinagkistrodon acutus | 25 kDa, homodimeric SVSP | Inhibition of ADP-induced platelet aggregation | In vitro and in vivo | [127] | |
| Eptifibatide-PLGA | Sistrurus miliarius barbouri | 0.8 kDa, peptide conjugated to nanoparticles | Integrin αIIbβ3 antagonist | In vitro | [135] | |
| GVB-PA | Gloydius brevicaudus | 32.6 kDa, SVSP | Inhibition of ADP-induced platelet aggregation | In vitro and in vivo | [147] | |
| Jerdonibitin | Trimeresurus jerdonii | 25 kDa, heterodimeric snaclec | Inhibition of ristocetin-induced platelet aggregation, integrin GPIb antagonist | In vitro and in vivo | [215] | |
| KT-6.9 | Naja kaouthia | 6.9 kDa, peptide | Inhibition of arachidonic acid-, thrombin- and ADP-induced platelet aggregation | In vitro | [216] | |
| Metalloproteinase SP | Agkistrodon acutus | 22.9 kDa, SVMP | Inhibition of arachidonic acid-, collagen- and ADP-induced platelet aggregation | In vitro and in vivo | [198] | |
| NKV 66 | Naja kaouthia | 66 kDa, SVMP | Inhibition of collagen- and ADP-induced platelet aggregation | In vitro | [199] | |
| NN-PF3 | Naja naja | 67.81 kDa, SVMP | Inhibition of epinephrine-, collagen- and ADP-induced platelet aggregation | In vitro | [217] | |
| r-Cam-dis | Crotalus adamanteus | 14 kDa, recombinant SVMP | Inhibition of collagen- and ADP-induced platelet aggregation | In vitro | [218] | |
| Rusvikunin and Rusvikunin-II | Daboia russelii | 6.9 and 7.1 kDa, KSPI | Inhibition of collagen- and ADP-induced platelet aggregation, integrin αIIbβ3 antagonist | In vitro | [136] | |
| Ruviapyrase | Daboia russelii | 79.4 kDa, apyrase | Inhibition of ADP-induced platelet aggregation | In vitro | [219] | |
| SLPC | Deinagkistrodon acutus | 14 kDa, snaclec-like | Inhibition of arachidonic acid- and ADP-induced platelet aggregation | In vitro and in vivo | [203] | |
| SV-PAD-2 | Protobothrops elegans | 110 kDa, homodimeric SVMP | Inhibition of collagen- and ADP-induced platelet aggregation | In vitro | [220] | |
| TFV-1 | Trimeresurus flavoviridis | 7.3 kDa, disintegrin | Inhibition of collagen-, thrombin- and ADP-induced platelet aggregation, integrin αIIbβ3 antagonist | In vitro and in vivo | [133] | |
| Troα6 and Troα10 | Tropidolaemus wagleri | 0.78 and 1.15 kDa, peptides from the snaclec Trowaglerix | Inhibition of collagen-induced platelet aggregation, integrin GPVI antagonist | In vitro and in vivo | [139] | |
| VP-i | Vipera palaestinae | Hetero-oligomer complex of 13, 17, 19 and 20 kDa snaclecs | Inhibition of collagen-induced platelet aggregation, integrin α2β1 antagonist | In vitro | [132] | |
| 3. Thrombolytic /fibrinolytic agents (clot bursting) | Agkihpin | Gloydius halys Pallas | 25.5 kDa, SVSP | Fibrin(ogen)olytic enzyme by cleaving Aα and Bβ chains of fibrinogen and fibrin. Inhibits in vivo thrombus formation in murine models | In vitro and in vivo | [146] |
| AHPM | Agkistrodon halys Pallas | 110 kDa, dimeric SVMP | Fibrin(ogen)olytic and thrombolytic activities | In vitro and in vivo | [204] | |
| Barnettlysin-I | Bothrops barnetti | 23.386 kDa, single-chain SVMP | Dissolves fibrin clots and devoid of haemorrhagic activity | In vitro | [143] | |
| Batroxase | Bothrops atrox | 22.9 kDa, P1-SVMP | Cleaves both Aα and Bβ-chains of the fibrinogen, dissolve fibrin clots, and shows plasminogen activation, in vitro thrombolytic activity whereas in vivo it shows thrombolytic activity in a concentration-dependent manner. | In vitro and in vivo | [140], [141] | |
| BbrzSP-32 | Bothrops brazili | 32.52 kDa, SVSP | Fibrinolytic activity and degrades the Aα-chain of fibrinogen | In vitro | [221] | |
| BjSP | Bothrops jararaca | 28.0 kDa, SVSP | Degrading fibrin clots the Aα and Bβ chains of fibrinogen | In vitro | [222] | |
| BpirMP | Bothrops pirajai | 23.1 kDa, P-1 SVMP | Fibrin(ogen)olytic and thrombolytic activities | In vitro | [144] | |
| BpirSP27 and BpirSP41 | Bothrops pirajai | 27.12 and 40.64 kDa, respectively. SVSP | Degrade fibrin and blood clots | In vitro | [223] | |
| Colombienase-1 and 2 | Bothrops colombiensis | 25.0 kDa, SVMP | A direct acting fibrinolytic enzyme without activating the fibrinolytic system (plasminogen/plasmin), but devoid of hemorrhagic, hemolytic, cytotoxic, plasminogen activation and coagulant activities. A promising thrombolytic agent | In vitro and in vivo | [142] | |
| GBV-PA | Gloydius brevicaudus | 32.6 kDa, SVSP | Exhibits thrombolytic, plasminogen activation, and antiplatelet activity. Does not show fibrinolytic activity. | In vitro and In vivo | [147] | |
| rGBV-PA | Gloydius brevicaudus | 32.6 kDa, recombinant SVSP (plasminogen activator) | rGBV-PA, like tPA, can prevent the formation of thromboses in the inferior vena cava of rats. This is devoid of hemorrhagic activity | In vitro and in vivo | [148] | |
| NN-PF3 | Naja naja | 67.81 kDa, SVMP | Fibrinolytic activity and preferentially hydrolysed the alpha polymer of fibrin | In vitro and in vivo | [145] | |
| PT-H2 protease | Protobothrops tokarensis | 22.5 kDa, non hemorrhagic SVMP | Potent fibrinolytic activity | In vitro | [224] |
Fig. 3.
A schematic diagram of the different components of the hemostatic system that are affected by snake venom proteins with antithrombotic potential. Venom components exhibit antithrombotic action by virtue of their anticoagulant, antiplatelet, and thrombolytic activities. Anticoagulant venom proteins can inhibit one or more coagulation factors of the blood coagulation cascade. Antiplatelet agents suppress platelet aggregation induced by agonist, or via interaction with platelet receptors (integrins) that blocks platelet activation and aggregation. Thrombolytic venom proteins can cause dissolution of fibrin or blood clots.
4.1. Anticoagulant drug prototypes from snake venom
The anticoagulant mechanism of snake venom toxins is primarily attributed to the non-enzymatic inhibition of one or more blood clotting factors. However, phospholipid hydrolysis by PLA2 enzymes and fibrinogenolysis by proteases (snake venom metalloprotease, SVMP, and snake venom serine protease, SVSP) also result in an anticoagulant action (Fig. 3). It has been observed that the anticoagulant action of many venom toxins is through targeting factor Xa and/or thrombin, the key components of the blood coagulation cascade. The tenase complex consisting of tissue factor, factor VIIa, and Ca2+ (extrinsic pathway), or factor IXa and factor VIIa (intrinsic pathways) converts the inactivated factor X to Xa [102], and several venom toxins primarily from the PLA2 (Daboxin P), snaclec (Cc-Lec), protease (Atrase B, VaaSPH-1), and three-finger toxin (Exactin, Ringhalexin) families attenuate this activation [103], [104], [105], [106], [107], [108]. As a result, the coagulation cascade is stalled, and the formation of the prothrombinase complex is abrogated, resulting in blood anticoagulation. Similarly, the prothrombinase complex that comprises the serine protease, factor Xa, a cofactor Va, and Ca2+ converts the inactive zymogen prothrombin (factor II) to thrombin (factor IIa) [109]. This first committed step is very critical in the blood coagulation cascade for thrombus formation, and venom proteins from PLA2 (Cc1-PLA2, Cc2-PLA2, Daboxin P, Nk-PLA2α, RVVA-PLA2-I), Kunitz-type serine protease inhibitors (KSPI; Rusvikunin II), and snaclec (ACF isoforms, Cc-Lec, RVsnaclec) families, and a few peptides (ACH-11, Ruviprase) inhibit the catalytic activation of prothrombin [94], [103], [104], [110], [111], [112], [113], [114], [115], [116], [117]. Consequently, thrombin, which catalyzes fibrin clot formation from fibrinogen, is hindered, resulting in inhibition of thrombus formation.
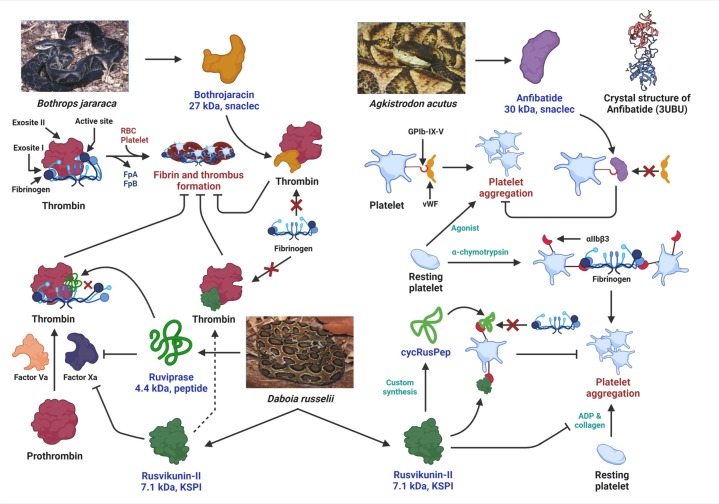
Several venom toxins such as Bothrojaracin (snaclec), Nk-PLA2β, NnPLA2-I and RVAPLA2 (PLA2), Rusvikunin, and Rusvikunin II (KSPI), and the peptide Ruviprase also inhibit fibrinogen clotting activity by directly binding to thrombin [94], [95], [111], [117], [118], [119]. Thrombin has three functional sites – an active site with serine protease activity, a fibrinogen-binding exosite I, and the heparin-binding exosite II [120]. The snaclec Bothrojaracin from the venom of Bothrops jararaca binds to exosite I of thrombin and, in turn, impairs the binding of fibrinogen to thrombin. Consequently, fibrinogen is not accessible to thrombin for catalysis, and fibrin clot formation is inhibited (Fig. 4 ) [119]. Notably, Ruviprase, a 4.4 kDa peptide, and Rusvikunin II, a 7.1 kDa KSPI from the venom of Russell's Viper, exhibit dual inhibition of thrombin and factor Xa, and are additional examples of potential anticoagulant drug prototypes (Fig. 4) [94], [117]. Thrombin and factor Xa, which catalyze the penultimate steps of the blood coagulation cascade, are crucial druggable targets for the prevention and/or cure of thrombosis. However, commercial thrombin inhibitors such as Argatroban, Dabigatran, etc., and factor Xa inhibitors including heparin, Fondaparinux are associated with life-threatening bleeding complications and other side-effects (Table 2). Under such circumstances, thrombin and factor Xa inhibitors derived from snake venoms capable of acting at a nanomolar concentration and possessing extreme target specificity have the potential to be developed as better anticoagulant agents for the prevention of COVID-19-associated thrombosis.
Fig. 4.
Antithrombotic mechanisms of Bothrojaracin, Anfibatide, Rusvikunin-II and Ruviprase. Bothrojaracin binds to exosite I of thrombin and interferes with the binding of fibrinogen to thrombin. Consequently, fibrinogen is not accessible to thrombin for catalysis and fibrin clot formation is inhibited. Anfibatide binds to the GPIb-IX-V platelet receptor and inhibits the association of vWF with this platelet receptor which subsequently inhibits platelet aggregation. Ruviprase and Rusvikunin II exhibit dual inhibition of thrombin and factor Xa. While Ruviprase binds to the active site of thrombin, Rusvikunin II occupies thrombin exosite I and inhibits the conversion of fibrinogen to fibrin. In addition, the synthetic peptide cycRusPep derived from Rusvikunin II binds to the αIIbβ3 receptor and inhibits platelet aggregation mediated by the association of fibrinogen to this receptor.
Apart from inhibiting blood coagulation factors, phospholipid hydrolysis by PLA2 enzymes also contributes to their anticoagulant action [112], [121], [122]. Phospholipids play an essential role in the blood coagulation cascade and are required during the catalytic conversion of prothrombin to thrombin [109]. Therefore, most of the PLA2 enzymes exhibit anticoagulant action by their plasma phospholipid hydrolytic activity. Notably, concomitant non-enzymatic inhibition of other blood coagulation factors by these PLA2 enzymes (as described above) potentiates their anticoagulant property. In addition, snake venom proteases including SVMPs (Atrase B, BpMP-I, Moojenin, etc.) and SVSPs (Bhalternin, DAnase, Russelobin) demonstrate preferential degradation of the Aα and/or Bβ chains of fibrinogen [105], [123], [124], [125], [126], [127]. Consequently, due to the unavailability of functional fibrinogen molecules in the bloodstream, fibrin clot formation is impaired, resulting in blood anticoagulation.
4.2. Antiplatelet agents from snake venom
While platelet activation and aggregation is an integral step in thrombus formation, to date, their role in COVID-19 remains unclear. Thrombocytopenia has been reported in several COVID-19 patients [128], [129], whereas platelet count and size were average in others [80], [130]. Increased platelet activation has also been observed in severe COVID-19 patients but not those with mild symptoms [80], [81]. Furthermore, platelets from COVID-19 patients demonstrated increased adhesion and spreading on fibrinogen [80], a primary ligand for αIIbβ3. Notably, several snake venom toxins inhibit platelet aggregation induced by an array of agonists, including ADP, thrombin, collagen, epinephrine, and ristocetin (Fig. 3). While a majority of the toxins belonging to SVMP, SVSP, PLA2, nucleotidase, and KSPI snake venom protein families suppress platelet aggregation induced by the above agonist, disintegrins and snaclecs can specifically interact with platelet receptors (integrins) and block platelet activation and aggregation. For example, Agkisacucetin (trade name Anfibatide), a homodimeric snaclec of 30 kDa isolated from the venom of Agkistrodon acutus binds to the GPIb-IX-V platelet receptor and inhibits the association of vWF with this platelet receptor (Fig. 4) [131]. Similarly, the hetero-oligomeric snaclec VP-i is an integrin α2β1 (collagen receptor) antagonist that inhibits collagen-induced platelet aggregation [132]. In addition, the disintegrins TFV-1 (purified from Trimeresurus flavoviridis venom) and Cerastategrin (purified from Cerastes cerastes venom), and disintegrin derivatives Tirofiban (a peptide mimetic from Echis carinatus venom), Eptifibatide (a peptide from the venom of Sistrurus miliarius barbouri), and Rusvikunins from Daboia russelii venom (Fig. 4) act as integrin αIIbβ3 antagonist and block the platelet-platelet adhesion mediated by binding of fibrinogen to the αIIbβ3 receptor [133], [134], [135], [136], [137]. Notably, while disintegrins interact with platelet receptors primarily via a conserved RGD motif [138], the binding of Rusvikunins and their peptides (RusPep and cycRusPep) to the αIIbβ3 receptor was found to be RGD-independent [136]. Furthermore, the Troα6 and Troα10 peptides derived from the snaclec Trowaglerix (isolated from the venom of Tropidolaemus wagleri) function as antagonists to integrin GPVI, the primary collagen receptor and thereby inhibit collagen-induced platelet aggregation [139]. These antagonistic activities of the snake venom components on different platelet receptors affect platelet activation that ultimately inhibits thrombus formation; therefore, these venom-derived toxins are potential antithrombotic agents.
4.3. Thrombolytic proteins from snake venom
The thrombolytic potential of snake venom components, especially the weak or non-hemorrhagic SVMPs, and SVSPs are well appreciated. Among them, the PI-SVMP Batroxase purified from Bothrops atrox venom deserves special attention. Under in vitro conditions, the weakly hemorrhagic PI-SVMP Batroxase directly dissolved fibrin clots without activating the endogenous fibrinolytic system (plasminogen/plasmin), whereas, in an animal model of venous thrombosis, the PI-SVMP exhibited potent antithrombotic activity [140], [141]. Other weak or non-hemorrhagic PI-SVMPs, including Colombienase-1 and 2, Barnettlysin-I, and BpirMP, have also demonstrated potent fibrinolysis under in vitro conditions [142], [143], [144]; however, these studies lack in vivo evidence of their thrombolytic potential.
A non-hemorrhagic high molecular weight (67.8 kDa) PIII-SVMP NN-PF3 purified from the venom of Naja naja exhibited potent fibrinolytic activity under in vitro conditions and caused defibrinogentaion, prolongation of bleeding time, and reduction in the blood fibrinogen level in the Swiss albino mice model [145]. Apart from the SVMPs mentioned above, the in vivo thrombolytic potential of a few SVSPs was also demonstrated in rat and canine thrombosis models. For example, Agkihpin, a 25.5 kDa SVSP isolated from the venom of Gloydius halys exhibited fibrinolysis under in vitro conditions and reduced thrombin-induced venous thrombosis in a Sprague Dawley rat model [146]. Another SVSP GBV-PA, a 32.6 kDa plasminogen activator from the venom of Gloydius brevicaudus exhibited hydrolysis of rabbit blood clots under in vitro conditions. More importantly, intravenous injection of GBV-PA significantly reduced the thrombus weight and length in rat inferior vena cava (thrombin-induced venous thrombosis model) and ferric chloride-induced carotid artery thrombosis models. Furthermore, GBV-PA demonstrated antithrombotic potential in a canine (dog) acute ischemia-reperfusion stroke model by augmenting arterial thrombosis recanalization rates. Notably, GBV-PA was reported with a lower hemorrhagic effect and a longer antithrombotic half-life than tissue plasminogen activator and urokinase [147].
Considering the tremendous potential yet low abundance of this snake venom plasminogen activator, the venom toxin was cloned into a prokaryotic expression vector (pET-42a) and expressed in Escherichia coli. The recombinant molecule (rGBV-PA) was functional and it replicated the pharmacological activities exhibited by the parent toxin [148], suggesting the prospect of utilizing recombinant DNA technology for the large-scale production of therapeutically important snake venom toxins. Moreover, considering the life-threatening bleeding complications associated with current clot bursting drugs such as Alteplase, Urokinase, and Streptokinase (Table 2), the development of snake venom-derived thrombolytic agents against COVID-19-related cardiovascular complications seems promising.
5. Strategies for augmenting the therapeutic application and commercialization of antithrombotic proteins and peptides derived from snake venoms: a road map
5.1. An update of the status of a few snake venom toxins showing antithrombotic property
Despite the plethora of antithrombotic drug candidates characterized from snake venom, a considerable gap exists between preliminary in vitro and pre-clinical laboratory findings and their translation for clinical applications, mainly due to cost, time, efficacy, and long term side-effects [98]. Nevertheless, a few snake venom toxins or their derived counterparts with antithrombotic potential have completed rigorous pre-clinical and clinical investigations and are approved by the US Food and Drug Administration (FDA) for commercialization. Table 4 summarizes the commercialization status or clinical trials of a few venom toxins and their derivatives showing antithrombotic properties. Notably, with the advancement in drug development technologies, significant effort has been dedicated to improving and successfully commercializing antithrombotic snake venom agents.
Table 4.
Current status of commercialization or clinical trials of snake venom-based antithrombotic agents.
| Snake venom toxins/peptides | Nature of molecule | Source snake species | Therapeutic target | Current status (FDA approved/clinical trial) |
|---|---|---|---|---|
| Aggrastat (Tirofiban) | An analog of venom disintegrin | Echis carinatus | Integrin αIIbβ3 antagonist | FDA approved |
| Anfibatide | Snaclec | Agkistrodon acutus | Inhibitor of GPIb-VWF interaction | Phase 2 clinical trial |
| Captopril | Synthetic analog of an ACE-inhibiting peptide | Bothrops jararaca | Angiotensin-converting enzyme inhibitor | FDA approved |
| Defibrase/Reptilase (Batroxobin) | Thrombin-like enzyme | Bothrops atrox and B. moojeni | Defibrinogenation | FDA approved |
| Enalapril (Vasopril) | Synthetic analog of an ACE-inhibiting peptide | Bothrops jararaca | Angiotensin-converting enzyme inhibitor | FDA approved |
| Integrilin (Eptifibatide) | Peptide | Sistrurus miliarus | Integrin αIIbβ3 antagonist | FDA approved |
5.2. Commercialization strategies for antithrombotic snake venom proteins and peptides
5.2.1. Large-scale production of snake venom antithrombotic proteins by recombinant DNA technology will improve their commercial potential
Isolation and purification of many snake venom enzymes and proteins for their commercial application are cumbersome and costly and may not be feasible because of the scarcity of commercial snake venoms. However, the production of a large number of enzymes and the antithrombotic efficacy can be achieved by recombinant DNA technology and site-directed mutagenesis, respectively.
Boldrini-França et al. (2015) reported the heterologous expression of an SVSP named collinein-1 from the venom of Crotalus durissus collilineatus in Pichia pastoris (methyltropic yeast) that resulted in the production of 56 mg/L of recombinant collinein-1 (rCollinein-1), and importantly, retained its functional integrity to hydrolyze bovine fibrinogen. A subsequent study has shown that rCollinein-1 may have therapeutic potential in preventing thrombus formation [149]. An acute and repeated dose (28 days) toxicity study of a recombinant thrombin-like defibrinogenating enzyme, batroxobin expressed in Pichia pastoris, showed no adverse effects at a dose of 2.5 NIH u/kg in rats and 1 NIH u/kg in dogs, indicating clinical potential in the treatment of hemostatic disturbances [150]. In another approach, a novel thrombin-like enzyme with fibrinogenolytic activity from Deinagkistrodon acutus venom was expressed in soluble form in E. coli. The recombinant enzyme retained its biological activity, reinforcing its large-scale industrial production for commercial exploitation as a therapeutic molecule [151].
5.2.2. Synthetic and peptidomimetics approaches for snake venom antithrombotic molecules to improve their commercialization
Several of the low molecular mass polypeptides from snake venoms (see Table 3) have demonstrated appreciable antithrombotic activity. With the advancement of cost-effective automated protein synthesis technology, a synthetic biology approach for the production of antithrombotic proteins and enzymes will overcome the cumbersome task of isolating and purifying protein/polypeptide from natural resources [152], [153], [154]. A similar approach may also be applied for the production of antithrombotic snake venom proteins and polypeptides.
A small peptide that has been designed to mimic a particular region of a protein or enzyme yet displays its biological function is known as a peptidomimetic. Several of such peptidomimetic inhibitors that target blood coagulation factors and platelet receptors or regulate various stages of the coagulation cascade to produce an antithrombotic effect have been projected as drug prototypes to treat thrombotic disorders [155], [156], [157], [158]. The peptidomimetics of a small stretch of snake venom toxins that target and inhibit the function of blood coagulation factors and/or platelet receptors [136] will be a unique approach for inventing improved antithrombotic drug prototypes to treat coagulation disorders in COVID-19.
5.3. Augmenting the antithrombotic efficacy and oral delivery of recombinant antithrombotic proteins
5.3.1. Site-directed mutagenesis for enhancing the antithrombotic potency of recombinant proteins
Improving the efficacy of snake venom antithrombotic proteins can be achieved via a site-directed mutagenesis approach. Results of several experiments have demonstrated enhanced antithrombotic efficacy of proteins by site-directed mutagenesis, which is considered a new approach for treating or preventing acute thrombotic and thromboembolic conditions [159], [160]. Similarly, it has been demonstrated that site-specific mutagenesis of Fasxiator, a KSPI from Bungarus fasciatus venom, increased its potency to inhibit factor XIa both in vitro and in vivo conditions by approximately 1000 times [161]. The authors suggest that the development of Fasxiator as a novel anticoagulant candidate seems promising. A review article also highlighted the importance of recombinant and chimeric snake venom disintegrins in preclinical research [162]. Therefore, it is anticipated that there is enough scope for the improvement of therapeutic efficiency and potency of other snake venom antithrombotic molecules by in silico alanine scanning mutagenesis, followed by structural stability analysis of native protein, heterologous expression of mutated recombinant protein in a suitable host, and finally comparing the potency of the mutated recombinant protein with that of the native protein. Such improved antithrombotic proteins will have more commercialization potential.
5.3.2. Improvement of therapeutic efficiency by combination of two antithrombotic drugs
In treated animals, a therapeutic combination of two antithrombotic drugs demonstrated improvement of broad preclinical efficacy against thrombotic disorders without enhancing bleeding complications [163]. A similar approach may also be adopted by combining two or more snake venom antithrombotic drugs to enhance their antithrombotic potency.
5.3.3. Nanoparticle conjugation and PEGylation of snake venom antithrombotic drugs to improve their efficacy as well as oral delivery
Applying a targeted nanoparticulate drug delivery system to improve the therapeutic efficacy of antithrombotic drugs has gained significant attention in recent years. Strategies such as nanoencapsulation, delivery via liposomes or inorganic nanoparticles, and PEGylation have been extensively investigated to deliver antithrombotic agents (Fig. 5 ). In a thrombus-targeting therapy approach, it has been demonstrated that activated platelet-homing liposomes containing tissue plasminogen activator (tPA) and cRGD peptides exhibit improved thrombolytic effects of tPA with low toxicity risk [164]. Mechanistically, upon incubation with activated platelets, cRGD peptides interacted with the platelet receptor integrin αIIbβ3, which resulted in the fusion of platelet membrane and liposomes and a subsequent release of tPA within one hour of incubation (Fig. 5) [164]. Inorganic nanoparticles such as mesoporous silica, gold, and carbon nanotubes have also been tested for cellular delivery to enhance the bioavailability and circulation time of antithrombotic drugs [165].
Fig. 5.
Improvement of efficacy and targeted delivery of antithrombotic agents using diverse strategies. A. Nanoencapsulation of venom proteins/peptides aids effective delivery to the target. B. Activated platelet-homing liposomes harboring tissue plasminogen activator (tPA) and cRGD peptides exhibit targeted delivery and improved thrombolytic effects of tPA with low toxicity risk. C. PEGylated [KGDRR]trimucrin (an αIIbβ3 antagonist) inhibits fibrinogen-induced platelet aggregation at an IC50 value ~4 times lower and at a half-life ~1.3 fold longer than the parent molecule.
PEGylation, i.e. the process of attaching polyethylene glycol (PEG) to molecules and macrostructures, is yet another widely used approach to enhance the systemic circulation time of antithrombotic agents. Kuo et al. demonstrated that the PEGylated antiplatelet disintegrin derivative [KGDRR]trimucrin (an αIIbβ3 antagonist purified from the venom of Trimeresurus mucrosquamatus) inhibited platelet aggregation at an IC50 value which was ~4 times lower than the parent molecule. Furthermore, the in vivo half-life of the PEGylated form was marginally longer (~1.3 fold) than [KGDRR]trimucrin (Fig. 5) [166]. Apart from increasing the circulation time of antithrombotic drugs, nanoparticle-based polymers have been employed to enhance the bioavailability of drugs via the oral route. For example, the oral bioavailability of heparin conjugated to hydroxypropyl methylcellulose phthalate (HPMCP)-modified nanoparticles was ~2.5 fold higher than in pure nanoparticles [167]. Therefore, considering the remarkable progress in developing antithrombotic nanomedicines in recent years [165], [168], [169], application of a targeted nanoparticulate drug delivery system to improve the antithrombotic efficiency of snake venom toxins seems promising.
6. Conclusion
SARS-CoV-2 can result in adverse health conditions among infected individuals around the world. Apart from the common respiratory illness, increasing reports have pointed towards cardiovascular disorders in COVID-19 patients. Although several drugs have been approved for the treatment and/or prevention of cardiovascular disorders, the clinical management of COVID-19-associated thrombosis has become challenging in terms of use, dose, and choice of anticoagulants. Under these circumstances, developing antithrombotic agents from snake venoms with high specificity and lower toxicity for the prevention and/or treatment of cardiovascular disorders in COVID-19 patients seems promising. Further, selecting an appropriate delivery system will augment the antithrombotic potential and bioavailability of such candidate snake venom molecules.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
Acknowledgement
BK acknowledges the financial support from the DBT-RA Program in Biotechnology and Life Sciences. Figures were created with BioRender under a paid subscription.
Data and materials availability
All data associated with this study are present in the paper.
References
- 1.Fauci A.S., Lane H.C., Redfield R.R. Covid-19 - navigating the uncharted. N. Engl. J. Med. 2020;382(13):1268–1269. doi: 10.1056/NEJMe2002387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO Pneumonia of unknown cause – China. Disease outbreak news, 5 January 2020. Geneva. 2020. www.who.int/csr/don/05january-2020-pneumonia-of-unkown-cause-china/en/
- 3.Wu F., Zhao S., Yu B., Chen Y.M., Wang W., Song Z.G., Hu Y., Tao Z.W., Tian J.H., Pei Y.Y., Yuan M.L., Zhang Y.L., Dai F.H., Liu Y., Wang Q.M., Zheng J.J., Xu L., Holmes E.C., Zhang Y.Z. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma J. Coronavirus: China’s first confirmed Covid-19 case traced back to November 17, South China Morning Post, 13 March 2020. 2020. www.scmp.com/news/china/society/article/3074991/coronavirus-chinas-first-confirmed-covid-19-casetraced-back
- 5.Pekar J., Worobey M., Moshiri N., Scheffler K., Wertheim J.O. Timing the SARS-CoV-2 index case in Hubei province. Science. 2021;372(6540):412–417. doi: 10.1126/science.abf8003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Munster V.J., Koopmans M., van Doremalen N., van Riel D., de Wit E. A novel coronavirus emerging in China - key questions for impact assessment. N. Engl. J. Med. 2020;382(8):692–694. doi: 10.1056/NEJMp2000929. [DOI] [PubMed] [Google Scholar]
- 7.Sohrabi C., Alsafi Z., O'Neill N., Khan M., Kerwan A., Al-Jabir A., Iosifidis C., Agha R. World Health Organization declares global emergency: a review of the 2019 novel coronavirus (COVID-19) Int. J. Surg. 2020;76:71–76. doi: 10.1016/j.ijsu.2020.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO WHO timeline - COVID-19. 2020. https://www.who.int/news/item/29-06-2020-covidtimeline
- 9.Cucinotta D., Vanelli M. WHO declares COVID-19 a pandemic. 2020;91(1):157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO WHO Coronavirus (COVID-19) Dashboard. 2021. https://covid19.who.int/
- 11.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., Zhao Y., Li Y., Wang X., Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan,China. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zaim S., Chong J.H., Sankaranarayanan V., Harky A. COVID-19 and multiorgan response. Curr. Probl. Cardiol. 2020;45(8) doi: 10.1016/j.cpcardiol.2020.100618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rudrapal M., Khairnar S.J., Borse L.B., Jadhav A.G. Coronavirus Disease-2019 (COVID-19): an updated review. Drug Res. 2020;70(9):389–400. doi: 10.1055/a-1217-2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levi M., Thachil J., Iba T., Levy J.H. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;7(6):e438–e440. doi: 10.1016/S2352-3026(20)30145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McFadyen J.D., Stevens H., Peter K. The emerging threat of (micro) thrombosis in COVID-19 and its therapeutic implications. Circ. Res. 2020;127(4):571–587. doi: 10.1161/CIRCRESAHA.120.317447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shen B. A new Golden age of natural products drug discovery. Cell. 2015;163(6):1297–1300. doi: 10.1016/j.cell.2015.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harvey A.L. Natural products in drug discovery. Drug Discov. Today. 2008;13(19–20):894–901. doi: 10.1016/j.drudis.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 19.Matsui T., Fujimura Y., Titani K. Snake venom proteases affecting hemostasis and thrombosis. Biochim. Biophys. Acta. 2000;1477(1–2):146–156. doi: 10.1016/s0167-4838(99)00268-x. [DOI] [PubMed] [Google Scholar]
- 20.Yamazaki Y., Morita T. Snake venom components affecting blood coagulation and the vascular system: structural similarities and marked diversity. Curr. Pharm. Des. 2007;13(28):2872–2886. doi: 10.2174/138161207782023775. [DOI] [PubMed] [Google Scholar]
- 21.King G.F. Venoms as a platform for human drugs: translating toxins into therapeutics. Expert. Opin. Biol. Ther. 2011;11(11):1469–1484. doi: 10.1517/14712598.2011.621940. [DOI] [PubMed] [Google Scholar]
- 22.Koh C.Y., Kini R.M. From snake venom toxins to therapeutics–cardiovascular examples. Toxicon. 2012;59(4):497–506. doi: 10.1016/j.toxicon.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 23.Saviola A.J., Peichoto M.E., Mackessy S.P. Rear-fanged snake venoms: an untapped source of novel compounds and potential drug leads. Toxin Rev. 2014;33(4):185–201. [Google Scholar]
- 24.Mohamed Abd El-Aziz T., Soares A.Garcia, Stockand J.D. Snake venoms in drug discovery: valuable therapeutic tools for life saving. Toxins. 2019;11(10) doi: 10.3390/toxins11100564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalita B., Saviola A.J., Mukherjee A.K. From venom to drugs: a review and critical analysis of indian snake venom toxins envisaged as anticancer drug prototypes. Drug Discov. Today. 2021;26(4):993–1005. doi: 10.1016/j.drudis.2020.12.021. [DOI] [PubMed] [Google Scholar]
- 26.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., Mehra M.R., Schuepbach R.A., Ruschitzka F., Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levi M., Scully M. How I treat disseminated intravascular coagulation, blood, the journal of the american society of. Hematology. 2018;131(8):845–854. doi: 10.1182/blood-2017-10-804096. [DOI] [PubMed] [Google Scholar]
- 28.Danzi G.B., Loffi M., Galeazzi G., Gherbesi E. Acute pulmonary embolism and COVID-19 pneumonia: a random association? Eur. Heart J. 2020;41(19):1858. doi: 10.1093/eurheartj/ehaa254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y., Xiao M., Zhang S., Xia P., Cao W., Jiang W., Chen H., Ding X., Zhao H., Zhang H., Wang C., Zhao J., Sun X., Tian R., Wu W., Wu D., Ma J., Chen Y., Zhang D., Xie J., Yan X., Zhou X., Liu Z., Wang J., Du B., Qin Y., Gao P., Qin X., Xu Y., Zhang W., Li T., Zhang F., Zhao Y., Li Y., Zhang S. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N. Engl. J. Med. 2020;382(17) doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klok F.A., Kruip M., van der Meer N.J.M., Arbous M.S., Gommers D., Kant K.M., Kaptein F.H.J., van Paassen J., Stals M.A.M., Huisman M.V., Endeman H. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poissy J., Goutay J., Caplan M., Parmentier E., Duburcq T., Lassalle F., Jeanpierre E., Rauch A., Labreuche J., Susen S., Lille ICU Haemostasis COVID-19 Group Pulmonary embolism in patients with COVID-19: awareness of an increased prevalence. Circulation. 2020;142(2):184–186. doi: 10.1161/CIRCULATIONAHA.120.047430. [DOI] [PubMed] [Google Scholar]
- 32.Léonard-Lorant I., Delabranche X., Séverac F., Helms J., Pauzet C., Collange O., Schneider F., Labani A., Bilbault P., Molière S. Acute pulmonary embolism in patients with COVID-19 at CT angiography and relationship to D-dimer levels. Radiology. 2020;296(3):E189–E191. doi: 10.1148/radiol.2020201561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oxley T.J., Mocco J., Majidi S., Kellner C.P., Shoirah H., Singh I.P., De Leacy R.A., Shigematsu T., Ladner T.R., Yaeger K.A. Large-vessel stroke as a presenting feature of Covid-19 in the young. N. Engl. J. Med. 2020;382(20) doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ranucci M., Ballotta A., Di Dedda U., Bayshnikova E., Dei Poli M., Resta M., Falco M., Albano G., Menicanti L. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J. Thromb. Haemost. 2020;18(7):1747–1751. doi: 10.1111/jth.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Panigada M., Bottino N., Tagliabue P., Grasselli G., Novembrino C., Chantarangkul V., Pesenti A., Peyvandi F., Tripodi A. Hypercoagulability of COVID-19 patients in intensive care unit: a report of thromboelastography findings and other parameters of hemostasis. J. Thromb. Haemost. 2020;18(7):1738–1742. doi: 10.1111/jth.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li T., Lu H., Zhang W. Clinical observation and management of COVID-19 patients. Emerg. Microbes Infect. 2020;9(1):687–690. doi: 10.1080/22221751.2020.1741327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin L., Lu L., Cao W., Li T. Hypothesis for potential pathogenesis of SARS-CoV-2 infection–a review of immune changes in patients with viral pneumonia. Emerg. Microbes Infect. 2020;9(1):727–732. doi: 10.1080/22221751.2020.1746199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou M., Zhang X., Qu J. Coronavirus disease 2019 (COVID-19): a clinical update. Front. Med. 2020;14(2):126–135. doi: 10.1007/s11684-020-0767-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sebag S.C., Bastarache J.A., Ware L.B. Therapeutic modulation of coagulation and fibrinolysis in acute lung injury and the acute respiratory distress syndrome. Curr. Pharm. Biotechnol. 2011;12(9):1481–1496. doi: 10.2174/138920111798281171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ware L.B., Fang X., Matthay M.A. Protein C and thrombomodulin in human acute lung injury, american journal of physiology-lung cellular and molecular. Physiology. 2003;285(3):L514–L521. doi: 10.1152/ajplung.00442.2002. [DOI] [PubMed] [Google Scholar]
- 42.Frantzeskaki F., Armaganidis A., Orfanos S.E. Immunothrombosis in acute respiratory distress syndrome: cross talks between inflammation and coagulation. Respiration. 2017;93(3):212–225. doi: 10.1159/000453002. [DOI] [PubMed] [Google Scholar]
- 43.Colling M.E., Kanthi Y. COVID–19-associated coagulopathy: an exploration of mechanisms. Vasc. Med. 2020;25(5):471–478. doi: 10.1177/1358863X20932640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fox S.E., Akmatbekov A., Harbert J.L., Li G., Brown J.Q., Vander Heide R.S. Pulmonary and cardiac pathology in african american patients with COVID-19: an autopsy series from New Orleans, the lancet. Respir. Med. 2020;8(7):681–686. doi: 10.1016/S2213-2600(20)30243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barnes B.J., Adrover J.M., Baxter-Stoltzfus A., Borczuk A., Cools-Lartigue J., Crawford J.M., Daßler-Plenker J., Guerci P., Huynh C., Knight J.S. Targeting potential drivers of COVID-19: neutrophil extracellular traps. J. Exp. Med. 2020;217(6) doi: 10.1084/jem.20200652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yadav V., Chi L., Zhao R., Tourdot B.E., Yalavarthi S., Jacobs B.N., Banka A., Liao H., Koonse S., Anyanwu A.C. ENTPD-1 disrupts inflammasome IL-1β–driven venous thrombosis. J. Clin. Invest. 2019;129(7):2872–2877. doi: 10.1172/JCI124804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ali R.A., Gandhi A.A., Meng H., Yalavarthi S., Vreede A.P., Estes S.K., Palmer O.R., Bockenstedt P.L., Pinsky D.J., Greve J.M. Adenosine receptor agonism protects against NETosis and thrombosis in antiphospholipid syndrome. Nat. Commun. 2019;10(1):1–12. doi: 10.1038/s41467-019-09801-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kambas K., Mitroulis I., Ritis K. The emerging role of neutrophils in thrombosis—the journey of TF through NETs. Front. Immunol. 2012;3:385. doi: 10.3389/fimmu.2012.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liberale L., Holy E.W., Akhmedov A., Bonetti N.R., Nietlispach F., Matter C.M., Mach F., Montecucco F., Beer J.H., Paneni F. Interleukin-1β mediates arterial thrombus formation via NET-associated tissue factor. J. Clin. Med. 2019;8(12):2072. doi: 10.3390/jcm8122072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Noubouossie D.F., Reeves B.N., Strahl B.D., Key N.S. Neutrophils: back in the thrombosis spotlight. Blood. 2019;133(20):2186–2197. doi: 10.1182/blood-2018-10-862243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thålin C., Hisada Y., Lundström S., Mackman N., Wallén H. Neutrophil extracellular traps: villains and targets in arterial, venous, and cancer-associated thrombosis. Arterioscler. Thromb. Vasc. Biol. 2019;39(9):1724–1738. doi: 10.1161/ATVBAHA.119.312463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zuo Y., Yalavarthi S., Shi H., Gockman K., Zuo M., Madison J.A., Blair C., Weber A., Barnes B.J., Egeblad M. Neutrophil extracellular traps in COVID-19. JCI Insight. 2020;5(11) doi: 10.1172/jci.insight.138999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zuo Y., Zuo M., Yalavarthi S., Gockman K., Madison J.A., Shi H., Woodard W., Lezak S.P., Lugogo N.L., Knight J.S. Neutrophil extracellular traps and thrombosis in COVID-19. J. Thromb. Thrombolysis. 2021;51(2):446–453. doi: 10.1007/s11239-020-02324-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.He Y., Zhou Y., Liu S., Kou Z., Li W., Farzan M., Jiang S. Receptor-binding domain of SARS-CoV spike protein induces highly potent neutralizing antibodies: implication for developing subunit vaccine. Biochem. Biophys. Res. Commun. 2004;324(2):773–781. doi: 10.1016/j.bbrc.2004.09.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li W., Greenough T.C., Moore M.J., Vasilieva N., Somasundaran M., Sullivan J.L., Farzan M., Choe H. Efficient replication of severe acute respiratory syndrome coronavirus in mouse cells is limited by murine angiotensin-converting enzyme 2. J. Virol. 2004;78(20):11429–11433. doi: 10.1128/JVI.78.20.11429-11433.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xiao X., Chakraborti S., Dimitrov A.S., Gramatikoff K., Dimitrov D.S. The SARS-CoV S glycoprotein: expression and functional characterization. Biochem. Biophys. Res. Commun. 2003;312(4):1159–1164. doi: 10.1016/j.bbrc.2003.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.-H., Nitsche A. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.-L., Abiona O., Graham B.S., McLellan J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.He L., Ding Y., Zhang Q., Che X., He Y., Shen H., Wang H., Li Z., Zhao L., Geng J. Expression of elevated levels of pro-inflammatory cytokines in SARS-CoV-infected ACE2+ cells in SARS patients: relation to the acute lung injury and pathogenesis of SARS. 2006;210(3):288–297. doi: 10.1002/path.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McGonagle D., O'Donnell J.S., Sharif K., Emery P., Bridgewood C. Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. Lancet Rheumatol. 2020;2(7):e437–e445. doi: 10.1016/S2665-9913(20)30121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bone R.C., Francis P.B., Pierce A.K. Elsevier; 1976. Intravascular coagulation associated with the adult respiratory distress syndrome. [DOI] [PubMed] [Google Scholar]
- 63.Blondonnet R., Constantin J.-M., Sapin V., Jabaudon M. A pathophysiologic approach to biomarkers in acute respiratory distress syndrome. Dis. Markers. 2016;2016 doi: 10.1155/2016/3501373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tomashefski J.F., Jr. Pulmonary pathology of acute respiratory distress syndrome. Clin. Chest Med. 2000;21(3):435–466. doi: 10.1016/s0272-5231(05)70158-1. [DOI] [PubMed] [Google Scholar]
- 65.Vesconi S., Rossi G.P., Pesenti A., Fumagalli R., Gattinoni L. Pulmonary microthrombosis in severe adult respiratory distress syndrome. Crit. Care Med. 1988;16(2):111–113. doi: 10.1097/00003246-198802000-00002. [DOI] [PubMed] [Google Scholar]
- 66.Colling M.E., Kanthi Y. COVID–19-associated coagulopathy: an exploration of mechanisms. Vasc. Med. 2020;25(5):471–478. doi: 10.1177/1358863X20932640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Koupenova M., Clancy L., Corkrey H.A., Freedman J.E. Circulating platelets as mediators of immunity, inflammation, and thrombosis. Circ. Res. 2018;122(2):337–351. doi: 10.1161/CIRCRESAHA.117.310795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mackman N. The many faces of tissue factor. J. Thromb. Haemost. 2009;7:136–139. doi: 10.1111/j.1538-7836.2009.03368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van der Poll T., Herwald H. The coagulation system and its function in early immune defense. Thromb. Haemost. 2014;112(10):640–648. doi: 10.1160/TH14-01-0053. [DOI] [PubMed] [Google Scholar]
- 70.Lefrançais E., Mallavia B., Zhuo H., Calfee C.S., Looney M.R. Maladaptive role of neutrophil extracellular traps in pathogen-induced lung injury. JCI Insight. 2018;3(3) doi: 10.1172/jci.insight.98178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Glas G., Van Der Sluijs K., Schultz M., Hofstra J.J., Van Der Poll T., Levi M. Bronchoalveolar hemostasis in lung injury and acute respiratory distress syndrome. J. Thromb. Haemost. 2013;11(1):17–25. doi: 10.1111/jth.12047. [DOI] [PubMed] [Google Scholar]
- 72.Casari I., Manfredi M., Metharom P., Falasca M. Dissecting lipid metabolism alterations in SARS-CoV-2. Prog. Lipid Res. 2021;82 doi: 10.1016/j.plipres.2021.101092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chignard M., Le Couedic J.P., Tence M., Vargaftig B.B., Benveniste J. The role of platelet-activating factor in platelet aggregation. Nature. 1979;279(5716):799–800. doi: 10.1038/279799a0. [DOI] [PubMed] [Google Scholar]
- 74.Uchiyama S., Yamazaki M., Maruyama S. Role of platelet-activating factor in aggregation of leukocytes and platelets in cerebral ischemia. Lipids. 1991;26(12):1247–1249. doi: 10.1007/BF02536541. [DOI] [PubMed] [Google Scholar]
- 75.Abdol Razak N., Elaskalani O., Metharom P. 2017;18(3) doi: 10.3390/ijms18030487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Elaskalani O., Abdol Razak N.B., Metharom P. Neutrophil extracellular traps induce aggregation of washed human platelets independently of extracellular DNA and histones. 2018;16(1):24. doi: 10.1186/s12964-018-0235-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yost C.C., Cody M.J., Harris E.S., Thornton N.L., McInturff A.M., Martinez M.L., Chandler N.B., Rodesch C.K., Albertine K.H., Petti C.A., Weyrich A.S., Zimmerman G.A. Impaired neutrophil extracellular trap (NET) formation: a novel innate immune deficiency of human neonates. Blood. 2009;113(25):6419–6427. doi: 10.1182/blood-2008-07-171629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fuchs T.A., Brill A., Duerschmied D., Schatzberg D., Monestier M., Myers D.D., Jr., Wrobleski S.K., Wakefield T.W., Hartwig J.H., Wagner D.D. Extracellular DNA traps promote thrombosis. Proc. Natl. Acad. Sci. U. S. A. 2010;107(36):15880–15885. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rao G.H., White J.G. Role of arachidonic acid metabolism in human platelet activation and irreversible aggregation. Am. J. Hematol. 1985;19(4):339–347. doi: 10.1002/ajh.2830190404. [DOI] [PubMed] [Google Scholar]
- 80.Manne B.K., Denorme F., Middleton E.A., Portier I., Rowley J.W., Stubben C., Petrey A.C., Tolley N.D., Guo L., Cody M., Weyrich A.S., Yost C.C., Rondina M.T., Campbell R.A. Platelet gene expression and function in patients with COVID-19. Blood. 2020;136(11):1317–1329. doi: 10.1182/blood.2020007214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hottz E.D., Azevedo-Quintanilha I.G., Palhinha L., Teixeira L., Barreto E.A., Pao C.R.R., Righy C., Franco S., Souza T.M.L., Kurtz P., Bozza F.A., Bozza P.T. Platelet activation and platelet-monocyte aggregate formation trigger tissue factor expression in patients with severe COVID-19. Blood. 2020;136(11):1330–1341. doi: 10.1182/blood.2020007252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Middleton E.A., He X.Y., Denorme F., Campbell R.A., Ng D., Salvatore S.P., Mostyka M., Baxter-Stoltzfus A., Borczuk A.C., Loda M., Cody M.J., Manne B.K., Portier I., Harris E.S., Petrey A.C., Beswick E.J., Caulin A.F., Iovino A., Abegglen L.M., Weyrich A.S., Rondina M.T., Egeblad M., Schiffman J.D., Yost C.C. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood. 2020;136(10):1169–1179. doi: 10.1182/blood.2020007008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Leppkes M., Knopf J., Naschberger E., Lindemann A., Singh J., Herrmann I., Sturzl M., Staats L., Mahajan A., Schauer C., Kremer A.N., Volkl S., Amann K., Evert K., Falkeis C., Wehrfritz A., Rieker R.J., Hartmann A., Kremer A.E., Neurath M.F., Munoz L.E., Schett G., Herrmann M. Vascular occlusion by neutrophil extracellular traps in COVID-19. EBioMedicine. 2020;58 doi: 10.1016/j.ebiom.2020.102925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Thachil J., Tang N., Gando S., Falanga A., Cattaneo M., Levi M., Clark C., Iba T. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J. Thromb. Haemost. 2020;18(5):1023–1026. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chandra A., Chakraborty U., Ghosh S., Dasgupta S. Anticoagulation in COVID-19: current concepts and controversies. Postgrad. Med. J. 2021 doi: 10.1136/postgradmedj-2021-139923. In press. [DOI] [PubMed] [Google Scholar]
- 86.Zhai Z., Li C., Chen Y., Gerotziafas G., Zhang Z., Wan J., Liu P., Elalamy I., Wang C. Prevention and treatment of venous thromboembolism associated with coronavirus disease 2019 infection: a consensus statement before guidelines. Thromb. Haemost. 2020;120(06):937–948. doi: 10.1055/s-0040-1710019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bikdeli B., Madhavan M., Jimenez D., Chuich T., Dreyfus I., Driggin E., Nigoghossian C., Ageno W., Madjid M., Guo Y. Global COVID-19 thrombosis collaborative group, endorsed by the ISTH, NATF, ESVM, and the IUA, supported by the ESC working group on pulmonary circulation and right ventricular function. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2020;75(23):2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Miesbach W., Makris M. COVID-19: coagulopathy, risk of thrombosis, and the rationale for anticoagulation. Clin. Appl. Thromb. Hemost. 2020;26 doi: 10.1177/1076029620938149. (1076029620938149) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wijaya I., Andhika R., Huang I. The use of therapeutic-dose anticoagulation and its effect on mortality in patients with COVID-19: a systematic review. Clin. Appl. Thromb. Hemost. 2020;26 doi: 10.1177/1076029620960797. (1076029620960797) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang Y., Ao G., Nasr B., Qi X. Effect of antiplatelet treatments on patients with COVID-19 infection: a systematic review and meta-analysis. Am. J. Emerg. Med. 2021;43:27–30. doi: 10.1016/j.ajem.2021.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mackessy S.P., Saviola A.J. Oxford University Press; 2016. Understanding Biological Roles of Venoms Among the Caenophidia: The Importance of Rear-fanged Snakes. [DOI] [PubMed] [Google Scholar]
- 92.Banerjee Y., Mizuguchi J., Iwanaga S., Kini R.M. Hemextin AB complex–a snake venom anticoagulant protein complex that inhibits factor VIIa activity. Pathophysiol. Haemost. Thromb. 2005;34(4–5):184–187. doi: 10.1159/000092420. [DOI] [PubMed] [Google Scholar]
- 93.Dutta S., Sinha A., Dasgupta S., Mukherjee A.K. Binding of a Naja naja venom acidic phospholipase A2 cognate complex to membrane-bound vimentin of rat L6 cells: implications in cobra venom-induced cytotoxicity. 2019;1861(5):958–977. doi: 10.1016/j.bbamem.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 94.Mukherjee A.K., Mackessy S.P. Pharmacological properties and pathophysiological significance of a kunitz-type protease inhibitor (Rusvikunin-II) and its protein complex (Rusvikunin complex) purified from Daboia russelii russelii venom. Toxicon. 2014;89:55–66. doi: 10.1016/j.toxicon.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 95.Mukherjee A.K., Mackessy S.P., Dutta S. Characterization of a kunitz-type protease inhibitor peptide (Rusvikunin) purified from Daboia russelii russelii venom. Int. J. Biol. Macromol. 2014;67:154–162. doi: 10.1016/j.ijbiomac.2014.02.058. [DOI] [PubMed] [Google Scholar]
- 96.Kalita B., Mukherjee A.K. Recent advances in snake venom proteomics research in India: a new horizon to decipher the geographical variation in venom proteome composition and exploration of candidate drug prototypes. J. Proteins Proteomics. 2019:1–16. [Google Scholar]
- 97.Chanda A., Mukherjee A.K. Mass spectrometric analysis to unravel the venom proteome composition of indian snakes: opening new avenues in clinical research. Expert Rev. Proteomics. 2020;17(5):411–423. doi: 10.1080/14789450.2020.1778471. [DOI] [PubMed] [Google Scholar]
- 98.Bordon K.C.F., Cologna C.T., Fornari-Baldo E.C., Pinheiro-Junior E.L., Cerni F.A., Amorim F.G., Anjolette F.A.P., Cordeiro F.A., Wiezel G.A., Cardoso I.A., Ferreira I.G., de Oliveira I.S., Boldrini-Franca J., Pucca M.B., Baldo M.A., Arantes E.C. From animal poisons and venoms to medicines: achievements,challenges and perspectives in drug discovery. 2020;11:1132. doi: 10.3389/fphar.2020.01132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.White J. Snake venoms and coagulopathy. Toxicon. 2005;45(8):951–967. doi: 10.1016/j.toxicon.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 100.Alvarez-Flores M., Faria F., de Andrade S., Chudzinski-Tavassi A. Springer Netherlands; Dordrecht: 2016. pp. 1–20. [Google Scholar]
- 101.Thakur R., Mukherjee A. A brief appraisal on Russell’s viper venom (Daboia russelii russelii) proteinases. Snake Venoms. 2015:1–18. [Google Scholar]
- 102.Autin L., Miteva M., Lee W., Mertens K., Radtke K.P., Villoutreix B. Molecular models of the procoagulant factor VIIIa–factor IXa complex. J. Thromb. Haemost. 2005;3(9):2044–2056. doi: 10.1111/j.1538-7836.2005.01527.x. [DOI] [PubMed] [Google Scholar]
- 103.Sharma M., Iyer J.K., Shih N., Majumder M., Mattaparthi V.S., Mukhopadhyay R., Doley R. Daboxin P, a major phospholipase A2 Enzyme from the Indian Daboia russelii russelii Venom Targets Factor X and Factor Xa for Its Anticoagulant Activity. PLoS One. 2016;11(4) doi: 10.1371/journal.pone.0153770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Samah S., Fatah C., Jean-Marc B., Safia K.T., Fatima L.D. Purification and characterization of cc-lec, C-type lactose-binding lectin: a platelet aggregation and blood-clotting inhibitor from Cerastes cerastes venom. Int. J. Biol. Macromol. 2017;102:336–350. doi: 10.1016/j.ijbiomac.2017.04.018. [DOI] [PubMed] [Google Scholar]
- 105.Sun Q.-Y., Wang C.-E., Li Y.-N., Bao J. Inhibition of platelet aggregation and blood coagulation by a P-III class metalloproteinase purified from Naja atra venom. Toxicon. 2020;187:223–231. doi: 10.1016/j.toxicon.2020.09.009. [DOI] [PubMed] [Google Scholar]
- 106.Latinović Z., Leonardi A., Kovačič L., Koh C.Y., Šribar J., Bakija A.T., Venkateswarlu D., Kini R.M., Križaj I. The first intrinsic tenase complex inhibitor with serine protease structure offers a new perspective in anticoagulant therapy. Thromb. Haemost. 2018;118(10):1713–1728. doi: 10.1055/s-0038-1669785. [DOI] [PubMed] [Google Scholar]
- 107.Girish V.M., Kini R.M. Exactin: a specific inhibitor of factor X activation by extrinsic tenase complex from the venom of Hemachatus haemachatus. Sci. Rep. 2016;6:32036. doi: 10.1038/srep32036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Barnwal B., Jobichen C., Girish V.M., Foo C.S., Sivaraman J., Kini R.M. Ringhalexin from Hemachatus haemachatus: a novel inhibitor of extrinsic tenase complex. Sci. Rep. 2016;6:25935. doi: 10.1038/srep25935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Krishnaswamy S. The transition of prothrombin to thrombin. J. Thromb. Haemost. 2013;11:265–276. doi: 10.1111/jth.12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fatah C., Samah S., Fatima L.D. Antiplatelet and anticoagulant activities of two phospholipase A2s purified from Cerastes cerastes venom: structure-function relationship. J. Biochem. Mol. Toxicol. 2018;32(12) doi: 10.1002/jbt.22219. [DOI] [PubMed] [Google Scholar]
- 111.Mukherjee A.K., Kalita B., Thakur R. Two acidic, anticoagulant PLA2 isoenzymes purified from the venom of monocled cobra Naja kaouthia exhibit different potency to inhibit thrombin and factor xa via phospholipids independent, non-enzymatic mechanism. PloS one. 2014;9(8) doi: 10.1371/journal.pone.0101334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Saikia D., Thakur R., Mukherjee A.K. An acidic phospholipase A2 (RVVA-PLA2-I) purified from Daboia russelli venom exerts its anticoagulant activity by enzymatic hydrolysis of plasma phospholipids and by non-enzymatic inhibition of factor xa in a phospholipids/Ca2+ independent manner. Toxicon. 2011;57(6):841–850. doi: 10.1016/j.toxicon.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 113.Mukherjee A.K., Dutta S., Mackessy S.P. A new C-type lectin (RVsnaclec) purified from venom of Daboia russelii russelii shows anticoagulant activity via inhibition of FXa and concentration-dependent differential response to platelets in a Ca2+-independent manner. Thromb. Res. 2014;134(5):1150–1156. doi: 10.1016/j.thromres.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 114.Shen D.K., Xu X.L., Zhang Y., Song J.J., Yan X.C., Guo M.C. Ca2+-induced binding of anticoagulation factor II from the venom of Agkistrodon acutus with factor IX. Biopolymers. 2012;97(10):818–824. doi: 10.1002/bip.22078. [DOI] [PubMed] [Google Scholar]
- 115.Zhang Y., Xu X., Shen D., Song J., Guo M., Yan X. Anticoagulation factor I, a snaclec (snake C-type lectin) from Agkistrodon acutus venom binds to FIX as well as FX: Ca2+ induced binding data. Toxicon. 2012;59(7–8):718–723. doi: 10.1016/j.toxicon.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 116.Chen M., Ye X., Ming X., Chen Y., Wang Y., Su X., Su W., Kong Y. A novel direct factor xa inhibitory peptide with anti-platelet aggregation activity from Agkistrodon acutus venom hydrolysates. Sci. Rep. 2015;5(1):1–11. doi: 10.1038/srep10846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Thakur R., Kumar A., Bose B., Panda D., Saikia D., Chattopadhyay P., Mukherjee A.K. A new peptide (Ruviprase) purified from the venom of Daboia russelii russelii shows potent anticoagulant activity via non-enzymatic inhibition of thrombin and factor Xa. Biochimie. 2014;105:149–158. doi: 10.1016/j.biochi.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 118.Dutta S., Gogoi D., Mukherjee A.K. Anticoagulant mechanism and platelet deaggregation property of a non-cytotoxic, acidic phospholipase A2 purified from indian cobra (Naja naja) venom: inhibition of anticoagulant activity by low molecular weight heparin. Biochimie. 2015;110:93–106. doi: 10.1016/j.biochi.2014.12.020. [DOI] [PubMed] [Google Scholar]
- 119.Assafim M., Frattani F.S., Ferreira M.S., Silva D.M., Monteiro R.Q., Zingali R.B. Exploiting the antithrombotic effect of the (pro) thrombin inhibitor bothrojaracin. Toxicon. 2016;119:46–51. doi: 10.1016/j.toxicon.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 120.Davie E.W., Kulman J.D. Seminars in thrombosis and hemostasis. Copyright© 2006 by Thieme Medical Publishers, Inc.; 333 Seventh Avenue, New …: 2006. An overview of the structure and function of thrombin; pp. 003–015. [DOI] [PubMed] [Google Scholar]
- 121.Mukherjee A.K. A major phospholipase A2 from Daboia russelii russelii venom shows potent anticoagulant action via thrombin inhibition and binding with plasma phospholipids. Biochimie. 2014;99:153–161. doi: 10.1016/j.biochi.2013.11.026. [DOI] [PubMed] [Google Scholar]
- 122.Saikia D., Majumdar S., Mukherjee A.K. Mechanism of in vivo anticoagulant and haemolytic activity by a neutral phospholipase A2 purified from Daboia russelii russelii venom: correlation with clinical manifestations in Russell's viper envenomed patients. Toxicon. 2013;76:291–300. doi: 10.1016/j.toxicon.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 123.de Souza D.L.N., Gomes M.S.R., Ferreira F.B., Rodrigues R.S., Achê D.C., Richardson M., Borges M.H., Rodrigues V.M. Biochemical and enzymatic characterization of BpMP-I, a fibrinogenolytic metalloproteinase isolated from Bothropoides pauloensis snake venom. Comp. Biochem. Physiol. B: Biochem. Mol. Biol. 2012;161(2):102–109. doi: 10.1016/j.cbpb.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 124.de Morais N.C., Mamede C.C.N., Fonseca K.C., de Queiroz M.R., Gomes-Filho S.A., Santos-Filho N.A., de CF Bordon K., Beletti M.E., Sampaio S.V., Arantes E.C. Isolation and characterization of moojenin, an acid-active, anticoagulant metalloproteinase from Bothrops moojeni venom. Toxicon. 2012;60(7):1251–1258. doi: 10.1016/j.toxicon.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 125.Costa J.d.O., Fonseca K.C., Mamede C.C.N., Beletti M.E., Santos-Filho N.A., Soares A.M., Arantes E.C., Hirayama S.N., Selistre-de-Araújo H.S., Fonseca F. Bhalternin: functional and structural characterization of a new thrombin-like enzyme from Bothrops alternatus snake venom. Toxicon. 2010;55(7):1365–1377. doi: 10.1016/j.toxicon.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 126.Mukherjee A.K., Mackessy S.P. Biochemical and pharmacological properties of a new thrombin-like serine protease (Russelobin) from the venom of Russell's viper (Daboia russelii russelii) and assessment of its therapeutic potential. Biochim. Biophys. Acta. 2013;1830(6):3476–3488. doi: 10.1016/j.bbagen.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 127.Wang S., Xu X., Gao S., Zhu S., Rong R., Li B. Purification and partial characterization of a novel fibrinogenase from the venom of Deinagkistrodon acutus: inhibition of platelet aggregation. Protein Expr. Purif. 2014;99:99–105. doi: 10.1016/j.pep.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 128.Lippi G., Plebani M., Henry B.M. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin. Chim. Acta. 2020;506:145–148. doi: 10.1016/j.cca.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yang X., Yang Q., Wang Y., Wu Y., Xu J., Yu Y., Shang Y. Thrombocytopenia and its association with mortality in patients with COVID-19. J. Thromb. Haemost. 2020;18(6):1469–1472. doi: 10.1111/jth.14848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fan B.E., Ng J., Chan S.S.W., Christopher D., Tso A.C.Y., Ling L.M., Young B.E., Wong L.J.L., Sum C.L.L., Tan H.T. COVID-19 associated coagulopathy in critically ill patients: a hypercoagulable state demonstrated by parameters of haemostasis and clot waveform analysis. J. Thromb. Thrombolysis. 2021;51(3):663–674. doi: 10.1007/s11239-020-02318-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lei X., Reheman A., Hou Y., Zhou H., Wang Y., Marshall A.H., Liang C., Dai X., Li B.X., Vanhoorelbeke K. Anfibatide, a novel GPIb complex antagonist, inhibits platelet adhesion and thrombus formation in vitro and in vivo in murine models of thrombosis. Thromb. Haemost. 2014;112(02):279–289. doi: 10.1160/TH13-06-0490. [DOI] [PubMed] [Google Scholar]
- 132.Arlinghaus F.T., Momic T., Ammar N.A., Shai E., Spectre G., Varon D., Marcinkiewicz C., Heide H., Lazarovici P., Eble J.A. Identification of α2β1 integrin inhibitor VP-i with anti-platelet properties in the venom of Vipera palaestinae. Toxicon. 2013;64:96–105. doi: 10.1016/j.toxicon.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 133.Kuo Y.-J., Chung C.-H., Pan T.-Y., Chuang W.-J., Huang T.-F. A novel αIIbβ3 antagonist from snake venom prevents thrombosis without causing bleeding. Toxins. 2020;12(1):11. doi: 10.3390/toxins12010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ameziani M., Chérifi F., Kiheli H., Saoud S., Hariti G., Kellou-Taîri S., Laraba-Djebari F. Isolation and functional identification of an antiplatelet RGD-containing disintegrin from Cerastes cerastes venom. Protein J. 2020;39(5):574–590. doi: 10.1007/s10930-020-09915-y. [DOI] [PubMed] [Google Scholar]
- 135.Reczyńska K., Major R., Kopernik M., Pamuła E., Imbir G., Plutecka H., Bruckert F., Surmiak M. Surface modification of polyurethane with eptifibatide-loaded degradable nanoparticles reducing risk of blood coagulation. Colloids Surf. B: Biointerfaces. 2021;201 doi: 10.1016/j.colsurfb.2021.111624. [DOI] [PubMed] [Google Scholar]
- 136.Kalita B., Dutta S., Mukherjee A.K. RGD-independent binding of Russell's viper venom kunitz-type protease inhibitors to platelet GPIIb/IIIa receptor. Sci. Rep. 2019;9(1):8316. doi: 10.1038/s41598-019-44767-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cannon C.P., Weintraub W.S., Demopoulos L.A., Vicari R., Frey M.J., Lakkis N., Neumann F.-J., Robertson D.H., DeLucca P.T., DiBattiste P.M. Comparison of early invasive and conservative strategies in patients with unstable coronary syndromes treated with the glycoprotein IIb/IIIa inhibitor tirofiban. N. Engl. J. Med. 2001;344(25):1879–1887. doi: 10.1056/NEJM200106213442501. [DOI] [PubMed] [Google Scholar]
- 138.Sánchez-Cortés J., Mrksich M. The platelet integrin αIIbβ3 binds to the RGD and AGD motifs in fibrinogen. Chem. Biol. 2009;16(9):990–1000. doi: 10.1016/j.chembiol.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chang C.-H., Chung C.-H., Tu Y.-S., Tsai C.-C., Hsu C.-C., Peng H.-C., Tseng Y.J., Huang T.-F. Trowaglerix venom polypeptides as a novel antithrombotic agent by targeting immunoglobulin-like domains of glycoprotein VI in platelet. Arterioscler. Thromb. Vasc. Biol. 2017;37(7):1307–1314. doi: 10.1161/ATVBAHA.116.308604. [DOI] [PubMed] [Google Scholar]
- 140.Cintra A., De Toni L., Sartim M., Franco J., Caetano R., Murakami M., Sampaio S. Batroxase, a new metalloproteinase from B. atrox snake venom with strong fibrinolytic activity. Toxicon. 2012;60(1):70–82. doi: 10.1016/j.toxicon.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 141.Jacob-Ferreira A.L., Menaldo D.L., Bernardes C.P., Sartim M.A., de Angelis C.D., Tanus-Santos J.E., Sampaio S.V. Evaluation of the in vivo thrombolytic activity of a metalloprotease from Bothrops atrox venom using a model of venous thrombosis. Toxicon. 2016;109:18–25. doi: 10.1016/j.toxicon.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 142.Girón M.E., Rodríguez-Acosta A., Salazar A.M., Sánchez E.E., Galán J., Ibarra C., Guerrero B. Isolation and characterization of two new non-hemorrhagic metalloproteinases with fibrinogenolytic activity from the mapanare (Bothrops colombiensis) venom. Arch. Toxicol. 2013;87(1):197–208. doi: 10.1007/s00204-012-0914-3. [DOI] [PubMed] [Google Scholar]
- 143.Sanchez E.F., Richardson M., Gremski L.H., Veiga S.S., Yarleque A., Niland S., Lima A.M., Estevao-Costa M.I., Eble J.A. A novel fibrinolytic metalloproteinase, barnettlysin-I from Bothrops barnetti (Barnett s pitviper) snake venom with anti-platelet properties. 2016;1860(3):542–556. doi: 10.1016/j.bbagen.2015.12.021. [DOI] [PubMed] [Google Scholar]
- 144.Bernardes C.P., Menaldo D.L., Camacho E., Rosa J.C., Escalante T., Rucavado A., Lomonte B., Gutiérrez J.M., Sampaio S.V. Proteomic analysis of Bothrops pirajai snake venom and characterization of BpirMP, a new PI metalloproteinase. J. Proteome. 2013;80:250–267. doi: 10.1016/j.jprot.2013.01.021. [DOI] [PubMed] [Google Scholar]
- 145.Kumar M., Devaraj V., Vishwanath B., Kemparaju K. Anti-coagulant activity of a metalloprotease: further characterization from the indian cobra (Naja naja) venom. J. Thromb. Thrombolysis. 2010;29(3):340–348. doi: 10.1007/s11239-009-0379-2. [DOI] [PubMed] [Google Scholar]
- 146.Xie H., Huang M., Hu Q., Sun K., Wu H., Shu W., Li X., Fang L. Agkihpin, a novel SVTLE from Gloydius halys Pallas, promotes platelet aggregation in vitro and inhibits thrombus formation in vivo in murine models of thrombosis. Toxicon. 2016;122:78–88. doi: 10.1016/j.toxicon.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 147.Zhang Z., Lin L., Zhang J., Zhang Q., Zhao N., Wu L., Chen J., Wu Z., Wu G., Lin J. Thrombolytic and antiplatelet effects of a novel plasminogen activator from the venom of Gloydius brevicaudus viper. J. Atheroscler. Thromb. 2015;27649 doi: 10.5551/jat.27649. [DOI] [PubMed] [Google Scholar]
- 148.Zhang J., Meng W., Wang C., Wu Z., Wu G., Xu Y. Expression, purification and characterization of recombinant plasminogen activator from Gloydius brevicaudus venom in Escherichia coli. Protein Expr. Purif. 2013;91(1):85–90. doi: 10.1016/j.pep.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 149.Boldrini-França J., Pinheiro-Junior E.L., Arantes E.C. Functional and biological insights of rCollinein-1, a recombinant serine protease from Crotalus durissus collilineatus. J. Venomous Anim. Toxins Incl. Trop. Dis. 2019;25 doi: 10.1590/1678-9199-JVATITD-1471-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kim O.H., Cho K.-S., Seomun Y., Kim J.-T., Chung K.-H. Acute and repeated dose (28 days) toxicity studies in rats and dogs of recombinant batroxobin, a snake venom thrombin-like enzyme expressed from Pichia pastoris. Toxicon. 2017;129:153–163. doi: 10.1016/j.toxicon.2017.01.023. [DOI] [PubMed] [Google Scholar]
- 151.Li A., Zhang C., Wang J., Wang J., Jiang H., Li J., Ma X., Zhang W., Lu Y. Cloning, expression, purification and bioactivity evaluation of a thrombin-like enzyme from Deinagkistrodon acutus venom gland library. Biotechnol. Lett. 2018;40(1):93–102. doi: 10.1007/s10529-017-2441-z. [DOI] [PubMed] [Google Scholar]
- 152.Kelly A.B., Maraganore J.M., Bourdon P., Hanson S.R., Harker L.A. Antithrombotic effects of synthetic peptides targeting various functional domains of thrombin. Proc. Natl. Acad. Sci. U. S. A. 1992;89(13):6040–6044. doi: 10.1073/pnas.89.13.6040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bacher P., Walenga J.M., Iqbal O., Bajusz S., Breddin K., Fareed J. The antithrombotic and anticoagulant effects of a synthetic tripeptide and recombinant hirudin in various animal models. Thromb. Res. 1993;71(4):251–263. doi: 10.1016/0049-3848(93)90195-t. [DOI] [PubMed] [Google Scholar]
- 154.McDaniel J.K., Abdelgawwad M.S., Hargett A., Renfrow M.B., Bdeir K., Cao W., Cines D.B., Zheng X.L. Human neutrophil peptide-1 inhibits thrombus formation under arterial flow via its terminal free cysteine thiols. 2019;17(4):596–606. doi: 10.1111/jth.14407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kikelj D. Peptidomimetic thrombin inhibitors. Pathophysiol. Haemost. Thromb. 2003;33(5–6):487–491. doi: 10.1159/000083850. [DOI] [PubMed] [Google Scholar]
- 156.Momic T., Katzhendler J., Shai E., Noy E., Senderowitz H., Eble J.A., Marcinkiewicz C., Varon D., Lazarovici P. Vipegitide: a folded peptidomimetic partial antagonist of α2β1 integrin with antiplatelet aggregation activity. Drug Des. Develop. Ther. 2015;9:291. doi: 10.2147/DDDT.S72844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Nellenbach K., Brown A.C. Peptide mimetic drugs for modulating thrombosis and hemostasis. Drug Dev. Res. 2017;78(6):236–244. doi: 10.1002/ddr.21407. [DOI] [PubMed] [Google Scholar]
- 158.Pasternack R., Büchold C., Jähnig R., Pelzer C., Sommer M., Heil A., Florian P., Nowak G., Gerlach U., Hils M. Novel inhibitor ZED3197 as potential drug candidate in anticoagulation targeting coagulation FXIIIa (F13a) J. Thromb. Haemost. 2020;18(1):191–200. doi: 10.1111/jth.14646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Griffin J.H., Zlokovic B.V., Mosnier L.O. Activated protein C, protease activated receptor 1, and neuroprotection. Blood. 2018;132(2):159–169. doi: 10.1182/blood-2018-02-769026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tucker E.I., Verbout N.G., Markway B.D., Wallisch M., Lorentz C.U., Hinds M.T., Shatzel J.J., Pelc L.A., Wood D.C., McCarty O.J.T., Di Cera E., Gruber A. The protein C activator AB002 rapidly interrupts thrombus development in baboons. Blood. 2020;135(9):689–699. doi: 10.1182/blood.2019002771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chen W., Carvalho L.P., Chan M.Y., Kini R.M., Kang T.S. Fasxiator, a novel factor XIa inhibitor from snake venom, and its site-specific mutagenesis to improve potency and selectivity. J. Thromb. Haemos. 2015;13(2):248–261. doi: 10.1111/jth.12797. [DOI] [PubMed] [Google Scholar]
- 162.David V., Succar B.B., de Moraes J.A., Saldanha-Gama R.F.G., Barja-Fidalgo C., Zingali R.B. Recombinant and chimeric disintegrins in preclinical research. Toxins. 2018;10(8) doi: 10.3390/toxins10080321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wong P.C., Crain E.J., Watson C.A., Wexler R.R., Lam P.Y., Quan M.L., Knabb R.M. Razaxaban, a direct factor xa inhibitor, in combination with aspirin and/or clopidogrel improves low-dose antithrombotic activity without enhancing bleeding liability in rabbits. J. Thromb. Thrombolysis. 2007;24(1):43–51. doi: 10.1007/s11239-007-0017-9. [DOI] [PubMed] [Google Scholar]
- 164.Huang Y., Yu L., Ren J., Gu B., Longstaff C., Hughes A.D., Thom S.A., Xu X.Y., Chen R. An activated-platelet-sensitive nanocarrier enables targeted delivery of tissue plasminogen activator for effective thrombolytic therapy. 2019;300:1–12. doi: 10.1016/j.jconrel.2019.02.033. [DOI] [PubMed] [Google Scholar]
- 165.Villegas M.R., Baeza A., Vallet-Regi M. Nanotechnological strategies for protein delivery. Molecules. 2018;23(5) doi: 10.3390/molecules23051008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kuo Y.J., Chang Y.T., Chung C.H., Chuang W.J., Huang T.F. Improved antithrombotic activity and diminished bleeding side effect of a PEGylated alphaIIbbeta3 antagonist, disintegrin. Toxins. 2020;12(7) doi: 10.3390/toxins12070426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Fan B., Xing Y., Zheng Y., Sun C., Liang G. pH-responsive thiolated chitosan nanoparticles for oral low-molecular weight heparin delivery: in vitro and in vivo evaluation. Drug Deliv. 2016;23(1):238–247. doi: 10.3109/10717544.2014.909908. [DOI] [PubMed] [Google Scholar]
- 168.Suk J.S., Xu Q., Kim N., Hanes J., Ensign L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016;99(Pt A):28–51. doi: 10.1016/j.addr.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zhao Z., Yang F., Zhang X., Sun J., He Z., Luo C. Emerging nanotherapeutics for antithrombotic treatment. Biomaterials. 2020;255 doi: 10.1016/j.biomaterials.2020.120200. [DOI] [PubMed] [Google Scholar]
- 170.Kaide C.G., Gulseth M.P. Current strategies for the management of bleeding associated with direct oral anticoagulants and a review of investigational reversal agents. J.Emerg. Med. 2020;58(2):217–233. doi: 10.1016/j.jemermed.2019.10.011. [DOI] [PubMed] [Google Scholar]
- 171.Maura G., Billionnet C., Coste J., Weill A., Neumann A., Pariente A. Non-bleeding adverse events with the use of direct oral anticoagulants: a sequence symmetry analysis. Drug Saf. 2018;41(9):881–897. doi: 10.1007/s40264-018-0668-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Seshadri M., Jasimuddin Ahamed J.L. Intervention in COVID-19 linked hypercoaguable states characterized by circuit thrombosis utilizing a direct thrombin inhibitor. Thromb. Update. 2020;1:100009. doi: 10.1016/j.tru.2020.100009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Tomo S., Kumar K.P., Roy D., Sankanagoudar S., Purohit P., Yadav D., Banerjee M., Sharma P., Misra S. Complement activation and coagulopathy-an ominous duo in COVID19. Expert. Rev. Hematol. 2021;14(2):155–173. doi: 10.1080/17474086.2021.1875813. [DOI] [PubMed] [Google Scholar]
- 174.Ellenberg S.S. Clinical trials in the time of a pandemic. Clin. Trials. 2020;17(5):467–471. doi: 10.1177/1740774520939871. [DOI] [PubMed] [Google Scholar]
- 175.Alban S. Heparin-a century of progress. 2012. Adverse effects of heparin; pp. 211–263. [Google Scholar]
- 176.Huang F., Hong E. Platelet glycoprotein IIb/IIIa inhibition and its clinical use. 2004;2(3):187–196. doi: 10.2174/1568016043356309. [DOI] [PubMed] [Google Scholar]
- 177.Mousa S.A., Jeske W.P., Fareed J. Antiplatelet therapy prasugrel: a novel platelet ADP P2Y12 receptor antagonist. Clin. Appl. Thromb. Hemost. 2010;16(2):170–176. doi: 10.1177/1076029609355589. [DOI] [PubMed] [Google Scholar]
- 178.Tedeschi D., Rizzi A., Biscaglia S., Tumscitz C. Acute myocardial infarction and large coronary thrombosis in a patient with COVID-19. Catheter. Cardiovasc. Interv. 2021;97(2):272–277. doi: 10.1002/ccd.29179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Jones W.S., Tricoci P., Huang Z., Moliterno D.J., Harrington R.A., Sinnaeve P.R., Strony J., Van de Werf F., White H.D., Held C. Vorapaxar in patients with peripheral artery disease and acute coronary syndrome: insights from thrombin receptor antagonist for clinical event reduction in acute coronary syndrome (TRACER) Am. Heart J. 2014;168(4):588–596. doi: 10.1016/j.ahj.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 180.Uslu A.B. Effect of dipyridamole on random pattern skin flap viability in rats. J. Plastic Surg. Hand Surg. 2020;54(4):240–247. doi: 10.1080/2000656X.2020.1756836. [DOI] [PubMed] [Google Scholar]
- 181.Liu X., Li Z., Liu S., Sun J., Chen Z., Jiang M., Zhang Q., Wei Y., Wang X., Huang Y.-Y. Potential therapeutic effects of dipyridamole in the severely ill patients with COVID-19. Acta Pharm. Sin. B. 2020;10(7):1205–1215. doi: 10.1016/j.apsb.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wang J., Hajizadeh N., Moore E.E., McIntyre R.C., Moore P.K., Veress L.A., Yaffe M.B., Moore H.B., Barrett C.D. Tissue plasminogen activator (tPA) treatment for COVID-19 associated acute respiratory distress syndrome (ARDS): a case series. J. Thromb. Haemost. 2020;18(7):1752–1755. doi: 10.1111/jth.14828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Goyal A., Saigal S., Niwariya Y., Sharma J., Singh P. Successful use of tPA for thrombolysis in COVID related ARDS: a case series. J. Thromb. Thrombolysis. 2021;51(2):293–296. doi: 10.1007/s11239-020-02208-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Briedis K., Aldujeli A., Aldujeili M., Briede K., Zaliunas R., Hamadeh A., Stoler R.C., McCullough P.A. Considerations for management of acute coronary syndromes during the SARS-CoV-2 (COVID-19) pandemic. Am. J. Cardiol. 2020;131:115–119. doi: 10.1016/j.amjcard.2020.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ebrahiminik H., Yazdi H.R., Mohammadi A., Mirza-Aghazadeh-Attari M. Successful vascular interventional management of superior mesenteric vein thrombosis in a patient with COVID-19: a case report and review of literature. Radiol. Case Rep. 2021;16(6):1539–1542. doi: 10.1016/j.radcr.2021.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.López A.C., Cartaya C.H., González E.C., Reinoso D.G., Font J.A.C., Vera N.S., Lizano M.E.N., Roque A.V., Valdera A.M., Ramos T.C. Seminars in thrombosis and hemostasis. Thieme Medical Publishers, Inc.; 2020. Pulmonary thrombosis in COVID-19 treated by thrombolysis: a small case series using streptokinase. [DOI] [PubMed] [Google Scholar]
- 187.Warach S.J., Saver J.L. Stroke thrombolysis with tenecteplase to reduce emergency department spread of coronavirus disease 2019 and shortages of alteplase. JAMA Neurol. 2020;77(10):1203–1204. doi: 10.1001/jamaneurol.2020.2396. [DOI] [PubMed] [Google Scholar]
- 188.Garcia Denegri M.E., Acosta O.C., Huancahuire-Vega S., Martins-de-Souza D., Marangoni S., Marunak S.L., Teibler G.P., Leiva L.C., Ponce-Soto L.A. Isolation and functional characterization of a new acidic PLA2 Ba SpII RP4 of the Bothrops alternatus snake venom from Argentina. Toxicon. 2010;56(1):64–74. doi: 10.1016/j.toxicon.2010.02.031. [DOI] [PubMed] [Google Scholar]
- 189.Ding L., Hao J., Luo X., Zhu W., Wu Z., Qian Y., Hu F., Liu T., Ruan X., Li S., Li J., Chen Z. The Kv1.3 channel-inhibitory toxin BF9 also displays anticoagulant activity via inhibition of factor XIa. Toxicon. 2018;152:9–15. doi: 10.1016/j.toxicon.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 190.Gomes M.S., de Queiroz M.R., Mamede C.C., Mendes M.M., Hamaguchi A., Homsi-Brandeburgo M.I., Sousa M.V., Aquino E.N., Castro M.S., de Oliveira F., Rodrigues V.M. Purification and functional characterization of a new metalloproteinase (BleucMP) from Bothrops leucurus snake venom, comparative biochemistry and physiology. Toxicol. Pharmacol. 2011;153(3):290–300. doi: 10.1016/j.cbpc.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 191.Torres F.S., Rates B., Gomes M.T., Salas C.E., Pimenta A.M., Oliveira F., Santoro M.M., de Lima M.E. Bmoo FIBMP-I: a new fibrinogenolytic metalloproteinase from Bothrops moojeni snake venom. ISRN Toxicol. 2012;2012 doi: 10.5402/2012/673941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.de Oliveira F., de Sousa B.B., Mamede C.C., de Morais N.C., de Queiroz M.R., da Cunha Pereira D.F., Matias M.S., Homi Brandeburgo M.I. Biochemical and functional characterization of BmooSP, a new serine protease from Bothrops moojeni snake venom. Toxicon. 2016;111:130–138. doi: 10.1016/j.toxicon.2016.01.055. [DOI] [PubMed] [Google Scholar]
- 193.Naves de Souza D.L., Gomes M.S., Ferreira F.B., Rodrigues R.S., Ache D.C., Richardson M., Borges M.H., Rodrigues V.M. Biochemical and enzymatic characterization of BpMP-I, a fibrinogenolytic metalloproteinase isolated from bothropoides pauloensis snake venom. 2012;161(2):102–109. doi: 10.1016/j.cbpb.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 194.Cherifi F., Rousselle J.C., Namane A., Laraba-Djebari F. CCSV-MPase, a novel procoagulant metalloproteinase from Cerastes cerastes venom: purification, biochemical characterization and protein identification. Protein J. 2010;29(7):466–474. doi: 10.1007/s10930-010-9273-1. [DOI] [PubMed] [Google Scholar]
- 195.Sekhar C.C., Chakrabarty D. Fibrinogenolytic toxin from indian monocled cobra (Naja kaouthia) venom. J. Biosci. 2011;36(2):355–361. doi: 10.1007/s12038-011-9068-3. [DOI] [PubMed] [Google Scholar]
- 196.Damico D.C., Vassequi-Silva T., Torres-Huaco F.D., Nery-Diez A.C., de Souza R.C., Da Silva S.L., Vicente C.P., Mendes C.B., Antunes E., Werneck C.C., Marangoni S. LmrTX, a basic PLA2 (D49) purified from Lachesis muta rhombeata snake venom with enzymatic-related antithrombotic and anticoagulant activity. Toxicon. 2012;60(5):773–781. doi: 10.1016/j.toxicon.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 197.Kumar R.V., Yariswamy M., Joshi V., Dharmappa K.K., Venkatesha S.H., Sharath B.K., Vishwanath B.S. Malabarase, a serine protease with anticoagulant activity from Trimeresurus malabaricus venom. 2013;164(2):111–116. doi: 10.1016/j.cbpb.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 198.Huang J., Fan H., Yin X., Huang F. Isolation of a novel metalloproteinase from agkistrodon venom and its antithrombotic activity analysis. Int. J. Mol. Sci. 2019;20(17) doi: 10.3390/ijms20174088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Chanda C., Sarkar A., Chakrabarty D. Thrombolytic protein from cobra venom with anti-adhesive properties. Arch. Biochem. Biophys. 2016;590:20–26. doi: 10.1016/j.abb.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 200.Du Q.S., Trabi M., Richards R.S., Mirtschin P., Madaras F., Nouwens A., Zhao K.-N., De Jersey J., Lavin M.F., Guddat L.W. Characterization and structural analysis of a potent anticoagulant phospholipase A2 from Pseudechis australis snake venom. Toxicon. 2016;111:37–49. doi: 10.1016/j.toxicon.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 201.Deka A., Sharma M., Sharma M., Mukhopadhyay R., Doley R. Purification and partial characterization of an anticoagulant PLA2 from the venom of indian Daboia russelii that induces inflammation through upregulation of proinflammatory mediators. J. Biochem. Mol. Toxicol. 2017;31(10) doi: 10.1002/jbt.21945. [DOI] [PubMed] [Google Scholar]
- 202.Mukherjee A.K. The pro-coagulant fibrinogenolytic serine protease isoenzymes purified from Daboia russelii russelii venom coagulate the blood through factor V activation: role of glycosylation on enzymatic activity. PLoS One. 2014;9(2) doi: 10.1371/journal.pone.0086823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Huang J., Song W., Hua H., Yin X., Huang F., Alolga R.N. Antithrombotic and anticoagulant effects of a novel protein isolated from the venom of the Deinagkistrodon acutus snake. Biomed. Pharmacother. 2021;138 doi: 10.1016/j.biopha.2021.111527. [DOI] [PubMed] [Google Scholar]
- 204.Song J., Xu X., Zhang Y., Guo M., Yan X., Wang S., Gao S. Purification and characterization of AHPM, a novel non-hemorrhagic P-IIIc metalloproteinase with alpha-fibrinogenolytic and platelet aggregation-inhibition activities, from Agkistrodon halys pallas venom. Biochimie. 2013;95(4):709–718. doi: 10.1016/j.biochi.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 205.Matias M.S., de Sousa B.B., da Cunha Pereira D.F., Dias E.H.V., Mamede C.C.N., de Queiroz M.R., Silva A.C.A., Dantas N.O., Soares A.M., de Oliveira Costa J., de Oliveira F. BaltDC: purification, characterization and infrared spectroscopy of an antiplatelet DC protein isolated from Bothrops alternatus snake venom. J. Venomous Anim. Toxins Incl. Trop. Dis. 2017;23:36. doi: 10.1186/s40409-017-0126-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Dias E.H.V., Dos Santos Paschoal T., da Silva A.P., da Cunha Pereira D.F., de Sousa Simamoto B.B., Matias M.S., Santiago F.M., Rosa J.C., Soares A., Santos-Filho N.A., de Oliveira F., Mamede C.C.N. BaltPLA2: a new phospholipase A2 from Bothrops alternatus Snake venom with antiplatelet aggregation activity. Protein Pept. Lett. 2018;25(10):943–952. doi: 10.2174/0929866525666181004101622. [DOI] [PubMed] [Google Scholar]
- 207.de Queiroz M.R., Mamede C.C., de Morais N.C., Fonseca K.C., de Sousa B.B., Migliorini T.M., Pereira D.F., Stanziola L., Calderon L.A., Simoes-Silva R., Soares A.M., de Oliveira F. Purification and characterization of BmooAi: a new toxin from Bothrops moojeni snake venom that inhibits platelet aggregation. Biomed. Res. Int. 2014;2014 doi: 10.1155/2014/920942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.de Queiroz M.R., Mamede C.C., Fonseca K.C., de Morais N.C., de Sousa B.B., Santos-Filho N.A., Beletti M.E., Arantes E.C., Stanziola L., de Oliveira F. Rapid purification of a new P-I class metalloproteinase from Bothrops moojeni venom with antiplatelet activity. Biomed. Res. Int. 2014;2014 doi: 10.1155/2014/352420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Silveira L.B., Marchi-Salvador D.P., Santos-Filho N.A., Silva F.P., Jr., Marcussi S., Fuly A.L., Nomizo A., da Silva S.L., Stábeli R.G., Arantes E.C. Isolation and expression of a hypotensive and anti-platelet acidic phospholipase A2 from Bothrops moojeni snake venom. J. Pharm. Biomed. Anal. 2013;73:35–43. doi: 10.1016/j.jpba.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 210.Teixeira S.S., Silveira L.B., da Silva F.M., Marchi-Salvador D.P., Silva F.P., Izidoro L.F.M., Fuly A.L., Juliano M.A., dos Santos C.R., Murakami M.T. Molecular characterization of an acidic phospholipase A2 from Bothrops pirajai snake venom: synthetic C-terminal peptide identifies its antiplatelet region. Arch. Toxicol. 2011;85(10):1219–1233. doi: 10.1007/s00204-011-0665-6. [DOI] [PubMed] [Google Scholar]
- 211.Ferreira F.B., Gomes M.S.R., De Souza D.L.N., Gimenes S.N.C., Castanheira L.E., Borges M.H., Rodrigues R.S., Yoneyama K.A.G., Brandeburgo M.I.H., Rodrigues V.M. Molecular cloning and pharmacological properties of an acidic PLA2 from Bothrops pauloensis snake venom. Toxins. 2013;5(12):2403–2419. doi: 10.3390/toxins5122403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Saoud S., Chérifi F., Benhassine T., Laraba-Djebari F. Purification and characterization of a platelet aggregation inhibitor and anticoagulant cc 5_NTase, CD 73-like, from Cerastes cerastes venom. J. Biochem. Mol. Toxicol. 2017;31(5) doi: 10.1002/jbt.21885. [DOI] [PubMed] [Google Scholar]
- 213.Kiheli H., Cherifi F., Ameziani M., Saoud S., Hariti G., Laraba-Djebari F. Isolation and characterization of CD39-like phosphodiesterase (Cc-PDE) from Cerastes cerastes venom: molecular inhibitory mechanism of antiaggregation and anticoagulation. Protein Pept. Lett. 2021;28(4):426–441. doi: 10.2174/0929866527666200813200148. [DOI] [PubMed] [Google Scholar]
- 214.Kong Y., Sun Q., Zhao Q., Zhang Y. Purification and characterization of a novel antiplatelet peptide from Deinagkistrodon acutus venom. Toxins. 2018;10(8):332. doi: 10.3390/toxins10080332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Chen Z., Wu J., Zhang Y., Yang X., Yu G., Zhu S., Lee W., Lu Q., Zhang Y. A novel platelet glycoprotein ib-binding protein with human platelet aggregation-inhibiting activity from Trimeresurus jerdonii venom. Toxicon. 2011;57(5):672–679. doi: 10.1016/j.toxicon.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 216.Chanda C., Sarkar A., Sistla S., Chakrabarty D. Anti-platelet activity of a three-finger toxin (3FTx) from indian monocled cobra (Naja kaouthia) venom. Biochem. Biophys. Res. Commun. 2013;441(3):550–554. doi: 10.1016/j.bbrc.2013.10.125. [DOI] [PubMed] [Google Scholar]
- 217.Kumar M., Girish K., Vishwanath B., Kemparaju K. The metalloprotease, NN-PF3 from Naja naja venom inhibits platelet aggregation primarily by affecting α2β1 integrin. Ann. Hematol. 2011;90(5):569–577. doi: 10.1007/s00277-010-1103-1. [DOI] [PubMed] [Google Scholar]
- 218.Suntravat M., Jia Y., Lucena S.E., Sánchez E.E., Pérez J.C. cDNA cloning of a snake venom metalloproteinase from the eastern diamondback rattlesnake (Crotalus adamanteus), and the expression of its disintegrin domain with anti-platelet effects. Toxicon. 2013;64:43–54. doi: 10.1016/j.toxicon.2012.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Kalita B., Patra A., Jahan S., Mukherjee A.K. First report of the characterization of a snake venom apyrase (Ruviapyrase) from indian Russell's viper (Daboia russelii) venom. Int. J. Biol. Macromol. 2018;111:639–648. doi: 10.1016/j.ijbiomac.2018.01.038. [DOI] [PubMed] [Google Scholar]
- 220.Oyama E., Takahashi H. Purification and characterization of two high molecular mass snake venom metalloproteinases (P-III SVMPs), named SV-PAD-2 and HR-Ele-1, from the venom of Protobothrops elegans (Sakishima-habu) Toxicon. 2015;103:30–38. doi: 10.1016/j.toxicon.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 221.Zaqueo K.D., Kayano A.M., Domingos T.F., Moura L.A., Fuly A.L., da Silva S.L., Acosta G., Oliveira E., Albericio F., Zanchi F.B. BbrzSP-32, the first serine protease isolated from Bothrops brazili venom: purification and characterization. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2016;195:15–25. doi: 10.1016/j.cbpa.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 222.Carone S.E., Menaldo D.L., Sartim M.A., Bernardes C.P., Caetano R.C., da Silva R.R., Cabral H., Barraviera B., Junior R.S.F., Sampaio S.V. BjSP, a novel serine protease from Bothrops jararaca snake venom that degrades fibrinogen without forming fibrin clots. Toxicol. Appl. Pharmacol. 2018;357:50–61. doi: 10.1016/j.taap.2018.08.018. [DOI] [PubMed] [Google Scholar]
- 223.Menaldo D.L., Bernardes C.P., Santos-Filho N.A., de Andrade Moura L., Fuly A.L., Arantes E.C., Sampaio S.V. Biochemical characterization and comparative analysis of two distinct serine proteases from Bothrops pirajai snake venom. Biochimie. 2012;94(12):2545–2558. doi: 10.1016/j.biochi.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 224.Oyama E., Kitagawa Y., Takahashi H. Primary structure and characterization of a non hemorrhagic metalloproteinase with fibrinolytic activity, from the snake venom of Protobothrops tokarensis (Tokara-habu) Toxicon. 2013;70:153–161. doi: 10.1016/j.toxicon.2013.04.004. [DOI] [PubMed] [Google Scholar]