Abstract
Double-stranded RNA (dsRNA) accumulates in virus-infected mammalian cells and signals the activation of host defense pathways of the interferon system. We describe here a novel form of dsRNA-triggered signaling that leads to the stimulation of the p38 mitogen-activated protein kinase (p38 MAPK) and the c-Jun NH2-terminal kinase (JNK) and of their respective activators MKK3/6 and SEK1/MKK4. The dsRNA-dependent signaling to p38 MAPK was largely intact in cells lacking both RNase L and the dsRNA-activated protein kinase (PKR), i.e., the two best-characterized mediators of dsRNA-triggered antiviral responses. In contrast, activation of both MKK4 and JNK by dsRNA was greatly reduced in cells lacking RNase L (or lacking both RNase L and PKR) but was restored in these cells when introduction of dsRNA was followed by inhibition of ongoing protein synthesis or transcription. These results are consistent with the notion that the role of RNase L and PKR in the activation of MKK4 and JNK is the elimination, via inhibition of protein synthesis, of a labile negative regulator(s) of the signaling to JNK acting upstream of SEK1/MKK4. In the course of these studies, we identified a long-sought site of RNase L-mediated cleavage in the 28S rRNA, which could cause inhibition of translation, thus allowing the activation of JNK by dsRNA. We propose that p38 MAPK is a general participant in dsRNA-triggered cellular responses, whereas the activation of JNK might be restricted to cells with reduced rates of protein synthesis. Our studies demonstrate the existence of alternative (RNase L- and PKR-independent) dsRNA-triggered signaling pathways that lead to the stimulation of stress-activated MAPKs. Activation of p38 MAPK (but not of JNK) was demonstrated in mouse fibroblasts in response to infection with encephalomyocarditis virus (ECMV), a picornavirus that replicates through a dsRNA intermediate. Fibroblasts infected with EMCV (or treated with dsRNA) produced interleukin-6, an inflammatory and pyrogenic cytokine, in a p38 MAPK-dependent fashion. These findings suggest that stress-activated MAPKs participate in mediating inflammatory and febrile responses to viral infections.
Double-stranded RNA (dsRNA) produced during viral infections triggers stress response pathways that lead to elimination of infected cells by apoptosis. Two complementary but independent cellular dsRNA-detecting systems have been implicated in the translational inhibition in response to viral infection: the 2-5A system and the dsRNA-activated protein kinase (PKR) (for a recent review, see reference 55). The 2-5A system is composed of a family of dsRNA-dependent enzymes known as 2′-5′ oligoadenylate synthetases (OAS) (5) and the dormant cytosolic RNase L (64) (for recent reviews on the 2-5A system and RNase L, see references 45 and 52, respectively). Upon dsRNA binding, OAS produce unusual second messengers, short 2′-5′-linked oligoadenylates (2-5A) (32), which, in turn, specifically bind to and activate RNase L (64). Activated RNase L cleaves diverse RNA substrates, including 18S and 28S rRNAs, thus inhibiting cellular protein synthesis (53, 61). PKR (41) is also a dormant enzyme directly activated by binding of dsRNA (for recent reviews, see references 8, 10, 11, 16, 30, 46, 55, and 60). A major substrate of PKR is the α subunit of the eukaryotic translation initiation factor 2 (eIF-2α) (38). Phosphorylation of eIF-2α greatly reduces the rate of initiation of translation (9). While certain viruses (e.g., encephalomyocarditis virus [EMCV]) trigger activation of RNase L and PKR, other viruses (e.g., vaccinia virus) are able to evade the antiviral action of these enzymes (55).
The p38 mitogen-activated protein kinases (p38 MAPKs) and the c-Jun NH2-terminal kinases (JNKs) define the stress-responsive family of the MAPK superfamily of protein kinases (for recent reviews, see references 12, 18, 27, and 49). These kinases are strongly activated in cells subjected to osmotic stress (15, 20), UV radiation (22, 23, 26, 44), disregulated K+ currents (24), RNA-damaging agents (25), and a multitude of other stresses, as well as inflammatory cytokines (47, 59), endotoxin (19, 20), and withdrawal of a trophic factor (37, 63). The stress-responsive MAPKs mediate a plethora of cellular responses to such stressful stimuli, including apoptosis (7, 31, 37, 43, 50, 63) and production of inflammatory and immunoregulatory cytokines (1, 6, 29, 34, 36, 42, 48, 56, 62) in diverse cell systems. All MAPKs are regulated via phosphorylation at both threonine and tyrosine residues by dual-specificity upstream kinases, designated MAPK kinases (MKK) (for a review, see reference 49). MKK3 and MKK6 are specific p38 MKK (13, 21), whereas MKK4 and MKK7 are specific JNK kinases (13, 58). MKK4 has the ability to activate p38 MAPK as well (13). A major specific downstream effector of activated p38 MAPK is another protein kinase, MAPKAP kinase 2 (2). The activity of p38 MAPK and JNK kinases is potently stimulated by some agents that inhibit protein synthesis but is unaffected by others (24–26). In this study, we have addressed for the first time the possibility of a cross talk between the dsRNA-induced signal transduction pathways that lead to inhibition of protein synthesis and the stress-responsive signal transduction pathways leading to activation of p38 MAPK and JNK. Our results provide evidence for the existence of such a cross talk, in which RNase L, PKR, and a novel, alternative, dsRNA-dependent effector system play different roles to mediate synergistically the activation of p38 MAPK and JNK by dsRNA.
MATERIALS AND METHODS
Chemicals.
Lipofectin reagent was from Gibco Bethesda Research Laboratories/Life Technologies. Polyinosinic · polycytidylic acid (pI · pC), polyinosinic acid (pI), and polycytidylic acid (pC) were from Midland Certified Reagent Co. pI · pC, pI, and pC were stored at −20°C as 10-mg/ml stock solutions in double-distilled deionized water. SB203580 (Calbiochem) was stored as a 10 mM stock solution in dimethyl sulfoxide at −20°C. Hybrid recombinant human alpha interferon (IFN-α) BBDB was a gift from H. K. Hochkeppel (17). All commonly used chemicals, emetine, and actinomycin D were from Sigma Chemical Company. Both emetine and actinomycin D were stored at −70°C in lightproof containers as 1,000× stock solutions in dimethyl sulfoxide. All radiochemicals were from DuPont NEN Research Products.
Cell culture.
All cells were maintained in Dulbecco modified Eagle's medium (DMEM) supplemented with 10% calf serum (HyClone, Logan, Utah). The 3T3(neo) and 3T3(RNaseL) cell lines have been described previously (66). The RNase L+/+ PKR+/+, RNase L−/− PKR+/+, and RNase L−/− PKR−/− fibroblasts have been described previously (65).
Lipofectin-mediated delivery of pI · pC.
The procedure for treatment of cells with pI · pC outlined below applies for all experiments presented in Fig. 1 to 5 and 7. Per milliliter of the final volume (4/4) of Lipofectin mixture, an initial concentrated mixture (containing Lipofectin and pI · pC) was prepared in 1/4 of the final volume (250 μl). To this end, 10 μl of Lipofectin (1 mg/ml) was added to serum- and antibiotic-free DMEM and mixed, and the desired amount of pI · pC was added (in a volume of 250 μl). This mixture was left for 10 min at room temperature. Finally, the remaining 3/4 of the final volume (750 μl) was added. Before the application of the Lipofectin-pI · pC mixtures, the cells were washed once with serum-free DMEM.
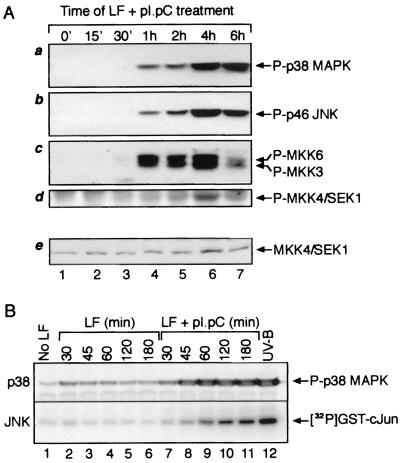
FIG. 1.
Phosphorylation of p38 MAPK, JNK, MKK3, MKK6, and SEK1/MKK4 and increased kinase activity of JNK in response to dsRNA. (A) Immunoblot analysis. HeLa cells were grown to ∼80% confluence in normal growth medium. The cells were then treated with pI · pC (3 μg/ml) in the presence of Lipofectin (LF; 10 μg/ml). At indicated times, the cells were harvested and cell lysates representing equal number of cells were subjected to immunoblot analyses with antibodies specific for the phosphorylated forms of p38 MAPK, JNK, MKK3, MKK6, and SEK1/MKK4 (see Materials and Methods). Panel e shows an immunoblot analysis of the levels of total (phosphorylated and nonphosphorylated) SEK1/MKK4 run in a parallel gel. (B) Analogous analysis of pI · pC action in Rat-1 fibroblasts, demonstrating also that pI · pC, and not Lipofectin, is the kinase-activating agent. A direct determination of JNK activity (rather than phosphorylation of JNK) after pI · pC treatment is presented. JNK1 was immunoprecipitated from Rat-1 cells, and the activity of the kinase was determined in immunocomplex kinase reactions using glutathione S-transferase–c-Jun as the substrate for phosphorylation (see Materials and Methods).
FIG. 5.
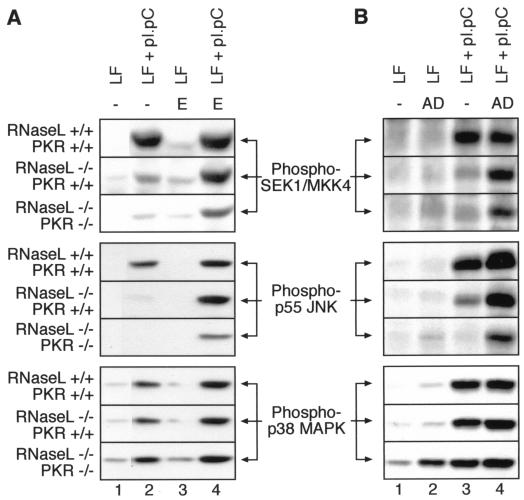
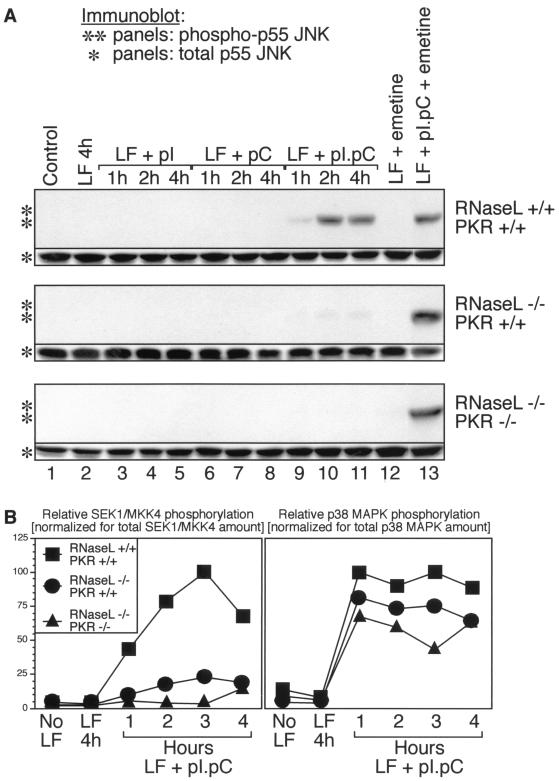
Effects of emetine and actinomycin D posttreatments on the dsRNA-induced phosphorylation of SEK/MKK4, JNK, and p38 MAPK in RNase L+/+ PKR+/+, RNase L−/− PKR+/+, and RNase L−/− PKR−/− cells determined by immunoblot analyses. The cells were grown and treated with pI · pC for 4 h as for Fig. 4A. Where indicated, emetine (E; 100 μg/ml) or actinomycin D (AD; 25 μg/ml) was added either 1 h after (emetine) or 15 min after (actinomycin D) the treatment with pI · pC. Note the lack of effect of both emetine and actinomycin D on dsRNA-induced phosphorylation of p38 MAPK. The mechanism of actinomycin D-induced p38 MAPK phosphorylation in RNase L−/− PKR−/− cells (B, bottom panel, lane 2) is unknown. LF, lipofectin.
FIG. 7.
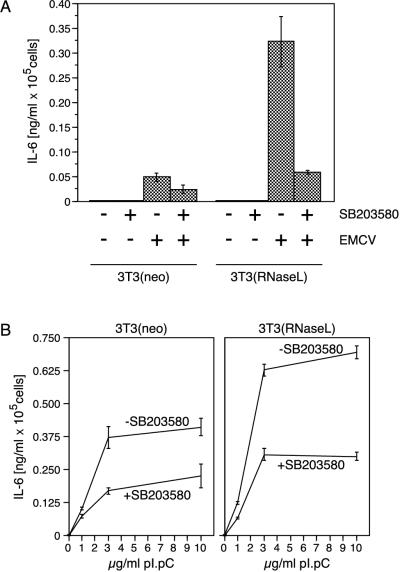
p38 MAPK-dependent expression of IL-6 in fibroblasts in response to EMCV infection or dsRNA. (A) IL-6 detection by ELISA. 3T3(neo) and 3T3(RNaseL) cells were grown and infected as described in Materials and Methods, and the appearance of IL-6 in the cell culture medium was assessed by ELISA as described in Materials and Methods. Error bars indicate standard deviation from experimental points in triplicate determinations. (B) Identical assessment of IL-6 expression 24 h after pI · pC treatment (0, 1, 3, or 10 μg/ml). Error bars indicate standard deviation from experimental points in triplicate determinations.
Counting of cells.
Whenever two or more cell lines were compared in immunoblot procedures or immunocomplex kinase assays (see Fig. 2A and B, 4, and 5), cells from parallel 10-cm-diameter plates representing each cell line were trypsinized and counted in a cell counter. The averaged cell numbers (from triplicate determinations) were used to calculate the volumes of cell lysates to be processed in each assay in such a way that each assay was performed with a volume of cell lysate representing the same number of cells. Typically, 1 × 105 to 5 × 105 cells were used per assay (immunoblot analyses or immunocomplex kinase assays).
FIG. 2.
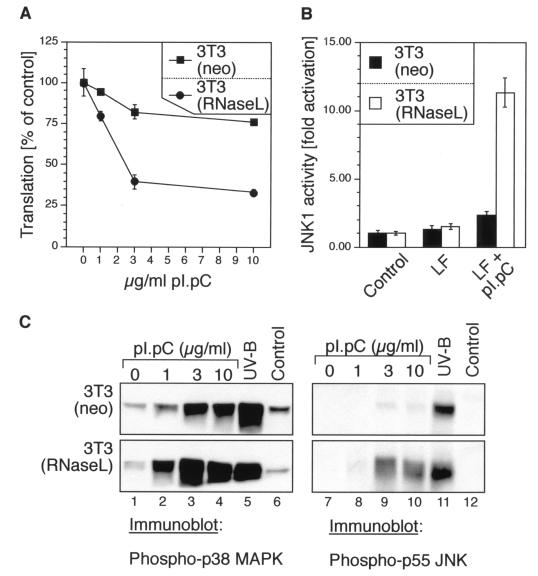
dsRNA-induced inhibition of protein synthesis, activation of JNK, and phosphorylation of p38 MAPK and JNK in 3T3(neo) and 3T3(RNaseL) cells. (A) The cells were grown in 12-well plates to ∼70% confluence in normal growth medium and then leucine deprived for 1.5 h in leucine- and serum-free DMEM. Lipofectin mixes were made in leucine- and serum-free DMEM to contain 0, 1, 3, or 10 μg of pI · pC per ml and were given to the cells. Between 2.5 and 3 h after the pI · pC addition, the cells were pulse-labeled with 1 μCi of [3H]leucine per ml and processed further as described in Materials and Methods. Error bars indicate standard deviation from experimental points in triplicate determinations. (B) Immunocomplex kinase assay. The cells were grown in 10-cm-diameter plates to ∼70% confluence in normal growth medium. The growth medium was then exchanged, where indicated, with serum-free DMEM (control) or Lipofectin (LF)-containing serum-free DMEM, without or with 10 μg of pI · pC per ml. JNK activity was determined 3 h later in immunocomplex kinase assays as described for Fig. 1B. Error bars indicate standard deviation from experimental points in triplicate determinations. (C) Immunoblot analyses of p38 MAPK and JNK phosphorylation, using phosphoepitope-specific antibodies. The cells were grown and treated as for panel B with the indicated concentrations of pI · pC. For the UV-B irradiation, the cells were given a 1,200-J/m2 dose of UV-B as described previously (26) and harvested 30 min later.
Antibodies and immunoblot analyses.
The antibodies against the phosphorylated forms of p38 MAPK, JNK, MKK3/6, and SEK1/MKK4 were from New England BioLabs. The immunoprecipitating anti-JNK1 antibody (C-17) used for the immunocomplex kinase assays shown in Fig. 1B and 2B was from Santa Cruz Biotechnology. The antibodies against total (phosphorylated and nonphosphorylated) SEK1/MKK4 (K-18), p55 JNK and p46 JNK (JNK-FL), and p38 MAPK (C-20 and N-20) were from Santa Cruz Biotechnology. Separation of proteins by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (13% gel) and electrotransfer onto polyvinylidene difluoride membranes (Millipore) were performed by standard procedures. Immunodetections with phosphoepitope-specific antibodies were performed as instructed by the manufacturer.
Immunocomplex kinase assays.
Immunoprecipitations and immunocomplex kinase assays to determine JNK1 activity were performed as described previously (25).
Measurement of protein synthesis via incorporation of [3H]leucine.
Incorporation of [3H]leucine was performed as described previously (25).
Preparation of RNA and Northern blot procedures.
Total RNA was prepared by using an RNeasy kit from Qiagen as directed by the manufacturer. Two micrograms of total RNA was resolved in 1% denaturing agarose gels and transferred onto a Hybond-N membrane (Amersham-Pharmacia). The hybridizations with 28S rRNA-specific oligonucleotide probes were performed by using ExpressHyb hybridization solution (Clontech) as directed by the manufacturer. All probes used have been listed elsewhere (26). The following probes were used for the Northern blot presented in Fig. 8A: 5′-CAGAAGGATCGTGAGG-3′ (panel b) and 5′-TAGGTTGACATCGTTTC-3′ (panel c).
FIG. 8.
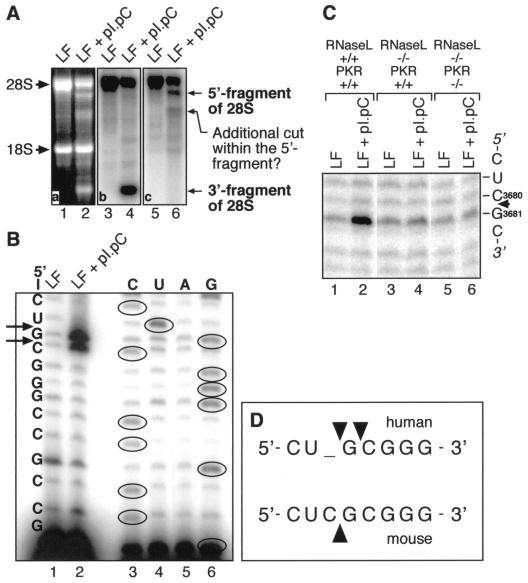
Identification of a major site of dsRNA-induced cleavage of 28S rRNA in human and mouse cells. (A) Northern blot analysis. HeLa cells were treated for 3 h with pI · pC (3 μg/ml). Total RNA was isolated, and 2-μg aliquots were used for Northern blot detection of cleavage fragments of 28S rRNA, using DNA oligonucleotides hybridizing specifically to 28S rRNA and fragments thereof. One probe hybridized to the fragment located 3′ of the site of cleavage (panel b), whereas the other hybridized to the fragment located 5′ of the site of cleavage (panel c). LF, Lipofectin. (B) The RNA preparation used for panel A was subjected to a reverse transcriptase-driven primer extension using the primer 5′-GAGTAGTGGTATTTCAC-3′. Direct RNA sequencing using the same primer is shown to the right. The sites of cleavage are represented by arrows. (C) Analysis identical to that shown in panel B was performed with RNase L+/+ PKR+/+, RNase L−/− PKR+/+, and RNase L−/− PKR−/−cells, except that 10 μg of pI · pC per ml was used. (D) Alignment of the human and mouse sequences within the region of dsRNA-induced cleavage of the 28S rRNA. The sites of cleavage are represented by arrowheads.
Reverse transcription of rRNA by primer extension.
The reverse transcription method used has been described in detail previously (25, 26). The primer used in the primer extensions shown in Fig. 8B and C was 5′-GAGTAGTGGTATTTCAC-3′.
Infection of cells with EMCV.
3T3(neo) and 3T3(RNaseL) cells were grown in 10-cm-diameter dishes and incubated with IFN-α BBDB (2,000 U/ml) for 20 h. The medium was replaced twice with serum-free, antibiotic-free DMEM, and cells were infected with EMCV at a multiplicity of infection (MOI) of 10 and incubated at 37°C. Cells were harvested at different times by scraping, washed in phosphate-buffered saline, and pelleted by centrifugation at 4°C. Cell pellets were stored at −70°C.
Determination of IL-6 production.
3T3(neo) and 3T3(RNaseL) cells were grown in 12-well plates until confluent (∼2.5 × 105 cells/well). The medium was replaced twice with serum-free DMEM, and cells were left uninfected or were infected with EMCV at an MOI of 40 in a volume of 1 ml per well, in the presence or in the absence of 10 μM SB203580 given 1 h before the infection. The cells were then incubated at 37°C for 18 h. Alternatively, cells were treated with pI · pC as described above for 24 h, also in the presence or in the absence of an SB203580 pretreatment. Conditioned media were collected and cleared of cell debris by centrifugation, and the presence of interleukin-6 (IL-6) was determined quantitatively, using a Quantikine M mouse IL-6 enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems) as instructed by the manufacturer.
RESULTS
Activation of MKK3/6-p38 MAPK and SEK1/MKK4-JNK kinase cascades by dsRNA.
To determine whether accumulation of dsRNA in cells would activate p38 MAPK and JNK, we investigated the phosphorylation of p38 MAPK and JNK (or measured JNK activity) in cell lysates prepared from HeLa (Fig. 1A) or Rat-1 (Fig. 1B) cells treated with pI · pC, a synthetic dsRNA, in the presence of the cationic lipid vehicle Lipofectin (see Materials and Methods). One hour after treatment of HeLa cells with pI · pC, p38 MAPK was dually phosphorylated at threonine-180 and tyrosine-182 (amino acid numbering corresponding to the sequence of human p38α MAPK) (Fig. 1A, panel a). Similar to p38 MAPK, there was a clear pattern of dual phosphorylation of p46 JNK at threonine-183 and tyrosine-185 (amino acid numbering corresponding to the sequence of human JNK2), as detected by immunoblotting with an antibody recognizing only the dually phosphorylated form of JNK (Fig. 1A, panel b). The phosphorylations of p38 MAPK and JNK were persistent and well pronounced 6 h after the pI · pC treatment (Fig. 1A, panels a and b, lanes 4 to 7). Immunocomplex kinase activity assays (done as described previously [25]) using JNK1 immunoprecipitated from pI · pC-treated cells revealed consistently that JNK was functionally activated up to 100-fold over the basal activity of the kinase in control HeLa cells (not shown) and similarly in Rat-1 cells (Fig. 1B, lower panel, lanes 7 to 11). The following criteria were used to conclude that the response of p38 MAPK and JNK to pI · pC was a bona fide reaction of cells to intracellular accumulation of dsRNA: (i) p38 MAPK and JNK were not activated in cells treated with Lipofectin alone (Fig. 1B, 2C, 3A, and 3B), (ii) p38 MAPK and JNK were not activated in cells treated with pI · pC without Lipofectin (not shown), indicating thus the need for intracellular delivery of pI · pC, and (iii) p38 MAPK and JNK were not activated in cells treated in the presence of Lipofectin with either single-stranded RNA (ssRNA) (pI or pC) or dsDNA {p[D(IC)]} (see Fig. 3A for ssRNA; not shown for dsDNA). To investigate whether dsRNA activated p38 MAPK and JNK through an activation of their upstream kinases, we performed immunoblot analyses using antibodies specific for the phosphorylated (active) forms of MKK3, MKK6, and SEK1/MKK4. The substantial degree of amino acid identity between the phosphorylated epitopes of MKK3 and MKK6 allows their simultaneous identification with the same antibody. All three kinases displayed dsRNA-induced phosphorylations (Fig. 1A, panels c and d). Although the phosphorylation of SEK1/MKK4 was not easily detectable in HeLa cells treated with dsRNA (but also with other strong activators of JNK [Fig. 1A, panel d, and data not shown]), the dsRNA-induced phosphorylation of SEK1/MKK4 in wild-type mouse fibroblasts was very potent (see Fig. 5A, top panel, lanes 1 and 2). Identical analyses using antibodies specific for the phosphorylated (active) forms of ERK and MEK1/2 failed to detect dsRNA-induced phosphorylation of these kinases (not shown), indicating that the stress-activated members of the MAPK superfamily are specific targets for activation by intracellular accumulation of dsRNA.
FIG. 3.
dsRNA-induced inhibition of protein synthesis in RNase L+/+ PKR+/+, RNase L−/− PKR+/+, and RNase L−/− PKR−/− cells. The cells were grown in 12-well plates to ∼100% confluence in normal growth medium and then serum deprived for 24 hours in serum-free DMEM. Lipofectin (LF) mixes were made in leucine- and serum-free DMEM to contain 10 μg of pI · pC per ml and were given to the cells. Between 2.5 and 3 h after the pI · pC addition, the cells were pulse-labeled with 1 μCi of [3H]leucine per ml and processed further as described in Materials and Methods. Error bars indicate standard deviation from experimental points in triplicate determinations.
Differential responsiveness of p38 MAPK and JNK to dsRNA in cells overexpressing RNase L.
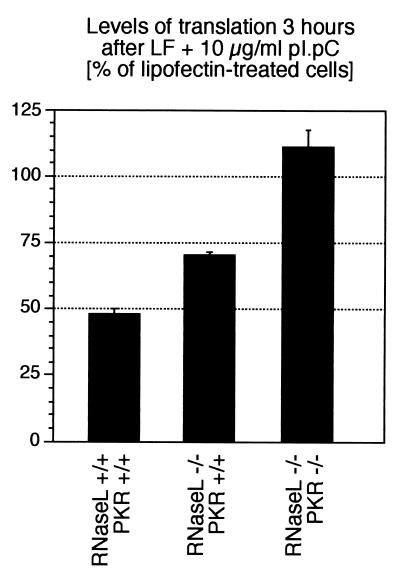
To investigate the possible involvement of RNase L in the dsRNA-induced signal transduction cascades to p38 MAPK and JNK, we used an NIH 3T3 fibroblast-derived cell line [3T3(RNaseL) cells] that overexpress RNase L approximately 100-fold over the endogenous levels of this RNase in the parental cells [3T3(neo)] (66). As measured by [3H]leucine incorporation, in the parental 3T3(neo) cells, dsRNA (10 μg/ml) treatment for 3 h inhibited total protein synthesis by only ∼20 to 25%, whereas in the 3T3(RNaseL) cells, protein synthesis was inhibited by ∼70% after 3 h of dsRNA treatment (Fig. 2A). Interestingly, after 3 h of dsRNA (10 μg/ml) treatment, JNK1 activity was increased only 2-fold in the 3T3(neo) cells, whereas the activity of the kinase in the 3T3(RNaseL) cells was increased 11-fold (Fig. 2B). The difference between the two cell lines in their dsRNA-induced JNK activities was also clearly detected at the level of phosphorylation of JNK (Fig. 2C, lanes 7 to 10, compare upper and lower panels), whose levels of phosphorylation in the 3T3(RNaseL) cells were substantially greater than in the 3T3(neo) cells. Importantly, RNase L overexpression affected specifically the response of JNK to dsRNA, as JNK was activated equally well in the two cell lines by UV radiation (Fig. 2C, lane 11, compare upper and lower panels). dsRNA, however, displayed an ability to cause a robust phosphorylation of p38 MAPK in the parental 3T3(neo) cells; furthermore, in the 3T3(RNaseL) cells, there was only a minor potentiation of p38 MAPK response to dsRNA (Fig. 2C, lanes 1 to 4, compare upper and lower panels). Thus, the ability of dsRNA to activate JNK appeared to be sensitive to the levels of RNase L, whereas the levels of RNase L were not important for the activation by dsRNA of p38 MAPK.
Differential responsiveness of p38 MAPK and JNK to dsRNA in cells lacking RNase L.
To investigate further the requirement for RNase L in the dsRNA-induced activation of stress-activated MAPKs, we used mouse embryonic fibroblasts (MEF) with the following three genotypes: RNase L+/+ PKR+/+ (wild-type MEF), RNase L−/− PKR+/+, and RNase L−/− PKR−/−. As demonstrated in Fig. 3, RNase L and PKR are solely responsible (required and sufficient) for the inhibition of protein synthesis in MEF exposed to dsRNA, each apparently contributing about 50% of the overall inhibition of protein synthesis by dsRNA. We investigated whether the response of JNK to pI · pC in MEF was a bona fide dsRNA response. Indeed, neither pI nor pC was able to cause JNK phosphorylation in the MEF with any of the three genotypes (Fig. 4A, all panels, lanes 3 to 8). However, when the response of JNK to pI · pC (dsRNA) was assessed, striking differences among the three genotypes were observed: (i) JNK was phosphorylated strongly (in a time-dependent manner) in the RNase L+/+ PKR+/+cells (Fig. 4A, top “**” panel, lanes 9 to 11), (ii) the response of JNK to dsRNA was severely reduced in the RNase L−/− PKR+/+cells (Fig. 4A, middle “**” panel, lanes 9 to 11; Fig. 5A, middle panel, lane 2; Fig. 5B, middle panel, lane 3), and (iii) the activation of JNK by dsRNA was undetectable in the RNase L−/− PKR−/−cells (Fig. 4A, lower “**” panel, lanes 9 to 11; Fig. 5A, middle panel, lane 2; Fig. 5B, middle panel, lane 3). These differences among the three genotypes were specific for the dsRNA treatment, since the levels of JNK expression in the three cell lines were comparable (Fig. 4A, all * panels) and the responsiveness of JNK to UV radiation or to anisomycin was very similar in the three cell lines (not shown). SEK1/MKK4 displayed behavior identical to that of JNK in the three cell lines (Fig. 4B, left). In contrast, p38 MAPK remained generally well responsive to dsRNA in all of the cell lines (Fig. 4C, right; Fig. 5), although a tendency toward reduced responsiveness (RNase L+/+ PKR+/+ > RNase L−/− PKR+/+ > RNase L−/− PKR−/−) was observed (Fig. 4C). In conclusion, the results presented in Fig. 2 and 4 demonstrated that the presence and activity of RNase L and PKR strongly influence the ability of dsRNA to activate JNK but that they do not appear to play a major role in the ability of dsRNA to activate p38 MAPK.
FIG. 4.
dsRNA-induced phosphorylation of p38 MAPK, JNK, and SEK/MKK4 in RNase L+/+ PKR+/+, RNase L−/− PKR+/+, and RNase L−/− PKR−/− cells. (A) Immunoblot analyses of JNK phosphorylation (∗∗ panels) and total levels of JNK (∗ panels). The cells were grown in 10-cm-diameter plates to ∼100% confluence in normal growth medium and then serum deprived for 24 h in serum-free DMEM. Lipofectin (LF) mixes were made in serum-free DMEM to contain no nucleic acid or 10 μg of either pI, pC, or pI · pC per ml and were given to the cells for the indicated times. Where indicated, the cells were treated with emetine (100 μg/ml) 1 h after the addition of Lipofectin mixes. (B) Graphic presentation of immunoblot analyses. Cells were treated as indicated in the graphs and as for panel A, and analyses were performed with antibodies against the phosphorylated forms of SEK1/MKK4 and p38 MAPK. The membranes were stripped and rehybridized with antibodies recognizing total (phosphorylated and nonphosphorylated) SEK1/MKK4 and p38 MAPK. For each immunoblot, appropriately nonsaturated film exposures were selected and scanned, and the scanned images were imported into IP Lab Gel software for quantification. The maximum level of phosphorylation of each kinase (after normalization for total amount of the kinase) was expressed as 100%.
Neither RNase L nor PKR is absolutely required for the activation of JNK by dsRNA.
We noticed that a major difference among the five MEF cell lines used in Fig. 2 to 4 [3T3(neo), 3T3(RNase L), RNase L+/+ PKR+/+, RNase L−/− PKR+/+, and RNase L−/−] was the varied degree of dsRNA-induced inhibition of protein synthesis (Fig. 2A and 3). In each case, a stronger inhibition of protein synthesis correlated well with stronger activation of JNK, suggesting the possibility of an important role for the inhibition of translation in the dsRNA-induced activation of JNK. We undertook, therefore, to examine the responsiveness of JNK to dsRNA in the RNase L+/+ PKR+/+, RNase L−/− PKR+/+, and RNase L−/− PKR−/−cells under conditions of protein synthesis being inhibited to the same degree in each of the three cell lines. To this end, we treated the cells with dsRNA for 4 h (Fig. 4A, all ** panels, lanes 11 and 13). After the dsRNA addition, the cells were either left without further treatment (Fig. 4A, all ** panels, lanes 11) or treated with emetine 1 h after the dsRNA treatment (Fig. 4A, all ** panels, lanes 13). Emetine irreversibly inhibits protein synthesis by more than 98% within the first minute after addition and, importantly, it does not activate JNK in confluent, serum-deprived rodent fibroblast cells (reference 25 and Fig. 4A, all ** panels, lanes 12). In striking contrast to the results described above (Fig. 4A, all ** panels, lanes 9 to 11), when dsRNA treatment was followed by a complete inhibition of protein synthesis (emetine addition), the cells of all three genotypes appeared able to activate JNK equally well (Fig. 4A, all ** panels, lanes 13). Furthermore, under conditions of emetine posttreatment, dsRNA also became able to activate SEK1/MKK4 in the RNase L−/− PKR+/+and RNase L−/− PKR−/−cells (Fig. 5A, top panels, compare lanes 2 and 4). To test whether this striking effect of emetine was indeed attributable to its ability to block protein synthesis, we performed identical experiments using pactamycin and T-2 toxin, two unrelated antibiotic inhibitors of translation that do not activate JNK (25); the use of pactamycin or T-2 toxin led to results identical to those observed in assays using emetine (not shown). We next tested whether the inhibition of protein synthesis required for the activation of JNK by dsRNA in the RNase L−/− PKR+/+and RNase L−/− PKR−/−cells could be mimicked by inhibition of transcription. To this end, we used a posttreatment of cells with actinomycin D 15 min after treatment with dsRNA. Similar to the effects of emetine, pactamycin, and T-2 toxin, the effect of actinomycin D was to restore a wild-type cell-like responsiveness of JNK and SEK1/MKK4 to dsRNA in the RNase L−/− PKR+/+and RNase L−/− PKR−/−cells (Fig. 5B, top and middle panels, compare lanes 3 and 4). In conclusion, the results shown in Fig. 4A and 5 suggest that the roles of both RNase L and PKR in the signal transduction pathway(s) generated by dsRNA that lead to the activation of JNK are indirect (see Discussion).
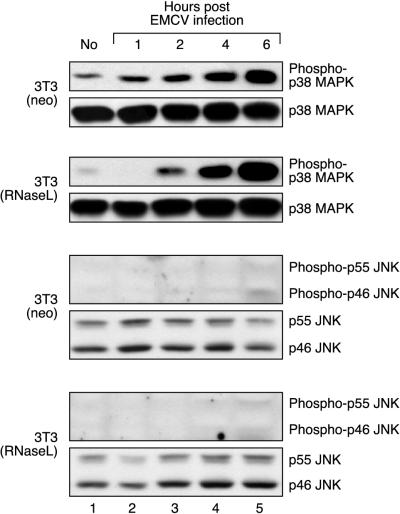
Activation of p38 MAPK, but not of JNK, in mouse fibroblasts infected with EMCV.
To begin to understand the biological role of this newly identified dsRNA-triggered signal transduction pathway that leads to the activation of stress-activated MAPKs, we investigated whether infection of cells by EMCV, a virus known for its ability to trigger dsRNA-dependent cellular reactions, would lead to activation of p38 MAPK and/or JNK. Indeed, both in the 3T3(neo) and in the 3T3(RNaseL) cells, EMCV infection caused a clear pattern of p38 MAPK phosphorylation that was detectable as early as 2 h postinfection and persisted at least for the next 4 h (Fig. 6, lanes 3 and 4). In contrast, we failed to detect in these cells an EMCV-induced phosphorylation of either SEK1/MKK4 (not shown) or JNK (Fig. 6, lanes 3 and 4). One possible explanation for the inability of EMCV to trigger JNK activation could be that in the cell type used, the virus fails to ensure a sufficient inhibition of protein synthesis to permit the activation of JNK (see Discussion). In support of this hypothesis, we were consistently unable to detect a decrease of [3H]leucine incorporation in EMCV-infected fibroblasts (up to 6 h after EMCV infection at an MOI of ≥10 [not shown]).
FIG. 6.
Phosphorylation of p38 MAPK, but not JNK, in EMCV-infected cells. 3T3(neo) and 3T3(RNaseL) cells were grown in 10-cm-diameter dishes and incubated with IFN-α BBDB (2,000 U/ml) for 20 h. The medium was replaced twice with serum-free, antibiotic-free DMEM; cells were infected with EMCV at an MOI of 10 and incubated at 37°C. At indicated times, the cell were harvested and processed for immunoblot analyses. Appropriate positive controls (not shown) were performed for the immunoblot analysis of JNK phosphorylation to demonstrate that, when present, phosphorylated JNK was detectable.
Both EMCV infection and dsRNA trigger the expression of the inflammatory and pyrogenic cytokine IL-6 through a mechanism that involves p38 MAPK.
We speculated that one possible biological consequence of p38 MAPK activation in EMCV-infected cells may be the production of inflammatory and pyrogenic cytokines that, in turn, may participate in the inflammatory and febrile responses accompanying viral infections. To test this hypothesis, we determined whether EMCV infection of fibroblasts leads to the production of IL-6, a cytokine whose expression has been previously found to depend on the presence of a functional MKK3→p38 MAPK→MAPKAP kinase 2 signaling cascade (2, 34, 62). In noninfected 3T3(neo) or 3T3(RNaseL) fibroblasts, the levels of IL-6 released into the medium were below the detection ability of the method used (≤3 pg/ml). However, 18 h after infection of these fibroblasts with EMCV (MOI of 40), there was a substantial increase in IL-6 levels in the medium (Fig. 7A). The 3T3(RNaseL) cells were significantly more active in producing IL-6 than the parental 3T3(neo) cells. Furthermore, whereas the secretion of IL-6 by the 3T3(neo) cells was maximal after EMCV infection at an MOI of 40, the peak of IL-6 secretion by the 3T3(RNaseL) cells was achieved at a 100-fold-lower viral concentration (MOI of 0.4 [not shown]). We are unable to determine whether these differences result from the overexpression of RNase L or reflect a clonal difference. Importantly, however, SB203580 (10 μM), a specific inhibitor of p38 MAPK (36a), was able to inhibit the EMCV-induced IL-6 production in either cell line by more than 50% (Fig. 7A), suggesting the involvement of p38 MAPK in virus-induced IL-6 expression. Similarly, treatment of the same cells with pI · pC led to the appearance of IL-6 in the cell culture medium, which was also inhibited by the p38 MAPK inhibitor (Fig. 7B).
Identification of the major dsRNA-induced site of cleavage in the 28S rRNA: (i) localization of the site of cleavage in the L1 protuberance and (ii) mediation of the cleavage by RNase L.
Since the dsRNA-induced, RNase L-mediated inhibition of protein synthesis appeared crucial for the ability of dsRNA to activate JNK, we attempted to identify mechanisms of this inhibition. Previously, RNase L was observed to cleave rRNA in intact ribosomes, producing discrete and characteristic cleavage products (54, 61). We observed that treatment of HeLa cells with pI · pC led to a specific pattern of 28S rRNA cleavage, as demonstrated by an electrophoretic analysis in denaturing agarose gels and ethidium bromide-UV visualization (Fig. 8A, compare lanes 1 and 2). Of all rRNAs and tRNA, 28S rRNA was the preferred target for a dsRNA-induced cleavage in HeLa cells (Fig. 8A, lane 2; not shown for 5.8S rRNA, 5S rRNA, and tRNA). Northern blot analyses using six oligonucleotide probes specific for different parts of the 28S rRNA revealed the presence of two major nucleolytic fragments in dsRNA-treated HeLa cells: one fragment contained the 3′ end of the 28S rRNA (Fig. 8A, lane 4), and the other contained the 5′ end of the 28S rRNA (Fig. 8A, lane 6). Some further fragmentation of the 5′-end fragment was also evident (Fig. 8A, lane 6). Since the 3′ end of the 28S rRNA contains two structural motifs directly involved in peptidyl transfer and ribosomal translocation (the peptidyl transferase ring and the sarcin/ricin loop [reference 25 and references therein]), we set out to determine whether the dsRNA-induced cleavage that leads to the formation of the 3′-end fragment occurs within any of these functionally essential motifs. Precise mapping of the 3′ end of the 28S rRNA was therefore undertaken, using a reverse transcriptase-driven primer extension (described previously [25, 26]). This analysis allowed the identification within the whole region of a single sequence 5′-C3998-U3999-G4000-C4001-G4002-3′ (nucleotide numbering corresponding to the human 28S rDNA sequence, GenBank accession no. M11167) as the exact target for cleavage, with two cuts (between 5′-U3999-G4000-3′ and between 5′-G4000-C4001-3′) occurring with approximately equal intensity (Fig. 8B, lanes 1 and 2). The thus identified region of dsRNA-induced cleavage is located neither in the peptidyltransferase ring nor in the sarcin/ricin loop. Rather, the site of cleavage was determined to belong to the so-called L1 protuberance of the ribosome that is believed to be involved in the formation of the exit site (E site) of the ribosome (3, 14).
The following criteria were used to conclude that the dsRNA-induced 28S rRNA cleavage is mediated by RNase L: (i) treatment of cells with 2-5A, a specific RNase L activator, induced 28S rRNA cleavage at the same site (not shown), and (ii) the dsRNA-induced cleavage to the 28S rRNA was detected in the RNase L+/+ PKR+/+ MEF but not in the RNase L−/− PKR+/+ or RNase L−/− PKR−/− MEF (Fig. 8C, compare lanes 2, 4, and 6). (Note that due to an additional C nucleotide present in the mouse 28S rRNA but absent in the human 28S rRNA, only a single site of cleavage appears in the region of the mouse 28S rRNA in question [Fig. 8D]).
DISCUSSION
Cooperation of RNase L, PKR, and a novel dsRNA-sensing effector system in the activation of stress-responsive MAPKs.
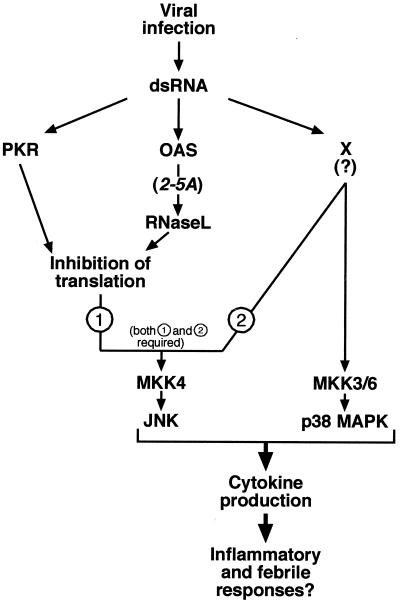
In this work, we used a cell culture-based approach to identify and characterize novel participants in the cellular signal transduction responses to dsRNA. Our study found that p38 MAPK and JNK, representatives of the stress-activated MAPKs, are potently activated in dsRNA-treated cells of both mesenchymal (fibroblasts) and epithelial (HeLa cells) origins (Fig. 1, 2, 4, and 5). PKR and the OAS/2-5A/RNase L system are the best characterized effectors of dsRNA known to trigger bona fide signal transduction events in dsRNA-treated cells (reference 55 and references therein). Using cells lacking both PKR and RNase L alleles (RNase L−/− PKR−/−), we now demonstrate conclusively the existence of an additional cellular dsRNA-sensing effector system that triggers the activation of p38 MAPK and (under conditions of protein synthesis inhibition; see below) of JNK. A hypothetical model summarizing the findings of this study is presented in Fig. 9. According to this model, intracellular accumulation of dsRNA occurs as a result of a viral infection. dsRNA causes inhibition of protein synthesis via PKR and the OAS/2-5A/RNase L system. A yet to be identified dsRNA-sensing effector system (X), different from PKR and RNase L, couples the signal (dsRNA) to signal transduction cascades leading ultimately to the activation of MKK3/6 and p38 MAPK, on one hand, and of SEK1/MKK4 and JNK, on the other hand. A major difference between the mechanisms of activation of p38 MAPK and JNK by dsRNA is the requirement for an RNase L- and PKR-mediated inhibition of protein synthesis for the successful activation of JNK by dsRNA. dsRNA-activated stress-activated MAPK may mediate the production of proinflammatory and immunoregulatory cytokines by virally infected cells. In some cases, dsRNA-activated p38 MAPK and JNK may mediate apoptosis of virally infected cells. Our data, however, do not allow us to rule out the possibility that p38 MAPK and JNK are activated by dsRNA through two completely independent dsRNA-induced effector systems. Since we consider this possibility less likely, it is not indicated in the model proposed in Fig. 9.
FIG. 9.
Model for the mechanisms of activation of stress-activated MAP kinases by dsRNA. See the text for explanation.
dsRNA is a genuine signaling molecule that triggers upstream signal transduction pathways to activate p38 MAPK and JNK.
Most cellular stressors that activate p38 MAPK and JNK do so by engaging signal transduction cascades that act upstream of their MKKs. In some instances, however, an action of a cellular stressor on a MAPK has been attributed to the stress-induced inactivation of a negative regulator of a MAPK. For instance, the activation of JNK by sodium arsenite has been proposed to result, in part, from inactivation of a JNK phosphatase without a strong potentiation by arsenite of SEK1/MKK4 activity (4, 40). A major effect of dsRNA accumulation in mammalian cells is the inhibition of protein synthesis. This creates the possibility for a dsRNA-induced activation of p38 MAPK and JNK through the disappearance of a short-lived MAPK phosphatase (e.g., MKP-1 [57]). Such a hypothesis, however, is not supported by the experimental evidence shown here and in our previous work: (i) dsRNA triggers the phosphorylation of MKK3/6 and SEK1/MKK4 (Fig. 1A and 5), (ii) inhibition of protein synthesis per se cannot activate stress-activated MAPKs (25, 26) (Fig. 4A and 5A), and (iii) the dsRNA-induced activation of p38 MAPK is readily observable in the RNase L−/− PKR−/− cells that do not show any inhibition of protein synthesis in response to dsRNA (Fig. 3, 4B, and 5). Thus, it seems safe to conclude that dsRNA is a genuine signaling molecule that triggers upstream signal transduction pathways to activate p38 MAPK and JNK.
Involvement of a putative labile repressor(s) in the dsRNA-induced activation of JNK but not p38 MAPK.
What role does the dsRNA-induced, RNase L- and PKR-mediated inhibition of translation play in the activation of p38 MAPK and JNK, respectively? Figures 2C, 4B, and 5A demonstrate that p38 MAPK is responsive to dsRNA treatment independent of the levels of protein synthesis in the dsRNA-treated cells. However, JNK could be activated by dsRNA in the RNase L−/− PKR−/− cells only if the dsRNA treatment was followed by inhibition of protein synthesis (Fig. 4A and 5B) or by inhibition of ongoing transcription (actinomycin D posttreatment [Fig. 5B]). Under the conditions tested, actinomycin D did not affect translation, as measured by [3H]leucine incorporation into actinomycin D-treated cells (not shown). Taken together, these findings are consistent with the notion that the role of dsRNA-induced inhibition of translation (and thus, by implication, the role of RNase L and PKR) is to ensure, through inhibition of protein synthesis, the disappearance of a labile negative regulator(s) of the signaling to JNK. Only after the elimination of the negative regulator(s) is the positively acting dsRNA-triggered upstream signaling cascade to JNK able to potentiate JNK activity (Fig. 9). Interestingly, our results seem to eliminate the most obvious candidate for the role of such labile repressor of the JNK pathway, namely, the MAPK phosphatase MKP-1: the putative repressor(s) in question must act upstream of SEK1/MKK4 (Fig. 5A), whereas MKP-1 directly regulates JNK activity and lies downstream of SEK1/MKK4 (39). Identification of the dsRNA-sensitive negative regulator(s) of SEK1/MKK4-JNK cascade remains, therefore, a goal of future experimental work.
Mechanism of dsRNA-triggered, RNase L-mediated inhibition of protein synthesis.
The identification of the major site of dsRNA-induced, RNase L-mediated cleavage of 28S rRNA provides a possible mechanism for one of the modes of inhibition of translation in response to intracellular accumulation of dsRNA. The target site for RNase L in the 28S rRNA is part of the L1 protuberance, which has been proposed to participate in the formation of the E site of the ribosome (3, 14). dsRNA-induced cleavage in the 28S rRNA is likely to compromise the structural and functional integrity of the E site, thus possibly interfering with the release of deacylated tRNA after the peptidyl transfer and ribosomal translocation.
Possible role of dsRNA in the activation of p38 MAPK (and, under certain conditions, of JNK) by viral infections.
Figure 6 demonstrates the potent ability of EMCV to trigger the activation of p38 MAPK in fibroblasts. Based on the results presented here, it is our hypothesis that one of the triggering events for this EMCV-induced p38 MAPK activation is the intracellular accumulation of viral dsRNA. In contrast, SEK1/MKK4 and JNK phosphorylation was not detected in the EMCV-infected fibroblasts (Fig. 6). In view of the previously demonstrated requirement for a substantial reduction of the rate of ongoing protein synthesis to activate JNK via dsRNA accumulation, it is possible that such a robust inhibition of translation was not achieved in the fibroblasts infected with EMCV. Thus, the cells were able to respond to the infection by triggering the activation of p38 MAPK but not of JNK. In support of this notion is the observation that the virus apparently efficiently replicated and lysed the cells within 18 h postinfection (not shown). It therefore seems plausible for EMCV to activate both p38 MAPK and JNK in cell types that have low levels of the above-postulated negative regulator(s) of JNK activation or in cell types in which the actions of both PKR and RNase L can ensure sufficient levels of protein synthesis inhibition. It must be noted, however, that alternative explanations for the activation of p38 MAPK by EMCV are also possible; the kinase may be activated, for instance, in response to a particular viral antigen. Our data are not inconsistent with such a scenario.
Possible role of stress-activated MAPK in the cellular responses to viral infections.
Whatever the mechanisms of activation of stress-responsive kinases by EMCV, the question of the role of this activation in the responses of mammals to viral infections is, in our view, of importance. Based on our results shown in Fig. 7A, we propose that a major possible role of p38 MAPK activation in EMCV-infected cells is the production of proinflammatory and pyrogenic cytokines, such as IL-6, and perhaps others such as tumor necrosis factor alpha, IL-1β, and the immunoregulatory IFN-γ. The role of the MKK3→p38 MAPK→MAPKAP kinase 2 signaling cascade in the production of these cytokines has been well established (1, 29, 34, 42, 48, 62). Experimental observations on EMCV-induced experimental myocarditis and dilated cardiomyopathy in mice have shown increased production of these cytokines (28, 51). Indirect evidence suggests a possible role of proinflammatory cytokines in combating viruses. IL-6-deficient mice, for instance, fail to control efficiently infections with vaccinia virus and vesicular stomatitis virus (33), two viruses that also activate dsRNA-mediated cellular responses. Tumor necrosis factor alpha treatment of HeLa cells has been shown to inhibit vesicular stomatitis virus replication by accelerating the apoptotic response of the infected cells (35). Our findings reported here suggest, therefore, that p38 and JNK activation by viral dsRNA might constitute a newly recognized host defense mechanism.
ACKNOWLEDGMENTS
We thank Olga Ryabinina, Thanh-Hoai Dinh, and Jennifer Magun for excellent technical assistance.
This work was supported by Public Health Service grants CA-39360 and ES-08456 to B.E.M., CA-44059 to R.H.S., and AI-34039 to B.R.G.W. and by an N. L. Tartar Research Fund fellowship to M.S.I.
REFERENCES
- 1.Baldassare J J, Bi Y, Bellone C J. The role of p38 mitogen-activated protein kinase in IL-1 beta transcription. J Immunol. 1999;162:5367–5373. [PubMed] [Google Scholar]
- 2.Beyaert R, Cuenda A, Vanden Berghe W, Plaisance S, Lee J C, Haegeman G, Cohen P, Fiers W. The p38/RK mitogen-activated protein kinase pathway regulates interleukin-6 synthesis response to tumor necrosis factor. EMBO J. 1996;15:1914–1923. [PMC free article] [PubMed] [Google Scholar]
- 3.Brimacombe R. The structure of ribosomal RNA: a three-dimensional jigsaw puzzle. Eur J Biochem. 1995;230:365–383. [PubMed] [Google Scholar]
- 4.Cavigelli M, Li W W, Lin A, Su B, Yoshioka K, Karin M. The tumor promoter arsenite stimulates AP-1 activity by inhibiting a JNK phosphatase. EMBO J. 1996;15:6269–6279. [PMC free article] [PubMed] [Google Scholar]
- 5.Chebath J, Benech P, Hovanessian A, Galabru J, Revel M. Four different forms of interferon-induced 2′,5′-oligo(A) synthetase identified by immunoblotting in human cells. J Biol Chem. 1987;262:3852–3857. [PubMed] [Google Scholar]
- 6.Chen C Y, Del Gatto-Konczak F, Wu Z, Karin M. Stabilization of interleukin-2 mRNA by the c-Jun NH2-terminal kinase pathway. Science. 1998;280:1945–1949. doi: 10.1126/science.280.5371.1945. [DOI] [PubMed] [Google Scholar]
- 7.Chen Y R, Wang X, Templeton D, Davis R J, Tan T H. The role of c-Jun N-terminal kinase (JNK) in apoptosis induced by ultraviolet C and gamma radiation. Duration of JNK activation may determine cell death and proliferation. J Biol Chem. 1996;271:31929–31936. doi: 10.1074/jbc.271.50.31929. [DOI] [PubMed] [Google Scholar]
- 8.Clemens M J. PKR—a protein kinase regulated by double-stranded RNA. Int J Biochem Cell Biol. 1997;29:945–949. doi: 10.1016/s1357-2725(96)00169-0. [DOI] [PubMed] [Google Scholar]
- 9.Clemens M J. Regulation of eukaryotic protein synthesis by protein kinases that phosphorylate initiation factor eIF-2. Mol Biol Rep. 1994;19:201–210. doi: 10.1007/BF00986962. [DOI] [PubMed] [Google Scholar]
- 10.Clemens M J, Bommer U A. Translational control: the cancer connection. Int J Biochem Cell Biol. 1999;31:1–23. doi: 10.1016/s1357-2725(98)00127-7. [DOI] [PubMed] [Google Scholar]
- 11.Clemens M J, Elia A. The double-stranded RNA-dependent protein kinase PKR: structure and function. J Interferon Cytokine Res. 1997;17:503–524. doi: 10.1089/jir.1997.17.503. [DOI] [PubMed] [Google Scholar]
- 12.Davis R J. Signal transduction by the c-Jun N-terminal kinase. Biochem Soc Symp. 1999;64:1–12. doi: 10.1515/9781400865048.1. [DOI] [PubMed] [Google Scholar]
- 13.Derijard B, Raingeaud J, Barrett T, Wu I H, Han J, Ulevitch R J, Davis R J. Independent human MAP-kinase signal transduction pathways defined by MEK and MKK isoforms. Science. 1995;267:682–685. doi: 10.1126/science.7839144. . (Erratum, 269:17.) [DOI] [PubMed] [Google Scholar]
- 14.Dube P, Bacher G, Stark H, Mueller F, Zemlin F, van Heel M, Brimacombe R. Correlation of the expansion segments in mammalian rRNA with the fine structure of the 80 S ribosome; a cryoelectron microscopic reconstruction of the rabbit reticulocyte ribosome at 21 A resolution. J Mol Biol. 1998;279:403–421. doi: 10.1006/jmbi.1998.1804. [DOI] [PubMed] [Google Scholar]
- 15.Galcheva-Gargova Z, Derijard B, Wu I H, Davis R J. An osmosensing signal transduction pathway in mammalian cells. Science. 1994;265:806–808. doi: 10.1126/science.8047888. [DOI] [PubMed] [Google Scholar]
- 16.Gale M, Jr, Katze M G. Molecular mechanisms of interferon resistance mediated by viral-directed inhibition of PKR, the interferon-induced protein kinase. Pharmacol Ther. 1998;78:29–46. doi: 10.1016/s0163-7258(97)00165-4. [DOI] [PubMed] [Google Scholar]
- 17.Gangemi J D, Matter A, Poncioni B, Hochkeppel H K. Significant differences in therapeutic responses to a human interferon-alpha B/D hybrid in Rauscher or Friend murine leukemia virus infections. J Interferon Res. 1989;9:275–283. doi: 10.1089/jir.1989.9.275. [DOI] [PubMed] [Google Scholar]
- 18.Garrington T P, Johnson G L. Organization and regulation of mitogen-activated protein kinase signaling pathways. Curr Opin Cell Biol. 1999;11:211–218. doi: 10.1016/s0955-0674(99)80028-3. [DOI] [PubMed] [Google Scholar]
- 19.Hambleton J, Weinstein S L, Lem L, DeFranco A L. Activation of c-Jun N-terminal kinase in bacterial lipopolysaccharide-stimulated macrophages. Proc Natl Acad Sci USA. 1996;93:2774–2778. doi: 10.1073/pnas.93.7.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han J, Lee J D, Bibbs L, Ulevitch R J. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265:808–811. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- 21.Han J, Lee J D, Jiang Y, Li Z, Feng L, Ulevitch R J. Characterization of the structure and function of a novel MAP kinase kinase (MKK6) J Biol Chem. 1996;271:2886–2891. doi: 10.1074/jbc.271.6.2886. [DOI] [PubMed] [Google Scholar]
- 22.Hibi M, Lin A, Smeal T, Minden A, Karin M. Identification of an oncoprotein- and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes Dev. 1993;7:2135–2148. doi: 10.1101/gad.7.11.2135. [DOI] [PubMed] [Google Scholar]
- 23.Iordanov M, Bender K, Ade T, Schmid W, Sachsenmaier C, Engel K, Gaestel M, Rahmsdorf H J, Herrlich P. CREB is activated by UVC through a p38/HOG-1-dependent protein kinase. EMBO J. 1997;16:1009–1022. doi: 10.1093/emboj/16.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iordanov M S, Magun B E. Loss of cellular K+ mimics ribotoxic stress. Inhibition of protein synthesis and activation of the stress kinases SEK1/MKK4, stress-activated protein kinase/c-Jun NH2-terminal kinase 1, and p38/HOG1 by palytoxin. J Biol Chem. 1998;273:3528–3534. doi: 10.1074/jbc.273.6.3528. [DOI] [PubMed] [Google Scholar]
- 25.Iordanov M S, Pribnow D, Magun J L, Dinh T H, Pearson J A, Chen S L, Magun B E. Ribotoxic stress response: activation of the stress-activated protein kinase JNK1 by inhibitors of the peptidyl transferase reaction and by sequence-specific RNA damage to the α-sarcin/ricin loop in the 28S rRNA. Mol Cell Biol. 1997;17:3373–3381. doi: 10.1128/mcb.17.6.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iordanov M S, Pribnow D, Magun J L, Dinh T H, Pearson J A, Magun B E. Ultraviolet radiation triggers the ribotoxic stress response in mammalian cells. J Biol Chem. 1998;273:15794–15803. doi: 10.1074/jbc.273.25.15794. [DOI] [PubMed] [Google Scholar]
- 27.Ip Y T, Davis R J. Signal transduction by the c-Jun N-terminal kinase (JNK)—from inflammation to development. Curr Opin Cell Biol. 1998;10:205–219. doi: 10.1016/s0955-0674(98)80143-9. [DOI] [PubMed] [Google Scholar]
- 28.Iwasaki A, Matsumori A, Yamada T, Shioi T, Wang W, Ono K, Nishio R, Okada M, Sasayama S. Pimobendan inhibits the production of proinflammatory cytokines and gene expression of inducible nitric oxide synthase in a murine model of viral myocarditis. J Am Coll Cardiol. 1999;33:1400–1407. doi: 10.1016/s0735-1097(98)00692-5. [DOI] [PubMed] [Google Scholar]
- 29.Jackson J R, Bolognese B, Hillegass L, Kassis S, Adams J, Griswold D E, Winkler J D. Pharmacological effects of SB 220025, a selective inhibitor of P38 mitogen-activated protein kinase, in angiogenesis and chronic inflammatory disease models. J Pharmacol Exp Ther. 1998;284:687–692. [PubMed] [Google Scholar]
- 30.Jagus R, Joshi B, Barber G N. PKR, apoptosis and cancer. Int J Biochem Cell Biol. 1999;31:123–138. doi: 10.1016/s1357-2725(98)00136-8. [DOI] [PubMed] [Google Scholar]
- 31.Kasibhatla S, Brunner T, Genestier L, Echeverri F, Mahboubi A, Green D R. DNA damaging agents induce expression of Fas ligand and subsequent apoptosis in T lymphocytes via the activation of NF-kappa B and AP-1. Mol Cell. 1998;1:543–551. doi: 10.1016/s1097-2765(00)80054-4. [DOI] [PubMed] [Google Scholar]
- 32.Kerr I M, Brown R E. pppA2′p5′A2′p5′A: an inhibitor of protein synthesis synthesized with an enzyme fraction from interferon-treated cells. Proc Natl Acad Sci USA. 1978;75:256–260. doi: 10.1073/pnas.75.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kopf M, Baumann H, Freer G, Freudenberg M, Lamers M, Kishimoto T, Zinkernagel R, Bluethmann H, Kohler G. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature. 1994;368:339–342. doi: 10.1038/368339a0. [DOI] [PubMed] [Google Scholar]
- 34.Kotlyarov A, Neininger A, Schubert C, Eckert R, Birchmeier C, Volk H-D, Gaestel M. MAPKAP kinase 2 is essential for LPS-induced TNF-alpha biosynthesis. Nat Cell Biol. 1999;1:94–97. doi: 10.1038/10061. [DOI] [PubMed] [Google Scholar]
- 35.Koyama A H, Arakawa T, Adachi A. Acceleration of virus-induced apoptosis by tumor necrosis factor. FEBS Lett. 1998;426:179–182. doi: 10.1016/s0014-5793(98)00338-x. [DOI] [PubMed] [Google Scholar]
- 36.Krause A, Holtmann H, Eickemeier S, Winzen R, Szamel M, Resch K, Saklatvala J, Kracht M. Stress-activated protein kinase/Jun N-terminal kinase is required for interleukin (IL)-1-induced IL-6 and IL-8 gene expression in the human epidermal carcinoma cell line KB. J Biol Chem. 1998;273:23681–23689. doi: 10.1074/jbc.273.37.23681. [DOI] [PubMed] [Google Scholar]
- 36a.Lee J C, Laydon J T, McDonnell P C, Gallagher T F, Kumar S, Green D, McNulty D, Blumenthal M J, Heys J R, Landvatter S W, et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372:739–746. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- 37.Le-Niculescu H, Bonfoco E, Kasuya Y, Claret F X, Green D R, Karin M. Withdrawal of survival factors results in activation of the JNK pathway in neuronal cells leading to Fas ligand induction and cell death. Mol Cell Biol. 1999;19:751–763. doi: 10.1128/mcb.19.1.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levin D, London I M. Regulation of protein synthesis: activation by double-stranded RNA of a protein kinase that phosphorylates eukaryotic initiation factor 2. Proc Natl Acad Sci USA. 1978;75:1121–1125. doi: 10.1073/pnas.75.3.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Y, Gorospe M, Yang C, Holbrook N J. Role of mitogen-activated protein kinase phosphatase during the cellular response to genotoxic stress. Inhibition of c-Jun N-terminal kinase activity and AP-1-dependent gene activation. J Biol Chem. 1995;270:8377–8380. doi: 10.1074/jbc.270.15.8377. [DOI] [PubMed] [Google Scholar]
- 40.Meriin A B, Yaglom J A, Gabai V L, Zon L, Ganiatsas S, Mosser D D, Zon L, Sherman M Y. Protein-damaging stresses activate c-Jun N-terminal kinase via inhibition of its dephosphorylation: a novel pathway controlled by HSP72. Mol Cell Biol. 1999;19:2547–2555. doi: 10.1128/mcb.19.4.2547. . (Erratum, 19:5235.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meurs E, Chong K, Galabru J, Thomas N S, Kerr I M, Williams B R, Hovanessian A G. Molecular cloning and characterization of the human double-stranded RNA-activated protein kinase induced by interferon. Cell. 1990;62:379–390. doi: 10.1016/0092-8674(90)90374-n. [DOI] [PubMed] [Google Scholar]
- 42.Miyazawa K, Mori A, Miyata H, Akahane M, Ajisawa Y, Okudaira H. Regulation of interleukin-1beta-induced interleukin-6 gene expression in human fibroblast-like synoviocytes by p38 mitogen-activated protein kinase. J Biol Chem. 1998;273:24832–24838. doi: 10.1074/jbc.273.38.24832. [DOI] [PubMed] [Google Scholar]
- 43.Nagata Y, Todokoro K. Requirement of activation of JNK and p38 for environmental stress-induced erythroid differentiation and apoptosis and of inhibition of ERK for apoptosis. Blood. 1999;94:853–863. [PubMed] [Google Scholar]
- 44.Pandey P, Raingeaud J, Kaneki M, Weichselbaum R, Davis R J, Kufe D, Kharbanda S. Activation of p38 mitogen-activated protein kinase by c-Abl-dependent and -independent mechanisms. J Biol Chem. 1996;271:23775–23779. doi: 10.1074/jbc.271.39.23775. [DOI] [PubMed] [Google Scholar]
- 45.Player M R, Torrence P F. The 2-5A system: modulation of viral and cellular processes through acceleration of RNA degradation. Pharmacol Ther. 1998;78:55–113. doi: 10.1016/S0163-7258(97)00167-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Proud C G. PKR: a new name and new roles. Trends Biochem Sci. 1995;20:241–246. doi: 10.1016/s0968-0004(00)89025-8. [DOI] [PubMed] [Google Scholar]
- 47.Raingeaud J, Gupta S, Rogers J S, Dickens M, Han J, Ulevitch R J, Davis R J. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J Biol Chem. 1995;270:7420–7426. doi: 10.1074/jbc.270.13.7420. [DOI] [PubMed] [Google Scholar]
- 48.Rincon M, Enslen H, Raingeaud J, Recht M, Zapton T, Su M S, Penix L A, Davis R J, Flavell R A. Interferon-gamma expression by Th1 effector T cells mediated by the p38 MAP kinase signaling pathway. EMBO J. 1998;17:2817–2829. doi: 10.1093/emboj/17.10.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schaeffer H J, Weber M J. Mitogen-activated protein kinases: specific messages from ubiquitous messengers. Mol Cell Biol. 1999;19:2435–2444. doi: 10.1128/mcb.19.4.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shifrin V I, Anderson P. Trichothecene mycotoxins trigger a ribotoxic stress response that activates c-Jun N-terminal kinase and p38 mitogen-activated protein kinase and induces apoptosis. J Biol Chem. 1999;274:13985–13992. doi: 10.1074/jbc.274.20.13985. [DOI] [PubMed] [Google Scholar]
- 51.Shioi T, Matsumori A, Sasayama S. Persistent expression of cytokine in the chronic stage of viral myocarditis in mice. Circulation. 1996;94:2930–2939. doi: 10.1161/01.cir.94.11.2930. [DOI] [PubMed] [Google Scholar]
- 52.Silverman R H. 2-5A dependent RNase L: a regulated endoribonuclease in the interferon system. In: Riordan J F, D'Alessio G, editors. Ribonucleases: structures and functions. San Diego, Calif: Academic Press, Inc.; 1997. pp. 515–551. [Google Scholar]
- 53.Silverman R H, Cayley P J, Knight M, Gilbert C S, Kerr I M. Control of the ppp(a2′p)nA system in HeLa cells. Effects of interferon and virus infection. Eur J Biochem. 1982;124:131–138. doi: 10.1111/j.1432-1033.1982.tb05915.x. [DOI] [PubMed] [Google Scholar]
- 54.Silverman R H, Skehel J J, James T C, Wreschner D H, Kerr I M. rRNA cleavage as an index of ppp(A2′p)nA activity in interferon-treated encephalomyocarditis virus-infected cells. J Virol. 1983;46:1051–1055. doi: 10.1128/jvi.46.3.1051-1055.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stark G R, Kerr I M, Williams B R, Silverman R H, Schreiber R D. How cells respond to interferons. Annu Rev Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- 56.Su B, Jacinto E, Hibi M, Kallunki T, Karin M, Ben-Neriah Y. JNK is involved in signal integration during costimulation of T lymphocytes. Cell. 1994;77:727–736. doi: 10.1016/0092-8674(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 57.Sun H, Charles C H, Lau L F, Tonks N K. MKP-1 (3CH134), an immediate early gene product, is a dual specificity phosphatase that dephosphorylates MAP kinase in vivo. Cell. 1993;75:487–493. doi: 10.1016/0092-8674(93)90383-2. [DOI] [PubMed] [Google Scholar]
- 58.Tournier C, Whitmarsh A J, Cavanagh J, Barrett T, Davis R J. Mitogen-activated protein kinase kinase 7 is an activator of the c-Jun NH2-terminal kinase. Proc Natl Acad Sci USA. 1997;94:7337–7342. doi: 10.1073/pnas.94.14.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Westwick J K, Weitzel C, Minden A, Karin M, Brenner D A. Tumor necrosis factor alpha stimulates AP-1 activity through prolonged activation of the c-Jun kinase. J Biol Chem. 1994;269:26396–26401. [PubMed] [Google Scholar]
- 60.Williams B R. Role of the double-stranded RNA-activated protein kinase (PKR) in cell regulation. Biochem Soc Trans. 1997;25:509–513. doi: 10.1042/bst0250509. [DOI] [PubMed] [Google Scholar]
- 61.Wreschner D H, James T C, Silverman R H, Kerr I M. Ribosomal RNA cleavage, nuclease activation and 2-5A(ppp(A2′p)nA) in interferon-treated cells. Nucleic Acids Res. 1981;9:1571–1581. doi: 10.1093/nar/9.7.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wysk M, Yang D D, Lu H T, Flavell R A, Davis R J. Requirement of mitogen-activated protein kinase kinase 3 (MKK3) for tumor necrosis factor-induced cytokine expression. Proc Natl Acad Sci USA. 1999;96:3763–3768. doi: 10.1073/pnas.96.7.3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xia Z, Dickens M, Raingeaud J, Davis R J, Greenberg M E. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 64.Zhou A, Hassel B A, Silverman R H. Expression cloning of 2-5A-dependent RNase: a uniquely regulated mediator of interferon action. Cell. 1993;72:753–765. doi: 10.1016/0092-8674(93)90403-d. [DOI] [PubMed] [Google Scholar]
- 65.Zhou A, Paranjape J M, Der S D, Williams B R, Silverman R H. Interferon action in triply deficient mice reveals the existence of alternative antiviral pathways. Virology. 1999;258:435–440. doi: 10.1006/viro.1999.9738. [DOI] [PubMed] [Google Scholar]
- 66.Zhou A, Paranjape J M, Hassel B A, Nie H, Shah S, Galinski B, Silverman R H. Impact of RNase L overexpression on viral and cellular growth and death. J Interferon Cytokine Res. 1998;18:953–961. doi: 10.1089/jir.1998.18.953. [DOI] [PubMed] [Google Scholar]