Abstract
Autoimmune lymphoproliferative syndrome (ALPS) is an inherited non-malignant and non-infectious lymphoproliferative syndrome caused by mutations in genes affecting the extrinsic apoptotic pathway (FAS, FASL, CASP10). The resulting FAS-mediated apoptosis defect accounts for the expansion and accumulation of autoreactive (double-negative) T cells leading to cytopenias, splenomegaly, lymphadenopathy, autoimmune disorders, and risk of lymphoma. However, there are other monogenetic disorders known as ALPS-like syndromes that can be clinically similar to ALPS but are genetically and biologically different, such as observed in patients with immune checkpoint deficiencies, particularly cytotoxic T-lymphocyte antigen 4 (CTLA-4) insufficiency and lipopolysaccharide-responsive beige-like anchor protein LRBA deficiency. CTLA-4 insufficiency is caused by heterozygous mutations in CTLA-4, an essential negative immune regulator that is constitutively expressed on regulatory T (Treg) cells. Mutations in CTLA-4 affect CTLA-4 binding to CD80-CD86 costimulatory molecules, CTLA-4 homodimerization, or CTLA-4 intracellular vesicle trafficking upon cell activation. Abnormal CTLA-4 trafficking is also observed in patients with LRBA deficiency, a syndrome caused by biallelic mutations in LRBA that abolishes the LRBA protein expression. Both immune checkpoint deficiencies are biologically characterized by low levels of CTLA-4 protein on the cell surface of Tregs, accounting for the autoimmune manifestations observed in CTLA4-insufficient and LRBA-deficient patients. In addition, both immune checkpoint deficiencies present with an overlapping but heterogeneous clinical picture despite the difference in inheritance and penetrance. In this review, we describe the most prominent clinical features of ALPS, CTLA-4 insufficiency and LRBA deficiency, emphasizing their corresponding biological mechanisms. We also provide some clinical and laboratory approaches to diagnose these three rare immune disorders, together with therapeutic strategies that have worked best at improving prognosis and quality life of patients.
Keywords: ALPS, FAS, LRBA, CTLA-4, Primary immunodeficiency, Immune checkpoints
The generation of the enormous BCR (B cell receptor) and TCR (T cell receptor) repertoire through a randomized gene rearrangement and somatic mutation process, allows the immune system to recognize and remember specific molecular structures from pathogens, providing long-lasting protection against recurrent infections. However, the inevitable consequence of this receptor diversity process is the creation of lymphocytes reactive to self-antigens. To control these auto-reactive cell clones, two main mechanisms have been developed: The first is known as central tolerance, consisting of the deletion of self-reactive T and B cells in the thymus and bone marrow, respectively. The second system is known as peripheral tolerance involving the deletion of self-reactive immune cells that evaded central tolerance. Peripheral tolerance is also important once the immune response against a pathogen has been initiated, by guaranteeing immune homeostasis, a balance between co-stimulatory and inhibitory signals (also known as immune checkpoints) that controls the durability of the T cell immune response. Immune homeostasis is essential for host survival, since an uncontrolled immune response against invading pathogens or self-antigens can cause tissue damage, autoinflammatory, and autoimmune diseases. Breakdowns in either central or peripheral tolerance due to genetic mutations have been described to cause a plethora of immunodeficiency and immune dysregulation syndromes. Particularly, mutations in the death receptor FAS (or CD95) and its ligand FASL, a mechanism for apoptosis in central and in peripheral tolerance, result in a lymphoproliferative syndrome termed ALPS (Autoimmune LymphoProliferative Syndrome) [1,2]. This primary immunodeficiency (PID) is characterized by an increased amount of double negative (DN) T cells in blood and by an uncontrolled lymphoproliferation observed as lymphocyte infiltration in tissues and lymph nodes, due to an overall inability to efficiently deplete lymphocytes through apoptosis [3]. This general inability to send lymphocytes efficiently into apoptosis not only applies to lymphocytes reactive to antigens from pathogens, but also affects the apoptosis of self-reactive cells. Notably, homozygous mutations in other members of the FAS/FASL pathway such as FADD (Fas Associated Via Death Domain) or CAPS8 (Caspase 8), or heterozygous mutations in CASP10 (Caspase 10) also cause ALPS. In addition, breakdowns in immune checkpoint mechanisms, are observed in patients with heterozygous mutations in CTLA4 (Cytotoxic T Lymphocyte-associated Antigen 4) and in patients with biallelic mutations in LRBA (Lipopolysaccharide Responsive Beige-like Anchor protein) [4,5]. Mutations in either of those two genes result in an overlapping clinical phenotype characterized by autoimmunity, respiratory infections and enteropathy [6,7]. These diseases, known as CTLA-4 insufficiency or LRBA deficiency respectively, have been mainly attributed to a defective suppressive activity of regulatory T cells (Tregs), as in both conditions a reduced overall expression of CTLA-4 leads to their functional impairment. In LRBA deficiency however, other cellular mechanisms involving other cell types might also be impaired, resulting in a more severe disease with an earlier onset of symptoms and a full penetrance of the mutations. Other abnormalities in genes that encode important proteins for maintaining immune tolerance and/or immune homeostasis, such as mutations in FOXP3 (Forkhead box P3), CD25, BACH2 (BTB Domain And CNC Homolog 2), STAT3 (Signal Transducer And Activator Of Transcription 3, only GOF- Gain-Of-Function mutations), and recently in DEF6 (Guanine Nucleotide Exchange Factor), result in similar clinical diseases [8]. In this review, however, we will focus specifically on ALPS, CTLA-4 insufficiency, and LRBA deficiency. Herein, we summarize their pathomechanisms and their clinical and laboratory characteristics. Moreover, we discuss their differential diagnosis and treatment options.
Breakdowns of immune tolerance mechanisms: autoimmune proliferation syndrome (ALPS)
Peter Medawar – Noble prize winner in 1960 – described immunological tolerance “as a state of indifference or non-reactivity towards a substance that would normally be expected to excite an immunological response”. Immunological tolerance is required to prevent self-destructive immune responses (and thereby tissue damage) and is induced through three different mechanisms: i) Clonal deletion, the main mechanism of central tolerance, refers to the physical elimination of autoreactive cells, ii) clonal anergy is induced by the lack of co-stimulatory molecules, thereby preventing full T cell activation, and iii) suppression of cellular activation by Tregs [9,10]. Clonal deletion or negative selection is the elimination via apoptosis of B and T cells that express high affinity receptors to a broad self-tissue-specific antigen set. In the thymus, autoantigens are expressed by the thymic medullary epithelial cells under the control of AIRE (autoimmune regulator) [11]. Notably, autosomal recessive mutations in AIRE result in the APECED syndrome (Autoimmune PolyEndocrinopathy-Candidiasis-Ectodermal Dystrophy) characterized by widespread autoimmune manifestations in humans and mice [12,13]. Similarly, self-reactive B cells are removed in the bone marrow, but some of them have further chances to express alternative BCR receptors to avoid apoptosis, through a gene rearrangement process known as receptor editing [14]. Although a substantial proportion of self-reactive cells are eliminated in the corresponding primary lymphoid organs, many escape from the central tolerance mechanisms and are found in the blood circulation. Nevertheless, additional tolerance mechanisms operating in the periphery are capable of deleting autoreactive lymphocytes. These peripheral tolerance mechanisms involve apoptosis, anergy and immune deviation. The apoptosis of the autoreactive clones either in central or peripheral tolerance occurs via the Fas-FasL pathway, which is also required for the contraction of lymphocytes following an immune response against a pathogen. Additionally, anergic B cells are as well eliminated through the Fas-FasL pathway. Other cellular immune functions such as cytotoxicity of NK and CD8 T cells, regulation of myeloid suppressor cells' turnover [15], activation of macrophages, maintenance of immune privileged sites and maternal tolerance are also regulated by the Fas-FasL pathway [16].
Fas, also known as CD95, is a type I membrane receptor, and a member of the tumor necrosis factor receptor superfamily (TNFR), and operates as a homotrimeric complex which binds to its cognate ligand Fas (FasL) [17]. Following ligation, the Fas-associated death domain (FADD) adaptor protein is recruited to the intracellular death domain (DD) of Fas. In turn, FADD recruits caspase 8 and 10, which subsequently activate downstream effector caspases, leading eventually to proteolysis, DNA degradation, and apoptosis of the respective cell [18]. The role of FAS in maintaining lymphocyte homeostasis and peripheral immune tolerance was initially elucidated by studies of mice with deficient fas (MRL/lpr knockout mice) or fasL (MRL/gld) [19]. These mice had a massive expression of double negative T cells (DNT) that accumulated in secondary lymphoid organs, hypergammaglobulinemia and glomerulonephritis [19,20]. Later, the identification of mutations in human genes encoding any of the proteins involved in the Fas signalling pathway were identified as monogenetic cause for ALPS. ALPS however, presents with variable penetrance and severity. Specifically, mutations in FAS cover about 80% of total ALPS patients, including autosomal dominant germline mutations (70%, also known as ALPS-FAS) and somatic FAS mutations in hematopoietic cells (10%, also known as ALPS-sFAS). Mutations in FASL cover about 1% of ALPS patients whereas mutations in either FADD or CASP10 occur in less than 1% [2,3,21]. The mode of inheritance for the majority of mutations is autosomal dominant but ALPS-FAS and ALPS-FASL can also be inherited in an autosomal recessive manner. These mutations are fully penetrant and especially severe [22]. Interestingly, a cohort of 150 ALPS-FAS patients revealed that 63 healthy FAS mutation carriers who had an abnormal apoptosis also had elevated DNTs, sFASL and IL-10 in a similar degree to affected patients. This observation suggests that FAS mutations causing defective apoptosis alone, are not sufficient to cause clinical ALPS [21]. Therefore, a modifier gene and/or environmental factors are suggested to be involved in the pathogenesis of ALPS [23].
Clinically, ALPS patients present with a broad range of symptoms of variable severity, including chronic lymphoproliferation associated with the accumulation of DNTs (around 40% of total lymphocytes), manifesting as lymphadenopathy (>95% of patients) and/or splenomegaly (>90% of patients). Although the reason for the DNTs accumulation in ALPS is not well established, multiple studies have demonstrated an association with hyperactivation of the mTOR pathway [24]. Antibody-mediated autoimmune cytopenias, including autoimmune hemolytic anemia, autoimmune thrombocytopenia, or neutropenia and increased susceptibility to lymphoid malignancies, including both Hodgkin and non-Hodgkin lymphoma are also characteristically observed in ALPS-FAS patients [2,21]. However, these clinical features are commonly found in other PIDs, such in patients with mutations in LRBA [6], CTLA-4 [7], gain of function (GOF) mutations in STAT3 [25], PIK3CD [26], and NF-kB1 [27] and NF-kB2 [28]. The identification of mutations in those above genes supports the rationale of using inhibitors of their corresponding pathways in ALPS patients. Details on the treatment options as well as differential diagnostic approaches are described later in this review.
Breakdowns of immune homeostasis: immune checkpoint deficiencies
Immune control mechanisms are critical once T cells have been fully activated through at least two signals. The first signal is initiated by the TCR on naïve T cells through the recognition of their corresponding specific antigen presented by the MHC molecules on the antigen-presenting cells (APCs). The second signal requires the formation of an immunological synapse, which includes the binding of the costimulatory T-cell receptor CD28 on the T cell surface to CD80 (B7.1) and CD86 (B7.2) on the APCs (i.e dendritic cells, macrophages and B cells). Once activated, T cells proliferate and migrate to sites of inflammation where they attack cells expressing relevant antigens, destroying them either directly (CD8 T cells) or indirectly (CD4 T cells) through other effector cells by secreting cytokines [29]. Although an effective immune response is crucial in defending the host against invading pathogens and malignant cells, a tight regulation of the immune response regarding its duration and extent is essential to minimize collateral tissue damage that might result in chronic inflammation or autoimmunity. This immune regulation is executed by inhibitory signals, so called immune checkpoints. To date, more than twenty immune checkpoint pairs (receptor and ligand), both co-stimulatory and co-inhibitory, have been discovered, including TIGIT/CD155, LAG-3/MHCII, and TIM3/Gal-9, which are variably expressed not only by T cells but also by other cells of both the myeloid and the lymphoid linage [30]. However, the two inhibitory receptor/ligand pairs which have received the most attention are the cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) receptor binding to the B7 ligands (CD80 and CD86), and the programmed cell death protein 1 (PD1) receptor with PD1-L1 as ligand [31]. CTLA-4 (or CD152) was first reported to be constitutively expressed on Tregs in 1987. However, its cellular function as an inhibitor of T cell activity (showing opposing effects of CD28 upon T cell activation), was expounded in 1995 by James Allison et al. [32]. In the same decade, PD-1 (programmed cell death protein 1) was identified as a second immune checkpoint by Tasuko Honjo et al. [33]. Importantly, experiments blocking the negative regulators CTLA-4 and PD-1 using monoclonal CTLA-4 and PD-1 antibodies, respectively, resulted in tumor regression in mice [34,35]. These observations opened a new concept of cancer therapy, allowing the development of multiple clinical studies that have proven a great medical success. In 2018, Allison and Honjo were awarded with the Nobel Prize in Medicine for the discovery of a novel principle for tumor therapy based on the removal of the “brakes” in T cells. Thus, immune checkpoints are considered as master regulators of the immune peripheral tolerance, where regulatory T cells are considered the major executers. Forkhead-box-protein-3 (FOXP3) is the linage defining transcription factor for Tregs. A fatal autoimmune dysregulation syndrome called IPEX (immune dysregulation, polyendocrinopathy, enteropathy, X-linked) is observed in patients and mouse models (scurfy mice) carrying mutations in FOXP3, characterized by an overall loss of Tregs, highlighting the essential role of these cells in maintaining immune tolerance and immune homeostasis [36]. Of note, scurfy mice, as well as untreated IPEX newborns, die within weeks from severe inflammation and autoimmunity [37,38].
Tregs may exert their immune control through mechanisms that are both independent or dependent of CTLA-4. The first mechanism includes i) release of inhibitory chemokines such as IL-10 and TGFβ (transforming growth factor beta) that induce the expression of Foxp3 provoking the conversion of naive Tconv (conventional T cells) into Tregs in the periphery; ii) release of granzym A, which enters through perforin pores in target cells, activating intracellular caspases and causing apoptosis; and iii) metabolic disruption, i.e the effects of IL-2 and adenosine, essential mediators for T cell proliferation [39]. The second set of inhibitory mechanisms of Tregs is mediated via CTLA-4: Under steady state, CTLA-4 is located in intracellular vesicles in the naive T cells or constitutively expressed on Tregs. However, once the second signal of T cell activation is triggered (CD28/B7binding), CTLA-4 containing vesicles are mobilized to the cell surface where CTLA-4 outcompetes CD28 with higher affinity and avidity to bind the B7 molecules, which are expressed on the surface of APCs. The CTLA-4/B7 complex controls the T cell response by i) anergy of T cells due to the lack of CD28/B7 synapse formation and ii) removal of B7 ligands from the APCs surface in a cellular event known as trogocytosis (or transendocytosis), resulting in a significant depletion of available B7 co-stimulatory ligands on APCs [40,41].
While CD28 is expressed in the membrane of the cell's surface, CTLA-4 is found in intracellular vesicles in Tregs and activated Tconv, which are cycled within 5 min to the cell surface upon cell activation through the coordination of two proteins with opposing functions [42]. On one hand, AP-1 acts as a negative post-translational regulator of CTLA-4 by biding to a four-aminoacid (YVKM)-motif on the cytoplasmic tail of CTLA-4, allowing the trafficking of CTLA4-containing vesicles to lysosomes for further degradation [41]. On the contrary, LRBA allows the recycling and further cell surface shuttling of CTLA4-containing vesicles by directly binding CTLA-4 through its BEACH domain at the same YVKM motif as AP-1, thereby blocking the AP1-binding site and preventing CTLA-4 from lysosomal degradation [43]. In the absence of LRBA (as seen in LRBA deficiency), diminished CTLA-4 protein levels are observed due to enhanced AP-1 binding to CTLA-4, which results in an increased lysosomal degradation of CTLA-4. Therefore, while LRBA is not considered an immune checkpoint, it acts as one via CTLA-4. Low CTLA-4 levels are observed in patients with i) heterozygous mutations in CTLA-4, so called CTLA-4 insufficiency, or ii) homozygous mutations in LRBA also known as LRBA deficiency. Although LRBA deficiency is autosomal recessive and presents with an earlier onset of the disease and it is more severe than the autosomal dominant CTLA-4 insufficiency (due to potential other cellular functions of LRBA), both clinical syndromes overlap considerably. They both present with a defective regulation of the immune response resulting in the development of autoimmune manifestations and lymphoproliferation, demonstrating the essential function of CTLA-4 [6,7]. Clinical overlap of LRBA deficiency and CTLA-4 insufficiency is also observed with ALPS [44]. Since the initial discovery of these two PIDs, multiple reports have been published highlighting the broad spectrum of the clinical picture, but also shedding insights into the biological roles of CTLA-4 and LRBA. Notably, mutations in other genes including CD25, FOXP3 and DEF6 are also considered to cause monogenic autoimmune disorders involving defective Treg function, resulting in similar clinical characteristics [8]. However, for the purpose of this review, we will focus on abnormalities of CTLA-4 and its interacting protein LRBA.
Features of CTLA-4 insufficiency
Although CTLA-4 has been a research focus for decades, it was not only until 2014 when heterozygous mutations in CTLA-4 were identified to cause a dysregulation and immunodeficiency syndrome in humans known today as CTLA-4 insufficiency [4,45]. CTLA-4 insufficient-patients suffer from a broad spectrum of clinical manifestations including, autoimmune cytopenias, enteropathy, lymphocytic infiltrations of multiple nonlymphoid organs and recurrent infections caused by a progressive loss of circulating B cells and antibody levels [4,7]. The latter defects in the humoral compartment frequently results in CTLA-4 insufficient patients being diagnosed as Common variable immunodeficiency (CVID). In addition, CTLA-4 insufficient patients might also present with autoimmunity only, enteropathy only and in few cases with EBV-associated Hodgkin lymphoma [46].
CTLA-4 insufficiency presents with variable disease expressivity and the absence of an association between the type of the mutation and disease severity of the clinical phenotype. Moreover, as commonly seen in human autosomal dominant disorders, CTLA-4 insufficiency has an incomplete clinical penetrance resulting in 30% of mutation carriers having one or few clinical manifestations [7]. The cellular penetrance, however, is fully complete since all mutations in CTLA-4 lead to reduced CTLA-4 function including ligand binding to B7 molecules, transendocytosis of B7 ligands, and an overall Treg suppression activity in both affected and unaffected mutation carriers [7]. Although, PIDs are in general categorized as Mendelian disorders, CTLA-4 insufficiency is just one example of many PIDs exhibiting incomplete penetrance. This phenomenon might be explained by the presence of additional genetic factors such as gene modifiers, or epigenetic changes. Alternatively, the environment, including microbiota composition between individuals, has been previously found to be a risk factor in the development of autoimmunity [47]. Genetic and environmental modifiers might also explain the discrepancies between human and the Ctla4+/− mouse model, which in contrast to humans, appear completely healthy [48]. Ctla4−/− mice, however, present with splenomegaly, generalized lymphadenopathy, and massive lymphocytic organ infiltrations mostly into thymus, lymph nodes and spleen, resulting in a fatal autoimmune lymphoproliferative syndrome at four weeks of age [48]. Although the presence of definitive modifying factors is currently unknown, a rare heterozygous mutation in JAK3 (R840C variant) provided in vitro experimental evidence as a contributor of exacerbated lymphoproliferation in the context of CTLA-4 insufficiency. However, screening for the JAK3-R840C variant in a cohort of 42 CTLA4-insufficient patients did not identify any another case, leaving the search for modifying factors still open [49].
The progressive loss of B cells over time, characteristically observed in CTLA4-insufficient patients, has been attributed to a gradual B cell exhaustion as a consequence of chronic infections supported by the presence of large germinal centers and increased CD21low B cells [50]. Similar evidence of B cell exhaustion has been seen in patients with autoimmune disease and chronic infections [51]. Since CD21low B cells are enriched in autoreactive clones that are unresponsive to BCR stimulation [52], it is suggested that CTLA-4 might be important in maintaining B cell homeostasis and in preventing the development of autoantibodies [53].
Features of LRBA deficiency
LRBA deficiency is caused by deleterious biallelic mutations in LRBA [5]. Clinically, LRBA deficiency resembles the broad set of symptoms of CTLA-4 insufficiency, such as immune dysregulation, recurrent infections, and hypogammaglobulinemia. This clinical overlap is due to the overall reduced expression of CTLA-4 in both diseases. Immunologically, LRBA-deficient patients are characterized by a reduced number and function of regulatory T cells (Tregs), low switched memory B cells, and the lack of plasmablasts [6]. Despite their B cell switch defect, LRBA-deficient subjects may mount an intense autoantibody response. Autoreactive clones were found enriched in mature naive B cells from patients with LRBA deficiency [54]. This may indicate that LRBA might play a discrete role in preventing the accumulation of autoreactive cells in the blood [54]. Moreover, recent reports have shown increased levels of CD21low B cells related to concomitant autoimmune complication, and dysregulated T follicular helper T cells (Tfh) responses reflected by high frequency of cTFH, which play a role in disease-related autoimmunity [55]. Noteworthy, Tregs control antigen-specific expansion of Tfh cell numbers and humoral responses via CTLA-4, thereby controlling the B cell response [53,56].
Although LRBA deficiency presents with almost full penetrance, patients show a highly variable disease expressivity, which was observed in siblings carrying the same LRBA homozygous mutation while presenting with different disease severity [6,57]. This suggests the potential influence of modifier genes. In fact, mutated LRBA has been considered to exacerbate the disease severity of NEIL3 deficiency, since neil3−/− mice as well as individuals carrying a loss-of-function homozygous mutation in NEIL3 (D132V) were either asymptomatic or with elevated levels of autoantibodies without overt autoimmune disease, whereas patients with the same NEIL3 variant plus a loss-of-protein mutation in LRBA suffered from severe infections, autoimmune cytopenias, chronic diarrhea and early death. However, a genetic screen on LRBA-deficient patients would be required to confirm whether or not the NEIL3-D132V variant acts as a genetic modifier, since the carrier rate of this particular variant was found to be approximately 2% in healthy individuals [54].
In general, regardless of the type of mutation, almost all LRBA mutations cause a loss of LRBA protein, thereby impeding a genotype–phenotype correlation [6,58]. Few patients however, have been reported to present with either normal or reduced LRBA protein expression, who appeared to be at a lower risk of a severe disease course [59]. Therefore, quantification of LRBA protein expression might be useful to i) screen for LRBA-deficient patients from cohorts of CVID and/or with autoimmune manifestations, ii) establish the prognosis, iii) distinguish patients with LRBA deficiency from CTLA4 insufficiency, iv) guide treatment decisions, or v) monitor treatment success after HSCT.
Biologically, LRBA is widely expressed in all tissues, but immune cells need to be activated to express LRBA [60]. Although in murine macrophages, LRBA is located in the cytosol, endoplasmic reticulum, Golgi apparatus, and plasma membrane [60], in human T cells LRBA is found in recycling endosomes, supporting its function in the vesicle trafficking of CTLA-4 and transferrin receptor (CD71) but not of CD28, ICOS, PD-1, and CD154-containing vesicles, further indicating a role of LRBA in specific trafficking routes [43]. Similar functions are exerted by the LRBA protein homologue LYST (lysosomal trafficking regulator) [61]. Biallelic mutations in LYST cause a PID known as Chediak-Higashi syndrome (CHS) [62], which in contrast to LRBA deficiency does not present with immune dysregulation features, suggesting that LRBA and LYST act in different vesicle trafficking pathways with cellular specificity. Therefore, protein compensation by other LRBA homologues in LRBA-deficient patients with less severe clinical phenotype might be interesting to address. Besides the role of LRBA as a regulator of CTLA-4 trafficking in Tregs, LRBA has been suggested to play a role in autophagy, since LRBA-deficient B cells had a reduced autophagy flux upon serum starvation, leading to higher apoptosis and to an accumulation of organelles in the cytosol. As autophagy is a catabolic process required for the formation and/or maintenance of the long-lived plasma cell pool [5,63], abnormal autophagy might explain the low number of switched memory B cells and plasmablasts, resulting in hypogammaglobulinemia and the recurrent infections frequently observed in LRBA-deficient patients. Further experiments to elucidate the specific role of LRBA in autophagy are, however, required.
LRBA-deficient mouse models have shown some limitations and discrepancies to reproduce the human disease. In 2017, two different research groups reported rather healthy mice upon acute and chronic infections, despite carrying a homozygous truncating mutation in Lrba and having a decreased CTLA-4 expression [64,65]. However, in 2019 Wang et al. reported that LRBA-null mice had an enhanced susceptibility to DSS-induced colitis [66]. Interestingly, they conclude that the excessive intestinal inflammation of LRBA-null mice to DSS was caused by a hyperactivation of the endosomal TLR signaling, resulting in increased expression of IRF3/7-dependent genes such as IL-8, CXCL10, RANTES and CCL3, as well as an elevated IL-23 in response to endosomal TLR stimulation. These observations suggest that LRBA functions in the endosomal pathway to limit the TLR response but the underlying mechanisms remain to be clarified. Moreover, in a study by Park et al. murine LRBA-deficient NK cells exhibited impaired signalling by the key NK activating receptors, NKp46 and NKG2D, which interact with ligands expressed on the surface of virally infected, stressed or malignant cell targets [67]. This abnormality might result in the inability to respond to viruses but also in the inability to reject allogenic BM grafts, making LRBA-deficient patients highly susceptible to tumors and viral infections but good candidates for HSCT due to their possible resistance to acute GvHD as observed in LRBA-null mice [66]. However, according to the published LRBA deficiency cohorts, patients do not show susceptibility to any specific microorganism or virus. An increased risk of cancer, however, should be determined.
With regards to the humoral response in mice, there is no evidence that LRBA is required for normal B cell development and functionality. This discrepancy between the murine model and the human disease have generated three hypotheses: First, some species-specific compensatory mechanisms where LRBA homologue proteins overtake the LRBA function and compensate for its absence in mice. Second, the B cell phenotype observed in LRBA-deficient patients as a consequence of CTLA-4 loss (in mice it was 40% reduced), which is supported by the fact that patients with CTLA4 insufficiency also present with an abnormal humoral immune system [4]. Third, an intrinsic B cell defect in humans, as evidenced in a defective autophagy observed in human B cells lacking LRBA [5]. Autophagy is not only essential for plasma cell differentiation but also for B1a B cell survival. Interestingly, B1a B cells were significantly reduced in the peritoneum of LRBA-null mice [64,65].
Malignancy in ALPS, CTLA-4 insufficiency and LRBA deficiency
Malignant transformation is often observed in patients with PID. This high frequency might result from a defective DNA repair mechanism or a deficient cancer immunosurveillance and hence a poor control of oncogenic viruses that frequently produce chronic tissue inflammation. CTLA-4 particularly helps in restricting lymphocytic proliferation and preventing inflammatory damage, since low CTLA-4 expression results in uncontrolled proliferation of T cells with a possible overgrowth of autoreactive clones over e.g. EBV-specific T cell clones suggesting a role of CTLA-4 in CD8+ T cells and NK cells. In the large cohort of 131 patients with CTLA-4 insufficiency, 13% of affected CTLA4 mutation carriers had a malignant cell growth [7,46]. Lymphomas and gastric cancers were the most predominant type of malignancy and more than half of them were associated to EBV (Epstein-Barr-Virus). Therefore, the importance of monitoring EBV and CMV virus replication in blood of patients by PCR has been highlighted in several reports. The high predisposition to gastric cancer suggests the presence of a gut trigger resulting in gastrointestinal dysregulation that favours recurrent infections as with Helicobacter pylori [46]. Despite LRBA deficiency leading to a secondary loss of CTLA4, less malignancy prevalence and frequency has been reported. Of note, absence of LRBA protein expression causes LRBA deficiency, but overexpression of LRBA is associated with cancer cell growth, particularly in kidney, pancreas, colon, rectum, and lung [68]. However, the expression levels of LRBA varied greatly depending on the tumor type and developmental stage. These results indicate that LRBA might play a role in the suppression of apoptosis, thereby facilitating cell proliferation and cell survival [68]. Additionally, an increased expression of LRBA has been observed in erythroid progenitor cells suggesting the possible involvement of LRBA in hematopoietic disorders [69]. Finally, in ALPS patients, lymphoma continues to be the most common type of malignancy. However, other types of cancer have been observed, suggesting a broader cancer predisposition in the context of ALPS, as 22 malignancies were noted in 200-ALPS patients with intracellular mutations in FAS [21].
Approaches for differential diagnosis
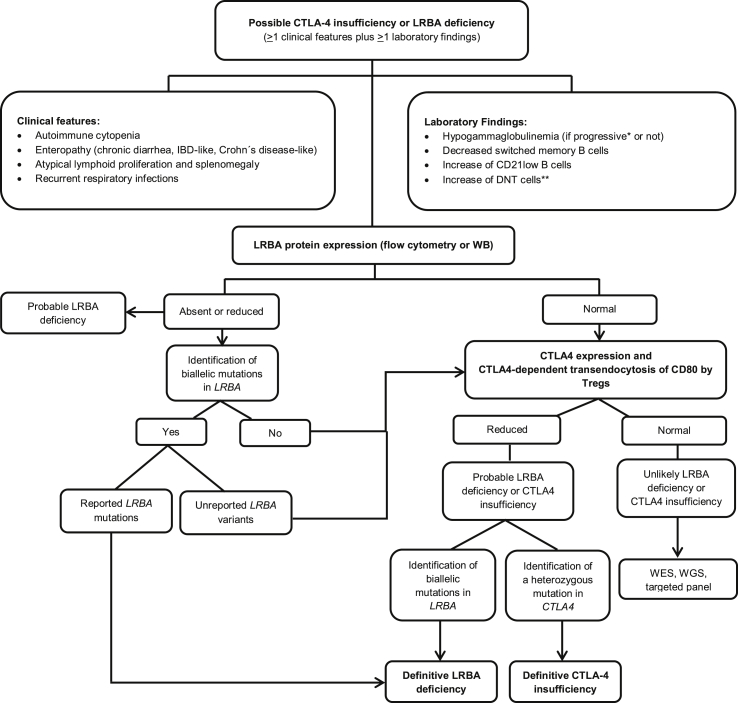
Genetic identification of a disease-pathogenic variant is required for the definitive diagnosis of LRBA deficiency or CTLA-4 insufficiency. However, some laboratory tests can be performed in order to screen for these PIDs. Particularly in ALPS, patients present with three classic signs i) chronic lymphadenopathy (>6 months), ii) elevation of DNTs to over 1.5% of total lymphocytes or 2.5% of T lymphocytes, and iii) defective in vitro lymphocyte apoptosis [70]. Other tests including elevated soluble Fas ligand, IL-18, IL-10 and/or Vitamin B12 accompanied by a family history of a non-malignant/non-infections lymphoproliferation with or without autoimmunity, help to reinforce the suspicious of ALPS [70]. Thus, ALPS patients can be frequently identified in CVID and Evan's syndromes cohorts, where immune checkpoint deficiencies are also commonly present. Particularly, LRBA deficiency should be considered mostly in children with early-onset hypogammaglobulinemia, severe autoimmune manifestations, enteropathy, lymphoproliferation and recurrent tract infections. Autoimmune manifestations are often the first clinical symptom. LRBA deficiency and CTLA-4 insufficiency frequently present in patients with a CVID-like phenotype or with multi-organ autoimmunity. However, these clinical features might be frequently found in several PIDs such as in IPEX (mutations in FOXP3), or IPEX-like disease, including defects in CD25, NF-κB1, NF-κB2, STAT1 GOF, STAT3 GOF, and in the recently described DEF6 deficiency. Although some immune cell frequencies are characteristic of a particular PID, for instance increased DNTs in ALPS, functional testing or screening tests based on mRNA or protein detection are currently being implemented for differential diagnosis or when the identified variant has not yet been reported as pathogenic. Specifically, the detection of LRBA protein by flow cytometry in either PHA-stimulated PBMCs or in unstimulated whole blood cells have been reported useful to discriminate with over 90% specificity and sensitivity LRBA-deficient patients harbouring LRBA biallelic mutations from LRBA-like patients with wild type LRBA sequence [71,72]. Although, the LRBA protein is found to be absent in most LRBA-deficient patients, the detection of it will not exclude LRBA deficiency as some patients present with normal or residual LRBA expression. Therefore, additional functional testing such as CTLA-4 expression or transendocytosis might be useful. Particularly, CTLA-4 expression has shown to be higher upon CD3/CD28 stimulation in T cells from LRBA-deficient patients. This increase is even higher in response to lysosomal inhibitors following T cell stimulation [73]. These observations are consistent with the fact that there is no defect in the synthesis of CTLA-4 itself but rather in its trafficking. The transendocytosis or ligand uptake test is based in the ability of CTLA-4 to capture and remove the co-stimulatory ligands CD80/CD86 from the membrane of APCs followed by its internalization into the Tregs for further lysosomal degradation. In the laboratory, this process is measured by the amount of GFP signal detected inside isolated CD4+ T cells from patients after co-culturing them with CD80-GFP-expressing CHO cells, which act here as APCs [74]. Transendocytosis is expected to be abnormal in patients with mutations in CTLA-4 as well as in LRBA-deficient patients. This functional efficacy test is frequently used in newly identified CTLA-4 variants. As with LRBA, DEF6 has been determined to be essential for the trafficking of CTLA-4, an abnormal transendocytosis test in DEF6 deficiency has been also observed [75] [Fig. 1 and Table 1].
Fig. 1.
Differential diagnosis for LRBA deficiency and CTLA4 insufficiency.
Table 1.
Characteristics of ALPS, CTLA-4 insufficiency, and LRBA deficiency.
| PID |
ALPS-FAS/ALPS-FasL | CTLA-4 insufficiency | LRBA deficiency |
|---|---|---|---|
| Characteristic | |||
| Gene affected | TNFRSF6 (FAS)/FAS-L | CTLA-4 | LRBA |
| Hereditary pattern | germline or somatic, AD or AR | germline, AD | germline, AR |
| Penetrance | Missense mutations in intracellular domain: 90% Truncated mutations in intracellular domain: 70% Any mutation in extracellular domain: 30% |
Incomplete (70%) | Almost complete (Only one individual stayed healthy) |
| Genotype-phenotype correlation | Yes (Heterogeneous syndrome) |
No (Heterogeneous syndrome) |
No (Heterogeneous syndrome) |
| Onset | Neonatal | Early adulthood | Childhood |
| Autoimmune cytopenias | Yes | Yes | Yes |
| Enteropathy | Not frequent | Yes | Yes |
| Organomegaly | Yes | Yes | Yes |
| Hypogammaglobulinemia | Few cases; mostly hyper-IgG and hyper-IgA. | Yes (around 80%) | Yes (around 60%) |
| Recurrent infections | Not frequent (mostly bacterial infections) | Yes (not to a specific pathogen) | Yes (not to a specific pathogen) |
| Lymphoproliferation | Yes | Yes | Yes |
| Endocrinopathy | No | Yes | T1DM, thyroiditis |
| Main laboratory findings | Increased circulating DNT cells Increased plasma levels of FasL Increased plasma levels of IL-10 Decrease in vitro T-cell apoptosis |
Decrease of CTLA-4 expression Abnormal CTLA-4 transendocytosis Increase of CD21low B cells Increased DNT cells |
Decrease of T regs (∼60% patients) Decreased switched B cells Decreased plasmablasts Decreased CTLA-4 expression Decreased CTLA-4 transendocytosis Absence of LRBA expression |
| Mimics murine model | Yes | No | No |
Abbreviations: AD: Autosomal dominant; AR: Autosomal recessive.
Treatment options
The management of ALPS, LRBA deficiency and CTLA-4 insufficiency focuses mostly on treating their clinical manifestations and complications using corticosteroids and immunosuppressive agents, immunoglobulin replacement and antibiotics. Corticosteroids and immunosuppressants are used to control the autoimmune manifestations. Among them, sirolimus (rapamycin), an mTOR inhibitor and an autophagy inducer, has demonstrated to lead to a successful reduction of lymphoproliferation and an overall improvement of autoimmune manifestations. Especially in ALPS patients, researchers have shown a concomitant decrease of DNTs supported by increased apoptosis and reduced proliferation of DTNs in vitro [76]. In addition, sirolimus has shown good control of disease symptoms as autoimmunity and enteropathy, specifically weight loss and diarrhoea in patients with CTLA-4 insufficiency [45] and in LRBA deficiency [77]. Despite its side effects, sirolimus is considered as a relatively well tolerated drug, especially in children and it is an attractive option. Immunoglobulin substitution (IgG-RT) is given to manage the immunodeficiency and to mitigate the recurrent respiratory infections as patients present with hypogammaglobulinemia in 60% of cases in LRBA deficiency and in 80% of the CTLA-4 insufficiency cohort. IgG-RT however, is insufficient to control the gut involvement, which might be ameliorated with the use of local corticosteroids, gluten-free diet, and several immunosuppressive agents [78].
The only potential curative treatment for these PIDs is hematopoietic stem cell transplantation (HSCT). However, HSCT is currently recommended only in patients with severe manifestations and non-responsiveness to conventional therapy, including those who have developed complications after long-term immunosuppressive treatment. In fact, a study cohort of 18 patients with CTLA-4 insufficiency who required HSCT presented with an overall survival of 72% at 26 months post-transplantation [79]. Recently, an international retrospective analysis of 24 patients with LRBA deficiency that underwent HSCT reported a mortality rate of 29.2% [59]. The transplantation outcome was clearly better in patients with a less severe disease and thus better pre-HSCT clinical conditions. Additional studies are however necessary to determine the best timing of HSCT and long-term survival following HSCT in these immunodysregulation syndromes. Importantly, lung involvement could be ameliorated in a percentage of patients under abatacept or sirolimus treatment and resolved in many patients after HSCT, but patients with uncontrolled pulmonary disease should be considered at highest risk.
Finally, patients with CTLA-4 insufficiency and LRBA deficiency may be successfully treated with abatacept to control their T cell activation. Abatacept, a fusion protein formed of a Fc-region of human IgG1 linked to the extracellular domain of CTLA-4, mimics the CTLA-4 function as a immunosuppressant [80]. Abatacept has successfully shown clinical improvement in enteropathy, lymphoproliferation, and autoimmune cytopenias in 11 of 14 patients with the diagnosis of CTLA-4-insufficiency. In addition, abatacept reduced the frequencies of circulating follicular T helper cells (cTfh) and autoantibody levels in accordance with the hypothesis that the loss of CTLA-4 is responsible for the dysregulation. Similarly, LRBA-deficient patients appear to have a marked clinical improvement with long-term use of abatacept, demonstrating control of immune dysregulation and lymphoproliferation with a very favourable side–effect profile [43,59]. cTfh cells were noted to be a reliable marker for disease activity (and monitoring for effectiveness of abatacept) in LRBA-deficient patients [81].
Summary
-
•
Breakdowns in the extrinsic apoptotic pathway due to genetic mutations in FAS, FASL, CAPS8, CAPS10 cause ALPS. Apoptosis through FAS-FASL pathway is essential to eliminate autoreactive clones.
-
•
Breakdowns in immune checkpoint mechanisms are observed in patients with heterozygous mutations in CTLA4 and in patients with biallelic mutations in LRBA. Both disorders present with a reduced overall abundance of CTLA-4 leading to a defective suppressive activity of Tregs.
-
•
ALPS, CTLA-4 insufficiency, and LRBA deficiency clinically overlap in chronic lymphoproliferation, organomegaly and antibody-mediated autoimmune cytopenias. In contrast to ALPS, CTLA-4 insufficiency and LRBA deficiency may additionally present with hypogammaglobulinemia, enteropathy and recurrent respiratory tract infections.
-
•
Incomplete clinical penetrance as well as variable severity are observed in ALPS with autosomal dominant mode of inheritance and in CTLA-4 insufficiency. Up to date, a gene and/or environmental modifier accounting for the incomplete clinical penetrance, and the variable severity and expressivity is still unknown.
-
•
Laboratory tests for differential diagnosis include: i) elevation of DNTs, ii) defective in vitro lymphocyte apoptosis, and iii) elevated soluble Fas ligand, to identify ALPS patients. CTLA-4 patients present with abnormal CTLA-4-dependent transendocytosis, whereas LRBA-deficient patients present with absence or reduction of LRBA protein expression upon in vitro stimulation.
-
•
The management of ALPS, LRBA deficiency and CTLA-4 insufficiency mostly consist of corticosteroids and immunosuppressive agents, immunoglobulin replacement and antibiotics. In particular, sirolimus has shown good control of disease symptoms. Patients with CTLA-4 insufficiency and LRBA deficiency may be successfully treated with abatacept to control their T cell activation. However, the only potential curative treatment is HSCT.
Conflict of interest
None.
Acknowledgement
Our research work on LRBA deficiency and CTLA-4 insufficiency receives support by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – Project-ID 403222702 – SFB 1381 and SFB1160/2_B5; under Germany's Excellence Strategy (CIBSS – EXC-2189 – Project ID 390939984, and RESIST – EXC 2155 – Project ID 390874280); by the E-rare program of the EU, managed by the DFG, grant code GR1617/14-1/iPAD; by the German Federal Ministry of Education and Research (BMBF) through a grant to the German Auto-Immunity Network (GAIN), grant code 01GM1910A; and by the Fritz Thyssen Stiftung. Our work is also supported in part by the Center for Chronic Immunodeficiency (CCI), Freiburg Center for Rare Diseases (FZSE).
Footnotes
Peer review under responsibility of Chang Gung University.
Contributor Information
Laura Gámez-Díaz, Email: laura.gamez@uniklinik-freiburg.de.
Bodo Grimbacher, Email: bodo.grimbacher@uniklinikfreiburg.de.
References
- 1.Bettinardi A., Brugnoni D., Quiròs-Roldan E., Malagoli A., La Grutta S., Correra A. Missense mutations in the Fas gene resulting in autoimmune lymphoproliferative syndrome: a molecular and immunological analysis. Blood. 1997;89:902–909. [PubMed] [Google Scholar]
- 2.Rieux-Laucat F., Le Deist F., Hivroz C., Roberts I.A., Debatin K.M., Fischer A. Mutations in Fas associated with human lymphoproliferative syndrome and autoimmunity. Science. 1995;268:1347–1349. doi: 10.1126/science.7539157. [DOI] [PubMed] [Google Scholar]
- 3.Fisher G.H., Rosenberg F.J., Straus S.E., Dale J.K., Middleton L.A., Lin A.Y. Dominant interfering Fas gene mutations impair apoptosis in a human autoimmune lymphoproliferative syndrome. Cell. 1995;81:935–946. doi: 10.1016/0092-8674(95)90013-6. [DOI] [PubMed] [Google Scholar]
- 4.Schubert D., Bode C., Kenefeck R., Hou T.Z., Wing J.B., Kennedy A. Autosomal dominant immune dysregulation syndrome in humans with CTLA4 mutations. Nat Med. 2014;20:1410–1416. doi: 10.1038/nm.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lopez-Herrera G., Tampella G., Pan-Hammarstrom Q., Herholz P., Trujillo-Vargas C.M., Phadwal K. Deleterious mutations in LRBA are associated with a syndrome of immune deficiency and autoimmunity. Am J Hum Genet. 2012;90:986–1001. doi: 10.1016/j.ajhg.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gámez-Díaz L., August D., Stepensky P., Revel-Vilk S., Seidel M.G., Noriko M. The extended phenotype of LPS-responsive beige-like anchor protein (LRBA) deficiency. J Allergy Clin Immunol. 2016;137:223–230. doi: 10.1016/j.jaci.2015.09.025. [DOI] [PubMed] [Google Scholar]
- 7.Schwab C., Gabrysch A., Olbrich P., Patino V., Warnatz K., Wolff D. Phenotype, penetrance, and treatment of 133 cytotoxic T-lymphocyte antigen 4-insufficient subjects. J Allergy Clin Immunol. 2018;142:1932–1946. doi: 10.1016/j.jaci.2018.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cepika A.M., Sato Y., Liu J.M., Uyeda M.J., Bacchetta R., Roncarolo M.G. Tregopathies: monogenic diseases resulting in regulatory T-cell deficiency. J Allergy Clin Immunol. 2018;142:1679–1695. doi: 10.1016/j.jaci.2018.10.026. [DOI] [PubMed] [Google Scholar]
- 9.Gregersen P.K., Behrens T.W. Genetics of autoimmune diseases--disorders of immune homeostasis. Nat Rev Genet. 2006;7:917–928. doi: 10.1038/nrg1944. [DOI] [PubMed] [Google Scholar]
- 10.Bluestone J.A. Mechanisms of tolerance. Immunol Rev. 2011;241:5–19. doi: 10.1111/j.1600-065X.2011.01019.x. [DOI] [PubMed] [Google Scholar]
- 11.Fierabracci A. Recent insights into the role and molecular mechanisms of the autoimmune regulator (AIRE) gene in autoimmunity. Autoimmun Rev. 2011;10:137–143. doi: 10.1016/j.autrev.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 12.Aaltonen J., Björses P., Perheentupa J., Horelli-Kuitunen N., Palotie A., Peltonen L. An autoimmune disease, APECED, caused by mutations in a novel gene featuring two PHD-type zinc-finger domains. Nat Genet. 1997;17:399–403. doi: 10.1038/ng1297-399. [DOI] [PubMed] [Google Scholar]
- 13.Anderson M.S., Venanzi E.S., Chen Z., Berzins S.P., Benoist C., Mathis D. The cellular mechanism of Aire control of T cell tolerance. Immunity. 2005;23:227–239. doi: 10.1016/j.immuni.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Casellas R., Shih T.A., Kleinewietfeld M., Rakonjac J., Nemazee D., Rajewsky K. Contribution of receptor editing to the antibody repertoire. Science. 2001;291:1541–1544. doi: 10.1126/science.1056600. [DOI] [PubMed] [Google Scholar]
- 15.Sinha P., Chornoguz O., Clements V.K., Artemenko K.A., Zubarev R.A., Ostrand-Rosenberg S. Myeloid-derived suppressor cells express the death receptor Fas and apoptose in response to T cell-expressed FasL. Blood. 2011;117:5381–5390. doi: 10.1182/blood-2010-11-321752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bride K., Teachey D. Autoimmune lymphoproliferative syndrome: more than a FAScinating disease. F1000Res. 2017;6:1928. doi: 10.12688/f1000research.11545.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lenardo M.J. Fas and the art of lymphocyte maintenance. J Exp Med. 1996;183:721–724. doi: 10.1084/jem.183.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe-Fukunaga R., Brannan C.I., Copeland N.G., Jenkins N.A., Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992;356:314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- 20.Martina M.N., Noel S., Saxena A., Rabb H., Hamad A.R. Double negative (DN) alphabeta T cells: misperception and overdue recognition. Immunol Cell Biol. 2015;93:305–310. doi: 10.1038/icb.2014.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Price S., Shaw P.A., Seitz A., Joshi G., Davis J., Niemela J.E. Natural history of autoimmune lymphoproliferative syndrome associated with FAS gene mutations. Blood. 2014;123:1989–1999. doi: 10.1182/blood-2013-10-535393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van der Burg M., de Groot R., Comans-Bitter W.M., den Hollander J.C., Hooijkaas H., Neijens H.J. Autoimmune lymphoproliferative syndrome (ALPS) in a child from consanguineous parents: a dominant or recessive disease? Pediatr Res. 2000;47:336–343. doi: 10.1203/00006450-200003000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Rieux-Laucat F., Casanova J.L. Immunology. Autoimmunity by haploinsufficiency. Science. 2014;345:1560–1561. doi: 10.1126/science.1260791. [DOI] [PubMed] [Google Scholar]
- 24.Völkl S., Rensing-Ehl A., Allgäuer A., Schreiner E., Lorenz M.R., Rohr J. Hyperactive mTOR pathway promotes lymphoproliferation and abnormal differentiation in autoimmune lymphoproliferative syndrome. Blood. 2016;128:227–238. doi: 10.1182/blood-2015-11-685024. [DOI] [PubMed] [Google Scholar]
- 25.Fabre A., Marchal S., Barlogis V., Mari B., Barbry P., Rohrlich P.S. Clinical aspects of STAT3 gain-of-function germline mutations: a systematic review. J Allergy Clin Immunol Pract. 2019;7:1958–19569. doi: 10.1016/j.jaip.2019.02.018. e9. [DOI] [PubMed] [Google Scholar]
- 26.Coulter T.I., Chandra A., Bacon C.M., Babar J., Curtis J., Screaton N. Clinical spectrum and features of activated phosphoinositide 3-kinase delta syndrome: a large patient cohort study. J Allergy Clin Immunol. 2017;139:597–606. doi: 10.1016/j.jaci.2016.06.021. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tuijnenburg P., Lango Allen H., Burns S.O., Greene D., Jansen M.H., Staples E. Loss-of-function nuclear factor kappaB subunit 1 (NFKB1) variants are the most common monogenic cause of common variable immunodeficiency in Europeans. J Allergy Clin Immunol. 2018;142:1285–1296. doi: 10.1016/j.jaci.2018.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klemann C., Camacho-Ordonez N., Yang L., Eskandarian Z., Rojas-Restrepo J.L., Frede N. Clinical and immunological phenotype of patients with primary immunodeficiency due to damaging mutations in NFKB2. Front Immunol. 2019;10:297. doi: 10.3389/fimmu.2019.00297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bretscher P.A. A two-step, two-signal model for the primary activation of precursor helper T cells. Proc Natl Acad Sci U S A. 1999;96:185–190. doi: 10.1073/pnas.96.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pardoll D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Canc. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buchbinder E.I., Desai A. CTLA-4 and PD-1 pathways: similarities, differences, and implications of their inhibition. Am J Clin Oncol. 2016;39:98–106. doi: 10.1097/COC.0000000000000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krummel M.F., Allison J.P. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182:459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ishida Y., Agata Y., Shibahara K., Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kwon E.D., Hurwitz A.A., Foster B.A., Madias C., Feldhaus A.L., Greenberg N.M. Manipulation of T cell costimulatory and inhibitory signals for immunotherapy of prostate cancer. Proc Natl Acad Sci U S A. 1997;94:8099–8103. doi: 10.1073/pnas.94.15.8099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iwai Y., Terawaki S., Honjo T. PD-1 blockade inhibits hematogenous spread of poorly immunogenic tumor cells by enhanced recruitment of effector T cells. Int Immunol. 2005;17:133–144. doi: 10.1093/intimm/dxh194. [DOI] [PubMed] [Google Scholar]
- 36.Park J.H., Lee K.H., Jeon B., Ochs H.D., Lee J.S., Gee H.Y. Immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome: a systematic review. Autoimmun Rev. 2020;19:102526. doi: 10.1016/j.autrev.2020.102526. [DOI] [PubMed] [Google Scholar]
- 37.Hori S., Nomura T., Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 38.Khattri R., Cox T., Yasayko S.A., Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 39.Vignali D.A., Collison L.W., Workman C.J. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walker L.S., Sansom D.M. The emerging role of CTLA4 as a cell-extrinsic regulator of T cell responses. Nat Rev Immunol. 2011;11:852–863. doi: 10.1038/nri3108. [DOI] [PubMed] [Google Scholar]
- 41.Sansom D.M. IMMUNOLOGY. Moving CTLA-4 from the trash to recycling. Science. 2015;349:377–378. doi: 10.1126/science.aac7888. [DOI] [PubMed] [Google Scholar]
- 42.Linsley P.S., Bradshaw J., Greene J., Peach R., Bennett K.L., Mittler R.S. Intracellular trafficking of CTLA-4 and focal localization towards sites of TCR engagement. Immunity. 1996;4:535–543. doi: 10.1016/s1074-7613(00)80480-x. [DOI] [PubMed] [Google Scholar]
- 43.Lo B., Zhang K., Lu W., Zheng L., Zhang Q., Kanellopoulou C. AUTOIMMUNE DISEASE. Patients with LRBA deficiency show CTLA4 loss and immune dysregulation responsive to abatacept therapy. Science. 2015;349:436–440. doi: 10.1126/science.aaa1663. [DOI] [PubMed] [Google Scholar]
- 44.Cagdas D., Halaçli S.O., Tan Ç., Lo B., Çetinkaya P.G., Esenboğa S. A spectrum of clinical findings from ALPS to CVID: several novel LRBA defects. J Clin Immunol. 2019;39:726–738. doi: 10.1007/s10875-019-00677-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuehn H.S., Ouyang W., Lo B., Deenick E.K., Niemela J.E., Avery D.T. Immune dysregulation in human subjects with heterozygous germline mutations in CTLA4. Science. 2014;345:1623–1627. doi: 10.1126/science.1255904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Egg D., Schwab C., Gabrysch A., Arkwright P.D., Cheesman E., Giulino-Roth L. Increased risk for malignancies in 131 affected CTLA4 mutation carriers. Front Immunol. 2018;9:2012. doi: 10.3389/fimmu.2018.02012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yurkovetskiy L.A., Pickard J.M., Chervonsky A.V. Microbiota and autoimmunity: exploring new avenues. Cell Host Microbe. 2015;17:548–552. doi: 10.1016/j.chom.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tivol E.A., Borriello F., Schweitzer A.N., Lynch W.P., Bluestone J.A., Sharpe A.H. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 49.Sic H., Speletas M., Cornacchione V., Seidl M., Beibel M., Linghu B. An activating janus kinase-3 mutation is associated with cytotoxic T lymphocyte antigen-4-dependent immune dysregulation syndrome. Front Immunol. 2017;8:1824. doi: 10.3389/fimmu.2017.01824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lo B., Abdel-Motal U.M. Lessons from CTLA-4 deficiency and checkpoint inhibition. Curr Opin Immunol. 2017;49:14–19. doi: 10.1016/j.coi.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 51.Baeyens A., Saadoun D., Billiard F., Rouers A., Gregoire S., Zaragoza B. Effector T cells boost regulatory T cell expansion by IL-2, TNF, OX40, and plasmacytoid dendritic cells depending on the immune context. J Immunol. 2015;194:999–1010. doi: 10.4049/jimmunol.1400504. [DOI] [PubMed] [Google Scholar]
- 52.Rakhmanov M., Keller B., Gutenberger S., Foerster C., Hoenig M., Driessen G. Circulating CD21low B cells in common variable immunodeficiency resemble tissue homing, innate-like B cells. Proc Natl Acad Sci U S A. 2009;106:13451–13456. doi: 10.1073/pnas.0901984106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sage P.T., Paterson A.M., Lovitch S.B., Sharpe A.H. The coinhibitory receptor CTLA-4 controls B cell responses by modulating T follicular helper, T follicular regulatory, and T regulatory cells. Immunity. 2014;41:1026–1039. doi: 10.1016/j.immuni.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Massaad M.J., Zhou J., Tsuchimoto D., Chou J., Jabara H., Janssen E. Deficiency of base excision repair enzyme NEIL3 drives increased predisposition to autoimmunity. J Clin Invest. 2016;126:4219–4236. doi: 10.1172/JCI85647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Azizi G., Mirshafiey A., Abolhassani H., Yazdani R., Ghanavatinejad A., Noorbakhsh F. The imbalance of circulating T helper subsets and regulatory T cells in patients with LRBA deficiency: correlation with disease severity. J Cell Physiol. 2018;233:8767–8777. doi: 10.1002/jcp.26772. [DOI] [PubMed] [Google Scholar]
- 56.Wing J.B., Ise W., Kurosaki T., Sakaguchi S. Regulatory T cells control antigen-specific expansion of Tfh cell number and humoral immune responses via the coreceptor CTLA-4. Immunity. 2014;41:1013–1025. doi: 10.1016/j.immuni.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 57.Azizi G., Abolhassani H., Mahdaviani S.A., Chavoshzadeh Z., Eshghi P., Yazdani R. Clinical, immunologic, molecular analyses and outcomes of iranian patients with LRBA deficiency: a longitudinal study. Pediatr Allergy Immunol. 2017;28:478–484. doi: 10.1111/pai.12735. [DOI] [PubMed] [Google Scholar]
- 58.Habibi S., Zaki-Dizaji M., Rafiemanesh H., Lo B., Jamee M., Gámez-Diaz L. Clinical, immunologic, and molecular spectrum of patients with LPS-responsive beige-like anchor protein deficiency: a systematic review. J Allergy Clin Immunol Pract. 2019;7:2379–23786 e5. doi: 10.1016/j.jaip.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 59.Tesch V.K., Abolhassani H., Shadur B., Zobel J., Mareika Y., Sharapova S. Long-term outcome of LRBA deficiency in 76 patients after various treatment modalities as evaluated by the immune deficiency and dysregulation activity (IDDA) score. J Allergy Clin Immunol. 2020;145:1452–1463. doi: 10.1016/j.jaci.2019.12.896. [DOI] [PubMed] [Google Scholar]
- 60.Wang J.W., Howson J., Haller E., Kerr W.G. Identification of a novel lipopolysaccharide-inducible gene with key features of both A kinase anchor proteins and chs1/beige proteins. J Immunol. 2001;166:4586–4595. doi: 10.4049/jimmunol.166.7.4586. [DOI] [PubMed] [Google Scholar]
- 61.Cullinane A.R., Schäffer A.A., Huizing M. The BEACH is hot: a LYST of emerging roles for BEACH-domain containing proteins in human disease. Traffic. 2013;14:749–766. doi: 10.1111/tra.12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Introne W., Boissy R.E., Gahl W.A. Clinical, molecular, and cell biological aspects of Chediak-Higashi syndrome. Mol Genet Metab. 1999;68:283–303. doi: 10.1006/mgme.1999.2927. [DOI] [PubMed] [Google Scholar]
- 63.Pengo N., Scolari M., Oliva L., Milan E., Mainoldi F., Raimondi A. Plasma cells require autophagy for sustainable immunoglobulin production. Nat Immunol. 2013;14:298–305. doi: 10.1038/ni.2524. [DOI] [PubMed] [Google Scholar]
- 64.Gámez-Díaz L., Neumann J., Jäger F., Proietti M., Felber F., Soulas-Sprauel P. Immunological phenotype of the murine Lrba knockout. Immunol Cell Biol. 2017;95:789–802. doi: 10.1038/icb.2017.52. [DOI] [PubMed] [Google Scholar]
- 65.Burnett D.L., Parish I.A., Masle-Farquhar E., Brink R., Goodnow C.C. Murine LRBA deficiency causes CTLA-4 deficiency in Tregs without progression to immune dysregulation. Immunol Cell Biol. 2017;95:775–788. doi: 10.1038/icb.2017.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang K.W., Zhan X., McAlpine W., Zhang Z., Choi J.H., Shi H. Enhanced susceptibility to chemically induced colitis caused by excessive endosomal TLR signaling in LRBA-deficient mice. Proc Natl Acad Sci U S A. 2019;116:11380–11389. doi: 10.1073/pnas.1901407116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Park M.Y., Sudan R., Srivastava N., Neelam S., Youngs C., Wang J.W. LRBA is essential for allogeneic responses in bone marrow transplantation. Sci Rep. 2016;6:36568. doi: 10.1038/srep36568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang J.W., Gamsby J.J., Highfill S.L., Mora L.B., Bloom G.C., Yeatman T.J. Deregulated expression of LRBA facilitates cancer cell growth. Oncogene. 2004;23:4089–4097. doi: 10.1038/sj.onc.1207567. [DOI] [PubMed] [Google Scholar]
- 69.Fujishima N., Hirokawa M., Aiba N., Ichikawa Y., Fujishima M., Komatsuda A. Gene expression profiling of human erythroid progenitors by micro-serial analysis of gene expression. Int J Hematol. 2004;80:239–245. doi: 10.1532/ijh97.04053. [DOI] [PubMed] [Google Scholar]
- 70.Oliveira J.B., Bleesing J.J., Dianzani U., Fleisher T.A., Jaffe E.S., Lenardo M.J. Revised diagnostic criteria and classification for the autoimmune lymphoproliferative syndrome (ALPS): report from the 2009 NIH International Workshop. Blood. 2010;116:e35–e40. doi: 10.1182/blood-2010-04-280347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gámez-Díaz L., Sigmund E.C., Reiser V., Vach W., Jung S., Grimbacher B. Rapid flow cytometry-based test for the diagnosis of lipopolysaccharide responsive beige-like anchor (LRBA) deficiency. Front Immunol. 2018;9:720. doi: 10.3389/fimmu.2018.00720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Meshaal S., El Hawary R., Adel R., Abd Elaziz D., Erfan A., Lotfy S. Clinical phenotypes and immunological characteristics of 18 Egyptian LRBA deficiency patients. J Clin Immunol. 2020;40:820–832. doi: 10.1007/s10875-020-00799-2. [DOI] [PubMed] [Google Scholar]
- 73.Hou T.Z., Verma N., Wanders J., Kennedy A., Soskic B., Janman D. Identifying functional defects in patients with immune dysregulation due to LRBA and CTLA-4 mutations. Blood. 2017;129:1458–1468. doi: 10.1182/blood-2016-10-745174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hou T.Z., Qureshi O.S., Wang C.J., Baker J., Young S.P., Walker L.S. A transendocytosis model of CTLA-4 function predicts its suppressive behavior on regulatory T cells. J Immunol. 2015;194:2148–2159. doi: 10.4049/jimmunol.1401876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Serwas N.K., Hoeger B., Ardy R.C., Stulz S.V., Sui Z., Memaran N. Human DEF6 deficiency underlies an immunodeficiency syndrome with systemic autoimmunity and aberrant CTLA-4 homeostasis. Nat Commun. 2019;10:3106. doi: 10.1038/s41467-019-10812-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bleesing J.J.H., Nagaraj C.B., Zhang K. In: GeneReviews((R)). Seattle (WA): University of Washington, Seattle. Adam M.P., Ardinger H.H., Pagon R.A., Wallace S.E., Bean L.J.H., Stephens K., editors. 1993-2020. Autoimmune lymphoproliferative syndrome. [PubMed] [Google Scholar]
- 77.Azizi G., Abolhassani H., Yazdani R., Mohammadikhajehdehi S., Parvaneh N., Negahdari B. New therapeutic approach by sirolimus for enteropathy treatment in patients with LRBA deficiency. Eur Ann Allergy Clin Immunol. 2017;49:235–239. doi: 10.23822/EurAnnACI.1764-1489.22. [DOI] [PubMed] [Google Scholar]
- 78.Serwas N.K., Kansu A., Santos-Valente E., Kuloğlu Z., Demir A., Yaman A. Atypical manifestation of LRBA deficiency with predominant IBD-like phenotype. Inflamm Bowel Dis. 2015;21:40–47. doi: 10.1097/MIB.0000000000000266. [DOI] [PubMed] [Google Scholar]
- 79.Slatter M.A., Engelhardt K.R., Burroughs L.M., Arkwright P.D., Nademi Z., Skoda-Smith S. Hematopoietic stem cell transplantation for CTLA4 deficiency. J Allergy Clin Immunol. 2016;138:615–619 e1. doi: 10.1016/j.jaci.2016.01.045. [DOI] [PubMed] [Google Scholar]
- 80.Alten R., Märker-Hermann E. [Selective co-stimulation blockade. CTLA4-Ig (Abatacept)] Z Rheumatol. 2010;69:601–607. doi: 10.1007/s00393-009-0533-4. German. [DOI] [PubMed] [Google Scholar]
- 81.Lu Yang, X X., Chen Xuemei, Wu Junfeng, Yang Xi, Xu Li. Abatacept is effective in Chinese patients with LRBA and CTLA4 deficiency. Gene Dis. Forthcoming. 2020 doi: 10.1016/j.gendis.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]