Abstract
Background
Intravascular large B-cell lymphoma (IVLBCL) is a rare subtype of non-Hodgkin lymphoma with uncommon clinical presentations and poor prognosis. The purpose of this study is to report the clinical features and outcome of IVLBCL in a single institution of Taiwan.
Methods
Ten patients with IVLBCL diagnosed from June 2006 to January 2018 were retrospectively reviewed.
Results
The median age was 61 (range 39–88) years. The most common presentation was fever (90%), cytopenia (90%), and confusion (50%). For all patients, the median progression free survival (PFS) and overall survival (OS) were 12.6 (95% confidence interval [CI] 0.0–76.1) and 18.8 (95% CI 0–59.3) months, respectively. Six patients received rituximab combined chemotherapy, and the other one patient was treated with chemotherapy alone. Six of seven (85.7%) patients achieved complete response after chemotherapy. The median PFS and OS for six patients who completed treatment were not reached. Three-year PFS and OS rates were 80% and 75%, respectively.
Conclusion
Our study showed that patients might achieve durable remission after rituximab-based chemotherapy. The outcome of IVLBCL patients may further improve if early diagnosis and prompt treatment were made.
Keywords: Intravascular large B-Cell lymphoma, Hemophagocytosis, Rituximab
At a glance of commentary
Scientific background on the subject
Intravascular large B-cell lymphoma is rare and aggressive hematologic disease. Three different types of variants (classical, cutaneous, hemophagocytic syndrome-associated) have been described. Early diagnosis is difficult due to uncommon clinical presentations and heterogeneity among patients. Most patients are associated with poor prognosis.
What this study adds to the field
Although rituximab-based chemotherapy had improved the outcome of patients with intravascular large B-cell lymphoma but the long-term survival still remains dismal. Some studies had showed that intensive strategy with autologous hematopoietic stem cell transplantation may further prolong the overall survival of those patients.
Intravascular large B-cell lymphoma (IVLBCL) is a rare subtype of Non-Hodgkin lymphoma which has been updated and revised in 2017 World Health Organization (WHO) classification of lymphoid neoplasms [1]. IVLBCL is an aggressive and disseminated disease with the presence of large lymphoma cells in the lumen of vessels [2].
Previously, two patterns of IVLBCL manifestations (western and Asian form) have been introduced according to the geography [3,4]. However, three different types of variants (classical, cutaneous, hemophagocytic syndrome-associated) have been described recently because of differences in the clinical features and outcome [5,6]. The “classical form” was characterized by organ involved symptoms. The “cutaneous variant” limited to skin lesions without other systemic involvement and it is associated with better prognosis. The “hemophagocytic syndrome-associated form” has the worst prognosis due to associated with hemophagocytosis and multiorgan failure. IVLBCL patients are suggested to be described in these three variants according to their clinical features rather than by their geographical distribution based on the recently revised 2017 WHO classification [1,5].
Other than these defined variants of IVLBCL, several case reports described unusual clinical features with difficulties in diagnosis [[7], [8], [9]]. Therefore, the diagnosis of IVLBCL still remains a challenge because of heterogeneity among patients. Many studies investigated the approaches for early diagnosis of IVLBCL, including random skin biopsy, bone marrow biopsy or FDG PET/CT [[10], [11], [12], [13], [14]]. Yet, consensus on the strategies for early diagnosis of IVLBCL has not been met. The addition of rituximab to chemotherapy has been reported to improve the outcome of IVLBCL patients [[15], [16], [17]].
Due to the rarity of IVLBCL, we aim to describe the presenting symptoms, clinical variants, therapeutic management and outcome of IVLBCL patients in a single institution of Taiwan.
Patients and methods
Patient characteristics
Between June 2006 and January 2018, ten patients with newly diagnosed IVLBCL were retrospectively reviewed. The patient list was retrieved from the databank of our institution. The Investigational Review Broad of our institution delivered the approval for this study. The diagnosis of IVLBCL was discussed in a combined conference meeting of lymphoma, in which the pathology was reviewed and confirmed by experienced pathologists in hematopoietic malignancies according to the World Health Organization (WHO)classification in 2008 [18].
Variants of IVLBCL were classified according to the clinical features defined by reported studies, especially “hemophagocytic syndrome-associated form”. Both the criteria of clinical/laboratory and histopathological must be met [3,5,6,19]. However, an international consensus meeting of IVLBCL in 2007 can consider IVLBCL patients as “hemophagocytic syndrome-associated form” with a less strict criteria by presence of clinical features of fever, hepatosplenomegaly and thrombocytopenia [20]. Any of our patients who classified as “hemophagocytic syndrome-associated form” was according to the criteria of international consensus meeting of IVLBCL in 2007. Data collection was retrospectively analyzed from the medical records.
A series of staging work up including complete history taking, physical examination, complete blood cell counts, liver and kidney profiles, lactate dehydrogenase, beta 2 microglobulin, bone marrow trephine biopsy, Computed tomography (CT) of brain, chest, abdomen, and pelvis, and fluorine-18–2-fluoro-2-deoxy-d-glucose positron emission tomography/computed tomography (FDG PET/CT) were performed. FDG PET/CT (n = 7) and whole body computed tomography (WBCT) (n = 2) were done for initial staging work up. The disease staging is defined according to the 2014 Lugano classification [21]. Performance status was evaluated according to the Eastern Cooperative Oncology Group (ECOG) scale (0–4). Risk stratification was assigned according to International Prognostic Index (IPI) [22].
Treatment and response evaluation
The main chemotherapy regimens were R–CHOP (Rituximab 375 mg/m2, Cyclophosphamide 750 mg/m2 on day 1, Doxorubicin 50 mg/m2 on day 1, Vincristine 1.4 mg/m2, maximum 2 mg on day 1 and Prednisolone 100 mg/day on days 1–5), R–COP (Rituximab 375 mg/m2, Cyclophosphamide 750 mg/m2 on day 1, Vincristine 1.4 mg/m2, maximum 2 mg on day 1 and Prednisolone 60 mg/day on days 1–7), or CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) with or without intrathecal methotrexate chemotherapy. Treatment response was evaluated by WBCT (n = 4) or FDG PET/CT (n = 2) three months after starting the treatment, one month after the completion of treatment, and at an interval of three to six months thereafter. Additional bone marrow study was performed for the evaluation of treatment response in cases with bone marrow involvement.
Statistical analysis
Overall survival (OS) was calculated from the date of diagnosis to death (die of any causes) or to the last date of follow-up. Progression-free survival (PFS) was calculated from the date of diagnosis to disease relapse or death. Both OS and PFS curves were generated by Kaplan–Meier method. Comparisons of estimated survival curves were analyzed by the log-rank test. SPSS software version 17.0 (SPSS Inc.) was used for statistical analysis.
Results
Patient characteristics and clinical features
The median age of ten patients (6 male and 4 female) with IVLBCL was 61 years (range 39–88). Most of the patients initially presented as prolonged fever (n = 9, 90%). Neurologic symptoms such as seizure (n = 2), confusion (n = 5), hallucination (n = 1) or facial numbness (n = 1) were found in five patients as one of the initial symptoms, but only two patients (20%) were confirmed as IVLBCL with central nervous system (CNS) involvement by brain biopsy. Three patients manifested with skin lesions over thigh, left axilla and abdomen, respectively. IVLBCL with cutaneous involvement were confirmed by skin biopsy.
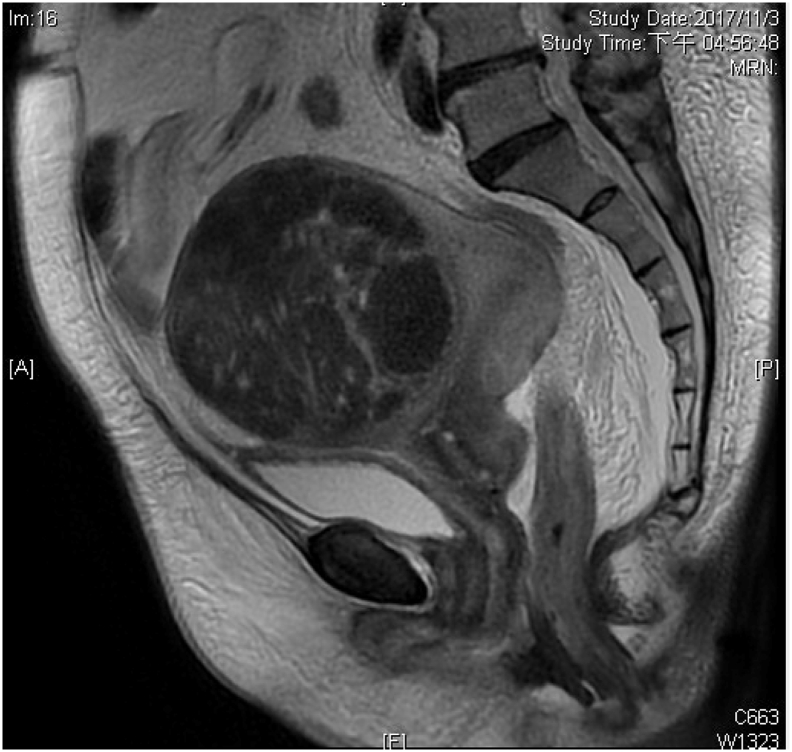
One patient presented with unusual manifestations of lower limbs edema due to uterine mass complicated with lymphomatous obstruction, and uterus IVLBCL was confirmed after hysterectomy. The feature of magnetic resonance imaging and pathology findings were shown in Fig. 3, Fig. 4, respectively.
Fig. 3.
Sagittal view of magnetic resonance imaging (T2 weighted image) of IVLBCL patient presented with uterine mass (a 11 cm mass noted over anterior fundus) complicated with lymphomatous obstruction.
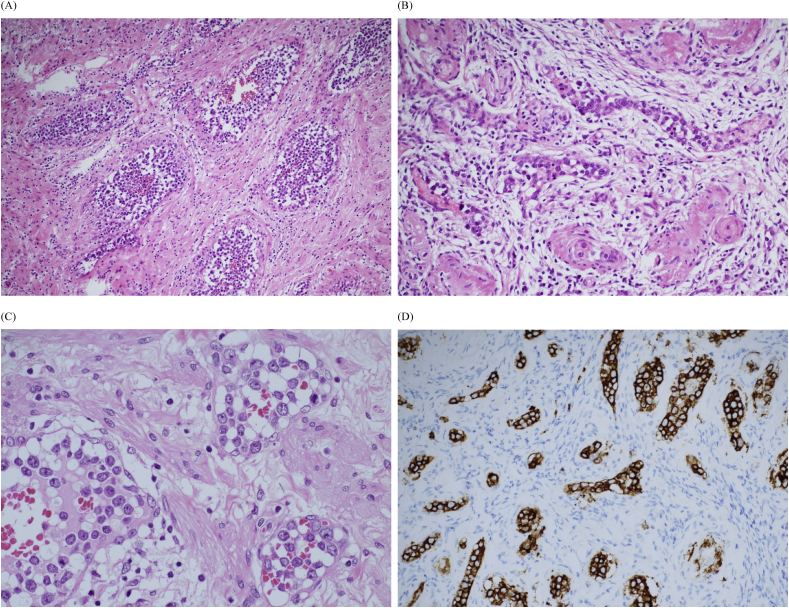
Fig. 4.
(A) Microscopically, the tumor cells filled with medium-sized veins (H&E stain; original magnification x100) and (B) capillaries (H&E stain; original magnification x 200). (C) The tumor cells were large lymphoid cells with prominent nucleoli (H&E stain; original magnification x 400), and (D) they were strongly positive for CD20 (immunohistochemistry; original magnification x 200).
Nine patients (90%) presented with cytopenia including anemia (n = 9, 90%), thrombocytopenia (n = 7, 70%), and leukopenia (n = 3, 30%). Pancytopenia was noted in 30% of patients. Elevated LDH and beta 2 microglobulin were noted in nine (90%) and eight (80%) patients, respectively.
The median time from the first presentation to definite pathological diagnosis of IVLBCL was 42.5 (range 15–240) days. Poor performance status (ECOG >2) was noted in six (60%) patients. All the ten patients had stage IV disease and they were assigned into high-intermediate or high-risk category of IPI. Seven patients had bone marrow examination and six of them were proven with lymphoma involvement. Additionally, all the seven patients demonstrated hemophagocytosis in their bone marrow pathology.
In summary, the involving sites included bone marrow (n = 6, 60%), spleen (n = 9, 90%), skin (n = 3, 30%), liver (n = 3, 30%), adrenal gland (n = 3, 30%), brain (n = 2, 20%), lung (n = 2, 20%), bone (n = 1, 10%), kidney (n = 1, 10%) and uterus (n = 1, 10%). None of the patients had nodal involvement. Those involved sites were diagnosed by either tissue biopsies or FDG PET/CT surveillances. The clinical features of patients were summarized in Table 1.
Table 1.
Patients characteristics and clinical manifestations.
| Case no. | Age | Sex | Clinical features | HLH | HS | CNS symptoms | Biopsy site | Involving site | Elevated LDH | Elevated b2M | IPI | ECOG | Lugano classification | Therapy (cycles) | Response | Relapse | OS (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 64 | M | Fever, anemia, thrombocytopenia | – | + | – | BM | BM, spleen, adrenal gland | N/A | + | 3 | 1 | IV | R–CHOP x6 | CR | 10 months | 18.8 |
| 2 | 58 | F | Fever, anemia | – | + | – | BM | BM, liver, spleen, kidney, lung, adrenal gland | + | + | 4 | 4 | IV | supportive | – | 0.2 | |
| 3 | 39 | M | Fever, anemia, thrombocytopenia, skin lesions | – | + | – | BM, skin (thigh) | BM, skin, spleen | + | + | 3 | 1 | IV | R–CHOP x8 | CR | – | 126.2+ |
| 4 | 71 | F | Fever, pancytopenia, seizure, confusion | – | + | + | BM | BM, spleen | + | + | 4 | 4 | IV | supportive | – | 0.1 | |
| 5 | 59 | M | Fever, BW loss, pancytopenia, confusion, skin lesions | – | + | + | BM, skin (left axilla) | Skin, spleen, liver | + | N/A | 3 | 0 | IV | R–COP x8 | CR | 44 months | 61.6 |
| 6 | 60 | M | Fever, skin lesions, confusion, visual hallucination, facial numbness | – | – | + | Brain, skin (abdomen) | Brain, skin, spleen | + | + | 4 | 4 | IV | R–CHOP x1 | Early mortality | 0.7 | |
| 7 | 71 | M | Fever, anemia | – | + | – | BM | BM, spleen | + | + | 4 | 1 | IV | CHOP x6 | CR | – | 75+ |
| 8 | 62 | F | Fever, limbs edema, anemia, thrombocytopenia | – | – | – | Uterus | Uterus, liver, spleen | + | + | 5 | 3 | IV | R–CHOP + IT x6 | CR | – | 13.3+ |
| 9 | 47 | M | Confusion, seizure, anemia, thrombocytopenia | – | – | + | Brain | Brain, adrenal gland, bone, lung | + | + | 4 | 3 | IV | supportive | – | 1.0 | |
| 10 | 88 | F | Fever, fatigue, pancytopenia, confusion | – | + | + | BM | BM, spleen | + | N/A | 5 | 3 | IV | R–CVOP x6 | CR | – | 10.8+ |
Abbreviations: BW: body weight; HLH: hemophagocytic lymphohistiocytosis; HS: hemophagocytosis in bone marrow; CNS: central nervous system; BM: bone marrow; LDH: lactate dehydrogenase; b2M: beta 2 microglobulin; IPI: international prognostic index; R–CHOP: rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone; CHOP: cyclophosphamide, doxorubicin, vincristine, prednisone; R–COP: rituximab, cyclophosphamide, vincristine, prednisone; IT: intrathecal therapy with methotrexate; R–CVOP: rituximab, cyclophosphamide, etoposide, vincristine, prednisolone; CR: complete response; CCR: clinical complete response; OS: overall survival.
Based on the clinical features, two patients were categorized into classical type, while eight patients were categorized into hemophagocytic syndrome-associated variant.
Pathological and cytogenetic features of IVLBCL
The biopsy sites were obtained from bone marrow (n = 6), skin (n = 3), brain (n = 2) and uterus (n = 1). Reviewing the pathology report, large lymphoid cells were found diffusely or clustered inside the vessels or sinusoids among all patients. Immunohistochemical (IHC) study showed all patients were positive for CD20. Other positivity of IHC were CD5 (3 of 6), CD45 (2 of 2), CD10 (2 of 5), CD79a (2 of 2), MUM1 (4 of 4), BCL-6 (1 of 2), MYC/BCL-2 (1 of 1). They are negative for CD2, CD3, CD30, CD4, CD8, CD15, CD23, CD56, MPO, cyclin CD1, SOX11, TIA-1 and LMP-1. In our study, only 3 patients had cytogenetic analysis. All of them have complex karyotype, with all involving both chromosome 1 and 6q, while 2 of the 3 patients involving chromosome 18. The chromosome results of three patients were listed in Table 2.
Table 2.
Cytogenetic results of bone marrow aspirates from three analyzed patients.
| Patients Number | Cytogenetic analysis |
|---|---|
| 2 | 83, XXX, -X, t(1:?) (p13; ?), −2, −4, add(4) (q31), −6, add(6) (p23), del(6) (q11q24), −7, −8, −9, −11, −11, −12, −13, −14, −15, −15, add(17) (p13), +18, −19, add(19) (p13), −20, −21,-21, −22, +9mar [19]/46, XX [6] |
| 4 | 94, XX, -X, -X, −1, +i(3) (q10), +add(3) (p25), del(4) (q31.3)x2, del(6) (q16), −10, −13, −15, +16, −17, −17, +18 × 4, +20, +20, −22, −22, +3mar [20]/97, XX, -X, -X, −1, +i(3) (q10), +add(3) (p25), del(4) (q31.3)x2, +del(6) (q16), +9, −10, −13, −15, +16, −17, −17, +18 × 5, +20, −22, −22, +4mar [6] |
| 7 | 45, Y, -X, add(1) (p36.1), add(3) (q21), der(3)add(3) (p25)add(3) (q21), −6, add(6) (q25), del(7) (p15), del(8) (p21), add(9) (p220, add(11) (q23), add(17) (q25), add(19) (q13.3), +mar [5]/45, X, -Y [5]/46, XY [7] |
Treatment and responses
Seven patients received chemotherapy, and three patients received supportive care only. The chemotherapy delivered to patients were R–CHOP (n = 4), R–COP (n = 2), and CHOP (n = 1). The reason for patient who only received CHOP is because of severe allergic reaction during first rituximab infusion. One patient who was treated with R–CHOP received 6 courses of concomitant intrathecal therapy with methotrexate 12 mg for CNS prophylaxis. Of seven patients who received chemotherapy, six patients completed treatment courses, while the remaining one patient received only one course of R–CHOP and died due to disease progression.
The first treatment date is defined by the day starting of steroid therapy. The date of confirmed diagnosis is defined by the day of pathology report. The median time from confirmed diagnosis of IVLBCL to the first treatment was 4 (range, 1–11) days. For six patients who have completed treatment, the complete response (CR) rate was 100%. Four patients remained in complete remission and were still alive at 126.2 months, 75 months, 13.3 months, and 10.8 months, respectively. The remaining two patients eventually relapsed at 10 and 44 months, respectively. The relapse sites were brain and diffuse lymphadenopathy which is different from the initial involved sites. The one with brain relapse previously do not received intrathecal therapy prophylaxis.
Three patients with supportive care alone because of unfit status and multiple comorbidities had early mortality due to rapid disease progression at 3, 7, and 31 days, respectively.
Outcome
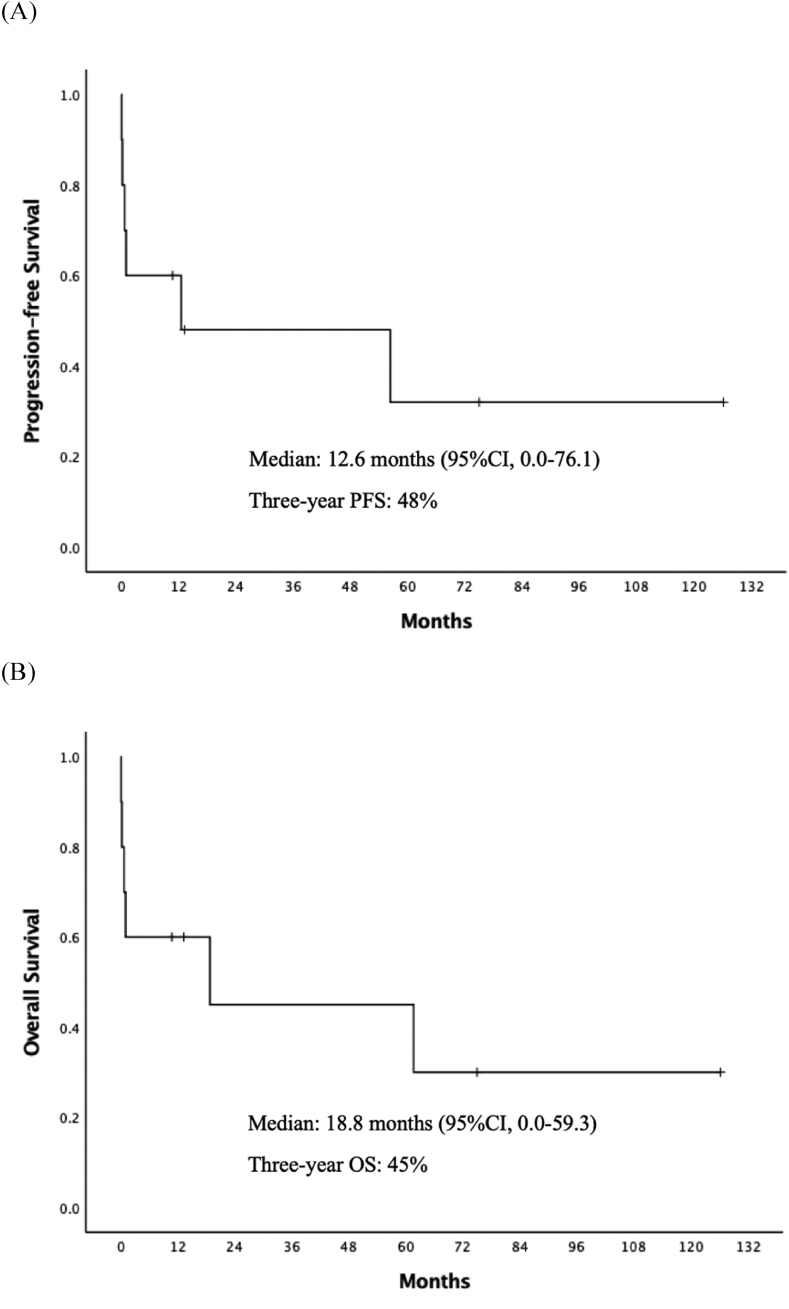
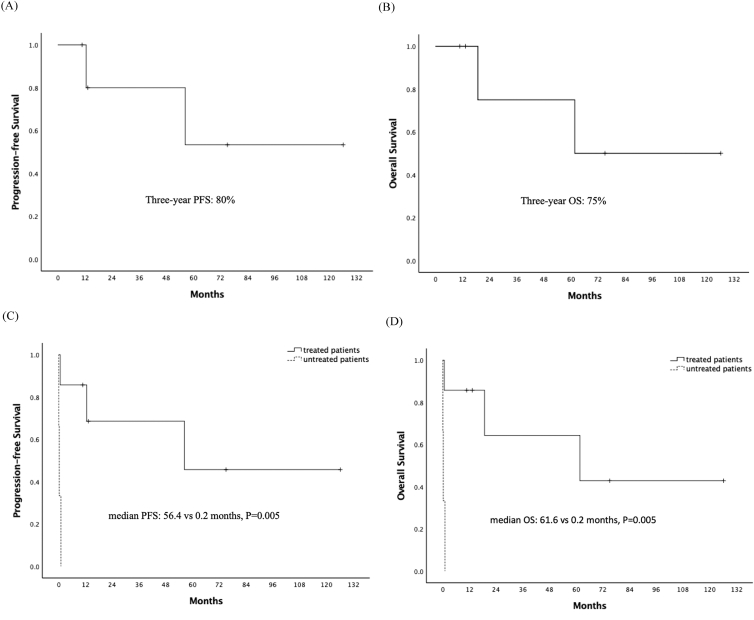
For all ten patients, the median OS time was 18.8 months (95% confidence interval [CI] 0–59.3) and the three-year OS rate was 45% (Fig. 1). Of the six patients who have completed chemotherapy, the three-year PFS and OS was 80% and 75%, respectively (Fig. 2A and B) The median PFS (56.4 vs 0.2 months, p = 0.005) and OS (61.6 vs 0.2 months, p = 0.005) of IVLBCL patients were improved for patients who received chemotherapy compared to patients without treatment (Fig. 2C and D).
Fig. 1.
(A) Progression-free survival and (B) overall survival of ten IVLBCL patients.
Fig. 2.
(A) Progression-free survival and (B) overall survival of six completely treated IVLBCL patients. Comparison of (C) progression-free survival and (D) overall survival of treated and untreated patients.
Discussion
Here we reported 10 patients with newly diagnosed IVLBCL in a single institution of Taiwan. Most of the clinical data of IVLBCL with large cohort were derived from Japanese, United State, and European studies [3,4,23,24]. Some studies with smaller series from other countries contributed valuable clinical information of IVLBCL [17,25].
Hemophagocytic lymphohistiocytosis (HLH) is a rare and potentially life-threatening disorder characterized by fever, cytopenia, hepatosplenomegaly, hemophagocytosis, hypertriglyceridemia, hyperferritinemia, low natural killer (NK) cell activity and elevated soluble CD25 (sCD25). At least five criteria have to be fulfilled for diagnosis of HLH [26]. In our patients, the diagnosis of HLH could not be fully established as the laboratory assay of NK cell activity and sCD25 were not available. However, most of them could meet four criteria (fever, cytopenia, hemophagocytosis and hepatosplenomegaly), suggesting high probability of HLH.
The majority of our patients (n = 8, 80%) are categorized as “hemophagocytic syndrome-associated form”, which was more similar to Japanese studies. Bone marrow involvement is associated with poor prognosis [27]. Spleen (n = 90%), liver (n = 30%) and bone marrow (n = 6, 60%) involvement are common in our study. These findings were similar to eastern studies. Hepatosplenomegaly and bone marrow involvement in Japanese study were 77% and 75%, respectively [4]. Spleen, liver and bone marrow involvements in Korean study were 75%, 70% and 50%, respectively [17]. Furthermore, fever is the most common clinical manifestations found in present study. This finding is compatible with reported studies [6,23,28].
The clinical features of IVLBCL are heterogeneous among patients which lead to difficulty in early diagnosis. Early treatment by early diagnosis might improve the outcome of IVLBCL [23,25,28,29]. Although currently there is no consensus on best diagnosis protocol, however random skin biopsy remains the most common strategy for patients suspected with IVLBCL [[10], [11], [12],14,30].
In our case series, skin biopsy was only performed for 3 patients with cutaneous lesions. Other patients without skin lesions were diagnosed by bone marrow biopsies mainly because of FUO with or without cytopenia. FDG PET/CT may be helpful for the early diagnosis of IVLBCL. In one case report, skin biopsy was obtained through abnormal FDG uptake lesions found by FDG PET/CT and IVLBCL was successful diagnosed [13]. Few case series have revealed successful in diagnosis of IVLBCL through tissue biopsy guided by FDG PET/CT [28,31]. Therefore, for those patients with suspicion of IVLBCL and without obvious skin involvement, FDG PET/CT can be considered as a tool of surveillance before random skin biopsies.
Liquid biopsy may be another diagnostic stool for IVLBCL, but this method is restricted to institutions that have the facilities to analyze it [32]. In our opinions, both bone marrow and skin biopsy should be performed early for patients with prolonged fever with cytopenia or cutaneous lesions because IVLBCL with bone marrow or cutaneous involvement is quite common. FDG PET/CT may have a role in guiding biopsy sites and as an assist in the diagnosis of IVLBCL. Random skin biopsy should be considered if no cutaneous lesion is found in patients suspected of IVLBCL.
Studies have shown that anthracycline-based chemotherapy can improve the outcome of IVLBCL patients compared to those without any treatment [6,25,33]. Addition of rituximab to chemotherapy further improves their outcome [[15], [16], [17],23,24,34]. The reported CR and OS rate in western studies were 53%–90% and 42%–89%, respectively [15,23]. IVLBCL patients in Japan have the CR and three-year OS rates of 82% and 66%, respectively [16]. In this study, we treated six patients with rituximab-based chemotherapy with CR rate and three-year OS rate of 100% and 75%, respectively. Our result is similar or better than previous reported series.
Despite rituximab has improved the outcome of IVLBCL patients, but the long-term survival still remains dismal. More intensive therapy such as autologous hematopoietic stem cell transplantation (auto-HSCT) has been pursued to overcome this problem. Auto-HSCT has been reported to result in prolonged survival for IVLBCL patients, with three-year OS of 91%–100% [[35], [36], [37], [38]]. Selected studies in the treatment and outcome of IVLBCL patients were summarized in Table 3. None of patients in our study underwent auto-HSCT. Thus, the impact of auto-HSCT cannot be evaluated. Risk factors for identifying IVLBCL patients with high relapse rate have not been determined. Based on the results of previous studies, auto-HSCT may be an option for selected feasible IVLBCL patients.
Table 3.
Studies in treating IVLBCL.
| Authors | Patient number | Treatment | Response rate | Survival |
|---|---|---|---|---|
| Ferreri et al. 2004 [33] | 38 (22 treated, 16 untreated) | CHOP, CEOP, CNOP, MACOP-B | 10 CR, 3 PR, 7 PD, 2 TD | 3-year OS: 33% |
| Shimada et al. 2008 [16] | 106 | R-CT (n = 49) CT (n = 57) |
CR rate: 82% for R-CT, 51% for CT | 2-year PFS: 56% for R-CT, 27% for CT 2-year OS: 66% for R-CT, 46% for CT. |
| Ferreri et al. 2008 [15] | 30 | R-CT (n = 10) CT (n = 20) |
CR rate: 90% for R-CT, 50% for CT | 3-year EFS: 89% for R-CT, 35% for CT 3-year OS: 89% for R-CT, 38% for CT |
| Hong et al. 2014 [17] | 20 | R–CHOP (n = 12) CHOP (n = 7) VP (n = 1) |
CR rate: 93.3% for 15 evaluable patients (9 R–CHOP, 6 CHOP); 100% for R–CHOP (6 relapsed); 83.3% for CHOP | median PFS: 30.8 months median OS: 38.9 months 3-year-OS: 71.4% for R–CHOP, 25% for CHOP 3-year-OS: with HLH 29.6%, without HLH 75% |
| Brunet et al. 2017 [23] | 29 (18 treated, 11 untreated) | R–CHOP (n = 17) CHOP (n = 1) |
8 CR, 3 PR, 4 SD/PD for 15 patients completed first line treatment | 3-year OS: 42.7% 3-year EFS: 64.2% |
| Rajyaguru et al. 2017 [24] | 344 (245 treated, 99 untreated) | R-CT or CT ± RT | Not reported | 1-year OS: 67.6% 3-year OS: 52.5% 5-year OS: 46.4% |
| Kato et al. 2014 [37] | 6 | R–CHOP or R-THP-COP, followed by autoHSCT | CR rate: 100% | median OS since autoHSCT: 56.5 (12–99) months |
| Meissner et al. 2016 [38] | 11 | R–CHOP/DHAP/HCVAD/R–CHP, followed by autoHSCT | 8 CR, 2 PD, 1 TD | 2-year PFS: 81% 2-year OS: 91% |
| Our study | 10 (7 treated, 3 untreated) | R–CHOP(n = 4), CHOP(n = 1), R–COP (n = 1), R–CVOP(n = 1) | 6 CR with 2 relapsed; 1 early death | median PFS: 12.6 months median OS: 18.8 months 3-year OS: 45% |
Abbreviations: CHOP: cyclophosphamide, doxorubicin, vincristine, prednisone; CEOP: cyclophosphamide, epidoxorubicin, vincristine, prednisone; CNOP: cyclophosphamide, mitoxantrone, vincristine, prednisone; MACOP-B: methotrexate, doxorubicin, cyclophosphamide, vincristine, prednisone, bleomycin; R-CT: rituximab, chemotherapy; CT: chemotherapy; R–CHOP: rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone; VP: vincristine, prednisolone; RT: radiotherapy; R-THP-COP: rituximab, pirarubicin, cyclophosphamide, vincristine, prednisone; DHAP: dexamethasone, high-dose cytarabine, cisplatin; HCVAD: cyclophosphamide, vincristine, doxorubicin, dexamethasone, methotrexate, cytarabine; R–CHP: rituximab, cyclophosphamide, doxorubicin and prednisone; R–CVOP: rituximab, cyclophosphamide, etoposide, vincristine, prednisolone; CR: complete response; PR: partial response; PD: progression disease; SD: stable disease; TD: treatment related death; OS: overall survival; PFS: progression free survival; EFS: event free survival; RFS: relapsed free survival; HLH: hemophagocytic lymphohistiocytosis; autoHSCT: autologous hematopoietic stem cell transplantation.
In our study, three patients had cytogenetic analysis for bone marrow samples. All of them had complex karyotype and both chromosome 1 and 6q involved. Two of three patients had chromosome 18 involved. These findings were similar to the study of Klairmont et al. which showed most of IVLBCL patients were associated with complex karyotypes with recurrent cytogenetic abnormalities commonly involving chromosomes 1, 6q, and 18 [39]. Early mortality was found in two of these patients in our study, but the remaining one was still alive to the date of last follow-up. It is still unclear whether complex karyotypes or cytogenetic abnormalities have prognostic implications, and further studies are warranted.
The limitation of our study was mainly due to the retrospective feature and small sample size. It is difficult to conduct a prospective study owning to the rarity of IVLBCL. In our experience, rituximab combined chemotherapy was effective in treating IVLBCL patients. The CR rate was 100% for patients who completed treatment courses, but with a significant relapse rate which compromised the long-term survival of IVLBCL patients. Consolidation therapy with auto-HSCT may be considered for selected patients to achieve a durable remission and long-term survival.
Conclusion
In conclusion, IVLBCL is a rare and aggressive disease with unusual presentations and difficulties in early diagnosis. Therefore, we should consider IVLBCL as one of the differential diagnosis in patients with prolonged fever or cytopenia. Early diagnosis and prompt treatment with rituximab-combined chemotherapy may play a key role in improving the outcome of IVLBCL patients.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Acknowledgements
This work was supported by grants from Chang Gung Memorial Hospital (CORPG3F0701, CORPG3F0671, CORPG3G0811).
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Nakamura S., Ponzoni M., Campo E. In: WHO classification of tumours of haematopoietic and lymphoid tissues, Revised. 4th ed. Swerdlow S.H., Campo E., Harris N.L., Jaffe E.S., Pileri S.A., Stein H., editors. IARC; Lyon, France: 2017. Intravascular large B-cell lymphoma; pp. 317–318. [Google Scholar]
- 2.Grimm K.E., O'Malley D.P. Aggressive B cell lymphomas in the 2017 revised WHO classification of tumors of hematopoietic and lymphoid tissues. Ann Diagn Pathol. 2018;38:6–10. doi: 10.1016/j.anndiagpath.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 3.Ferreri A.J., Dognini G.P., Campo E., Willemze R., Seymour J.F., Bairey O. Variations in clinical presentation, frequency of hemophagocytosis and clinical behavior of intravascular lymphoma diagnosed in different geographical regions. Haematologica. 2007;92:486–492. doi: 10.3324/haematol.10829. [DOI] [PubMed] [Google Scholar]
- 4.Murase T., Yamaguchi M., Suzuki R., Okamoto M., Sato Y., Tamaru Ji. Intravascular large B-cell lymphoma (IVLBCL): a clinicopathologic study of 96 cases with special reference to the immunophenotypic heterogeneity of CD5. Blood. 2007;109:478–485. doi: 10.1182/blood-2006-01-021253. [DOI] [PubMed] [Google Scholar]
- 5.Ponzoni M., Campo E., Nakamura S. Intravascular large B-cell lymphoma: a chameleon with multiple faces and many masks. Blood. 2018;132:1561–1567. doi: 10.1182/blood-2017-04-737445. [DOI] [PubMed] [Google Scholar]
- 6.Ferreri A.J., Campo E., Seymour J.F., Willemze R., Ilariucci F., Ambrosetti A. Intravascular lymphoma: clinical presentation, natural history, management and prognostic factors in a series of 38 cases, with special emphasis on the 'cutaneous variant'. Br J Haematol. 2004;127:173–183. doi: 10.1111/j.1365-2141.2004.05177.x. [DOI] [PubMed] [Google Scholar]
- 7.Emmanuela Obiorah I., Ozdemirli M. Intravascular large B-cell lymphoma mimicking temporal arteritis. Case Rep Rheumatol. 2018;2018:5364985. doi: 10.1155/2018/5364985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Katib S., Colvin R., Sokhandon F. Intravascular large B-cell lymphoma presenting with diffuse gallbladder wall thickening: a case report and literature review. Case Rep Radiol. 2018;2018:2494207. doi: 10.1155/2018/2494207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pearce C., Hope S., Butchart J. Intravascular lymphoma presenting with postural hypotension. BMJ Case Rep. 2018;2018 doi: 10.1136/bcr-2017-221803. bcr-2017-221803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higashi Y., Kawai K., Yonekura K., Takeda K., Kanzaki T., Utsunomiya A. Indication for random skin biopsy for the diagnosis of intravascular large B cell lymphoma. Dermatology. 2012;224:46–50. doi: 10.1159/000336885. [DOI] [PubMed] [Google Scholar]
- 11.Matsue K., Asada N., Odawara J., Aoki T., Kimura SI, Iwama KI. Random skin biopsy and bone marrow biopsy for diagnosis of intravascular large B cell lymphoma. Ann Hematol. 2010;90:417–421. doi: 10.1007/s00277-010-1101-3. [DOI] [PubMed] [Google Scholar]
- 12.Asada N., Odawara J., Kimura SI, Aoki T., Yamakura M., Takeuchi M. Use of random skin biopsy for diagnosis of intravascular large B-cell lymphoma. Mayo Clin Proc. 2007;82:1525–1527. doi: 10.1016/s0025-6196(11)61097-5. [DOI] [PubMed] [Google Scholar]
- 13.Matsukura K., Hokkoku K., Shiraoka A., Yang L., Takahashi Y., Hatanaka Y. Increased uptake on (18) F-fluorodeoxyglucose positron emission tomography/computed tomography is indicative of occult skin lesions in a patient with intravascular large B-cell lymphoma. J Dermatol. 2018;45:e254–e255. doi: 10.1111/1346-8138.14302. [DOI] [PubMed] [Google Scholar]
- 14.Pongpudpunth M., Rattanakaemakorn P., Fleischer A.B., Jr. Usefulness of random skin biopsy as a diagnostic tool of intravascular lymphoma presenting with fever of unknown origin. Am J Dermatopathol. 2015;37:686–690. doi: 10.1097/DAD.0000000000000321. [DOI] [PubMed] [Google Scholar]
- 15.Ferreri A.J., Dognini G.P., Bairey O., Szomor A., Montalbán C., Horvath B. The addition of rituximab to anthracycline-based chemotherapy significantly improves outcome in ‘Western’ patients with intravascular large B-cell lymphoma. Br J Haematol. 2008;143:253–257. doi: 10.1111/j.1365-2141.2008.07338.x. [DOI] [PubMed] [Google Scholar]
- 16.Shimada K., Matsue K., Yamamoto K., Murase T., Ichikawa N., Okamoto M. Retrospective analysis of intravascular large B-cell lymphoma treated with rituximab-containing chemotherapy as reported by the IVL study group in Japan. J Clin Oncol. 2008;26:3189–3195. doi: 10.1200/JCO.2007.15.4278. [DOI] [PubMed] [Google Scholar]
- 17.Hong J.Y., Kim H.J., Ko Y.H., Choi J.Y., Jung C.W., Kim S.J. Clinical features and treatment outcomes of intravascular large B-cell lymphoma: a single-center experience in korea. Acta Haematol. 2014;131:18–27. doi: 10.1159/000351060. [DOI] [PubMed] [Google Scholar]
- 18.Swerdlow S.H., Campo E., Harris N.L., Jaffe E.S., Pileri S.A., Stein H. IARC; Lyon, France: 2008. WHO classification of tumours of haematopoietic and lymphoid tissues. [Google Scholar]
- 19.Murase T., Nakamura S., Kawauchi K., Matsuzaki H., Sakai C., Inaba T. An Asian variant of intravascular large B-cell lymphoma: clinical, pathological and cytogenetic approaches to diffuse large B-cell lymphoma associated with haemophagocytic syndrome. Br J Haematol. 2000;111:826–834. [PubMed] [Google Scholar]
- 20.Ponzoni M., Ferreri A.J., Campo E., Facchetti F., Mazzucchelli L., Yoshino T. Definition, diagnosis, and management of intravascular large B-cell lymphoma: proposals and perspectives from an international consensus meeting. J Clin Oncol. 2007;25:3168–3173. doi: 10.1200/JCO.2006.08.2313. [DOI] [PubMed] [Google Scholar]
- 21.Cheson B.D., Fisher R.I., Barrington S.F., Cavalli F., Schwartz L.H., Zucca E. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059–3068. doi: 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.The International Non-Hodgkin's Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin's lymphoma. N Engl J Med. 1993;329:987–994. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 23.Brunet V., Marouan S., Routy J.P., Hashem M.A., Bernier V., Simard R. Retrospective study of intravascular large B-cell lymphoma cases diagnosed in Quebec: a retrospective study of 29 case reports. Medicine (Baltim) 2017;96 doi: 10.1097/MD.0000000000005985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rajyaguru D.J., Bhaskar C., Borgert A.J., Smith A., Parsons B. Intravascular large B-cell lymphoma in the United States (US): a population-based study using surveillance, epidemiology, and end results program and national cancer database. Leuk Lymphoma. 2017;58:1–9. doi: 10.1080/10428194.2017.1287363. [DOI] [PubMed] [Google Scholar]
- 25.Sukpanichnant S., Visuthisakchai S. Intravascular lymphomatosis: a study of 20 cases in Thailand and a review of the literature. Clin Lymphoma Myeloma. 2006;6:319–328. doi: 10.3816/CLM.2006.n.007. [DOI] [PubMed] [Google Scholar]
- 26.Jordan M.B., Allen C.E., Weitzman S., Filipovich A.H., McClain K.L. How I treat hemophagocytic lymphohistiocytosis. Blood. 2011;118:4041–4052. doi: 10.1182/blood-2011-03-278127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang J., Ding W., Gao L., Yao W., Chen M., Zhao S. High frequency of bone marrow involvement in intravascular large B-cell lymphoma. Int J Surg Pathol. 2017;25:118–126. doi: 10.1177/1066896916665203. [DOI] [PubMed] [Google Scholar]
- 28.Hadjadj J., Nielly H., Piekarski E., Cuccuini W., Deau-Fischer B., Hourseau M. Uterine intravascular lymphoma as a cause of fever of unknown origin. Ann Hematol. 2017;96:1891–1896. doi: 10.1007/s00277-017-3117-4. [DOI] [PubMed] [Google Scholar]
- 29.Masaki Y., Dong L., Nakajima A., Iwao H., Miki M., Kurose N. Intravascular large B cell lymphoma: proposed of the strategy for early diagnosis and treatment of patients with rapid deteriorating condition. Int J Hematol. 2009;89:600–610. doi: 10.1007/s12185-009-0304-7. [DOI] [PubMed] [Google Scholar]
- 30.Banjongjit A., Chiratikarnwong K., Saelue P., Sangmala S., Auepemkiate S., Kayasut K. Random skin biopsy for diagnosis of intravascular large B-cell lymphoma in a patient with hypoxemia and normal lung imaging. JAAD Case Rep. 2018;4:149–151. doi: 10.1016/j.jdcr.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miura Y., Tsudo M. Fluorodeoxyglucose-PET/CT for diagnosis of intravascular large B-cell lymphoma. Mayo Clin Proc. 2010;85:e56–e57. doi: 10.4065/mcp.2009.0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suehara Y., Sakata-Yanagimoto M., Hattori K., Nanmoku T., Itoh T., Kaji D. Liquid biopsy for the identification of intravascular large B-cell lymphoma. Haematologica. 2018;103:e241–e244. doi: 10.3324/haematol.2017.178830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferreri A.J., Campo E., Ambrosetti A., Ilariucci F., Seymour J.F., Willemze R. Anthracycline-based chemotherapy as primary treatment for intravascular lymphoma. Ann Oncol. 2004;15:1215–1221. doi: 10.1093/annonc/mdh274. [DOI] [PubMed] [Google Scholar]
- 34.Shimizu I., Ichikawa N., Yotsumoto M., Sumi M., Ueno M., Kobayashi H. Asian variant of intravascular lymphoma: aspects of diagnosis and the role of rituximab. Intern Med. 2007;46:1381–1386. doi: 10.2169/internalmedicine.46.0066. [DOI] [PubMed] [Google Scholar]
- 35.Yamaguchi M., Kimura M., Watanabe Y., Taniguchi M., Masuya M., Kageyama S. Successful autologous peripheral blood stem cell transplantation for relapsed intravascular lymphomatosis. Bone Marrow Transplant. 2001;27:89–91. doi: 10.1038/sj.bmt.1702735. [DOI] [PubMed] [Google Scholar]
- 36.Koizumi M., Nishimura M., Yokota A., Munekata S., Kobayashi T., Saito Y. Successful treatment of intravascular malignant lymphomatosis with high-dose chemotherapy and autologous peripheral blood stem cell transplantation. Bone Marrow Transplant. 2001;27:1101–1103. doi: 10.1038/sj.bmt.1703038. [DOI] [PubMed] [Google Scholar]
- 37.Kato K., Ohno Y., Kamimura T., Kusumoto H., Tochigi T., Jinnouchi F. Long-term remission after high-dose chemotherapy followed by auto-SCT as consolidation for intravascular large B-cell lymphoma. Bone Marrow Transplant. 2014;49:1543–1544. doi: 10.1038/bmt.2014.189. [DOI] [PubMed] [Google Scholar]
- 38.Meissner J., Finel H., Dietrich S., Boumendil A., Kanfer E., Laboure G. Autologous hematopoietic stem cell transplantation for intravascular large B-cell lymphoma: the European Society for Blood and Marrow Transplantation experience. Bone Marrow Transplant. 2017;52:650–652. doi: 10.1038/bmt.2016.339. [DOI] [PubMed] [Google Scholar]
- 39.Klairmont M.M., Cheng J., Martin M.G., Gradowski J.F. Recurrent cytogenetic abnormalities in intravascular large B-cell lymphoma. Am J Clin Pathol. 2018;150:18–26. doi: 10.1093/ajcp/aqy023. [DOI] [PubMed] [Google Scholar]