Abstract
Nocardia asteroides complex is an important opportunistic agent in immunocompromised hosts. Usually, primary pulmonary infection occurs and is followed by dissemination of the pathogen to the central nervous system and soft tissues. As described in the literature, almost every organ can be infected, but to our knowledge, Nocardia has been described as a pathogen responsible for thyroid abscess in only one report, which was published in 1993. The present report is the second case report of Nocardia thyroiditis. The patient was under immunosuppressor treatment following a combined liver-kidney transplant and presented with a preexisting nodular goiter which was probably a predisposing factor to the start and development of the thyroid infection.
Nocardiae are aerobic actinomycetes and are branching, filamentous, and ubiquitous soil saprophytes. They are gram positive and partially acid fast and are known as opportunistic pathogens in immunocompromised patients (13, 18), including AIDS patients. The species Nocardia asteroides is heterogeneous, and therefore, most taxonomists agree that it should be called Nocardia asteroides complex. This complex includes two established species, Nocardia nova and Nocardia farcinica, as well as several other taxa that are unnamed (13, 23). The organism has been reported to cause a variety of infections, but primary pulmonary infection occurs most often (5, 18). Other localized extrapulmonary infections may also occur, especially disseminated infections to the central nervous system and soft tissues (7, 14), and more rarely, infections of the pericardium (20), cornea (21), and urinary tract (17) are involved. Recently, pseudo-outbreaks of infections caused by N. asteroides have been reported and investigated (8, 12). The thyroid location of Nocardia infection is unusual. However, a case report of a thyroid Nocardia infection in an immunocompromised patient with systemic lupus erythematosus was published in 1993 (11). We report here on a second case of Nocardia thyroiditis in the recipient of a combined liver-kidney transplant undergoing immunosuppressor treatment.
Case report.
A 58-year-old man was admitted to the Saint Eloi Hospital in April 1998 for a combined liver-kidney transplant following 2 years of hemodialysis for polycystic liver-kidney disease. The initial immunosuppressor treatment was a combination of azathioprine, prednisone, and an antilymphocyte preparation. After 8 days, the antilymphocyte preparation was replaced by tacrolimus. There was no sign of acute rejection. One month later, the patient presented with a fever associated with pericardial and pleural effusions, but searches for bacteria in those effusions were negative. He was placed on empiric therapy consisting of a combination of intravenous imipenem and vancomycin. After 1 week of treatment, complete resolution of the pericardial and pleural effusions was observed. Approximately 30 days after this episode, the patient was readmitted to the hospital with a fever and a thyromegaly. He was known to have a previous history of a small right nodular goiter. The clinical examination of the neck revealed a painful thyroid nodule in the right lobe without cutaneous induration. The sonographic features of the gland were strongly suggestive of the presence of a thyroid abscess containing fluid. The diagnosis was confirmed by ultrasound-guided aspiration of the liquid. A few milliliters of very viscous, granular, and cream-colored purulent drainage was obtained and was sent to the bacteriology laboratory, where the microbiological diagnosis of Nocardia infection was made. Antibiotic therapy was started empirically with a combination of intravenous imipenem and amikacin. Despite this treatment, there was no clinical improvement of the thyroid nodule after 10 days. Moreover, a perirenal nodule and a perirenal effusion were found by ultrasonography of the transplanted kidney. This clinical evolution was strongly suggestive of Nocardia dissemination. The cerebral scan was normal. No evidence of a pulmonary source was found; indeed, no respiratory symptoms were present, the chest radiograph was normal, and direct examinations and cultures of sputum for Nocardia remained negative. Surgical drainage of the pus of the thyroid nodule was then performed, and a combination of oral amoxicillin-clavulanic acid and co-trimoxazole treatment was prescribed. With this treatment, the patient became afebrile and the thyroid nodule decreased in size in a few days. A complete resolution of the renal nodule and the perirenal effusion was also observed. After 1 week, the amoxicillin-clavulanic acid was stopped and co-trimoxazole alone was prescribed for 5 months.
Laboratory identification and susceptibility testing.
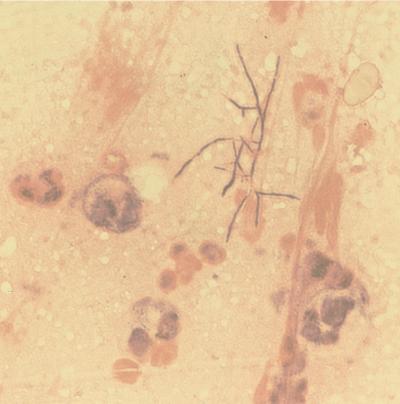
The microbiological diagnosis was made by isolation of Nocardia from the thyroid abscess by puncture. Samples were examined by direct microscopic observation of preparations stained with Gram stain. A primary smear made directly from the pus showed organisms with the classical appearance of Nocardia organisms and offered a rapid means of diagnosis (Fig. 1). We noted by Gram staining many irregular, gram-positive, branching, filamentous rods and numerous leukocytes with 90% neutrophilic polynuclear cells. The specimen was cultured on blood agar plates, brain heart infusion agar, and blood-chocolate plates that were incubated in a CO2 atmosphere at 37°C. After a incubation for only 2 days, typical wrinkled, dull, rough, white colonies appeared on all media. We also processed the sample through the BACTEC 460 TB system (Becton Dickinson Diagnostic Instrument Systems, Sparks, Md.) and Loewenstein-Jensen medium for the isolation of mycobacteria since coinfection with N. asteroides and Mycobacterium spp. has been reported previously (9, 15). No organism other than Nocardia was isolated, and the Nocardia isolate grew very well in the BACTEC system. We identified Nocardia spp. to the genus level in our laboratory using standard manual methods based on biochemical tests and enzymatic activities detected with the API ZYM system (BioMérieux). Enzymatic tests were performed with the following principal enzymes, which all gave positive reactions: alkaline phosphatase, caprylate esterase, leucine arylamidase, valine arylamidase, phosphatase acid, phosphohydrolase, α-glucosidase, and β-glucosidase. The β-galactosidase reaction was positive within 3 h, but the test was not performed with API ZYM system since it gives results which are consistently negative for Nocardia (6). We could exclude N. farcinica because it is usually butyrate esterase positive, but any positive or negative result by enzymatic tests could exclude N. nova. The species N. asteroides was determined by the National Reference Center for Mycosis and Antifungal Agents (P. Boiron, Institut Pasteur, Paris, France), on the basis of complementary tests, as the capacity of the strain to decompose certain substrates (6). Antibiotic susceptibility testing of the strain was performed by the disk diffusion assay on Mueller-Hinton blood agar plates. Indeed, this agar diffusion technique is the most widely used technique since no standardized method for studying the sensitivity of Nocardia to antibiotics exists (6). The results were read after 48 h, and the strain was scored as susceptible, intermediate, or resistant according to the recommendations of the Comité Français de l’Antibiogramme of the French Society for Microbiology (1). Beta-lactamase production was detected by the Cefinase (BBL) test. The strain was susceptible to imipenem, trimethoprim-sulfamethoxazole, gentamicin, amikacin, tobramycin, cefamandole, cefotaxime, minocycline, and amoxicillin-clavulanic acid. It was resistant to amoxicillin, ceftriaxone, erythromycin, vancomycin, pefloxacin, and ciprofloxacin.
FIG. 1.
Gram-stained specimen from the thyroid abscess, obtained by puncture, showing irregular, gram-positive, branching, filamentous rods. Magnification, ×950.
Discussion.
Nocardia species are organisms associated with environmental materials. They are ubiquitous saprophytes, including soil saprophytes, and occasionally cause disease in humans. Members of the N. asteroides complex cause most infections in humans, especially in immunocompromised patients. The lung is the most commonly affected primary site (73%); it is also the site of the initial infection in the majority of patients with disseminated cases of infection. The dissemination can occur in almost every organ, but we report in this paper on an unusual location of Nocardia infection: the thyroid gland. Pyogenic thyroiditis is a rare inflammatory disease which most commonly affects women with preexisting thyroid disease as a nodular goiter (2, 3). The most common etiologic agents isolated are Streptococcus pyogenes, Staphylococcus aureus, and Streptococcus pneumoniae, although other bacteria including Escherichia coli, Haemophilus influenzae, meningococcal organisms, and anaerobes have been reported as causes of infection (2). To our knowledge, Nocardia has been described as a pathogen responsible for thyroid abscess in only one patient in 1993 (11). The patient was a 20-year-old woman who had a 2-year history of lupus nephritis and who had been treated with prednisone and cyclophosphamide. She developed in the right anterior triangle of the neck a diffuse tender swelling that extended from the level of the thyroid cartilage to the suprasternal notch. A large amount of pus was drained from the lateral pharyngeal space. Gram staining of the pus was negative, and Nocardia was obtained from a culture of the pus. Although the first stage of diagnosis of nocardiosis involves direct examination of the specimen, microscopic observation occasionally reveals the presence of gram-positive, branched bacterial filaments. In our report, diagnosis by microscopic examination of the pus was easily made because of the large amount of organisms in the sample. The development of Nocardia in culture media is rather slow; colonies are usually visible after 3 to 5 days, and the delay can sometimes be as long as 2 to 3 weeks. In our study bacterial growth in either the usual media or Lowenstein-Jensen and BACTEC 460 TB media was fast (2 days). In relation to the portal of entry, the thyroid infection was probably due to hematogenous spread of Nocardia from the lung, which would have been the primary site of infection. Indeed, according to the literature, because lung lesions may be small or obscured by underlying pulmonary abnormalities, infections with no known primary site may actually be of pulmonary origin (2). The origin of infection in the only report of Nocardia thyroiditis (11) was unknown, and the investigators also suspected a primary pulmonary infection. The preexisting nodular goiter in our patient was probably a predisposing factor to the start and development of the thyroid infection. The patient was also at increased risk of infection because he was the recipient of a combined liver-kidney transplant and was undergoing immunosuppressor treatment.
With regard to the treatment of thyroiditis, an aspiration with a thick needle is usually recommended as the first line of management. For our patient, the pus was so thick that incision and surgical drainage were more appropriate. Once the diagnosis of nocardiosis was made, a combination of imipenem and amikacin was prescribed without in vitro test results. As we could see from the antibiotic susceptibility pattern, the strain was susceptible to imipenem and amikacin, but despite this treatment, the patient did not improve clinically, probably because the antibiotics could not reach the bacteria because of the thickness of the pus. For this reason surgical drainage was necessary. As has already been described, we noted that the mechanism of penicillin resistance of our strain implicated the production of beta-lactamase (16). The beta-lactamase was detected as published previously (19) by the addition of clavulanic acid to commercial amoxicillin disks and observation of a zone of inhibition greater than that achieved with amoxicillin alone. Moreover, the Cefinase test for beta-lactamase production was positive. The strain was sensitive to trimethoprim-sulfamethoxazole, which is often the drug of choice for the treatment of nocardiosis (10, 22). On the other hand, our isolate was resistant to the two quinolones tested, pefloxacin and ciprofloxacin. In general agreement with previous studies (4, 10), ciprofloxacin has been shown to have intermediate activity against Nocardia.
In conclusion, we believe that the case of infection described here represents the second reported case of thyroiditis due to N. asteroides complex infection. This observation suggests that the thyroid should be added to the list of tissues that can be the site of infection in patients with disseminated nocardiosis.
Acknowledgments
We thank M. J. Bardou for technical help, A. Portela for bibliographic research, B. Gay for Gram staining photographs, and C. Legraverend for critical reading of the manuscript.
REFERENCES
- 1.Acar J, Carret G, Cavallo J D, Chardon H, Choutet P, Courvalin P, Dabernat H, Drugeon H, Dubreuil L, Goldstein F, Jarlier V, Leclercq R, Nicolas-Chanoine M H, Philippon A, Rouveix B, Sirot J, Soussy J C, Thabaut A. Antibiogram Committee of the French Society for Microbiology 1998 Statement. Pathol Biol. 1998;46:I–XVI. [PubMed] [Google Scholar]
- 2.Agarwal A, Mishra S K, Sharma A K. Acute suppurative thyroiditis with demonstrable distant primary focus: a report of two cases. Thyroid. 1998;8:399–401. doi: 10.1089/thy.1998.8.399. [DOI] [PubMed] [Google Scholar]
- 3.Berger S A, Zonszein J, Villamena P, Mittman N. Infectious diseases of the thyroid gland. Rev Infect Dis. 1983;5:108–122. doi: 10.1093/clinids/5.1.108. [DOI] [PubMed] [Google Scholar]
- 4.Berkey P, Moore D, Rolston K. In vitro susceptibilities of Nocardia species to newer antimicrobial agents. Antimicrob Agents Chemother. 1988;32:1078–1079. doi: 10.1128/aac.32.7.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boiron P, Provost F, Chevrier G, Dupont B. Review of nocardial infections in France 1987 to 1990. Eur J Clin Microbiol Infect Dis. 1992;11:709–714. doi: 10.1007/BF01989975. [DOI] [PubMed] [Google Scholar]
- 6.Boiron P, Provost F, Dupont B, editors. Laboratory methods for the diagnosis of nocardiosis. Paris, France: Institut Pasteur; 1993. [Google Scholar]
- 7.Boscaro M, Fallo F, Sonino N. Disseminated nocardiosis in a patient with Cushing’s syndrome. J Endocrinol Invest. 1994;17:443–445. doi: 10.1007/BF03347735. [DOI] [PubMed] [Google Scholar]
- 8.Exmelin L, Malbruny B, Vergnaud M, Prosvost F, Boiron P, Morel C. Molecular study of nosocomial nocardiosis outbreak involving heart transplant recipients. J Clin Microbiol. 1996;34:1014–1016. doi: 10.1128/jcm.34.4.1014-1016.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holt R I G, Kwan J T C, Sefton A M, Cunningham J. Successful treatment of concomitant pulmonary nocardiosis and aspergillosis in an immunocompromised renal patient. Eur J Clin Microbiol Infect Dis. 1993;12:110–112. doi: 10.1007/BF01967584. [DOI] [PubMed] [Google Scholar]
- 10.Khardori N, Shawar R, Gupta R, Rosembaum B, Rolston K. In vitro antimicrobial susceptibilities of Nocardia species. Antimicrob Agents Chemother. 1993;37:882–884. doi: 10.1128/aac.37.4.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lewin S R, Street A C, Snider J. Suppurative thyroiditis due to Nocardia asteroides. J Infect. 1993;26:339–340. doi: 10.1016/0163-4453(93)95937-e. [DOI] [PubMed] [Google Scholar]
- 12.Louie L, Louie M, Simor A E. Investigation of a pseudo-outbreak of Nocardia asteroides infection by pulsed-field gel electrophoresis and randomly amplified polymorphic DNA PCR. J Clin Microbiol. 1997;35:1582–1584. doi: 10.1128/jcm.35.6.1582-1584.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McNeil M M, Brown J M. The medically important aerobic actinomycetes: epidemiology and microbiology. Clin Microbiol Rev. 1994;7:357–417. doi: 10.1128/cmr.7.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McNeil M M, Brown J M, Herbert C, Shearlock K T, Saul R A, Ajello L. Disseminated Nocardia transvalensis infection: an unusual opportunistic pathogen in severely immunocompromised patients. J Infect Dis. 1992;165:175–178. doi: 10.1093/infdis/165.1.175. [DOI] [PubMed] [Google Scholar]
- 15.Monteforte J S, Wood C A. Pneumonia caused by Nocardia nova and Aspergillus fumigatus after cardiac transplantation. Eur J Clin Microbiol Infect Dis. 1993;12:112–114. doi: 10.1007/BF01967585. [DOI] [PubMed] [Google Scholar]
- 16.Provost F, Laurent F, Camacho Uzcategui L R, Boiron P. Molecular study of persistence of Nocardia asteroides and Nocardia otitidiscaviarum strains in patients with long-term nocardiosis. J Clin Microbiol. 1997;35:1157–1160. doi: 10.1128/jcm.35.5.1157-1160.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salahuddin F, Sen P, Chechko S. Urinary tract infection with an unusual pathogen (Nocardia asteroides) J Urol. 1996;155:654–655. [PubMed] [Google Scholar]
- 18.Simpson G L, Stinson E B, Egger M J, Remington J S. Nocardial infections in the immunocompromised host: a detailed study in a defined population. Rev Infect Dis. 1981;3:492–506. doi: 10.1093/clinids/3.3.492. [DOI] [PubMed] [Google Scholar]
- 19.Steingrube V A, Wallace R J, Jr, Brown B A, Zhang Y, Steele L C, Young G, Nash D R. Partial characterization of Nocardia farcinica β-lactamases. Antimicrob Agents Chemother. 1993;37:1850–1855. doi: 10.1128/aac.37.9.1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tabrizi S J. Nocardia pericarditis. BMJ. 1994;309:1495–1497. doi: 10.1136/bmj.309.6967.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tendolkar U M, Varaiya A, Ahuja A S, Motwane S A, Gogate A S. Corneal ulcer caused by Nocardia asteroides in a patient with leprosy. J Clin Microbiol. 1998;36:1154–1156. doi: 10.1128/jcm.36.4.1154-1156.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tokumoto J I N, Jacobs R A. Case report: Nocardia osteomyelitis. Am J Med Sci. 1994;307:428–432. doi: 10.1097/00000441-199406000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Wallace R J, Jr, Brown B A, Tsukamura M, Brown J M, Onyi G O. Clinical and laboratory features of Nocardia nova. J Clin Microbiol. 1991;29:2407–2411. doi: 10.1128/jcm.29.11.2407-2411.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]