Abstract
Background
The ongoing SARS-CoV-2 pandemic, which is dramatically spreading worldwide, is well known for its respiratory sequelae. Besides cases of Guillain-Barré Syndrome, encephalitis, hyposmia, the whole range of neurological complications due to SARSCoV-2 is still not well known.
Methods and findings
Herein, we report a new case of COVID-19, associated with mononeuropathy with reversible conduction block (CB). After SARS-CoV-2 infection, the patient developed acute weakness of left peroneal muscles. He underwent an endovenous immunoglobulin treatment, and symptoms improved. Two electroneurographic exam (before and after treatment), showed a reversible CB on left peroneal nerve. Dosage of serum antiganglioside antibodies showed anti-GM1 IgM positivity.
Conclusions
The present case gives new informations about reversible CB neuropathy as an acute presentation of SARS-CoV-2. Besides, antiganglioside antibodies evaluation could be useful to understand etiology of the increasing number of neurological manifestations related to SARS-CoV-2.
Keywords: Neuroimmunology, Peripheral neuropathy, CIDP, Antiganglioside antibodies, SARS-CoV-2, COVID-19
The ongoing SARS-CoV-2 pandemic, which is dramatically spreading worldwide, is well known for its respiratory sequelae [1]. Patients with COVID-19 disease typically present with severe viral pneumonia, often life-threatening, and other complications (gastrointestinal, renal, cardiovascular, endocrine, and hepatic systems can be hit) [2]. Neurological complications are also described: hyposmia, ageusia, headache, encephalitis, Guillain-Barré syndrome (GBS) [3].
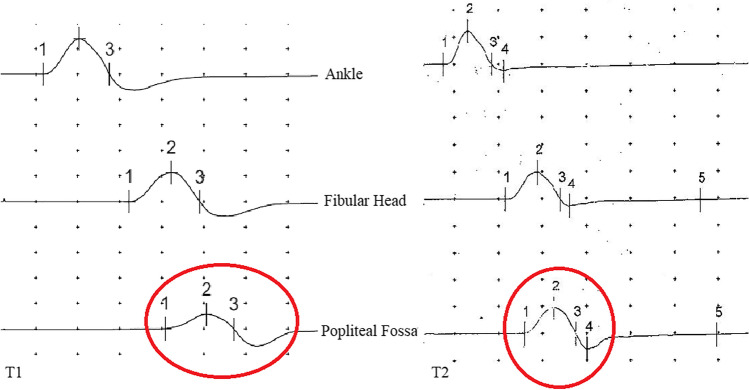
Herein, we report a new case of COVID-19, associated with mononeuropathy with reversible conduction block (CB). On 11 November 2020, a 59-year-old man, presenting with fever (Tmax 38.7 °C–101.66°F), dry cough, diarrhea, and asthenia, went to his family doctor, who sent him to the COVID-19 test center at our hospital in the suspect of SARS-CoV-2. He had no neurological sign or symptom. A real-time polymerase chain reaction (RT-PCR) nasopharyngeal swab (NPS) for SARS-CoV-2 was performed, resulting positive. Since the mild COVID-19-related symptoms, the family doctor decided to treat the patient at home, in quarantine regimen, and started a therapy with antibiotics, steroids, and low molecular weight heparin. The patient did not develop SARS-CoV-2 pneumonia, thus was not admitted to Intensive Care Unit (ICU). COVID-19-related symptoms lasted for 4 days. On 12 December 2020, after a new RT-PCR NPS for SARS-CoV-2 resulted negative, the patient came at our attention for clumsy gait with left steppage which appeared 12 days earlier (01 December 2020). After admission at our neurorehabilitation department, neurological examination disclosed weakness of the left anterior tibial, toe extensors, and peroneal muscles, with residual strength grade 3/5 according to the Medical Research Council (MRC) scale. Laboratory results on admission were unremarkable. Cerebrospinal fluid (CSF)’s test showed cell count, protein, and glucose levels in normal range; RT-PCR assay for SARS-CoV-2 on CSF resulted negative. Screening tests for Epstein–Barr virus, Campylobacter jejuni, cytomegalovirus, Herpes viruses panel, Varicella zoster virus, influenza A virus, Haemophilus influenzae, HIV, and culture examinations were negative. On 17 December 2020 (T1), nerve conduction studies showed a CB of the left peroneal nerve at the popliteal fossa (Table 1; Fig. 1). Dosage of serum antiganglioside antibodies with indirect ELISA test showed anti-GM1 IgM positivity (index 53.4; normal value 0–50).
Table 1.
Values from the peroneal nerves’ conduction studies both at T1 and T2. Values describing reversible conduction block are written in bold (decrease in amplitude - greater than 50% - and CV recorded by the left peroneal nerve at T1, not evidenced at T2), compared with the healthy peroneal nerve
| Distal latency | Amplitude | Fibular head-ankle CV | F waves mean | |
|---|---|---|---|---|
| Left peroneal nerve (T1) | 4.69 ms | Ankle 6.9 mV | 54.8 ms | |
| Fib head 5.7 mV | Fib head – ankle 41 m/s | |||
| Pop fossa 2.8 mV | Pop fossa – fib head 29 m/s | |||
| Left peroneal nerve (T2) | 3.75 ms | Ankle 7.4 mV | 51.9 ms | |
| Fib Head 6.3 mV | Fib head – Ankle 45.5 m/s | |||
| Pop fossa 7.6 mV | Pop fossa – Fib head 45.7 m/s | |||
| Right peroneal nerve (T1) | 3.76 ms | Ankle 8.3 mV | 52.2 ms | |
| Fib head 8.1 mV | Fib head – ankle 46 m/s | |||
| Pop fossa 8.9 mV | Pop fossa – fib head 50 m/s | |||
| Right peroneal nerve (T2) | 3.73 ms | Ankle 8.2 mV | 52 ms | |
| Fib head 8.2 mV | Fib head – ankle 47.1 m/s | |||
| Pop fossa 8.7 mV | Pop fossa – fib head 49 m/s |
Fig. 1.
Amplitude 5 mV; duration 50 ms. In red circles, a 50% increase in amplitude of distal compound muscle action potential at T2 (from 2.8 to 8.7 mV) can be seen
Clinical, laboratory, and neurophysiological findings were consistent with mononeuropathy with reversible CB associated with anti-GM1 antibodies. However, we had to exclude other pathologic situations such as compressive causes, or Slimmer’s paralysis [4]. These etiologies were unlikely, since the patient did not assume prolonged positions which could cause compression of peroneal nerve [5], nor had traumas, hematomas, or loss of weight (Slimmer’s paralysis). Also, systemic causes of peroneal neuropathy were excluded since the normalcy of laboratory results. Thus, the patient was treated with a 5-day protocol of intravenous immunoglobulins (IVIg) at the dosage of 0.4 g/kg/day, with subsequent improvement. On 03 February 2021, the patient underwent a follow-up neurophysiologic exam (T2), which was normal (Table 1; Fig. 1).
To date, several cases of SARS-CoV-2-associated GBS have been reported [6]; although, no other cases of SARS-CoV-2 infection associated with mononeuropathy with reversible CB have been described. It is possible that the aforedescribed event may remain isolated, and a relapse may not occur, but also it could represent the onset of a disease belonging to the symmetric or asymmetric CIDPs’ spectrum (such as multifocal motor neuropathy, or Lewis-Sumner neuropathy, or “classical” CIDP) [7], further considering the remarkable improvement with IVIg therapy. Although, we cannot totally exclude the possibility of spontaneous improvement. To be sure, regular clinical and neurophysiological follow-ups will need to be carried out, to define if the present case represents an isolated one, or any of the abovementioned relapsing–remitting dysimmune neuropathies. Seen the novelty of COVID-19 immunopathology, anti-GM1 antibodies may represent a new etiopathogenic mechanism underlining COVID19-related reversible CB neuropathy, although in our case we have mildly increased levels of antibodies, given their low level (i.e., 53.4; upper value 50). During SARS-CoV-2 disease, complexes of gangliosides are targeted by autoantibodies in GBS and its variants, being the GD1a/GD1b one of the most frequent [6]. Also, anosmia in COVID-19 could be explained by antiganglioside antibodies, seen the conspicuous expression of GD1a gangliosides in the olfactory bulb [8].
The present case gives a new information about reversible CB neuropathy as a possible acute presentation of SARS-CoV-2. Besides, antiganglioside antibody evaluation could be useful to understand etiology of the increasing number of neurological manifestations related to SARS-CoV-2.
Author contribution
Material preparation, data collection, and analysis were performed by Ettore Cioffi, Alessandro Polidoro, Luigi Iuliano, and Davide Dilenola. The first draft of the manuscript was written by Ettore Cioffi. All authors read and approved the final manuscript. Mariano Serrao and Carlo Casali supervised the final version of the manuscript. Ettore Cioffi and Davide Dilenola contributed equally to the paper.
Data availability
Raw data and materials are available upon request.
Declarations
Ethical approval
This study has been approved by our appropriate ethics committee (Ethical Committee of Sapienza University—Comitato Etico Lazio 2) and has been performed in accordance with the ethical standards laid down in the Declaration of Helsinki of 1964 and its later amendments.
Conflict of interest
None.
Informed consent
was obtained from the patient. Also, the patient signed informed consent regarding publishing its data.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Attaway AH, Scheraga RG, Bhimraj A, Biehl M, HatipoÄŸlu U. Severe COVID-19 pneumonia: pathogenesis and clinical management. BMJ. 2021;372:n436. doi: 10.1136/bmj.n436. [DOI] [PubMed] [Google Scholar]
- 2.Mahajan RK, Paul G, Mahajan R, Gautam PL, Paul B. Systemic manifestations of COVID-19. J Anaesthesiol Clin Pharmacol. 2020;36(4):435–442. doi: 10.4103/joacp.JOACP_359_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whittaker A, Anson M, Harky A. Neurological manifestations of COVID-19: a systematic review and current update. Acta Neurol Scand. 2020;142(1):14–22. doi: 10.1111/ane.13266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sotaniemi KA. Slimmer’s paralysis–peroneal neuropathy during weight reduction. J Neurol Neurosurg Psychiatry. 1984;47(5):564–566. doi: 10.1136/jnnp.47.5.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang LG, Zar S, Seidel B, Kurra A, Gitkind A. COVID-19 proned ventilation and its possible association with foot drop: a case series. Cureus. 2021;13(4):e14374. doi: 10.7759/cureus.14374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abu-Rumeileh S, Abdelhak A, Foschi M, Tumani H, Otto M. Guillain-Barré syndrome spectrum associated with COVID-19: an up-to-date systematic review of 73 cases. J Neurol. 2021;268(4):1133–1170. doi: 10.1007/s00415-020-10124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lehmann HC, Burke D, Kuwabara S. Chronic inflammatory demyelinating polyneuropathy: update on diagnosis, immunopathogenesis and treatment. J Neurol Neurosurg Psychiatry. 2019;90(9):981–987. doi: 10.1136/jnnp-2019-320314. [DOI] [PubMed] [Google Scholar]
- 8.Cutillo G, Saariaho A-H, Meri S. Physiology of gangliosides and the role of antiganglioside antibodies in human diseases. Cell Mol Immunol. 2020;17:313–322. doi: 10.1038/s41423-020-0388-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw data and materials are available upon request.