Abstract
The RAS-RAF-MEK-ERK signaling pathway is vital for different cellular mechanisms including cell proliferation, differentiation and apoptosis. This importance is highlighted by the high prevalence of mutations in RAS or related proteins of the pathway in cancers. More recently, development abnormalities have been linked to various germline mutations in this pathway and called RASopathies. Interestingly, rare disorders such as RAS-associated leukoproliferative diseases and histiocytosis have also been recently linked to multiple mutations in the same pathway, sometimes with the same mutation. This review will focus on germline RASopathies and rare somatic RASopathies and focus on how gain-of-function mutations in the same pathway can lead to various diseases.
Keywords: RAS, Somatic, Germline, Monocytes, Histiocytes, Lymphoproliferation
It's almost 60 years since discovering the human RAS proteins as normal cellular counterparts of the Harvey and Kirstein viral rat sarcoma oncogenes and almost 40 years since the discovery of mutations in these proteins contributing to the development of human cancers [1,2]. Ever since, RAS proteins and the associated pathways have been extensively studied for their contribution to cancer development, unveiling its regulation and importance in cellular proliferation, differentiation and survival, as well as the high prevalence of these mutations in human cancers (∼30% of all cancers) [3]. RAS genes encode a family of small GTPases (HRAS, NRAS and two isoforms of KRAS: KRASA, KRASB). This family of GTPases controls the activation of the downstream RAF-MEK-ERK proteins constituting the RAS/mitogen-activated protein kinase (MAPK) signaling pathway. They function as binary on-off switches between an inactive GDP-bound form and an active GTP-bound form after stimulation by various signals, including growth factors binding to receptor tyrosine kinases (RTKs), G-protein coupled receptors, cytokine receptors and extracellular matrix receptors [4]. The switch between GDP/GTP-RAS is tightly regulated by guanosine exchange factors (GEFs) participating in its activation and GTPases activating proteins (GAPs) facilitating GTP release, thus inactivating RAS. Growth factors binding to RTKs leads to the receptors' autophosphorylation. Adaptor proteins, such as growth factor receptor-bound protein 2 (GRB2), can then interact with RTKs. GRB2 is attached to Son of Sevenless proteins (SOS), a guanosine exchange factor, increasing the RAS nucleotide exchange rate from GDP to GTP, thereby amplifying the RAS activation. GTP-bound activated RAS is then able to phosphorylate downstream transducers: RAF proteins (BRAF, ARAF and CRAF), which then phosphorylate and activate MEK1 and/or MEK2, culminating to the phosphorylation and activation of ERK1 and/or ERK2 [Fig. 1] [5]. Phosphorylated-ERK1/2 exert numerous vital cellular functions in the nucleus and cytosol, including cellular growth, regulation of apoptosis and differentiation [6]. Multiple regulators of this pathway have been identified, and disruption, by genetic alterations, in one of the myriads of proteins in the signaling pathway can lead to a global gain-of-function in the RAS-associated pathways contributing to various diseases [6]. Considering its importance in cell differentiation, growth and survival, it is not surprising that germline mutations in this pathway lead to several congenital developmental disorders named “RASopathies” [4]. Interestingly, most of these conditions are known risk-factor of developing secondary cancers of various origins (both hematopoietic and non-hematopoietic neoplasm) but are not fully penetrant in this matter, although the same mutations can be found somatically in various cancers [7]. This review will focus on congenital diseases, so-called “germline RASopathies”, and discuss what we could call “somatic RASopathies” and how similar genetic backgrounds can lead to different conditions.
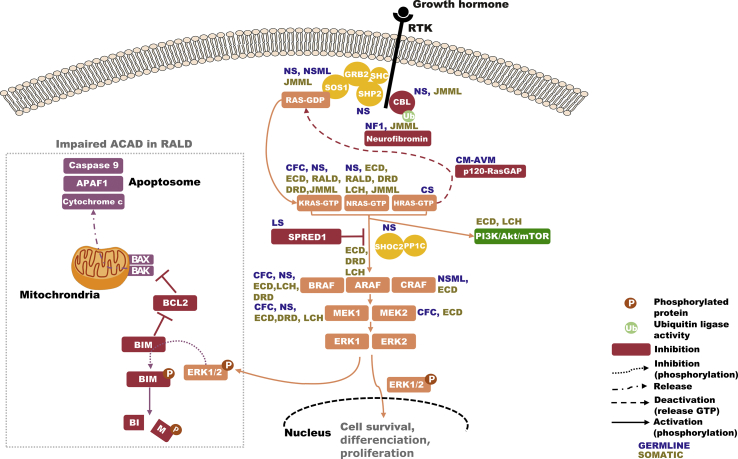
Fig. 1.
RAS/MAPK pathway and proteins involved in RAS-associated diseases. Overview of the RAS-MAPK pathway activation process from the detection of stimuli (here growth factor) by tyrosine kinase receptors (RTK) to the phosphorylation of ERK1 and 2. Diseases associated with mutations in the pathway are indicated in dark blue for germline diseases and dark yellow for somatic ones. Increased phosphorylated ERK1/2 in RAS-associated autoimmune leukoproliferative disorders (RALD) participate in BIM degradation, impairing activation autonomous cell-death (ACAD). NS: Noonan syndrome, NF1: Neurofibromatosis type 1, CFC: Cardio-facio-cutaneous syndrome, NSML: Noonan syndrome with multiple lentigines, CM-AVM: capillary malformation-arteriovenous malformation syndrome, LS: Legius syndrome, ECD: Erdheim-Chester disease, RDD: Destombes-Rosai-Dorfman, LCH: Langerhans-cell histiocytosis.
Germline rasopathies
The “RASopathies” referred to a clinical spectrum of disorders caused by germline mutations in components or regulators of the RAS/MAPK pathway. They include neurofibromatosis 1 (NF1), Noonan syndrome (NS), Noonan syndrome with multiple lentigines (NSML), Costello syndromes (CS), Legius syndrome (LS), cardiofaciocutaneous syndrome (CFC), capillary malformation-arteriovenous malformation syndrome (CM-AVM) and autosomal dominant intellectual disability type 5 [4] [Table 1]. Each of these diseases has unique clinical features. Still, the common underlying genetic conditions lead to an overlapping phenotype, including cardio-facio-cutaneous anomalies, a known risk of secondary malignancies and variable neurocognitive impairment [8]. Altogether, these syndromes account for most congenital malformation disorders, with 1 in 1000 newborns affected.
Table 1.
Germline mutations in RASopathies and associated somatic diseases.
| Diseases | Mutated genes | Protein function | Example of somatic hematopoietic disease associated with mutations in each gene |
|---|---|---|---|
| Neurofibromatosis type 1 (NF1) | NF1 | RAS-GAP | JMML, M-group histiocytosis |
| Noonan syndrome (NS) | PTPN11 | Phosphatase | M-group histiocytosis |
| SOS1 | GEF | JMML | |
| KRAS | GTPase | RALD, JMML, ECD, RDD, M-group histiocytosis | |
| NRAS | GTPase | RALD, JMML, ECD, RDD, LCH, M-group histiocytosis | |
| SHOC2 | Scaffolding | ||
| CBL | E3 ubiquitin ligase | JMML, M-group histiocytosis | |
| MAP2K1 | Kinase | ECD, RDD, LCH | |
| Noonan syndrome with multiple lentigines (NSML) | RAF1 | Kinase | ECD |
| PTPN11 | Phosphatase | M-group histiocytosis | |
| Costello syndrome (CS) | HRAS | GTPase | M-group histiocytosis |
| Cardio-facio-cutaneous syndrome (CFC) | KRAS | GTPase | RALD, JMML, ECD, RDD, M-group histiocytosis |
| BRAF | Kinase | ECD, LCH, RDD, M-group histiocytosis, hairy cell leukemia | |
| MAP2K1 | Kinase | ECD, RDD, LCH, M-group histiocytosis | |
| MAP2K2 | Kinase | ECD | |
| Legius syndrome (LS) | SPRED1 | Sprouty-related, EVH1 domain-containing protein 1 (inhibitor of RAS/MAPK activation) | |
| Capillary malformation arteriovenous malformation Syndrome (CM-AVM) | RASA1 | RAS-GAP | |
| Autosomal dominant intellectual disability type 5 | SYNGAP1 | Synaptic RAS-GAP |
Abbreviations: JMML: juvenile Myelo-Monocytic leukemia; RDD: Destombes-Rosai-Dorfman; ECD: Erdheim-Chester disease; LCH: Langerhans cell histiocytosis; RALD: RAS-associated leukoproliferative disease.
Neurofibromatosis type 1 (NF1)
NF1 was the first description of a congenital malformation caused by a germline mutation in the RAS/MAPK pathway. It is an autosomal dominant disorder affecting approximately 1 in 3000 newborns. The clinical diagnosis relies on identifying one or more of the following features: café-au-lait spots on the skin, intertriginous freckling, neurofibromas, plexiform neurofibromas, Iris Lisch nodules, skeletal dysplasia, optic pathway gliomas [9]. Patients can also present with craniofacial dysmorphia reminiscent of Noonan syndrome, cardiac malformations and other cardiovascular involvement, brain malformation, seizures and mild cognitive impairment [9]. The disease is caused by heterozygous mutations in the NF1 gene located on chromosome 17 leading to haploinsufficiency of neurofibromin. Half of the mutations are de novo [10,11]. Neurofibromin being a negative regulator of RAS, its haploinsufficiency leads to a decrease GTPase activity of neurofibromin and thus to an excess of active GTP-bound RAS [9]. This condition is a known risk factor for cancers including optic pathway glioma but also rhabdomyosarcoma, neuroblastoma and juvenile myelomonocytic leukemia (JMML) in childhood along with peripheral nerve sheath tumors, gastrointestinal stromal tumors, somatostatinomas, pheochromocytomas, breast cancer, colon cancer, lung cancers, in adulthood [4,9]. The appearance of a JMML in NF1 is frequently linked to a biallelic inactivation of the NF1 gene due to uniparental disomy or less frequently to compound heterozygous mutations and occasionally to interstitial deletions or other additional events [[12], [13], [14]]. The clinical course of JMML in such cases is aggressive and allogenic hematopoietic stem-cell transplantation is often mandatory. Despite these second genetic events, there is still a lack of understanding why some patients will develop a secondary malignancy and others not. Further understanding of this specific matter would be of interest in the follow-up of patients.
Noonan syndrome (NS) and related disorders
NS is one of the most prevalent RASopathies with 1 in 1000 people affected worldwide cited in most publications even though there are no dedicated epidemiological studies to date [15,16]. In 1963, Jacqueline Noonan first reported nine patients with pulmonary valve stenosis, craniofacial anomalies such as hypertelorism, high forehead, epicantal folds, ptosis, short neck and growth retardation, significant chest deformities and mild cognitive impairment [17]. The disorder spectrum further extended, then adding clinical heterogeneity (reviewed in Ref. [18]). The diagnosis relies on clinical features standard in all Noonan patients, including craniofacial dysmorphic features evolving with age, congenital heart defects in 90% of cases, with the most prevalent being pulmonic stenosis and hypertrophic cardiomyopathy, afterbirth growth impairment, bleeding disorders, cryptorchidism, variable cognitive deficits and less frequently predisposition to juvenile myelomonocytic leukemia (JMML) [19]. As clinical features evolve with age, some adult patients have been diagnosed after the birth of a more severely affected child.
NS is a clinically diagnosed, genetically based, heterogeneous autosomal dominant disorder with some de novo mutations reported [18]. Almost 50% of Noonan patients carry a heterozygous mutation in PTPN11 encoding SHP2 [20]. SHP2 is a nonreceptor protein tyrosine phosphatase composed of two SH2 domains and a catalytic PTP domain that dephosphorylates tyrosine-phosphorylated signaling proteins. The vast majority of the missense mutations in PTPN11 lead to constitutive activation of the catalytic domain of the protein product conducting an excess of RAS/MAPK signaling [20].
In addition to the PTPN11 mutations, many other genes have been linked to NS and NS-like disorders: SOS1, RAF1, KRAS, NRAS, SHOC2, CBL, SOS2, MRAS, RRAS2, MAP2K1 [16]. Mutations of these genes lead to the RAS/MAPK pathway activation through different mechanisms and thus to different phenotypes.
SOS1 heterozygous hypermorphic missense mutations are the second NS-causing mutations accounting for almost 20% of cases [[21], [22], [23]]. SOS1 is an essential RAS guanosine exchange factor (GEF) involved in the RAS-GDP to RAS-GTP conversion after translocation to the membrane [24]. Once translocated to the membrane, changes of SOS1-conformation unmask the allosteric binding site for RAS, whereas at the baseline SOS1 is in a catalytically inactive conformation. Almost all the mutations affect codons altering residues responsible for this inactive conformation leading to a sustained and prolonged ligand-dependent activation of RAS [22,23]. Of note, SOS1-mutated NS patients have some distinctive clinical features such as ectodermal abnormalities and absence of cognitive impairment [21].
Some Noonan patients carry mutations in the KRAS gene [25,26]. KRAS encodes two isoforms, KRASA, with tissue-specific expression and KRASB ubiquitously expressed and highly implicated in normal development. Mutations in this gene lead to the activation of the Ras/MAPK pathway in two different ways: decreased intrinsic or GAP-dependent GTPase activity (interfering with the inherent ability to hydrolyze GTP in GDP). Secondly, mutations can interfere with the binding of guanine nucleotide to KRAS. Patients harboring mutations in KRAS tend to have a more severe phenotype, including the occurrence of a severe form of JMML [8].
Mutations in NRAS have also been reported in some cases with a weaker capacity of activating MAPK cascade than the NRAS mutations found in cancer, especially the G12V substitution [27].
Mutations in RAF1 encoding CRAF, part of a small family of serine–threonine kinase including BRAF and ARAF, is rarely seen in NS [28,29]. These proteins have different kinase activity regarding their substrate MEK1 and MEK2 and thus participate differentially in the RAS/MAPK pathway [5,30]. The RAF1 gene encodes a protein with three functional domains (conserved region 1 (CR1), CR2 and CR3). The N-terminal CR1 is involved in GTP-RAS binding and the interaction with the cytosolic surface of the cell membrane. CR2 controls an autoinhibition conformation of the protein whereas CR3 contains the kinase domain. Germline mutations cluster in CR2 or CR3 residues. According to their location, mutations can differentially perturb the protein function with amino-acid changes in the CR2 domain, disturbing the protein's inactive conformational state, leading to enhanced MAPK signaling. In contrast, mutations affecting the kinase domain are loss-of-function mutations, implying a yet undetermined mechanism leading to enhanced MAPK activation [16]. These RAF1 mutations have been linked to NS and found in some patients with LS. They seem to be characterized by a high prevalence of hypertrophic cardiomyopathy (∼75%) compared to the prevalence in the general population of NS (∼18%) and a high proportion of patients with multiple nevi lentigines and café-au-lait spots. BRAF mutations have also been found in some patients with features of NS or LS (<2% of NS) [31]. These mutations do not overlap with the cardio-facio-cutaneous syndrome (CFC) in which this gene is frequently involved [31].
A single de novo heterozygous missense mutation in the gene SHOC2, resulting in an S2G substitution, leads to an excess of SHOC2 N-myristoylation. Consequently, the SHOC2 aberrant localization to the internal membrane drives the excessive MAPK signaling [32]. This mutation was associated with a unique phenotype of Noonan syndrome with loss of anagen hair, an NS-like disease [32,33].
MAP2K1 germline mutations have been rarely reported in NS [34]. In the study of Nava and colleagues, three mutations affecting highly conserved residues have been reported. Still, one mutation was inherited from a healthy mother and the other mutations were not characterized functionally but were predicted deleterious and located in a hotspot of known pathogenic mutations [34].
Deleterious heterozygous mutations in Cas-Br-M (murine) ecotropic retroviral transforming sequence (CBL) have been detected in patients carrying clinical features fitting or partially overlapping NS [35,36]. CBL belongs to a small family of E3 ubiquitin ligase contributing to the endocytosis and thus negative regulation of RTKs and participate in signal flow of the MAPK cascade through its adaptor function [37]. Mutations affect the well-conserved region of its tyrosine kinase domain and RING-finger domain responsible for its ubiquitin ligase activity leading to a gain-of-function in the MAPK pathway [35].
Costello syndrome (CS)
CS is one of the rarest RASopathies with many overlapping features with previous syndromes and an estimated incidence of 1/300 000 births [38,39]. Patients present in early life with characteristic dysmorphic facial features, failure to thrive, musculoskeletal, ectodermal and ocular abnormalities along with hypotonia and variable cognitive impairment [40]. Typical facial features include, among others, macrocephaly, epicanthal folds and a short nose. Patients also present with cardiac malformations consisting of hypertrophic cardiomyopathy, valve anomalies, septal defect and arrhythmia. A primary concern in these patients is the increased risk of secondary neoplasm, both benign and malignant. Papilloma is highly prevalent, with more than 70% of patients harboring different localizations, mainly to the nose, throughout life. Interestingly this particular neoplasm is not seen in the other RASopathies and can help to make the diagnosis with other dermatological features such as soft skin with excessive wrinkling along with deep creases [41]. Malignant neoplasms concern up to 20% of patients and consist of rhabdomyosarcoma, transitional cell carcinoma and neuroblastoma. Contrary to NS, Costello syndrome is caused by different mutations in a single gene, namely HRAS, with the G12S substitution being the most prevalent, followed by G12A [40]. These mutations lead to a constitutive activation state of HRAS, increasing RAS/MAPK signaling. Most of these mutations are de novo, thus siblings are rarely affected although gonad mosaicism in a father has been once reported [42]. Of note, loss of heterozygosity has been frequently observed in rhabdomyosarcoma, explaining in part that malignancies occurrence is not fully penetrant and illustrating the frequent need for second events in germline RASopathies to develop neoplasia [4,43]. Genotype-phenotype correlation has been reviewed elsewhere and highlights the importance of a genetic diagnosis in these patients and in RASopathies in general to improve the clinical follow-up based on known predicted evolution [40].
Cardio-facio-cutaneous syndrome (CFC)
CFC is another syndrome of the large family of RASopathies, thus sharing some clinical features of NS and CS with some specific characteristics [44]. Patients have sparse, brittle, curly hair, a macrocephaly with a prominent forehead, among other features. Cardiac malformations are as common as in CS and NS with a high prevalence of pulmonic stenosis. Heterozygous gain-of-function mutations accounting for this phenotype have been found in 4 genes: BRAF, MAP2K1 (MEK1), MAP2K2 (MEK2) and less frequently KRAS. Heterozygous BRAF mutations account for 75% of the genetic background in CFC patients for whom a mutation is identified whereas MAP2K1 and MAP2K2 account for 25% [34,44]. Almost all the mutations in BRAF, encoding a serine–threonine kinase, are gain-of-function with increased kinase activity. However, some other mutations are associated with an impaired kinase activity but still lead to a global gain-of-function on the MAPK pathway through CRAF [34]. Interestingly, although gain-of-function mutations in CFC patients belong to a well-known oncogenic pathway, patients do not appear to have an increased risk of cancer such as NS, CS and NF1, adding a yet unexplained genotype-phenotype heterogeneity.
Capillary malformation-arteriovenous malformation syndrome (CM-AVM)
CM-AVM is a rare autosomal dominant disorder clinically characterized by many disseminated capillary malformations and fistulas [45]. Heterozygous loss-of-function mutations in RASA1, encoding a RasGAP (p120-GAP) like NF1, cause the activation of the RAS/MAPK pathway by losing a RasGAP-mediated negative regulation [46]. Almost 30% of patients carry a de novo mutation and nearly all the mutations are frameshift, nonsense or affect a splice region leading to p120-GAP haploinsufficiency. Some mutations in RASA1 have been found in Parkes Weber syndrome and vein of Galens malformations. Some patients have additional clinical features such as cardiac malformations (tetralogy de Fallot, valve anomalies, septal defects) [45].
Legius syndrome (LS)
LS is an NF1-like syndrome caused by heterozygous loss-of-function mutations in SPRED1. SPRED1 encodes the SPRED1 protein, a negative regulator of the RAS/MAPK pathway by inhibiting RAS phosphorylation [47]. Patients have standard features of NF1 such as café-au-lait spots, intertriginous freckling, mild neurocognitive impairment but do not present with neurofibromas or central nervous system tumors seen in classical NF1 [48].
Interestingly, although all these syndromes have in common germline mutations activating the RAS/MAPK cascade, consequences regarding the hematopoietic system are highly heterogeneous. Some patients may present JMML during their life (especially NS with CBL, NRAS, KRAS, PTPN11 mutation and NF1 patients). In contrast, other germline syndromes with mutations in BRAF or MAP2K1 are not associated with an increased risk of hematopoietic disorders such as histiocytosis, where somatic mutations in BRAF and MAP2K1 account for almost 70% of the diseases. Moreover, mutations in KRAS or NRAS causing NS do not necessarily lead to hematopoietic or solid cancers. One hypothesis is that the affected residues lead to a weaker activation of the pathway in germline versus somatic mutations.
RAS/MAPK events and the hematopoietic system
Mutations in the RAS/MAPK pathway have been implicated in numerous diseases since its discovery in humans 60 years ago. Somatic mutations in cancer have been extensively studied and more recently, germline mutations leading to an overall activation of the pathway have been linked to what is now called RASopathies, comprising ectodermal and mesodermal development abnormalities and various neoplasia. Interestingly, although mutations in RASopathies are germline and may affect all tissues, the consequence in the different cellular subsets does not necessarily fit with the phenotype seen in patients with somatic mutations. This discrepancy could be explained by other affected residues and by second events, epigenetic, environmental and yet undetermined factors as some patients with different phenotypes carry the same mutation.
The next part of the review will focus on somatic events in the RAS/MAPK pathway that have consequences in the hemopoietic system and we will discuss overlapping phenotypes between germline and somatic mutations.
RAS-associated autoimmune leukoproliferative disorder (RALD)
RAS-associated autoimmune leukoproliferative disorder, so-called RALD, has been recently recognized as a non-malignant and non-infectious clinical syndrome sharing characteristics with the autoimmune lymphoproliferative syndrome (ALPS) [49]. Before classifying the disease as RALD, patients were classified as ALPS-like patients (ALPS type IV) of unknown origin because they shared common ALPS clinical and biological features without mutations in known genes associated with this condition (namely FAS or FASLG). Indeed, patients with RALD present with early-onset splenomegaly, chronic lymphadenopathy, monocytosis, multilineage cytopenia and hypergammaglobulinemia [49,50]. These cytopenia are highly frequent and mostly immune-mediated, like in ALPS, immune-mediated thrombocytopenia, autoimmune hemolytic anemia, or both [51]. In a recent literature review, Neven and colleagues also reported neutropenia in 3 cases (probably auto-immune mediated) [52]. In this study, 27 patients are discussed, giving an overview of RALD cases published so far. The median age at disease-onset was 2 years but ranged from 3 months to 36 years indicating that this disease can be seen in adults. The most frequent feature was splenomegaly (26/27), followed by immune-mediated cytopenia (15/16) and monocytosis (18/24). Some patients present with pericarditis, reported in 6 cases, and skin involvement, reported in 4, with various findings such as unspecified erythematosus and Henoch-Schonlein purpura. Gastrointestinal tract manifestations have been reported with colonoscopy biopsies showing B-cell lymphoproliferation in one case, colic inflammation in another and ulcerations in 2 patients. Glomerulonephritis, arthralgias with or without inflammatory arthritis were also reported. Even though RALD shares clinical features with ALPS, biomarkers are quite different since an increased TCRab + CD4-CD8- “double negative T cells count”, the hallmark of ALPS, and increased plasma levels of vitamin B12, soluble FASLG (sFASLG), IL-10 and IL-18 are not always found in RALD patients. Of note, the persistence of an increased monocyte absolute count or relative percentage seems to be a hallmark of RALD in a compatible context [53]. Despite this, clinicians facing the differential diagnosis of patients presenting with splenomegaly, generalized lymphadenopathy with or without autoimmunity must evoke an extensive list of possible diseases, both rare and frequent, including infections, various type of malignancies such as lymphoma, JMML or CMML, and rare diseases such as ALPS, RALD or Destombes-Rosai-Dorfman (RDD). Thus, reliable markers delineating and helping differentiate these disorders are essential to guide therapeutic strategies and follow-up.
The genetic basis of these patients presenting with ALPS-like features has been resolved at the beginning of the 21st century with the discovery of recurrent somatic mutations in KRAS and NRAS. In the first description of ALPS type IV, Oliveira and colleagues described a patient with an activating NRAS mutation impairing apoptosis of T cells after withdrawal of IL-2 [50]. In normal lymphocytes, growth factor withdrawal (such as IL-2 for T cells) leads to BIM release, a pro-apoptotic member of the B cell lymphoma 2 family (BCL2). By interacting with BCL2, BIM allows the oligomerization of BAX and BAK at the mitochondrion surface, leading to their permeabilization and the release of cytochrome c in the cytoplasm where it triggers apoptosis via the formation of a multimolecular complex, containing APAF1 and Caspase 9, and called the apoptosome [54]. This intrinsic apoptosis pathway, named ACAD for activated cell-autonomous death, is involved in the contraction of the lymphocyte pool, with the cytokine production decreasing once the targeted cells or antigens are eliminated. AICD, for activation-induced cell death, is another way of triggering apoptosis in chronically stimulated lymphocytes. AICD involves the activation of “death receptors”, such as FAS, upon interaction with its cognate ligand (FASLG). FAS is expressed at the surface of activated lymphocytes and the chronic antigen-receptor stimulation leads to FASLG expression and thus to the apoptosis of unwanted, mainly autoreactive, lymphocytes [[54], [55], [56]]. Contrary to RALD, the ALPS patients' lymphocytes exhibit an AICD defect but normal ACAD, thus stratifying these two diseases in terms of pathophysiology.
The discovery of a distinct apoptosis defect in patients with ALPS-like features (impaired ACAD, normal AICD) prompted Oliveira and colleagues to look for mutations in genes encoding proteins involved in ACAD regulation, such as the BH3-only BCL-2-interacting mediator of cell death (BIM), ERK1/2, JNK1/2. Still, they found no mutation [50]. By looking at differentially expressed genes between healthy donors' lymphocytes and patient's lymphocytes, the authors discovered a signature suggestive of constitutive activation of the RAS/MAPK pathway. The sequencing of NRAS evidenced a single heterozygous mutation leading to a non-conservative amino acid change from aspartic acid to glycine at codon 13 (NRAS p. G13D). Interestingly, this amino acid change had already been described in various malignant conditions (human pediatric cancers, adult myeloid and lymphoid malignancies) and was known to stabilize RAS in an activate conformation leading to an overflow of the RAS/MAPK pathway. Later, additional patients have been recognized as RALD and all of them carry a gain-of-function somatic mutation in NRAS or KRAS [52]. These RAS genes activating mutations lead to the constitutive phosphorylation of ERK1/2, which then phosphorylate the pro-apoptotic protein BIM leading to its degradation and thus impairing ACAD [Fig. 1] [50,57]. In the most recent review of the literature, 16 patients had a somatic mutation in KRAS (59%) and 11 patients a mutation in NRAS (41%) [52]. These mutations are thought to appear in early hematopoietic progenitors and have been detected in various immune subsets (lymphocytes, monocytes) but not in skin-derived fibroblasts or saliva swab specimens. Since then, RALD has been classified as a distinct clinical entity from ALPS presenting with non-malignant lymphoproliferation, persistent monocytosis, leukocytosis, and frequently associated variable autoimmune features. The most frequent autoimmune feature is autoimmune cytopenia, but systemic lupus erythematosus (SLE) has also been described [58]. A key clinical message from the reported pediatric patients carrying a somatic NRAS mutation and SLE is that they presented with lymphoproliferative syndrome (enlarged lymph nodes, splenomegaly, or hepatosplenomegaly), which is possible in SLE but should call attention, especially in a pediatric setting. Plus, all patients had persistent monocytosis. Another case of RALD has been described with both features of SLE and Destombes-Rosai-Dorfman (RDD) linked to a de novo somatic mutation in KRAS (p.G13C), reported in cancer, RALD and NS [59].
Interestingly, in bona fide germline RASopathies, some patients may present with autoimmunity reminiscent of SLE, especially NS patients, although it is rare [60]. These cases of RALD or germline RASopathies associated with SLE highlight the usefulness of looking for monogenic forms of autoimmunity in pediatric SLE to better understand the pathophysiology and eventually propose personalized therapeutic strategies. It is, for example, not known if patients with SLE and/or RALD could benefit from MEK inhibitors such as cobimetinib or trametinib, used in both malignant (melanoma) and non-malignant diseases (histiocytosis) as tested in pre-clinical models of RAS-mutated iPSCs [[61], [62], [63]].
Juvenile myelo-monocytic leukemia (JMML)
The clinical and biological features of RALD patients are reminiscent of those of JMML making the differential diagnosis sometimes difficult [64]. JMML is a rare myeloproliferative/myelodysplastic neoplasm of early childhood reminiscent of CMML in adults. Clinical features include splenomegaly, fever, thrombocytopenia, monocytosis and myelo-monocytic infiltration of the skin and sometimes vital organs such as the lungs. Sixty-five percent of JMML patients also have hypergammaglobulinemia and 22% have features of autoimmunity, two features also seen in RALD [65]. It represents between 20 and 30% of myeloproliferative/myelodysplastic syndrome seen in childhood with a poor median survival of less than one year in untreated patients [65]. The risk of evolution toward acute myeloid leukemia (1/3 of the patients) has made hematopoietic stem-cell transplantation the standard of care in these patients even though some rare “long-term survivors” with spontaneous remission have been described [66]. Thus, differentiating between RALD and JMML is of the utmost importance in terms of clinical management. Guidelines for the diagnosis of JMML have been reviewed in the WHO classification of myeloid malignancies in 2016 but RALD can fit with these criteria [67]. This neoplasm shares a genetic basis with both RALD and germline RASopathies as it can be both initiated by germline or somatic mutation in RAS genes (NRAS, KRAS) or regulators of the pathway (PTPN11, CBL, NF1) defining syndromic JMML or sporadic JMML, respectively [64,68]. More than 90% of patients with JMML now have a molecular diagnosis. Indeed, contrary to other cancers in children, it has been shown that JMML requires fewer oncogenic genetic events to develop [69]. Interestingly, it has been demonstrated in a large cohort of JMML that additional mutations could distinguish between long-term survivors' patients and those who will evolve toward a very aggressive form of the disease such as transformation to acute myeloid leukemia. These mutations include additional hits in the RAS pathway (disproving the concept of mutually exclusive RAS pathway mutations), duplication of the oncogenic mutation due to acquired uniparental disomy and mutations in epigenetic regulators (SETBP1, ASXL1 for example). These second events were not found in NS or CBL syndrome with JMML suggesting that the germline hit was sufficient on itself to develop the disease contrary to NF1 patients where additional events were found in 100% of cases, including acquired uniparental disomy. This observation brings grain to the mill for different activation levels of the RAS/MAPK pathway induced by germline versus somatic mutations. This is exemplified by the publication of two PTPN11 and one NRAS germline variants in neo-natal or pre-natal lethal Noonan syndrome. These variants had been previously described in isolated JMML and argued that somatic variants found in JMML lead to a gain-of-function in the RAS/MAPK pathway rarely compatible with life if germline [70].
Nevertheless, second events could account for RALD evolution to JMML. This risk of malignancy following long-term non-malignant lymphoproliferation in RALD is exemplified by the case of a p.G13C KRAS-mutated patient with more than 10 years of indolent disease evolving toward aggressive leukemia. This patient was diagnosed at 5 years with lymphoproliferation and autoimmune cytopenia requiring splenectomy and rituximab therapy and then presented at age 20 with chest pain and leukocytosis, subsequently evolving to a fatal acute respiratory distress syndrome linked to leukostasis [57]. A better understanding of indolent RALD to severe JMML evolution would be of great interest to stratify patients at risk.
Other non-malignant hematopoietic proliferation linked to genetic-based RAS-activated pathway
Histiocytosis is a large group of rare diseases characterized by the accumulation of histiocytes in various organs recently revised in their classification [71]. Five groups are now delineated: R-group with different subtypes of Destombes-Rosai-Dorfman (familial monogenic, associated with auto-immune diseases or malignancies, nodal and extra-nodal), L-group with Erdheim-Chester disease (ECD), Langerhans cell histiocytosis (LCH), and mixed histiocytosis (ECD and LCH features), C-group with cutaneous non-Langerhans cell histiocytosis (non-LCH), H-group for primary and secondary hemophagocytic lymphohistiocytosis and finally M-group for malignant histiocytosis, previously named histiocytic sarcoma. In the past decade, a breakthrough in understanding these diseases was the discovery of recurrent mutations in the RAS/MAPK pathway. First, in LCH, with the discovery of recurrent somatic BRAF V600E mutation in more than 50% of patients, followed by the same discovery in ECD with more than 50% of patients harboring the same mutation [72,73]. The characterization of these variants led to the revised classification of histiocytosis in 2016, bringing in the same group LCH and ECD based on a shared genetic landscape and a frequent association between the two diseases (called mixed histiocytosis) [74]. Following this discovery, several other mutations in this particular pathway were found, both in LCH and ECD and to a lesser extent in R-group and M-group (NRAS, KRAS, MAP2K1, MAP2K2, ARAF, PTPN11, among others) [[75], [76], [77], [78]]. Interestingly, many of these mutations have been described in RALD, JMML and germline RASopathies, such as NRAS p.G12D or KRAS p.G13C. More strikingly, as said before, a lymph node biopsy in a RALD patient with a KRAS p.G13C mutation has shown compatible histology with RDD [57]. This type of RDD is classified as immune-related RDD in the 2016 classification but asks questions in delineating these two diseases. Other patients with a diagnosis of RALD have been reported with a lymph node biopsy showing sinus histiocytosis without specifying if these images were compatible with emperipolesis and thus a diagnosis of RDD. Why some patients with a somatic mutation in hematopoietic precursors will develop an L-group or R-group histiocytosis or a RALD has yet to be explained [79]. The age of onset of the diseases, late adulthood for Erdheim-Chester disease and childhood for RALD argue for a link with different second events and somatic mutations arising at a different age or in different precursors.
Even more interesting was the discovery that, as seen in RALD, patients with an L-group histiocytosis were at increased risk of developing secondary myeloid neoplasm such as CMML [80]. More recently, Cohen-Aubart and colleagues reported a high frequency of what is now called clonal hematopoiesis of undetermined significance in addition to activating mutations in the RAS/MAPK pathway in Erdheim-Chester disease [81]. The most frequent mutations were found in TET2, ASXL1, DNMT3A and NRAS and were detectable in CD34 + CD38-derived bone marrow cells as well as in peripheral blood but to a lesser extent in B and T lymphocytes [81]. Interestingly, mutations in these genes have been found in JMML patients and classified as a second hit that could result in disease progression toward a more aggressive phenotype [82]. Even though the clinical phenotype of histiocytosis does not necessarily fit with RALD, it would be of interest to dissect this heterogeneity of presentation using new tools such as single-cell epitope and RNA and/or DNA sequencing to go deeper in the understanding of how the same somatic event can lead to such different diseases. Reminiscent of the high frequency of autoimmunity seen in RALD, a recent report highlight the high frequency of associated auto-immune diseases in L-group histiocytosis (Erdheim-Chester disease or mixed histiocytosis) and demonstrated how targeted therapies such as the MEK inhibitor cobimetinib could decrease the amount of autoantibodies (especially anticardiolipin) [83].
Concluding remarks (outlook)
Germline and somatic mutations in the RAS/MAPK pathway can lead to heterogeneous diseases. On the one hand, germline mutations lead to developmental abnormalities and susceptibility to various cancers, including JMML, on the other hand, somatic mutations have been well described in more than 30% of cancers including hematopoietic disorders. In particular, hematopoietic diseases related to a RAS/MAPK pathway hyperactivation include various rare disorders such as different types of histiocytosis, hairy cell leukemia, RALD and JMML with or without associated autoimmunity. The reason why some patients may develop RALD or histiocytosis with or without autoimmunity while carrying the same somatic mutation remains to be elucidated. Second, not all patients with germline mutations develop JMML or other hematopoietic disorders, while cells are affected by the mutation. It is not known either why and when some RALD or histiocytosis patients will develop, sometimes fatal, malignant hematopoietic diseases. One explanation is the acquisition of second events as shown in JMML and histiocytosis, participating in the development of the hematopoietic disease [69,81]. How the first hit in the RAS/MAPK pathway is linked to the appearance of second events is not known. One of the possibilities to decipher all these discrepancies would be to take advantage of expanding new technologies such as single-cell epitope and transcriptomic analysis. Such studies would hopefully lead to a better comprehension of RAS/MAPK gain-of-function consequences in a cell-specific manner. For example, these techniques explain the appearance of mixed cryoglobulinemic vasculitis in primary Sjögren Syndrome (pSS). In the study of Singh and colleagues, the multi-omics analysis revealed that acquisition of known lymphoma driver somatic mutations (CARD11, TNFAIP3, KLHL6 among others) in B-cells was correlated to the appearance of a pathogenic rheumatoid factor contributing to the onset of a mixed cryoglobulinemic vasculitis [84]. Interestingly, like in histiocytosis or RALD, patients with pSS can evolve toward a lymphoma (especially mucosa-associated lymphoid tissue lymphoma, MALT, but not exclusively). This study, using multi-omics, leads to a more comprehensive overview of the pathophysiology of these rare events.
The possibility to stratify patients with somatic RAS/MAPK mutations at risk of developing secondary hematologic cancers would be of great interest to adapt the follow-up and why not the treatments. Patients with histiocytosis carrying mutations in the RAS/MAPK pathway have been treated with success for almost a decade now, with various specific inhibitors such as BRAF and MEK inhibitors [62,63,85]. Whether these treatments can be repositioned in RALD, JMML or some germline RASopathies remained to be determined.
Conflicts of interest
Authors declare no conflicts of interest.
Acknowledgements
This work was supported by the Institut National de la Santé et de la Recherche Médicale (INSERM) and by government grants managed by the Agence National de la Recherche as part of the “Investment for the Future” program (Institut Hospitalo-Universitaire Imagine, grant ANR-10-IAHU-01, Recherche Hospitalo-Universitaire, grant ANR-18-RHUS-0010), the Centre de Référence Déficits Immunitaires Héréditaires (CEREDIH), the Agence National de la Recherche (ANR-14-CE14-0026-01 “Lumugene”; ANR-18-CE17-0001 “Action”), the Ligue Contre le Cancer – Comité de Paris, Fondation ARC pour la recherche sur le CANCER, the Fondation pour la Recherche Médicale (FRM: EQU202103012670). NGS was supported by a funding program of the Fondation Maladies Rares (AAP2012 High throughput sequencing).
Q.R. is a recipient of an Institut Imagine MD-PhD fellowship (program supported by the Fondation Bettencourt Schueller) and a Société National Française de Médecine Interne (SNFMI) fellowship.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Parada L.F., Tabin C.J., Shih C., Weinberg R.A. Human EJ bladder carcinoma oncogene is homologue of Harvey sarcoma virus ras gene. Nature. 1982;297:474–478. doi: 10.1038/297474a0. [DOI] [PubMed] [Google Scholar]
- 2.Santos E., Tronick S.R., Aaronson S.A., Pulciani S., Barbacid M. T24 human bladder carcinoma oncogene is an activated form of the normal human homologue of BALB- and Harvey-MSV transforming genes. Nature. 1982;298:343–347. doi: 10.1038/298343a0. [DOI] [PubMed] [Google Scholar]
- 3.Cox A.D., Der C.J. Ras history: The saga continues. Small GTPases. 2010;1:2–27. doi: 10.4161/sgtp.1.1.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rauen K.A. The RASopathies. Annu Rev Genom Hum Genet. 2013;14:355–369. doi: 10.1146/annurev-genom-091212-153523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leicht D.T., Balan V., Kaplun A., Singh-Gupta V., Kaplun L., Dobson M. Raf kinases: function, regulation and role in human cancer. Biochim Biophys Acta. 2007;1773:1196–1212. doi: 10.1016/j.bbamcr.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jimenez G. ERK signaling: methods and protocols. Springer New York; New York: 2017. [Internet] [cité 16 mars 2021]. (Methods in Molecular Biology; vol. 1487). [Google Scholar]
- 7.Aoki Y., Matsubara Y. Ras/MAPK syndromes and childhood hemato-oncological diseases. Int J Hematol. 2013;97:30–36. doi: 10.1007/s12185-012-1239-y. [DOI] [PubMed] [Google Scholar]
- 8.Niemeyer C.M. RAS diseases in children. Haematologica. 2014;99:1653–1662. doi: 10.3324/haematol.2014.114595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gutmann D.H., Ferner R.E., Listernick R.H., Korf B.R., Wolters P.L., Johnson K.J. Neurofibromatosis type 1. Nat Rev Dis Primers. 2017;3:17004. doi: 10.1038/nrdp.2017.4. [DOI] [PubMed] [Google Scholar]
- 10.Wallace M.R., Marchuk D.A., Andersen L.B., Letcher R., Odeh H.M., Saulino A.M. Type 1 neurofibromatosis gene: identification of a large transcript disrupted in three NF1 patients. Science. 1990;249:181–186. doi: 10.1126/science.2134734. [DOI] [PubMed] [Google Scholar]
- 11.Viskochil D., Buchberg A.M., Xu G., Cawthon R.M., Stevens J., Wolff R.K. Deletions and a translocation interrupt a cloned gene at the neurofibromatosis type 1 locus. Cell. 1990;62:187–192. doi: 10.1016/0092-8674(90)90252-a. [DOI] [PubMed] [Google Scholar]
- 12.Stephens K., Weaver M., Leppig K.A., Maruyama K., Emanuel P.D., Le Beau M.M. Interstitial uniparental isodisomy at clustered breakpoint intervals is a frequent mechanism of NF1 inactivation in myeloid malignancies. Blood. 2006;108:1684–1689. doi: 10.1182/blood-2005-11-011486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flotho C., Steinemann D., Mullighan C.G., Neale G., Mayer K., Kratz C.P. Genome-wide single-nucleotide polymorphism analysis in juvenile myelomonocytic leukemia identifies uniparental disomy surrounding the NF1 locus in cases associated with neurofibromatosis but not in cases with mutant RAS or PTPN11. Oncogene. 2007;26:5816–5821. doi: 10.1038/sj.onc.1210361. [DOI] [PubMed] [Google Scholar]
- 14.Steinemann D., Arning L., Praulich I., Stuhrmann M., Hasle H., Stary J. Mitotic recombination and compound-heterozygous mutations are predominant NF1-inactivating mechanisms in children with juvenile myelomonocytic leukemia and neurofibromatosis type 1. Haematologica. 2010;95:320–323. doi: 10.3324/haematol.2009.010355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nora J.J., Nora A.H., Sinha A.K., Spangler R.D., Lubs H.A. The Ullrich-Noonan syndrome (Turner phenotype) Am J Dis Child. 1974;127:48–55. doi: 10.1001/archpedi.1974.02110200050007. [DOI] [PubMed] [Google Scholar]
- 16.Tartaglia M., Zampino G., Gelb B.D. Noonan syndrome: clinical aspects and molecular pathogenesis. Mol Syndromol. 2010;1:2–26. doi: 10.1159/000276766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noonan J.A. Hypertelorism with Turner phenotype. A new syndrome with associated congenital heart disease. Am J Dis Child. 1968;116:373–380. doi: 10.1001/archpedi.1968.02100020377005. [DOI] [PubMed] [Google Scholar]
- 18.Roberts A.E., Allanson J.E., Tartaglia M., Gelb B.D. Noonan syndrome. Lancet. 2013;381:333–342. doi: 10.1016/S0140-6736(12)61023-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kruszka P., Porras A.R., Addissie Y.A., Moresco A., Medrano S., Mok G.T.K. Noonan syndrome in diverse populations. Am J Med Genet A. 2017;173:2323–2334. doi: 10.1002/ajmg.a.38362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tartaglia M., Mehler E.L., Goldberg R., Zampino G., Brunner H.G., Kremer H. Mutations in PTPN11, encoding the protein tyrosine phosphatase SHP-2, cause Noonan syndrome. Nat Genet. 2001;29:465–468. doi: 10.1038/ng772. [DOI] [PubMed] [Google Scholar]
- 21.Zenker M., Horn D., Wieczorek D., Allanson J., Pauli S., van der Burgt I. SOS1 is the second most common Noonan gene but plays no major role in cardio-facio-cutaneous syndrome. J Med Genet. 2007;44:651–656. doi: 10.1136/jmg.2007.051276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tartaglia M., Pennacchio L.A., Zhao C., Yadav K.K., Fodale V., Sarkozy A. Gain-of-function SOS1 mutations cause a distinctive form of Noonan syndrome. Nat Genet. 2007;39:75–79. doi: 10.1038/ng1939. [DOI] [PubMed] [Google Scholar]
- 23.Roberts A.E., Araki T., Swanson K.D., Montgomery K.T., Schiripo T.A., Joshi V.A. Germline gain-of-function mutations in SOS1 cause Noonan syndrome. Nat Genet. 2007;39:70–74. doi: 10.1038/ng1926. [DOI] [PubMed] [Google Scholar]
- 24.Quilliam L.A., Rebhun J.F., Castro A.F. A growing family of guanine nucleotide exchange factors is responsible for activation of Ras-family GTPases. Prog Nucleic Acid Res Mol Biol. 2002;71:391–444. doi: 10.1016/s0079-6603(02)71047-7. [DOI] [PubMed] [Google Scholar]
- 25.Schubbert S., Zenker M., Rowe S.L., Böll S., Klein C., Bollag G. Germline KRAS mutations cause Noonan syndrome. Nat Genet. 2006;38:331–336. doi: 10.1038/ng1748. [DOI] [PubMed] [Google Scholar]
- 26.Carta C., Pantaleoni F., Bocchinfuso G., Stella L., Vasta I., Sarkozy A. Germline missense mutations affecting KRAS Isoform B are associated with a severe Noonan syndrome phenotype. Am J Hum Genet. 2006;79:129–135. doi: 10.1086/504394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cirstea I.C., Kutsche K., Dvorsky R., Gremer L., Carta C., Horn D. A restricted spectrum of NRAS mutations causes Noonan syndrome. Nat Genet. 2010;42:27–29. doi: 10.1038/ng.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pandit B., Sarkozy A., Pennacchio L.A., Carta C., Oishi K., Martinelli S. Gain-of-function RAF1 mutations cause Noonan and LEOPARD syndromes with hypertrophic cardiomyopathy. Nat Genet. 2007;39:1007–1012. doi: 10.1038/ng2073. [DOI] [PubMed] [Google Scholar]
- 29.Razzaque M.A., Nishizawa T., Komoike Y., Yagi H., Furutani M., Amo R. Germline gain-of-function mutations in RAF1 cause Noonan syndrome. Nat Genet. 2007;39:1013–1017. doi: 10.1038/ng2078. [DOI] [PubMed] [Google Scholar]
- 30.Wellbrock C., Karasarides M., Marais R. The RAF proteins take centre stage. Nat Rev Mol Cell Biol. 2004;5:875–885. doi: 10.1038/nrm1498. [DOI] [PubMed] [Google Scholar]
- 31.Lee Y., Choi Y., Seo G.H., Kim G.H., Choi I.H., Keum C. Clinical and molecular spectra of BRAF-associated RASopathy. J Hum Genet. 2021;66:389–399. doi: 10.1038/s10038-020-00852-3. [DOI] [PubMed] [Google Scholar]
- 32.Cordeddu V., Di Schiavi E., Pennacchio L.A., Ma’ayan A., Sarkozy A., Fodale V. Mutation of SHOC2 promotes aberrant protein N-myristoylation and causes Noonan-like syndrome with loose anagen hair. Nat Genet. 2009;41:1022–1026. doi: 10.1038/ng.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Komatsuzaki S., Aoki Y., Niihori T., Okamoto N., Hennekam R.C., Hopman S. Mutation analysis of the SHOC2 gene in Noonan-like syndrome and in hematologic malignancies. J Hum Genet. 2010;55:801–809. doi: 10.1038/jhg.2010.116. [DOI] [PubMed] [Google Scholar]
- 34.Nava C., Hanna N., Michot C., Pereira S., Pouvreau N., Niihori T. Cardio-facio-cutaneous and Noonan syndromes due to mutations in the RAS/MAPK signalling pathway: genotype-phenotype relationships and overlap with Costello syndrome. J Med Genet. 2007;44:763–771. doi: 10.1136/jmg.2007.050450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martinelli S., De Luca A., Stellacci E., Rossi C., Checquolo S., Lepri F. Heterozygous germline mutations in the CBL tumor-suppressor gene cause a Noonan syndrome-like phenotype. Am J Hum Genet. 2010;87:250–257. doi: 10.1016/j.ajhg.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Niemeyer C.M., Kang M.W., Shin D.H., Furlan I., Erlacher M., Bunin N.J. Germline CBL mutations cause developmental abnormalities and predispose to juvenile myelomonocytic leukemia. Nat Genet. 2010;42:794–800. doi: 10.1038/ng.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Swaminathan G., Tsygankov A.Y. The Cbl family proteins: ring leaders in regulation of cell signaling. J Cell Physiol. 2006;209:21–43. doi: 10.1002/jcp.20694. [DOI] [PubMed] [Google Scholar]
- 38.Goriely A., Wilkie A.O. Paternal age effect mutations and selfish spermatogonial selection: causes and consequences for human disease. Am J Hum Genet. 2012;90:175–200. doi: 10.1016/j.ajhg.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abe Y., Aoki Y., Kuriyama S., Kawame H., Okamoto N., Kurosawa K. Prevalence and clinical features of Costello syndrome and cardio-facio-cutaneous syndrome in Japan: findings from a nationwide epidemiological survey. Am J Med Genet A. 2012;158A:1083–1094. doi: 10.1002/ajmg.a.35292. [DOI] [PubMed] [Google Scholar]
- 40.Gripp K.W., Morse L.A., Axelrad M., Chatfield K.C., Chidekel A., Dobyns W. Costello syndrome: clinical phenotype, genotype, and management guidelines. Am J Med Genet A. 2019;179:1725–1744. doi: 10.1002/ajmg.a.61270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Siegel D.H., Mann J.A., Krol A.L., Rauen K.A. Dermatological phenotype in Costello syndrome: consequences of ras dysregulation in development. Br J Dermatol. 2012;166:601–607. doi: 10.1111/j.1365-2133.2011.10744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gripp K.W., Stabley D.L., Geller P.L., Hopkins E., Stevenson D.A., Carey J.C. Molecular confirmation of HRAS p.G12S in siblings with Costello syndrome. Am J Med Genet A. 2011;155A:2263–2268. doi: 10.1002/ajmg.a.34150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gripp K.W. Tumor predisposition in Costello syndrome. Am J Med Genet C Semin Med Genet. 2005;137C:72–77. doi: 10.1002/ajmg.c.30065. [DOI] [PubMed] [Google Scholar]
- 44.Pierpont M.E., Magoulas P.L., Adi S., Kavamura M.I., Neri G., Noonan J. Cardio-facio-cutaneous syndrome: clinical features, diagnosis, and management guidelines. Pediatrics. 2014;134:e1149–e1162. doi: 10.1542/peds.2013-3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boon L.M., Mulliken J.B., Vikkula M. RASA1: variable phenotype with capillary and arteriovenous malformations. Curr Opin Genet Dev. 2005;15:265–269. doi: 10.1016/j.gde.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 46.Eerola I., Boon L.M., Mulliken J.B., Burrows P.E., Dompmartin A., Watanabe S. Capillary malformation-arteriovenous malformation, a new clinical and genetic disorder caused by RASA1 mutations. Am J Hum Genet. 2003;73:1240–1249. doi: 10.1086/379793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pasmant E., Sabbagh A., Hanna N., Masliah-Planchon J., Jolly E., Goussard P. SPRED1 germline mutations caused a neurofibromatosis type 1 overlapping phenotype. J Med Genet. 2009;46:425–430. doi: 10.1136/jmg.2008.065243. [DOI] [PubMed] [Google Scholar]
- 48.Spurlock G., Bennett E., Chuzhanova N., Thomas N., Jim H.P., Side L. SPRED1 mutations (Legius syndrome): another clinically useful genotype for dissecting the neurofibromatosis type 1 phenotype. J Med Genet. 2009;46:431–437. doi: 10.1136/jmg.2008.065474. [DOI] [PubMed] [Google Scholar]
- 49.Oliveira J.B., Bleesing J.J., Dianzani U., Fleisher T.A., Jaffe E.S., Lenardo M.J. Revised diagnostic criteria and classification for the autoimmune lymphoproliferative syndrome (ALPS): report from the 2009 NIH International Workshop. Blood. 2010;116:e35–e40. doi: 10.1182/blood-2010-04-280347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oliveira J.B., Bidère N., Niemela J.E., Zheng L., Sakai K., Nix C.P. NRAS mutation causes a human autoimmune lymphoproliferative syndrome. Proc Natl Acad Sci U S A. 2007;104:8953–8958. doi: 10.1073/pnas.0702975104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takagi M., Shinoda K., Piao J., Mitsuiki N., Takagi M., Matsuda K. Autoimmune lymphoproliferative syndrome-like disease with somatic KRAS mutation. Blood. 2011;117:2887–2890. doi: 10.1182/blood-2010-08-301515. [DOI] [PubMed] [Google Scholar]
- 52.Neven Q., Boulanger C., Bruwier A., de Ville de Goyet M., Meyts I., Moens L. Clinical spectrum of ras-associated autoimmune leukoproliferative disorder (RALD) J Clin Immunol. 2021;41:51–58. doi: 10.1007/s10875-020-00883-7. [DOI] [PubMed] [Google Scholar]
- 53.Rieux-Laucat F. What's up in the ALPS. Curr Opin Immunol. 2017;49:79–86. doi: 10.1016/j.coi.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 54.Hildeman D.A., Zhu Y., Mitchell T.C., Kappler J., Marrack P. Molecular mechanisms of activated T cell death in vivo. Curr Opin Immunol. 2002;14:354–359. doi: 10.1016/s0952-7915(02)00335-7. [DOI] [PubMed] [Google Scholar]
- 55.Arnold R., Brenner D., Becker M., Frey C.R., Krammer P.H. How T lymphocytes switch between life and death. Eur J Immunol. 2006;36:1654–1658. doi: 10.1002/eji.200636197. [DOI] [PubMed] [Google Scholar]
- 56.Rieux-Laucat F., Le Deist F., Hivroz C., Roberts I.A., Debatin K.M., Fischer A. Mutations in Fas associated with human lymphoproliferative syndrome and autoimmunity. Science. 1995;268:1347–1349. doi: 10.1126/science.7539157. [DOI] [PubMed] [Google Scholar]
- 57.Lanzarotti N., Bruneau J., Trinquand A., Stolzenberg M.C., Neven B., Fregeac J. RAS-associated lymphoproliferative disease evolves into severe juvenile myelo-monocytic leukemia. Blood. 2014;123:1960–1963. doi: 10.1182/blood-2014-01-548958. [DOI] [PubMed] [Google Scholar]
- 58.Wang W., Zhou Y., Zhong L., Wang L., Tang X., Ma M. RAS-associated Autoimmune Leukoproliferative disease (RALD) manifested with early-onset SLE-like syndrome: a case series of RALD in Chinese children. Pediatr Rheumatol. 2019;17:55. doi: 10.1186/s12969-019-0346-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ragotte R.J., Dhanrajani A., Pleydell-Pearce J., Del Bel K.L., Tarailo-Graovac M., van Karnebeek C. The importance of considering monogenic causes of autoimmunity: a somatic mutation in KRAS causing pediatric Rosai-Dorfman syndrome and systemic lupus erythematosus. Clin Immunol. 2016;175:143–146. doi: 10.1016/j.clim.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 60.Bader-Meunier B., Cavé H., Jeremiah N., Magerus A., Lanzarotti N., Rieux-Laucat F. Are RASopathies new monogenic predisposing conditions to the development of systemic lupus erythematosus? Case report and systematic review of the literature. Semin Arthritis Rheum. 2013;43:217–219. doi: 10.1016/j.semarthrit.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 61.Kubara K., Yamazaki K., Ishihara Y., Naruto T., Lin H.T., Nishimura K. Status of KRAS in iPSCs impacts upon self-renewal and differentiation propensity. Stem Cell Reports. 2018;11:380–394. doi: 10.1016/j.stemcr.2018.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cohen Aubart F., Emile J.F., Maksud P., Galanaud D., Cluzel P., Benameur N. Efficacy of the MEK inhibitor cobimetinib for wild-type BRAF Erdheim-Chester disease. Br J Haematol. 2018;180:150–153. doi: 10.1111/bjh.14284. [DOI] [PubMed] [Google Scholar]
- 63.Diamond E.L., Durham B.H., Ulaner G.A., Drill E., Buthorn J., Ki M. Efficacy of MEK inhibition in patients with histiocytic neoplasms. Nature. 2019;567:521–524. doi: 10.1038/s41586-019-1012-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Calvo K.R., Price S., Braylan R.C., Oliveira J.B., Lenardo M., Fleisher T.A. JMML and RALD (Ras-associated autoimmune leukoproliferative disorder): common genetic etiology yet clinically distinct entities. Blood. 2015;125:2753–2758. doi: 10.1182/blood-2014-11-567917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Niemeyer C.M., Arico M., Basso G., Biondi A., Cantu Rajnoldi A., Creutzig U. Chronic myelomonocytic leukemia in childhood: a retrospective analysis of 110 cases. European Working Group on Myelodysplastic Syndromes in Childhood (EWOG-MDS) Blood. 1997;89:3534–3543. [PubMed] [Google Scholar]
- 66.Dvorak C.C., Loh M.L. Juvenile myelomonocytic leukemia: molecular pathogenesis informs current approaches to therapy and hematopoietic cell transplantation. Front Pediatr. 2014;2:25. doi: 10.3389/fped.2014.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Arber D.A., Orazi A., Hasserjian R., Thiele J., Borowitz M.J., Le Beau M.M. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–2405. doi: 10.1182/blood-2016-03-643544. [DOI] [PubMed] [Google Scholar]
- 68.Meynier S., Rieux-Laucat F. After 95 years, it's time to eRASe JMML. Blood Rev. 2020;43:100652. doi: 10.1016/j.blre.2020.100652. [DOI] [PubMed] [Google Scholar]
- 69.Caye A., Strullu M., Guidez F., Cassinat B., Gazal S., Fenneteau O. Juvenile myelomonocytic leukemia displays mutations in components of the RAS pathway and the PRC2 network. Nat Genet. 2015;47:1334–1340. doi: 10.1038/ng.3420. [DOI] [PubMed] [Google Scholar]
- 70.Mason-Suares H., Toledo D., Gekas J., Lafferty K.A., Meeks N., Pacheco M.C. Juvenile myelomonocytic leukemia-associated variants are associated with neo-natal lethal Noonan syndrome. Eur J Hum Genet. 2017;25:509–511. doi: 10.1038/ejhg.2016.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Emile J.F., Abla O., Fraitag S., Horne A., Haroche J., Donadieu J. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood. 2016;127:2672–2681. doi: 10.1182/blood-2016-01-690636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Haroche J., Charlotte F., Arnaud L., von Deimling A., Hélias-Rodzewicz Z., Hervier B. High prevalence of BRAF V600E mutations in Erdheim-Chester disease but not in other non-Langerhans cell histiocytoses. Blood. 2012;120:2700–2703. doi: 10.1182/blood-2012-05-430140. [DOI] [PubMed] [Google Scholar]
- 73.Badalian-Very G., Vergilio J.A., Degar B.A., MacConaill L.E., Brandner B., Calicchio M.L. Recurrent BRAF mutations in Langerhans cell histiocytosis. Blood. 2010;116:1919–1923. doi: 10.1182/blood-2010-04-279083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hervier B., Haroche J., Arnaud L., Charlotte F., Donadieu J., Néel A. Association of both Langerhans cell histiocytosis and Erdheim-Chester disease linked to the BRAFV600E mutation. Blood. 2014;124:1119–1126. doi: 10.1182/blood-2013-12-543793. [DOI] [PubMed] [Google Scholar]
- 75.Diamond E.L., Durham B.H., Haroche J., Yao Z., Ma J., Parikh S.A. Diverse and targetable kinase alterations drive histiocytic neoplasms. Canc Discov. 2016;6:154–165. doi: 10.1158/2159-8290.CD-15-0913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Abla O., Jacobsen E., Picarsic J., Krenova Z., Jaffe R., Emile J.F. Consensus recommendations for the diagnosis and clinical management of Rosai-Dorfman-Destombes disease. Blood. 2018;131:2877–2890. doi: 10.1182/blood-2018-03-839753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Goyal G., Heaney M.L., Collin M., Cohen-Aubart F., Vaglio A., Durham B.H. Erdheim-Chester disease: consensus recommendations for evaluation, diagnosis, and treatment in the molecular era. Blood. 2020;135:1929–1945. doi: 10.1182/blood.2019003507. [DOI] [PubMed] [Google Scholar]
- 78.Egan C., Nicolae A., Lack J., Chung H.J., Skarshaug S., Pham T.A. Genomic profiling of primary histiocytic sarcoma reveals two molecular subgroups. Haematologica. 2020;105:951–960. doi: 10.3324/haematol.2019.230375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Durham B.H., Roos-Weil D., Baillou C., Cohen-Aubart F., Yoshimi A., Miyara M. Functional evidence for derivation of systemic histiocytic neoplasms from hematopoietic stem/progenitor cells. Blood. 2017;130:176–180. doi: 10.1182/blood-2016-12-757377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Papo M., Diamond E.L., Cohen-Aubart F., Emile J.F., Roos-Weil D., Gupta N. High prevalence of myeloid neoplasms in adults with non-Langerhans cell histiocytosis. Blood. 2017;130:1007–1013. doi: 10.1182/blood-2017-01-761718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cohen Aubart F., Roos-Weil D., Armand M., Marceau-Renaut A., Emile J.F., Duployez N. High frequency of clonal hematopoiesis in Erdheim-Chester disease. Blood. 2021;137:485–492. doi: 10.1182/blood.2020005101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lasho T., Patnaik M.M. Juvenile myelomonocytic leukemia - a bona fide RASopathy syndrome. Best Pract Res Clin Haematol. 2020;33:101171. doi: 10.1016/j.beha.2020.101171. [DOI] [PubMed] [Google Scholar]
- 83.Roeser A., Cohen-Aubart F., Breillat P., Miyara M., Emile J.-F., Charlotte F. Autoimmunity associated with Erdheim-Chester disease improves with BRAF/MEK inhibitors. Haematologica. 2019;104:e502–e505. doi: 10.3324/haematol.2018.214007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Singh M., Jackson K.J.L., Wang J.J., Schofield P., Field M.A., Koppstein D. Lymphoma driver mutations in the pathogenic evolution of an iconic human autoantibody. Cell. 2020;180:878–894. doi: 10.1016/j.cell.2020.01.029. e19. [DOI] [PubMed] [Google Scholar]
- 85.Haroche J., Cohen-Aubart F., Emile J.F., Arnaud L., Maksud P., Charlotte F. Dramatic efficacy of vemurafenib in both multisystemic and refractory Erdheim-Chester disease and Langerhans cell histiocytosis harboring the BRAF V600E mutation. Blood. 2013;121:1495–1500. doi: 10.1182/blood-2012-07-446286. [DOI] [PubMed] [Google Scholar]