Summary
Hematopoietic stem and progenitor cell (HSPC) mobilization into the blood occurs under normal physiological conditions and is stimulated in the clinic to enable the isolation of HSPCs for transplantation therapies. In the present study, we identify the tetraspanin CD82 as a novel regulator of HSPC mobilization. Using a global CD82 knockout (CD82KO) mouse, we measure enhanced HSPC mobilization after granulocyte-colony stimulating factor (G-CSF) or AMD3100 treatment, which we find is promoted by increased surface expression of the sphingosine 1-phosphate receptor 1 (S1PR1) on CD82KO HSPCs. Additionally, we identify a disruption in S1PR1 internalization in CD82-deficient HSPCs, suggesting that CD82 plays a critical role in S1PR1 surface regulation. Finally, combining AMD3100 and anti-CD82 treatments, we detect enhanced mobilization of mouse HSPCs and human CD34+ cells in animal models. Together, these data provide evidence that CD82 is an important regulator of HSPC mobilization and suggests exploiting the CD82 scaffold as a therapeutic target to enhance stem cell isolation.
Keywords: hematopoietic stem and progenitor cells, CD82, tetraspanins, mobilization, sphingosine-1-phosphate receptor
Graphical abstract
Highlights
-
•
CD82 knockout enhances hematopoietic stem and progenitor cell (HSPC) mobilization
-
•
CD82 knockout promotes HSPC mobilization through an S1PR1-mediated mechanism
-
•
CD82 knockout increases S1PR1 expression by disrupting internalization
-
•
Anti-CD82 treatment enhances mobilization of mouse HSPCs and human CD34+ cells
Saito-Reis et al. discovers that loss of the tetraspanin CD82 enhances hematopoietic stem and progenitor cell (HSPC) mobilization into blood through an S1PR1-mediated mechanism. Moreover, they demonstrate that anti-CD82 treatments promote increased mobilization of mouse HSPCs and human CD34+ cells in animal models. Collectively, this work suggests exploiting the CD82 scaffold as a novel therapeutic target to enhance HSPC isolation.
Introduction
Hematopoietic stem cell transplant is a routine treatment for malignant and non-malignant hematological diseases. Successful transplant depends on multiple factors, including the number and fitness of transplanted hematopoietic stem and progenitor cells (HSPCs). Under static and stress conditions, HSPCs are mobilized into the vasculature from the bone marrow (BM). Transplantation therapies exploit this mobilization process by using treatments such as granulocyte-colony stimulating factor (G-CSF) to enhance the mobilization response, thereby increasing the number of HSPCs available in the blood for harvest. However, studies suggest that 5%–25% of patients mobilize poorly with G-CSF alone (Jantunen et al., 2012). Thus, identifying novel molecules and mechanisms that regulate HSPC mobilization is crucial for the improvement of transplant therapies.
HSPC mobilization is mediated by a variety of key molecules, such as chemokines, cytokines, and proteolytic enzymes, that promote egress into the peripheral blood. In particular, the chemokine receptor, CXCR4, which is highly expressed on the surface of HSPCs, facilitates BM migration toward the chemoattractant, CXCL12. The clinical drugs AMD3100 and G-CSF both target the CXCR4 receptor in order to induce mobilization (Broxmeyer et al., 2005). In addition to the CXCR4/CXCL12 signaling axis, the lysophospholipid sphingosine-1-phosphate (S1P) ligand, produced by mature red blood cells, binds to the sphingosine-1-phosphate receptor family of G protein-coupled receptors (GPCRs) (S1PR1-5) (Bendall and Basnett, 2013). In particular, S1PR1, which is expressed on the surface of HSPCs, mediates mobilization toward a high S1P gradient within the blood and lymph (Seitz et al., 2005). Thus, the S1P/S1PR1 signaling axis facilitates HSPC trafficking and is an essential element of HSPC mobilization.
The tetraspanin family of scaffold proteins modulates a variety of cellular processes, including cell adhesion and signaling via their regulation of surface molecules, such as GPCRs, adhesion receptors, and receptor tyrosine kinases (Termini and Gillette, 2017). Previous work from our laboratory identified the tetraspanin CD82 as a critical regulator of HSPC migration and adhesion within the BM (Saito-Reis et al., 2018). However, the specific contribution of CD82 to HSPC mobilization has not been explored. Using a global CD82KO mouse model, we find that CD82 mediates HSPC mobilization through the regulation of S1PR1 expression and internalization. Furthermore, our data indicate that antibody (Ab) targeting of CD82 promotes the mobilization of HSPCs, suggesting that CD82 may be a novel target to enhance the release of HSPCs for transplant therapies.
Results
Enhanced mobilization potential of CD82KO HSPCs
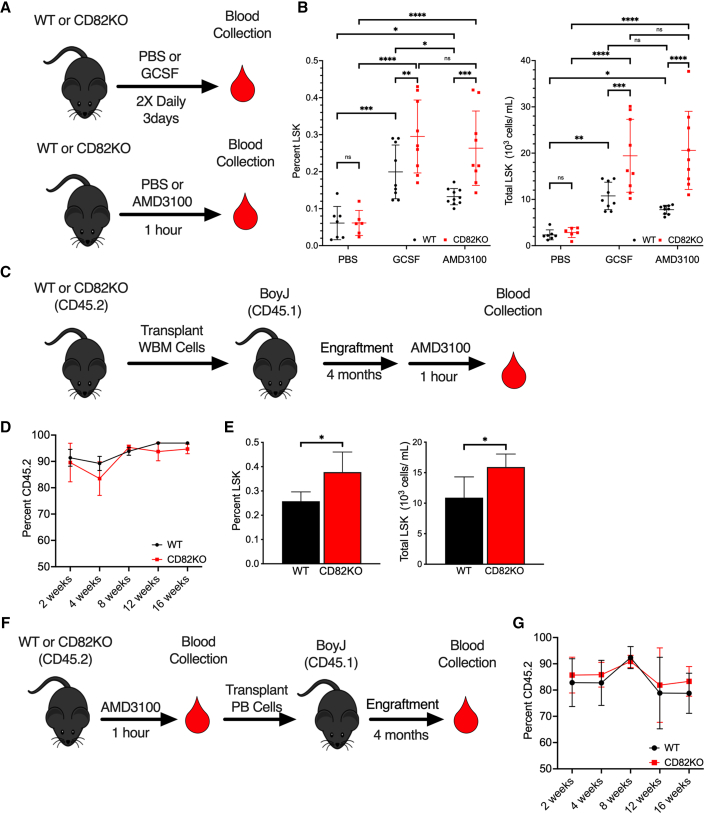
To determine how the CD82 scaffold affects HSPC mobilization, we utilized the CD82KO mouse previously described (Wei et al., 2014). Wild-type (WT) and CD82KO mice, which have comparable complete blood counts (Table S1), were injected with vehicle control, G-CSF, or AMD3100, therapeutics used clinically to mobilize HSPCs. Following treatment, blood was harvested and the Lin−Sca1+Kit+ (LSK) HSPC population was identified by flow cytometry (Figure 1A). Under control treatment, a minimal number of HSPCs are detected in the blood, with no difference identified between WT and CD82KO animals (Figure 1B). As expected, G-CSF increases the quantity of mobilized HSPCs measured in the blood; however, an even greater increase in HSPC mobilization is detected in the CD82KO mice compared with WT (Figure 1B). Similarly, AMD3100 treatment increases the number of LSKs mobilized from CD82KO mice compared with WT (Figure 1B), further confirming an increased mobilization potential of CD82KO HSPCs.
Figure 1.
CD82KO HSPCs display enhanced mobilization
(A) WT and CD82KO mice were treated with PBS, G-CSF, or AMD3100 prior to peripheral blood collection.
(B) Flow cytometry analysis of %LSK and total LSK cells in peripheral blood collected from WT and CD82KO mice treated with PBS, AMD3100, or G-CSF (n = 5–9 mice/group, four independent experiments; ∗∗∗∗p < 0.0001, ∗∗∗p < 0.001, ∗p < 0.05, two-way ANOVA).
(C) BM cells from WT or CD82KO mice were transplanted into lethally irradiated BoyJ mice and engrafted for 4 months prior to AMD3100 mobilization.
(D) Percentage of donor cells (CD45.2) repopulated in peripheral blood over 4 months measured by flow cytometry.
(E) Flow cytometry analysis of %LSK and total LSK cells in peripheral blood collected after AMD3100-induced HSPC mobilization of WT and CD82KO transplanted BoyJ mice (n = 4–5 mice/group, three independent experiments,∗p < 0.05, unpaired t test).
(F) Mobilized PBMCs from WT or CD82KO mice were transplanted into lethally irradiated BoyJ mice and engrafted for 4 months.
(G) Percentage of donor cells (CD45.2) repopulated in peripheral blood over 4 months measured by flow cytometry (n = 4–5 mice/group, three independent experiments,∗p < 0.05, unpaired t test). Error bars, SD.
As this is a global CD82KO mouse, we next confirmed that the enhanced mobilization observed is due to loss of CD82 in the HSPCs rather than an effect of CD82KO within the BM microenvironment. Total BM cells from WT or CD82KO HSPCs (CD45.2) were transplanted into lethally irradiated B6.SJL-Ptprca Pepcb/BoyJ (BoyJ) (CD45.1) recipient mice, which provide a WT BM niche (Figure 1C). Blood chimerism analyses indicate equivalent engraftment over 4 months prior to mobilization with AMD3100 (Figure 1D). Similar to our previous observation, we detect an increased mobilization of transplanted CD82KO HSPCs compared with WT cells, indicating a cell-intrinsic defect of the CD82KO HSPCs (Figure 1E). Finally, to determine if mobilized CD82KO HSPCs engraft with efficiency equal to WT HSPCs, we completed reconstitution experiments (Figure 1F). Blood chimerism analyses of animals injected with equal numbers of AMD3100 mobilized WT and CD82KO HSPCs demonstrate functional reconstitution out to 4 months, indicating that CD82KO HSPCs engraft with the same capacity as WT HSPCs (Figure 1G). Collectively, these data suggest the loss of CD82 enhances HSPC mobilization and implicates the CD82 scaffold as a regulator of HSPC egress.
Increased S1PR1 expression promotes CD82KO HSPC mobilization
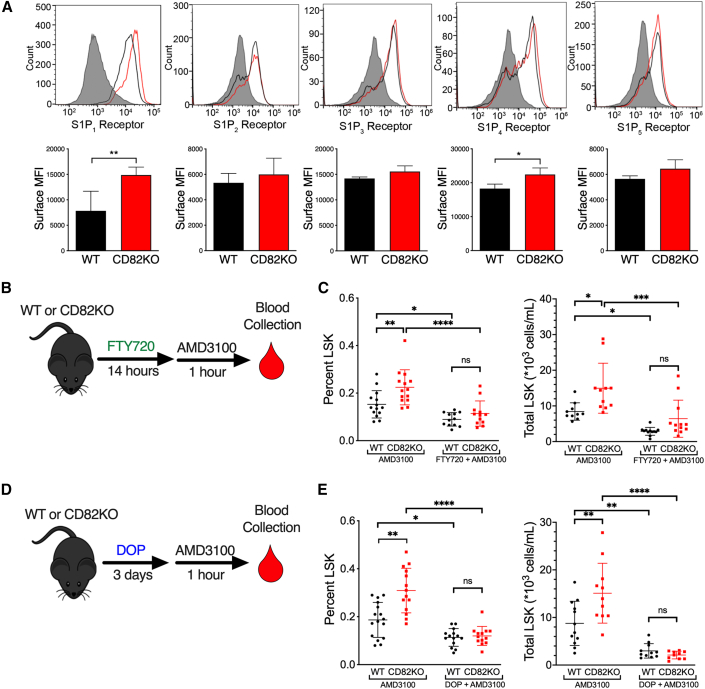
HSPC mobilization is critically dependent upon S1PR signaling in response to the S1P ligand gradient. Thus, we asked whether CD82 regulates HSPC egress by modulating S1PR surface expression. Using flow cytometry, we characterized the surface expression of the five S1PR family members (S1PR1–5). While we detected no difference in the surface expression of S1PR2, S1PR3, or S1PR5 between WT and CD82KO HSPCs, we measured a significant increase in the surface expression of S1PR1 and a more modest increase of S1PR4 on CD82KO HSPCs (Figure 2A).
Figure 2.
Increased S1PR1 expression on CD82KO HSPCs promotes enhanced mobilization
(A) MFI of S1PR1-5 surface expression on WT and CD82KO LSK cells (n = 4 mice/group ∗∗p < 0.01 and ∗p < 0.05, unpaired t test).
(B) FTY720, which activates and internalizes S1PR1, was injected into WT or CD82KO mice 14 h prior to AMD3100 treatment followed by peripheral blood collection.
(C) Flow cytometry analysis of %LSK and total LSK cells in peripheral blood collected from WT and CD82KO mice treated with AMD3100 or AMD3100/FTY720.
(D) The S1P lyase inhibitor, DOP, increases S1P resulting in S1PR1 internalization. Drinking water for WT and CD82KO mice was supplemented with DOP for 3 days. AMD3100 treatment occurred 1 h prior to peripheral blood collection.
(E) Flow cytometry analysis of %LSK and total LSK cells in peripheral blood collected from WT and CD82KO mice treated with AMD3100 or AMD3100/DOP. (B–E) n = 12–16 mice/group, four independent experiments, ∗∗∗∗p < 0.0001, ∗∗p < 0.01, ∗p < 0.05, two-way ANOVA. Error bars, SD.
Since trafficking of HSPCs and their egress from extramedullary tissues was shown previously to depend on S1PR1 expression, we set out to determine if the increased expression of S1PR1 mediates the increased mobilization of CD82KO HSPCs. To desensitize S1P receptors, mice were injected with FTY720, which can stimulate persistent internalization of S1PR1 downstream of activation (Sykes et al., 2014). As indicated previously, AMD3100 treatment of animals increases the number of mobilized HSPCs from CD82KO animals compared with WT. However, upon FTY720 treatment in combination with AMD3100, the number of mobilized HSPCs is decreased compared with AMD3100 treatment alone with no difference in mobilization detected between WT and CD82KO mice (Figure 2B and 2C). Additionally, we disrupted the S1P gradient by treating mice with 4-deoxypyridoxine (DOP), which inhibits S1P lyase, causing a BM increase in S1P that also results in S1PR1 downregulation (Schwab et al., 2005). Similarly, we found a significant decrease in mobilization of HSPCs following DOP treatment with no difference measured between WT and CD82KO mice (Figure 2D and 2E). Together, these experiments demonstrate that S1PR1 expression and signaling significantly contribute to the enhanced blood mobilization of CD82KO HSPCs.
CD82 regulates S1PR1 internalization and signaling
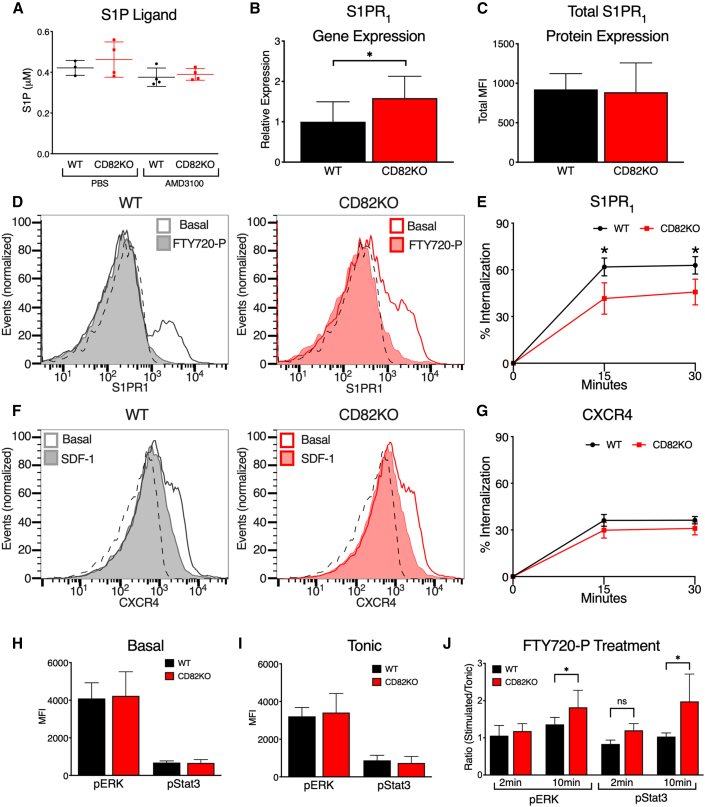
Since S1P concentrations within the blood can modulate HSPC egress as well as S1PR1 surface expression, we also quantified the S1P ligand within the plasma of WT and CD82KO mice. Under control and AMD3100 treatment conditions, we measure no significant difference in S1P ligand (Figure 3A). We also analyzed the mRNA and total S1PR1 protein levels by qRT-PCR and flow cytometry. While we detected a modest increase of S1PR1 mRNA expression in HSPCs from CD82KO mice, we found no overall difference in S1PR1 total protein expression between CD82KO and WT HSPCs (Figures 3B and 3C).
Figure 3.
CD82 regulates S1PR1 internalization and signaling
(A) S1P plasma levels of WT and CD82KO mice post PBS or AMD3100 treatment (n = 3–4 mice/group).
(B) Quantitative PCR analysis of relative S1PR1 gene expression compared with GAPDH in WT and CD82KO LSK cells (n = 4 mice/group ∗p < 0.05, unpaired t test).
(C) MFI of total S1PR1 expression of fixed and permeabilized WT and CD82KO LSK cells (n = 4 mice/group ∗p < 0.05, unpaired t test).
(D) Representative histograms of S1PR1 surface MFI at 0 (basal) and 30 min post 10 μM FTY720-P treatment in WT and CD82KO LSK cells. Isotype indicated by dotted line.
(E) Percentage internalization of S1PR1 at 15 and 30 min post 10 μM FTY720-P treatment (n = 3 independent experiments; ∗p < 0.05, unpaired t test).
(F) Representative histograms of CXCR4 surface MFI at 0 (basal) and 30 min post 100 ng/mL SDF-1 treatment in WT and CD82KO LSK cells. Isotype indicated by dotted line.
(G–I) (G) Percentage internalization of CXCR4 at 15 and 30 min post 100 ng/mL SDF-1 treatment (n = 3 independent experiments). Phosphoflow cytometry analysis of (H) basal and (I) tonic conditions to assess MFI of pERK and pStat3 signaling in the LSK population (n = 3–4 mice/group; ∗∗∗p < 0.001, unpaired t test).
(J) Phosphoflow cytometry analysis of LSK pERK and pSTAT3 signaling after 10 μM FTY720-P treatment at 2 and 10 min (n = 3 independent experiments; ∗p < 0.05; ns, non-significant, unpaired t test). Error bars, SD.
Recognizing that tetraspanins, including CD82, are known to modulate receptor endocytosis, we next examined S1PR1 internalization using flow cytometry. Following activation with the phosphorylated form of FTY720 (FTY720-P), we find that HSPCs from CD82KO animals have reduced S1PR1 internalization at 15 and 30 min compared with WT HSPCs (Figures 3D and 3E). To determine whether the effect of CD82KO affects GPCRs in general, we completed the endocytosis assay for CXCR4 following treatment with the ligand SDF-1. In Figures 3F and 3G, we find equivalent CXCR4 internalization when comparing HSPCs from WT and CD82KO mice, which suggests that CD82KO HSPCs do not demonstrate a global GPCR internalization defect.
In addition to measuring S1PR1 internalization, we also evaluated the downstream signal transduction from ligand activation. S1PR1 activates multiple intracellular signaling cascades, including the extracellular signal-regulated kinase (ERK) and the STAT3 pathways (Rosen et al., 2009). Using phosphoflow, we detect no change in basal or tonic levels of pERK or pSTAT3 in HSPCs from CD82KO animals compared with WT (Figures 3H and 3I; Figure S1). However, following FTY720-P stimulation, we detect a significant increase in pERK and pSTAT3 expression in CD82KO HSPCs at 10 min (Figure 3J; Figure S1). Collectively, these data suggest that the increased S1PR1 surface expression on CD82KO HSPCs results in enhanced signal transduction downstream of ligand engagement.
Anti-CD82 treatment enhances HSPC mobilization
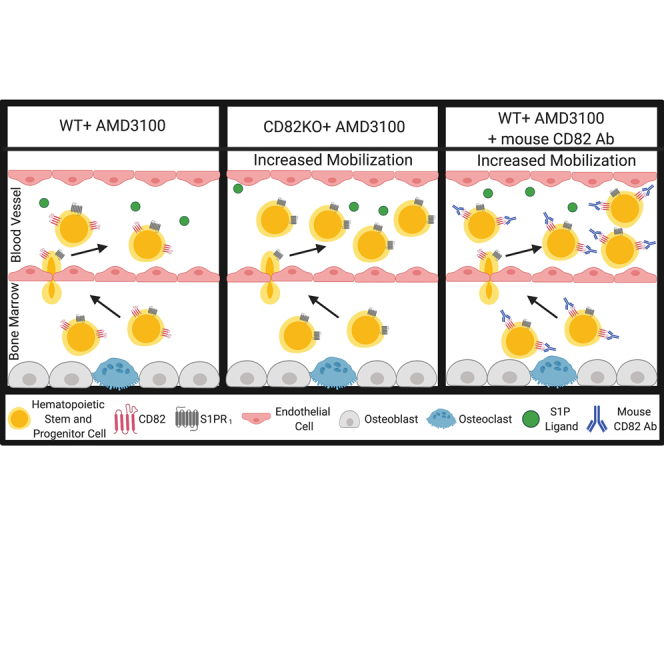
The observation that HSPCs are more readily released into the peripheral circulation of CD82KO mice led us to ask whether CD82 could be a novel target to promote HSPC mobilization. Previous studies have used antibodies to illustrate a critical role for specific integrins in HSPCs mobilization (Craddock et al., 1997). As such, we set out to determine if pretreatment with anti-CD82 antibodies induces HSPC mobilization in mice. Quality antibodies specific to mouse CD82 have been a limitation to the field, but the anti-CD82 Ab (clone M35) has been well characterized (Custer et al., 2006). Using this Ab, we measured CD82 expression on HSPCs from CD82KO and WT mice, validating the loss of CD82 surface expression on CD82KO HSPCs (Figure 4A). Next, we intravenously injected WT mice with either 2 mg/kg of anti-CD82 or control immunoglobulin G (IgG) for 2 h and then treated the animals for 1 h with control saline or AMD3100 (Figure 4B). Blood was isolated and analyzed by flow cytometry to quantify peripheral-blood-mobilized HSPCs. While anti-CD82 alone has no impact on mobilization, mice treated with anti-CD82 in combination with AMD3100 display increased total and percentage LSK (%LSK) cell mobilization compared with controls (Figure 4C). However, if AMD3100 was administered 24 h after anti-CD82 injection, we detected no enhanced mobilization (Figures 4D and 4E), suggesting that the timing of the anti-CD82/AMD3100 treatment affects mobilization efficiency. Lastly, we evaluated anti-CD82 treatment in NSG mice humanized with CD34+ cells. Using a human anti-CD82 Ab, we also detect a similar increase in CD34+ cell mobilization when anti-CD82 is injected prior to AMD3100 treatment (Figures 4F and 4G). Therefore, anti-CD82 treatment when used in combination with AMD3100 stimulates enhanced mobilization of HSPCs and further suggests that CD82 is a key contributor to the BM retention of HSPCs.
Figure 4.
CD82 Ab treatment enhances HSPC mobilization
(A) MFI of CD82 surface expression on WT and CD82KO LSK cells (n = 4–5 mice/group; ∗∗∗p < 0.001, unpaired t test).
(B) WT mice were injected with either IgG control or CD82 Ab for 2 h followed by PBS or AMD3100 treatment for 1 h prior to blood collection.
(C) Flow cytometry analysis of %LSK and total LSK cells in peripheral blood collected from WT mice treated with IgG control or CD82 Ab (n = 4–5 mice/group, three independent experiments; ∗∗∗∗p < 0.0001, ∗∗∗p < 0.001, ∗∗p < 0.01, or ∗p < 0.05, two-way ANOVA).
(D) WT mice were injected with either IgG control or CD82 Ab once daily for 2 days, then injected with PBS or AMD3100 treatment the following day 1 h prior to blood collection.
(E) Flow cytometry analysis of %LSK and total LSK cells in peripheral blood collected from WT mice treated with IgG control or CD82 Ab (n = 5 mice/group).
(F) Lethally irradiated NSG mice were injected with human CD34+ cells and allowed to engraft for 6 weeks prior to IgG or human CD82 Ab injection and AMD3100 treatment.
(G) Flow cytometry analysis of %CD45+ and total CD45+ cells in peripheral blood collected from NSG mice treated with IgG control or CD82 (n = 5–6 mice/group, three independent experiments; ∗∗p < 0.01, unpaired t test). Error bars, SD.
Discussion
Decreased numbers of HSPCs harvested from the peripheral blood limits the success of BM transplants. In fact, standard methods for peripheral blood mobilization of HSPCs fail to collect sufficient stem cells in 5%–40% of patients (Jantunen et al., 2012). Therefore, identifying unique targets to promote HSPC mobilization and increase HSPC numbers within the peripheral blood is crucial for treatment of both non-hematological and hematological malignancies. The tetraspanin family of scaffold proteins function as molecular facilitators interacting with adhesion and signaling molecules at the plasma membrane to create tetraspanin-enriched microdomains, which contribute to a number of cellular functions, including migration, adhesion, and protein trafficking (Termini and Gillette, 2017; van Deventer et al., 2017). Specifically, within HSPCs, tetraspanins are described to affect homing, engraftment, migration, and quiescence (Larochelle et al., 2012; Saito-Reis et al., 2018), and, in the current study, we identify a novel role for the tetraspanin CD82 as a critical regulator of HSPC mobilization.
Under normal physiological conditions, HSPCs are found in circulation at very low numbers; however, increased numbers of HSPCs mobilize into the blood in response to injury, infection, or stress (Heidt et al., 2014). Additionally, treatments such as G-CSF and AMD3100, which target CXCR4, are used to induce peripheral blood mobilization of HSPCs for stem cell transplant. We identify an increase in the mobilization capacity of HSPCs from CD82KO mice following G-CSF and AMD3100 treatment that is HSPC intrinsic. Previous work from our laboratory thoroughly evaluated the expression and signaling potential of the CXCR4 receptor in the context of CD82KO HSPCs, finding no altered expression or signaling of CXCR4 (Saito-Reis et al., 2018). Therefore, we went on to explore S1PR1, which we find upregulated on the surface of CD82KO HSPCs.
S1P receptors are targets of the lipid signaling molecule S1P, and facilitate the egress of HSPCs toward the higher S1P concentration found within the blood upon HSPC release from the BM (Ratajczak et al., 2010; Seitz et al., 2005).Within the receptor family, S1PR1 is the most well characterized as an important mediator of HSPC mobilization. For example, treatment of mice with the S1PR1 agonist, FTY720, results in the rapid downregulation and degradation of the receptor and subsequently prevents HSPC mobilization (Mullershausen et al., 2009). Similarly, our data suggest that CD82KO HSPCs have an enhanced mobilization capacity, due in part to increased surface S1PR1, since treatment of CD82KO mice with FTY720 ablated the enhanced mobilization. Dynamin-2 and the clathrin-mediated endocytic pathway play a role in S1PR1 internalization, altering receptor surface expression and function (Reeves et al., 2016; Willinger et al., 2014). Similarly, several tetraspanins have also been described to modulate receptor internalization. In fact, CD82 was identified to regulate epidermal growth factor receptor endocytosis (Danglot et al., 2010) and the internalization and recycling of the α4 integrin (Termini et al., 2014). Here, we find that CD82 expression also modulates S1PR1 internalization, although whether CD82 interacts directly or indirectly with S1PR1 or modulates clathrin-mediated endocytosis remains unclear.
Improvements in mobilization efficacy continue to occur through the development and investigation of novel mobilization regimens, which are critical for successful stem cell transplants and hematopoietic recovery. A host of mobilization agents that target various signaling pathways have been identified and can be found in different phases of clinical trials (Luo et al., 2021). While some of these agents induce HSPC mobilization alone, many more enhance G-CSF and AMD3100-induced mobilization, which promote rapid mobilization, reducing the need for multiple treatment doses. In this study, we demonstrate CD82 antibodies to be a novel agent to enhance G-CSF and AMD3100-induced mobilization in animal models. We find that CD82 antibodies enhance HSPC egress when administered with treatments that perturb BM interactions, suggesting an acute impact of Ab treatment that coincides with other rapid mobilization regimens (Luo et al., 2021). Future studies will be needed to directly compare the targeting of CD82 with other rapid mobilization agents and to investigate how CD82 Ab treatment affects tetraspanin-enriched microdomain organization and specifically S1PR1 internalization and signaling. Taken together, our data provide compelling evidence that the CD82 scaffold represents a unique target that can be exploited to enhance HSPC mobilization for clinical therapies.
Experimental procedures
Mice
C57BL/6, B6.SJL-Ptprca Pepcb/BoyJ, and NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NSG) mice were obtained from Jackson. CD82KO mice were generated as previously described (Wei et al., 2014). All procedures were approved by the UNMHSC Institutional Animal Care and Use Committee. Mice were housed under pathogen-free conditions in the UNMHSC Animal Facility. Mice were sex and age matched for all experiments.
Cell isolation and flow cytometry
Peripheral blood was collected by cardiac puncture. BM cells were isolated from the front and back limb bones. Red blood cells from either blood or BM were lysed using ACK lysis buffer. Peripheral blood mononuclear cells (PBMCs) were isolated using Lympholyte Mammal. White blood cells (WBCs) were analyzed using the LSR Fortessa (BD Bioscience), Accuri C6 Flow Cytometer (BD Bioscience), or Attune NxT (Thermo Fisher Scientific). Mouse antibodies for LSK (lineage−, sca+, c-kit+) labeling include: allophycocyanin (APC) lineage cocktail, phycoerythrin (PE) c-kit, and PE-Cy7 Sca-1. Where indicated, the Lin− population was isolated using a lineage depletion kit. For cell surface expression, BM cells were labeled for LSK and S1PR1, S1PR2, S1PR3, S1PR4, S1PR5, or CD82. Histograms were created using FlowJo software.
HSPC mobilization
Mice were subcutaneously (SC) injected with 62.5 μg/kg G-CSF twice daily for 3 days or 5 mg/kg AMD3100 or PBS for 1 h prior to blood collection. WBCs were labeled for LSK and %LSK was calculated from total WBC. For HSPC transplant, either 5 × 106 BM cells or 1 × 106 PBMCs with 5 × 105 BM cells from CD82KO or WT (CD45.2) mice were intravenously (IV) injected into irradiated (9 Gy) recipient BoyJ mice (CD45.1). AMD3100 treatment was performed 4 months post transplant. FTY720 was injected intraperitoneal (IP) at 1 mg/kg 14 h prior to AMD3100 treatment. DOP was supplemented in the drinking water for 3 days at 30 mg/L with both DOP-treated and control mice receiving 10 g/L glucose. For mouse CD82 Ab mobilization, WT mice were IV injected with 2 mg/kg CD82 Ab or rabbit IgG for either 2 h or once daily for 2 days prior to AMD3100 treatment. For human CD82 Ab mobilization, 6 × 105 human CD34+ cells were IV injected into irradiated (0.9 Gy) NSG mice. After 6 weeks, mice were SC injected with either human CD82 Ab or IgG1 at 1 mg/kg for 2 h before AMD3100 treatment. Blood was isolated and WBCs were labeled with hCD45 and percentage/total CD45+ was calculated from total WBC.
S1P ELISA
Blood was collected 1h post PBS or ADM3100 treatment. Blood stood for 2 h, then was centrifuged at 2.0 × g for 20 min to isolate plasma. S1P ligand protein concentrations from WT and CD82KO plasma were determined by enzyme-linked immunosorbent assay (ELISA) per the manufacturer's instructions.
qRT-PCR
qRT-PCR was performed as in Saito-Reis et al. (2018). Primer sequences for S1PR1: F: 5′-ACTACACAACGGGAGCAACAG-3′, R: 5′-GATGGAAAGCAGGAGCAGAG-3′.
Internalization
Lin− BM cells were labeled with either S1PR1 Alexa 647 or CXCR4 Alexa 647. Cells were suspended in StemSpan serum-free expansion medium (SFEM) and treated with 10 μM FTY720-P or 100 ng/mL SDF-1. Samples were taken at 0 (basal), 15, and 30 min then labeled for the LSK population. Surface expression was analyzed by flow cytometry. Percentage internalization was calculated by the following: 100 − ((mean fluorescence intensity [MFI] at 15 or 30 min/basal MFI) × 100).
Phosphoflow
Lin− BM cells were analyzed for basal signaling and serum starved in SFEM for 30 min at 37°C to assess tonic signaling. Cells were treated for 2 and 10 min with 10 μM FTY720-P at 37°C, then fixed with 4% paraformaldehyde and permeabilized with acetone. Cells were labeled with LSK markers, pERK Alexa 488 and pSTAT3 (Tyr705) and analyzed by flow cytometry. The signaling ratio was calculated by dividing treated MFI by tonic MFI.
Statistical analysis
Utilizing GraphPad Prism 8 Software, statistical significance was calculated using a Student's t test or two-way ANOVA multiple comparisons corrected by Sidak. All experiments were performed with three or four independent experiments.
Reagents can be found in Table S2.
Author contributions
C.A.S.-R., V.D.B., E.M.P., M.J., and J.M.G. designed experiments and analyzed data. C.A.S.-R., V.D.B., E.M.P., and M.J. performed experiments. V.D.B., C.A.S.-R., and J.M.G. contributed to manuscript writing.
Conflicts of interest
The authors declare no competing interests.
Acknowledgments
We thank Mark Wright (Monash University, Australia) and Günter J. Hämmerling (German Cancer Research Center, Heidelberg, Germany) for the CD82KO mice. This study was supported by the following NIH grants: R01 HL12248301 (to J.M.G.), T32 HL007736 (to TCR for C.A.S.-R. and E.M.P.), K12 GM088021 (to AWN for V.D.B.), and P20GM103451. Data were collected using the Flow Cytometry and Animal Models Shared Resources supported by the UNM Comprehensive Cancer Center, NIH P30 CA118100 (to CLW).
Published: September 16, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.stemcr.2021.08.009.
Supplemental information
References
- Bendall L.J., Basnett J. Role of sphingosine 1-phosphate in trafficking and mobilization of hematopoietic stem cells. Curr. Opin. Hematol. 2013;20:281–288. doi: 10.1097/MOH.0b013e3283606090. [DOI] [PubMed] [Google Scholar]
- Broxmeyer H.E., Orschell C.M., Clapp D.W., Hangoc G., Cooper S., Plett P.A., Liles W.C., Li X., Graham-Evans B., Campbell T.B. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J. Exp. Med. 2005;201:1307–1318. doi: 10.1084/jem.20041385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock C.F., Nakamoto B., Andrews R.G., Priestley G.V., Papayannopoulou T. Antibodies to VLA4 integrin mobilize long-term repopulating cells and augment cytokine-induced mobilization in primates and mice. Blood. 1997;90:4779–4788. [PubMed] [Google Scholar]
- Custer M.C., Risinger J.I., Hoover S., Simpson R.M., Patterson T., Barrett J.C. Characterization of an antibody that can detect the Kai1/CD82 murine metastasis suppressor. Prostate. 2006;66:567–577. doi: 10.1002/pros.20386. [DOI] [PubMed] [Google Scholar]
- Danglot L., Chaineau M., Dahan M., Gendron M.C., Boggetto N., Perez F., Galli T. Role of TI-VAMP and CD82 in EGFR cell-surface dynamics and signaling. J. Cell Sci. 2010;123:723–735. doi: 10.1242/jcs.062497. [DOI] [PubMed] [Google Scholar]
- Heidt T., Sager H.B., Courties G., Dutta P., Iwamoto Y., Zaltsman A., von Zur Muhlen C., Bode C., Fricchione G.L., Denninger J. Chronic variable stress activates hematopoietic stem cells. Nat. Med. 2014;20:754–758. doi: 10.1038/nm.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantunen E., Varmavuo V., Juutilainen A., Kuittinen T., Mahlamaki E., Mantymaa P., Nousiainen T. Kinetics of blood CD34(+) cells after chemotherapy plus G-CSF in poor mobilizers: implications for pre-emptive plerixafor use. Ann. Hematol. 2012;91:1073–1079. doi: 10.1007/s00277-012-1411-8. [DOI] [PubMed] [Google Scholar]
- Larochelle A., Gillette J.M., Desmond R., Ichwan B., Cantilena A., Cerf A., Barrett A.J., Wayne A.S., Lippincott-Schwartz J., Dunbar C.E. Bone marrow homing and engraftment of human hematopoietic stem and progenitor cells is mediated by a polarized membrane domain. Blood. 2012;119:1848–1855. doi: 10.1182/blood-2011-08-371583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo C., Wang L., Wu G., Huang X., Zhang Y., Ma Y., Xie M., Sun Y., Huang Y., Huang Z. Comparison of the efficacy of hematopoietic stem cell mobilization regimens: a systematic review and network meta-analysis of preclinical studies. Stem Cell Res. Ther. 2021;12:310. doi: 10.1186/s13287-021-02379-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullershausen F., Zecri F., Cetin C., Billich A., Guerini D., Seuwen K. Persistent signaling induced by FTY720-phosphate is mediated by internalized S1P1 receptors. Nat. Chem. Biol. 2009;5:428–434. doi: 10.1038/nchembio.173. [DOI] [PubMed] [Google Scholar]
- Ratajczak M.Z., Lee H., Wysoczynski M., Wan W., Marlicz W., Laughlin M.J., Kucia M., Janowska-Wieczorek A., Ratajczak J. Novel insight into stem cell mobilization-plasma sphingosine-1-phosphate is a major chemoattractant that directs the egress of hematopoietic stem progenitor cells from the bone marrow and its level in peripheral blood increases during mobilization due to activation of complement cascade/membrane attack complex. Leukemia. 2010;24:976–985. doi: 10.1038/leu.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves P.M., Kang Y.L., Kirchhausen T. Endocytosis of ligand-Activated sphingosine 1-phosphate receptor 1 mediated by the clathrin-pathway. Traffic. 2016;17:40–52. doi: 10.1111/tra.12343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H., Gonzalez-Cabrera P.J., Sanna M.G., Brown S. Sphingosine 1-phosphate receptor signaling. Annu. Rev. Biochem. 2009;78:743–768. doi: 10.1146/annurev.biochem.78.072407.103733. [DOI] [PubMed] [Google Scholar]
- Saito-Reis C.A., Marjon K.D., Pascetti E.M., Floren M., Gillette J.M. The tetraspanin CD82 regulates bone marrow homing and engraftment of hematopoietic stem and progenitor cells. Mol. Biol. Cell. 2018;29:2946–2958. doi: 10.1091/mbc.E18-05-0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab S.R., Pereira J.P., Matloubian M., Xu Y., Huang Y., Cyster J.G. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science. 2005;309:1735–1739. doi: 10.1126/science.1113640. [DOI] [PubMed] [Google Scholar]
- Seitz G., Boehmler A.M., Kanz L., Mohle R. The role of sphingosine 1-phosphate receptors in the trafficking of hematopoietic progenitor cells. Ann. N Y Acad. Sci. 2005;1044:84–89. doi: 10.1196/annals.1349.011. [DOI] [PubMed] [Google Scholar]
- Sykes D.A., Riddy D.M., Stamp C., Bradley M.E., McGuiness N., Sattikar A., Guerini D., Rodrigues I., Glaenzel A., Dowling M.R. Investigating the molecular mechanisms through which FTY720-P causes persistent S1P1 receptor internalization. Br. J. Pharmacol. 2014;171:4797–4807. doi: 10.1111/bph.12620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Termini C.M., Cotter M.L., Marjon K.D., Buranda T., Lidke K.A., Gillette J.M. The membrane scaffold CD82 regulates cell adhesion by altering alpha4 integrin stability and molecular density. Mol. Biol. Cell. 2014;25:1560–1573. doi: 10.1091/mbc.E13-11-0660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Termini C.M., Gillette J.M. Tetraspanins function as regulators of cellular signaling. Front. Cell Dev. Biol. 2017;5:34. doi: 10.3389/fcell.2017.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Deventer S.J., Dunlock V.E., van Spriel A.B. Molecular interactions shaping the tetraspanin web. Biochem. Soc. Trans. 2017;45:741–750. doi: 10.1042/BST20160284. [DOI] [PubMed] [Google Scholar]
- Wei Q., Zhang F., Richardson M.M., Roy N.H., Rodgers W., Liu Y., Zhao W., Fu C., Ding Y., Huang C. CD82 restrains pathological angiogenesis by altering lipid raft clustering and CD44 trafficking in endothelial cells. Circulation. 2014;130:1493–1504. doi: 10.1161/CIRCULATIONAHA.114.011096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willinger T., Ferguson S.M., Pereira J.P., De Camilli P., Flavell R.A. Dynamin 2-dependent endocytosis is required for sustained S1PR1 signaling. J. Exp. Med. 2014;211:685–700. doi: 10.1084/jem.20131343. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.