Abstract
This study aimed to determine the impact of tacrolimus (TAC) trough level (C0) intrapatient variability (IPV) over a period of 2 years after kidney transplantation (KT) on allograft outcomes. In total, 1,143 patients with low immunologic risk were enrolled. The time-weighted coefficient variability (TWCV) of TAC-C0 was calculated, and patients were divided into tertile groups (T1: < 24.6%, T2: 24.6%–33.7%, T3: ≥ 33.7%) according to TAC-C0-TWCV up to post-transplant 1st year. They were classified into the low/low, low/high, high/low, and high/high groups based on a TAC-C0-TWCV value of 33.7% during post-transplant 0–1st and 1st–2nd years. The allograft outcomes among the three tertile and four TAC-C0-TWCV groups were compared. The T3 group had the highest rate of death-censored allograft loss (DCGL), and T3 was considered an independent risk factor for DCGL. The low/low group had the lowest and the high/high group had the highest risk for DCGL. Moreover, patients with a mean TAC-C0 of ≥5 ng/ml in the high/high group were at the highest risk for DCGL. Thus, TAC-IPV can significantly affect allograft outcomes even with a high mean TAC-C0. Furthermore, to improve allograft outcomes, a low TAC-IPV should be maintained even after the first year of KT.
Keywords: allograft, rejection, transplant, graft survival, tacrolimus
Introduction
Tacrolimus (TAC) is the most widely used immunosuppressant drug, and it has better allograft outcomes than other drugs used after kidney transplantation (KT) (1–4). However, it has a narrow therapeutic range. Hence, the monitoring of optimal TAC levels is strongly recommended. At low doses, TAC is associated with high acute rejection rates due to an insufficient immunosuppressive effect (5, 6). Meanwhile, at high doses, it is correlated with adverse events such as infection, malignancy, and nephrotoxicity (7, 8). Therefore, TAC should be administered at an appropriate dose, and patients should undergo therapeutic drug level monitoring (8). Among the indicators for predicting the area under the curve of blood TAC concentration, trough level (C0) has been mainly used for monitoring TAC concentrations in clinical practice (9). Several studies showed that the mean TAC-C0 was significantly associated with clinical outcomes in KT recipients (10, 11).
Meanwhile, variable absorption, first-pass effect, unpredictable metabolism, and, most importantly, nonadherence to TAC can cause fluctuations in TAC-C0 (12, 13). Although the mean TAC-C0 is stable within the target range, there is a risk of extremely low or high drug exposure if there are high fluctuations. In relation to this, previous studies have shown that TAC-C0 intrapatient variability (IPV) can complicate the proper maintenance of the TAC level. Some studies reported that a high TAC-IPV was significantly associated with poor allograft outcomes (14–16). However, most studies have focused on the impact of TAC-IPV during a relatively early post-transplant period, mostly only up to 1 year after KT (14, 16–20).
Only a few studies have reported on TAC-IPV after the first year of KT. In a large-scale study of 6,638 KT recipients, allograft outcomes were poorer if the TAC-IPV was higher according to TAC-C0 at post-transplant first, second, and third years (21). However, because TAC-IPV was calculated using TAC-C0 only at three time points, whether the actual TAC-IPV was represented has been a cause of concern. In another study, TAC-IPV was analyzed at 6-month intervals after KT during a median follow-up period of 3.5 years. The results showed that a high TAC-IPV was correlated with a high risk of allograft loss (22). However, this study used TAC-IPV calculated using the TAC level during the entire study period. Hence, the effect of TAC-IPV during the early versus late post-transplant period cannot be differentiated.
Therefore, not only TAC-IPV up to post-transplant first year but also TAC-IPV thereafter may have an important impact on allograft outcomes. However, previous studies about TAC-IPV after the first year of KT had limitations, as mentioned previously. Therefore, the present study aimed to investigate comprehensive allograft outcomes according to TAC-IPV not only up to post-transplant first year but also up to post-transplant second year.
Materials and Methods
Study Design
This was a single-center, retrospective observational cohort study that used information collected from a clinical data warehouse system. From January 1996 to December 2018, 1,779 patients received KT at Seoul St. Mary’s Hospital. Patients who experienced allograft loss within 1 year (n = 63), those who died within 1 year (n = 20), those who were lost to follow-up within 1 year (n = 47), those who underwent TAC-C0 measurements <3 times within 1 year (n = 225), those whose treatment was changed from TAC to other drugs within 1 year (n = 166), and those who were sensitized to donor human leukocyte antigen (HLA) before transplantation (n = 115) were excluded. Sensitization to donor HLA was defined as positivity to the complement-dependent cytotoxicity crossmatch test, the flow cytometry crossmatch test, or the presence of donor-specific anti-HLA antibody (HLA-DSA) with a median fluorescence intensity (MFI) of ≥3,000 before transplantation. Finally, 1,143 patients were included in this study. The mean duration of follow-up was 5.7 years.
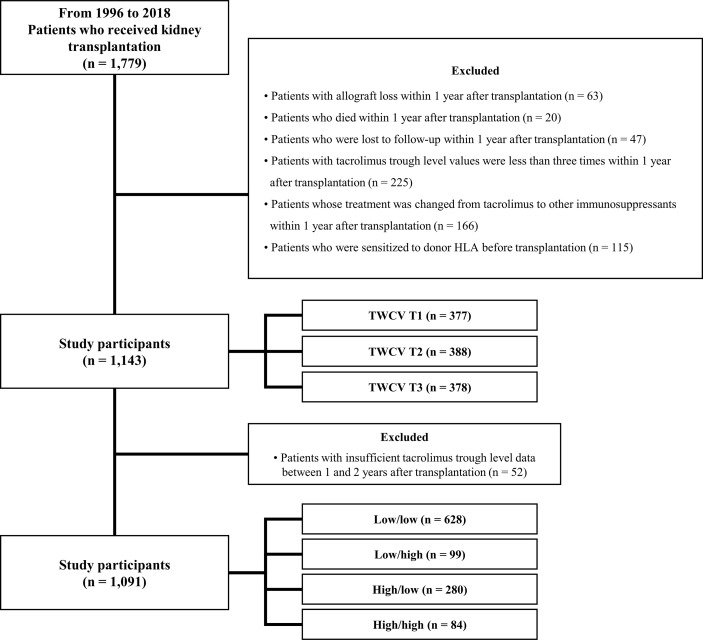
TAC-IPV was calculated using the time-weighted coefficient of variability (TWCV), which is described later. The patients were divided into tertile groups (T1, T2, and T3) according to TAC-C0-TWCV up to post-transplant first year. In addition, based on a high TAC-C0-TWCV cutoff value, patients were classified into the low/low, low/high, high/low, and high/high groups according to TAC-C0-TWCV during post-transplant 0–1st and 1st–2nd years ( Figure 1 ).
Figure 1.
Distribution of patients according to TAC-C0-TWCV. Of 1,779 patients who underwent KT, 636 were excluded. Hence, from January 1996 to December 2018, 1,143 patients were finally included in this study. They were classified into tertile groups according to TAC-C0-TWCV up to post-transplant first year. Fifty-two patients had missing TAC-C0 data during the post-transplant 1st–2nd year. In total, 1,091 patients with complete data up to post-transplant second year were classified into four groups according to TAC-C0-TWCV during post-transplant 0–1st and 1st–2nd years. HLA, human leukocyte antigen; KT, kidney transplantation; TAC-C0, tacrolimus trough level; TWCV, time-weighted coefficient variability.
This study was performed in accordance with the principles of the Declaration of Helsinki and was approved by the institutional review board of Seoul St. Mary’s Hospital (XC20WIDI0024K).
TAC-C0-TWCV Calculation
TAC level measurement was performed using the automated Dimension TAC method (Siemens Healthcare Diagnostics Inc, Deerfield, IL), which is an affinity chrome-mediated immunoassay (23). The results of the tests performed in the outpatient department and those conducted just before the next TAC dose in fasting status were used. TAC-C0-TWCV was calculated using a previously reported method (15). Briefly, the time-weighted average (TWA) of TAC-C0 was calculated using the following formula: . The time-weighted standard deviation was calculated using the following formula: , where i is the patient’s visit to the ith outpatient clinic after transplantation, xi is the TAC-C0 (ng/ml) during the interval period, ti is the time interval (days), and t is the total duration of drug exposure (days). TAC-C0-TWCV was calculated using the formula .
Immunosuppressive Regimen
The maintenance immunosuppressive therapy comprised TAC, mycophenolate mofetil (MMF), and glucocorticoid (prednisolone or deflazacort). The initial TAC was 0.1 mg/kg in two divided doses 2 days before KT. The target TAC-C0 was 8–12 ng/ml until 3 months after KT and 5–8 ng/ml thereafter. The initial MMF was 1,500 mg in two divided doses 2 days before KT. In case of enteric-coated mycophenolate sodium formulation, it was administered at 1,080 mg in two divided doses. Intravenous (IV) glucocorticoid was administered at a high dose during the perioperative period. Then, the dose was reduced (prednisolone 5 mg or deflazacort 6 mg once daily within 3 months after KT). Based on the patient’s immunologic risk (retransplant or positivity to panel reactive antibodies), IV rabbit antithymocyte globulin at a dose of 1.5 mg/kg for 5 consecutive days from day 0 to 4 or anti-interleukin-2 receptor antagonist (basiliximab) at a dose of 20 mg was administered on days 0 and 4. Patients with ABO-incompatible KT received desensitization therapy, as previously reported (24).
Clinical Parameters
All data were extracted from a clinical data warehouse system. Information about baseline characteristics, including KT donors’ age, sex, and body mass index (BMI); recipients’ age, sex, and BMI; and dialysis- and transplant-related factors, was collected. The concentration-to-dose ratio (CDR) was obtained by dividing TAC-C0 by the previously administered TAC dosage and was used as the average up to post-transplant first year. CDR values were calculated based on the result of the second visit at the outpatient clinic after transplantation. Therefore, the specific period for the CDR values was from a median of 29 days (interquartile range, 27–33 days) to 1 year after transplantation, and the average of these values was obtained and used for analysis. In terms of allograft outcome parameters, data regarding the development of de novo DSA, biopsy-proven allograft rejection (BPAR), calcineurin inhibitor (CNI) toxicity, cytomegalovirus (CMV) DNAemia, BK viremia, and death-censored allograft loss (DCGL), as well as mortality rates, were collected.
Clinical Outcomes
The primary outcome of this study was DCGL, and the secondary outcomes were the development of de novo DSA, BPAR, CNI toxicity, and mortality rates.
DCGL was defined as redialysis or retransplantation, excluding patient death with functioning allograft. Mortality was attributed to any cause after transplantation. Allograft kidney biopsy was performed in the case of unexpected allograft dysfunction (serum creatinine level that is 20% above the baseline), unexpected development of proteinuria, and occurrence of de novo DSA. Allograft kidney biopsy findings were interpreted according to the 2019 Banff classification. Biopsy-proven rejection was diagnosed via allograft biopsy for acute T-cell-mediated rejection (TCMR), acute antibody-mediated rejection (ABMR), chronic active TCMR, and chronic active ABMR. CNI toxicity was diagnosed based on the Banff classification (25, 26). HLA-DSAs were detected using Lifecodes LSA Class I and II kits or LABScreen Single Antigen kit, as previously described (27). A positive result was defined as an MFI of ≥1,000. HLA-DSA monitoring (post-transplant 3–6 and 12 months and annually thereafter) was performed for all patients from January 2010. Moreover, HLA-DSA detection was performed according to the judgment of the clinician when unexpected allograft dysfunction or proteinuria occurred. CMV DNAemia and BK viremia were screened using CMV real-time quantitative (RQ) polymerase chain reaction (PCR) and BK virus real-time (RT) PCR through blood tests at 1- to 2-month intervals up to post-transplant first year. After post-transplant first year, screening was performed with CMV RQ-PCR and BKV RT-PCR every 6 months to 1 year (28, 29). In addition, CMV RQ-PCR and BKV RT-PCR tests were conducted if renal function deteriorated or when the tests were considered necessary per the clinician’s discretion.
Statistical Analysis
Continuous variables were expressed as mean ± standard deviation. If the variables had a normal distribution, one-way analysis of variance was performed. If the variables had a non-normal distribution, the Kruskal–Wallis test was performed. The independent t-test or Wilcoxon’s rank-sum test followed by the Bonferroni method was performed for post hoc analysis. All categorical variables were compared using the chi-square test or Fisher’s exact test and were expressed as proportions. Analysis of death-censored graft survival and patient survival was conducted using Kaplan–Meier curves, and a between-group comparison was performed using the log-rank test. The effect of TAC-C0-TWCV on DCGL was analyzed via a Cox proportional hazards regression analysis. We developed a multivariate model with all significant baseline characteristics among the groups. Then, backward selection (likelihood ratio) was applied to eliminate nonsignificant variables (P-value of >0.010). Missing data were censored from the last follow-up date. P-values of <0.05 were considered statistically significant. All statistical analyses were performed using the SAS® version 9.4 software (SAS Institute, Inc., Cary, NC, USA).
Results
Comparison of Baseline Characteristics According to TAC-C0-TWCV Tertiles up to Post-transplant First Year
Table 1 shows the baseline characteristics according to the tertile groups of TWCV calculated using TAC-C0 up to post-transplant first year. In the group classification according to the tertile of TAC-C0-TWCV, the cutoff values of each tertile were 24.6% and 33.7%. The frequency of TAC-C0 measurement was significantly higher in the T3 group than in the other groups. TAC-C0-TWA was highest in the T1 group and lowest in the T3 group. The CDR was highest in the T1 group and lowest in the T3 group (1.99 ± 1.04 in T1, 1.85 ± 1.06 in T2, and 1.77 ± 1.03 in T3, P < 0.001). The T3 group had the lowest proportion of male recipients. The proportion of recipients with positivity to panel reactive antibody (PRA) was highest in the T1 group and lowest in the T3 group.
Table 1.
Baseline characteristics according to TAC-C0-TWCV tertiles up to post-transplant first year.
| T1 | T2 | T3 | P-value | |
|---|---|---|---|---|
| (n = 377) | (n = 388) | (n = 378) | ||
| Donor factors | ||||
| Age (years) | 44.2 ± 12.7 | 44.6 ± 12.6 | 44.0 ± 12.9 | 0.821 |
| Male sex | 189 (50.1%) | 207 (53.4%) | 199 (52.7%) | 0.647 |
| BMI (kg/m2) | 23.5 ± 3.4 | 23.5 ± 3.2 | 23.6 ± 3.6 | 0.874 |
| Recipient factors | ||||
| Tacrolimus measurement times | 14.3 ± 2.7‡ | 14.4 ± 2.6‡ | 15.1 ± 2.7*† | <0.001 |
| TAC-C0-TWA (ng/ml) | 6.76 ± 1.36†‡ | 6.31 ± 1.62*‡ | 6.03 ± 1.80*† | <0.001 |
| TAC-C0-TWCV (%) | 19.9 ± 3.4†‡ | 28.9 ± 2.6*‡ | 43.3 ± 12.2*† | <0.001 |
| CDR | 1.99 ± 1.04‡ | 1.85 ± 1.06 | 1.77 ± 1.03* | <0.001 |
| Age (years) | 47.2 ± 11.3 | 46.6 ± 11.3 | 45.1 ± 11.7 | 0.046 |
| Male sex | 237 (62.9%) | 250 (64.4%) | 207 (54.8%) | 0.014 |
| BMI (kg/m2) | 23.1 ± 3.6 | 22.9 ± 3.3 | 22.9 ± 3.5 | 0.551 |
| Cause of ESKD | ||||
| DM | 70 (18.6%) | 82 (21.1%) | 66 (17.5%) | 0.413 |
| HTN | 59 (15.7%) | 52 (10.8%) | 61 (16.1%) | 0.066 |
| CGN | 63 (16.7%) | 80 (20.6%) | 55 (14.6%) | 0.079 |
| Others | 116 (30.8%) | 123 (31.7%) | 117 (31.0%) | 0.957 |
| Unknown | 69 (18.3%) | 61 (15.7%) | 79 (20.9%) | 0.180 |
| Dialysis modality | ||||
| Hemodialysis | 236 (62.6%) | 250 (64.4%) | 253 (66.9%) | 0.458 |
| Peritoneal dialysis | 62 (16.5%) | 60 (15.5%) | 59 (15.6%) | 0.923 |
| Preemptive KT | 79 (21.0%) | 78 (20.1%) | 66 (17.5%) | 0.450 |
| Dialysis vintage (months) | 52.6 ± 6 2.3 | 43.6 ± 50.6 | 53.9 ± 60.2 | 0.145 |
| Transplant information | ||||
| Deceased donor KT | 129 (34.2%) | 131 (33.8%) | 149 (39.4%) | 0.196 |
| ABO incompatible KT | 49 (13.0%) | 52 (13.4%) | 44 (11.6%) | 0.746 |
| Previous KT history | 47 (12.5%) | 39 (10.1%) | 33 (8.7%) | 0.234 |
| PRA positive | 130 (36.4%)‡ | 104 (28.6%) | 85 (25.5%)* | 0.005 |
| Mismatch number | 3.49 ± 1.56 | 3.55 ± 1.56 | 3.44 ± 1.50 | 0.426 |
| Induction therapy | ||||
| Antithymocyte globulin | 63 (16.7%) | 67 (17.3%) | 48 (12.7%) | 0.166 |
| Basiliximab | 310 (82.2%) | 322 (83.0%) | 330 (87.3%) | 0.119 |
Continuous variables are shown as mean ± standard deviation, and categorical variables are shown as proportions. *P < 0.017 versus tertile 1, †P < 0.017 versus tertile 2, ‡P < 0.017 versus tertile 3.
BMI, body mass index; CDR, concentration-to-dose ratio; CGN, clinical glomerulonephritis; DM, diabetes mellitus; ESKD, end-stage kidney disease; HTN, hypertension; KT, kidney transplantation; PRA, panel reactive antibody; TAC-C0, tacrolimus trough level; TWA, time-weighted average; TWCV, time-weighted coefficient variability.
Comparison of the Incidences of BPAR and Other Complications According to TAC-C0-TWCV Tertiles up to Post-transplant First Year
Table 2 shows the incidence rates of BPAR and other complications according to TAC-C0-TWCV tertiles up to post-transplant first year. The overall BPAR rate was significantly higher in the T3 group. Interestingly, the incidence of acute TCMR significantly differed among the TAC-C0-TWCV tertile groups, whereas that of acute ABMR did not differ among the groups. The incidence of chronic active TCMR did not significantly differ among the groups. However, that of chronic active ABMR significantly differed. The incidence of CNI toxicity was higher with increasing TAC-C0-TWCV tertiles. However, the incidence of de novo DSA did not significantly differ among the groups.
Table 2.
Incidences of BPAR and other complications according to TAC-C0-TWCV tertiles up to post-transplant first year.
| T1 | T2 | T3 | P-value | |
|---|---|---|---|---|
| (n = 377) | (n = 388) | (n = 378) | ||
| Overall biopsy-proven rejection | 50 (13.6%)† | 63 (16.7%) | 83 (24.1%)* | <0.001 |
| Acute TCMR | 39 (10.6%)† | 49 (13.0%) | 69 (20.1%)* | 0.010 |
| Acute ABMR | 9 (2.4%) | 11 (2.9%) | 11 (3.2%) | 0.826 |
| Chronic active TCMR | 4 (1.1%) | 5 (1.3%) | 3 (0.9%) | 0.935 |
| Chronic active ABMR | 2 (0.5%)† | 11 (2.9%) | 18 (5.2%)* | <0.001 |
| De novo DSA positive | 43 (12.2%) | 45 (12.8%) | 30 (11.0%) | 0.787 |
| Non-DQ DSA positive | 35 (9.9%) | 40 (11.3%) | 27 (9.9%) | 0.772 |
| DQ DSA positive | 14 (4.0%) | 7 (2.0%) | 4 (1.5%) | 0.101 |
| Calcineurin inhibitor toxicity | 50 (13.6%) | 68 (18.0%) | 70 (20.4%) | 0.049 |
| CMV DNAemia | 63 (16.7%) | 71 (18.3%) | 79 (20.9%) | 0.328 |
| BK viremia | 50 (13.3%) | 73 (18.8%) | 56 (14.8%) | 0.092 |
Categorical variables are shown as proportions. *P < 0.017 versus tertile 1, †P < 0.017 versus tertile 3.
ABMR, antibody-mediated rejection; CMV, cytomegalovirus; DSA, donor-specific antibody; TAC-C0, tacrolimus trough level; TCMR, T-cell mediated rejection; TWCV, time-weighted coefficient variability.
Comparison of the Incidences of BPAR and Other Complications According to TAC-C0-TWCV During Post-transplant 0–1st and 1st–2nd Years
Table 3 shows the incidence rates of BPAR and complications according to TAC-C0-TWCV during post-transplant 0–1st and 1st–2nd years. The overall incidence of BPAR was highest in the high/high group and was higher in the low/high and high/low groups than in the low/low group. In a sub-analysis according to rejection type, the incidence of acute ABMR and chronic active TCMR did not significantly differ among the groups. However, the incidence of acute TCMR and chronic active ABMR was significantly higher in the high/high group than in the other groups. The incidence of CNI toxicity was higher in groups with a high TWCV at least once either during post-transplant 0–1st or 1st–2nd year (low/high, high/low, and high/high groups vs. low/low group).
Table 3.
Incidences of BPAR and other complications according to TAC-C0-TWCV during post-transplant 0–1st and 1st–2nd years.
| Low/low | Low/high | High/low | High/high | P-value | |
|---|---|---|---|---|---|
| (n = 628) | (n = 99) | (n = 280) | (n = 84) | ||
| Overall biopsy-proven rejection | 88 (14.3%)† | 21 (22.8%) | 57 (22.0%) | 23 (31.9%)* | <0.001 |
| Acute TCMR | 70 (11.4%)† | 15 (16.3%) | 45 (17.4%) | 21 (29.2%)* | <0.001 |
| Acute ABMR | 15 (2.4%) | 3 (3.3%) | 10 (3.9%) | 1 (1.4%) | 0.592 |
| Chronic active TCMR | 7 (1.1%) | 2 (2.2%) | 1 (0.4%) | 2 (2.8%) | 0.170 |
| Chronic active ABMR | 10 (1.6%) | 2 (2.2%) | 13 (5.0%) | 4 (5.6%) | 0.014 |
| De novo DSA positive | 68 (11.5%) | 12 (16.0%) | 26 (12.6%) | 3 (5.6%) | 0.321 |
| Non-DQ DSA positive | 57 (9.6%) | 11 (14.7%) | 23 (11.1%) | 3 (5.6%) | 0.341 |
| DQ DSA positive | 17 (2.9%) | 1 (1.3%) | 4 (1.9%) | 0 (0%) | 0.694 |
| Calcineurin inhibitor toxicity | 88 (14.3%) | 22 (23.9%) | 54 (20.9%) | 14 (19.4%) | 0.026 |
| CMV DNAemia | 118 (18.8%) | 14 (14.1%) | 60 (21.4%) | 18 (21.4%) | 0.414 |
| BK viremia | 95 (15.1%) | 22 (22.2%) | 39 (13.9%) | 14 (16.7%) | 0.254 |
Categorical variables are shown as proportions. *P < 0.0083 versus low/low group, †P < 0.0083 versus high/high group.
ABMR, antibody-mediated rejection; CMV, cytomegalovirus; DSA, donor-specific antibody; TAC-C0, tacrolimus trough level; TCMR, T-cell mediated rejection; TWCV, time-weighted coefficient variability.
Comparison of the Incidence of DCGL and Mortality Rates According to TAC-C0-TWCV Tertiles up to Post-transplant First Year
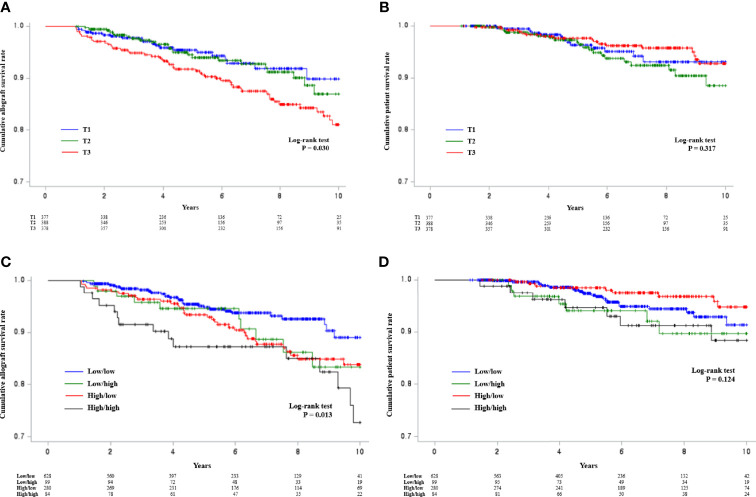
The incidence of DCGL according to TAC-C0-TWCV tertiles up to post-transplant first year was 6.1% (n = 23) in the T1 group, 7.0% (n = 27) in the T2 group, and 14.8% (n = 56) in the T3 group. The incidence of DCGL was significantly higher in the T3 group than in the other groups (P < 0.001, Supplementary Table S1 ). The mortality rates were 3.7% (n = 14) in the T1 group, 5.7% (n = 22) in the T2 group, and 5.0% (n = 19) in the T3 group. However, the results did not significantly differ among the groups (P = 0.437, Supplementary Table S1 ). Using Kaplan–Meier curves, the cumulative allograft survival rate was found to be significantly decreased in the T3 group ( Figure 2A ). The cumulative patient survival rate did not significantly differ among the groups ( Figure 2B ).
Figure 2.
Kaplan–Meier analysis of allograft survival according to TAC-C0-TWCV tertiles up to post-transplant first year and TAC-C0-TWCV tertiles during the post-transplant 0–1st and 1st–2nd years. (A) The cumulative allograft survival rate was significantly lower in the T3 group than in the other groups. However, there was no difference between the T1 and T2 groups. (B) The cumulative patient survival rates did not differ according to the TAC-C0-TWCV tertiles up to post-transplant first year. (C) The high/high group had a significantly low cumulative allograft survival rate. In terms of intermediate outcomes, the allograft survival rates of the low/high and high/low groups were similar, and the low/low group had the highest allograft survival rate. (D) The cumulative patient survival rates did not differ according to the TAC-C0-TWCV tertiles during the post-transplant 0–1st and 1st–2nd years. TAC-C0, tacrolimus trough level; TWCV, time-weighted coefficient variability.
Comparison of the Incidence of DCGL and Mortality Rates According to TAC-C0-TWCV During Post-transplant 0–1st and 1st–2nd Years
The incidence rates of DCGL according to TAC-C0-TWCV during post-transplant 0–1st and 1st–2nd years were 5.4% (n = 34) in the low/low group, 14.1% (n = 14) in the low/high group, 13.2% (n = 37) in the high/low group, and 20.2% (n = 17) in the high/high group. The high/high group had the highest incidence of DCGL, followed by the low/high or high/low group. Meanwhile, the low/low group had the lowest incidence (P < 0.001, Supplementary Table S2 ). Using Kaplan–Meier curves, the cumulative allograft survival rate was found to be lowest in the high/high group, and the low/high and high/low groups had a similar allograft survival rate, which is an intermediate outcome. The low/low group had the highest allograft survival rate ( Figure 2C ). The mortality rates were 3.5% (n = 22) in the low/low group, 7.1% (n = 7) in the low/high group, 3.2% (n = 9) in the high/low group, and 8.3% (n = 7) in the high/high group. However, the results did not significantly differ among the groups (P = 0.067, Supplementary Table S2 ). Moreover, there was no significant difference in the cumulative patient survival rate among the groups ( Figure 2D ).
Comparison of Allograft Survival Rates According to TAC-C0-TWA and TAC-C0-TWCV During Post-transplant 0–1st and 1st–2nd Years in Each TAC-C0-TWA Subgroup
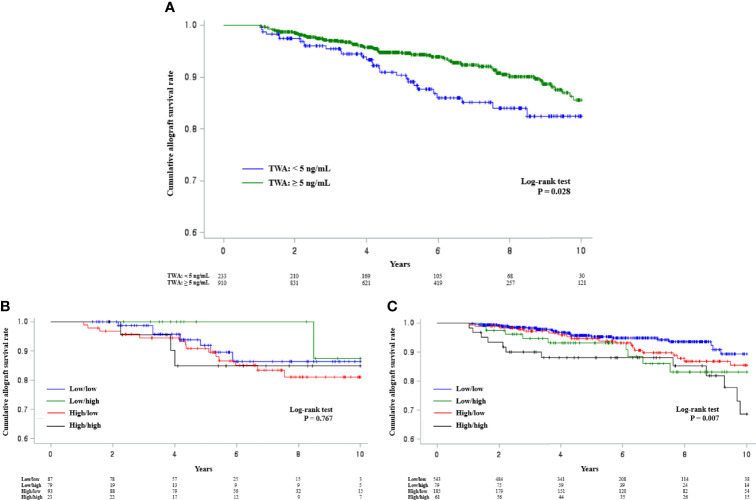
According to a previous report, the risk of allograft loss can increase with a mean TAC-C0 of <5 ng/ml up to post-transplant first year (30). Hence, patients were assessed and then divided into two groups based on a TAC-C0-TWA value of 5 ng/ml up to post-transplant first year. The results showed that the allograft survival rate was significantly poor in the group with a TAC-C0-TWA of <5 ng/ml ( Figure 3A ). The allograft survival rates were analyzed according to TAC-C0-TWCV during post-transplant 0–1st and 1st–2nd years in each subgroup. In patients with a TAC-C0-TWA of <5 ng/ml, allograft survival did not differ according to TAC-C0-TWCV levels ( Figure 3B ). However, in patients with a TAC-C0-TWA of ≥5 ng/ml, allograft survival was the worst in the high/high group and the best in the low/low group ( Figure 3C ).
Figure 3.
Kaplan–Meier survival analysis of allograft survival according to TAC-C0-TWA and TAC-C0-TWCV during post-transplant 0–1st and 1st–2nd years. (A) The group with a TAC-C0-TWA of <5 ng/ml had a lower cumulative allograft survival rate than the group with a TAC-C0-TWA of ≥5 ng/ml. (B) The cumulative allograft survival rates did not differ among the groups in the subgroup with a TAC-C0-TWA of <5 ng/ml. (C) The high/high group in the subgroup with a TWA of ≥5 ng/ml had the lowest cumulative allograft survival rate. These findings were similar to those of the entire patient cohort. TAC-C0, tacrolimus trough level; TWA, time-weighted average; TWCV, time-weighted coefficient variability.
Multivariate Cox Proportional Hazards Regression Analysis of DCGL
Table 4 shows the results of multivariate Cox regression analysis of DCGL according to TAC-C0-TWCV up to post-transplant first year (T1, T2, and T3). T3 was an independent risk factor for DCGL, with a hazard ratio (HR) of 1.853 (P = 0.029) after adjusting for the recipient’s age and sex, PRA positivity, CDR, and mismatch number. In the subgroup analysis, in the model for patients with TAC-C0-TWA of ≥5 ng/ml, T3 remained a significant risk factor with HR of 1.932 (P = 0.047) for DCGL. However, in the model for patients with TAC-C0-TWA of <5 ng/ml, it was not observed as a significant risk factor for DCGL. Previous studies have reported that low CDR increases the risk of graft loss (31, 32). There was a significant difference in CDR values among tertile groups at baseline. In this regard, multivariate model analysis including CDR values was performed. In our cohort, CDR was not observed as a significant risk factor for DCGL.
Table 4.
Multivariate Cox proportional hazard ratio model analysis for DCGL including TAC-C0-TWCV tertiles up to post-transplant first year.
| Univariate HR | P-value | Multivariate HR | P-value | ||
|---|---|---|---|---|---|
| (95% confidence interval) | (95% confidence interval) | ||||
| Entire patient cohort* | |||||
| TWCV T1 | Reference | – | Reference | – | |
| TWCV T2 | 1.051 (0.603–1.834) | 0.860 | 1.162 (0.629–2.148) | 0.632 | |
| TWCV T3 | 1.614 (0.988–2.638) | 0.056 | 1.869 (1.074–3.251) | 0.027 | |
| Mismatch number | 1.155 (1.010–1.321) | 0.036 | 1.158 (1.002–1.338) | 0.047 | |
| Age | 0.996 (0.978–1.013) | 0.627 | 0.997 (0.978–1.017) | 0.768 | |
| Sex (female) | Reference | – | Reference | – | |
| Sex (male) | 0.884 (0.602–1.299) | 0.532 | 0.904 (0.585–1.398) | 0.651 | |
| PRA positive | 0.636 (0.378–1.070) | 0.088 | 0.647 (0.377–1.110) | 0.114 | |
| CDR | 0.906 (0.735–1.116) | 0.353 | 0.955 (0.769–1.186) | 0.680 | |
| TAC-C0-TWA ≥ 5 ng/ml † | |||||
| TWCV T1 | Reference | – | Reference | – | |
| TWCV T2 | 1.132 (0.603–2.126) | 0.700 | 1.357 (0.682–2.700) | 0.385 | |
| TWCV T3 | 1.630 (0.916–2.900) | 0.097 | 1.932 (1.009–3.701) | 0.047 | |
| Mismatch number | 1.141 (0.974–1.338) | 0.103 | 1.161 (0.977–1.379) | 0.090 | |
| Age | 0.985 (0.965–1.006) | 0.172 | 0.987 (0.964–1.010) | 0.259 | |
| Sex (female) | Reference | – | Reference | – | |
| Sex (male) | 0.659 (0.419–1.037) | 0.072 | 0.724 (0.437–1.200) | 0.210 | |
| PRA positive | 0.702 (0.386–1.277) | 0.247 | 0.704 (0.378–1.312) | 0.270 | |
| CDR | 1.000 (0.800–1.251) | 0.997 | 1.035 (0.820–1.307) | 0.772 | |
| TAC-C0-TWA < 5 ng/ml ‡ | |||||
| TWCV T1 | Reference | – | Reference | – | |
| TWCV T2 | 0.681 (0.207–2.242) | 0.527 | 0.570 (0.140–2.326) | 0.433 | |
| TWCV T3 | 0.966 (0.362–2.584) | 0.946 | 0.959 (0.316–2.908) | 0.942 | |
| Mismatch number | 1.205 (0.931–1.560) | 0.157 | 1.074 (0.815–1.414) | 0.612 | |
| Age | 1.024 (0.990–1.058) | 0.164 | 1.020 (0.982–1.059) | 0.300 | |
| Sex (female) | Reference | – | Reference | – | |
| Sex (male) | 2.346 (1.099–5.004) | 0.027 | 1.867 (0.741–4.706) | 0.186 | |
| PRA positive | 0.497 (0.170–1.449) | 0.200 | 0.632 (0.199–2.008) | 0.436 | |
| CDR | 0.680 (0.289–1.600) | 0.377 | 0.830 (0.349–1.971) | 0.672 | |
Multivariate model was adjusted with parameters showing significant differences among the groups according to TWCV tertiles during post-transplant first year. *Excluding patients with missing values, 1048 (91.7%) patients were included in the model. †836 (73.1%) patients with TAC-C0-TWA of ≥5 ng/ml were included in the model. ‡212 (18.5%) patients with TAC-C0-TWA of <5 ng/ml were included in the model.
CDR, concentration-to-dose ratio; DCGL, death-censored graft loss; PRA, panel reactive antibody; TAC-C0, tacrolimus trough level; TWA, time-weighted average; TWCV, time-weighted coefficient variability.
Table 5 shows the results of multivariate Cox regression analysis of DCGL according to TAC-C0-TWCV during post-transplant 0–1st and 1st–2nd years (low/low, high/low, low/high, and high/high). Supplementary Table S3 shows the baseline characteristics among the groups, and significant factors were included in the multivariate model (recipient’s age and sex, PRA positivity, basiliximab as an induction therapy, CDR, and mismatch number). The HR of the low/high, high/low, and high/high groups were 2.054 (P = 0.050), 1.818 (P = 0.020), and 2.468 (P = 0.010), respectively, and was the highest in the high/high group. In the subgroup analysis, in the model for patients with TAC-C0-TWA of ≥5 ng/ml, TAC-C0-TWCV during post-transplant 0–1st and 1st–2nd years remained a significant risk factor for DCGL [HR of 2.384 (P = 0.027) in the low/high group and HR of 3.084 (P = 0.003) in the high/high group]. However, in the model for patients with TAC-C0-TWA of <5 ng/ml, it was not observed as a significant risk factor for DCGL.
Table 5.
Multivariate Cox proportional hazard ratio model analysis for DCGL including TAC-C0-TWCV during post-transplant 0–1st and 1st–2nd years.
| Univariate HR | P-value | Multivariate HR | P-value | |
|---|---|---|---|---|
| (95% confidence interval) | (95% confidence interval) | |||
| Entire patient cohort* | ||||
| Low/low | Reference | – | Reference | – |
| Low/high | 1.941 (1.038–3.631) | 0.038 | 2.054 (1.000–4.220) | 0.050 |
| High/low | 1.597 (0.997–2.560) | 0.052 | 1.818 (1.098–3.009) | 0.020 |
| High/high | 2.563 (1.424–4.615) | 0.002 | 2.468 (1.243–4.900) | 0.010 |
| Mismatch number | 1.182 (1.030–1.357) | 0.017 | 1.197 (1.032–1.389) | 0.018 |
| Age | 0.996 (0.978–1.014) | 0.633 | 0.995 (0.976–1.016) | 0.657 |
| Sex (female) | Reference | – | Reference | – |
| Sex (male) | 0.909 (0.614–1.346) | 0.633 | 0.937 (0.599–1.467) | 0.777 |
| PRA positive | 0.574 (0.332–0.993) | 0.047 | 0.573 (0.320–1.028) | 0.062 |
| Basiliximab | 1.067 (0.579–1.968) | 0.834 | 0.888 (0.413–1.912) | 0.762 |
| CDR | 0.922 (0.748–1.137) | 0.447 | 0.972 (0.784–1.205) | 0.794 |
| TAC-C0-TWA ≥ 5 ng/ml † | ||||
| Low/low | Reference | – | Reference | – |
| Low/high | 2.150 (1.064–4.346) | 0.033 | 2.384 (1.103–5.155) | 0.027 |
| High/low | 1.454 (0.819–2.579) | 0.201 | 1.674 (0.904–3.102) | 0.102 |
| High/high | 3.116 (1.622–5.987) | <0.001 | 3.084 (1.467-6.482) | 0.003 |
| Mismatch number | 1.153 (0.982–1.353) | 0.082 | 1.195 (1.004–1.422) | 0.045 |
| Age | 0.986 (0.966–1.008) | 0.207 | 0.987 (0.674–1.010) | 0.270 |
| Sex (female) | Reference | – | Reference | – |
| Sex (male) | 0.651 (0.412–1.027) | 0.065 | 0.759 (0.454–1.266) | 0.291 |
| PRA positive | 0.705 (0.387–1.283) | 0.253 | 0.681 (0.357–1.298) | 0.243 |
| Basiliximab | 0.915 (0.452–1.853) | 0.805 | 0.777 (0.334–1.808) | 0.559 |
| CDR | 0.991 (0.791–1.242) | 0.938 | 1.008 (0.793–1.281) | 0.948 |
| TAC-C0-TWA < 5 ng/ml ‡ | ||||
| Low/low | Reference | – | Reference | – |
| Low/high | 1.152 (0.284–4.676) | 0.843 | 0.676 (0.077–5.930) | 0.724 |
| High/low | 1.310 (0.528–3.249) | 0.560 | 1.312 (0.496–3.469) | 0.584 |
| High/high | 1.010 (0.255–4.008) | 0.988 | 0.514 (0.060–4.393) | 0.543 |
| Mismatch number | 1.279 (0.968–1.689) | 0.083 | 1.107 (0.812–1.509) | 0.520 |
| Age | 1.023 (0.987–1.059) | 0.215 | 1.016 (0.976–1.058) | 0.441 |
| Sex (female) | Reference | – | Reference | – |
| Sex (male) | 2.902 (1.272–6.622) | 0.011 | 2.263 (0.793–6.452) | 0.127 |
| PRA positive | 0.260 (0.061–1.112) | 0.069 | 0.451 (0.093–2.174) | 0.321 |
| Basiliximab | 1.542 (0.444–5.359) | 0.495 | 1.767 (0.228–13.704) | 0.586 |
| CDR | 0.687 (0.279–1.689) | 0.413 | 0.883 (0.360–2.164) | 0.786 |
Multivariate model was adjusted with parameters showing significant differences among the groups according to high or low TWCV during post-transplant 1st and 2nd years. *Excluding patients with missing values, 998 (91.5%) patients were included in the model. †796 (73.0%) patients with TAC-C0-TWA of ≥5 ng/mL were included in the model. ‡202 (18.5%) patients with TAC-C0-TWA of <5 ng/mL were included in the model.
CDR, concentration-to-dose ratio; DCGL, death-censored graft loss; PRA, panel reactive antibody; TAC-C0, tacrolimus trough level; TWCV, time-weighted coefficient variability.
Discussion
This study showed that a high TAC-C0-TWCV not only up to post-transplant first year but also post-transplant first to second year is associated with adverse clinical outcomes such as acute TCMR, chronic ABMR, and DCGL in immunologically low-risk KTs. The results strongly suggest that TAC-IPV should be maintained at least over a period of post-transplant 2 years to improve allograft outcomes.
During the early post-transplant period, the interval of outpatient visits is commonly short. However, over time, the visit interval gradually increases. If the time interval is not kept constant when calculating TAC-IPV, the results might be inaccurate. Therefore, previous studies were generally conducted using TAC-C0 between 6 months and 1 year after KT, when the interval of outpatient clinic visits was constant after KT (14, 17–20). To overcome this limitation, the TAC-C0-TWCV formula was applied to correct for different follow-up intervals. Hence, TAC-C0 during the early period after KT can be used (15). Therefore, in the present study, this formula was employed to analyze parameters, including TAC-C0 in the early period after KT, which can reflect a more accurate TAC-IPV.
Interestingly, the T1 group had the highest TAC-C0-TWA, followed by the T2 group; the T3 group had the lowest TAC-C0-TWA. This may be attributed to the low CDR in the T3 group. A low CDR indicates a fast TAC metabolizer (33). The T3 group had a lower TAC-C0-TWA than the other groups, and this might be attributable to the large proportion of fast TAC metabolizers in this group. In addition, the frequency of TAC-C0 measurement was significantly higher in the T3 group than in the other groups because it may be difficult to reach the therapeutic target range due to large fluctuations in TAC-C0. Hence, more frequent measurements could be performed to assess whether TAC-C0 reached the therapeutic target range. Another possible reason is the high incidences of acute rejection and allograft dysfunction in the T3 group, which could shorten the interval of outpatient clinic visits and resulted in the frequent measurement of TAC-C0 in the T3 group.
Second, we compared the incidence of BPAR and other complications according to TAC-C0-TWCV. The overall BPAR rate was significantly higher in the T3 group than in the other groups. In terms of rejection type, the incidence of acute TCMR significantly differed according to TAC-C0-TWCV tertiles, which was consistent with the results of previous studies (34, 35). Interestingly, the incidence of acute ABMR did not differ, and this might be attributed to the fact that the patients in our study were at low immunological risk. The incidence of chronic ABMR was significantly higher in the T3 group than in the other groups. This result is consistent with previous studies showing that high TAC-IPV was a significant risk factor for composite outcomes, including transplant glomerulopathy and high chronicity scores (16, 19). In addition, the incidence of CNI toxicity was higher in patients with higher TAC-C0-TWCV tertiles, even though the T3 group had a lower TAC-C0-TWA than the T1 group. Therefore, high TAC-IPV is associated with a high risk of not only rejection due to an insufficient immunosuppressive effect but also drug toxicity during exposure to high TAC levels.
In contrast to our expectation, the incidence of de novo DSA did not significantly differ according to TAC-C0-TWCV. This could be because not all de novo DSA were detected in our study. We previously reported that de novo anti-HLA-DQ antibody has the most significant impact on the development of chronic active ABMR (27). However, we could not determine whether the detected anti-HLA-DQ antibody was donor specific before the year 2016 because HLA-DQ typing in kidney donors was started after 2016. The incidence of de novo DSA after KT is approximately 15%–25% (36, 37). However, in the present study, the incidence of de novo DSA was only 12.0%. Moreover, in the sub-analysis of non-HLA-DQ and HLA-DQ antibodies, the incidence of HLA-DQ antibody was lower than that generally known (38, 39). Thus, the rate of de novo anti-HLA-DQ antibody may be significantly underestimated, which might affect the incidence of de novo DSA.
The main finding of this study is the impact of TAC-C0-TWCV during post-transplant 0–1st and 1st–2nd years on long-term DCGL. Based on an analysis up to post-transplant first year, TAC-C0-TWCV T3 was considered an independent risk factor for DCGL, and the value for defining TAC-C0-TWCV T3 (≥33.7%) was similar to the previously reported TAC-IPV value (≥30%) of poor allograft outcomes (14, 20). Based on this value (≥33.7%), we divided the patients into four groups according to TAC-C0-TWCV during post-transplant 0–1st and 1st–2nd years. The patients with a sustained high TAC-C0-TWCV over 2 years after KT (high/high group) had the highest risk for DCGL. Of note, the changes in TAC-C0-TWCV during post-transplant 1st–2nd year had a significant influence on allograft outcomes. Indeed, the TAC-C0-TWCV was low up to post-transplant first year, but when it increased during post-transplant 1st–2nd year (low/high group), there was a greater risk of DCGL. Conversely, when TAC-C0-TWCV reduced during post-transplant 1st–2nd year, even though it was high up to post-transplant first year (high/low group), the risk of DCGL decreased compared with those of the high/high group. Therefore, TAC-IPV even after the first year of KT can significantly influence long-term allograft outcomes.
The Collaborative Transplant Study Registry previously reported that a mean TAC-C0 of <5 ng/ml up to post-transplant first year was associated with a higher incidence of graft loss (30). Expectedly, a TAC-C0-TWA of <5 ng/ml was associated with lower allograft survival in our study. Because TAC-C0-TWA was lowest in the T3 group, whether a lower TAC-C0-TWA worsens allograft outcomes in this group should be considered. However, the TAC-C0-TWA of the T3 group was 6.03 ng/ml, which was higher than the target level of 5 ng/ml. Additionally, in a subgroup analysis of patients with a TAC-C0-TWA of ≥5 ng/ml, allograft survival was the poorest in the high/high group ( Figure 3C ), as shown in the results of the entire patient cohort. In the multivariate analysis of patients with TAC-C0-TWA of ≥5 ng/ml, similar to the results of the entire patient cohort, TAC-C0-TWCV was observed as an independent risk factor for DCGL. In contrast, when only patients with a TAC-C0-TWA of <5 ng/ml were analyzed, there was no significant difference in terms of allograft survival according to TAC-C0-TWCV during post-transplant 0–1st and 1st–2nd years ( Figure 3B ). Similarly, in the multivariable analysis of patients with TAC-C0-TWA of <5 ng/ml, TAC-C0-TWCV was not observed as a significant risk factor. The above findings suggest that TAC-C0 should be kept higher than the target level, but even if the mean TAC-C0 was higher than the target level, a high TAC-C0-TWCV can have adverse effects on allograft outcomes. Therefore, not only the TAC-C0 level but also TAC-IPV should be controlled properly.
The present study has several strengths. In a previous study, TAC-C0 was assessed only at three time points (first, second, and third year) after KT (21). Moreover, another research did not utilize TAC-IPV according to a specific period after KT (22). However, the present study calculated TAC-IPV in each period after KT. Notably, the average number of TAC-C0 measurements used to calculate TAC-IPV during the post-transplant 1st–2nd year was 7.8 ( Supplementary Table S3 ). Hence, the number of measurements was sufficient in reflecting an accurate TAC-IPV. Therefore, our study can provide relatively objective information about the importance of TAC-IPV after the first year of KT.
The present study has several limitations. First, because this study included only immunologically low-risk patients, there is a limit in applying the results of this study to all patients. A previous study reported that TAC-IPV was important even in highly sensitized patients (40). Therefore, we briefly analyzed whether TAC-IPV had an effect even in highly sensitized patients in our cohort. Similar to the previous study, the allograft survival rate tended to be worse in the highest tertile group of TAC-C0-TWCV, but there was no statistical significance owing to the small number of patients ( Supplementary Table S4 , Supplementary Figure S1 ). We are currently conducting further research on this. Second, we did not present the causes for high TAC-C0-TWCV in each patient because the study was retrospective in nature. In this regard, we cannot propose a clear solution for decreasing TAC-C0-TWCV. Third, the directionality between TAC-IPV and treatments remains unclear. Treatments given for rejection might impact TAC-IPV, and the observed higher TAC-IPV might be partially the result of rejection treatment rather than the cause of rejection. However, this is a common limitation of all studies related to TAC-IPV that have been reported to date. High TAC-IPV can be caused by various factors, including drug absorption (41), metabolism (42), formulation (43), concurrent medications (44), and nonadherence among patients (18). Additional studies must be performed to determine whether some interventions, such as patient education, strict monitoring of drug adherence, or use of extended-release TAC formulation, can decrease TAC-IPV and improve allograft outcomes.
In conclusion, TAC-IPV is an important factor that can significantly affect comprehensive allograft outcomes, including rejection, drug toxicity, and allograft loss. In addition, TAC-IPV after the first year of KT was considered an important factor for allograft outcomes. Therefore, continuous control of TAC-IPV, regardless of the post-transplant period, is important in improving allograft outcomes. Further research and clinical efforts to control this IPV are considered to be necessary.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Seoul St. Mary’s Hospital (XC20WIDI0024K). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
YP and BC wrote the manuscript. YP, CY, and BC designed the study. YP performed the experiments. HL, SE, HK, and EK analyzed the data. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by a grant from the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HI20C0317). And this study was supported by Research Fund of Seoul St. Mary's Hospital, the Catholic University of Korea. This study is an investigator led project with no specific participation by the funders.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank for supporting by Research Fund of Seoul St. Mary's Hospital, the Catholic University of Korea. And we thank Medical Excellence Inc. and PYEONGHWA IS Co. for their help in performing this research.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2021.746013/full#supplementary-material
References
- 1. Ekberg H, Tedesco-Silva H, Demirbas A, Vítko S, Nashan B, Gürkan A, et al. Reduced Exposure to Calcineurin Inhibitors in Renal Transplantation. New Engl J Med (2007) 357(25):2562–75. doi: 10.1056/NEJMoa067411 [DOI] [PubMed] [Google Scholar]
- 2. Webster AC, Woodroffe RC, Taylor RS, Chapman JR, Craig JC. Tacrolimus Versus Ciclosporin as Primary Immunosuppression for Kidney Transplant Recipients: Meta-Analysis and Meta-Regression of Randomised Trial Data. BMJ (Clin Res ed) (2005) 331(7520):810. doi: 10.1136/bmj.38569.471007.AE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Samaniego M, Becker BN, Djamali A. Drug Insight: Maintenance Immunosuppression in Kidney Transplant Recipients. Nat Clin Pract Nephrol (2006) 2(12):688–99. doi: 10.1038/ncpneph0343 [DOI] [PubMed] [Google Scholar]
- 4. Bertram LK, Martin GZ, Jonathan CC, Henrik E, Catherine AG, et al. Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group, KDIGO Clinical Practice Guideline for the Care of Kidney Transplant Recipients. Am J Transplant Off J Am Soc Transplant Am Soc Transplant Surgeons (2009) 9 Suppl 3:S1–155. doi: 10.1111/j.1600-6143.2009.02834.x [DOI] [PubMed] [Google Scholar]
- 5. Undre NA, van Hooff J, Christiaans M, Vanrenterghem Y, Donck J, Heeman U, et al. Low Systemic Exposure to Tacrolimus Correlates With Acute Rejection. Transplant Proc (1999) 31(1-2):296–8. doi: 10.1016/S0041-1345(98)01633-9 [DOI] [PubMed] [Google Scholar]
- 6. Charpentier B, Rostaing L, Berthoux F, Lang P, Civati G, Touraine JL, et al. A Three-Arm Study Comparing Immediate Tacrolimus Therapy With Antithymocyte Globulin Induction Therapy Followed by Tacrolimus or Cyclosporine A in Adult Renal Transplant Recipients. Transplantation (2003) 75(6):844–51. doi: 10.1097/01.TP.0000056635.59888.EF [DOI] [PubMed] [Google Scholar]
- 7. Kershner RP, Fitzsimmons WE. Relationship of FK506 Whole Blood Concentrations and Efficacy and Toxicity After Liver and Kidney Transplantation. Transplantation (1996) 62(7):920–6. doi: 10.1097/00007890-199610150-00009 [DOI] [PubMed] [Google Scholar]
- 8. Schiff J, Cole E, Cantarovich M. Therapeutic Monitoring of Calcineurin Inhibitors for the Nephrologist. Clin J Am Soc Nephrol CJASN (2007) 2(2):374–84. doi: 10.2215/CJN.03791106 [DOI] [PubMed] [Google Scholar]
- 9. Undre NA . Pharmacokinetics of Tacrolimus-Based Combination Therapies. Nephrol dialysis Transplant Off Publ Eur Dialysis Transplant Assoc - Eur Renal Assoc (2003) 18 Suppl 1:i12–5. doi: 10.1093/ndt/gfg1029 [DOI] [PubMed] [Google Scholar]
- 10. Opelz G, Döhler B. Effect on Kidney Graft Survival of Reducing or Discontinuing Maintenance Immunosuppression After the First Year Posttransplant. Transplantation (2008) 86(3):371–6. doi: 10.1097/TP.0b013e31817fdddb [DOI] [PubMed] [Google Scholar]
- 11. Yin S, Song T, Jiang Y, Li X, Fan Y, Lin T. Tacrolimus Trough Level at the First Month May Predict Renal Transplantation Outcomes Among Living Chinese Kidney Transplant Patients: A Propensity Score-Matched Analysis. Ther Drug Monit (2019) 41(3):308–16. doi: 10.1097/FTD.0000000000000593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lampen A, Christians U, Guengerich FP, Watkins PB, Kolars JC, Bader A, et al. Metabolism of the Immunosuppressant Tacrolimus in the Small Intestine: Cytochrome P450, Drug Interactions, and Interindividual Variability. Drug Metab Dispos: Biol fate Chem (1995) 23(12):1315–24. [PubMed] [Google Scholar]
- 13. van Gelder T. Within-Patient Variability in Immunosuppressive Drug Exposure as a Predictor for Poor Outcome After Transplantation. Kidney Int (2014) 85(6):1267–8. doi: 10.1038/ki.2013.484 [DOI] [PubMed] [Google Scholar]
- 14. Rodrigo E, Segundo DS, Fernández-Fresnedo G, López-Hoyos M, Benito A, Ruiz JC, et al. Within-Patient Variability in Tacrolimus Blood Levels Predicts Kidney Graft Loss and Donor-Specific Antibody Development. Transplantation (2016) 100(11):2479–85. doi: 10.1097/TP.0000000000001040 [DOI] [PubMed] [Google Scholar]
- 15. Rozen-Zvi B, Schneider S, Lichtenberg S, Green H, Cohen O, Gafter U, et al. Association of the Combination of Time-Weighted Variability of Tacrolimus Blood Level and Exposure to Low Drug Levels With Graft Survival After Kidney Transplantation. Nephrol dialysis Transplant Off Publ Eur Dialysis Transplant Assoc - Eur Renal Assoc (2017) 32(2):393–9. doi: 10.1093/ndt/gfw394 [DOI] [PubMed] [Google Scholar]
- 16. Sapir-Pichhadze R, Wang Y, Famure O, Li Y, Kim SJ. Time-Dependent Variability in Tacrolimus Trough Blood Levels is a Risk Factor for Late Kidney Transplant Failure. Kidney Int (2014) 85(6):1404–11. doi: 10.1038/ki.2013.465 [DOI] [PubMed] [Google Scholar]
- 17. Shuker N, Shuker L, van Rosmalen J, Roodnat JI, Borra LC, Weimar W, et al. A High Intrapatient Variability in Tacrolimus Exposure is Associated With Poor Long-Term Outcome of Kidney Transplantation. Transplant Int Off J Eur Soc Organ Transplant (2016) 29(11):1158–67. doi: 10.1111/tri.12798 [DOI] [PubMed] [Google Scholar]
- 18. Borra LC, Roodnat JI, Kal JA, Mathot RA, Weimar W, van Gelder T. High Within-Patient Variability in the Clearance of Tacrolimus is a Risk Factor for Poor Long-Term Outcome After Kidney Transplantation. Nephrol dialysis Transplant Off Publ Eur Dialysis Transplant Assoc - Eur Renal Assoc (2010) 25(8):2757–63. doi: 10.1093/ndt/gfq096 [DOI] [PubMed] [Google Scholar]
- 19. Mo H, Kim SY, Min S, Han A, Ahn S, Min SK, et al. Association of Intrapatient Variability of Tacrolimus Concentration With Early Deterioration of Chronic Histologic Lesions in Kidney Transplantation. Transplant direct (2019) 5(6):e455. doi: 10.1097/TXD.0000000000000899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vanhove T, Vermeulen T, Annaert P, Lerut E, Kuypers DRJ. High Intrapatient Variability of Tacrolimus Concentrations Predicts Accelerated Progression of Chronic Histologic Lesions in Renal Recipients. Am J Transplant Off J Am Soc Transplant Am Soc Transplant Surgeons (2016) 16(10):2954–63. doi: 10.1111/ajt.13803 [DOI] [PubMed] [Google Scholar]
- 21. Süsal C, Döhler B. Late Intra-Patient Tacrolimus Trough Level Variability as a Major Problem in Kidney Transplantation: A Collaborative Transplant Study Report. Am J Transplant Off J Am Soc Transplant Am Soc Transplant Surgeons (2019) 19(10):2805–13. doi: 10.1111/ajt.15346 [DOI] [PubMed] [Google Scholar]
- 22. Rahamimov R, Tifti-Orbach H, Zingerman B, Green H, Schneider S, Chagnac A, et al. Reduction of Exposure to Tacrolimus Trough Level Variability is Associated With Better Graft Survival After Kidney Transplantation. Eur J Clin Pharmacol (2019) 75(7):951–8. doi: 10.1007/s00228-019-02643-y [DOI] [PubMed] [Google Scholar]
- 23. Bargnoux AS, Sutra T, Badiou S, Kuster N, Dupuy AM, Mourad G, et al. Evaluation of the New Siemens Tacrolimus Assay on the Dimension EXL Integrated Chemistry System Analyzer: Comparison With an Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry Method. Ther Drug Monit (2016) 38(6):808–12. doi: 10.1097/FTD.0000000000000331 [DOI] [PubMed] [Google Scholar]
- 24. Chung BH, Joo YY, Lee J, Kim HD, Kim JI, Moon IS, et al. Impact of ABO Incompatibility on the Development of Acute Antibody-Mediated Rejection in Kidney Transplant Recipients Presensitized to HLA. PloS One (2015) 10(4):e0123638. doi: 10.1371/journal.pone.0123638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Loupy A, Haas M, Roufosse C, Naesens M, Adam B, Afrouzian M, et al. The Banff 2019 Kidney Meeting Report (I): Updates on and Clarification of Criteria for T Cell- and Antibody-Mediated Rejection. Am J Transplant Off J Am Soc Transplant Am Soc Transplant Surgeons (2020) 20(9):2318–31. doi: 10.1111/ajt.15898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jeong HJ. Diagnosis of Renal Transplant Rejection: Banff Classification and Beyond. Kidney Res Clin Pract (2020) 39(1):17–31. doi: 10.23876/j.krcp.20.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lee H, Min JW, Kim JI, Moon IS, Park KH, Yang CW, et al. Clinical Significance of HLA-DQ Antibodies in the Development of Chronic Antibody-Mediated Rejection and Allograft Failure in Kidney Transplant Recipients. Medicine (2016) 95(11):e3094. doi: 10.1097/MD.0000000000003094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vanichanan J, Udomkarnjananun S, Avihingsanon Y, Jutivorakool K. Common Viral Infections in Kidney Transplant Recipients. Kidney Res Clin Pract (2018) 37(4):323–37. doi: 10.23876/j.krcp.18.0063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Park WY, Kang SS, Jin K, Park SB, Choe M, Han S. Long-Term Prognosis of BK Virus-Associated Nephropathy in Kidney Transplant Recipients. Kidney Res Clin Pract (2018) 37(2):167–73. doi: 10.23876/j.krcp.2018.37.2.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Opelz G. CTS Collaborative Transplant Study Newsletter. (2014) 1:5–8. Available at: https://www.ctstransplant.org/public/newsletters/2014/2014-1.html. [Google Scholar]
- 31. Jouve T, Fonrose X, Noble J, Janbon B, Fiard G, Malvezzi P, et al. The TOMATO Study (Tacrolimus Metabolization in Kidney Transplantation): Impact of the Concentration-Dose Ratio on Death-Censored Graft Survival. Transplantation (2020) 104(6):1263–71. doi: 10.1097/TP.0000000000002920 [DOI] [PubMed] [Google Scholar]
- 32. Thölking G, Fortmann C, Koch R, Gerth HU, Pabst D, Pavenstädt H, et al. The Tacrolimus Metabolism Rate Influences Renal Function After Kidney Transplantation. PloS One (2014) 9(10):e111128. doi: 10.1371/journal.pone.0111128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schütte-Nütgen K, Thölking G, Steinke J, Pavenstädt H, Schmidt R, Suwelack B, et al. Fast Tac Metabolizers at Risk ¯ It is Time for a C/D Ratio Calculation. J Clin Med (2019) 8(5):587. doi: 10.3390/jcm8050587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ro H, Min SI, Yang J, Moon KC, Kim YS, Kim SJ, et al. Impact of Tacrolimus Intraindividual Variability and CYP3A5 Genetic Polymorphism on Acute Rejection in Kidney Transplantation. Ther Drug Monit (2012) 34(6):680–5. doi: 10.1097/FTD.0b013e3182731809 [DOI] [PubMed] [Google Scholar]
- 35. Taber DJ, Su Z, Fleming JN, McGillicuddy JW, Posadas-Salas MA, Treiber FA, et al. Tacrolimus Trough Concentration Variability and Disparities in African American Kidney Transplantation. Transplantation (2017) 101(12):2931–8. doi: 10.1097/TP.0000000000001840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wiebe C, Gibson IW, Blydt-Hansen TD, Karpinski M, Ho J, Storsley LJ, et al. Evolution and Clinical Pathologic Correlations of De Novo Donor-Specific HLA Antibody Post Kidney Transplant. Am J Transplant Off J Am Soc Transplant Am Soc Transplant Surgeons (2012) 12(5):1157–67. doi: 10.1111/j.1600-6143.2012.04013.x [DOI] [PubMed] [Google Scholar]
- 37. Everly MJ, Rebellato LM, Haisch CE, Ozawa M, Parker K, Briley KP, et al. Incidence and Impact of De Novo Donor-Specific Alloantibody in Primary Renal Allografts. Transplantation (2013) 95(3):410–7. doi: 10.1097/TP.0b013e31827d62e3 [DOI] [PubMed] [Google Scholar]
- 38. Willicombe M, Brookes P, Sergeant R, Santos-Nunez E, Steggar C, Galliford J, et al. De Novo DQ Donor-Specific Antibodies are Associated With a Significant Risk of Antibody-Mediated Rejection and Transplant Glomerulopathy. Transplantation (2012) 94(2):172–7. doi: 10.1097/TP.0b013e3182543950 [DOI] [PubMed] [Google Scholar]
- 39. Tambur AR. HLA-DQ Antibodies: Are They Real? Are They Relevant? Why So Many? Curr Opin Organ Transplant (2016) 21(4):441–6. doi: 10.1097/MOT.0000000000000325 [DOI] [PubMed] [Google Scholar]
- 40. Kim EJ, Kim SJ, Huh KH, Kim BS, Kim MS, Kim SI, et al. Clinical Significance of Tacrolimus Intra-Patient Variability on Kidney Transplant Outcomes According to Pre-Transplant Immunological Risk. Sci Rep (2021) 11(1):12114. doi: 10.1038/s41598-021-91630-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bekersky I, Dressler D, Mekki QA. Effect of Low- and High-Fat Meals on Tacrolimus Absorption Following 5 Mg Single Oral Doses to Healthy Human Subjects. J Clin Pharmacol (2001) 41(2):176–82. doi: 10.1177/00912700122009999 [DOI] [PubMed] [Google Scholar]
- 42. Andrews LM, Hesselink DA, van Schaik RHN, van Gelder T, de Fijter JW, Lloberas N, et al. A Population Pharmacokinetic Model to Predict the Individual Starting Dose of Tacrolimus in Adult Renal Transplant Recipients. Br J Clin Pharmacol (2019) 85(3):601–15. doi: 10.1111/bcp.13838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wu MJ, Cheng CY, Chen CH, Wu WP, Cheng CH, Yu DM, et al. Lower Variability of Tacrolimus Trough Concentration After Conversion From Prograf to Advagraf in Stable Kidney Transplant Recipients. Transplantation (2011) 92(6):648–52. doi: 10.1097/TP.0b013e3182292426 [DOI] [PubMed] [Google Scholar]
- 44. Christians U, Jacobsen W, Benet LZ, Lampen A. Mechanisms of Clinically Relevant Drug Interactions Associated With Tacrolimus. Clin Pharmacokinet (2002) 41(11):813–51. doi: 10.2165/00003088-200241110-00003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.