Abstract
After more than a year with COVID-19, it becomes increasingly clear that certain variants of concern have the potential to be game changers, determining the future of our aviation. These variants pose significant health threats and possibly undermine ongoing vaccination efforts. Recent research showed that flight bans on the initial SARS-CoV-2 outbreak in January 2020 were implemented too late and therefore, turned out to be largely ineffective, enabling a swift turn into a fully-blown pandemic. In this study, we investigate the following question: How effective were existing flight bans against the newly emerged variants of concern? In other words: Do airlines and countries happen to repeat the same mistake again? We analyze the spread of the three most prevalent variants of concern right now: B.1.1.7 (known as the UK variant), B.1.351 (known as the South African variant), and P.1 (known as the Brazilian variant). We find that many countries, again, implemented flights bans once the mutated virus had enough time to be imported via air transportation. To support our empirical analysis further, we designed and implemented a compartmental network spreading model on top of worldwide flight data for the years 2020 and 2021. We observe that the model predictions are rather accurate and confirm our findings. Overall, we hope that our study encourages air transportation stakeholders and policy makers to avoid repeating earlier mistakes in the future, with the ultimate goal to overcome COVID-19 entirely.
Keywords: Air transportation, COVID-19, Variants of concern
1. Introduction
The corona virus disease, COVID-19, has hit our society hard, with more than 150 million cases worldwide reported and more than three million fatalities directly related to the disease. Air transportation is an essential driver for turning an epidemic outbreak into a pandemic, given that it enables crossing extreme distances in a short time. This has been known for several years; based on data-driven research for a collection of earlier diseases (Brockmann and Helbing, 2013). Such diseases include, for instance, the severe acute respiratory syndrome (SARS) (Likhacheva, 2006, Bowen and Laroe, 2006) in 2003, the Middle East respiratory syndrome coronavirus (MERS-Cov) (Zaki et al., 2012, Gardner et al., 2016) in 2012, and an Ebola outbreak (Bogoch et al., 2015, Read et al., 2014) in 2014. These three examples share a commonality: They were contained before being able to spread at planet-scale, by an efficient combination of strict flight restrictions and local health checks. For COVID-19, the critical time window for containing the spread from the virus origin was missed (Lau et al., 2020, Nakamura and Managi, 2020, Sun et al., 2021a), spurring the development of one of the worst pandemics in recent human history.
Given the scale of COVID-19 as a year-long pandemic, thousands of SARS-CoV-2 variants have emerged over time. While mutations are common and often lead to rather insignificant changes of the virus’ epidemiological characteristics, some of the variants have received increasing importance, due to their inherent potential of faster spread, more severe associated health risks, or indications for a resistance against existing vaccines. These variants are generally referred to as variants of concern in the literature. The most noteworthy variants of concern are listed below; for a detailed survey on SARS-CoV-2 variants see Mahase (2021):
-
1.
Lineage B.1.1.7 (commonly referred to as the UK variant): This variant has been first reported in the United Kingdom in September 2020 and led to a significant increase in the public awareness of mutations. Given modifications in the receptor-binding domain, epidemiologists argued that this variant is easier to spread and more lethal (Volz et al., 2021).
-
2.
Lineage B.1.351 (commonly referred to as the South Africa variant): This variant has been first reported in South Africa in October 2020. It causes more severe illness and is particularly prevalent across younger and healthier people. Lineage B.1.351 has been frequently used in the literature as an example for a variant that might evade vaccination (Singh et al., 2021).
-
3.
Lineage P.1 (commonly referred to as the Brazil variant): This variant has been first reported in Manaus, Brazil in December 2020. The fact that Brazil is one of the hardest-hit countries by COVID-19 can partially be attributed to the emergence and spread of this variant. Particularly, P.1 is believed to be significantly easier to transmit than other variants (Faria et al., 2021).
In this study, we aim to evaluate the reactions of countries towards these emerging variants of concern. Especially, we are interested in answering the question of whether countries have possibly reacted too late with flight bans; as it seems to have occurred with uncoordinated border closures during the early phases of COVID-19 (Sun et al., 2021a); see Sun et al. (2021b) for a recent survey of the impact SARS-CoV-2 had on air transportation. Table 1 reports selected countries’ reactions towards the emergence of SARS-CoV-2 mutations. We will show that many of these flight ban announcements can be directly linked to the first reported cases in the announcing/banning countries.
Table 1.
Overview on reported country actions regarding the spread variants of concern.
| Date | Country | Action |
|---|---|---|
| 12/20/2020 | Netherlands | Banned flights with Great Britain for B.1.1.7. |
| 12/20/2020 | Canada | Restricted travel from Great Britain due to COVID lineage. |
| 12/20/2020 | Belgium | Suspended flight and trains from Great Britain over B.1.1.7. |
| 12/21/2020 | Saudi Arabia | Suspended international flights over B.1.1.7. |
| 12/21/2020 | Germany | Banned flights from South Africa over B.1.351. |
| 12/29/2020 | Japan | Suspended entry of non-resident foreigners due to B.1.1.7. |
| 1/5/2021 | Vietnam | Suspended flights from Britain over B.1.1.7 and B.1.351. |
| 1/7/2021 | Great Britain | Banned travel from 11 countries due to B.1.351. |
| 1/14/2021 | Great Britain | Imposed travel ban on Brazil and other South American countries due to P.1. |
| 1/25/2021 | United States | Banned travelers from South Africa and Brazil for B.1.351 and P.1. |
| 2/8/2021 | Rwanda | Suspended its flights to South Africa and Zimbabwe over B.1.351. |
| 4/13/2021 | France | Suspended all Brazil flights due to P.1. |
| 4/15/202 | Russia | Extended suspension of Great Britain flights due to B.1.1.7. |
This study provides an in-depth, data-driven discussion of the three major lineages prevalent first: B.1.1.7, B.1.351, and P.1. A comparative assessment of these three lineages is interesting specifically, given their different time of initial reports (September 2020, October 2020, and December 2020, respectively) and emerging locations on three distinct continents. Implicit to our analysis is the question whether countries have learned from the earlier outbreaks. We create a network-based Susceptible–Exposed–Infectious–Recovered (SEIR) model, inspired by the GLobal Epidemic and Mobility Model (Balcan et al., 2009), using flight data for the years 2020 and 2021. We show that the predictions of the model are rather accurate for the spread of the variants of concern. Based on the findings in our study, we strongly encourage policy makers and operators to use the existing modeling tools, combined with up-to-date data, in order to avert further damage from our society and in an attempt to not make history repeat itself further. This is in particular relevant, and timely, given the new variants emerging in India and their significant impacts in India and around the world.
The remainder of this study is structured as follows. Section 2 introduces the network-based SEIR model. Section 3 provides a preliminary data-driven analysis of the three major active SARS-CoV-2 lineages and reports the results of our simulations. Section 4 concludes this study and provides a set of recommendations for future work.
2. Model
In this section, we first revisit a classical compartmental Susceptible–Exposed–Infectious–Recovered (SEIR) model and then extend it to a network structure, following the ideas behind the GLobal Epidemic and Mobility (GLEaM) Model (Balcan et al., 2009). The SEIR model was designed for modeling the transmission of diseases between humans (Kermack and McKendrick, 1927). The classical variant manages four compartments, distinguishing individuals into the set of: Susceptible, Exposed, Infectious, and Recovered/Removed. Initially, all individuals are set to be susceptible. Individuals move from the Susceptible compartment to the Exposed compartment at the transmission rate (). The exposed individuals, who are infected but not yet infectious, go to the infectious stage with a rate inversely proportional to the mean latency period , and the infectious individuals proceed to the Recovered/Removed compartment with a rate inversely proportional to the mean infectious period . Many extensions of the SEIR model have been developed, especially considering COVID-19. With the global scale of our study, facing a) the lack of more detailed epidemiological data and b) limited computational resources, we have decided to choose the elementary model over more complex ones. Future work could investigate more results on extended models, in presence of richer region-based epidemiological data. The evolutionary dynamics of the four epidemiological states are described by the following set of equations:
| (1) |
| (2) |
| (3) |
| (4) |
The epidemiological status of all individuals is initially set to susceptible, where all individuals can contract the virus through contacts with individuals in the infectious compartment and proceed to the exposed compartment at the transmission rate ; we set as proposed by Zou et al. (2020) for COVID-19. The exposed individuals proceed to the infectious stage with a rate inversely proportional to the mean latency period ; we set as proposed by Chinazzi et al. (2020) for COVID-19. The infectious individuals proceed to the recovered/removed compartment with a rate inversely proportional to the mean infectious period ; we set as proposed by Chinazzi et al. (2020) for COVID-19. The adaptation of SEIR model into GLEaM model requires two more parameters, and . The probability of an infected individual to become asymptomatic is (Zou et al., 2020), and its probability to travel and further spread the disease is (Balcan et al., 2009). In this SEIR model, the population of a vertex at time is the sum of individuals in all four compartments, i.e., . The transmission rate reflects the speed of individuals in proceeding to and represents the density of infected individuals in the population. The expected cumulative number of confirmed cases of a vertex at time is the sum of the infectious and recovered, i.e., , which indicates the total number of cases that have been diagnosed up to a certain point in time regardless of whether they have recovered.
This model is extended to a network representation as follows. Given the SEIR model for a single node, a generic epidemic network is considered, which is composed of a set of populated vertices and a set of transportation edges connecting the vertices. Intuitively, the set represents the movement of population with the time unit of a single day. The weight of an edge represents the passenger flow per day. The flow of a specific compartment traveling from vertex to vertex at time is given by:
| (5) |
where expression denotes the population of compartment in vertex at time , , and the time-independent flow is the flow traveling from vertex to vertex per day, which satisfies , with .
We refer to GLEaM (Balcan et al., 2010) and adapt the following details. The initial populations are created based on multiple airport regions (Sun et al., 2017), where larger airports aggregated surrounding smaller airports (measured by the total number of flights). This process is necessary in order to make the computation tractable; as too large networks are rather time-consuming to simulate. The locations of the 3,227 subpopulations are reported in Fig. 1. With airports aggregated into subpopulations we compute the flights between subpopulations as follows. The number of flights is obtained from Flightradar24. The commuting flow data among subpopulations is calculated through gravity law, calibrated by the real-world data of countries in five continents (Balcan et al., 2010). The commuting flow between subpopulation and per day is given by:
| (6) |
where is the distance (in km) between the two major airports of subpopulations and . The gravity law has four free parameters: the exponents and , the inverse characteristic distance and the proportionality constant . A multivariate regression analysis is applied to obtain the values of the parameters that better fit the data as well as an estimation of their statistical significance. The values estimated for these parameters are reported in Table 2 along with their p-values and the regression coefficients (Balcan et al., 2010).
Fig. 1.
Overview on the 3,227 subpopulations. The top 20 largest subpopulations are highlighted by the IATA code of their largest airport. For airport names of the subpopulations, please refer to the notes in Appendix.
Table 2.
Exponents of gravity law as obtained by applying a multivariate analysis to global commuting data.
| d | Parameter | Estimation | Standard Error | -value | |
|---|---|---|---|---|---|
| 0.46 | 0.01 | 0.7972 | |||
| 0.64 | 0.01 | ||||
| 0.0122 | 0.0002 | ||||
| 0.35 | 0.06 | 0.5369 | |||
| 0.37 | 0.06 | ||||
3. Results
We provide a brief overview on three major SARS-CoV-2 lineages first: B.1.1.7, B.1.351, and P.1. While there are other lineages emerging, these three variants of concern are most relevant since they are prevalent for several months now and have been confirmed in multiple countries. Table 3 provides a comparison of the initial spreading patterns over time for three selected variants of concern: B.1.1.7 (left), B.1.351 (center), and P.1 (right). The first row of each sub-table reports the first observation of a mutation inside the (presumable) source country. The three lineages were discovered in September 2020, October 2020, and December 2020, respectively. After the initial discovery and verification of the first variant, it took one to two months until the variant was confirmed by sequencing in another country: for B.1.1.7 it took 55 days to reach Denmark (DNK), for B.1.351 it took 24 days to reach Mozambique (MOZ), and for P.1 it took 26 days to reach South Korea (KOR). Additional countries are reached quickly after the first one, except for B.1.351, where a significant spread is observed after another month, where the variant reportedly reached China (CHN) and Great Britain (GBR). While the actual country order is different for the three examples in Table 3, there exists similarities among the three variants, concerning the temporal evolution.
Table 3.
Evolution of first reported cases for three selected variants of concern: B.1.1.7 (left), B.1.351 (center), and P.1 (right). Each row in the three subtables corresponds to the first confirmation of a variant in a country. Rows as sorted by days since onset; the top 20 countries are shown for each of the three variants.
| B.1.1.7 (UK lineage) |
B.1.351 (South Africa lineage) |
P.1 (Brazil lineage) |
||||||
|---|---|---|---|---|---|---|---|---|
| Earliest report | Country | Days | Earliest report | Country | Days | Earliest report | Country | Days |
| 9/20/2020 | GBR | 0 | 10/8/2020 | ZAF | 0 | 12/15/2020 | BRA | 0 |
| 11/14/2020 | DNK | 55 | 11/1/2020 | MOZ | 24 | 1/10/2021 | KOR | 26 |
| 11/16/2020 | ARE | 57 | 12/8/2020 | CHN | 61 | 1/12/2021 | JPN | 28 |
| 11/27/2020 | DEU | 68 | 12/12/2020 | GBR | 65 | 1/12/2021 | FRO | 28 |
| 12/4/2020 | CHN | 75 | 12/14/2020 | CHE | 67 | 1/17/2021 | ITA | 33 |
| 12/17/2020 | LCA | 88 | 12/15/2020 | KEN | 68 | 1/22/2021 | DEU | 38 |
| 12/18/2020 | IND | 89 | 12/17/2020 | BWA | 70 | 1/22/2021 | FRA | 38 |
| 12/19/2020 | FRA | 90 | 12/19/2020 | FIN | 72 | 1/25/2021 | USA | 41 |
| 12/19/2020 | MLT | 90 | 12/19/2020 | JPN | 72 | 1/29/2021 | NLD | 45 |
| 12/20/2020 | AUS | 91 | 12/21/2020 | DEU | 74 | 1/30/2021 | NLD | 45 |
| 12/20/2020 | BEL | 91 | 12/22/2020 | AUS | 75 | 1/31/2021 | BEL | 47 |
| 12/20/2020 | ITA | 91 | 12/22/2020 | NLD | 75 | 2/3/2021 | TUR | 50 |
| 12/20/2020 | NLD | 91 | 12/24/2020 | ESP | 77 | 2/5/2021 | PER | 52 |
| 12/20/2020 | SWE | 91 | 12/26/2020 | KOR | 79 | 2/5/2021 | ESP | 52 |
| 12/20/2020 | CYP | 91 | 12/26/2020 | ARE | 79 | 2/5/2021 | IND | 52 |
| 12/20/2020 | URY | 91 | 12/30/2020 | ZMB | 83 | 2/7/2021 | CAN | 54 |
| 12/21/2020 | ISL | 92 | 12/31/2020 | FRA | 84 | 2/8/2021 | ARG | 55 |
| 12/21/2020 | LBN | 92 | 12/31/2020 | FRA | 84 | 2/9/2021 | CHE | 56 |
| 12/21/2020 | BRA | 92 | 1/3/2021 | SWE | 87 | 2/10/2021 | PRT | 57 |
| 12/21/2020 | JAM | 92 | 1/4/2021 | AUT | 88 | 2/10/2021 | VEN | 57 |
Data source: https://cov-lineages.org.
The relationship between the spreading of the three variants and the international flights is analyzed next. Specifically, based on flight data for the years 2020 and 2021, we have created weekly country networks (Wandelt and Sun, 2015), where nodes are countries and two countries are connected if there is at least one direct flight between the two countries. The number of flights between two countries is obtained by aggregation over all participating airport pairs. We have 0/1-normalized the number of flights over time to perform a cross comparison among countries. The results are shown in Fig. 2 to Fig. 4; for each lineage the evolution of the normalized number of flights to the top 10 earliest virus destinations from Table 3 are reported.
Fig. 2.
Evolution of the normalized number of flights from GBR to the ten countries that reported the first cases of Lineage B.1.1.7. The shaded area highlights the time period between the first reported case in GBR (start of the interval) and the first reported case in the destination country (end of the interval). Flight restrictions come very late; often after the first reported case in the destination.
Fig. 4.
Evolution of the normalized number of flights from BRA to the ten countries that reported the first cases of Lineage P.1. The shaded area highlights the time period between the first reported case in BRA (start of the interval) and the first reported case in the destination country (end of the interval). Stable number of flights or absence of direct connections (for KOR, JPN, and FRO) is observed.
Fig. 2 shows the results for Lineage B.1.1.7. Here, Denmark (DNK) was the first-hit country, 55 days after the initial outbreak in Great Britain. It can be observed that the number of flights between Great Britain and Denmark (DNK) reached a local maximum just at the time when B.1.1.7 emerged in Great Britain, and the number of flights was reduced significantly only just before the first case was reported in Denmark (DNK). Presumably, at that time it was already too late for cutting ties with Great Britain.1 Similar observations can be made for most of the other nine destination countries: Flight connections were often reduced rather late; close to or after the first reported local infection. Notable exceptions to this pattern include Malta (MLT), France (FRA), and Australia (AUS). The former two countries had gradually reduced their flight frequencies with Great Britain. Australia (AUS) is a special case: Throughout the pandemic, Australia followed a rather strict isolation strategy; reducing the incoming flights to a minimum. Yet, Australian citizens could return to Australia, once they were able to catch a rare flight ticket due to the daily quota for the number of Australians allowed to fly back home. Presumably, one of these passengers carried the mutation to Australia.
Fig. 3 reports the results for Lineage B.1.351. The results are slightly different compared to B.1.1.7. We still find several countries with insufficient flight connections to South Africa (ZAF) after the confirmation of the first B.1.351 case in ZAF; these countries include China (CHN), Great Britain (GBR), and Switzerland (CHE). Other countries even re-opened their flight connectivity in the critical time window, e.g., Mozambique (MOZ), Botswana (BWA), Kenya (KEN), Germany (DEU), and partially Australia (AUS). The remaining two countries, Finland (FIN) and Japan (JPN), did not have direct flight connections to South Africa according to our data, which suggests the possibility of transmittal over a third country, e.g., a transitive link. Such an indirect transmission is a real threat, particularly with a larger number of cases emerging and passengers desperate to travel to their destinations.
Fig. 3.
Evolution of the normalized number of flights from ZAF to the ten countries that reported the first cases of Lineage B.1.351. The shaded area highlights the time period between the first reported case in ZAF (start of the interval) and the first reported case in the destination country (end of the interval). Surprisingly, flight bans were often being relaxed in the critical period of spreading.
Fig. 4 reports the results for Lineage P.1. Three countries did not have direct flights with Brazil, according to our data: Korea (KOR), Japan (JPN), and the Faroe Islands (FRO). It is interesting to note that these countries were just the first to confirm the new variant. This can possibly be explained by transitive flight links, the absence of regular screening for the emerging type of variant, and possibly a much earlier presence of P.1 in Brazil. Otherwise, from a purely epidemiological perspective, it seems unlikely that these countries recognized the variant first. Similar observations can be made for Italy (ITA) and Belgium (BEL). For the remaining countries, we cannot identify clear flight bans during the major period of interest.
Next, by investigating different perspectives, time points, and parameters, we aim to better understand the spreading process, using real flight data throughout the years 2020 and 2021. Our major indicator of interest is the expected arrival time for the disease in another country, i.e., the time that is required until the first international case could be recorded. Given that the underlying simulations are non-deterministic by design, we perform all experiments 100 times and aggregate the results as described in individual experiments below.
In a first experiment, we are interested in the change of international propagation time, starting from the top 25 countries based on population. The subpopulation with an initial case is chosen randomly among all subpopulations of a country. Fig. 5 reports the results for five different periods of time: January 2020, April 2020, July 2020, October 2020, and January 2021. For each of the five periods, we report the expected arrival times with the source being one of the 25 countries. Overall, it can be observed that the expected time is mostly between 25 and 60 days, which is consistent with the data obtained for the three prevalent lineages (see Table 3 above). This confirms that the parameters of our model, which were obtained based on the original SARS-CoV-2, are applicable to the analysis of interest. A second observation is that the expected propagation time does not change significantly for most countries, when cross-comparing these five different time periods. For April 2020, the propagation time increases by 10–15 days, but is gradually reduced for the later time periods. Another important observation concerns the Democratic Republic of Congo (COD), which has a significantly longer propagation time towards other countries. One explanation for this phenomenon is that COD has, despite its large population, less-frequent air travel connections to other countries. Moreover, the subpopulation of COD has strong influence on the propagation time. An initial outbreak at the capital Kinshasa will be significantly faster propagated internationally (within 25 days), compared to outbreaks in more rural areas.
Fig. 5.
Comparison of spreading times from one of the top 25 countries with the largest populations towards any other country. Five points of time are compared from top to bottom: January 2020, April 2020, July 2020, October 2020, and January 2021. For each origin country along the -axis, the bar shows the frequency distribution of days until the first international infection is recorded.
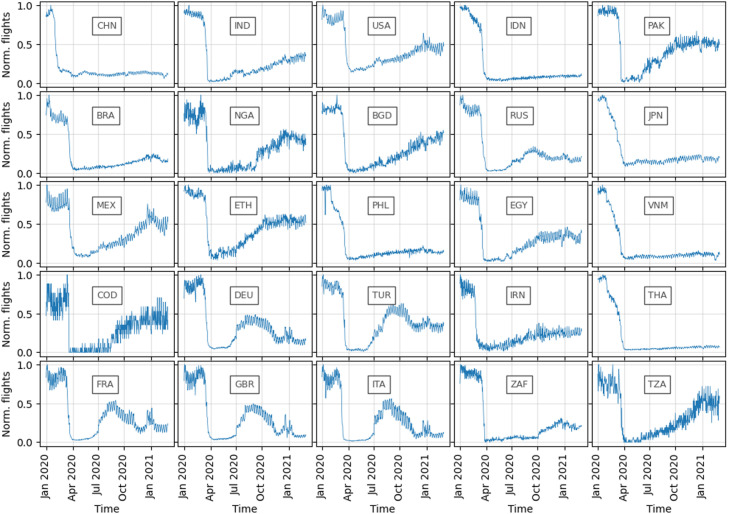
In the following, we aim to better understand the shifts in expected propagation time, by investigating the flight restrictions implemented in the top 25 countries. Fig. 6 reports the evolution of the daily number of international flights from the 25 selected countries to other countries. The curves are normalized relatively to the maximum number of flights for each country, to allow for cross-comparison. We can identify a wide range of patterns, which deserve further explanation; most country reactions can be categorized into three groups. The first group of countries have performed extensive international flight bans in early 2020, without obvious signs of recovery; these countries include most Asian countries: China (CHN), Indonesia (IDN), Japan (JPN), the Philippines (PHL), Vietnam (VNM), and Thailand (THA). A second group of countries has – after a strict flight ban – quickly started to recover their international flights selectively throughout the year 2020, without recognizable setback; these countries include India (IND), the United States (USA), Pakistan (PAK), Nigeria (NGA), Bangladesh (BGD), Egypt (EGY), the Democratic Republic of the Congo (COD), and Tanzania (TZA). The remaining countries often underwent a setback during their recovery phase, leading to a recovery peak in summer 2020, followed by another valley; these countries include: Brazil (BRA), Germany (DEU), Turkey (TUR), France (FRA), Great Britain (GBR), and Italy (ITA).
Fig. 6.
Evolution of the number of daily international flights for the top 25 countries since January 2020. The flights are normalized for better comparison between countries. The text label in the center of each subplot represents the ISO3 country code of the origin country.
With the next experiment, we aim to answer the following question: How much do international flight restrictions postpone the arrival time of a COVID-19-like virus? For this experiment, we start with the flight data for January 2020 as a baseline, which consists of largely unaffected international flights. We multiply this baseline with a flight reduction factor , which simulates a globally-orchestrated response to COVID-19, i.e., affects all international travel links at the same time. We perform a sensitivity analysis on variable , ranging from 0.00 (equal to not cutting any flights) and 0.99 (equal to cutting all flights by 99%). The results are shown in Fig. 7. The influence of is rather small for a wide range of values. Only once reaches values close to 0.9, a significant increase in the expected propagation time can be observed. For instance, when reaching , the time for propagation is roughly doubled. Nevertheless, it should be emphasized again, that this assumes that all international air transport worldwide would be reduced by 95%, for all country pairs; a rather extreme assumption, given the experiences in the year 2020. These results show that half-hearted flight restrictions are just postponing the epidemic spread, and only for a short period of time. To solve the challenges surrounding emerging variants of concern, policy makers need to find better strategies. China could be used as a leading example here. With the establishment of strict and controlled entry quarantine and screenings, it has been able to get the COVID-19 spread effectively under control, while allowing a limited amount of travel exchanges with other countries; with flight bans of around 90%.
Fig. 7.
Simulation results for the effect of different flight reductions on the number of days until the first international case. The country of origin is set as one of the top 25 largest countries, as reported in each subplot. The -axis represents the flight reduction from 0.0 (baseline before COVID-19) and 1.0 (no international flights).
In the following we explore a different facet of the ongoing flight bans: We are interested in the change of first-ranked international destinations when disease outbreaks are simulated starting from the top 25 countries based on population. For each of the 25 countries, we chose random subpopulations and simulate the spreading process until first ten other countries have received their first case. We compute an aggregated country ranking over these random instances and obtain one top 10 ranking for each origin country. Intuitively, this measure tells us which countries are most threatened, once an outbreak occurs at one of the top 25 countries. The experiments are executed for two different periods of time: January 2020 and January 2021, i.e., with a one-year gap. The results are reported in Fig. 8. For each of the 25 origin countries, we visualize one map as a subplot. Intuitively, this visualization helps to see how much different the underlying epidemiological situation is one year apart. In each subplot, we visualize the change in the top 10 rankings with three colors. First, countries highlighted in black are top 10 destinations in January 2020 and January 2021, i.e., their susceptibility has not changed throughout COVID-19. Countries colored in green have left the top 10 ranking in January 2021, most likely due to significant flight restrictions. Finally, red countries are newcomers to the top 10, which become critical to monitor in January 2021. The top 10 ranked countries are usually spatially close, often located on the same continent. Moreover, it is interesting to note that China remains to have a critical role in receiving first infections. In addition, European countries seem to be over-represented here.
Fig. 8.
Comparisons of early spreading destinations with the top 25 largest countries as source, comparing January 2021 with January 2020. Countries are highlighted by color: countries being ranked among the first 10 in both scenarios (black), countries among the first 10 in January 2020 only (green), and countries among the first 10 in January 2021 only (red). The number counts the black-highlighted countries. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
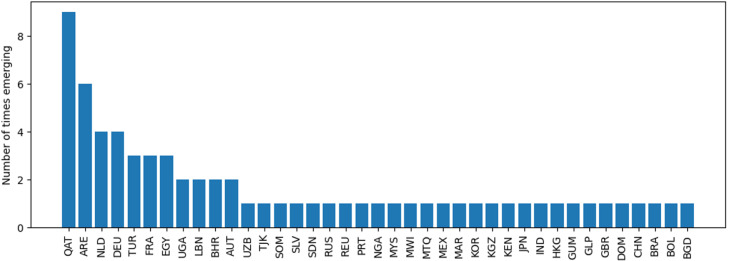
To better understand the changes in the top 10-ranked countries, we have computed the frequencies of countries being newcomers in January 2021. The results are reported in Fig. 9. Two countries stand out in our experiments: Qatar (QAT) and the United Arab Emirates (ARE). These countries are newly emerging in 9/25 and 6/25 cases, respectively. They are followed by two European countries, the Netherlands (NLD) and Germany (DEU). It is those countries that – from a simulation perspective – are most likely to be hit unexpectedly by emerging transmissions in 2021, compared to the analysis performed in the year 2020. Accordingly, the drivers for these countries appearing early in transmissions should be better understood. We report additional results for Qatar (QAT) and the United Arab Emirates (ARE) below. Fig. 10 reports the normalized number of international flights per day for QAT on the left and ARE on the right. It can be seen that both countries have steadily recovered their flight bans, reaching 60% and 40% of their international flight baseline, respectively. This is in line with several other countries which we reported in Fig. 6. The question remains why these two countries stand out from others. In Fig. 11, we report the number of destination countries that Qatar (QAT) and the United Arab Emirates (ARE) are connected to over time, respectively. Both countries have almost fully recovered their international markets, with levels up to 80%, presumably to remain international hub status. From a passenger perspective, these hubs were important for crossing extreme distances, since such hubs lead to economies of scale. From an epidemiological perspective, however, hubs open the opportunity for virus mutations to quickly spread throughout the world. This highlights the ambivalent role of air transportation throughout COVID-19. Only the existence of efficient long-distance connections enables the emergence of a pandemic at the scales of COVID-19.
Fig. 9.
Frequency of countries which are emergent early destinations comparing January 2021 with January 2020.
Fig. 10.
Normalized number of international flights per day for Qatar (left) and the United Arab Emirates (right).
Fig. 11.
Number of reachable destination countries per day for Qatar (left) and the United Arab Emirates (right).
4. Discussion
This study investigated the relationship between the emerging variants of SARS-CoV-2 concerns and air travel reductions in the years 2020 and 2021. To the best of our knowledge, this is the first study to specifically target the spread of such variants with recent air travel data. As part of this study, we have mainly investigated the evolution of three prevalent lineages: B.1.1.7, B.1.351, and P.1. In addition, we implemented a compartmental disease-spreading model with the goal to confirm the observations made for the selected variants of concern on real data.
As of April 2021, a new variant of concern is found to be emerging in India, labeled Lineage B.1.617. Several countries have announced the introduction of flight bans towards the end of April; yet again, after having identified the first cases of the known mutation in sequence screenings among their own populations. The evolution of the total number of cases in India is reported in Fig. 12. It is apparent that the number of confirmed cases is in an exponential growth phase since the end of March. Such a tremendous number of infections per day does provide a hotbed for the emergence of new variants of concern. Nevertheless, other countries have waited almost one month, again, until the predictable event happened. To discuss a specific example, the first verified case reported in Belgium was associated with a group of Indian students who entered Europe via an international flight to Paris and then proceeded to Belgium by bus.2 Accordingly, it seems like history does repeat itself. We hope that our study contributes to a better understanding of the dynamics coming with variants of concerns and that air transportation stakeholders and policy makers aim to make decisions within the short, critical time window. A global pandemic needs global, well-orchestrated responses.
Fig. 12.
Number of daily confirmed COVID-19 cases for India.
Data source: https://ourworldindata.org.
4.1. Major findings and policy implications
The experiments and analyses performed in our study lead to a few key insights, which we discuss in detail below. First, our data-driven analysis revealed that countries did react too late when facing emerging variants of concern. This holds for all three investigated lineages; and seems to also hold for the newly emerging Lineage B.1.617. It is important to reiterate an important insight from epidemiological studies: Air transportation restrictions only help to avoid the initial spread. Without a travel connection, the virus is not able to cross long distances in a short time. This holds for the original SARS-CoV-2, as well as for current and future variants.
Second, our analysis revealed that some countries did not have direct flight connections with the (presumable) origin of variants of concern. There are several plausible explanations. On one hand, information about variants of concern relies heavily on accurate data and early sequencing at high coverage. Without such information, it is next to impossible to track the dynamics of variants. On the other hand, the effect of transitivity is often neglected, as an indirect source for infections. This can be seen when countries announce flight bans on specific origin countries (e.g., specifically to Brazil or India). The problem is that air transportation is a global system, built with the goal of efficiency and high connectivity. As the example with Indian cases showed; as long as there is a feasible connection via third countries, there will be passengers that are willing to choose these options. Similar effects could be observed when China suddenly closed its international borders in February 2020, canceling about 90% of all travel options. Chinese citizens literally flew across the planet, having tickets for multiple backups. While this sounds like an extreme event, it needs to be understood that a single infected passenger is sufficient to carry a mutation from one country to another. Accordingly, there needs to be a strategic view on how to allocate travel bans effectively. We also found that Qatar and the United Arab Emirates seem to have increasingly strengthened their role as international hubs during the pandemic. Such a development requires careful attention from policy makers and researchers. One might argue that the United Arab Emirates have one of the highest vaccination rates in the world (about 50% as of April 2021) and, therefore, are possibly less susceptible to new infections. Nevertheless, the problems here are transit passengers. Economic benefits for either country should not be put on top of social harm for the global society.
Third, our simulations with a networked SEIR compartmental model showed that partial flight reductions are not as effective as one might expect. The major reason is that once a country hits an exponential growth phase, variants are produced at high rates. And the more variants are produced with random mutations, the more likely that there is a variant of concern emerging. Moreover, with an increasing size of an infected population, the likelihood for a random traveler to choose air transportation increases significantly. Therefore, half-heartedly reducing flight capacities with specific countries will only postpone the arrival time of a new variant in a country; partially due to the issue of transitivity discussed above. Notably, the predicted international arrival time of our model, based on data for the original COVID-19, is rather accurate for all three variants of concern. We conclude that – unless disease parameters change significantly – the expected arrival time window is less than one month.
Finally, and possibly most important, the ongoing vaccination efforts are being seen as a road to release and freedom. This might be only true temporally. With a larger disparity of vaccination and infections in different parts of the world, it becomes increasingly likely that a so-called escape mutation (Kupferschmidt, 2021) emerges, which means that proven vaccines may, at some point, become ineffective or need an update. Given the extraordinary ongoing efforts to perform a worldwide inoculation, the least one would hope for is another round of vaccinations just because history repeated itself again.
4.2. Limitations and future work
Our study is conducted based on available data for diseases and air travel. Naturally, the findings in this study are directly connected to the quality of the underlying data. For instance, the flight data we used does not provide information about load factors and it does not distinguish passenger aircraft temporally turned into cargo aircraft. As a consequence, the connectivity we measured may not necessarily represent the connectivity for passenger mobility alone. Future research with better data would overcome these limitations. Particularly, the availability of (global) air passenger data, would allow to perform experiments on the actual flow of people and lead to more specific insights, contrary to the approach based on a proxy like the number of flights. Despite extraordinary contributions from various scientific disciplines, the processes underlying the infections are not fully understood. The reported occurrences of variants and their time-line are subject to country-specific epidemiological measures, e.g., including the testing strategy, tightness of checking international flights, sequencing efforts, and many more. These variables are largely hidden; and we are not aware of globally available and consistent data regarding these issues. Table 5 in the Appendix provides an overview on travel entry restrictions for selected countries and dates. As it can be seen, these variables are subject to temporal evolution and often-unclear degree of implementation and verification — despite official policies for specific countries. Future studies on this issue could build up on this limitation, by performing richer experiments, once more detailed and reliable data is available. If a country requires a negative PCR test, the likelihood of importing a variants could be significantly reduced, assuming that the tests are actually checked upon arrival. Finally, future studies could elaborate explicitly on selective counter measures and how to find a sustainable trade-off between restrictions of movement and up-keeping of economic ties.
Table 5.
Travel entry restrictions for selected countries shown in Fig. 5. Abbreviations: BHR = Ban from high-risk regions, CLOS = Closed, QHR = Quarantine from high-risk regions, and SCREEN = Screening.
| Code | January 2020 | April 2020 | July 2020 | October 2020 | January 2021 |
|---|---|---|---|---|---|
| BGD | None | BHR | BHR | BHR | BHR |
| BRA | None | CLOS | CLOS | SCREEN | SCREEN |
| CHN | None | BHR | BHR | BHR | BHR |
| COD | None | CLOS | CLOS | SCREEN | SCREEN |
| EGY | None | CLOS | None | SCREEN | SCREEN |
| ETH | None | BHR | BHR | QHR | QHR |
| FRA | None | BHR | BHR | BHR | BHR |
| DEU | None | CLOS | BHR | BHR | BHR |
| IND | None | CLOS | CLOS | CLOS | BHR |
| IDN | None | CLOS | CLOS | BHR | BHR |
| IRN | None | None | None | BHR | BHR |
| ITA | None | BHR | BHR | BHR | BHR |
| JPN | None | QHR | BHR | SCREEN | CLOS |
| MEX | None | BHR | BHR | SCREEN | SCREEN |
| NGA | None | CLOS | CLOS | QHR | QHR |
| PAK | None | CLOS | QHR | QHR | QHR |
| PHL | None | CLOS | CLOS | QHR | QHR |
| RUS | None | CLOS | CLOS | BHR | BHR |
| ZAF | None | CLOS | CLOS | BHR | SCREEN |
| TZA | None | BHR | None | SCREEN | SCREEN |
| THA | None | CLOS | BHR | BHR | QHR |
| TUR | None | CLOS | BHR | SCREEN | QHR |
| GBR | None | None | QHR | QHR | BHR |
| USA | None | BHR | BHR | BHR | BHR |
| VNM | None | CLOS | BHR | CLOS | CLOS |
Data source: https://ourworldindata.org.
CRediT authorship contribution statement
Xiaoqian Sun: Conceptualization, Methodology, Writing – original draft. Sebastian Wandelt: Conceptualization, Writing – review & editing. Anming Zhang: Conceptualization, Validation, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This study is supported by the National Natural Science Foundation of China (Grant No. 61861136005, No. 61650110516, and No. 71731001).
Footnotes
One has to consider the actual time of infection and also that the first confirmed (= sequenced) is not necessarily the first case present in Denmark.
Appendix.
Table 4.
Notes for Fig. 1, containing full names of top 20 airports.
| IATA-Code | Airport |
|---|---|
| ATL | Hartsfield–Jackson Atlanta International Airport |
| CAN | Guangzhou Baiyun International Airport |
| CKG | Chongqing Jiangbei International Airport |
| CLT | Charlotte Douglas International Airport |
| DEL | Indira Gandhi International Airport |
| DEN | Denver International Airport |
| DFW | Dallas Fort Worth International Airport |
| HND | Tokyo International Airport |
| IAH | George Bush Intercontinental Airport |
| KMG | Kunming Wujiaba International Airport |
| LAX | Los Angeles International Airport |
| MEX | Mexico City Airport |
| MIA | Miami International Airport |
| ORD | Chicago O’Hare International Airport |
| PEK | Beijing Capital Airport |
| PHX | Phoenix Sky Harbor Airport |
| SEA | Seattle SEATAC Airport |
| SHA | Shanghai Hongqiao Airport |
| SZX | Shenzhen Bao’an International Airport |
| XIY | Xian Airport |
References
- Balcan D., Colizza V., Gonçalves B., Hu H., Ramasco J.J., Vespignani A. Multiscale mobility networks and the spatial spreading of infectious diseases. Proc. Natl. Acad. Sci. 2009;106(51):21484–21489. doi: 10.1073/pnas.0906910106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balcan D., Gonçalves B., Hu H., Ramasco J.J., Colizza V., Vespignani A. Modeling the spatial spread of infectious diseases: The global epidemic and mobility computational model. J. Comput. Sci. 2010;1(3):132–145. doi: 10.1016/j.jocs.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogoch I., Creatore M.I., Cetron M.S., et al. Assessment of the potential for international dissemination of ebola virus via commercial air travel during the 2014 west african outbreak. Lancet. 2015;385(9962):29–35. doi: 10.1016/S0140-6736(14)61828-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen J., Laroe C. Airline networks and the international diffusion of severe acute respiratory syndrome (SARS) The Geogr. J. 2006;172:130–144. doi: 10.1111/j.1475-4959.2006.00196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockmann D., Helbing D. The hidden geometry of complex, network-driven contagion phenomena. Science. 2013;342(6164):1337–1342. doi: 10.1126/science.1245200. [DOI] [PubMed] [Google Scholar]
- Chinazzi M., Davis J.T., Ajelli M., Gioannini C., Litvinova M., Merler S., y Piontti A.P., Mu K., Rossi L., Sun K., Viboud C., Xiong X., Yu H., Halloran M.E., Longini I.M., Vespignani A. The effect of travel restrictions on the spread of the 2019 novel coronavirus (COVID-19) outbreak. Science. 2020;368(6489):395–400. doi: 10.1126/science.aba9757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria N., Mellan T., Whittaker C., Claro I., Candido D., Mishra S., Crispim M., Sales F., Hawryluk I., McCrone J., Hulswit R., Franco L., Ramundo M., Jesus J., Andrade P., Coletti T., Ferreira G., Silva C., Manuli E., Sabino E. Genomics and epidemiology of the P.1 SARS-CoV-2 lineage in manaus, Brazil. Science. 2021:eabh2644. doi: 10.1126/science.abh2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner L., Chughtai A., Macintyre C. Risk of global spread of middle east respiratory syndrome coronavirus (MERS-CoV) via the air transport network. J. Travel Med. 2016;23:taw063. doi: 10.1093/jtm/taw063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kermack W.O., McKendrick A.G. A contribution to the mathematical theory of epidemics. Proc. R. Soc. London. Ser. A. 1927;115(772):700–721. [Google Scholar]
- Kupferschmidt K. New mutations raise specter of ‘immune escape’. Science. 2021;371:329–330. doi: 10.1126/science.371.6527.329. [DOI] [PubMed] [Google Scholar]
- Lau H., Khosrawipour V., Kocbach P., Mikolajczyk A., Ichii H., Zacharski M., Bania J., Khosrawipour T. The association between international and domestic air traffic and the coronavirus (COVID-19) outbreak. J. Microbiol., Immunol. Infection. 2020;53(3):467–472. doi: 10.1016/j.jmii.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likhacheva A. SARS Revisited. Am. Med. Assoc. J. Ethics. 2006;8(4):219–222. doi: 10.1001/virtualmentor.2006.8.4.jdsc1-0604. [DOI] [PubMed] [Google Scholar]
- Mahase E. Covid-19: What new variants are emerging and how are they being investigated? BMJ. 2021;372:n158. doi: 10.1136/bmj.n158. [DOI] [PubMed] [Google Scholar]
- Nakamura H., Managi S. Airport risk of importation and exportation of the COVID-19 pandemic. Transp Policy. 2020;96:40–47. doi: 10.1016/j.tranpol.2020.06.018. URL http://www.sciencedirect.com/science/article/pii/S0967070X20303516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read J., Diggle P., Chirombo J., Solomon T., Baylis M. Effectiveness of screening for Ebola at airports. Lancet. 2014;385 doi: 10.1016/S0140-6736(14)61894-8. [DOI] [PubMed] [Google Scholar]
- Singh J., Samal J., Sharma J., Agrawal U., Ehtesham N., Rahman S.A., Hira S., Hasnain S., Kumar V., Sundar D. Structure-function analyses of new SARS-CoV-2 variants b.1.1.7, b.1.351 and b.1.1.28.1: Clinical, diagnostic, therapeutic and public health implications. Viruses. 2021;13:439. doi: 10.3390/v13030439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X., Wandelt S., Hansen M., Li A. Multiple airport regions based on inter-airport temporal distances. Transp. Res. E: Logist. Transp. Rev. 2017;101:84–98. doi: 10.1016/j.tre.2017.03.002. URL https://www.sciencedirect.com/science/article/pii/S1366554516308109. [DOI] [Google Scholar]
- Sun X., Wandelt S., Zhang A. On the degree of synchronization between air transport connectivity and COVID-19 cases at worldwide level. Transp. Policy. 2021;105 doi: 10.1016/j.tranpol.2021.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X., Wandelt S., Zheng C., Zhang A. Covid-19 pandemic and air transportation: Successfully navigating the paper hurricane. J. Air Transp. Manag. 2021 doi: 10.1016/j.jairtraman.2021.102062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volz E., Mishra S., Chand M., Barrett J., Johnson R., Geidelberg L., Hinsley W., Laydon D., Dabrera G., O’Toole A., Amato R., Ragonnet-Cronin M., Harrison I., Jackson B., Ariani C., Boyd O., Loman N., McCrone J., GonΩ CÇcalves S., Ferguson N. Assessing transmissibility of SARS-CoV-2 lineage b.1.1.7 in England. Nature. 2021:1–17. doi: 10.1038/s41586-021-03470-x. [DOI] [PubMed] [Google Scholar]
- Wandelt S., Sun X. Evolution of the international air transportation country network from 2002 to 2013. Transp. Res. E: Logist. Transp. Rev. 2015;82:55–78. doi: 10.1016/j.tre.2015.08.002. URL http://www.sciencedirect.com/science/article/pii/S1366554515001581. [DOI] [Google Scholar]
- Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D., Fouchier R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012;367(19):1814–1820. doi: 10.1056/NEJMoa1211721. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- Zou L., Ruan F., Huang M., Liang L., Huang H., Hong Z., Yu J., Kang M., Song Y., Xia J., et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. New England J. Med. 2020;382(12):1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]