Abstract
Parvovirus B19 can be transmitted transplacentally from the infected mother to the fetus during pregnancy, and hydrops fetalis, abortion, or stillbirth can result. In our study we explored the use of chemiluminescence in situ hybridization to detect B19 DNA on cord blood cells, amniotic fluid cells, and pleuric fluid cells from several cases of hydrops fetalis. B19 DNA was detected by using digoxigenin-labeled probes immunoenzymatically visualized with the chemiluminescent adamantil-1,2-dioxetane phenyl phosphate substrate for alkaline phosphatase. The luminescent signal emitted from the hybridized probes was detected, analyzed, and measured with a high-performance, low-light-level imaging luminograph connected to an optical microscope and to a personal computer for the quantification and localization of the chemiluminescent emission inside individual cells.
Human parvovirus B19 is associated with a wide range of clinical manifestations, such as erythema infectiosum in children, acute arthritis in adults, and aplastic crisis in patients with chronic hemolytic anemias (21). Parvovirus B19 can be transmitted transplacentally from the infected mother to the fetus during pregnancy, and as B19 replicates in erythroid precursor cells and fetal tissues, a severe fetal anemia and hydrops fetalis can result (1, 4). Moreover, B19-associated hydrops fetalis can lead to abortion or stillbirth with an estimated incidence between 1.7 and 9% (9, 19). As B19 cannot be routinely grown in stable cell lines, the current diagnosis of B19 fetal infection mainly relies on the detection of B19 DNA in fetal cells or in fetal fluids by using in situ hybridization (ISH) with colorimetric detection or dot blot hybridization or by using PCR (10, 18, 20, 22). Serology is not probatory for a diagnosis of B19 fetal hydrops, since B19 immunoglobulin M can be detected in only about 50% of maternal sera at the time of clinical diagnosis of fetal hydrops, while in the hydropic fetus, immunoglobulin M is rarely found (21).
ISH is a successful method for the localization of specific viral nucleic acids inside individual cells with the preservation of cellular morphology, and it is the method of choice to detect B19 in fetal infections. In fact, in fetal hydrops at the time of clinical presentation, B19 can be detected inside fetal erythroid precursor cells and inside amniotic fluid cells, while in serum derived from cord blood or in amniotic fluid, the virus can be found mostly at very low titers or may be absent (22). Recently, as regards ISH, the need for increased sensitivity and specific and objective analysis achieved from digital imaging has led to the development of new methods such as chemiluminescence ISH, able to amplify ISH signals and give an image analysis and a quantification of the results (11, 12, 14, 15, 17). In chemiluminescence ISH, labeled probes are visualized with a final enzymatic reaction by using highly sensitive chemiluminescent substrates, which have been proposed as a more sensitive alternative to colorimetric substrates in various analytical techniques (13, 16). The spatial distribution of the light emitted from the hybridized probes is measured by using a high-performance, low-light-level imaging luminograph connected to an optical microscope and to a personal computer for the localization of the chemiluminescent emission inside individual cells and for the quantification of the photon fluxes at a single photon level. Since an improved, very sensitive assay for the prenatal diagnosis of B19-induced fetal hydrops would be very useful for a prompt diagnosis, for counseling and management of these infections, and for an improved comprehension of the role of B19 in some congenital pathologies, the aim of our work was to explore the use of chemiluminescence ISH on different cellular samples from hydrops fetalis by using B19 digoxigenin-labeled probes constructed in our laboratory. The B19 DNA probe was prepared from the molecular clone of a 5.0-kbp insert (nucleotides 282 to 5310), which represents the complete coding sequence of B19 DNA, cloned in vector pUC18 (7). The digoxigenin labeling of the B19 probe was performed as previously described (2) from the excised insert, by the randomly primed DNA labeling method.
For our study we selected 18 cases of nonimmune fetal hydrops from which specimens had been submitted to our laboratory with a special request to rule out parvovirus B19. Of these 18 cases, 12 were positive for B19 infection and 6 were negative. In the 12 B19-positive hydrops cases, the diagnosis of B19 fetal infection was determined by testing fetal cord blood or amniotic fluids with the three tests used in our laboratory for the detection of B19 DNA (dot blot hybridization, ISH with colorimetric detection, and nested PCR) (22). In the six B19-negative hydrops cases, negative results were obtained on amniotic fluids and fetal cord blood with the same tests. In our study we analyzed by chemiluminescence ISH: (i) heparinized fetal cord blood samples collected from the 12 positive and 6 negative hydropic fetuses, (ii) amniotic fluid cells collected from eight B19-positive and three B19-negative hydrops, and (iii) pleural fluid cells from two B19-positive hydropic fetuses. As positive controls, reference samples of B19-positive bone marrow cells from patients with B19 transient aplastic crisis were used. As negative controls, samples of cord blood cells and amniotic fluid cells from healthy fetuses were analyzed. Cell smears were prepared and fixed on silanized slides as previously described (22). For chemiluminescence ISH, fixed cell samples were hydrated in phosphate-buffered saline (PBS) and then placed in 0.02 N HCl for 10 min. After being washed with PBS, cells were treated with 0.01% Triton X-100 in PBS for 2 min. After three further washes with PBS, cells were treated with pronase (Boehringer, Mannheim, Germany) (0.5 mg/ml in 0.05 M Tris-HCl [pH 7.6]–5 mM EDTA) for 5 min. Cell preparations were then washed twice with PBS containing 2 mg of glycine per ml. After these treatments cells were postfixed with 4% paraformaldehyde in PBS and washed twice with PBS containing 2 mg of glycine per ml. Cellular samples were then dehydrated by ethanol washes (30, 60, 80, 95, and 100%) and then were overlaid with 10 μl of the hybridization mixture. The hybridization mixture consisted of 50% deionized formamide, 10% dextran sulfate, 250 ng of carrier calf thymus DNA per μl, and 2 ng of digoxigenin-labeled B19 DNA probe per μl in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) (pH 7.0). Cell samples and the hybridization mixture were denatured together by heating them in an 85°C water bath for 5 min and were then incubated at 37°C overnight. After hybridization cell samples were washed in stringent conditions (8).
For the detection of hybridized probes, samples were briefly washed in a 100 mM Tris-HCl buffer, pH 7.5, containing 150 mM NaCl. Cells were then incubated for 30 min with antidigoxigenin sheep polyclonal Fab fragments, conjugated to alkaline phosphatase, and diluted 1/500 in a blocking reagent (Boehringer). After incubation, cell samples were washed for two 15-min washes with Tris-HCl buffer and equilibrated for 2 min with an equilibration buffer (100 mM Tris-HCl, 100 mM NaCl, 50 mM MgCl2 [pH 9.5]). The chemiluminescent detection of alkaline phosphatase was performed by treating the cells with 20 μl of undiluted adamantil-1,2-dioxetane phenyl phosphate substrate (CDP Star) (Tropix, Inc., Bedford Mass.) at room temperature. After an optimized incubation of 30 min, the solution was removed and the luminescent signal from the hybrid formation was detected and analyzed with a system that consisted of the Luminograph model LB-980 (EG&G Berthold, Bad Wilbad, Germany), which is a high-performance, low-light-level imaging apparatus with a high dynamic range pickup tube (Saticon), combined with a video amplifier, and connected to a model BH-2 light microscope (Olympus Optical, Tokyo, Japan) and to a personal computer with a commercially available program for image analysis. The microscope was enclosed in a dark box to prevent contact with external light. The system operated in consecutive steps: first, fetal cells were recorded in transmitted light, and then the luminescent signal from the hybrid formation was measured; and then, after a computer elaboration of the luminescent signal with pseudocolors corresponding to the light intensity, an overlay of the two images on the screen provided by the transmitted light and by the luminescent signal allowed the spatial distribution of the target analyte to be localized and evaluated.
Digital images of the light emission from fetal cells were optimized at 2-s intervals of integration time for 1 min of total accumulation time. The light emission from each cell was quantified by defining a fixed area and calculating the total number of photon fluxes/second from within this area. The threshold background levels were provided for each run by analyzing a mean of 50 cells from control negative samples of cord blood cells and amniotic fluid cells from healthy fetuses. The average values ± standard deviations of the background light emission (expressed as photons/second/area) were then calculated. The average value of the background signal plus fivefold its standard deviation was considered the threshold value, above which the chemiluminescent signal resulting from the hybridized B19 DNA could be determined as positive. The net light signal of the sample was obtained automatically on the screen after subtraction of the threshold values with appropriate software. Corrections for instrumental background and flat field variations were automatically performed by the LB-980 apparatus.
In our study we analyzed by chemiluminescence ISH for the detection of B19 DNA, 12 fetal blood samples, eight amniotic fluid cell samples and two pleuric fluid cell samples from 12 cases of B19-positive fetal hydrops, and six fetal blood samples and three amniotic fluid samples from six fetal hydrops cases which had proved negative for B19. Of the 12 cord blood, eight amniotic fluid, and two pleuric fluid cell samples from fetuses which had previously proved positive for B19, all proved positive by chemiluminescence ISH for the detection of B19 DNA with a good preservation of cellular morphology and absence of aspecific photon emission (Fig. 1 and 2). In the positive samples, a mean of 64.5 positive cells/500 counted cells/sample were found in cord blood samples, a mean of 23.1 positive cells were found in amniotic fluid samples, and a mean of 70 positive cells were found in pleuric fluid cell specimens. The mean value of the net light signal of positive cells expressed as photon emission/second/cell was 1.413 × 103 with a range between 2.081 × 103 and 0.789 × 103. The number of emitted photons/second/positive cell, which corresponds to the presence of hybridized B19 parvovirus DNA, varied, presumably depending on the stage of B19 viral replication in infected cells; in fact, in a previous work regarding chemiluminescence ISH for the detection of cytomegalovirus (CMV) DNA we have demonstrated that increased values of emitted photons can be found in CMV-infected cells, fixed at 40, 62, 72, and 96 h after infection following CMV replication (15).
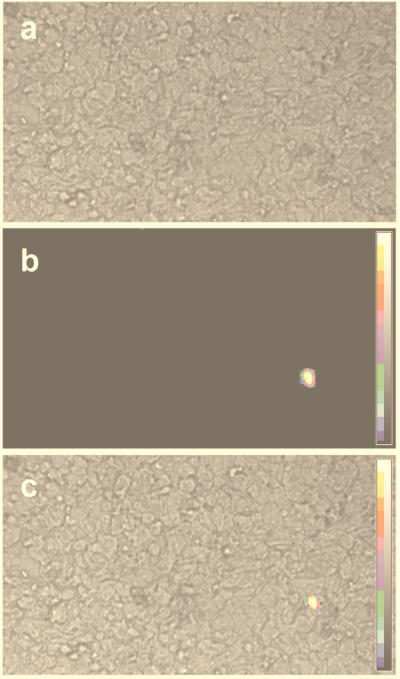
FIG. 1.
Chemiluminescence ISH revealing B19 parvovirus DNA in amniotic fluid cells, shown as a live image (a), a luminescent signal (b), and an overlay of the live image and luminescent signal (c).
FIG. 2.
Chemiluminescence ISH revealing B19 parvovirus DNA in fetal cord blood cells, shown as a live image (a), a luminescent signal (b), and an overlay of the live image and luminescent signal (c).
Of the six blood samples and three amniotic fluid cells from the hydropic fetuses who had previously been diagnosed as negative for B19 infection, the absence of B19 DNA was confirmed with chemiluminescence ISH. In fact, in these samples, no net light emission was detected since photon emission values were comparable with the background noise of cells obtained from normal fetuses.
The results achieved with chemiluminescence ISH were compared with results obtained by ISH with colorimetric detection with nitroblue tetrazolium chloride and 5-bromo-4-chloro-3-indolylphosphate toluidinum salt as the chromogenic alkaline phosphatase substrate. The same samples were analyzed by both chemiluminescence ISH and colorimetric ISH in the same run and with the same batch of probe, and concordant results were found in all cases but one. The discrepant result was obtained in an amniotic fluid sample which proved positive by chemiluminescence ISH (five positive cells/500 counted cells/sample) but negative by colorimetric detection (no positive cells in the whole sample). To assess if this chemiluminescence result was a true positive, the discrepant amniotic fluid was retested with both colorimetric and chemiluminescence detection, and the results previously obtained were confirmed. Nested PCRs performed on this amniotic fluid sample proved positive; moreover, a cord blood sample from the same fetus was retested, and the positivity obtained by both chemiluminescent and colorimetric ISHs was confirmed, demonstrating a fetal B19 infection. When a comparison between chemiluminescence ISH and colorimetric ISH was performed as regards the number of positive cells, the two methods showed a high correlation in the analysis both of cord blood samples and of amniotic fluids (P = 0.0071 and P = 0.0243, respectively, at Spearman rank correlation). In cord blood samples, by using the chemiluminescence method a mean of 64.5 positive cells/500 counted cells could be found versus a mean of 17.9 positive cells/500 counted cells with the colorimetric method. This difference was highly significant at a P value of 0.0022 (Wilcoxon signed test for paired data). In amniotic fluid cell samples, by using the chemiluminescence method a mean of 23.1 positive cells/500 counted cells could be found versus a mean of 5.6 positive cells/500 counted cells with the colorimetric method. This difference was highly significant at a P value of 0.0117 (Wilcoxon signed test for paired data). With chemiluminescence ISH, therefore, a higher number of positive cells/sample was detectable than with colorimetric ISH, thus permitting an easier evaluation of the sample.
When a comparison among chemiluminescence ISH, nested PCR, and dot blot hybridization was performed, chemiluminescence ISH proved very sensitive. In fact, of 12 fetal cord blood samples positive by chemiluminescence ISH, all 12 were confirmed positive by colorimetric ISH, 10 proved positive by nested PCR, and 2 proved positive by dot blot hybridization. Moreover, of the eight amniotic fluid samples which proved positive by chemiluminescence ISH, seven were confirmed positive by colorimetric ISH, five by nested PCR, and only one by dot blot hybridization assay. These results were in agreement with previous data showing that, in a comparative evaluation of virological methods for prenatal diagnosis of parvovirus B19 fetal hydrops, the most sensitive diagnostic system proved to be ISH for B19 DNA in fetal cells (22). ISHs permit the detection of viral infections which remain strictly associated with infected cells. This is particularly important for B19, since it has often been found to be strictly associated with infected cells, without being detectable by PCR in body fluids (3, 5, 6, 22). To assess the reproducibility of the chemiluminescence ISH, three positive and three negative samples were analyzed in triplicate in different runs, and the results were concordant in positivity and negativity with the expected data; positive samples showed a number of positive cells which was consistent with previous results and showed a net light emission in the same range of the original samples, while in negative samples no net light emission was detected in any cell.
Control experiments definitely proved that the chemiluminescence ISH was detecting parvovirus B19 sequences specifically. In fact, (i) a specific positive signal was detected when reference samples of B19-positive bone marrow cells from patients with B19 transient aplastic crisis were tested. In these control positive cells, the mean value of the net light signal of 50 cells determined to be positive was 1.864 × 103 photons/s/cell with a range between 2.267 × 103 and 1.112 × 103. (ii) No chemiluminescent signal was observed when positive fetal sample cells were hybridized with the plasmid pUC18 control DNA-labeled probe and treated with an antidigoxigenin Fab fragment conjugated with alkaline phosphatase and with the chemiluminescent substrate. (iii) No luminescent signal was detectable in positive fetal sample cells after hybridization with unlabeled B19 probes, followed by the immunoenzymatic chemiluminescent treatment. (iv) B19-positive fetal cells were completely negative after hybridization with the B19-labeled probes when the primary incubation with antidigoxigenin antibody was either omitted or replaced by an incubation with nonimmune sheep serum.
From our results, we think that chemiluminescence ISH because of its sensitivity and specificity can be useful to obtain better knowledge about infrequent diseases with difficult and multiple diagnostic problems such as nonimmune fetal hydrops. Nonimmune fetal hydrops, in fact, occurs in 1 of 3,000 births, and up to now, approximately 50% of cases are of an undetermined etiology. Chemiluminescence ISH would be also very helpful for an improved documentation of fetal parvovirus infections, in order to determine the real extent of fetal and congenital damage and the relative fetal risk in each trimester of pregnancy.
Acknowledgments
This work was supported by CNR Target Project on “Biotechnology,” MURST (Ministero della Università e della Ricerca Scientifica e Tecnologica), and University of Bologna Funds for Selected Research Topics.
REFERENCES
- 1.Anand A, Gray E S, Brown T, Clewley J P, Cohen B J. Human parvovirus infection in pregnancy and hydrops fetalis. N Engl J Med. 1987;316:183–187. doi: 10.1056/NEJM198701223160403. [DOI] [PubMed] [Google Scholar]
- 2.Azzi A, Zakrzewska K, Gentilomi G, Musiani M, Zerbini M. Detection of B19 parvovirus infections by a dot-blot hybridization assay using a digoxigenin-labelled probe. J Virol Methods. 1990;27:125–134. doi: 10.1016/0166-0934(90)90129-4. [DOI] [PubMed] [Google Scholar]
- 3.Brown K E, Green S W, Antunez de Mayolo J, Bellanti J A, Smith S D, Smith T J, Young N S. Congenital anaemia after transplacental B19 parvovirus infection. Lancet. 1994;343:895–896. doi: 10.1016/s0140-6736(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 4.Brown T, Anand A, Ritchie L D, Clewley J P, Reid T M S. Intrauterine parvovirus infection during pregnancy. Lancet. 1984;ii:1033–1034. doi: 10.1016/s0140-6736(84)91126-7. [DOI] [PubMed] [Google Scholar]
- 5.Cassinotti P, Weitz M, Siegl G. Human parvovirus B19 infections: routine diagnosis by a new nested polymerase chain reaction assay. J Med Virol. 1993;40:228–234. doi: 10.1002/jmv.1890400311. [DOI] [PubMed] [Google Scholar]
- 6.Foto F, Saag K G, Schatosch L L, Howard E J, Naides S J. Parvovirus B19 specific DNA in bone marrow from B19 arthropathy patients: evidence for B19 virus persistence. J Infect Dis. 1993;167:744–748. doi: 10.1093/infdis/167.3.744. [DOI] [PubMed] [Google Scholar]
- 7.Gallinella G, Musiani M, Zerbini M, Gentilomi G, Gibellini D, Venturoli S, La Placa M. Efficient parvovirus B19 DNA purification and molecular cloning. J Virol Methods. 1993;41:203–212. doi: 10.1016/0166-0934(93)90127-d. [DOI] [PubMed] [Google Scholar]
- 8.Gentilomi G, Zerbini M, Musiani M, Gallinella G, Gibellini D, Venturoli S, Re M C, Pileri S, Finelli C, La Placa M. In situ detection of B19 DNA in bone marrow of immunodeficient patients using a digoxigenin labelled probe. Mol Cell Probes. 1993;7:19–24. doi: 10.1006/mcpr.1993.1003. [DOI] [PubMed] [Google Scholar]
- 9.Gratacós E, Torres P J, Vidal J, Antolin E, Costa J, Jimenez de Anta M T, Carach V, Alonso P, Fortuny A. The incidence of human parvovirus B19 infection during pregnancy and its impact on perinatal outcome. J Infect Dis. 1995;171:1360–1363. doi: 10.1093/infdis/171.5.1360. [DOI] [PubMed] [Google Scholar]
- 10.Koch W C, Harger J H, Barnstein B, Adler S P. Serologic and virologic evidence for frequent intrauterine transmission of human parvovirus B19 with a primary maternal infection during pregnancy. Pediatr Infect Dis J. 1998;17:489–494. doi: 10.1097/00006454-199806000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Lorimier P, Lamarcq L, Labat-Molleur F, Guillermet C, Bethier R, Stoebner P. Enhanced chemiluminescence: a high-sensitivity detection system for in situ hybridization and immunohistochemistry. J Histochem Cytochem. 1993;41:1591–1597. doi: 10.1177/41.11.7691929. [DOI] [PubMed] [Google Scholar]
- 12.Lorimier P, Lamarcq L, Negoescu A, Robert C, Labat-Moleur F, Gras-Chappuis F, Durrant I, Brambilla E. Comparison of 35S and chemiluminescence for HPV in situ hybridization in carcinoma cell lines and on human cervical intraepithelial neoplasia. J Histochem Cytochem. 1996;44:665–671. doi: 10.1177/44.7.8675987. [DOI] [PubMed] [Google Scholar]
- 13.Martin C S, Butler L, Bronstein I. Quantitation of PCR products with chemiluminescence. BioTechniques. 1995;18:908–912. [PubMed] [Google Scholar]
- 14.Musiani M, Roda A, Zerbini M, Gentilomi G, Pasini P, Gallinella G, Venturoli S. Detection of parvovirus B19 DNA in bone marrow cells by chemiluminescence in situ hybridization. J Clin Microbiol. 1996;34:1313–1316. doi: 10.1128/jcm.34.5.1313-1316.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Musiani M, Roda A, Zerbini M, Pasini P, Gentilomi G, Gallinella G, Venturoli S. Chemiluminescent in situ hybridization for the detection of cytomegalovirus DNA. Am J Pathol. 1996;148:1105–1112. [PMC free article] [PubMed] [Google Scholar]
- 16.Musiani M, Zerbini M, Gibellini D, Gentilomi G, La Placa M, Ferri E, Girotti S. Chemiluminescent assay for the detection of viral and plasmid DNA using digoxigenin-labeled probes. Anal Biochem. 1991;194:394–398. doi: 10.1016/0003-2697(91)90247-q. [DOI] [PubMed] [Google Scholar]
- 17.Musiani M, Zerbini M, Venturoli S, Gentilomi G, Gallinella G, Manaresi E, La Placa M, Jr, D’Antuono A, Roda A, Pasini P. Sensitive chemiluminescence in situ hybridization for the detection of human papillomavirus genomes in biopsy specimens. J Histochem Cytochem. 1997;45:729–735. doi: 10.1177/002215549704500511. [DOI] [PubMed] [Google Scholar]
- 18.Nascimento J P, Hallam N F, Field A M, Clewley J P, Brown K E, Cohen B J. Detection of B19 parvovirus in human fetal tissue by in situ hybridization. J Med Virol. 1991;33:77–82. doi: 10.1002/jmv.1890330203. [DOI] [PubMed] [Google Scholar]
- 19.Public Health Laboratory Service Working Party on Fifth Disease. Prospective study of human parvovirus (B19) infection in pregnancy. Br Med J. 1990;300:1166–1170. doi: 10.1136/bmj.300.6733.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Török T J, Wang Q Y, Gary G W, Jr, Yang C F, Finch T M, Anderson L J. Prenatal diagnosis of intrauterine infection with parvovirus B19 by polymerase chain reaction technique. Clin Infect Dis. 1992;14:149–155. doi: 10.1093/clinids/14.1.149. [DOI] [PubMed] [Google Scholar]
- 21.Zerbini M, Musiani M. Human parvoviruses. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 7th ed. Washington, D.C: American Society for Microbiology; 1999. pp. 1089–1098. [Google Scholar]
- 22.Zerbini M, Musiani M, Gentilomi G, Venturoli S, Gallinella G, Morandi R. Comparative evaluation of virological and serological methods in prenatal diagnosis of parvovirus B19 fetal hydrops. J Clin Microbiol. 1996;34:603–608. doi: 10.1128/jcm.34.3.603-608.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]