Abstract
Antibody avidity is an important parameter to evaluate immune response, being useful to evaluate vaccine responses and helping to distinguish acute and latent infection. The antibody avidity can be measured by different methods, yet the most common is a modified ELISA. The utilization of commercial kits or in-house methods to evaluate antibody avidity have been adopted more and more, although the lack of standardization between different assays may generate a lot of variation in the process, making it hard to compare the results generated.
Keywords: Antibody avidity assay, Antibody, ELISA
Antibody avidity
Antibody production in response to infection or vaccination is an essential process to combat and prevent infectious diseases. The binding of the antibody to the antigen is a non-covalent interaction [1]. It has been shown that affinity of antibodies increases with the time, through a process named affinity maturation, which is a consequence of somatic hypermutation, this reaction occurs in the germinal centers and requires the support provided by follicular dendritic cells and T-helper cells, thus generating antibodies that bind with more strength to the antigen [2].
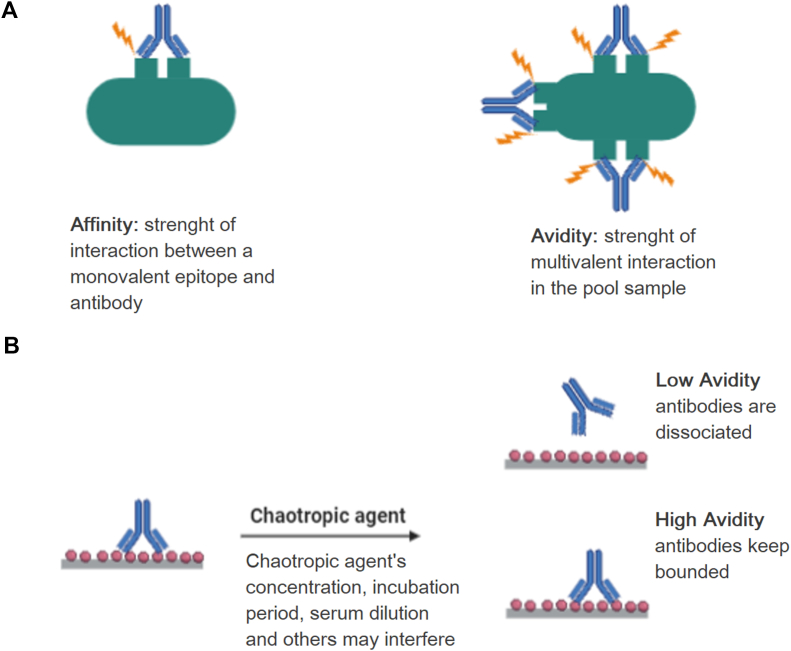
Affinity is defined as the strength of interaction between a monovalent epitope and a monovalent antibody, while bivalent and multivalent interactions are defined as avidity (Fig. 1) [3].
Fig. 1.
(A) Representation of affinity and avidity, (B) chaotropic agent activity in antibody binding to epitope.
In the germinal center also occurs events that lead to immunoglobulin class-switch and differentiation into plasma cells or memory B cells [4]. Thus, some authors investigate the generation of immunological memory by means of antibody avidity evaluation [[5], [6], [7], [8]]. Besides, high affinity antibodies display a better function and may be more indicative of protective antibodies [[9], [10], [11]], being useful in the evaluation of responses to vaccines. Hence, the antibody avidity is an important parameter to evaluate vaccine efficacy [12].
To analyze the antibody avidity two methodologies may be applied: the use of a protein-denaturing agent that is added in serum diluent to prevent antigen–antibody complex, known as dilution principle, or the utilization of this protein-denaturing agent after the formation of antigen–antibody complex, known as elution principle [13]. Considering that, the antibody avidity can be measured by different methods, such as ammonium sulphate precipitation, solid-phase radioimmunoassay, surface plasmon resonance and modified ELISA [13,14], the latter being the most common method to analyze the avidity index, due to its simplicity and quickness [1].
To perform the avidity assay, it can be used standard commercial kits or it can be developed an in house method, but the biggest problem concerning this topic is the variability between the results obtained when used different commercial kits and in house protocols, which makes it hard to compare the results obtained by each methodology. This review aims to discuss the use of modified ELISA to assess the avidity index of antibodies and how the lack of standardization may impair the interpretation and comparison of the results between the scientific community.
Indirect-enzyme-linked immunosorbent assay (Indirect-ELISA)
Before performing a modified ELISA to check the Avidity Index (AI) of antibodies, it is important to understand the conventional method, which has to be well standardized, given that several factors may influence its performance and, consequentially, the AI assay [15].
Firstly, despite the several ELISA methods, as indirect-ELISA, Sandwich-ELISA and Competition-ELISA [15], not all of them are suitable for assessing AI. The modified ELISA that is suggested in this review is based on the Indirect method. It was described by Lindström and Wager [16] and consists of immobilizing the antigen on the plate, then incubating the serum sample and use an enzyme-labelled anti-immunoglobulin to detect the presence of antibodies in the sample. When the AI assay is performed, the chaotropic agent is incubated after the serum and before the secondary antibody, in that way, the reaction reveals only the presence of high-avidity antibodies [17].
There are different types of plates for ELISA, made of rigid polyvinyl, polypropylene and polystyrene, with different binding capacities [18]. The nature of the antigen (complex with several structures, purified proteins, etc) and its structure (size, charge, etc) must be observed to choose the ideal plate for that antigen to bind. The binding reaction is also influenced by the pH, the incubation conditions and the concentration of the antigen [15,19].
The antigen–antibody reaction is mainly determined by the concentration of each one on the assay, along with the affinity constant of the antibody [20]. However, the incubation time and temperature are important issues to be considered. The most used temperatures are 4 °C, room temperature or 37 °C, usually, the lower the temperature, the longer the incubation period [15], however, stronger signals are usually obtained by longer serum incubation periods [21].
The diverse options of substrates also impact the final reading. Some substrates are described as more sensitive than others. For one of the most common enzymes used, HRP (horseradish peroxidase), TMB (3 3′ 5 5′-tetramethylbenzidine) is described as a more sensitive substrate when compared with OPD (o-phenylenediamine dihydrochloride) and ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)), for example [22]. Shorter periods of substrate incubation are usually associated with higher temperatures to accomplish maximum enzymatic reaction [23]. ELISA technique may differ between protocols and must be standardized to suit the conditions of the laboratory where it is performed and to provide trustworthy results. After all, once standardized, the protocol should be proceeded as established, avoiding changes that might impact on the results [21].
Modified ELISA for antibody avidity assay
The modified ELISA assay consists of adding a chaotropic agent, such as urea, thiocyanate or diethylamine, after the incubation of the plates with the serum, once these agents have the capacity to elute antibodies that bind weakly to the antigen (Fig. 1). These agents can disturb hydrophobic interaction, hydrogen bonds, van der Waals forces and, in the case of thiocyanate, even electrostatic interactions [24]. Moreover, when this assay is used to evaluate antibody avidity, it is necessary to consider some variants, such as antigen source, serial or single serum dilution, chaotropic agent used and its concentration, incubation period, serum dilution, calculation and interpretation of results, among others [24,25].
The use of standard commercial kits to evaluate antibody avidity has been adopted more and more, however, it is necessary to evaluate which kit will be the best for the analysis. Another important point is the possibility of comparison of results among different kits. Even if different kits are standardized to a specific pathogen, it is important to check if results obtained by different kits for the same pathogens can be compared.
The IgG avidity is an important parameter to evaluate the risk of congenital infections by Toxoplasma spp., human cytomegalovirus (HCMV) and rubella virus, for example, since IgM antibodies may persist for a long time and its measure in immunoassays may not be able to distinguish recent infections, in some cases. Low avidity IgG in the mother is associated with a recent primary infection with HCMV, Toxoplasma gondii and Rubella, presenting high risk of intrauterine transmission, on the other side, high avidity IgG in mother in the recurrent infection represents little risk of transmission to the fetus [[26], [27], [28]]. A study assessed the performance of eight commercial human cytomegalovirus IgG avidity assays (5 ELISA, 2 chemiluminescent and 1 enzyme-linked fluorescent assay) and verified a widely performance variance between the kits [26].
Bobić et al. [25] compared the performance of three Toxoplasma-specific IgG antibody avidity commercial tests, their results demonstrated that T. gondii IgG avidity enzyme immune assay (Ani Labsystems) and VIDAS Toxo IgG Avidity (bioMérieux) presented strong concordance, while an ELISA adapted for IgG avidity determination (EUROIMMUN) presented moderate or poor agreement with the other tests. Another study compared four anti-T. gondii IgG avidity kits. These kits are from different companies: VIDAS Toxo IgG Avidity (bioMérieux), EIA Toxoplasma IgG (TEST-LINE), PLATELIA Toxo IgG Avidity (Bio-rad) and Enzywell Toxoplasma IgG avidity (DIESSE). The kits demonstrated variable correlations between themselves and the results obtained using the same sera were divergent, which is worrying, since the results influence clinical decisions [29]. Mubareka et al. [28] compared five commercial rubella IgG avidity tests and showed that these assays presented variable correlation with each other.
These studies indicate that there is a difference in results of avidity assays performed with different kits, making it harder to compare results of assays using kits of different companies, in addition, it creates a discussion of which kit presents the most reliable results.
This fact can be seen in Table 1, which demonstrates the difference in result's interpretation of avidity assays performed with 5 different kits to HCMV, using urea or thiocyanate as chaotropic agents. This inter-variability among all commercial kits creates a variability in the final results and it implicates in many different result's conclusions for the same sample.
Table 1.
Interpretation of results of some avidity assay kits to HCMV of different companies according to the manufacturer's instruction.
| Manufacturer |
Dissociating agent | Interpretation of results according to manufacturer's | Ref. |
|---|---|---|---|
| Name of the kit | |||
| Diesse | Urea | <30% low avidity | |
| Enzywell Cytomegalovirus IgG avidity | [26] | ||
| >40% high avidity | |||
| Euroimmun | Urea | <40% low avidity | |
| CMV IgG avidity | [40] | ||
| >60% high avidity | |||
| Bio-Rad | Urea | <40% low avidity | |
| Platelia CMV IgG avidity | [26] | ||
| >55% high avidity | |||
| Radim | Urea | <35% low avidity | |
| Cytomegalovirus IgG avidity | [41] | ||
| >45% high avidity | |||
| Technogenetics | Potassium thiocyanate | <25% low avidity | |
| BEIA CMV IgG avidity |
There are few kits to evaluate antibody avidity, thus many assays are developed in-house, but the utilization of this method creates much more variables, like serum and chaotropic agents concentration, and which agent will be used, due to the lack of automatization and standardization of in-house methods [30], as it can be seen in Table 2. These variants in the assays may implicate in divergent results or even in a wrong interpretation of the results. So, the application of a protocol consensus would be ideal, making it possible to compare the results of different research groups and providing a more reliable interpretation of the results obtained.
Table 2.
Variations in chaotropic agent, its concentration and interpretation of results of different in-house antibody avidity assays for distinct pathogens.
| Chaotropic agent and its concentration | Interpretation of results de according to the author | Pathogen | Ref. |
|---|---|---|---|
| Urea 6 M | <50% low avidity | Cytomegalovirus | [42] |
| >60% high avidity | |||
| Urea 8 M | <50% low avidity | Cytomegalovirus | [43] |
| >60% high avidity | |||
| Urea 6 M | <70% low avidity | Rubella virus | [44] |
| >90% high avidity | |||
| Urea 5 M | <40% low avidity | Rubella virus | [45] |
| >60% high avidity | |||
| diethylamine 35 mM | <45% low avidity | Rubella virus | [46] |
| >60% high avidity | |||
| diethylamine 60 mM | <30% low avidity | Rubella virus | [47] |
| >70% high avidity | |||
| diethylamine 35 mM | <53% low avidity | Rubella virus | [48] |
| >53% high avidity | |||
| Urea 6 M | <50% low avidity | Toxoplasma gondii | [49] |
| >50% high avidity | |||
| Urea 6 M | <30% low avidity | Toxoplasma gondii | [50] |
| >40% high avidity | |||
| Urea 8 M | <30% low avidity | Toxoplasma gondii | [51] |
| >30% high avidity |
The need for standardization
Although knowing that is impossible for all in-house assays to use the same serum dilution or chaotropic agent concentration, the adoption of an universal parameter to choose the concentration of these reagents may decrease the method performance variation and enable a better comparison of results.
A study performed by Dimitrov et al. [24] demonstrated that antibody concentration influence quite a lot in the dissociation of antibodies in the presence of the chaotropic agent. The authors verified that when high IgG concentrations are used, above the plateau of titration curve, moderate reductions in binding couldn't be detected, inducing a misunderstanding of results. The results obtained suggested that only antibody concentration in the linear part of the titration curves allows an accurate estimative of antibody avidity [24]. Thus, the adoption of these parameters could be a good option for the standardization of the avidity assays.
Also, Perciani et al. [31] pointed out that the main cause of variation in antibody avidity assays is the necessity to choose a reference point in the ELISA titration curve. Therefore, the authors established a new method to calculate antibody avidity that considers the data of the whole titration curve, in which the avidity index is the average of each point of titration curve, thus, the author propose a method based on the ratio of the areas derived from the curves obtained by the plot of optical density (OD) and log of the sera dilution in the ELISA with and without KSCN treatment.
Antibody avidity index as a tool to infer protection against meningococcal disease
Neisseria meningitidis is one of the main etiological agents of bacterial meningitis; meningococcal disease can rapidly evolve to death and presents a high risk of developing sequelae, so its prevention is extremely important and appears to be cost-effective for public health [32,33]. Given the low overall incidence of meningococcal disease, the direct evaluation of meningococcal vaccine efficacy is not feasible in clinical trials. Instead, the efficacy of the vaccine is inferred based on the induction of serum bactericidal antibodies measured in vitro using the serum bactericidal activity assay (SBA) [34]. However, the SBA is a laborious method and requires the manipulation of meningococcus, thus there is an attempt to develop new trials that correlate with protection against disease [35]. The antibody avidity assays performed in the studies conducted by Granoff et al. [36] and Vermont et al. [37] presented a linear correlation between antibody avidity and SBA. In addition, it has been demonstrated that infants, whom vaccines based on OMVs against N. meningitidis B have low efficacy, produce antibodies of low avidity, which do not present bactericidal activity, whereas children older than 10 years have antibodies of greater avidity and that exhibit bactericidal activity [38].
Several modified ELISA assays were used to evaluate the avidity of antibodies produced after immunization against N. meningitidis. Vermont et al. [37] performed a modified ELISA, using serum diluted initially 1:100 and sodium thiocyanate (NaSCN) at 1.5 M as chaotropic agent, the avidity index was defined as the percentage of antibodies that remained bound at the antigen coat after the treatment with chaotropic agent:
Another study defined the serum dilution that resulted in an O.D. close to 1, ammonium thiocyanate was chosen as the chaotropic agent and incubated at various concentration between 0 and 1 M, the avidity index was defined as the concentration necessary to decrease the absorbance by 50% [39].
Conclusion
As it can be observed, the evaluation of antibody avidity is an important tool in both research and clinical areas, but we emphasize the need to standardize the in house methodologies and avidity assay kits to generate more reliable and comparable results between different laboratories.
Financial support
This work was supported by the São Paulo Research Foundation (FAPESP) [grants numbers 12/15568–0, 13/11147-2, 14/11172-0, 14/07182–0, 18/04202-0, 18/17945-1, 19/02042-9]; National Council for Scientific and Technological Development [grants number 132743/2014-6, 131412/2019-1] and Coordination of Improvement of Post-Graduate Level Personnel (CAPES).
Conflicts of interest
The authors declare that there are no conflicts of interest.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Brady A.M., Unger E.R., Panicker G. Description of a novel multiplex avidity assay for evaluating HPV antibodies. J Immunol Methods. 2017;447:31–36. doi: 10.1016/j.jim.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Victora G.D., Nussenzweig M.C. Germinal Centers. Annu Rev Immunol. 2012;30:429–457. doi: 10.1146/annurev-immunol-020711-075032. [DOI] [PubMed] [Google Scholar]
- 3.Eisen H.N. Affinity enhancement of antibodies: how low-affinity antibodies produced early in immune responses are followed by high-affinity antibodies later and in memory B-cell responses. Cancer Immunol Res. 2014;2:381–392. doi: 10.1158/2326-6066.CIR-14-0029. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y., Garcia-Ibanez L., Toellner K.M. Regulation of germinal center B-cell differentiation. Immunol Rev. 2016;270:8–19. doi: 10.1111/imr.12396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldblatt D., Borrow R., Miller E. Natural and vaccine-induced immunity and immunologic memory to Neisseria meningitidis serogroup C in young adults. J Infect Dis. 2002;185:397–400. doi: 10.1086/338474. [DOI] [PubMed] [Google Scholar]
- 6.Gioia C.A.C., De Sousa A.B., Cruz S.C., Junior F.C.S., Andrade A.F.B., Sassi R.M. Effect of a booster dose of serogroup B meningococcal vaccine on antibody response to Neisseria meningitidis in mice vaccinated with different immunization schedules. FEMS Immunol Med Microbiol. 2005;44:35–42. doi: 10.1016/j.femsim.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 7.Longworth E., Borrow R., Goldblatt D., Balmer P., Dawson M., Andrews N. Avidity maturation following vaccination with a meningococcal recombinant hexavalent PorA OMV vaccine in UK infants. Vaccine. 2002;20:2592–2596. doi: 10.1016/s0264-410x(02)00151-2. [DOI] [PubMed] [Google Scholar]
- 8.Alam M.M., Bhuiyan T.R., Qadri F. Short Communication on: Study of Avidity of Antigen-Specific Antibody as a Means of Understanding Development of Long-Term Immunological Memory after Vibrio cholerae O1 Infection. J Vaccines Vaccin. 2017;8:1000363. doi: 10.1128/CVI.00521-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reddy S.B., Anders R.F., Beeson J.G., Färnert A., Kironde F., Berenzon S.K. High affinity antibodies to plasmodium falciparum merozoite antigens are associated with protection from malaria. PloS One. 2012;7:e32242. doi: 10.1371/journal.pone.0032242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steward M.W., Stanley C.M., Dimarchi R., Mulcahy G., Doel T.R. High-affinity antibody induced by immunization with a synthetic peptide is associated with protection of cattle against foot-and-mouth disease. Immunology. 1991;72:99–103. [PMC free article] [PubMed] [Google Scholar]
- 11.Usinger W.R., Lucas A.H. Avidity as a determinant of the protective efficacy of human antibodies to pneumococcal capsular polysaccharides. Infect Immun. 1999;67:2366–2370. doi: 10.1128/iai.67.5.2366-2370.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anttila M., Voutilainen M., Jäntti V., Eskola J., Käyhty H. Contribution of serotype-specific IgG concentration, IgG subclasses and relative antibody avidity to opsonophagocytic activity against Streptococcus pneumoniae. Clin Exp Immunol. 1999;118:402–407. doi: 10.1046/j.1365-2249.1999.01077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hedman K., Lappalainen M., Söderlund M., Hedman L. Avidity of IgG in serodiagnosis of infectious diseases. Rev Med Microbiol. 1993;4:123–129. [Google Scholar]
- 14.Lynch H.E., Stewart S.M., Kepler T.B., Sempowski G.D., Alam S.M. Surface plasmon resonance measurements of plasma antibody avidity during primary and secondary responses to anthrax protective antigen. J Immunol Methods. 2014;404:1–12. doi: 10.1016/j.jim.2013.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crowther J.R. The ELISA guidebook. 2nd ed. Humana Press; New York: 2009. [Google Scholar]
- 16.LIindström P., Wager O. IgG autoantibody to human serum albumin studied by the ELISA-technique. Scand J Immunol. 1978;7:419–425. doi: 10.1111/j.1365-3083.1978.tb00472.x. [DOI] [PubMed] [Google Scholar]
- 17.Premjeet S., Deepika G., Sudeep B., Sonam J., Sahil K., Devashish R. Enzyme-Linked Immuno-Sorbent Assay ( ELISA ), basics and it's application: a comprehensive review. J Pharm Res. 2011;4:4581–4583. [Google Scholar]
- 18.Aydin S. A short history, principles, and types of ELISA, and our laboratory experience with peptide/protein analyses using ELISA. Peptides. 2015;72:4–15. doi: 10.1016/j.peptides.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 19.Blake C., Gould B.J. Use of enzymes in immunoassay techniques: a review. Analyst. 1984;109:533–547. [Google Scholar]
- 20.Yolken R.H., Leister F.J. Comparison of fluorescent and colorigenic substrates for enzyme immunoassays. J Clin Microbiol. 1982;15:757–760. doi: 10.1128/jcm.15.5.757-760.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hornbeck P.V. Enzyme-linked immunosorbent assays. Curr Protoc Im. 2015;110 doi: 10.1002/0471142735.im0201s110. 2.1.1–23. [DOI] [PubMed] [Google Scholar]
- 22.Porstmann T., Kiessig S.T. Enzyme immunoassay techniques an overview. J Immunol Methods. 1992;150:5–21. doi: 10.1016/0022-1759(92)90061-w. [DOI] [PubMed] [Google Scholar]
- 23.Porstmann B., Porstmann T., Gaede D., Nugel E., Egger E. Temperature dependent rise in activity of horseradish peroxidase caused by non-ionic detergents and its use in enzyme-immunoassay. Clin Chim Acta. 1981;109:175–181. doi: 10.1016/0009-8981(81)90332-6. [DOI] [PubMed] [Google Scholar]
- 24.Dimitrov J.D., Lacroix-Desmazes S., Kaveri S.V. Important parameters for evaluation of antibody avidity by immunosorbent assay. Anal Biochem. 2011;418:149–151. doi: 10.1016/j.ab.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 25.Bobić B., Klun I., Vujanić M., Nikolić A., Ivović V., Živković T. Comparative evaluation of three commercial Toxoplasma-specific IgG antibody avidity tests and significance in different clinical settings. J Med Microbiol. 2009;58:358–364. doi: 10.1099/jmm.0.006668-0. [DOI] [PubMed] [Google Scholar]
- 26.Revello M.G., Genini E., Gorini G., Klersy C., Piralla A., Gerna G. Comparative evaluation of eight commercial human cytomegalovirus IgG avidity assays. J Clin Virol. 2010;48:255–259. doi: 10.1016/j.jcv.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 27.Hedman K., Lappalainen M., Seppäiä I., Miikelä O. Recent primary Toxoplasma infection indicated by a low avidity of specific IgG. J Infect Dis. 1989;159:736–740. doi: 10.1093/infdis/159.4.736. [DOI] [PubMed] [Google Scholar]
- 28.Mubareka S., Richards H., Gray M., Tipples G.A. Evaluation of commercial rubella immunoglobulin G avidity assays. J Clin Microbiol. 2007;45:231–233. doi: 10.1128/JCM.01243-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horváth K.N., Szénási Z., Danka J., Kucsera I. Value of the IgG avidity in the diagnosis of recent toxoplasmosis: a comparative study of four commercially available anti-Toxoplasma gondii IgG avidity assays. Acta Parasitol. 2005;50:255–260. [Google Scholar]
- 30.Villard O., Breit L., Cimon B., Franck J., Fricker-Hidalgo H., Godineau N. Comparison of four commercially available avidity tests for Toxoplasma gondii-specific IgG antibodies. Clin Vaccine Immunol. 2013;20:197–204. doi: 10.1128/CVI.00356-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perciani C.T., Peixoto P.S., Dias W.O., Kubrusly F.S., Tanizaki M.M. Improved method to calculate the antibody avidity index. J Clin Lab Anal. 2007;21:201–206. doi: 10.1002/jcla.20172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Batista R.S., Gomes A.P., Dutra Gazineo J.L., Balbino Miguel P.S., Santana L.A., Oliveira L. Meningococcal disease, a clinical and epidemiological review. Asian Pac J Trop Med. 2017;10:1019–1029. doi: 10.1016/j.apjtm.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 33.Strelow V.L., Vidal J.E. Doença meningocócica invasiva. Arq Neuropsiquiatr. 2013;71:653–658. doi: 10.1590/0004-282X20130144. [DOI] [PubMed] [Google Scholar]
- 34.Goldschneider I., Gotschlich E.C., Artenstein M.S. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med. 1969;129:1307–1326. doi: 10.1084/jem.129.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vermont C., Dobbelsteen G. Neisseria meningitidis serogroup B: laboratory correlates of protection. FEMS Immunol Med Microbiol. 2002;34:89–96. doi: 10.1111/j.1574-695X.2002.tb00608.x. [DOI] [PubMed] [Google Scholar]
- 36.Granoff D.M., Maslanka S.E., Carlone G.M., Plikaytis B.D., Santos G.F., Mokatrin A. A modified enzyme-linked immunosorbent assay for measurement of antibody responses to meningococcal C polysaccharide that correlate with bactericidal responses. Clin Diagn Lab Immunol. 1998;5:479–485. doi: 10.1128/cdli.5.4.479-485.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vermont C.L., van Dijken H.H., van Limpt C.J.P., de Groot R., van Alphen L., van Den Dobbelsteen G.P.J.M. Antibody avidity and immunoglobulin G isotype distribution following immunization with a monovalent meningococcal B outer membrane vesicle vaccine. Infect Immun. 2002;70:584–590. doi: 10.1128/IAI.70.2.584-590.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pollard A.J., Levin M. Production of low-avidity antibody by infants after infection with serogroup B meningococci. Lancet. 2000;356:2065–2066. doi: 10.1016/S0140-6736(00)03405-X. [DOI] [PubMed] [Google Scholar]
- 39.Harris S.L., Finn A., Granoff D.M. Disparity in functional activity between serum anticapsular antibodies induced in adults by immunization with an investigational group A and C Neisseria meningitidis-diphtheria toxoid conjugate vaccine and by a polysaccharide vaccine. Infect Immun. 2003;71:3402–3408. doi: 10.1128/IAI.71.6.3402-3408.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prince H.E., Lapé-Nixon M. Role of cytomegalovirus (CMV) IgG avidity testing in diagnosing primary CMV infection during pregnancy. Clin Vaccine Immunol. 2014;21:1377–1384. doi: 10.1128/CVI.00487-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gentile M., Galli C., Pagnotti P., Di Marco P., Tzantzoglou S., Bellomi F. Measurement of the sensitivity of different commercial assays in the diagnosis of CMV infection in pregnancy. Eur J Clin Microbiol Infect Dis. 2009;28:977–981. doi: 10.1007/s10096-009-0738-0. [DOI] [PubMed] [Google Scholar]
- 42.Ikuta K., Koshizuka T., Kanno R., Inoue N., Kubo T., Koyano S. Evaluation of the indirect and IgM-capture anti-human cytomegalovirus IgM ELISA methods as confirmed by cytomegalovirus IgG avidity. Microbiol Immunol. 2019;63:172–178. doi: 10.1111/1348-0421.12683. [DOI] [PubMed] [Google Scholar]
- 43.Kourí V., Correa C.B., Verdasquera D., Martínez P.A., Alvarez A., Alemán Y. Diagnosis and screening for cytomegalovirus infection in pregnant women in Cuba as prognostic markers of congenital infection in newborns: 2007-2008. Pediatr Infect Dis J. 2010;29:1105–1110. doi: 10.1097/INF.0b013e3181eb7388. [DOI] [PubMed] [Google Scholar]
- 44.Vauloup-Fellous C., Grangeot-Keros L. Humoral immune response after primary rubella virus infection and after vaccination. Clin Vaccine Immunol. 2007;14:644–647. doi: 10.1128/CVI.00032-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wandinger K.P., Saschenbrecker S., Steinhagen K., Scheper T., Meyer W., Bartelt U. Diagnosis of recent primary rubella virus infections: significance of glycoprotein-based IgM serology, IgG avidity and immunoblot analysis. J Virol Methods. 2011;174:85–93. doi: 10.1016/j.jviromet.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 46.Agbede O.O., Adeyemi O.O., Olatinwo A.W.O. Significance of IgG-avidity in antenatal rubella diagnosis. J Fam Reprod Health. 2013;7:131–137. [PMC free article] [PubMed] [Google Scholar]
- 47.Mercader S., Garcia P., Bellini W.J. Measles virus IgG avidity assay for use in classification of measles vaccine failure in measles elimination settings. Clin Vaccine Immunol. 2012;19:1810–1817. doi: 10.1128/CVI.00406-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hamkar R., Jalilvand S., Mokhtari-Azad T., Nouri Jelyani K., Dahi-Far H., Soleimanjahi H. Assessment of IgM enzyme immunoassay and IgG avidity assay for distinguishing between primary and secondary immune response to rubella vaccine. J Virol Methods. 2005;130:59–65. doi: 10.1016/j.jviromet.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 49.Ferra B.T., Holec-Gąsior L., Gatkowska J., Dziadek B., Dzitko K., Grąźlewska W. The first study on the usefulness of recombinant tetravalent chimeric proteins containing fragments of SAG2, GRA1, ROP1 and AMA1 antigens in the detection of specific anti-Toxoplasma gondii antibodies in mouse and human sera. PloS One. 2019;14 doi: 10.1371/journal.pone.0217866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rahbari A.H., Keshavarz H., Shojaee S., Mohebali M., Rezaeian M. IgG avidity ELISA test for diagnosis of acute toxoplasmosis in humans. Kor J Parasitol. 2012;50:99–102. doi: 10.3347/kjp.2012.50.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rodrigues J.P., Frei F., Navarro I.T., Silva L.P., Marcelino M.Y., de Andrade-Junior H.F. Seroepidemiological analysis of toxoplasmosis in college students. J Venom Anim Toxins Incl Trop Dis. 2015;21:1. doi: 10.1186/1678-9199-21-1. [DOI] [PMC free article] [PubMed] [Google Scholar]