Abstract
Calcific uremic arteriolopathy, termed calciphylaxis, was previously considered a condition that developed mostly in patients requiring dialysis. It has now been described in kidney transplant patients, in advanced chronic kidney disease (CKD) patients not requiring dialysis, and in individuals with maintained kidney function. We describe an individual with CKD stage 3b with hypercalcemia who presented with features highly specific for calciphylaxis based on results of a skin biopsy. The condition has high morbidity and mortality, and thus prompts immediate cessation of the offending agents or treatment of the cause. The following case and literature review demonstrates a need for a detailed assessment of patients’ risks and exposures and expanding the differential diagnosis to include calciphylaxis in nonuremic patients with necrotic ulcers with a plan for early imaging and possible biopsy.
Index Words: Nonuremic calciphylaxis, calcific uremic arteriolopathy, vitamin D intoxication, hypercalcemia, kidney disease, case report, Sjögren syndrome, rheumatoid arthritis
Introduction
Calcific uremic arteriolopathy or calciphylaxis is a rare disorder in which calcium accumulates in the arterioles and capillaries of the dermis and subcutaneous adipose tissue, causing ischemic necrosis.1 This disorder is associated with a high morbidity and mortality with a 6-month survival of approximately 50%, depending on comorbidities and the severity of cutaneous involvement.2
Calciphylaxis occurs most often in end-stage kidney disease patients requiring dialysis2, 3, 4 but has been described in kidney transplant recipient patients,4 individuals with advanced chronic kidney disease (CKD), and those with maintained kidney function.5,6
Herein, we report a case of calciphylaxis in a patient with CKD not receiving dialysis associated with hypercalcemia, rheumatoid arthritis, and Sjögren syndrome. The patient’s necrotic skin lesions healed after stopping the offending agents with improvement in hypercalcemia, treatment with sodium thiosulfate, and aggressive wound care. Initially presumed to be due to vitamin D intoxication, lesions recurred 1 year later, at which time hypercalcemia was present, with low vitamin D levels suggesting that hypercalcemia and her rheumatologic disorders were associated with her calciphylaxis.
Case Report
A white woman in her late 40s with history of erosive rheumatoid arthritis, Sjögren syndrome, hypothyroidism, and CKD stage 3b managed with chronic glucocorticoids presented with a 10-day history of multiple escharotic painful skin lesions located on the left and right lower quadrants, right flank, and the right upper extremity (Fig 1). She did not report any recent medication changes, over-the-counter drug use, or toxic occupational exposure. She was empirically treated with linezolid and meropenem for a suspected skin infection. Biopsy of one of the skin lesions showed fat necrosis with fibrotic tissue. A review of her medications showed that she was taking ergocalciferol (vitamin D2) 50,000 units daily.
Figure 1.
Left lower quadrant, right upper extremity, right flank, and right lower quadrant eschar lesions (from left to right).
On admission, pertinent laboratory tests were as follows: serum urea nitrogen 33 mg/dL, creatinine 2.2 mg/dL, estimated glomerular filtration rate 26 mL/min/1.73 m2 (baseline serum creatinine was 1.6 mg/dL), calcium 11.6 mg/dL, corrected calcium 12.3 mg/dL, albumin 3.1 g/dL, and phosphorus 2.8 mg/dL. Hypercalcemia work-up was as follows: parathyroid hormone 20.9 pg/mL (normal 9-77 pg/mL), parathyroid hormone–related protein 0.8 pmol/L (<4.2 pmol/L), 25-hydroxy vitamin D 103 ng/mL (normal 30-100 ng/mL), 1,25-hydroxy vitamin D 49 pg/mL (20-79 pg/mL), and serum and urine electrophoresis and immunotyping showing no restriction bands in the gamma region and an unremarkable kappa-to-lambda ratio. A nuclear medicine (Tc-99m sestamibi) parathyroid scan with single-photon emission computed tomography / computed tomography showed a focus of persistent activity over the lower pole of the left thyroid lobe suggestive of an enlarged left inferior parathyroid gland, but no parathyroid adenoma was seen. An ultrasound of the thyroid showed diffuse enlargement of the gland with no discrete mass in the left lower pole. An ultrasound examination of her kidneys showed a 1 cm calcified stone in the right upper pole without evidence of hydronephrosis.
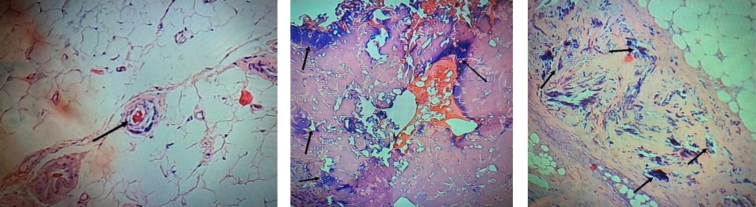
She received intravenous (IV) hydration with normal saline, intermittent IV furosemide, a single dose of IV calcitonin 4 units/kg, and oral cinacalcet 60 mg twice a day for treatment of her hypercalcemia. Owing to concerns about the diagnosis of calciphylaxis versus rheumatoid vasculitis, full-thickness skin biopsies from the left lower abdominal quadrant and left flank eschars were obtained. Pathology of these lesions showed focal fat necrosis and focal interstitial and arteriolar intimal calcium deposition most consistent with calciphylaxis (Fig 2). Direct immunofluorescence examination for anti-IgG, IgM, IgA, C3, fibrinogen, and C1q were negative.
Figure 2.
Skin biopsy (hematoxylin-eosin stain). Arrows show calcium deposits in arterioles and necrotic skin tissue, respectively.
She was treated for calciphylaxis with IV sodium thiosulfate 25 g twice weekly for 3 weeks for a total of 5 doses. She received aggressive wound care and debridement of necrotic lesions. At the time of her discharge serum calcium was 9.6 mg/dL, corrected calcium 10.6 mg/dL, albumin 2.8 g/dL, and 25-hydroxy vitamin D level 111.9 ng/mL. Her wounds showed decrease in size and increase in healthy granulation tissue. She was discharged to a long-term acute care facility with wound care management for 3 months with complete resolution thereafter.
One year later, she was readmitted to a hospital for recurrent calciphylaxis with a 3-cm wound on the lateral left breast and 3 superficial wounds on the inferior left axilla ranging from 0.3 to 0.7 cm in diameter. Her serum creatinine was 3.2 mg/dL, albumin 2.2 g/dL, calcium 12.6 mg/dL, phosphorus 3.4 mg/dL, and 25-hydroxy vitamin D level 25 ng/mL on presentation. Hypercalcemia improved with aggressive IV fluids and her lesions improved with wound care. At discharge, the serum creatinine was 2.28 mg/dL, serum albumin 2.2 g/dL, serum calcium 9 mg/dL, and corrected serum calcium 10.4 mg/dL. One of her medications, oral chlorthalidone 25 mg daily, was discontinued, as it may contribute to hypercalcemia and volume depletion.
Discussion
Nonuremic calcific uremic arteriolopathy, or calciphylaxis, is an uncommon but serious diagnosis. The first admission in this patient suggested vitamin D intoxication as the primary trigger for calciphylaxis. Vitamin D administration at high doses has been shown to induce calciphylaxis in experimental models7,8 and domestic animals.9 Vitamin D analogues may contribute to calciphylaxis through their effect on the vasculature or by increasing serum calcium and phosphate levels. Two case-controlled studies have shown an association between 1,25 dihydroxy vitamin D and 25 hydroxy vitamin D use with an increased odds ratio of calciphylaxis in hemodialysis patients.10,11 When calciphylaxis recurred, her vitamin D level was low, suggesting that hypercalcemia from possible thiazide use and underlying rheumatological diseases played a role rather than vitamin D.
The patient described in the case report likely had vitamin D–induced hypercalcemia on her first admission and most probably thiazide-induced hypercalcemia on her second admission. In between these episodes of calciphylaxis, her vitamin D levels remained normal. Additional risk factors that may have predisposed to calciphylaxis in this patient were female sex, autoimmune disease with a history of rheumatoid arthritis and Sjögren syndrome, and use of corticosteroids.15,20,21 A calcium phosphate product >70 mg2/dL2 was associated with an increased risk of calciphylaxis in a few uncontrolled studies.13,14 In this patient, the calcium phosphate product was 33 mg2/dL2 during her initial presentation and 42.8 mg2/dL2 with her second admission, levels not associated with calciphylaxis.
Historically, when Hans Seyle coined the term calciphylaxis, in his animal studies, he hypothesized causation via “sensitization” with an offending agent (high-dose vitamin D, parathyroid extract, induced kidney failure) followed by a “challenging agent” from local trauma, egg albumin, or metallic salts.1 In our patient, it could be postulated that the “sensitizing agent” was the vitamin D supplement, leading to hypercalcemia in conjunction with her autoimmune diseases as the “challenging agent” that led to calciphylaxis.
This case report teaches us that calciphylaxis can occur in nonuremic CKD patients who are not on dialysis. Calciphylaxis has been described in kidney transplant recipients,4 CKD patients not requiring dialysis, and individuals with maintained kidney function.5,6 In a review of patients with calciphylaxis from the Mayo Clinic, nearly 20% of patients had an estimated glomerular filtration rate >60 mL/min/1.73 m2.2 Since calciphylaxis is rare in non-dialysis-dependent patients, the diagnosis can be missed or delayed, as in our case. The occurrence of painful escharotic skin lesions that are often located in the lower abdomen and upper thighs should raise clinical suspicion for the diagnosis of calciphylaxis. Skin biopsy of the lesions may assist in the diagnosis, but it lacks sensitivity, as the tissue may not necessarily demonstrate calcification.16 When a biopsy is available, it helps rule out mimics but by itself does not confirm the diagnosis. If diagnosis, and thereby treatment, is delayed, both morbidity and mortality increase significantly.
Management of calciphylaxis includes elimination of suspected causative factors, treatment of hypercalcemia, and aggressive daily wound care. Typically, sodium thiosulfate is administered intravenously to all patients with calciphylaxis for several months until resolution of the skin lesions, but in our patient it was administered 2 days a week owing to intolerance. In vitro, sodium thiosulfate blocks the ability of adipocytes to induce calcification of vascular smooth muscle cells.12,17 The rationale for the use of sodium thiosulfate in calciphylaxis is the chelation of calcium to produce calcium thiosulfate, which may be more soluble than other calcium salts and, therefore, more readily cleared from the body.18 The optimal dose of sodium thiosulfate for the treatment of calciphylaxis in non-dialysis-dependent patients is not known. The recommended dose of 25 g IV 3 times a week is based on information gleaned from case reports and case series.19 The calciphylaxis lesions were fewer and smaller during the second episode and treatment consisted of stopping chlorthalidone and aggressive IV hydration, which resulted in resolution of hypercalcemia and improvement of the lesions.
In summary, we report a rare case of a patient with calciphylaxis associated with hypercalcemia and rheumatological diseases, who had CKD stage 3b. Prompt skin biopsy confirmed the diagnosis. Therapy was directed to resolving the hypercalcemia. The painful escharotic skin lesions began healing after lowering serum calcium and aggressive daily wound care.
Article Information
Authors’ Full Names and Academic Degrees
Athip Vatanapradith, MD, Ashwini Pujari, MD, Phani Morisetti, MD, Samina Hayat, MD, Kenneth Abreo, MD, and Bakhtiar M. Amin, MD.
Support
None.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Patient Protections
The authors declare that they have obtained consent from the patient reported in this article for publication of the information about her that appears within this Case Report and any associated supplementary material.
Peer Review
Received January 10, 2021. Evaluated by 1 external peer reviewer, with direct editorial input by the Editor-in-Chief. Accepted in revised form April 25, 2021.
Footnotes
Complete author and article information provided before references.
References
- 1.Nigwekar S.U., Kroshinsky D., Nazarian R.M. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66(1):133–146. doi: 10.1053/j.ajkd.2015.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCarthy J.T., El-Azhary R.A., Patzelt M.T. Survival, risk factors, and effect of treatment in 101 patients with calciphylaxis. Mayo Clin Proc. 2016;91(10):1384–1394. doi: 10.1016/j.mayocp.2016.06.025. [DOI] [PubMed] [Google Scholar]
- 3.Fine A., Zacharias J. Calciphylaxis is usually non-ulcerating: risk factors, outcome and therapy. Kidney Int. 2002;61(6):2210–2217. doi: 10.1046/j.1523-1755.2002.00375.x. [DOI] [PubMed] [Google Scholar]
- 4.Brandenburg V.M., Kramann R., Rothe H. Calcific uraemic arteriolopathy (calciphylaxis): data from a large nationwide registry. Nephrol Dial Transplant. 2017;32(1):126–132. doi: 10.1093/ndt/gfv438. [DOI] [PubMed] [Google Scholar]
- 5.Nigwekar S.U., Wolf M., Sterns R.H., Hix J.K. Calciphylaxis from nonuremic causes: a systematic review. Clin J Am Soc Nephrol. 2008;3(4):1139–1143. doi: 10.2215/CJN.00530108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bajaj R., Courbebaisse M., Kroshinsky D., Thadhani R.I., Nigwekar S.U. Calciphylaxis in patients with normal renal function: a case series and systematic review. Mayo Clin Proc. 2018;93(9):1202–1212. doi: 10.1016/j.mayocp.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Price P.A., Williamson M.K., Nguyen T.M., Than T.N. Serum levels of the fetuin-mineral complex correlate with artery calcification in the rat. J Biol Chem. 2004;279(3):1594–1600. doi: 10.1074/jbc.M305199200. [DOI] [PubMed] [Google Scholar]
- 8.Price P.A., Omid N., Than T.N., Williamson M.K. The amino bisphosphonate ibandronate prevents calciphylaxis in the rat at doses that inhibit bone resorption. Calcif Tissue Int. 2002;71(4):356–363. doi: 10.1007/s00223-002-1006-9. [DOI] [PubMed] [Google Scholar]
- 9.Bille N. Hypervitaminosis D og calciphylaxis hos husdyr [Vitamin D excess and calciphylaxis in domestic animals] NordisckVeterinaermedicin. 1970;22:218–233. [in Danish] [Google Scholar]
- 10.Nigwekar S.U., Bhan I., Turchin A. Statin use and calcific uremic arteriolopathy: a matched case-control study. Am J Nephrol. 2013;37(4):325–332. doi: 10.1159/000348806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nigwekar S.U., Zhao S., Wenger J. A nationally representative study of calcific uremic arteriolopathy risk factors. J Am Soc Nephrol. 2016;27(11):3421–3429. doi: 10.1681/ASN.2015091065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sowers K.M., Hayden M.R. Calcific uremic arteriolopathy: pathophysiology, reactive oxygen species and therapeutic approaches. Oxid Med Cell Longev. 2010;3(2):109–121. doi: 10.4161/oxim.3.2.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Angelis M., Wong L.L., Myers S.A., Wong L.M. Calciphylaxis in patients on hemodialysis: a prevalence study. Surgery. 1997;122(6):1083–1090. doi: 10.1016/s0039-6060(97)90212-9. [DOI] [PubMed] [Google Scholar]
- 14.Weenig R.H., Sewell L.D., Davis M.D.P. Calciphylaxis: natural history, risk factor analysis and outcome. J Am Acad Dermatol. 2007;56(4):569–579. doi: 10.1016/j.jaad.2006.08.065. [DOI] [PubMed] [Google Scholar]
- 15.Costa-Silva M., Vide J., Cruz M.J., Baudrier T., Azevedo F. Nonuremic calciphylaxis: four cases associated with autoimmune diseases. Skinmed. 2018;16(4):235–237. [PubMed] [Google Scholar]
- 16.Baby D., Upadhyay M., Joseph M.D. Calciphylaxis and its diagnosis: a review. J Family Med Prim Care. 2019;8(9):2763–2767. doi: 10.4103/jfmpc.jfmpc_588_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen N.X., O'Neill K., Akl N.K., Moe S.M. Adipocyte induced arterial calcification is prevented with sodium thiosulfate. Biochem Biophys Res Commun. 2014;449(1):151–156. doi: 10.1016/j.bbrc.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 18.Baker B.L., Fitzgibbons C.A., Buescher L.S. Calciphylaxis responding to sodium thiosulfate therapy. Arch Dermatol. 2007;143(2):269–270. doi: 10.1001/archderm.143.2.269. [DOI] [PubMed] [Google Scholar]
- 19.Generali J.A., Dennis J.C. Sodium thiosulfate: calciphylaxis. Hosp Pharm. 2015;50(11):975–977. doi: 10.1310/hpj5011-975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bouchemla N., Laamani A., Chettai M., Fadili W., Laouad I. Nonuremic calciphylaxis in a patient with multiple myeloma and rheumatoid arthritis. Saudi J Kidney Dis Transpl. 2020;31(2):556–560. doi: 10.4103/1319-2442.284038. [DOI] [PubMed] [Google Scholar]
- 21.Kusari A., Cotter D., Hinds B., Paravar T. Non-uremic calciphylaxis in a patient with multiple rheumatologic diseases. Dermatol J Online. 2019;25(2) [PubMed] [Google Scholar]