Abstract
Severe acute kidney injury is a common complication in critically ill patients, often necessitating support with a modality of kidney replacement therapy. Continuous kidney replacement therapies (CKRTs) have become a mainstay in the management of patients with acute kidney injury in the intensive care unit. Understanding the fundamentals of CKRT is necessary to safely and effectively prescribe treatment. In this narrative review, we summarize critical aspects of CKRT management, including selection of the mode of therapy; choice of hemofilter/hemodialyzer used; determination of the blood flow rate, composition and flow rates of dialysate and/or replacement fluids, and the ultrafiltration rate; and use and methods of anticoagulation. Requirements for vascular access and appropriate monitoring and dose adjustment of medications and a plan for monitoring the delivery of therapy and ensuring appropriate nutritional management are also discussed.
Index Words: Acute Kidney Injury, Continuous renal replacement therapy, continuous kidney replacement therapy, hemodialysis, hemofiltration, continuous venovenous hemofiltration, hemodiafiltration, ultrafiltration, continuous venovenous hemodialysis, continuous venovenous hemodiafiltration
Acute kidney injury (AKI), defined by an abrupt decrease in glomerular filtration rate over a matter of hours to days, occurs in >50% of critically ill patients and is associated with an in-hospital mortality rate of 20% to 25%.1,2 Among patients requiring kidney replacement therapy (KRT), mortality is 40% to 70%, with 5% to 30% of survivors remaining dialysis dependent at hospital discharge.2, 3, 4 Indications for KRT include severe hypervolemia unresponsive to diuretics, electrolyte abnormalities including severe hyperkalemia and refractory metabolic acidosis, drug intoxications, and overt uremic symptoms.
Although these broad indications for KRT are widely accepted, there has been a lack of consensus on precise criteria for KRT initiation. Thus, decisions to initiate KRT are often subjective, based on the individual patient's clinical condition as determined by the treating nephrologist and/or intensivist. Because AKI must be considered potentially reversible, a key goal in the management of KRT is to not interfere with the recovery of kidney function while preventing the morbidity and mortality associated with the acute loss of kidney function.
Modalities of KRT in the Intensive Care Unit
Multiple modalities of KRT can be used in the management of AKI, including intermittent hemodialysis (IHD), continuous KRTs (CKRTs), prolonged intermittent KRTs (also known as hybrid therapy, extended duration dialysis, or sustained low-efficiency dialysis), and peritoneal dialysis. These modalities provide solute clearance using varying proportions of diffusion (dialysis) and advection∗ (hemofiltration) based on the specifics of the chosen treatment.
In 1977, Kramer et al5 published the initial description of continuous arteriovenous hemofiltration, followed soon thereafter by a description of slow continuous ultrafiltration (SCUF) using a similar arteriovenous circuit by Paganini et al.6 These initial arteriovenous modes of CKRT were associated with complications of arterial cannulation, including both thrombosis and hemorrhage, as well as with relatively low blood flows in the extracorporeal circuit. Arteriovenous CKRT was supplanted by venovenous therapies, which eliminated the need for prolonged arterial cannulation and provided more consistent blood flow but necessitated additional safety monitoring, including air detectors and pressure monitors.7 During the past 3 decades there has been a rapid proliferation of dedicated CKRT machines, replacing early jury-rigged systems assembled from blood pumps and other components scavenged from dialysis machines and intravenous pumps, with integrated blood and fluid pumps, pressure monitors, air detectors, and ultrafiltration control mechanisms to permit the safe and efficient delivery of a wide variety of CKRT modes in the intensive care unit.8
Multiple randomized controlled trials (RCTs) and meta-analyses have compared CKRT and IHD and found them to be comparable in terms of mortality, recovery of kidney function, and length of stay.9, 10, 11, 12, 13, 14 However, it should be recognized that the IHD prescription in hemodynamically unstable patients was often modified in these trials and treatment duration was prolonged to enhance hemodynamic tolerance and safety.10 CKRT has been associated with a greater ability to achieve negative fluid balance15 as compared with IHD and is suggested by the KDIGO (Kidney Disease: Improving Global Outcomes) AKI clinical practice guidelines to be superior in hemodynamically unstable patients.3 CKRT has slower solute removal, which diminishes osmotic shifts that can exacerbate cerebral edema and potentially less treatment-related hypotension, resulting in greater preservation of cerebral perfusion. Thus, CKRT is preferred over IHD in patients with increased intracerebral pressure, including patients with fulminant hepatic failure, cerebral edema, and head trauma.16,17
The benefit of CKRT as compared to IHD with regard to recovery of kidney function has been debated. Although multiple observational studies have shown a higher probability of dialysis independence among surviving patients treated with CKRT as compared with IHD,18 this finding has not been confirmed in RCTs.9,18
Timing of Initiation of KRT
Although an in-depth discussion of when to initiate KRT is beyond the scope of this review of CKRT prescription, the decision to start therapy undergirds the prescription of therapy. Indications for CKRT are similar to those for other modalities of KRT, including volume overload unresponsive to diuretic therapy; metabolic acidosis, hyperkalemia, and other electrolyte abnormalities refractory to medical management; and uremic manifestations. Although CKRT has been used in the management of drug intoxications, IHD provides more rapid drug clearance and is generally preferred to CKRT in this setting, even in hemodynamically unstable patients.19, 20, 21
The role of earlier KRT in patients without an absolute indication for initiation of therapy has been debated. Although observational studies suggested a survival advantage with earlier initiation of KRT, most of these studies excluded patients with early AKI who never received KRT, introducing significant bias.22 Five recent RCTs have examined this question, with most concluding that a moderately delayed strategy of KRT initiation was not inferior to earlier initiation of therapy and was associated with decreased health care use and higher rates of recovery of kidney function.23, 24, 25, 26, 27
CKRT Prescription
In developing a CKRT prescription for a patient, a number of factors need to be considered, beginning with selection of the mode of treatment and corresponding operational parameters including blood flow, selection of dialysate and/or replacement fluid, dosing of therapy, anticoagulation, and fluid management (Table 1). In addition, consideration must also be given to monitoring of treatment efficacy, medication dosing, and nutrition.
Table 1.
Components of CKRT Orders
| CKRT Mode |
||||
|---|---|---|---|---|
| SCUF | CVVH | CVVHD | CVVHDF | |
| Hemofilter/hemodialyzer | X | X | X | X |
| Blood flow | X | X | X | X |
| Replacement fluid composition | X | X | ||
| Replacement fluid flow rate | X | X | ||
| Replacement fluid infusion site | X | X | ||
| Dialysate composition | X | X | ||
| Dialysate flow rate | X | X | ||
| Net ultrafiltration rate | X | X | X | X |
| Anticoagulation | X | X | X | X |
Abbreviations: CKRT, continuous renal replacement therapy; CVVH, continuous venovenous hemofiltration; CVVHD, continuous venovenous hemodialysis; CVVHDF, continuous venovenous hemodiafiltration; SCUF, slow continuous ultrafiltration.
Selection of Mode of CKRT
Multiple modes of CKRT are available, varying with regard to configuration of the extracorporeal circuit (arteriovenous or venovenous) and by the primary mechanism of solute transfer across the membrane (predominantly diffusion, predominantly advection, or a balanced combination). Given the virtual abandonment of the use of arteriovenous circuits for CKRT, our remaining discussion focuses exclusively on the venovenous modes of CKRT.
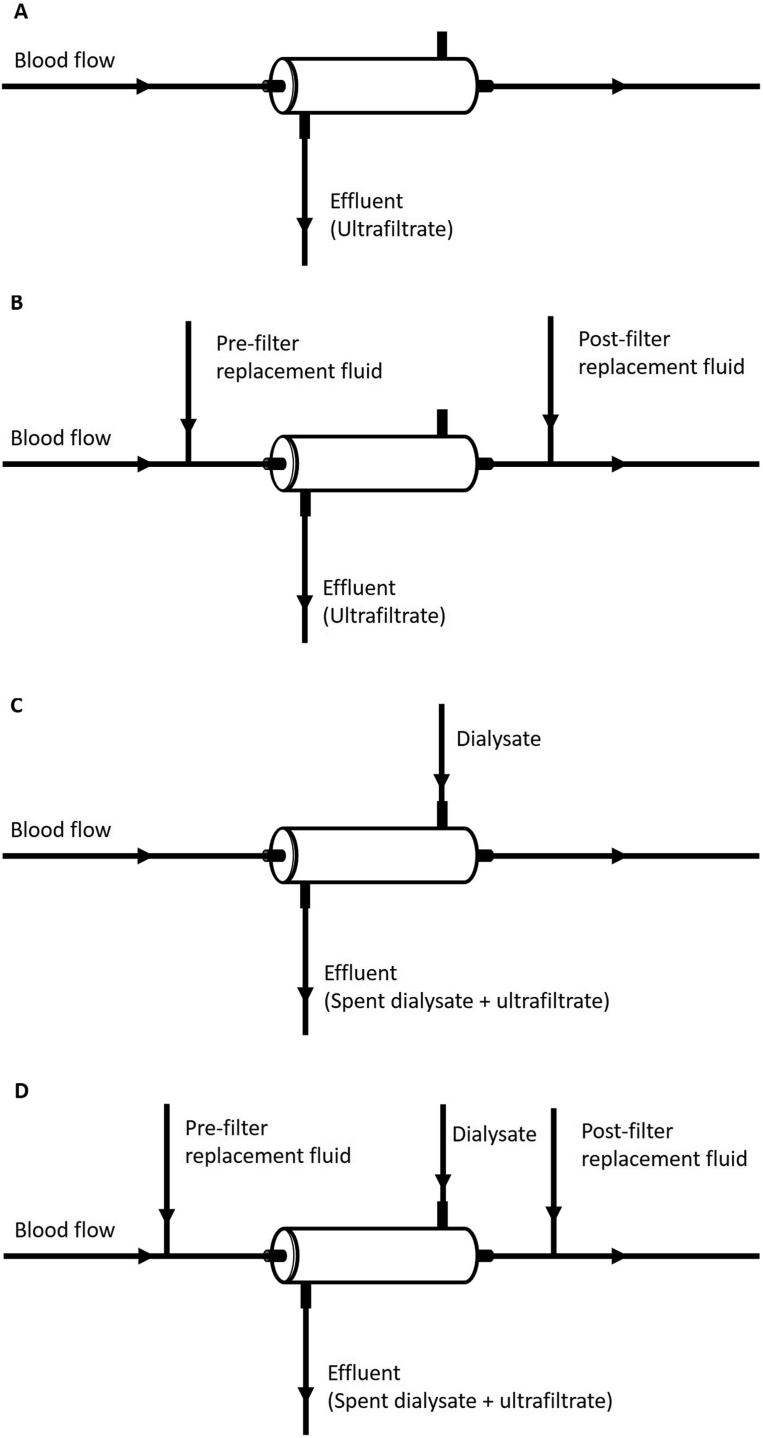
SCUF provides isolated ultrafiltration with minimal solute removal (Fig 1A). The primary role of SCUF is the treatment of volume overload in hemodynamically unstable patients. It has also been used as an alternative to high-dose diuretic therapy in patients with decompensated heart failure, although the relative benefit of ultrafiltration as compared with diuretics is controversial.28,29 Given the low blood flow rates required to perform SCUF, it can be performed using a peripheral access capable of sustaining a blood flow of only 50 mL/min as compared with the other modes of CKRT that require central or femoral venous access, although substantial anticoagulation may be needed to maintain circuit patency.
Figure 1.
Schematics of modes of continuous kidney replacement therapy. (A) Slow continuous ultrafiltration (SCUF): blood in the extracorporeal circuit is perfused through a hemofilter and an ultrafiltrate is generated equal to the desired rate of fluid removal. (B) Continuous venovenous hemofiltration (CVVH): blood in the extracorporeal circuit is perfused through a hemofilter and a high volume of ultrafiltrate is generated. Ultrafiltrate volume in excess of the desired rate of fluid removal is replaced with crystalloid solution that may be infused prior to the hemofilter (prefilter replacement fluid), into the return line (postfilter replacement fluid), or both. The net ultrafiltration rate is equal to the difference between the effluent and the replacement fluids flow rates. (C) Continuous venovenous hemodialysis (CVVHD): blood in the extracorporeal circuit is perfused through a hemodialyzer, dialysate is perfused across the membrane, and an ultrafiltrate is generated equal to the desired rate of fluid removal. The effluent consists of both the spent dialysate and ultrafiltrate with the net ultrafiltration rate equal to the difference between the effluent and dialysate flow rates. (D) Continuous venovenous hemodiafiltration (CVVHDF): blood in the extracorporeal circuit is perfused through a hemofilter, dialysate is perfused across the membrane, and a high volume of ultrafiltrate is generated. Ultrafiltrate volume in excess of the desired rate of fluid removal is replaced with crystalloid solution that may be infused before the hemofilter (prefilter replacement fluid), into the return line (postfilter replacement fluid), or both. The effluent consists of both the spent dialysate and ultrafiltrate with the net ultrafiltration rate equal to the difference between the effluent flow rate and the sum of dialysate and replacement fluid flow rates.
Continuous venovenous hemofiltration (CVVH) provides solute clearance primarily by advection (Fig 1B). During CVVH, ultrafiltration is provided at rates higher than required for volume management and the excess ultrafiltrate is replaced with pre- or postfilter infusion of solutions with electrolyte composition similar to plasma water. Ultrafiltration is driven by the hydrostatic pressure across the hemofilter membrane; solutes with molecular diameter smaller than the size of the membrane pores will cross the membrane entrained in the flow of ultrafiltrate.
Continuous venovenous hemodialysis (CVVHD) resembles conventional intermittent hemodialysis in that solute removal across the dialyzer membrane occurs primarily by diffusion of solutes down their concentration gradients (Fig 1C). Because diffusion is highly dependent on the mobility of solutes in solution and is inversely related to the molecular weight of the solute, low-molecular-weight solutes are more readily cleared by diffusion than higher-molecular-weight solutes. Net ultrafiltration rates are set based on the desired rate of volume removal. Thus, unlike CVVH, CVVHD uses the perfusion of dialysate with an electrolyte composition resembling normal plasma water across the opposite side of the dialyzer membrane from the blood but does not require the infusion of intravenous replacement solutions. Although CVVHD is commonly referred to as a predominantly diffusive therapy, filtration from blood into the dialysate compartment and back filtration from dialysate into blood will occur as the result of the change of pressure gradients along the length of the membrane. As a result, when membranes with high hydraulic permeability are used, substantial rates of advective transport may also occur, permitting removal of solutes with higher molecular weights than would be expected by diffusion alone.
Continuous venovenous hemodiafiltration (CVVHDF) combines the higher ultrafiltration rates and intravenous replacement fluids used during CVVH with the dialysate flow of CVVHD (Fig 1D). This combination of hemodialysis and hemofiltration achieves solute clearance by both advection and diffusion.
Choosing a Mode of CKRT
The initial step in prescription of CKRT is selection of the mode of therapy. Hemofiltration is preferred by some practitioners because of the higher clearances provided for larger molecules as compared with the diffusive clearance provided by CVVHD, which has been postulated to provide modulation of inflammatory cytokines. Although this is a theoretical benefit, no difference in survival, recovery of kidney function, vasopressor use, or organ dysfunction has been demonstrated when outcomes of CVVH and CVVHD are compared.30 Potential reasons for this lack of benefit include the relatively low clearance achieved for inflammatory mediators as compared with their biological half-lives and the concomitant removal of anti-inflammatory cytokines.
The initial development of CVVHDF was driven by technological limitations of early CKRT equipment that restricted the maximal clearances that could be provided with either CVVH or CVVHD alone. Given the operational characteristics of current CKRT equipment, adequate solute clearance can be achieved with all 3 modalities, and no specific therapeutic benefit can be ascribed to CVVHDF. Thus, selection of mode of CKRT is generally a matter of individual or institutional preference rather than being driven by specific clinical outcomes.
Hemofilter/Hemodialyzer Selection
Hemofilters/hemodialyzers containing membranes with high hydrostatic permeability, to permit high ultrafiltration rates, are generally most appropriate for CKRT. Membrane permeability is dependent on the number of pores, pore size, and membrane thickness. High-flux membranes that have more or larger pores allow greater ultrafiltration. As pore diameter increases, larger solutes can be entrained in the ultrafiltrate and can cross the membrane by advection. Typically, membranes used for hemodialysis and hemofiltration permit solutes with molecular weights of up to 10 to 40 kDa to cross the membrane, but membranes with even larger pore sizes can be used. Membrane surface area determines the available area for diffusion and ultrafiltration. Although larger surface area membranes may increase maximal clearance, increasing membrane surface area poses an increased risk for clotting. Membranes with anionic or cationic residues may also bind solutes to the membrane surface; surface adsorption of midsized molecules including inflammatory mediators has been demonstrated, although generally with rapid saturation.31
Membrane biocompatibility is also an important consideration. Biocompatibility is defined by the degree of activation of humoral and cellular mediators on exposure to blood.32 Unmodified cellulosic membranes are generally less biocompatible than synthetic membranes and have been associated with impaired survival and delayed recovery of kidney function.33, 34, 35, 36 Thus, high-flux biocompatible membranes such as polyacrylonitrile and polysulfone membranes are generally preferred for CKRT.
Blood Flow
Selection of a blood flow rate for CKRT should optimize patency of the extracorporeal circuit, avoid hemodynamic stress, and provide sufficient flow to maintain solute clearance. Although it is often believed that higher blood flow rates are associated with increased risk for hemodynamic instability, when using hollow-fiber membranes there is little relationship between blood flow through the extracorporeal circuit and hemodynamic stability. Other membrane configurations that have been used for hemodialysis in the past (eg, parallel plate dialyzers and coils) were associated with substantial increases in extracorporeal volume as blood flow increased. In contrast, hollow-fiber membranes have low compliance and exhibit minimal change in extracorporeal volume with variation in blood flow, minimizing the risk for flow-related hemodynamic stress with higher blood flow rates. At the same time, very high blood flow rates, as frequently used during IHD, are not required to optimize solute clearance. Solute clearance during CVVHD is generally dialysate-flow rather than blood-flow limited and during CVVH is dependent on ultrafiltration.
The use of higher flow rates decreases filtration fraction during hemofiltration (discussed in Dialysate/Replacement Fluid Flow Rates), diminishing the risk for system clotting. However, when catheter function is poor, higher flow rates may trigger alarms, interrupting treatment and increasing clotting risk. Higher flow rates may also dilute anticoagulation and diminish its effectiveness. Conversely, if very low blood flow rates (eg, ≤100 mL/min) are used, equilibration with dialysate, particularly at higher dialysate flow rates, may be incomplete, resulting in lower-than-expected clearances. Similarly, low blood flow rates during CVVH with prefilter replacement may result in excessive solute dilution and decreased clearance. Thus, the optimal blood flow rate for most patients is between 150 and 250 mL/min, as permitted by catheter function.
Dialysate/Replacement Fluid Composition
Dialysate and replacement fluids should have an electrolyte composition approximating that of plasma water, with sufficient buffer to correct metabolic acidosis.37 Multiple commercial solutions are available with variable concentrations of calcium and potassium. Although dialysate does not need to be sterile, replacement fluids must meet the sterility standards for intravenous solutions. Glucose concentrations of solutions vary. Physiologic glucose concentrations are generally preferred, although glucose-free solutions can be used but are associated with an increased risk for hypoglycemia or euglycemic ketoacidosis.38, 39, 40 Solutions with high glucose concentration were used in the past and were associated with significant hyperglycemia.
Although non–bicarbonate-buffered solutions were frequently used in the past, these have now been supplanted by commercially-available bicarbonate-buffered fluids. Typically, a buffer concentration of 35 mmol/L is sufficient to provide control of acidosis; however, higher compositions may be necessary to compensate for marked hypercarbia. Lower concentrations are needed when citrate anticoagulation is used to adjust for citrate’s buffering capacity and may also be needed to prevent alkalemia when patients are maintained on CKRT for a prolonged duration.
The use of phosphate-free solutions is associated with significant risk for hypophosphatemia and need for phosphorus supplementation.41 The use of phosphate-containing fluids when hyperphosphatemia has resolved can prevent the development of hypophosphatemia and obviate the need for phosphorus replacement.42
Sodium concentrations can be adjusted to assist in the treatment of dysnatremia. Adding free water to dialysate or replacement fluid can mitigate the rate of increase in serum sodium levels in patients with severe hyponatremia.43 Alternatively, 5% dextrose in water can be administered post filter to control the rate of increase in plasma sodium concentration.
Dialysate/Replacement Fluid Flow Rates
Solute clearance during CKRT is determined by the effluent volume, which is the sum of the dialysate, replacement fluid, and ultrafiltration flow rates.44 Because dialysate flow is usually markedly lower than blood flow rates, almost complete equilibrium of low-molecular-weight molecules is achieved between plasma and dialysate during CVVHD. Similarly, the concentration of low-molecular-weight solutes in the ultrafiltrate during CVVH approximates the concentration in plasma water. Thus, the concentration of low-molecular-weight solutes in the effluent will correspond to the concentration in plasma water for all modes of CKRT, and clearance of these solutes approximates the effluent flow rate.
Several single-center trials suggested that effluent flow rates during CKRT of ≥35 mL/kg per hour were associated with improved survival.45,46 However, these results were not confirmed in 2 large multicenter RCTs, which did not find improved survival associated with effluent flow rates > 25 mL/kg per hour.47,48 In a meta-analysis, higher doses of therapy were also associated with delayed recovery of kidney function.49 Higher doses of CKRT are also associated with an increased risk for electrolyte abnormalities, particularly hypophosphatemia, which may have contributed to fewer ventilator-free days among patients treated with higher doses of CKRT.50 Higher doses of therapy also increase the likelihood of subtherapeutic antibiotic levels in patients with sepsis.51 For these reasons, the KDIGO guidelines recommend a target effluent flow of 20 to 25 mL/kg per hour, with the caveat that a higher prescribed dose may be required to deliver this ideal target dose.3,52
An additional consideration in prescribing replacement fluid during CVVH and CVVHDF is whether the fluid is infused between the blood pump and the hemofilter inlet (prefilter) or in the return line to the patient (postfilter). During hemofiltration, the high ultrafiltration rate can result in significant hemoconcentration within the hemofilter, increasing the risk for fiber occlusion and hemofilter failure. Prefilter administration of replacement fluid mitigates this hemoconcentration but at the expense of diluting the solute concentration within the hemofilter and reducing solute clearance. The ratio of ultrafiltration rate to plasma flow rate within the hemofilter when replacement fluid is infused postfilter is known as the filtration fraction and quantifies the degree of hemoconcentration occurring within the hemofilter.53 When the filtration fraction is >30%, there is an increased risk for hemofilter failure. Thus, the prescription should be adjusted during hemofiltration to maintain the filtration fraction at <20%. This can be achieved by shifting a portion or all of the replacement fluid from post- to prefilter or by increasing blood flow rate.
Net Ultrafiltration Rate
In addition to solute control, one of the major goals of CKRT is the management of volume overload. Critically ill patients have been shown to have poor outcomes with worsening fluid overload and the speed of fluid accumulation is an independent risk factor for hospital mortality.54 However, the optimal rate of net ultrafiltration (the difference between effluent flow rate and the sum of dialysate and replacement fluid administration) is uncertain and is likely dependent on whether the patient is in the resuscitation, optimization, stabilization, or de-escalation phase of volume management.55 During the initial resuscitation phase, any net ultrafiltration may be inappropriate; during optimization and stabilization, modest net ultrafiltration to mitigate further fluid overload may be appropriate, whereas more aggressive ultrafiltration may be indicated during the de-escalation or de-resuscitation phase. Failure to account for this temporal arc in the goals of fluid management may account in part for contradictory findings from observational studies attempting to define a single optimal rate of net ultrafiltration.56,57 Rather, individualization of prescribed ultrafiltration rates based on collaboration between intensivist and nephrologist is critical.
Anticoagulation
Clotting is the most common complication of CKRT. However, anticoagulation in the critically ill patient is associated with an increased risk for bleeding. Partial clotting of hemofilter/hemodialyzer fibers decreases solute clearance and ultrafiltration while complete clotting of the extracorporeal circuit contributes to treatment interruption and increased blood loss. Estimates suggest that CKRT is provided without anticoagulation 30% to 60% of the time.58 The most commonly used methods for anticoagulation are unfractionated heparin (UFH) and regional citrate anticoagulation (RCA). Less commonly, low-molecular-weight heparin, regional heparinization with protamine reversal, thrombin antagonists (eg, argatroban and bivalirudin), platelet-inhibiting agents, and prostacyclin have been used, but are not discussed here in detail.
Unfractionated Heparin
UFH is usually administered as an initial bolus of 10 to 30 IU/kg followed by a maintenance infusion of 5 to 10 IU/kg per hour into the arterial limb of the extracorporeal circuit. Heparin therapy can be monitored based on the activated partial thromboplastin time, with a target of 45 to 60 seconds (1.5-2 times normal), or by monitoring anti-factor Xa levels, with a target based on the low end of the specific laboratory’s therapeutic range. Bleeding episodes range from 10% to 50%, with mortality from bleeding ~15%.59 Heparin-induced thrombocytopenia may also develop and necessitates immediate discontinuation of heparin anticoagulation and consideration of systemic anticoagulation with argatroban.3
Regional Citrate Anticoagulation
Citrate chelates calcium, a necessary cofactor for the coagulation cascade. Infusion of citrate into the arterial limb of the extracorporeal circuit provides regional anticoagulation of the circuit with minimal systemic effects.60 Coagulation is inhibited when ionized calcium levels are <0.35 mmol/L in the extracorporeal circuit, which equates to a citrate concentration of 3 to 6 mmol/L in blood.61 When the blood is returned to the body, the citrate is metabolized and ionized calcium levels are restored. Because RCA is usually performed using calcium-free dialysate and replacement fluids, calcium needs to be infused systemically to replace the calcium lost in the effluent, although even when calcium-containing fluids are used, calcium infusion is required to replace calcium lost complexed to citrate.
Management of RCA requires careful titration of the citrate and calcium infusions to maintain the postfilter ionized calcium concentration between 0.25 and 0.40 mmol/L in the extracorporeal circuit while ensuring a normal systemic ionized calcium concentration. Because citrate levels are not readily measured, systemic citrate accumulation can be monitored for based on the ratio of total to ionized calcium, with a ratio > 2.5 indicative of significant citrate accumulation.62,63
Complications of citrate anticoagulation can include both metabolic alkalosis or, with impaired metabolism, a high anion gap metabolic acidosis, and symptomatic hypomagnesemia and hypocalcemia. The use of dialysate and replacement solutions with reduced buffer content may be necessary to mitigate the risk for citrate-induced metabolic alkalosis, and if a high-concentration citrate solution such as acid citrate dextrose is used, reducing the sodium concentration may be required to prevent hypernatremia. Because the required rate of citrate administration is proportional to the blood flow rate, the systemic effects of citrate can be mitigated by using lower blood flow rates, with optimal blood flow rates of 150 to 200 mL/min. As a result of impaired metabolic clearance, the use of citrate is relatively contraindicated in the setting of shock, liver failure, and muscle hypoperfusion.3,64 The use of RCA is associated with increased circuit lifespan and lower bleeding risks as compared with UFH.65,66 KDIGO recommends RCA over UFH in CKRT patients.3 However citrate is not approved by the US Food and Drug Administration for use in the United States.
Vascular Access
A well-functioning vascular access is essential to obtain adequate blood flows for CKRT. Most commonly, access is achieved using a large-bore nontunneled double-lumen catheter in an internal jugular, femoral, or subclavian vein. The right internal jugular vein is the preferred site because it provides the shortest and straightest course to the right atrium.3 Although femoral catheters are generally associated with higher rates of bacteremia than internal jugular catheters, the KDIGO guidelines recommend use of femoral catheters over the left internal jugular vein on the basis of the Cathedia trial, which demonstrated similar rates of infection with femoral and internal jugular vein catheters67 but with higher rates of catheter dysfunction using left internal jugular vein catheters.68 However, an important caveat is that the relative risk for femoral catheter bacterial colonization was higher in obese patients, defined based on a body mass index > 28.4 kg/m2.67 Thus, in many patients, use of left internal jugular venous catheters may be more appropriate. Although subclavian venous catheters are associated with the lowest rates of infection, their use is not recommended due to higher rates of insertion complications and risks for catheter induced subclavian vein stenosis and thrombosis.3 Proper positioning of the catheter tip is critical for adequate catheter function. For internal jugular catheters, depending on catheter design, the tip should be positioned at the junction of the superior vena cava and the right atrium or in the right atrium.69 Given the venous anatomy, a longer catheter is therefore required for insertions in the left than in the right internal jugular vein. Even longer catheters are required in the femoral position, with optimal positioning within or as close to the inferior vena cava as possible. Catheter malposition is associated with an increased risk for catheter malfunction leading to restricted blood flow and elevated access pressures, often leading to machine alarms, interruption of circuit flow, and increased circuit clotting. Although tunneled catheters are not recommended for routine use, they are associated with lower rates of infection and should be considered when the need for KRT is expected to be prolonged.3
Monitoring and Dose Adjustment of Medications
Drug dosing may need to be adjusted to maintain therapeutic blood levels, particularly in the setting of high effluent flow rates. Factors that affect clearance include the molecular weight, degree of protein binding, and volume of distribution of the drug; the mode of KRT (diffusion vs convection); and residual kidney function.51,70 Underdosing of medications, especially antibiotics, may lead to treatment failure, especially when higher doses of CKRT are used.51,71 Medications whose effect can be assessed immediately (eg, inotropes, vasopressors, and pain medications) should be titrated based on clinical response. Drugs with a high volume of distribution and affinity of protein binding may not need significant dose adjustments due to their poor extracorporeal clearance. For drugs that are removed by extracorporeal therapy, pharmacokinetic monitoring should be used when available to ensure adequate dosing.
Monitoring of Treatment Adequacy
Primary monitoring of treatment adequacy should be directed at ensuring appropriate prescription and delivery of a target low-molecular-weight solute clearance of 20 to 25 mL/kg per hour and minimization of time off therapy. Although the target dose of 20 to 25 mL/kg per hour is sufficient for most patients, individual patients who are highly catabolic may require higher doses to achieve adequate control of hyperkalemia, metabolic acidosis, and azotemia. Higher doses of therapy are also indicated when CKRT is used in the management of poisonings and drug intoxications and in some patients with hyperammonemia. Ongoing monitoring of serum urea nitrogen (SUN), electrolyte, calcium, phosphorus, and magnesium levels is essential to ensure treatment adequacy and avoid critical iatrogenic complications.
Filtrate Urea Nitrogen to SUN Ratio
The ratio between the filtrate or effluent urea nitrogen and SUN levels can be used as an index of hemofilter/hemodialyzer function. A primary assumption underlying the use of a fixed dose of CKRT of 20 to 25 mL/kg per hour is that filtrate urea nitrogen to SUN ratio approximates unity. In patients in whom the prescribed treatment is not achieving the desired degree of metabolic control, measuring the filtrate urea nitrogen to SUN ratio can provide an assessment of loss of filter efficacy over time.72 This may be of particular importance during CVVH and CVVHDF, in which higher rates of advection can result in protein polarization along the hemofilter membrane.
Nutrition
Dialysis patients are generally at a high protein catabolic state and undergo losses of amino acids and water-soluble vitamins. A daily caloric intake of 35 kcal/kg and protein of 1.5 g/kg provided through enteral feeding is recommended. Additional replacement of water-soluble vitamins is also recommended to account for CKRT losses, although the optimal dose is not defined and replacement has not been associated with improved survival.73
Ethical Considerations
The appropriateness of continuing CKRT treatment is always a consideration in critically ill patients. Consistent information needs to be presented to the patient and the family by both the primary teams and the other consulting teams. Alternative modalities of treatment should always be presented, while realistically explaining the overall prognosis. Identifying the appropriate surrogate decision maker is key if the patient is unable, as is often the case in critically ill patients, to participate in decision making regarding initiation and continuation or discontinuation of therapy. When patients are able to participate in decision making, they should be encouraged to include their families in the decision-making process. Given the uncertainty in establishing prognosis, time-limited trials of therapy with reassessment of disease trajectory should often be considered. Palliative care consultation is often beneficial in helping with difficult end-of-life decisions and in providing emotional support to patients and family during this difficult time.
Article Information
Authors’ Full Names and Academic Degrees
Siddharth Verma, MD and Paul M. Palevsky, MD.
Support
None
Financial Disclosure
The authors declare that they have no relevant financial interests.
Peer Review
Received February 11, 2021, in response to an invitation from the journal. Evaluated by 2 external peer reviewers, with direct editorial input by the Editor-in-Chief. Accepted in revised form May 23, 2021.
Footnotes
Complete author and article information provided before references.
References
- 1.Ostermann M., Joannidis M. Acute kidney injury 2016: diagnosis and diagnostic workup. Crit Care. 2016;20:299. doi: 10.1186/s13054-016-1478-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoste E.A., Bagshaw S.M., Bellomo R. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 2015;41:1411–1423. doi: 10.1007/s00134-015-3934-7. [DOI] [PubMed] [Google Scholar]
- 3.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1–138. [Google Scholar]
- 4.Bellomo R., Ronco C., Kellum J.A., Mehta R.L., Palevsky P. Acute Dialysis Quality Initiative workgroup. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kramer P., Wigger W., Rieger J., Matthaei D., Scheler F. [Arteriovenous haemofiltration: a new and simple method for treatment of over-hydrated patients resistant to diuretics] Klin Wochenschr. 1977;55:1121–1122. doi: 10.1007/BF01477940. [DOI] [PubMed] [Google Scholar]
- 6.Paganini E.P., Fouad F., Tarazi R.C., Bravo E.L., Nakamoto S. Hemodynamics of isolated ultrafiltration in chronic hemodialysis patients. Trans Am Soc Artif Intern Organs. 1979;25:422–425. doi: 10.1097/00002480-197902500-00081. [DOI] [PubMed] [Google Scholar]
- 7.Macias W.L., Mueller B.A., Scarim S.K., Robinson M., Rudy D.W. Continuous venovenous hemofiltration: an alternative to continuous arteriovenous hemofiltration and hemodiafiltration in acute renal failure. Am J Kidney Dis. 1991;18:451–458. doi: 10.1016/s0272-6386(12)80113-2. [DOI] [PubMed] [Google Scholar]
- 8.Ronco C. Continuous renal replacement therapy: forty-year anniversary. Int J Artif Organs. 2017;40:257–264. doi: 10.5301/ijao.5000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nash D.M., Przech S., Wald R., O'Reilly D. Systematic review and meta-analysis of renal replacement therapy modalities for acute kidney injury in the intensive care unit. J Crit Care. 2017;41:138–144. doi: 10.1016/j.jcrc.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 10.Vinsonneau C., Camus C., Combes A. Continuous venovenous haemodiafiltration versus intermittent haemodialysis for acute renal failure in patients with multiple-organ dysfunction syndrome: a multicentre randomised trial. Lancet. 2006;368:379–385. doi: 10.1016/S0140-6736(06)69111-3. [DOI] [PubMed] [Google Scholar]
- 11.Mehta R.L., McDonald B., Gabbai F.B. A randomized clinical trial of continuous versus intermittent dialysis for acute renal failure. Kidney Int. 2001;60:1154–1163. doi: 10.1046/j.1523-1755.2001.0600031154.x. [DOI] [PubMed] [Google Scholar]
- 12.Augustine J.J., Sandy D., Seifert T.H., Paganini E.P. A randomized controlled trial comparing intermittent with continuous dialysis in patients with ARF. Am J Kidney Dis. 2004;44:1000–1007. doi: 10.1053/j.ajkd.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 13.Bagshaw S.M., Berthiaume L.R., Delaney A., Bellomo R. Continuous versus intermittent renal replacement therapy for critically ill patients with acute kidney injury: a meta-analysis. Crit Care Med. 2008;36:610–617. doi: 10.1097/01.CCM.0B013E3181611F552. [DOI] [PubMed] [Google Scholar]
- 14.Pannu N., Klarenbach S., Wiebe N., Manns B., Tonelli M., Alberta Kidney Disease Network Renal replacement therapy in patients with acute renal failure: a systematic review. JAMA. 2008;299:793–805. doi: 10.1001/jama.299.7.793. [DOI] [PubMed] [Google Scholar]
- 15.Bouchard J., Soroko S.B., Chertow G.M. Fluid accumulation, survival and recovery of kidney function in critically ill patients with acute kidney injury. Kidney Int. 2009;76:422–427. doi: 10.1038/ki.2009.159. [DOI] [PubMed] [Google Scholar]
- 16.Davenport A., Will E.J., Davidson A.M. Improved cardiovascular stability during continuous modes of renal replacement therapy in critically ill patients with acute hepatic and renal failure. Crit Care Med. 1993;21:328–338. doi: 10.1097/00003246-199303000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Fulop T., Zsom L., Rodriguez R.D., Chabrier-Rosello J.O., Hamrahian M., Koch C.A. Therapeutic hypernatremia management during continuous renal replacement therapy with elevated intracranial pressures and respiratory failure. Rev Endocr Metab Disord. 2019;20:65–75. doi: 10.1007/s11154-019-09483-2. [DOI] [PubMed] [Google Scholar]
- 18.Schneider A.G., Bagshaw S.M. Effects of renal replacement therapy on renal recovery after acute kidney injury. Nephron Clin Pract. 2014;127:35–41. doi: 10.1159/000363671. [DOI] [PubMed] [Google Scholar]
- 19.Decker B.S., Goldfarb D.S., Dargan P.I. Extracorporeal treatment for lithium poisoning: systematic review and recommendations from the EXTRIP Workgroup. Clin J Am Soc Nephrol. 2015;10:875–887. doi: 10.2215/CJN.10021014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Juurlink D.N., Gosselin S., Kielstein J.T. Extracorporeal treatment for salicylate poisoning: systematic review and recommendations from the EXTRIP Workgroup. Ann Emerg Med. 2015;66:165–181. doi: 10.1016/j.annemergmed.2015.03.031. [DOI] [PubMed] [Google Scholar]
- 21.Patel N., Bayliss G.P. Developments in extracorporeal therapy for the poisoned patient. Adv Drug Deliv Rev. 2015;90:3–11. doi: 10.1016/j.addr.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 22.Seabra V.F., Balk E.M., Liangos O., Sosa M.A., Cendoroglo M., Jaber B.L. Timing of renal replacement therapy initiation in acute renal failure: a meta-analysis. Am J Kidney Dis. 2008;52:272–284. doi: 10.1053/j.ajkd.2008.02.371. [DOI] [PubMed] [Google Scholar]
- 23.Zarbock A., Kellum J.A., Schmidt C. Effect of early vs delayed initiation of renal replacement therapy on mortality in critically ill patients with acute kidney injury: the ELAIN randomized clinical trial. JAMA. 2016;315:2190–2199. doi: 10.1001/jama.2016.5828. [DOI] [PubMed] [Google Scholar]
- 24.Gaudry S., Hajage D., Schortgen F. Initiation strategies for renal-replacement therapy in the intensive care unit. N Engl J Med. 2016;375:122–133. doi: 10.1056/NEJMoa1603017. [DOI] [PubMed] [Google Scholar]
- 25.Barbar S.D., Clere-Jehl R., Bourredjem A. Timing of renal-replacement therapy in patients with acute kidney injury and sepsis. N Engl J Med. 2018;379:1431–1442. doi: 10.1056/NEJMoa1803213. [DOI] [PubMed] [Google Scholar]
- 26.The STARRT-AKI Investigators for the Canadian Critical Care Trials Group; the Australian and New Zealand Intensive Care Society Clinical Trials Group; the United Kingdom Critical Care Research Group Timing of initiation of renal-replacement therapy in acute kidney injury. N Engl J Med. 2020;383:240–251. doi: 10.1056/NEJMoa2000741. [DOI] [PubMed] [Google Scholar]
- 27.Gaudry S., Hajage D., Martin-Lefevre L. Comparison of two delayed strategies for renal replacement therapy initiation for severe acute kidney injury (AKIKI 2): a multicentre, open-label, randomised, controlled trial. Lancet. 2021;397:1293–1300. doi: 10.1016/S0140-6736(21)00350-0. [DOI] [PubMed] [Google Scholar]
- 28.Costanzo M.R., Guglin M.E., Saltzberg M.T. Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure. J Am Coll Cardiol. 2007;49:675–683. doi: 10.1016/j.jacc.2006.07.073. [DOI] [PubMed] [Google Scholar]
- 29.Bart B.A., Goldsmith S.R., Lee K.L. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med. 2012;367:2296–2304. doi: 10.1056/NEJMoa1210357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Friedrich J.O., Wald R., Bagshaw S.M., Burns K.E., Adhikari N.K. Hemofiltration compared to hemodialysis for acute kidney injury: systematic review and meta-analysis. Crit Care. 2012;16:R146. doi: 10.1186/cc11458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Onichimowski D., Ziolkowski H., Nosek K., Jaroszewski J., Rypulak E., Czuczwar M. Comparison of adsorption of selected antibiotics on the filters in continuous renal replacement therapy circuits: in vitro studies. J Artif Organs. 2020;23:163–170. doi: 10.1007/s10047-019-01139-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chanard J., Lavaud S., Randoux C., Rieu P. New insights in dialysis membrane biocompatibility: relevance of adsorption properties and heparin binding. Nephrol Dial Transplant. 2003;18:252–257. doi: 10.1093/ndt/18.2.252. [DOI] [PubMed] [Google Scholar]
- 33.Hakim R.M., Breillatt J., Lazarus J.M., Port F.K. Complement activation and hypersensitivity reactions to dialysis membranes. N Engl J Med. 1984;311:878–882. doi: 10.1056/NEJM198410043111403. [DOI] [PubMed] [Google Scholar]
- 34.Himmelfarb J., Tolkoff Rubin N., Chandran P., Parker R.A., Wingard R.L., Hakim R. A multicenter comparison of dialysis membranes in the treatment of acute renal failure requiring dialysis. J Am Soc Nephrol. 1998;9:257–266. doi: 10.1681/ASN.V92257. [DOI] [PubMed] [Google Scholar]
- 35.Subramanian S., Venkataraman R., Kellum J.A. Influence of dialysis membranes on outcomes in acute renal failure: a meta-analysis. Kidney Int. 2002;62:1819–1823. doi: 10.1046/j.1523-1755.2002.00608.x. [DOI] [PubMed] [Google Scholar]
- 36.Jaber B.L., Lau J., Schmid C.H., Karsou S.A., Levey A.S., Pereira B.J. Effect of biocompatibility of hemodialysis membranes on mortality in acute renal failure: a meta-analysis. Clin Nephrol. 2002;57:274–282. doi: 10.5414/cnp57274. [DOI] [PubMed] [Google Scholar]
- 37.Schetz M., Leblanc M., Murray P.T. The Acute Dialysis Quality Initiative--part VII: fluid composition and management in CRRT. Adv Ren Replace Ther. 2002;9:282–289. doi: 10.1053/jarr.2002.35572. [DOI] [PubMed] [Google Scholar]
- 38.Sriperumbuduri S., Clark E., Biyani M., Ruzicka M. High anion gap metabolic acidosis on continuous renal replacement therapy. Kidney Int Rep. 2020;5:1833–1835. doi: 10.1016/j.ekir.2020.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coutrot M., Hekimian G., Moulin T. Euglycemic ketoacidosis, a common and underecognized complication of continuous renal replacement therapy using glucose-free solutions. Intensive Care Med. 2018;44:1185–1186. doi: 10.1007/s00134-018-5118-8. [DOI] [PubMed] [Google Scholar]
- 40.Ting S., Chua H.-R., Cove M.E. Euglycemic ketosis during continuous kidney replacement therapy with glucose-free solution: a report of 8 cases. Am J Kidney Dis. 2021;78:305–308. doi: 10.1053/j.ajkd.2020.10.014. [DOI] [PubMed] [Google Scholar]
- 41.Bellomo R., Ernest D., Love J., Parkin G., Boyce N. Continuous arteriovenous haemodiafiltration: optimal therapy for acute renal failure in an intensive care setting? Aust N Z J Med. 1990;20:237–242. doi: 10.1111/j.1445-5994.1990.tb01027.x. [DOI] [PubMed] [Google Scholar]
- 42.Troyanov S., Geadah D., Ghannoum M., Cardinal J., Leblanc M. Phosphate addition to hemodiafiltration solutions during continuous renal replacement therapy. Intensive Care Med. 2004;30:1662–1665. doi: 10.1007/s00134-004-2333-2. [DOI] [PubMed] [Google Scholar]
- 43.Tandukar S., Kim C., Kalra K., Verma S., Palevsky P.M., Puttarajappa C. Severe hyponatremia and continuous renal replacement therapy: safety and effectiveness of low-sodium dialysate. Kidney Med. 2020;2:437–449. doi: 10.1016/j.xkme.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bouchard J., Weidemann C., Mehta R.L. Renal replacement therapy in acute kidney injury: intermittent versus continuous? How much is enough? Adv Chronic Kidney Dis. 2008;15:235–247. doi: 10.1053/j.ackd.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 45.Ronco C., Bellomo R., Homel P. Effects of different doses in continuous veno-venous haemofiltration on outcomes of acute renal failure: a prospective randomised trial. Lancet. 2000;356:26–30. doi: 10.1016/S0140-6736(00)02430-2. [DOI] [PubMed] [Google Scholar]
- 46.Saudan P., Niederberger M., De Seigneux S. Adding a dialysis dose to continuous hemofiltration increases survival in patients with acute renal failure. Kidney Int. 2006;70:1312–1317. doi: 10.1038/sj.ki.5001705. [DOI] [PubMed] [Google Scholar]
- 47.The VA/NIH Acute Renal Failure Trial Network. Palevsky P.M., Zhang J.H. Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med. 2008;359:7–20. doi: 10.1056/NEJMoa0802639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.RENAL Replacement Therapy Study Investigators. Bellomo R., Cass A. Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med. 2009;361:1627–1638. doi: 10.1056/NEJMoa0902413. [DOI] [PubMed] [Google Scholar]
- 49.Wang Y., Gallagher M., Li Q. Renal replacement therapy intensity for acute kidney injury and recovery to dialysis independence: a systematic review and individual patient data meta-analysis. Nephrol Dial Transplant. 2018;33:1017–1024. doi: 10.1093/ndt/gfx308. [DOI] [PubMed] [Google Scholar]
- 50.Sharma S., Kelly Y.P., Palevsky P.M., Waikar S.S. Intensity of renal replacement therapy and duration of mechanical ventilation: secondary analysis of the Acute Renal Failure Trial Network Study. Chest. 2020;158:1473–1481. doi: 10.1016/j.chest.2020.05.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mueller B.A., Pasko D.A., Sowinski K.M. Higher renal replacement therapy dose delivery influences on drug therapy. Artif Organs. 2003;27:808–814. doi: 10.1046/j.1525-1594.2003.07283.x. [DOI] [PubMed] [Google Scholar]
- 52.Lyndon W.D., Wille K.M., Tolwani A.J. Solute clearance in CRRT: prescribed dose versus actual delivered dose. Nephrol Dial Transplant. 2012;27:952–956. doi: 10.1093/ndt/gfr480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hatamizadeh P., Tolwani A., Palevsky P. Revisiting filtration fraction as an index of the risk of hemofilter clotting in continuous venovenous hemofiltration. Clin J Am Soc Nephrol. 2020;15:1660–1662. doi: 10.2215/CJN.02410220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Garzotto F., Ostermann M., Martin-Langerwerf D. The Dose Response Multicentre Investigation on Fluid Assessment (DoReMIFA) in critically ill patients. Crit Care. 2016;20:196. doi: 10.1186/s13054-016-1355-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Malbrain M., Van Regenmortel N., Saugel B. Principles of fluid management and stewardship in septic shock: it is time to consider the four D's and the four phases of fluid therapy. Ann Intensive Care. 2018;8:66. doi: 10.1186/s13613-018-0402-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Murugan R., Balakumar V., Kerti S.J. Net ultrafiltration intensity and mortality in critically ill patients with fluid overload. Crit Care. 2018;22:223. doi: 10.1186/s13054-018-2163-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Murugan R., Kerti S.J., Chang C.H. Association of net ultrafiltration rate with mortality among critically ill adults with acute kidney injury receiving continuous venovenous hemodiafiltration: a secondary analysis of the Randomized Evaluation of Normal vs Augmented Level (RENAL) of Renal Replacement Therapy Trial. JAMA Netw Open. 2019;2 doi: 10.1001/jamanetworkopen.2019.5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Uchino S., Bellomo R., Morimatsu H. Continuous renal replacement therapy: a worldwide practice survey. The beginning and ending supportive therapy for the kidney (B.E.S.T. kidney) investigators. Intensive Care Med. 2007;33:1563–1570. doi: 10.1007/s00134-007-0754-4. [DOI] [PubMed] [Google Scholar]
- 59.Oudemans-van Straaten H.M., de Pont A.-C.J.M., Davenport A., Gibney N. In: Acute Nephrology for the Critical Care Physician. Oudemans-van Straaten H.M., Forni L.G., Groeneveld A.B.J., Bagshaw S.M., Joannidis M., editors. Springer; 2015. Anticoagulation for continuous renal replacement therapy; pp. 187–202. [Google Scholar]
- 60.Davenport A., Tolwani A. Citrate anticoagulation for continuous renal replacement therapy (CRRT) in patients with acute kidney injury admitted to the intensive care unit. NDT Plus. 2009;2:439–447. doi: 10.1093/ndtplus/sfp136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tolwani A.J., Prendergast M.B., Speer R.R., Stofan B.S., Wille K.M. A practical citrate anticoagulation continuous venovenous hemodiafiltration protocol for metabolic control and high solute clearance. Clin J Am Soc Nephrol. 2006;1:79–87. doi: 10.2215/CJN.00040505. [DOI] [PubMed] [Google Scholar]
- 62.Meier-Kriesche H.U., Gitomer J., Finkel K., DuBose T. Increased total to ionized calcium ratio during continuous venovenous hemodialysis with regional citrate anticoagulation. Crit Care Med. 2001;29:748–752. doi: 10.1097/00003246-200104000-00010. [DOI] [PubMed] [Google Scholar]
- 63.Bakker A.J., Boerma E.C., Keidel H., Kingma P., van der Voort P.H. Detection of citrate overdose in critically ill patients on citrate-anticoagulated venovenous haemofiltration: use of ionised and total/ionised calcium. Clin Chem Lab Med. 2006;44:962–966. doi: 10.1515/CCLM.2006.164. [DOI] [PubMed] [Google Scholar]
- 64.James M., Bouchard J., Ho J. Canadian Society of Nephrology commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am J Kidney Dis. 2013;61:673–685. doi: 10.1053/j.ajkd.2013.02.350. [DOI] [PubMed] [Google Scholar]
- 65.Betjes M.G., van Oosterom D., van Agteren M., van de Wetering J. Regional citrate versus heparin anticoagulation during venovenous hemofiltration in patients at low risk for bleeding: similar hemofilter survival but significantly less bleeding. J Nephrol. 2007;20:602–608. [PubMed] [Google Scholar]
- 66.Zarbock A., Kullmar M., Kindgen-Milles D. Effect of regional citrate anticoagulation vs systemic heparin anticoagulation during continuous kidney replacement therapy on dialysis filter life span and mortality among critically ill patients with acute kidney injury: a randomized clinical trial. JAMA. 2020;324:1629–1639. doi: 10.1001/jama.2020.18618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Parienti J.J., Thirion M., Megarbane B. Femoral vs jugular venous catheterization and risk of nosocomial events in adults requiring acute renal replacement therapy: a randomized controlled trial. JAMA. 2008;299:2413–2422. doi: 10.1001/jama.299.20.2413. [DOI] [PubMed] [Google Scholar]
- 68.Parienti J.J., Megarbane B., Fischer M.O. Catheter dysfunction and dialysis performance according to vascular access among 736 critically ill adults requiring renal replacement therapy: a randomized controlled study. Crit Care Med. 2010;38:1118–1125. doi: 10.1097/CCM.0b013e3181d454b3. [DOI] [PubMed] [Google Scholar]
- 69.Morgan D., Ho K., Murray C., Davies H., Louw J. A randomized trial of catheters of different lengths to achieve right atrium versus superior vena cava placement for continuous renal replacement therapy. Am J Kidney Dis. 2012;60:272–279. doi: 10.1053/j.ajkd.2012.01.021. [DOI] [PubMed] [Google Scholar]
- 70.Pea F., Viale P., Pavan F., Furlanut M. Pharmacokinetic considerations for antimicrobial therapy in patients receiving renal replacement therapy. Clin Pharmacokinet. 2007;46:997–1038. doi: 10.2165/00003088-200746120-00003. [DOI] [PubMed] [Google Scholar]
- 71.Jiang S.P., Xu Y.Y., Ping Y. Improving antimicrobial dosing in critically ill patients receiving continuous venovenous hemofiltration and the effect of pharmacist dosing adjustment. Eur J Intern Med. 2014;25:930–935. doi: 10.1016/j.ejim.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 72.Claure-Del Granado R., Macedo E., Chertow G.M. Effluent volume in continuous renal replacement therapy overestimates the delivered dose of dialysis. Clin J Am Soc Nephrol. 2011;6:467–475. doi: 10.2215/CJN.02500310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Honore P.M., De Waele E., Jacobs R. Nutritional and metabolic alterations during continuous renal replacement therapy. Blood Purif. 2013;35:279–284. doi: 10.1159/000350610. [DOI] [PubMed] [Google Scholar]