Abstract
Anti-brush border antibody (ABBA) disease, also called anti–low-density lipoprotein receptor-related protein 2 (anti-LRP2) nephropathy, occurs due to the formation of antibodies against brush border antigens of the renal proximal convoluted tubule. We report a case of ABBA disease in a male farmer in his 30s who presented with 2 years of polyuria, dysuria, nocturia, and urinary urgency. He described a history of long-term occupational exposure to pesticides and silica, evolving into possible pneumoconiosis, and prior pulmonary tuberculosis. At presentation, he had reduced kidney function (serum creatinine 3.6 mg/dL) with hyponatremia, hypokalemia, hypophosphatemia, a normal anion gap, metabolic acidosis, and respiratory acidosis, and 2.2 g/day of urine proteinuria. The kidney biopsy was consistent with ABBA, showing amorphous immune-deposits in the tubular basement membrane and strong positivity on indirect immunofluorescence in the brush border of the proximal tubules. The trigger for production of ABBA is still unknown, but it may be associated with chronic conditions such as pulmonary tuberculosis and occupational exposures such as silica and pesticides, as seen in the patient in this report. Most cases do not respond to immunosuppression, and the prognosis is poor.
Index Words: Anti-brush border antibody disease, anti-LRP2 nephropathy, auto-antibodies, autoimmunity, chronic kidney disease, kidney biopsy, megalin
Introduction
Anti-brush border antibody (ABBA) disease/anti–low-density lipoprotein receptor-related protein 2 (anti-LRP2) nephropathy is a rare form of kidney disease that affects older patients and is characterized by acute kidney injury (AKI) and progressive renal tubular injury associated with immunoglobulin G (IgG) immune complex deposits along the basement membrane of proximal tubules, and circulating autoantibodies to the proximal tubule brush border protein LRP2 (megalin).1 Morrison et al2 first described the disease in 1981 with the case of a patient with myasthenia gravis associated with AKI, whose indirect immunofluorescence results showed positivity on the tubular basement membrane (TBM). Biopsy samples demonstrated advanced tubulointerstitial nephritis and indirect immunofluorescence positivity for immunoglobulins and C3 only in the proximal tubules. Since then, ABBA disease has been reported in the literature rarely; herein, we report a case of a man in his 30s with AKI and proteinuria, whose serum showed strong positivity in the proximal tubules’ brush border when applied on kidney tissue from a control.
Case Report
A male farmer in his 30s sought evaluation due to 2 years of urinary symptoms: polyuria, dysuria, nocturia, and urinary urgency. He confirmed long-term occupational exposure to pesticides and silica, evolving into possible pneumoconiosis. Spirometry showed severe mixed ventilatory disorder, and the pulmonary biopsy was inconclusive. He was in the last month of empirical treatment for pulmonary tuberculosis, did not report previous smoking, and had two siblings both with undiagnosed pneumopathy and with similar occupational exposures.
At physical examination, he was normotensive. The admission examinations showed creatinine at 3.6 mg/dL, hyponatremia (133 mEq/L), hypokalemia (2.7 mEq/L), hypophosphatemia (2.7 mg/dL), and mixed acidosis (pH 7.22, bicarbonate 18.1 mEq/L, and pCO2 43.2 mm Hg) with normal anion gap. The urine test revealed pH of 6.0, glucose (+), and 24-hour proteinuria was 2.2 g.
Hepatitis B, hepatitis C, human immunodeficiency virus, and autoimmune diseases tests (antinuclear-antibody, anti–smooth-muscle antibody, anti-DNA antibody, anti-glomerular basement membrane [GBM] antibody, anticardiolipin IgM and IgG, lupus anticoagulant, and antineutrophil cytoplasmic antibody) were negative. Serum complement was normal. Plasma protein electrophoresis showed an increase in the region of gamma globulins, but negative immunofixation.
Ultrasound showed a normal left kidney and a right kidney with reduced dimensions, high echogenicity, and with loss of corticomedullary differentiation. He had normal renal arteries by Doppler ultrasound, and his urodynamic was unremarkable. On kidney biopsy, the light microscopy showed 47 glomeruli: 16 were globally sclerosed, and some had segmental sclerosis. His arterial vessels showed slight thickening of the middle layer. There was fibrosis and tubular atrophy in about 20 % to 30 % of the parenchyma with interstitial mononuclear inflammatory infiltrate.
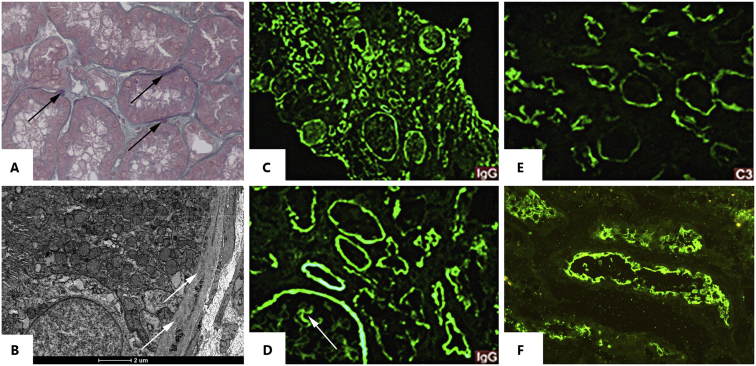
Indirect immunofluorescence was negative in the glomerular compartment and was positive in the segmental peripheral capillary wall and Bowman’s capsule staining. There was granular staining in TBM and in Bowman’s capsule for IgG (+++/3+), C3 (++/3+), and κ and λ (+++/3+). Electronic microscopy showed amorphous immune-deposits in the TBM. Indirect immunofluorescence was performed using the patient’s serum and kidney tissue from a control, which showed strong positivity in the proximal tubules’ brush border (Fig 1). After 4 months, the patient developed terminal respiratory failure and died.
Figure 1.
(A-B) Kidney needle biopsy, buffered 10 % formal paraffin process. (A) Fuchsinophilic “smudgy” appearance deposits on tubular basement membrane (Masson Trichrome stain; original magnification, ×40). (B) Electron microscopy shows mild tubular basement membrane deposits. (C-F) Kidney biopsy fixed in Michel’s solution. (C-D) Diffuse IgG deposits along Bowman capsule, tubular basement membrane, and segmental granular capillary wall (arrow). (E) Complement fraction C3 with weak stain along tubular membrane. (F) Indirect immunofluorescence microscopy from a separate patient’s normal kidney tissue. For the test, it was preincubated with serum from the studied patient and posteriorly incubated with IgG-FITC (fluorescein isothiocyanate). The staining along the brush border proves the presence of anti-brush border antibody in the serum.
Discussion
ABBA disease, or anti-LRP2 nephropathy, is a rare kidney disease associated with tubular dysfunction and progressive kidney failure, potentially manifesting with hyponatremia, hypokalemia, hypophosphatemia, proteinuria, and acidosis. This report describes a case of advanced chronic kidney disease associated with a decrease in serum levels of various electrolytes (sodium, potassium, magnesium, phosphorus, and loss of bicarbonate), which refers to the hypothesis of tubulopathy. The findings on kidney biopsy were consistent with ABBA disease/anti-LRP2 nephropathy.
After the first reports in 1981 and 1982,2, 3, 4 the next report was in 2016 when Rosales et al5 described a patient with end-stage kidney disease and negative serologies with kidney biopsy findings compatible with ABBA disease. Subsequently, in 2018 Larsen et al6 described the largest series of the disease: a cohort of 10 cases (including the report by Rosales et al5). All the patients were elderly and had reduced kidney function associated with subnephrotic proteinuria. Immunoprecipitation and mass spectrometry analysis showed that the serum from 9 of the 10 patients had an antigen-antibody reaction with a high-molecular-weight protein present on the renal tubular brush border known as megalin or LRP2, which formed a highly sensitive and disease-specific marker associated with ABBA disease. Therefore, a new nomenclature for the disease was proposed: anti-LRP2 nephropathy.6
In 2019, Dinesh et al7 published a case describing a novel feature of the disease: abundant IgG4-positive interstitial plasma cells. This feature was accompanied by the classic combination of TBM deposits, tubulointerstitial nephritis, and segmental glomerular subepithelial immune deposits. Light microscopy identified large TBM deposits, and IgG staining of apical aspects of proximal tubules using immunofluorescence microscopy pointed to the correct diagnosis of anti-LRP2 nephropathy.
More recently, authors have described ABBA disease/anti-LRP2 nephropathy associated with lupus,8 minimal change disease,9 and lymphoma,10 and highlighted the diagnostic difficulty faced by nephrologists and pathologists in the identification of this rare but likely underrecognized disease11,12 (Table 1).
Table 1.
Literature Review of ABBA Cases
| Case | Reference | Age, Sex | Comorbidities | Kidney Impairment | Autoimmunity | Kidney Biopsy | Treatment and Outcome |
|---|---|---|---|---|---|---|---|
| 1 | Morrison et al2 (1981) | 59, Male | Thymoma, myasthenia gravis |
|
|
|
|
| 2 | Douglas et al3 (1981) | 31, Male | None |
|
|
|
Not described |
| 3 | Rosales et al5 (2016) | 73, Male | DM, HTN, CAD |
|
|
|
|
| 4 | Larsen et al6 (2018) | 68, Male | Recent infection by bacillus cereus |
|
|
|
|
| 5 | Larsen et al6 (2018) | 78, Male | DM, HTN |
|
|
|
|
| 6 | Larsen et al6 (2018) | 76, Male | COPD |
|
|
|
|
| 7 | Larsen et al6 (2018) | 69, Female | DM |
|
|
|
|
| 8 | Larsen et al6 (2018) | 72, Male | DM |
|
|
|
|
| 9 | Larsen et al6 (2018) | 70, Male | HTN, gout, nephrolithiasis |
|
|
|
|
| 10 | Larsen et al6 (2018) | 66, Female | DM, HTN |
|
|
|
|
| 11 | Larsen et al6 (2018) | 77, Female | Sarcoidosis |
|
|
|
|
| 12 | Larsen et al6 (2018) | 80, Male | HTN |
|
|
|
|
| 13 | Dinesh et al7 (2019) | 90, Female | HTN, HLD, AV block, OP, PMR |
|
|
|
|
| 14 | Dvanajscak et al8 (2020) | 55, Male | DM, HTN, HLD |
|
|
|
|
| 15 | Caliskan et al9 (2020) | 79, Male | HTN, hemochromatosis, PMR, BPH |
|
|
|
|
| 16 | Gamayo et al10 (2019) | 74, Male | WM, LPL |
|
|
|
|
| 17 | Gamayo et al10 (2019) | 70, Male | HTN, DM, hypothyroidism, CLL |
|
|
|
|
| 18 | Gallan et al11 (2020) | 76, Male | HTN, DM, CKD |
|
|
|
|
| 19 | Zhu et al12 (2020) | 29, Female | None |
|
|
|
|
| 20 | Arcoverde Fechine Brito et al (2021) | 35, Male | Silicosis, pulmonary TB, exposure to pesticides |
|
|
|
|
Abbreviations: ABBA, anti-brush border antibody; AKI, acute kidney injury; ANA, antinuclear antibody; ANCA, antineutrophil cytoplasmic antibody; anti-GBM, antiglomerular basement membrane; AV block, atrioventricular block; BC, Bowman’s capsule; BPH, benign prostatic hyperplasia; CAD, coronary arterial disease; CKD, chronic kidney disease; CLL, chronic lymphocytic leukemia; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; dsDNA, double-stranded DNA; EM, electron microscopy; FU, follow-up; GBM, glomerular basement membrane; HLD, hyperlipidemia; HPF, high-power field; HTN, hypertension; IF, immunofluorescence; IgG, immunoglobulin G; IgM, immunoglobulin M; LPL, lymphoplasmacytic lymphoma; LRP2, low-density lipoprotein receptor-related protein 2 (megalin); OM, optical microscopy; OP, osteoporosis; PLA2R, M-type phospholipase A2 receptor; PMR, polymyalgia rheumatic; RBC, red blood cell; Scr, serum creatinine; SPEP, serum protein electrophoresis; TB, tuberculosis; TBM, tubular basement membrane; TIN, tubulointerstitial nephritis; UPCR, urinary protein-creatinine ratio; WM, Waldenström’s macroglobulinemia.
Our patient resembles the pattern of the cases described by Larsen et al6—a man with kidney failure and subnephrotic proteinuria who showed negative autoimmunity markers. In addition, he had a history of Mycobacterium bacillus infection, as did one of the patients in the case series in whom Bacillus cereus infection was documented. However, our patient contrasts with this cohort because he was not an elderly patient with ABBA disease.
In fact, the epidemiology of this disease is not well understood. It appears to affect mainly elderly people with comorbidities and subnephrotic proteinuria, situations in which kidney biopsy is hardly indicated. Thus, there is a high probability that this condition is underdiagnosed.
The trigger for the production of ABBA is unknown, although it may be associated with other autoimmune diseases and chronic infections.13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23 Our patient had no previous history of autoimmunity, but there was history of mycobacterial infection and occupational exposure to silica and pesticides. Mycobacterial infections are involved in mechanisms of “molecular mimicry” and consequent autoimmunity through cross reaction; therefore, it is possible that they are involved in the pathogenesis of diseases such as primary biliary cirrhosis, sarcoidosis, and systemic lupus erythematosus (SLE).14 A study published in 2007 demonstrated a significant increase in autoantibodies in 47 patients with active pulmonary tuberculosis when compared with a control group.15
Silicosis can be associated with positivity of several autoantibodies, suggesting that it may be involved in changes of immunoregulation.16 A meta-analysis involving 9 studies showed an increased risk of developing rheumatoid arthritis in individuals exposed to silica (combined relative risk of 3.43 [95 % CI, 2.25-5.22]), suggesting a causal relationship.17 A study by Parks and Cooper18 demonstrated a strong relationship between occupational exposure to silica and the appearance of autoimmune diseases such as SLE, scleroderma, rheumatoid arthritis, and others.
Regarding the correlation between exposure to pesticides and autoimmunity, in 1990 Broughton et al19 reported the association of organochlorines with the appearance of autoantibodies, including ABBA. A study performed in 1993 demonstrated that hexachlorobenzene, another pesticide, induces increased levels of autoantibodies such as anti-DNA and rheumatoid factor in animal models.20 Ten years later, Ezendam et al21 demonstrated that hexachlorobenzene led to the formation of lymph node germinal centers in mice, with an expressive increase in B-cell and T-cell subpopulations.
Serologies are not diagnostic for ABBA disease.22 Diagnostic confirmation occurs by kidney biopsy. On light microscopy, diffuse loss of the brush border of the proximal tubules is observed with signs of focal tubulitis and tubular atrophy; lymphocytic infiltrate is visible within the interstitium; the glomerular compartment has a normal pattern with a slight thickening of GBM.
On immunofluorescence, granular deposits of IgG, C3, and C4d are observed along the TBM and proportional light chain deposition, little to no deposition of IgA, IgM, C1q, and little to no immune deposits in the glomerular compartment are observed. Indirect immunofluorescence may show serum IgG reaction with antigens on a normal brush border.
On electronic microscopy, prominent amorphous electro-dense deposits are seen in TBM; discrete and limited glomerular deposits similar to the pattern of membranous glomerulonephritis can be seen in the glomerular compartment.22 Our patient showed these characteristics, and in the glomerular compartment the findings were suggestive of focal segmental glomerulosclerosis (FSGS). However, it is known that any chronic glomerular or tubular disease can reduce the nephron’s total function and result in adaptive FSGS superimposed on the primary disorder.23
Regarding indirect immunofluorescence, there was negativity in the glomerular compartment and strong positivity in the TBM and in the Bowman’s capsule for IgG, C3, and kappa and lambda. To confirm the hypothesis of ABBA disease, indirect immunofluorescence was performed using the patient’s serum and kidney tissue from a control, showing strong positivity in the proximal tubules’ brush border.
The differential diagnoses of ABBA disease mainly include (1) SLE,22 (2) IgG4 disease,24 and (3) idiopathic hypocomplementemic tubulointerstitial nephritis.25 In our patient, SLE was determined to be unlikely due to negative serologic markers and the fact that tubular deposits in the setting of lupus generally correlate with the presence of proliferative glomerular lesions,26 which were not seen in our patient with anti-LRP2 nephropathy. IgG4-related tubulointerstitial nephritis is the entity with the most morphologic overlap, because both it and anti-LRP2 nephropathy can exhibit interstitial inflammation and glomerular deposits in addition to the tubular deposits.6 However, systemic disease related to IgG4 was not clinically compatible with his presentation because of the absence of suggestive systemic signs. Finally, hypocomplementemic tubulointerstitial nephritis was ruled out because of the presence of normal serum C3 and C4 levels. Other common etiologies of tubulointerstitial nephropathy, such as drug-induced hypersensitivity reactions and sarcoidosis, do not show TBM IgG deposits in the majority of patients.6
Data are limited regarding therapeutics and outcomes. In the cases described before our report, one exhibited a decrease in the creatinine level after using cyclophosphamide and corticosteroids.4 Three cases had stabilization of the disease, despite the absence of a specific treatment.6 One of the 13 cases provided no description of the outcome.3 All the others developed unfavorably (Table 1).2,5,6 Rituximab was used in 2 cases unsuccessfully.6 In another patient, the disease recurred in the kidney graft despite immunosuppression; even after plasmapheresis and rituximab, there was progression of kidney disease. Pulse therapy with methylprednisolone and belatacept was attempted, but without success.5
ABBA disease is a rare and possibly underdiagnosed autoimmune disorder. There remains much to explore regarding its pathophysiology and predisposing factors. In addition, the cases described in the literature show variable evolution and reserved prognosis.
Article Information
Authors’ Full Names and Academic Degrees
Laíse Pereira Arcoverde Fechine Brito, MD, Felipe Leite Guedes, MD, Pedro Henrique Cavalcante Vale, MD, Rivaldo Pereira Santos, MD, José Bruno de Almeida, MD, PhD, Sílvia Queiroz Santos Martins, MD, Gleiko Yuri de Figueredo Dantas, MD, David Wanderley, MD, MSc, Stanley de Almeida Araújo, MD, MSc, and Gyl Eanes Barros Silva, MD, PhD.
Support
None.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Patient Protections
The authors declare that they have obtained consent from a relative with appropriate authority for publication of the information about the patient who appears within this Case Report and any associated supplementary material.
Peer Review
Received December 30, 2020. Evaluated by 1 external peer reviewer, with direct editorial input by the Editor-in-Chief. Accepted in revised form April 4, 2021.
Footnotes
Complete author and article information provided before references.
References
- 1.Carney E.F. Tubular disease: anti-LRP2 nephropathy. Nat Rev Nephrol. 2018;14(1):3. doi: 10.1038/nrneph.2017.159. [DOI] [PubMed] [Google Scholar]
- 2.Morrison E.B., Kozlowski E.J., McPhaul J.J., Jr. Primary tubulointerstitial nephritis caused by antibodies to proximal tubular antigens. Am J Clin Pathol. 1981;75:602–609. doi: 10.1093/ajcp/75.4.602. [DOI] [PubMed] [Google Scholar]
- 3.Douglas M.F., Rabideau D.P., Schwartz M.M. Evidence of autologous immune-complex nephritis. N Engl J Med. 1981:1326–1329. doi: 10.1056/NEJM198111263052206. [DOI] [PubMed] [Google Scholar]
- 4.Skogh T., Heuman R., Tagesson C. Anti-brush border antibodies (ABBA) in Crohn’s disease. J Clin Lab Immunol. 1982;9:147–150. [PubMed] [Google Scholar]
- 5.Rosales I.A., Collins A.B., do Carmo P.A. Immune complex tubulointerstitial nephritis due to autoantibodies to the proximal tubule brush border. J Am Soc Nephrol. 2016;27:380–384. doi: 10.1681/ASN.2015030334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larsen C.P., Trivin-Avillach C., Coles P. LDL Receptor-related protein 2 (megalin) as a target antigen in human kidney anti-brush border antibody disease. J Am Soc Nephrol. 2018;29:644–653. doi: 10.1681/ASN.2017060664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dinesh K.P., Raniele D., Michels K. Anti-LRP2 nephropathy with abundant IgG4-positive plasma cells: a case report. Am J Kidney Dis. 2019;74(1):132–137. doi: 10.1053/j.ajkd.2018.12.039. [DOI] [PubMed] [Google Scholar]
- 8.Dvanajscak Z., Murphy J.D., Larsen C.P., Padala S.A. Anti-brush border antibody disease (anti-LRP2 nephropathy) associated with lupus nephritis. Kidney Int Rep. 2020;5(9):1590–1594. doi: 10.1016/j.ekir.2020.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caliskan Y., Caza T., Mosman A. A case of immune complex mediated tubulointerstitial disease and nephrotic syndrome: anti LRP-2 nephropathy with diffuse podocyte effacement. J Nephrol. 2021;34(3):915–919. doi: 10.1007/s40620-020-00762-9. [DOI] [PubMed] [Google Scholar]
- 10.Gamayo A., Hecox D., Dicker L. Anti-LRP2 nephropathy with concurrent kidney infiltration by lymphoma. Clin Kidney J. 2019;13(3):468–472. doi: 10.1093/ckj/sfz166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gallan A.J., Garg A., Collins A.B. Anti-LRP2 nephropathy. Kidney Int Rep. 2020;5(12):2365–2370. doi: 10.1016/j.ekir.2020.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu X., Tu L., Liu S., You H., Xue J., Hao C. Complete remission of nephrotic syndrome in a young woman with anti-LRP2 nephropathy after immunosuppressive therapy. BMC Nephrol. 2020;21(1):364. doi: 10.1186/s12882-020-02027-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kivity S., Agmon-Levin N., Blank M. Infections and autoimmunity—friends or foes? Trends Immunol. 2009;30:409–414. doi: 10.1016/j.it.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Ribeiro F.M., Goldenberg T. Mycobacteria and autoimmunity. Lupus. 2015;24:374–381. doi: 10.1177/0961203314559634. [DOI] [PubMed] [Google Scholar]
- 15.Elkayam O., Caspi D., Lidgi M. Auto-antibody profiles in patients with active pulmonary tuberculosis. Int J Tuberc Lung Dis. 2007;11:306–310. [PubMed] [Google Scholar]
- 16.Lee S., Matsuzaki H., Kumagai-Takei N. Silica exposure and altered regulation of autoimmunity. Environ Health Prev Med. 2014;19:322–329. doi: 10.1007/s12199-014-0403-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khuder S.A., Peshimam A.Z., Agraharam S. Environmental risk factors for rheumatoid arthritis. Rev Environ Health. 2002;17:307–315. doi: 10.1515/reveh.2002.17.4.307. [DOI] [PubMed] [Google Scholar]
- 18.Parks C.G., Cooper G.S. Occupational exposures and risk of systemic lupus erythematosus. Autoimmunity. 2005;38:497–506. doi: 10.1080/08916930500285493. [DOI] [PubMed] [Google Scholar]
- 19.Broughton A., Thrasher J.D., Madison R. Chronic health effects and immunological alterations associated with exposure to pesticide. Comments Toxicol. 1990;4:59–71. [Google Scholar]
- 20.Schielen P., Schoo W., Tekstra J. Autoimmune effects of hexachlorobenzene in the rat. Toxicol App I Pharmacol. 1993;122:233–243. doi: 10.1006/taap.1993.1192. [DOI] [PubMed] [Google Scholar]
- 21.Ezendam J., Vissers I., Bleumink R. Immunomodulatory effects of tetrachlorobenzoquinone, a reactive metabolite of hexachlorobenzene. Chem Res Toxicol. 2003;16:688–694. doi: 10.1021/tx034016p. [DOI] [PubMed] [Google Scholar]
- 22.Colvin R.B., Chang A., editors. Diagnostic Pathology: Kidney Diseases. 2nd ed. Elsevier; 2016. [Google Scholar]
- 23.Rosenberg A.Z., Kopp J.B. Focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 2017;12:502–517. doi: 10.2215/CJN.05960616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cornell L.D. IgG4-related kidney disease. Curr Opin Nephrol Hypertens. 2012;21:279–288. doi: 10.1097/MNH.0b013e32835265ac. [DOI] [PubMed] [Google Scholar]
- 25.Kambham N., Markowitz G.S., Tanji N. Idiopathic hypocomplementemic interstitial nephritis with extensive tubulointerstitial deposits. Am J Kidney Dis. 2001;37:388–399. doi: 10.1053/ajkd.2001.21320. [DOI] [PubMed] [Google Scholar]
- 26.Park M.H., D’Agati V., Appel G.B., Pirani C.L. Tubulointerstitial disease in lupus nephritis: relationship to immune deposits, interstitial inflammation, glomerular changes, renal function, and prognosis. Nephron. 1986;44:309–319. doi: 10.1159/000184012. [DOI] [PubMed] [Google Scholar]