Abstract
A delayed Nellix (Endologix, Irvine, Calif) type 1a endoleak from endovascular aneurysm sealing (EVAS) is particularly challenging to treat owing to the restrictions and scarcity of the technical options available. We have described two viable endovascular solutions, with and without the availability of the Nellix endograft inventory. A Nellix-in-Nellix apparatus with multivisceral chimney, covered stent extensions and internal reinforcements can be used if Nellix endografts are available (patient 1). In the absence of Nellix endografts, we used a Viabahn-in-Nellix apparatus, also with multiple chimney stents, as an alternative and timely treatment for patient 2. Our patients remained well and free of endoleaks at 19 and 11 months after treatment.
Keywords: Delayed endoleak, Endovascular aortic sealing, NINA, Nellix, Type Ia endoleak
Endovascular aneurysm sealing (EVAS) with Nellix endografts (Endologix, Irvine, Calif) became another endovascular aneurysm repair (EVAR) option for abdominal aortic aneurysms (AAAs) in 2013. The EVAS Forward Global Registry showed low endoleak and complication rates at 12 months.1 The prevalence of a type Ia endoleak has ranged from 1.4% to 3.0% at 12 months.2,3 The Nellix design is not wholly reliant on proximal and distal stent fixation, making its application attractive for the treatment of certain AAA configurations unsuitable for conventional modular endografts. Krievins et al4 showed Nellix to be advantageous for AAAs with more hostile anatomy (ie, short proximal neck, wide neck diameter, severe angulations).
However, the real-world experience resulted in increasing reports of Nellix type Ia endoleaks (NTIaEs) after EVAS,2,5 leading to voluntary recall by Endologix as a safety measure and, consequently, CE Mark withdrawal in January 2019.6 One potential reason for NTIaEs is deployment of the endograft outside the instructions for use (IFU). Carpenter et al2 showed composite freedom from migration, NTIaEs, and aneurysm expansion of 95.9% within the IFU cohort compared with 85.1% in the off-IFU cohort. The EVAS Forward Global Registry showed that only 72% of EVAS cases had complied with the IFU.2
Owing to its double-barrel configuration, conventional proximal aortic cuff endograft extension with renal or visceral “chimney” stenting will not work to treat NTIaEs. Few treatment options are available, although embolization and proximal stent extensions have been described.2,7, 8, 9 Donselaar et al10 first described the use of a Nellix-in-Nellix apparatus (NINA) for NTIaEs, secondary to caudal stent migration, which was successfully performed in five cases with no procedural complications.
Endologix is conscientious in ensuring patient safety with stringent implementation of Nellix IFU compliance and preapproval investigation. However, restricted endograft availability and, ultimately, its recall has made the treatment of NTIaEs even more challenging. The present case series exemplifies two viable endovascular solutions for NTIaEs, with and without the availability of Nellix inventory. Our patients agreed to the use of their de-identified data for education and report of their cases as a part of the written informed consent process.
Case report
Patient 1
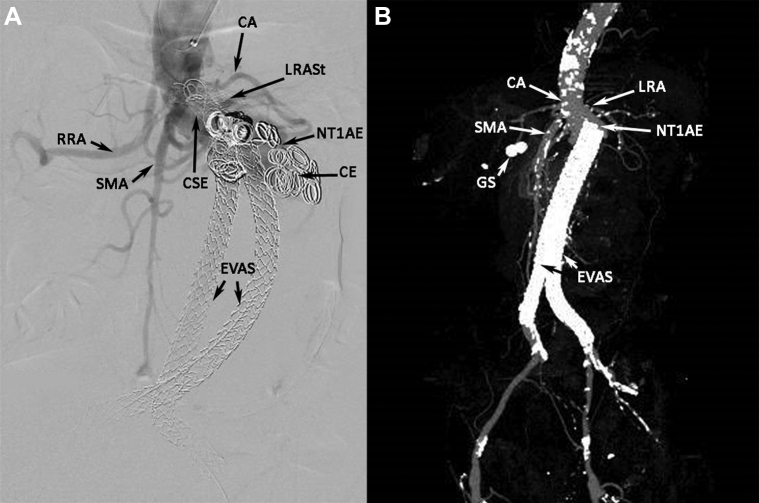
An 81-year-old man with a symptomatic, 8-cm-diameter, juxtarenal AAA and a 1.9-cm saccular right common iliac artery aneurysm underwent Nellix EVAS repair and treatment with a left renal artery (LRA) chimney covered stent. During polymer injection, the distal right common iliac artery ruptured and was treated successfully with a 10-mm × 59-mm BeGraft covered stent (Bentley Innomed, Hechingen, Germany). A computed tomography (CT) scan at the 14-month follow-up detected an NTIaE, an empty right endobag with caudal endograft migration, and interval occlusion of the left renal stent. An aortogram showed proximal covered stent extension of the right endograft plus coil embolization that was unsuccessful in obliterating the NTIaE (Fig 1, A).
Fig 1.
A, Aortogram showing Nellix type Ia endoleak (NTIaE) that was unsuccessfully treated with coil embolization (CE) and proximal covered stent extension (CSE). CA, Celiac artery, splenic artery branch; EVAS, endovascular aneurysm sealing with Nellix endografts; LRASt, left renal artery stent (occluded); RRA, right renal artery; SMA, superior mesenteric artery. B, Three-dimensional computed tomography aortogram (CTA) reconstruction showing new Nellix type Ia endoleak (NTIaE) with caudal migration of Nellix endografts (endovascular aneurysm sealing [EVAS]). CA, Celiac artery with severe ostial stenosis; GS, gall stones; LRA, left renal artery with proximal stenosis; SMA, superior mesenteric artery.
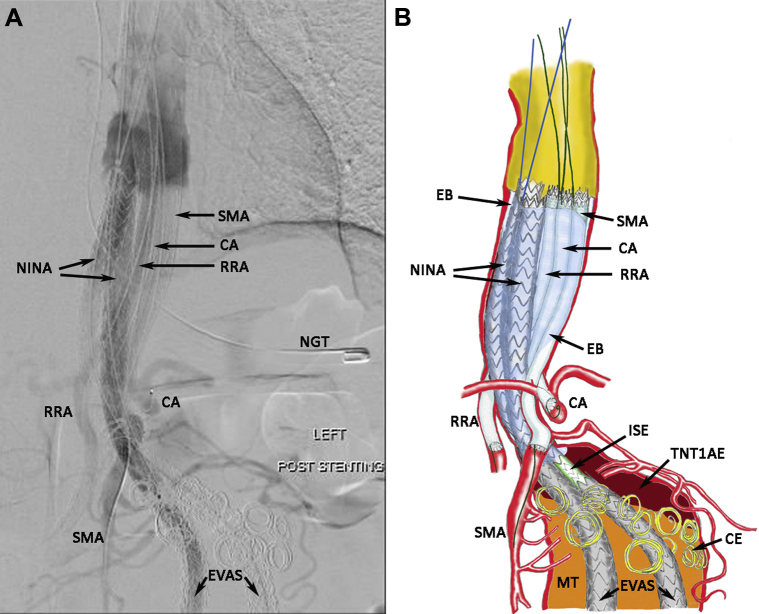
He underwent NINA, with right renal artery (RRA), superior mesentery artery (SMA), and celiac artery (CA), multivisceral chimney covered stent extensions with internal reinforcements. The goal was to extend the bilateral Nellix with additional Nellix endografts to bring their inflow supradiaphragmatically. The visceral arteries (CA, SMA, and RRA) were each treated with long covered chimney stents to bring their inflow at par with the Nellix extensions. Viabahn stents (W. L. Gore & Associates, Flagstaff, Ariz) would be ideal for their length; however, their modest radial strength would require internal reinforcement with matching length and diameter nitinol self-expanding stents (Absolute Pro; Abbott Laboratories, Abbott Park, Ill). The preexisting LRA stent was occluded and, thus, was abandoned. Once all the stent-grafts were in position, bilateral Nellix proximal extension endobags were filled with polymer to obliterate any “gutter” leaks that might occur between the multiple stents within the descending/paravisceral aorta. A completion aortogram after NINA showed resolution of the NTIaE (Fig 2, A). The graphic details of the multistent configuration are shown in Fig 2, B. Percutaneous common femoral artery access was used to deliver the Nellix extensions, and a left axillary artery cutdown with triple punctures was used to deliver the CA, SMA, and RRA stents. The following endografts were deployed:
-
1.
RRA: 7 mm × 150 mm Viabahn (W. L. Gore & Associates) and 7 mm × 100 mm and 7 mm × 60 mm Absolute Pro (Abbott Laboratories)
-
2.
CA: 8 mm × 150 mm Viabahn and 8 mm × 100 mm and 8 mm × 60 mm Absolute Pro
-
3.
SMA: 8 mm × 150 mm Viabahn and 8 mm × 100 mm and 8 mm × 40 mm (×2) Absolute Pro
-
4.
10 mm × 200 mm Nellix endografts to both iliac limbs
Fig 2.
A, Aortogram of Nellix-in-Nellix apparatus (NINA) with multivisceral chimney covered stent extensions and internal reinforcements. CA, Celiac artery stenting; EVAS, endovascular aneurysm sealing with Nellix endografts; NGT, nasogastric tube; RRA, right renal artery stenting; SMA, superior mesenteric artery stenting. B, Graphic illustration of aortogram (A). CE, coil embolization; EB, Endobags filled with polymer; ISE, intermediate stent extension; MT, mural thrombus; TNTIaE, thrombosed Nellix type Ia endoleak.
A CT aortogram (CTA) at 2 months and duplex ultrasound scan at 8 months showed no endoleak and a stable AAA sac size. The patient remained well and free of endoleaks at 19 months after NINA.
Patient 2
A 64-year-old man with severe cardiovascular comorbidities (ie, severe mitral regurgitation, ischemic heart disease, hypertension) underwent successful EVAS for a 5.2-cm-diameter AAA with a severe infrarenal neck angulation of 80°. He was well and followed up annually with CTAs for 2 years. He developed aortitis with presumed endograft infection without evidence of an endoleak at 30 months and was treated with intravenous antibiotics. At 32 months, the patient presented with pain. The CTA showed an NTIaE, an increased mycotic aneurysm size at the level of the RRA, and bilateral Nellix caudal migration (Fig 1, B).
Under emergent circumstances and with the nonavailability of Nellix inventory, the patient underwent a Viabahn-in-Nellix apparatus (VINA) procedure. The goals of the VINA procedure are similar to those for the NINA procedure: (1) proximal extension of the existing Nellix endografts to elevate the inflow to the supradiaphragmatic level; and (2) internally reinforced “chimney” covered stent proximal extensions to the remaining visceral arteries, with inflow at par with the Nellix extensions. The use of multiple long-length visceral covered stents running parallel and contained within a normal-diameter descending-aorta, with no AAA sac outflow, can lead to gutter and NTIaE thrombosis, despite the absence of endobags.
Bilateral common femoral artery percutaneous access was used to deliver the Viabahn-Nellix extensions, and RRA and SMA stenting was achieved via a left axillary artery cutdown and direct double punctures. The CA was critically stenosed and underwent coil embolization to prevent a type II endoleak. The LRA was sacrificed because it was diseased, could not be cannulated, and had a hypotrophic kidney. The following endografts were deployed:
-
1.
RRA: 6 mm × 150 mm Viabahn and 6 mm × 80 mm and 6 mm × 60 mm Absolute Pro
-
2.
SMA: 8 mm × 100 mm and 8 mm × 50 mm Viabahn and 8 mm × 100 mm and 8 mm × 60 mm Absolute Pro
-
3.
Left Nellix endograft: 9 mm × 150 mm Viabahn and 9 mm × 100 mm (×2) Absolute Pro
-
4.
Right Nellix endograft: 8 mm × 53 mm BeGraft interposition, 8 mm × 150 mm Viabahn, and 8 mm × 100 mm (×2) Absolute Pro
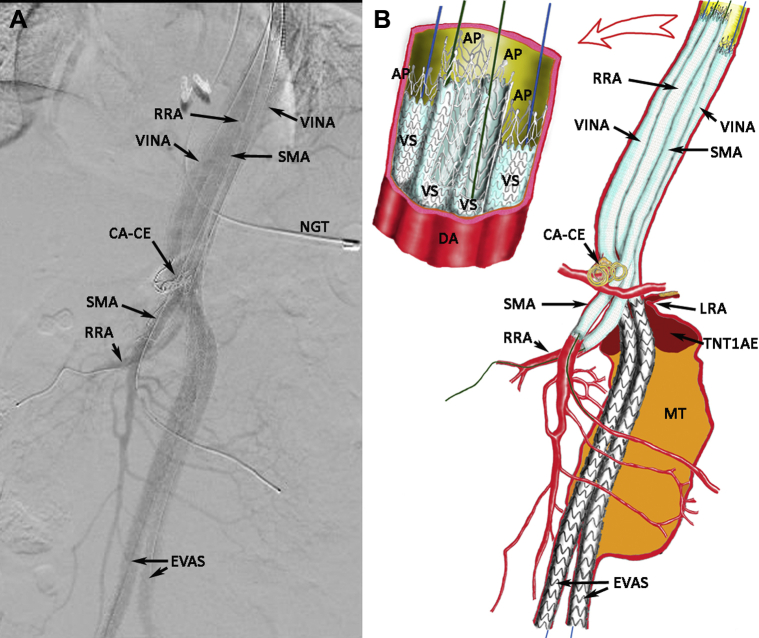
The completion aortogram showed resolution of the NTIaE and flow through all the stent grafts (Fig 3, A). The VINA multistent configuration is shown graphically in Fig 3, B. The postoperative CTA showed a small NTIaE from a gutter leak, which was successfully obliterated with adjunctive coil embolization. He was discharged well and remained free of endoleaks at 2 months by CTA, with negative ultrasound findings at 6 and 11 months after VINA.
Fig 3.
A, Aortogram of Viabahn-in-Nellix apparatus (VINA) with multivisceral chimney covered stent extensions and internal reinforcements. CA-CE, Celiac artery with coil embolization; EVAS, endovascular aneurysm sealing with Nellix endografts; NGT, nasogastric tube; RRA, right renal artery stenting; SMA, superior mesenteric artery stenting. B, Graphic illustration of aortogram (A), with details of proximal stent inflow configuration (including cut-away views of stents). AP, Absolute Pro stent internal reinforcement; DA, descending aorta; LRA, left renal artery, which was severely stenosed and sacrificed; MT, mural thrombus; TNTIaE, thrombosed Nellix type Ia endoleak; VS, Viabahn stent.
Discussion
NTIaEs continue to be a challenging problem owing to the delayed presentation. We believe that the NINA provides a better endovascular option for NTIaEs; however, its use has been limited by the unavailability of the Nellix. Despite CE Mark status reinstatement in June 2019, its availability will be restricted until the next iteration of endografts.11 In a retrospective cohort study of 41 patients, Zoethout et al12 reported a 97% and 100% technical success rate in both elective and emergency groups, making the NINA the most acceptable method. However, the aneurysm-related death rate was 9% in the elective group and 44.4% in the emergency group. The common causes of death were multiorgan failure and bleeding (retroperitoneal, gastrointestinal, intracranial). One mortality was attributed to endobag rupture, which led to multiorgan failure.
Our patients were not candidates for open surgery even at the index EVAS repair because of prohibitive pulmonary (patient 1) and cardiac (patient 2) risks. Both had had juxtarenal AAAs that had been treated with Nellix EVAS off-IFU with left renal chimney stents. Patient 1 likely had a ruptured right endobag with slow leeching of the polymer leading to endograft instability, caudal migration, and an NTIaE. In patient 2, the endograft had likely been seeded from an interim dental procedure, with a resultant mycotic pararenal AAA, NTIaE, and impending rupture. Our multidisciplinary vascular conferences concluded that neither patient would survive endograft explantation and aortic reconstruction; hence, although not ideal, an endovascular approach was the only therapeutic reintervention option. Both NINA and VINA involve “covering” long segments of healthy aorta in addition to a previous EVAR, thereby increasing the risk of spinal cord ischemia and paraplegia. Furthermore, endovascular treatment in the setting of aortic infection with implantation of additional endografts is contrary to the convention of explanting the infected prosthesis. Most experts consider endovascular treatment of aortitis as “bridging” therapy toward explantation. However, evidence is emerging showing the efficacy of EVAR after bacterial infection even in the medium to long term.13
We have followed the adage that all visceral and renal arteries should ideally be revascularized; thus, a complete NINA or VINA revascularization will contain six endografts within the descending aorta (two Nellix proximal extensions, plus the SMA, CA, and bilateral renal arteries). If complete revascularization cannot be achieved, we would recommend the following: the SMA should be prioritized over the CA and at least one mesenteric artery must be preserved, with coil embolization of the nonstented mesenteric artery to prevent a type II endoleak. In addition, at least one renal artery must be preserved. Visceral and renal stent diameter oversizing of 20% is recommended in accordance with the Viabahn IFU. The typical parallel stent diameters applicable are as follows: Nellix extensions, 10 mm (preferred); CA or SMA, 8 mm; and renal arteries, 6 to 7 mm. The number of “gutters” between parallel stents within the aorta depends on the number of stents used and their geometric configuration. By cross-sectional convention, circular stents within a circular aorta can have the following number of stents to gutters: 4 stents to 5 gutters, 5 stents to 8 gutters, and 6 stents to 8 to 10 gutters. The parallel overlap between the stents should be ≥12 cm when 15-cm-long Viabahn endografts are used, even when a 3-cm indwelling overlap with a Nellix is applied in the VINA. This is more than the 8-cm overlap shown effective in the thoracic aorta.14 The average luminal diameter (and cross-sectional area) of the descending/paravisceral aorta is calculated from the CTA, from the left inferior pulmonary ligament to the lowest renal artery using centerline measurements and any three-dimensional imaging software. The total cross-sectional area of the planned parallel stents (calculated from the known stent diameters) when referenced with the averaged aortic area will provide crucial information: (1) the estimated polymer volume injection required to obliterate the remaining gutter space in NINA; and (2) the adequacy of the parallel stent graft seal to prevent gutter leaks in VINA. We recommend that the total cross-section area of the parallel endografts equate the average area of the aorta treated for NINA and VINA. It can be oversized by ≤20% for VINA but should achieve not <80%. The polymer injection pressure for NINA should target 180 mm Hg. Oversizing the chimney grafts by 20% and minimizing the gutter areas to <7.5 mm2 has been shown to minimize endoleaks.15 Our VINA patient's average aortic diameter was 18 mm (area, 254.6 mm2), and the composite parallel stent area was 192.5 mm2 (75.6% of aorta), with an average gutter (five) area of 12.4 mm2, which might explain the occurrence of the small residual endoleak. This could have been mitigated by the deployment of larger 10-mm Viabahn stents for the Nellix extensions. However, the urgent nature of the repair and lack of consigned inventory resulted in the usage of 8-mm and 9-mm diameter stents. Residual endoleaks will not necessarily be obvious from on-table completion angiograms and mandatorily will require a postoperative CT scan for detection. We advocate vigilance in their detection and aggressive adjunctive interventions such as endovascular embolization to adequately resolve any NTIaEs. Both our patients recovered from contrast-induced nephropathy. Coincidentally, both previously stented left renal arteries had become occluded and were abandoned, thereby mitigating the need for additional steps in these already complicated endovascular procedures. Both patients had had prophylactic spinal drains placed perioperatively and experienced no neurologic deficits. All our NINA and VINA patients are instructed to continue clopidogrel indefinitely to prevent thrombosis in the extensive number of indwelling endografts. Our institution has followed up 39 patients with Nellix implants with a reintervention rate of >20%, mostly for NTIaEs.
VINA has no validation except for case reports such as ours. Because of the time and resource constraints, especially during emergency settings, innovative solutions such as VINA were used. The absence of endobag polymer filling in a VINA approach logically renders it more prone to the occurrence of gutter leaks and persistent NTIaEs and, hence, has a greater likelihood of requiring adjunctive coil embolization to achieve thrombosis. Despite their shortcomings, both NINA and VINA can be useful inclusions within vascular specialists' armamentarium because we are likely to see more NTIaEs secondary to progressive device failure and disappointing long-term follow-up outcomes.16
Conclusions
Type Ia endoleaks after EVAS have continued to be challenging and problematic to treat. NINA and VINA are technically feasible with reasonable results but require further and long-term validation.
Footnotes
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Thompson M., Heyligers J., Hayes P., Reijnen M., Bockler D., Schelzig H. Endovascular aneurysm sealing: early and midterm results from the EVAS Forward Global Registry. J Endovasc Ther. 2016;23:685–692. doi: 10.1177/1526602816664365. [DOI] [PubMed] [Google Scholar]
- 2.Carpenter J., Lane J., III, Trani J., Hussain S., Healey C., Buckley C. Refinement of anatomic indications for the Nellix® system for endovascular aneurysm sealing based on 2-year outcomes from the EVAS FORWARD IDE trial. J Vasc Surg. 2018;68:720–730.e721. doi: 10.1016/j.jvs.2018.01.031. [DOI] [PubMed] [Google Scholar]
- 3.Gossetti B., Martinelli O., Ferri M., Silingardi R., Verzini F. Preliminary results of endovascular aneurysm sealing from the multicenter Italian research on Nellix® endoprosthesis (IRENE) study. J Vasc Surg. 2018;67:1397–1403. doi: 10.1016/j.jvs.2017.09.032. [DOI] [PubMed] [Google Scholar]
- 4.Krievins D., Holden A., Savlovskis J., Calderas C., Donayre C., Moll F. EVAR using the Nellix® sac-anchoring endoprosthesis: treatment of favourable and adverse anatomy. Eur J Vasc Endovasc Surg. 2011;42:38–46. doi: 10.1016/j.ejvs.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 5.van den Ham L., Holden A., Savlovskis J., Witterbottom A., Ouriel K., Reijnen M. Editor's choice – occurrence and classification of proximal type I endoleaks after endovascular aneurysm sealing using the Nellix® device. Eur J Vasc Endovasc Surg. 2017;54:729–736. doi: 10.1016/j.ejvs.2017.09.016. [DOI] [PubMed] [Google Scholar]
- 6.Mahboob V. Endologix announces worldwide voluntary Nellix® system recall and field safety notification, January 4th, 2019. Loses the EC Certificate of Continuity (CE Mark), January 22, 2019. https://endologix.com/wp-content/uploads/2019/01/2019.01.22-Press-Release-Nellix-CE-Mark-FINAL.pdf Available at:
- 7.Ameli-Renani S., Das R., Weller A., Chung R., Morgan R. Embolisation of a proximal type I endoleak post-Nellix® aortic aneurysm repair complicated by reflux of Onyx into the Nellix endograft limb. Cardiovasc Intervent Radiol. 2015;38:747–751. doi: 10.1007/s00270-014-1044-5. [DOI] [PubMed] [Google Scholar]
- 8.Ameli-Renani S., Morgan R. Transcatheter embolisation of proximal type 1 endoleaks following endovascular aneurysm sealing (EVAS) using the Nellix® device: technique and outcomes. Cardiovasc Intervent Radiol. 2015;38:1137–1142. doi: 10.1007/s00270-015-1171-7. [DOI] [PubMed] [Google Scholar]
- 9.Harvey J., Stefan B., Hill A., Holden A. Transcatheter embolization of type Ia endoleak after Nellix® endovascular aortic aneurysm sealing with n-butyl cyanoacrylate: technique in three patients. J Vasc Interv Radiol. 2016;27:194–199. doi: 10.1016/j.jvir.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 10.Donselaar J., Holden A., Zoethout A., Zeebregts C., Reijnen M. Feasibility and technical aspects of proximal Nellix®-in-Nellix® extension for late caudal endograft migration. J Endovasc Ther. 2017;24:210–217. doi: 10.1177/1526602816677037. [DOI] [PubMed] [Google Scholar]
- 11.Mahboob V. Endologix announces reinstatement of CE Mark for its Nellix endovascular aneurysm sealing system. https://endologix.com/wp-content/uploads/2019/06/2019.06.06-Nellix-CE-Mark-Reinstatement.pdf Available at:
- 12.Zoethout A., Zerwes S., Zeebregts C., Heyligers J., De Vries J., Oberhuber A. Preliminary outcome of Nellix®-in-Nellix® extensions in patients treated with failed endovascular aneurysm sealing. J Vasc Surg. 2019;70:1099–1106. doi: 10.1016/j.jvs.2019.01.044. [DOI] [PubMed] [Google Scholar]
- 13.Mezzetto L., Veraldi G.F., Engelberger S., Giovannacci L., Van den Berg J., Rosso R. Successful endovascular repair of a penetrating aortic ulcer in bacterial aortitis. Ann Vasc Surg. 2016;35:205.e13–205.e17. doi: 10.1016/j.avsg.2016.01.030. [DOI] [PubMed] [Google Scholar]
- 14.Lobato A.C., Camacho-Lobato L. Endovascular treatment of complex aortic aneurysms using the sandwich technique. J Endovasc Ther. 2012;19:691–706. doi: 10.1583/JEVT-12-4023R.1. [DOI] [PubMed] [Google Scholar]
- 15.Fazzini S., Ronchey S., Orrico M., Martinelli O., Alberti V., Praquin B. “Over-SIRIX”: a new method for sizing aortic endografts in combination with the chimney grafts: early experience with aortic arch disease. Ann Vasc Surg. 2018;46:285–298. doi: 10.1016/j.avsg.2017.07.018. [DOI] [PubMed] [Google Scholar]
- 16.Singh A.A., Benaragam K.S., Pope T., Coughlin P., Winterbottom A.P., Harrison S.C. Progressive device failure at long term follow up of the Nellix endovascular aneurysm sealing (EVAS) system. Eur J Vasc Endovasc Surg. 2021;61:211–218. doi: 10.1016/j.ejvs.2020.11.004. [DOI] [PubMed] [Google Scholar]