Abstract
Introduction
We examine whether distinct brain atrophy patterns (using brain parenchymal fraction [BPF]) differentially predict functional performance and decline in Alzheimer's disease (AD), and are independently moderated by (1) a key AD genetic risk marker (apolipoprotein E [APOE]), (2) sex, and (3) high‐risk group (women APOE ɛ4 carriers).
Methods
We used a 2‐year longitudinal sample of AD patients (baseline N = 170; mean age = 71.3 [9.1] years) from the Sunnybrook Dementia Study. We applied latent class analysis, latent growth modeling, and path analysis. We aimed to replicate our findings (N = 184) in the Alzheimer's Disease Neuroimaging Initiative.
Results
We observed that high brain atrophy class predicted lower functional performance and steeper decline. This association was moderated by APOE, sex, and high‐risk group. Baseline findings as moderated by APOE and high‐risk group were replicated.
Discussion
Women APOE ɛ4 carriers may selectively be at a greater risk of functional impairment with higher brain atrophy.
Keywords: Alzheimer's disease, Alzheimer's Disease Neuroimaging Initiative, apolipoprotein E, brain parenchymal fraction, functional decline, sex, Sunnybrook Dementia Study
1. BACKGROUND
Functional decline is a key characteristic of dementia including Alzheimer's disease (AD). 1 Progressive functional decline in AD leads to increased caregiver burden 2 and loss of independence. 3 Changes in complex activities such as financial planning and housework are commonly observed, followed by deficits in self‐care activities. Instrumental activities of daily living (IADLs) are currently used to measure deficits in complex tasks in dementia. 4 Previous reports show a positive correlation between caregiver and patient reports on everyday cognitive decline, 5 and caregiver descriptions were more accurate for basic activities of daily living (ADLs) as cognition declined in older adults. 6 A recent study suggested that identifying adults with functional difficulties may serve as an informal screening tool for older adults with high dementia risk profiles, 7 who may benefit from a personalized medicine approach and intervention programs.
HIGHLIGHTS
Brain atrophy trajectories predict differential functional performance in Alzheimer's disease (AD).
This association is magnified in women apolipoprotein E (APOE) ɛ4 carriers.
Latent class analysis was applied to identify distinct patterns of brain atrophy trajectories.
Key baseline findings were replicated in an independent AD cohort.
Functional decline is accelerated in AD compared to prodromal stages of dementia (ie, mild cognitive impairment [MCI]), 8 and has been linked with several risk factors 9 including cognitive impairment, 10 brain atrophy, 11 increased white‐matter hyperintensity, 12 genetics, 13 sex, 14 and dementia status. 15 A multimodal risk approach integrating multiple domains 16 , 17 with modifiable and non‐modifiable risk factors is currently pursued in the field to predict accelerated cognitive trajectories in older adults 18 and dementia patients. 16 We apply a similar multimodal approach and extend previous work on cognitive changes to study differential functional trajectories in AD as predicted by three important and commonly studied risk domains (brain morphometry, genetics, and sex). Specifically, we examine whether brain atrophy (represented with brain parenchymal fraction [BPF]), key AD genetic risk marker (apolipoprotein E [APOE]), and sex in combination magnify functional decline (using IADL as a proxy) in AD.
RESEARCH IN CONTEXT
Systematic review: We reviewed the literature (eg, PubMed) on functional trajectories in dementia. We focused on studies applying a multidomain approach to study functional performance and decline. Specifically, the synergistic associations of three key risk domains: brain morphometry, genetics, and sex, in Alzheimer's disease (AD). There were no reports studying synergistic associations of brain atrophy, apolipoprotein E (APOE), and sex on functional trajectories in AD.
Interpretation: Our findings indicate that distinct classes of higher brain atrophy trajectories predict poorer functional performance in AD, and this is magnified in women APOE ɛ4 carriers. This was replicated in an independent AD cohort.
Future directions: Our findings provide a foundation to examine complex multimodal associations on functional trajectories in AD. Future work may benefit from (1) taking into account the vulnerability associated with APOE ɛ4+ risk and sex differences and (2) applying advanced statistical modeling to study brain atrophy and functional trajectories.
Brain atrophy, especially loss of brain parenchyma as a result of neurodegeneration, is a key feature in AD. 19 We use BPF to represent global brain atrophy. 20 Previous reports show whole brain volume measures of cerebral atrophy as a reliable source for measuring cognitive function, 21 and positive correlation for total brain volume trajectory with age and diagnosis of mild cognitive impairment (MCI). 22 The use of BPF is an intentional variable in our study, in that we sought to represent the overall brain atrophy trajectories in diagnosed AD cases commonly observed in real‐world clinical settings.
The APOE genetic polymorphism (chromosome 19q13.2) has been identified consistently and established as the strongest genetic risk factor for cognitive impairment 23 and functional decline. 24 APOE has three isoforms (ɛ2, ɛ3, and ɛ4); where the ɛ4 is considered to have the highest risk for AD and cognitive impairment, ɛ3 as neutral, and ɛ2 as protective. 23 , 25 APOE regulates lipid homeostasis and cholesterol metabolism important for amyloid beta (A) aggregation and metabolism leading to plaques and cerebral amyloid anigopathy in AD. 23 , 25 Previous work has shown that MCI adults with APOE ɛ4 allelic risk and higher brain atrophy may be at greater risk of functional decline. 11 Inconsistent findings have also been reported for APOE ɛ4 risk and functional decline. Specifically, APOE ɛ4/ɛ4 homozygotes showed a slower rate of cognitive and functional decline compared to their counterparts (APOE ɛ4+ and APOE ɛ4− groups). This finding implies potential underlying differences between rates of decline in the APOE ɛ4 homozygous group versus early diagnosis observed in APOE ɛ4 carriers alone. 26
Sex differences in AD showed that women with cognitive impairment (ie, executive function) had worse basic ADLs and IADLs over 6 years and increased mortality risk. 14 In addition, women with lower performance on IADLs were observed to be frailer with poor cognitive performance and greater falls. 27 APOE ɛ4 carriers also showed increased loss of cortical thickness and hippocampal volume linked to accelerated cognitive decline, 23 and functional impairment selectively in women. 13
To our knowledge this is the first study to examine whether the synergistic associations of brain atrophy, APOE, and sex influence functional performance and decline in AD. We test atrophy and functional associations as moderated by three separate risk moderations (1) APOE, (2) sex, and (3) high‐risk group (women APOE ɛ4 carriers). For our foundational analyses, we examine (1) 2‐year individual trajectories of global atrophy and (2) a latent growth model of functional performance and decline. We expect to observe two classes of atrophy trajectories corresponding to low and high atrophy progression and a random intercept and slope growth model for functional decline. We examine three sequential research goals (RGs).
RG1: We examine whether atrophy classes predict functional performance and decline. We expect to observe that higher atrophy class predicts poorer functional performance and steeper decline.
RG2: We test whether the observed association between atrophy class and functional performance and decline is moderated by APOE (ɛ4− vs ɛ4+) and sex (men vs women), independently. We expect that higher atrophy class will predict poorer functional performance and steeper decline in APOE ɛ4 carriers and women, separately.
RG3: We examine whether the observed atrophy class and functional performance and decline association is moderated by high APOE and sex risk combination (women APOE ɛ4 carriers) versus the low‐risk group (women in the APOE ɛ4− group and men in the APOE ɛ4− and ɛ4+ groups). We expect to observe worse functional performance and steeper decline in high‐risk group with higher atrophy class.
We aim to validate all our findings using the Alzheimer's Disease Neuroimaging Initiative (ADNI) as a replication sample.
2. METHOD
2.1. Participants
2.1.1. Sunnybrook Dementia Study (SDS)
We used data from the SDS (ClinicalTrials.gov NCT01800214), a large longitudinal observational prospective cohort study (1994 to the present) of dementia patients in Toronto, Canada. The SDS includes clinical data, standardized neuroimaging, neuropsychology, function, mood, behavior, and genetic assessments. All patients were recruited from the Sunnybrook Health Sciences Centre Cognitive Neurology Clinic, University of Toronto, Canada. All patients were enrolled through physician referrals to a tertiary memory clinic and older adults through word of mouth or advertisements. Institutional human research ethics guidelines were met in full for ongoing data collection procedures. Written informed consent was obtained from all participants. If participants were deemed too demented, their power of attorney provided consent on their behalf. For the present study, we included diagnosed AD patients tested across three waves (≈2 years). AD was diagnosed using the National Institute of Neurologic and Communicative Disorders and Stroke and Alzheimer's disease and Related Disorders Association criteria. 28 Mini‐Mental State Exam (MMSE) score of 16 is shown to be a key transition point for loss of IADLs 29 ; thus in the present study, we excluded patients with MMSE below 16 (n = 11) and those with missing APOE genotype data or unusable baseline magnetic resonance imaging (MRI) scans. Accordingly, 170 AD patients (age range = 46 to 89 years; mean age = 71.3 (9.1) years; n women = 93) were included (Table 1).
TABLE 1.
Baseline characteristics of AD patients in the Sunnybrook Dementia Study and Alzheimer's Disease Neuroimaging Initiative by apolipoprotein E (APOE) ɛ4 status
| Characteristics | APOE ɛ4− (SDS) | APOE ɛ4− (ADNI) | APOE ɛ4+ (SDS) | APOE ɛ4+ (ADNI) | Total (SDS) | Total (ADNI) |
|---|---|---|---|---|---|---|
| n | 61 | 61 | 109 | 123 | 170 | 184 |
| Age (years) | 72.6 (9.8) | 76.4 (8.5) | 70.6 (8.7) | 74.4 (6.9) | 71.3 (9.1) | 75.1 (7.5) |
| Sex (M/F) | 35/26 | 26/35 | 42/67 | 69/54 | 77/93 | 95/89 |
| Education (years) | 14.1 (4.1) | 15.0 (3.4) | 13.8 (3.8) | 14.5 (3.1) | 13.9 (3.9) | 14.7 (3.2) |
| MMSE | 24.2 (3.3) | 23.3 (2.0) | 24.0 (3.3) | 23.3 (2.0) | 24.1 (3.3) | 23.3 (2.0) |
| BPF (%) | 73.3 (4.2) | 65.9 (3.1) | 74.3 (4.8) | 66.1 (2.3) | 73.9 (4.6) | 66.0 (2.6) |
| BPF (%) range | 62.23‐80.83 | 59.84‐74.00 | 63.26‐86.37 | 60.35‐72.87 | 62.23‐86.37 | 59.84‐74.00 |
| IADL‐DAD (%) | 76.3 (21.6) | – | 75.6 (23.7) | – | 75.8 (22.9) | – |
| IADL‐DAD range | 30‐100 | – | 7‐100 | – | 7‐100 | – |
| FAQ | – | 14.0 (6.4) | – | 12.7 (7.1) | – | 13.1 (6.9) |
| FAQ range | – | 1‐30 | – | 0‐29 | – | 0‐30 |
Note. Means are represented with standard deviations in parentheses.
Abbreviations: ADNI, Alzheimer's Disease Neuroimaging Initiative; APOE, apolipoprotein E; BPF, brain parenchymal fraction; FAQ, Functional Activities Questionnaire.; IADL‐DAD, Instrumental Activities of Daily Living‐Disability Assessment Scale; MMSE, Mini‐Mental State Exam; n, sample size; SDS, Sunnybrook Dementia Study.
2.1.2. ADNI (replication sample)
Data used in our replication sample were obtained from the ADNI database (adni.loni.usc.edu). ADNI was launched in 2003 as a public‐private partnership, led by principal investigator Michael W. Weiner, MD. The primary goal of ADNI has been to test whether serial MRI, positron emission tomography (PET), other biological markers, and clinical and neuropsychological assessments can be combined to measure the progression of MCI and early AD. For up‐to‐date information, see www.adni‐info.org. We included 184 AD patients (mean age = 75.1 [7.5] years; n women = 89) 30 , 31 from ADNI‐1. Specifically, only AD patients with both clinical data in the “ADNIMERGE” table and imaging data from “UCSDVOL” table downloaded on March 26, 2020, were included. The selected patients were similar in severity and age of AD patients in the SDS (see Table 1). We included longitudinal structural imaging data from the “UCSDVOL” table across three time points (baseline, Years 1 and 2) and baseline data from “ADNIMERGE” table.
2.2. MRI acquisition protocols and processing
2.2.1. SDS
Structural brain images were obtained on a 1.5T GE Signa (Milwaukee, WI, USA) system. We examined global brain atrophy with the BPF measure using normal‐appearing gray matter (NAGM), normal‐appearing white matter (NAWM), and white matter hyperintensities (WMHs). Specifically, BPF = (NAGM + NAWM + WMH)/(supratentorial total intracranial volume [ST‐TIV]) x 100. Higher BPF corresponds to lower atrophy. Brain volumetrics were estimated from structural MRI using a previously published and validated segmentation algorithm. 32 , 33 , 34 , 35
2.2.2. ADNI (replication sample)
MRI data image acquisition and processing have previously been described. 31 , 36 To calculate BPF, we used BPF = (Whole brain volume/total intracranial volume [TIV]) x 100 from the “UCSDVOL” table. Higher BPF volume corresponds to lower atrophy. We note that the BPF in ADNI uses the supra‐ and infratentorial intracranial volume as denominator, whereas SDS uses only the supratentorial intracranial compartment. Although there are differences between the SDS and ADNI in calculating BPF, the analyses for each study were conducted independently; the larger denominator (TIV includes infratentorial volumes) in the ADNI sample should not exert any variance relative to the smaller denominator (ST‐TIV includes only supratentorial volume) in the SDS sample.
2.3. Genotyping
APOE ɛ4 genotyping was performed using DNA extraction in both the SDS 37 and ADNI. 38 All ɛ2/ɛ4 cases were excluded because of conflicting reports on ɛ2 protective effects versus ɛ4 risk associations. 39 APOE genotype frequencies did not deviate from Hardy‐Weinberg equilibrium in the SDS or ADNI.
2.4. Functional activities of daily living
2.4.1. SDS
We examined IADLs from the Disability Assessment for Dementia (DAD). 4 Patients’ caregivers are asked whether the patient performed certain activities to maintain an adequate lifestyle in the last 2 weeks. For example, “adequately plan a light meal or snack” or “show an interest in leisure activities” with a no (0) or yes (1) for each of initiation, planning, and action sections on the DAD form. From 46 total items, 27 items on the second half of the form measure instrumental activities. Specifically, 8 items for initiation, 6 items for planning, and 13 items for action. The total score was calculated using the 27 IADL items as a percentage of 100, 40 with higher scores representing greater functional activities.
2.4.2. ADNI (replication sample)
We used the Functional Activities Questionnaire (FAQ), 41 , 42 which measures IADLs (eg, preparing meals). The FAQ is ideal for following rate of functional impairment over time in clinical patients. The scores range from dependent (3) to normal (0) for a total score out of 30, with higher scores indicating greater impairment.
2.5. Statistical analyses
Descriptive statistics were calculated for all baseline characteristics in the SDS and ADNI. Continuous measures such as age were summarized using means and standard deviations, whereas categorical measures were summarized using counts and percentages. We used structural equation modeling in Mplus 7.4 43 to examine BPF latent growth model and class trajectories, latent growth model for functional activities, and the three RGs in the SDS and ADNI. Baseline age and education were added as covariates in all three RG analyses.
2.5.1. BPF latent growth model and class trajectories
First, we estimated the best latent growth model for BPF over 2 years using latent growth modeling. Second, we classified BPF into distinct groups by performing latent class growth analysis (LCGA). LCGA uses individual levels and slopes to calculate distinct classes (see Supplementary text).
2.5.2. Latent growth model for functional activities
We estimated the best latent growth model for functional activities (SDS: IADL‐DAD; ADNI: FAQ) over 2 years using latent growth modeling (see Supplementary text).
2.5.3. RG1: Brain atrophy classes predicting functional decline
We regressed functional activities (intercept) and 2‐year change (slope) on atrophy class.
2.5.4. RG2 and RG3: Moderation analysis with APOE ɛ4+, sex, and high‐risk group
Path analysis for functional activities on atrophy class (RG1) was repeated as stratified by (1) APOE (ɛ4‐/ɛ4+), (2) sex (men/women), and (3) high‐risk group (women APOE ɛ4 carriers/women in the APOE ɛ4− group and men in the APOE ɛ4− and ɛ4+ groups). Moderation effect was calculated using the D statistic between the unconstrained and constrained model of the interaction, 44 where a significant D statistic indicates moderation. 45
3. RESULTS
Our sample included 170 AD patients (age range = 46 to 89 years; mean age = 71.3 (9.1) years; n women = 93) in the SDS and 184 AD patients (age range = 55 to 91 years; mean age = 75.1 (7.5) years; n women = 89) in ADNI (replication sample) (Table 1). Standardized β coefficients are reported.
3.1. BPF growth model and class trajectories
The best latent growth model was obtained with random intercept and random slope model and the 3‐class LCGA model showed the best fit for BPF (see Table 2). We replicated these results in ADNI (see Table 2). Figure 1 shows the trajectories for BPF and the three distinct classes as represented by LCGA. In order of decreasing BPF, we define the classes as low (highest BPF), intermediate, and high global atrophy over 2 years.
TABLE 2.
Goodness of fit indexes for one‐to‐three class brain parenchymal fraction latent growth class models in the Sunnybrook Dementia Study (SDS) and the Alzheimer's Disease Neuroimaging Initiative (ADNI)
| Model | Class | AIC | BIC | −2LL | Entropy | Probability | Proportion | n |
|---|---|---|---|---|---|---|---|---|
| SDS: | ||||||||
| 1 | 1 | 1847.668 | 1863.347 | 1837.668 | – | 1.000 | 1.000 | 170 |
| 2 | 1 | 1728.791 | 1753.877 | 1712.790 | 0.738 | 0.914 | 0.441 | 75 |
| 2 | – | – | – | – | 0.932 | 0.559 | 95 | |
| 3a | 1 | 1689.287 | 1723.781 | 1667.286 | 0.741 | 0.905 | 0.394 | 67 |
| 2 | – | – | – | – | 0.903 | 0.265 | 45 | |
| 3 | – | – | – | – | 0.824 | 0.341 | 58 |
| ADNI: | ||||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 1958.626 | 1974.700 | 1948.626 | – | 1.000 | 1.000 | 184 |
| 2 | 1 | 1791.203 | 1816.923 | 1775.204 | 0.770 | 0.939 | 0.667 | 120 |
| 2 | – | – | – | – | 0.919 | 0.333 | 64 | |
| 3a | 1 | 1676.049 | 1711.413 | 1654.048 | 0.842 | 0.871 | 0.138 | 23 |
| 2 | – | – | – | – | 0.929 | 0.368 | 67 | |
| 3 | – | – | – | – | 0.944 | 0.495 | 94 |
Note. aBest fitting model.
Abbreviations: AIC, Akaike information criteria; BIC, Bayesian information criteria; −2LL, −2 log likelihood; Probability, probability of latent class membership; Proportion, proportion for the latent classes based on estimated model; n, sample size.
FIGURE 1.
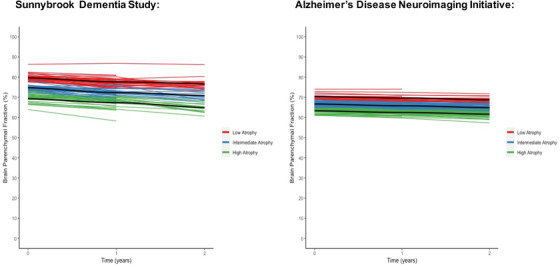
Global brain atrophy trajectories over 2 years (represented with brain parenchymal fraction [%]) in the Sunnybrook Dementia Study and the Alzheimer's Disease Neuroimaging Initiative. Three classes representing low (red), intermediate (blue), and high (green) atrophy were identified
3.2. Latent growth model for functional activities
The random intercept and random slope latent growth model provided the best fit for functional activities (see Table 3) in the SDS and ADNI.
TABLE 3.
Latent growth model fit statistics and chi‐square difference test for functional activities by wave in the Sunnybrook Dementia Study (SDS) and the Alzheimer's Disease Neuroimaging Initiative (ADNI)
| Functional activities (SDS) | ||||||
|---|---|---|---|---|---|---|
| Model | H0 value | Free parameters | −2LL | AIC | BIC | D () |
| Fixed intercept | −1207.452 | 4 | 2414.904 | 2422.905 | 2434.974 | – |
| Random intercept | −1196.456 | 5 | 2392.912 | 2402.913 | 2417.999 | 21.992 (1)** |
| Random intercept, fixed slope | −1171.758 | 6 | 2343.516 | 2355.516 | 2373.619 | 49.316 (1)** |
| Random intercept, random slope | −1167.190 | 6 | 2334.380 | 2346.379 | 2364.483 | 9.136 (0 a )* |
| Random intercept, random slope, fixed quadratic | −1166.535 | 7 | 2333.070 | 2347.071 | 2368.192 | 1.310 (1) |
| Functional activities (ADNI) | ||||||
|---|---|---|---|---|---|---|
| Model | H0 value | Free parameters | −2LL | AIC | BIC | D () |
| Fixed intercept | −1643.806 | 4 | 3287.612 | 3295.613 | 3308.472 | – |
| Random intercept | −1563.891 | 5 | 3127.782 | 3137.783 | 3153.857 | 159.830 (1) ** |
| Random intercept, fixed slope | −1484.460 | 6 | 2968.920 | 2980.921 | 3000.211 | 158.862 (1)** |
| Random intercept, random slope | −1477.591 | 7 | 2955.182 | 2969.182 | 2991.686 | 13.738 (1)* |
| Random intercept, random slope, fixed quadratic | −1474.203 | 8 | 2948.406 | 2964.405 | 2990.125 | 6.776 (1) |
Abbreviations: H0, Log Likelihood; ‐2LL, ‐2 Log Likelihood; AIC, Akaike Information Criteria; BIC, Bayesian Information Criteria; D, Deviance statistic; , Degrees of freedom for difference in deviance statistics.
= residuals for instrumental activities at a specific time point was constrained to zero for the model to work and difference of one was used to calculate the P‐value.
P < .05.
P < .001.
3.3. RG1: Brain atrophy classes predict functional decline
Higher atrophy class was associated with lower functional performance (intercept; β = −0.263, SE = 0.083, P = .002) and steeper decline (slope; β = −0.351, SE = 0.118, P = .003) in the SDS. Higher atrophy class was associated with lower functional performance (β = 0.243, SE = 0.087, P = .005) in ADNI (see Figure 2A). We note that higher FAQ performance (in ADNI) represents lower functional performance.
FIGURE 2.
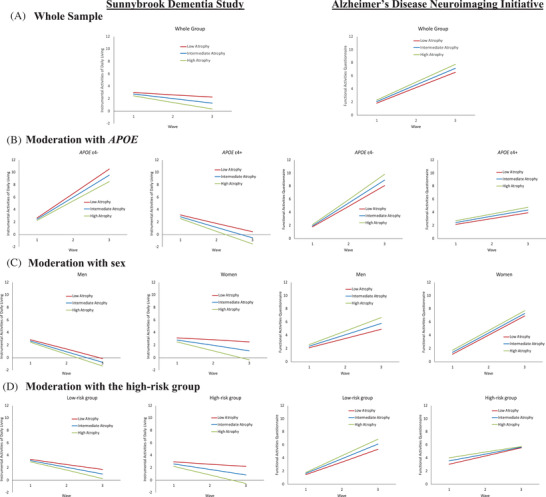
Predicted growth curve model of functional performance and decline with brain atrophy class as predictor. The three brain atrophy classes are represented as low (red), intermediate (blue), and high (green) atrophy. (A) Whole group: Higher brain atrophy class predicted poorer baseline functional performance in the Sunnybrook Dementia Study (SDS) and this was replicated in Alzheimer's Disease Neuroimaging Initiative (ADNI). We also observed steeper functional decline only in the SDS. (B) Moderation with apolipoprotein E (APOE): Higher brain atrophy class predicted poorer baseline functional performance in APOE ɛ4 carriers in the SDS, and this was replicated in ADNI. In ADNI, we also observed steeper functional decline in the APOE ɛ4− group. (C) Moderation with sex: Higher brain atrophy predicted poorer baseline functional performance and steeper decline in women in the SDS. In ADNI, we observed steeper functional decline in men. (D) Moderation with the high‐risk group: Higher brain atrophy class predicted poorer baseline functional performance in the high‐risk group (women APOE ɛ4 carriers) in the SDS, and this was replicated in ADNI. In ADNI, we also observed steeper functional decline in the low‐risk group (women APOE ɛ4− group and men APOE ɛ4− and ɛ4+ groups). Note all values represented for functional performance and decline are standardized. Higher instrumental activities of daily living indicate better functioning in the SDS and higher score on functional activities questionnaire in ADNI represents greater impairment.
3.4. RG2: Moderations with APOE and sex
First, we observed that APOE moderated the association between atrophy class and functional performance (see Figure 2B). In the SDS, higher atrophy class was associated with lower functional performance (intercept; β = −0.299, SE = 0.102, P = .003) in the APOE ɛ4+ group. In ADNI, higher atrophy class was associated with lower functional performance (intercept; β = 0.286, SE = 0.101, P = .005) in the APOE ɛ4+ group and steeper decline (slope; β = 0.362, SE = 0.159, P = .023) in the APOE ɛ4− group.
Second, we observed that sex moderated the association between atrophy class and functional performance (see Figure 2C). In the SDS, higher atrophy class was associated with lower functional performance (intercept; β = −0.340, SE = 0.104, P = .001) and steeper decline (slope; β = −0.548, SE = 0.137, P < .001) for women. In ADNI, higher atrophy class was associated with steeper functional decline (slope; β = 0.331, SE = 0.129, P = .010) in men.
3.5. RG3: Moderation with the high‐risk group
We observed that the high‐risk group moderated the association between atrophy class and functional performance and decline (see Figure 2D). In the SDS, higher atrophy class was associated with lower functional performance (intercept; β = −0.351, SE = 0.122, P = .004) and steeper decline (slope; β = −0.515, SE = 0.164, P = .002) in the high‐risk group (women APOE ɛ4 carriers). In ADNI, higher atrophy class was associated with lower functional performance (intercept; β = 0.499, SE = 0.183, P = .006) in the high‐risk group and steeper functional decline (slope; β = 0.309, SE = 0.107, P = .004) in the low‐risk group.
Significant moderations were observed with the D statistics for all three moderations (see Tables S1 and S2).
4. DISCUSSION
We observed that higher brain atrophy class predicted lower functional performance and steeper decline. This association was differentially moderated by APOE, sex, and high‐risk combination (women APOE ɛ4 carriers). Specifically, women APOE ɛ4 carriers showed exacerbated baseline functional performance. Key novel contributions and findings of our study include: (1) latent classes analyses identifying distinct 2‐year brain atrophy trajectories; (2) using brain atrophy classes to predict differential functional performance and decline in AD and as moderated with APOE, sex, and APOE and sex high‐risk combination; and (3) replicating baseline functional impairment is magnified in women APOE ɛ4 carriers with higher brain atrophy class in a large independent AD cohort (ADNI).
For RG1, higher atrophy class predicted lower functional performance and steeper decline. We replicated our baseline findings in ADNI. Recent study showed that a combined score representing WMH, lacunes, gray matter, and hippocampal volume may be a stronger predictor of cognitive and functional activities in cerebral small vessel disease 46 than specific brain regions. Our finding suggests that global brain atrophy patterns (using latent BPF classes) may identify distinct subgroups of AD patients at risk for accelerated functional impairment and those who may potentially benefit the most from personalized medicine (ie, tailored care and help to manage daily activities) and early intervention programs.
For RG2, APOE ɛ4 carriers with the higher brain atrophy class had lower functional performance. Previous studies have reported inconsistent results for APOE ɛ4+ and functional status in non‐demented older adults 47 , 48 and MCI participants. 11 Our finding extends prior work by (1) confirming APOE ɛ4+ as a risk factor for functional impairment 47 in a clinically diagnosed AD sample, and (2) replicating our findings in ADNI. As expected, higher global atrophy class and lower functional performance and steeper decline were observed selectively for women. Our results supplement previous work where women are observed to be at a higher risk overall. For example, women show (1) faster rates of atrophy, (2) steeper age‐related decline in cognition, and (3) longer survival rates leading to higher percentage of AD dementia diagnosis. 49
For RG3, we observed that women APOE ɛ4 carriers had worse functional performance with higher atrophy class than their low‐risk counterparts and this finding was replicated in ADNI. Previous work has shown that non‐demented women APOE ɛ4 carriers show decreased connectivity in the anterior cingulate cortex compared to women APOE ɛ3 carriers and men ɛ4 carriers, 50 and may have greater AD pathology, as detected at autopsy. 51 In addition, men have higher levels of sterol regulatory element‐binding protein (SREBP) 2 expression, where SREBP2 protein interacts with APOE to regulate lipid homeostasis 52 possibly contributing to an overall lower risk for men. To our knowledge this is the first study to confirm APOE and sex magnification for differential functional performance using latent global brain atrophy classes in AD. Future work examining the complex interactions between global brain atrophy, APOE, and sex should consider including asymptomatic older adults and other neurodegenerative groups (ie, vascular dementia) to identify adults with potentially high functional dependence risk profiles.
We note several strengths and limitations of the present study. For limitations, first, we used BPF to represent global brain atrophy and past studies have focused on specific brain region such as hippocampal volume, WMH, and cortical atrophy 53 , 54 , 55 to study functional impairment. Our aim was to focus on whether non‐modifiable risk factors (APOE and sex) magnify the risk of global brain atrophy. Future work should consider examining specific AD regions (ie, hippocampal volume) to target areas with greater AD‐related atrophy and to compare differences between specific brain regions associated with functional impairment. Second, we note several differences in findings between the SDS and ADNI replication sample: (1) in the SDS, higher brain atrophy class predicted steeper functional decline overall, in APOE ɛ4 carriers, women, and women APOE ɛ4 carriers but these associations were not observed in ADNI; (2) in ADNI, higher brain atrophy class predicted steeper functional decline in the APOE ɛ4− group, men, and low‐risk group, but these associations were not present in the SDS. These variations may be due to measurement differences in IADL (DAD‐IADL in SDS vs FAQ in ADNI), and other biomarkers and risk factors (ie, cerebrospinal fluid biomarkers) should be considered to elucidate this discrepancy. Future work with larger sample sizes and longer follow‐up of AD patients should be examined to confirm our findings. Third, we note that data on racial backgrounds were not available in the SDS and our ADNI replication sample was predominately White, not of Hispanic origin (93.5%). Future work should consider replicating our findings using diverse racial backgrounds. Fourth, we focused on global atrophy in AD patients so we did not explore other non‐AD pathologies (such as traumatic brain injury 56 ; stress and homocysteine levels 57 ) contributing to neurodegeneration.
Among strengths, first, our diagnosed AD sample is representative of dementia patients in a real‐world tertiary clinical setting. The SDS follows a research embedded in care approach and all recruited participants in our study are followed over time or as long as needed in our neurology clinic. Second, to our knowledge, this is first study to replicate such a complex magnification effect (brain atrophy, APOE, sex) associated with functional trajectories in AD. Third, we apply a novel approach by identifying distinct latent classes of brain atrophy trajectories and using this to predict functional performance and change.
In sum, distinct global brain atrophy patterns predicted functional trajectories in AD. Specifically, APOE ɛ4 carriers showed lower functional performance with higher brain atrophy class, and this association was magnified in women APOE ɛ4 carriers. Although women and APOE ɛ4+ risk are considered independent risk factors for functional decline, our findings suggest that the combined risk of women APOE ɛ4 carriers with global brain atrophy may be greater and highly influential than each risk domain separately. Such complex and dynamic multidomain interactions should be considered in intervention programs and clinical trial designs. Our study emphasizes the importance of applying a multidomain approach to identify patients with high functional dependence risk profiles who may benefit from early detection and personalized care.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
All of this research has been approved continuously by relevant institutional review boards. Certificates are available from and on file at Sunnybrook Health Sciences Centre. All participants have completed and signed informed consent forms.
CONFLICTS OF INTEREST
The authors declare that they have no competing interests.
Supporting information
Supporting information
ACKNOWLEDGMENTS
We gratefully acknowledge and thank all volunteer participants and the Sunnybrook Dementia Study (SDS) staff for their time and contributions. This work was supported by a Canadian Institutes of Health Research (MOP 13129) grant to Sandra E. Black and Mario Masellis. The authors also gratefully acknowledge financial support from the following sources. Sandra E. Black, Mario Masellis, and Joel Ramirez receive salary support from the Dr. Sandra Black Centre for Brain Resilience and Recovery, the Hurvitz Brain Sciences Research Program at Sunnybrook Research Institute, and the Department of Medicine at Sunnybrook Health Sciences Centre (SHSC). Mario Masellis receives salary support from the Department of Medicine at SHSC and the University of Toronto, as well as from the Sunnybrook Foundation and the Ontario Brain Institute. Joel Ramirez also acknowledges support from the LC Campbell Cognitive Neurology Research Institute and Ontario Neurodegeneration Research Initiative, Ontario Brain Institute. Shraddha Sapkota is supported by the Alzheimer Society of Canada/Canadian Consortium of Neurodegeneration in Aging Postdoctoral Fellowship. Data collection and sharing for this project were funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH‐12‐2‐0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer's Association; Alzheimer's Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol‐Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann‐La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California. The funding sources did not have role in the study design; data collection; statistical analysis; or results interpretation, reporting, or submission decisions.
Sapkota S, Ramirez J, Yhap V, Masellis M, Black SE, for the Alzheimer's Disease Neuroimaging Initiative . Brain atrophy trajectories predict differential functional performance in Alzheimer's disease: Moderations with apolipoprotein E and sex. Alzheimer's Dement. 2021;13:e12244. 10.1002/dad2.12244
Mario Masellis, Sandra E. Black equal contribution as co‐senior authors.
Trials Registration: ClinicalTrials.gov, NCT01800214. Registered on February 27, 2013.
REFERENCES
- 1. Brown PJ, Devanand DP, Liu X, Caccappolo E. Functional impairment in elderly patients with mild cognitive impairment and mild Alzheimer disease. Arch Gen Psychiatry. 2011;68:617–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kamiya M, Sakurai T, Ogama N, Maki Y, Toba K. Factors associated with increased caregivers’ burden in several cognitive stages of Alzheimer's disease. Geriatr Gerontol Int. 2014;14:45–55. [DOI] [PubMed] [Google Scholar]
- 3. Callahan CM, Boustani MA, Schmid AA, et al. Targeting functional decline in Alzheimer disease: a randomized trial. Ann Intern Med. 2017;166:164–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gauthier S, Gélinas I, Gauthier L. Functional disability in Alzheimer's disease. Int Psychogeriatrics. 1997;9:163–165. [DOI] [PubMed] [Google Scholar]
- 5. Bertrand RM, Willis SL. Everyday problem solving in Alzheimer's patients: a comparison of subjective and objective assessments. Aging Mental Health. 2010;3:281–293. [Google Scholar]
- 6. Miller LS, Brown CL, Mitchell MB, Williamson GM. Activities of daily living are associated with older adult cognitive status: caregiver versus self‐reports. J Appl Gerontol. 2011;32:3–30. [DOI] [PubMed] [Google Scholar]
- 7. Roehr S, Riedel‐Heller SG, Kaduszkiewicz H, et al. Is function in instrumental activities of daily living a useful feature in predicting Alzheimer's disease dementia in subjective cognitive decline?. Int J Geriatr Psychiatry. 2019;34:193–203. [DOI] [PubMed] [Google Scholar]
- 8. Farias ST, Chou E, Harvey DJ, et al. Longitudinal trajectories of everyday function by diagnostic status. Psychol Aging. 2013;28:1070–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang L, Van Belle G, Kukull WB, Larson EB. Predictors of functional change: a longitudinal study of nondemented people aged 65 and older. J Am Geriatr Soc. 2002;50:1525–1534. [DOI] [PubMed] [Google Scholar]
- 10. Mok WYW, Chu LW, Chung CP, Chan NY, Hui SL. The relationship between non‐cognitive symptoms and functional impairment in Alzheimer's disease. Int J Geriatr Psychiatry. 2004;19:1040–1046. [DOI] [PubMed] [Google Scholar]
- 11. Okonkwo OC, Alosco ML, Jerskey BA, Sweet LH, Ott BR, Tremont G. Cerebral atrophy, apolipoprotein e ε4, and rate of decline in everyday function among patients with amnestic mild cognitive impairment. Alzheimer's Dement. 2010;6:404–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ogama N, Sakurai T, Nakai T, et al. Impact of frontal white matter hyperintensity on instrumental activities of daily living in elderly women with Alzheimer disease and amnestic mild cognitive impairment. PLoS One. 2017;12:e0172484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Blazer DG, Fillenbaum G, Burchett B. The APOE‐E4 allele and the risk of functional decline in a community sample of African american and white older adults. J Gerontol A Biol Sci Med Sci. 2001;56:M785–M789. [DOI] [PubMed] [Google Scholar]
- 14. Johnson JK, Lui L‐Y, Yaffe K. Executive function, more than global cognition, predicts functional decline and mortality in elderly women. J Gerontol A Biol Sci Med Sci. 2007;62:1134–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sauvaget C, Yamada M, Fujiwara S, Sasaki H, Mimori Y. Dementia as a predictor of functional disability: a four‐year follow‐up study. Gerontology. 2002;48:226–233. [DOI] [PubMed] [Google Scholar]
- 16. Badhwar A, McFall GP, Sapkota S, et al. A multiomics approach to heterogeneity in Alzheimer's disease: focused review and roadmap. Brain. 2020;143:1315–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dixon RA, Lachman ME. Risk and protective factors in cognitive aging: advances in assessment, prevention, and promotion of alternative pathways. In: Samanez‐Larkin GR, ed. Editor Aging Brain Funct. Adapt. Across Adulthood. Washington DC: American Psychological Association; 2019:217–263. [Google Scholar]
- 18. Sapkota S, McFall GP, Masellis M, Dixon RA. A multimodal risk network predicts executive function trajectories in non-demented aging. Frontiers in Aging Neuroscience. 2021;13:621023, 10.3389/fnagi.2021.621023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Good CD, Scahill RI, Fox NC, et al. Automatic differentiation of anatomical patterns in the human brain: validation with studies of degenerative dementias. Neuroimage. 2002;17:29–46. [DOI] [PubMed] [Google Scholar]
- 20. Callahan BL, Ramirez J, Berezuk C, Duchesne S, Black SE. Predicting Alzheimer's disease development: a comparison of cognitive criteria and associated neuroimaging biomarkers. Alzheimer's Res Ther. 2015;7:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bigler ED, Neeley ES, Miller MJ, et al. Cerebral volume loss, cognitive deficit and neuropsychological performance: comparative measures of brain atrophy: i. Dementia. J Int Neuropsychol Soc. 2004;10:442–452. [DOI] [PubMed] [Google Scholar]
- 22. Erten‐Lyons D, Dodge HH, Woltjer R, et al. Neuropathologic basis of age‐associated brain atrophy. JAMA Neurol. 2013;70:616–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu C‐C, Liu C‐C, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol. 2013;9:106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Whitehair DC, Sherzai A, Emond J, et al. Influence of apolipoprotein e ε4 on rates of cognitive and functional decline in mild cognitive impairment. Alzheimer's Dement. 2010;6:412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Belloy ME, Napolioni V, Greicius MD. A quarter century of APOE and Alzheimer's disease: progress to date and the path forward. Neuron. 2019;101:820–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hoyt BD, Massman PJ, Schatschneider C, Cooke N, Doody RS. Individual growth curve analysis of APOE ε4–Associated cognitive decline in Alzheimer disease. Arch Neurol. 2005;62:454–459. [DOI] [PubMed] [Google Scholar]
- 27. Nourhashémi F, Andrieu S, Gillette‐Guyonnet S, Vellas B, Albarède JL, Grandjean H. Instrumental activities of daily living as a potential marker of frailty: a study of 7364 community‐dwelling elderly women (the EPIDOS Study). J Gerontol A Biol Sci Med Sci. 2001;56:M448–M453. [DOI] [PubMed] [Google Scholar]
- 28. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS‐ADRDA work group under the auspices of department of health and human services task force on Alzheimer's disease. Neurology. 1984;34:939–944. [DOI] [PubMed] [Google Scholar]
- 29. Feldman HH, Van Baelen B, Kavanagh SM, Torfs KEL. Cognition, function, and caregiving time patterns in patients with mild‐to‐moderate Alzheimer disease: a 12‐month analysis. Alzheimer Dis Assoc Disord. 2005;19:29–36. [DOI] [PubMed] [Google Scholar]
- 30. Crane PK, Carle A, Gibbons LE, et al. Development and assessment of a composite score for memory in the Alzheimer's Disease Neuroimaging Initiative (ADNI). Brain Imaging Behav. 2012;6:502–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Weiner MW, Veitch DP, Aisen PS, et al. Impact of the Alzheimer's disease neuroimaging initiative, 2004 to 2014. Alzheimer's Dement. 2015;11:865–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dade LA, Gao FQ, Kovacevic N, et al. Semiautomatic brain region extraction: a method of parcellating brain regions from structural magnetic resonance images. Neuroimage. 2004;22:1492–1502. [DOI] [PubMed] [Google Scholar]
- 33. Ramirez J, Gibson E, Quddus A, et al. Lesion explorer: a comprehensive segmentation and parcellation package to obtain regional volumetrics for subcortical hyperintensities and intracranial tissue. Neuroimage. 2011;54:963–973. [DOI] [PubMed] [Google Scholar]
- 34. Ramirez J, McNeely AA, Scott CJ, Stuss DT, Black SE. Subcortical hyperintensity volumetrics in Alzheimer's disease and normal elderly in the Sunnybrook Dementia Study: correlations with atrophy, executive function, mental processing speed, and verbal memory. Alzheimers Res Ther. 2014;6:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ntiri EE, Holmes MF, Forooshani PM, et al. Improved segmentation of the intracranial and ventricular volumes in populations with cerebrovascular lesions and atrophy using 3D CNNs. Neuroinformatics. 2021:1–22. [DOI] [PubMed] [Google Scholar]
- 36. Leung KK, Barnes J, Modat M, et al. Brain MAPS: an automated, accurate and robust brain extraction technique using a template library. Neuroimage. 2011;55:1091–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mirza SS, Saeed U, Knight J, et al. APOE ε4, white matter hyperintensities, and cognition in Alzheimer and Lewy body dementia. Neurology. 2019;93:e1807–e1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Saykin AJ, Shen L, Yao X, et al. Genetic studies of quantitative MCI and AD phenotypes in ADNI: progress, opportunities, and plans. Alzheimer's Dement. 2015;11:792–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Serrano‐Pozo A, Das S, Hyman BT. APOE and Alzheimer's disease: advances in genetics, pathophysiology, and therapeutic approaches. Lancet Neurol. 2021;20:68–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nadkarni NK, Levy‐Cooperman N, Black SE. Functional correlates of instrumental activities of daily living in mild Alzheimer's disease. Neurobiol Aging. 2012;33:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Juva K, Makela M, Erkinjuntti T, et al. Functional assessment scales in detecting dementia. Age Ageing. 1997;26:393–400. [DOI] [PubMed] [Google Scholar]
- 42. Pfeffer RI, Kurosaki TT, Harrah CH, Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37:323–329. [DOI] [PubMed] [Google Scholar]
- 43. Muthén L, Muthén B. Mplus User's Guide. 7th ed. Los Angeles, CA: Muthén, L Muthén, B; 1998. ‐2012. [Google Scholar]
- 44. McFall GP, Sapkota S, McDermott KL, Dixon RA. Risk‐reducing apolipoprotein E and Clusterin genotypes protect against the consequences of poor vascular health on executive function performance and change in nondemented older adults. Neurobiol Aging. 2016;42:91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Buckley JP, Doherty BT, Keil AP, Engel SM. Statistical approaches for estimating sex‐specific effects in endocrine disruptors research. Environ Health Perspect. 2017;125:067013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jokinen H, Koikkalainen J, Laakso HM, et al. Global burden of small vessel disease‐related brain changes on MRI predicts cognitive and functional decline. Stroke. 2020;51:170–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Albert SM, Gurland B, Maestre G, Jacobs DM, Stern Y, Mayeux R. APOE genotype influences functional status among elderly without dementia. Am J Med Genet Neuropsychiatr Genet. 1995;60:583–587. [DOI] [PubMed] [Google Scholar]
- 48. Kulminski A, Ukraintseva SV, Arbeev KG, et al. Association between APOE 2/3/ 4 polymorphism and disability severity in a national long‐term care survey sample. Age Ageing. 2008;37:288–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Riedel BC, Thompson PM, Brinton RD. Age, APOE and sex: triad of risk of Alzheimer's disease. J Steroid Biochem Mol Biol. 2016;160:134–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Reiman EM, Caselli RJ, Yun LS, et al. Preclinical evidence of Alzheimer's disease in persons homozygous for the ε4 allele for apolipoprotein E. N Engl J Med. 1996;334:752–758. [DOI] [PubMed] [Google Scholar]
- 51. Corder EH, Ghebremedhin E, Taylor MG, Thal DR, Ohm TG, Braak H. The biphasic relationship between regional brain senile plaque and neurofibrillary tangle distributions: modification by age, sex, and APOE polymorphism. Ann N Y Acad Sci. 2004;1019:24–28. [DOI] [PubMed] [Google Scholar]
- 52. De Marinis E, Martini C, Trentalance A, Pallottini V. Sex differences in hepatic regulation of cholesterol homeostasis. J Endocrinol. 2008;198:635–643. [DOI] [PubMed] [Google Scholar]
- 53. Vidoni ED, Honea RA, Burns JM. Neural correlates of impaired functional independence in early Alzheimer's disease. J Alzheimer's Dis. 2010;19:517–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wakefield DB, Moscufo N, Guttmann CR, et al. White matter hyperintensities predict functional decline in voiding, mobility, and cognition in older adults. J Am Geriatr Soc. 2010;58:275–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jutten RJ, Dicks E, Vermaat L, et al. Impairment in complex activities of daily living is related to neurodegeneration in Alzheimer's disease–Specific regions. Neurobiol Aging. 2019;75:109–116. [DOI] [PubMed] [Google Scholar]
- 56. Cole JH, Leech R, Sharp DJ. Prediction of brain age suggests accelerated atrophy after traumatic brain injury. Ann Neurol. 2015;77:571–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sachdev PS. Homocysteine and brain atrophy. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1152–1161. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information


