Abstract
Introduction
Objectively‐defined subtle cognitive decline (Obj‐SCD) and plasma phosphorylated‐tau181 (p‐tau181) are promising early Alzheimer's disease (AD) markers. However, associations between Obj‐SCD and p‐tau181, and their combined prognostic potential, are unknown.
Methods
Baseline and 4‐year longitudinal p‐tau181 changes were compared across cognitively unimpaired (CU; n = 402), Obj‐SCD (n = 199), and mild cognitive impairment (MCI; n = 346) groups. CU and Obj‐SCD participants were further classified as p‐tau181‐positive or negative.
Results
CU and Obj‐SCD has lower baseline p‐tau181 than MCI and did not differ from one another. Longitudinally, Obj‐SCD had the steepest p‐tau181 increase. Obj‐SCD/p‐tau181‐positive participants had the fastest rates of amyloid accumulation, cognitive decline, and functional decline.
Conclusions
Despite assumptions that cognitive changes invariably follow biomarker changes, early neuropsychological difficulties may emerge before/concurrently with plasma p‐tau181 changes. Combining Obj‐SCD and p‐tau181, two potentially accessible early markers, was associated with the faster declines in AD‐related outcomes.
Keywords: plasma biomarkers, preclinical AD, p‐tau181, subtle cognitive decline
1. INTRODUCTION
Cerebrospinal fluid (CSF) and positron emission tomography (PET) markers of Alzheimer's disease (AD) have proven to be relatively accurate and reliable methods of measuring in vivo AD pathology. 1 , 2 However, the availability of these methods is often limited by the invasiveness of lumbar puncture, exposure to radioactive ligands, high cost of PET imaging, access to an academic medical center, as well as some medical contraindications, limiting these technologies’ potential in clinical trial screening and making them less accessible to those most in need of diagnostic clarity. Blood‐based biomarkers of AD may be a potential solution to many of these barriers, and have garnered significant attention given recent advances and availability of assays for plasma biomarkers that correlate strongly with gold standard CSF and imaging markers. 3 , 4
Recent work has demonstrated that plasma phosphorylated tau at threonine 181 (p‐tau181) predicts poorer clinical outcomes and neurodegeneration, and is associated with both amyloid and tau biomarkers as well as AD neuropathology. 5 , 6 , 7 , 8 , 9 , 10 , 11 Longitudinally, plasma p‐tau181 changes were associated with a widespread tau‐PET signal increase 6 years later, particularly in temporoparietal cortical regions that are known predilection sites for neurofibrillary tangle pathology in “typical” Alzheimer's disease. 12 There were also stronger associations between p‐tau181 and both cross‐sectional and longitudinal β‐amyloid (Aβ)‐PET in participants with mild cognitive impairment (MCI) than cognitively unimpaired (CU) individuals, suggesting higher plasma p‐tau181 is associated with more widespread cortical Aβ. 12 Further, p‐tau181 has been shown to be higher in mutation carriers of familial AD, relative to non‐carriers, including up to 16 years before estimated year of symptom onset. 13 Unlike more general plasma markers of neurodegeneration such as neurofilament light (NfL), plasma p‐tau181 appears to be quite specific to AD pathology. 7 , 8 , 9 , 10
While studies thus far have examined plasma p‐tau181 in CU, MCI, and dementia clinical stages, and its relationship to future cognitive decline, little is known about the timing and utility of plasma p‐tau181 during the pre‐MCI stage. Subtle, but objectively measured cognitive changes, can be captured during the preclinical phase of AD using sensitive neuropsychological measures, which have been shown to improve prediction of cognitive decline above and beyond traditional AD biomarkers alone. 14 , 15 Neuropsychological “process scores” quantify the types of errors that an individual may produce on a neuropsychological test, or the approach and strategies that are used on a task, and are distinct from the traditionally used total score. Process scores have been used to detect cognitive inefficiencies associated with an AD trajectory prior to the onset of MCI and dementia. 15 , 16 , 17 Previous work using process scores and total scores to classify objectivelydefined subtle cognitive decline (Obj‐SCD) showed that individuals with Obj‐SCD have CSF and PET AD biomarker levels that fall in between those of CU and MCI participants. 18 , 19 Further, Obj‐SCD status predicts progression to MCI/dementia, decline in everyday functioning, Aβ accumulation, entorhinal cortex atrophy, and altered cerebral blood flow. 18 , 19 , 20 , 21 Recently, we have shown that participants with Obj‐SCD have plasma NfL levels that are higher than CU participants and do not differ from those of MCI participants. 22 Also, higher plasma NfL in participants with Obj‐SCD and MCI was associated with faster memory decline. However, the cross‐sectional and longitudinal relationship between Obj‐SCD and a more accessible and AD‐specific plasma p‐tau181 biomarker is unknown.
RESEARCH IN CONTEXT
Systematic review: Authors reviewed the literature using traditional (eg, PubMed) sources. Objective subtle cognitive decline (Obj‐SCD) and plasma phosporylated tau181 (p‐tau181) have been studied separately as they relate to Alzheimer's disease (AD) biomarkers and clinical outcomes; however, the relationships between Obj‐SCD and p‐tau181 were unknown.
Interpretation: Findings show that Obj‐SCD is an early marker of future AD biomarker changes and suggest that subtle cognitive changes can be detected coincident with plasma p‐tau181 changes. Further, results highlight the benefit of combining these two potentially accessible and cost‐effective early detection methods to improve prognostic value.
Future directions: A key benefit of both neuropsychological assessment and blood‐based biomarkers is the potential for implementation in community‐based research, so a critical next step is to examine these relationships in population‐based samples. Additionally, examining Obj‐SCD in the context of newer plasma p‐tau markers (p‐tau217, p‐tau231) may yield additional benefits to early detection.
HIGHLIGHTS
Objective subtle cognitive decline (Obj‐SCD) and plasma phosphorylated tau181 (p‐tau181) are promising early Alzheimer's disease (AD) markers.
Obj‐SCD is associated with a faster increase in plasma p‐tau181 over 4 years.
In combination, Obj‐SCD and elevated plasma p‐tau181 had the fastest declines in AD‐related outcomes.
Therefore, the aims of this study are to examine whether participants with Obj‐SCD show higher plasma p‐tau181 cross‐sectionally relative to CU participants and whether Obj‐SCD predicts future increases in plasma p‐tau181 concentrations. Additionally, given that plasma biomarkers and neuropsychological assessment are both potentially accessible methods of AD risk detection, and using combinations of markers may improve prediction compared to individual markers, 23 , 24 we examined whether the combination of Obj‐SCD plus elevated plasma p‐tau181 had added value in predicting longitudinal AD‐related outcomes such as amyloid accumulation, cognitive decline, and functional decline.
2. METHODS
2.1. The ADNI dataset
Data used in the preparation of this article were obtained from the Alzheimer's Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). The ADNI was launched in 2003 as a public‐private partnership, led by Principal Investigator Michael W. Weiner, MD. The primary goal of ADNI has been to test whether serial magnetic resonance imaging, PET, other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of MCI and early AD. For up‐to‐date information, see www.adni‐info.org.
2.2. Participants
Enrollment criteria for ADNI have been previously described in detail 25 and are included in the Supplemental Methods (in the Supporting Information). ADNI was approved by the institutional review boards at each of the participating institutions. Written informed consent was obtained from all participants or authorized representatives at each site. The current study included 947 participants without dementia from ADNI 1/GO/2 cohorts, for whom plasma p‐tau181 data are available. The initial visit in which plasma p‐tau181 was collected was considered the baseline visit (time = 0) and longitudinal timepoints included 1‐, 2‐, 3‐, and 4‐year annual follow‐up visits.
2.3. Cognitive groups
Participants who did not have an ADNI diagnosis of dementia at their baseline visit were classified into one of three diagnostic groups based on actuarial neuropsychological criteria: CU, Obj‐SCD, or MCI. 19 , 26 , 27 First, comprehensive neuropsychological MCI criteria were applied to all participants. 26 , 27 , 28 Participants were considered MCI if they performed > 1 SD below the age‐/education‐/sex‐adjusted mean on (1) two neuropsychological measures within the same cognitive domain, or (2) at least one measure across all three sampled cognitive domains. Six neuropsychological test scores were considered in the MCI criteria and included two memory measures (Rey Auditory Verbal Learning Test [AVLT] delayed free recall correct responses and AVLT recognition discrimination [hits minus false positives]); two language measures (30‐item Boston Naming Test total correct and Animal Fluency total score), and two attention/executive functioning measures (Trail Making Test Parts A and B times to completion).
Next, actuarial neuropsychological Obj‐SCD criteria were applied to the remaining participants not classified as MCI. Participants were considered to have Obj‐SCD if they performed > 1 SD below the age‐/education‐/sex‐adjusted mean on (1) one impaired total test score in two different cognitive domains (memory, language, attention/executive), or (2) two impaired neuropsychological process scores from the AVLT, or (3) one impaired total test score and one impaired process score. 18 , 19 The total test scores were the six neuropsychological variables used for determining MCI. The three process scores for the Obj‐SCD classification derived from the AVLT (see Supplemental Methods for details) were total intrusion errors (total number of non‐target words said across all recall trials), learning slope, and retroactive interference, which have been shown to differ between CU participants who remained stable and those who progressed to MCI within 5 years in ADNI. 15 If participants were classified as neither MCI nor Obj‐SCD, they were considered CU. 19 , 21
2.4. Plasma p‐tau181 measurements
Plasma p‐tau181 was analyzed by the Single Molecule array (Simoa) technique. The assay used was developed in the Clinical Neurochemistry Laboratory, University of Gothenburg, Sweden, and used a combination of two monoclonal antibodies (Tau12 and AT270) for measuring N‐terminal to mid‐domain forms of p‐tau181. Samples were analyzed in a single batch. Additional details of the methods can be found at adni.loni.usc.edu and in reports by Karikari et al. 11 , 29 Six outliers for plasma p‐tau181 were identified (0.6%) using a previously described approach of > 12 median absolute deviations above the median, and were excluded from subsequent analyses. 7 For analyses that examined plasma p‐tau181 continuously, plasma p‐tau181 was log‐transformed to improve normality prior to analyses. 29 For analyses examining plasma p‐tau181 positivity, a raw cutoff of > 17.3 pg/mL was used based on a previously derived threshold predicting flortaucapir PET positivity within ADNI. 30 This cutoff corresponded to a log‐transformed plasma p‐tau181 value of > 1.239.
2.5. Florbetapir PET
PET imaging using the 18F‐florbetapir AV‐45 tracer was used to quantify amyloid burden. The details of data acquisition and processing of ADNI florbetapir PET data are available at adni.loni.usc.edu and in the Supplemental Methods. A summary standardized uptake value ratio (SUVR) was then calculated by dividing the mean florbetapir uptake across four AD‐vulnerable cortical regions (frontal, anterior/posterior cingulate, lateral parietal, and lateral temporal cortices) by whole cerebellar (white and gray matter) florbetapir uptake. Greater retention of florbetapir is reflective of a greater cortical amyloid load. A threshold of 1.11 for cross‐sectional descriptive florbetapir analyses, using cerebellum as the reference region, was used to determine amyloid positivity. 31 , 32 Aβ‐PET was conducted every other year for most participants.
2.6. Cognitive outcomes
Changes in global cognitive functioning were measured using the Preclinical Alzheimer Composite Score (PACC), which has been shown to detect early cognitive changes associated with AD‐related pathology. 33 The ADNI‐modified PACC included the Mini‐Mental State Examination (MMSE), Logical Memory Delayed Recall, Digit Symbol Substitution Test, and the Delayed Word Recall from the Alzheimer's Disease Assessment Scale–Cognitive Subscale. Each of the four component scores has a mean of 0 and a standard deviation of 1. Lower PACC scores represent lower performance. This cognitive outcome was selected since measures included in the PACC do not overlap with the measures used for the actuarial classification of Obj‐SCD or MCI.
2.7. Everyday functioning outcomes
Changes in everyday functioning were measured via the Functional Activities Questionnaire (FAQ) and Clinical Dementia Rating—Sum of Boxes (CDR‐SB). The FAQ is an informant‐rated measure of difficulty across 10 instrumental activities of daily living. 34 Each participant's ability to perform the tasks was rated on a 4‐point scale: 0 (normal), 1 (has difficulty but does by self), 2 (requires assistance), or 3 (dependent), such that the FAQ total score ranges from 0 to 30 with higher scores indicating greater difficulty. The CDR is a semi‐structured informant and patient interview that assesses the degree of everyday impairment. The CDR‐SB provides a greater range of scores compared to the CDR global score and ranges from 0 to 18, with higher scores indicating worse functional status. 35
2.8. Additional covariates
Apolipoprotein E (APOE) ɛ4 allele frequency was the sum of the number of ɛ4 alleles (0‐2). Pulse pressure (systolic–diastolic blood pressure), a proxy measure for arterial stiffness and an index of vascular aging, has been associated with increased CSF p‐tau levels and progression to AD. 36 , 37 Pulse pressure was included as a covariate to determine whether associations between cognitive group and plasma p‐tau181 persisted above and beyond general vascular risk burden. Additional covariates included age and sex/gender (man or woman). Years of education was included as a covariate in models with cognitive and everyday functioning as an outcome. Baseline Aβ‐PET SUVR was included in the model with longitudinal Aβ‐PET as the outcome.
2.9. Statistical analyses
Analysis of variance (ANOVA) or chi‐squared tests examined baseline differences in demographic and clinical characteristics by cognitive group. At baseline, a one‐way ANOVA examined group differences in plasma p‐tau181. Next, a linear mixed effects (LME) model, adjusting for age, sex/gender, APOE ε4 allele frequency, and pulse pressure, examined the 4‐year p‐tau181 rate of change in Obj‐SCD and MCI participants relative to CU. Random intercept was included, but random slope did not improve model fit, so it was not included. Follow‐up analyses examined whether the group p‐tau181 x time trajectories differed by Aβ‐PET positivity status in a subset of participants with Aβ‐PET at the first plasma p‐tau181 visit (n = 824).
Given the potential for using a combination of cognition and plasma p‐tau181 markers in early detection, participants who were considered either CU or Obj‐SCD were determined to be either plasma p‐tau181 positive (p‐tau181+) or negative (p‐tau181–), creating four groups: CU/p‐tau181–, CU/p‐tau181+, Obj‐SCD/p‐tau181–, and Obj‐SCD/p‐tau181+. LME models that included random intercept and slope were then used to examine whether there were group differences in 4‐year trajectories of Aβ‐PET, global cognition (PACC), and everyday functioning (FAQ, CDR‐SB). The Aβ‐PET model was adjusted for age, sex/gender, baseline Aβ‐PET (given slightly higher Aβ in the Obj‐SCD group at baseline), APOE ε4 allele frequency, and pulse pressure. The PACC and everyday functioning models were adjusted for age, sex/gender, years of education, APOE ε4 allele frequency, and pulse pressure. The CU/p‐tau181– group was the reference group. Unstandardized estimates are reported to assist with interpretation and effect sizes are reported as r‐values. Description of missing data is included in Supplemental Methods; sample size for each outcome variable at each occasion is included in Supplemental Table S1.
3. RESULTS
3.1. Baseline characteristics
Table 1 shows the baseline demographic and clinical characteristics of participants by cognitive status. Briefly, the Obj‐SCD group tended to perform in between the CU and MCI groups on measures of cognitive and everyday functioning. They also had slightly higher levels of amyloid (P = .041) than the CU group, but had lower levels of amyloid (P < .001) and a lower frequency of APOE ε4 alleles (P < .001) than MCI participants. Notably, the CU (Mean = 15.56, SD = 10.19) and Obj‐SCD (Mean = 15.07, SD = 8.91) groups had lower baseline plasma p‐tau181 than the MCI group (Mean = 19.56, SD = 10.95; Ps < .001) and did not differ from one another (P = .580; Figure 1). Group differences in plasma p‐tau181 by amyloid status are described in the Supplemental Results.
TABLE 1.
Baseline demographic and clinical characteristics by cognitive group status
| Total Sample N = 947 | CU N = 402 | Obj‐SCD N = 199 | MCI N = 346 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean or % | SD or N | Range | Mean or % | SD or N | Range | Mean or % | SD or N | Range | Mean or % | SD or N | Range | F or χ2 | P | |
| Age | 73.60 | 7.43 | 55.00–93.60 | 73.40 | 7.30 | 55.00–91.40 | 73.87 | 7.43 | 55.00–90.40 | 73.68 | 7.60 | 55.00–93.60 | F = 0.29 | .747 |
| Education | 16.26 | 2.69 | 6–20 | 16.45 | 2.69 | 6–20 | 16.26 | 2.53 | 10–20 | 16.05 | 2.78 | 7–20 | F = 1.99 | .137 |
| Female/woman, % | 47.2% | N = 447 | 51.5% | N = 207 | 43.2% | N = 86 | 44.5% | N = 154 | χ2 = 5.25 | .073 | ||||
| Race | χ2 = 21.49 | .044 | ||||||||||||
|
American Indian/Alaska Native |
0.2% | N = 2 | 0.5% | N = 2 | 0.0% | N = 0 | 0.0% | N = 0 | ||||||
| Asian | 1.4% | N = 13 | 1.0% | N = 4 | 2.5% | N = 5 | 1.2% | N = 4 | ||||||
| Black | 4.0% | N = 38 | 2.5% | N = 10 | 5.0% | N = 10 | 5.2% | N = 18 | ||||||
|
Hawaiian/Pacific Islander |
0.2% | N = 2 | 0.0% | N = 0 | 0.0% | N = 0 | 0.6% | N = 2 | ||||||
| White | 92.6% | N = 877 | 94.5% | N = 380 | 92.5% | N = 184 | 90.5% | N = 313 | ||||||
| More than one | 1.3% | N = 12 | 1.5% | N = 6 | 0.0% | N = 0 | 1.7% | N = 6 | ||||||
| Unknown | 0.3% | N = 3 | 0.0% | N = 0 | 0.0% | N = 0 | 0.9% | N = 3 | ||||||
| Ethnicity | χ2 = 2.35 | .672 | ||||||||||||
| Hispanic | 3.5% | N = 33 | 2.7% | N = 11 | 3.0% | N = 6 | 4.6% | N = 16 | ||||||
| Non‐Hispanic | 96.1% | N = 910 | 96.8% | N = 389 | 96.5% | N = 192 | 95.1% | N = 329 | ||||||
| Unknown | 0.4% | N = 4 | 0.5% | N = 2 | 0.5% | N = 1 | 0.3% | N = 1 | ||||||
| MMSE | 28.46 | 1.69 | 23–30 | 28.98 | 1.44 | 24–30 | 28.47 | 1.57 | 24–30 | 27.84 | 1.82 | 21–30 | F = 46.07 [Link] , b , c | <.001 |
| Pulse pressure | 59.49 | 15.38 | 59.54 | 16.04 | 60.58 | 14.15 | 58.80 | 15.28 | F = 0.85 | .427 | ||||
| APOE ε4 frequency | χ2 = 43.79 b , c | <.001 | ||||||||||||
| 0 ε4 alleles | 61.6% | N = 583 | 70.1% | N = 282 | 65.3% | N = 130 | 49.4% | N = 171 | ||||||
| 1 ε4 allele | 32.6% | N = 309 | 27.1% | N = 109 | 30.7% | N = 61 | 40.2% | N = 139 | ||||||
| 2 ε4 alleles | 5.8% | N = 55 | 2.7% | N = 11 | 4.0% | N = 8 | 10.4% | N = 36 | ||||||
| Aβ‐PET SUVR* | 1.17 | 0.21 | 0.84–2.03 | 1.12 | 0.18 | 0.84–2.03 | 1.16 | 0.20 | 0.84–1.84 | 1.25 | 0.24 | 0.84–2.00 | F = 31.92 [Link] , b , c | <.001 |
| Plasma p‐tau181 (pg/mL) | 16.92 | 10.41 | 0.36–72.26 | 15.56 | 10.19 | 0.36–72.26 | 15.07 | 8.91 | 0.83–50.40 | 19.56 | 10.95 | 2.06–69.60 | F = 21.96 b , c | <.001 |
| PACC | −3.05 | 4.68 | −16.90–6.26 | −0.58 | 3.58 | −13.31–5.48 | −2.73 | 3.80 | −16.90–4.64 | −6.12 | 4.51 | −16.90–6.26 | F = 179.95 [Link] , b , c | <.001 |
| FAQ | 1.73 | 3.31 | 0–22 | 0.80 | 2.27 | 0–18 | 1.55 | 2.82 | 0–13 | 2.94 | 4.12 | 0–22 | F = 42.80 [Link] , b , c | <.001 |
| CDR‐SB | 0.91 | 1.10 | 0–5.5 | 0.51 | 0.93 | 0–3.5 | 0.86 | 1.08 | 0–3.5 | 1.40 | 1.11 | 0–5.5 | F = 69.77 [Link] , b , c | <.001 |
Note: F statistic reported for one‐way ANOVAs, χ2 statistic reported for chi‐square tests.
significant differences between CU and Obj‐SCD.
significant differences between CU and MCI.
significant difference between Obj‐SCD and MCI.
subset of participants (Total N = 824; CU n = 348; Obj‐SCD n = 177; MCI n = 299) had Aβ‐PET.
Abbreviations: CU, Cognitively normal; Obj‐SCD, Objectively‐defined subtle cognitive decline; MCI, mild cognitive impairment; MMSE, Mini‐Mental State Examination; APOE, apolipoprotein E; Aβ, amyloid beta; SUVR, standardized uptake value ratio; PACC, modified Preclinical Alzheimer Cognitive Composite; FAQ, Functional Activities Questionnaire; CDR‐SB, Clinical Dementia Rating–Sum of Boxes.
FIGURE 1.

Baseline plasma p‐tau181. Dot‐box plots show log‐transformed plasma p‐tau181 values by (A) cognitive group, and (B) cognitive groups subdivided for β‐amyloid (Aβ) positivity status on positron emission tomography. CU, cognitively unimpaired; Obj‐SCD, objectively‐defined subtle cognitive decline; MCI, mild cognitive impairment.
***P < .001. **P < .01.
3.2. Longitudinal plasma p‐tau181 by group
After adjusting for baseline age, sex/gender, APOE ε4 frequency, and pulse pressure, group main effects in plasma p‐tau levels remained, so that while the Obj‐SCD did not differ from the CU group, the MCI group had a higher level of plasma p‐tau181 than both groups. However, longitudinally, there was a small effect for the cognitive group x time interaction (Figure 2). Specifically, the Obj‐SCD group had the steepest increase in plasma p‐tau181 over 4 years and differed relative to both the CU participants (b = .014, 95% confidence interval [CI]: .002 to.027, P = .029, r = .064) and MCI participants (b = .017, 95% CI: .004 to.030, P = .012, r = .063; see Table 2 for model estimates). Participants with MCI did not differ from CU participants (b = –.003, 95% CI: –.014 to .008, P = .621, r = –.015) in the rate of p‐tau181 increase over 4 years.
FIGURE 2.

Trajectories of plasma p‐tau181 by baseline cognitive group. Model‐predicted values of log‐transformed plasma p‐tau181 adjusted for age, sex/gender, apolipoprotein E ε4 allele frequency, and pulse pressure are shown. Shaded area represents 95% confidence intervals. CU, cognitively unimpaired; Obj‐SCD, objectively‐defined subtle cognitive decline; MCI, mild cognitive impairment
TABLE 2.
Plasma p‐tau181 trajectories by cognitive group
| Estimate | SE | P | |
|---|---|---|---|
| Intercept | 0.443 | 0.077 | <.001 |
| Age | 0.008 | 0.001 | <.001 |
| Female/woman | −0.036 | 0.015 | .017 |
| APOE ε4 | |||
| 0 alleles (ref) | – | – | – |
| 1 allele | 0.109 | 0.016 | <.001 |
| 2 alleles | 0.198 | 0.033 | <.001 |
| Pulse pressure | 0.001 | 0.000 | .001 |
| Cognitive group | |||
| CU (ref) | – | – | – |
| Obj‐SCD | −0.022 | 0.021 | .309 |
| MCI | 0.084 | 0.018 | <.001 |
| Time | 0.011 | 0.004 | .003 |
| Cognitive group x Time | |||
| CU x Time (ref) | – | – | – |
| Obj‐SCD x Time | 0.014 | 0.007 | .029 |
| MCI x Time | −0.003 | 0.006 | .621 |
Abbreviations: APOE, apolipoprotein E; CU, Cognitively normal; Obj‐SCD Objectively‐defined subtle cognitive decline; MCI, mild cognitive impairment; .
In a follow‐up analysis examining whether the group p‐tau181 x time trajectories differed by Aβ‐PET positivity status, only the Aβ‐negative Obj‐SCD group showed a slightly faster increase in plasma p‐tau181 relative to the Aβ‐negative CU reference group (b = .019, 95% CI: .001 to .037, P = .042, r = .063) over 4 years (see Supplemental Table S2).
3.3. Longitudinal trajectories by cognitive group and p‐tau181 status
Next, participants were classified as described above into the four groups of CU/p‐tau181– (n = 275), CU/p‐tau181+ (n = 127), Obj‐SCD/p‐tau181– (n = 133), and Obj‐SCD/p‐tau181+ (n = 66; see Supplemental Table S3 for group characteristics). Four‐year changes in Aβ‐PET, global cognition (PACC), and everyday functioning (FAQ, CDR‐SB) by group were examined (See Figure 3 and Table 3 for model estimates).
FIGURE 3.
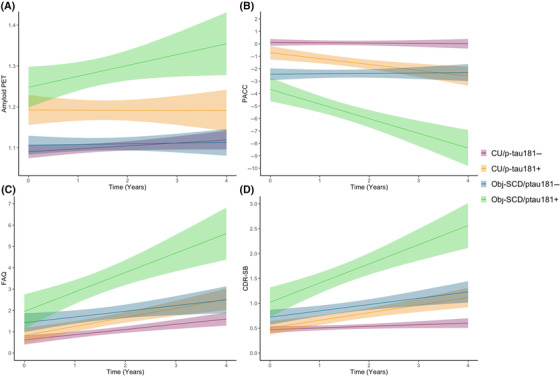
Trajectories of amyloid, cognition, and everyday function by baseline cognitive group/plasma p‐tau181 positivity classifications over 4 years. Model‐predicted values are shown for (A) amyloid positron emission tomography (PET) results, (B), modified Preclinical Alzheimer Cognitive Composite (PACC) scores, (C) Functional Activities Questionnaire (FAQ) scores, and (D) Clinical Dementia Rating–Sum of Boxes (CDR‐SB) scores, adjusted for age, sex/gender, apolipoprotein E ε4 allele frequency, and pulse pressure. The amyloid PET model is also adjusted for baseline amyloid level, and the PACC, FAQ, and CDR‐SB models for years of education. Shaded areas represent 95% confidence intervals. CU/p‐tau181–, cognitively unimpaired, p‐tau181‐negative; CU/p‐tau181+, cognitively unimpaired, p‐tau181‐positive; Obj‐SCD/p‐tau181–, objectively‐defined subtle cognitive decline, p‐tau181‐negative; Obj‐SCD/p‐tau181+, objectively‐defined subtle cognitive decline, p‐tau181‐positive.
TABLE 3.
Trajectories of Alzheimer's disease outcomes by cognitive group and plasma p‐tau181 status
| Amyloid PET | PACC | FAQ | CDR‐SB | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | SE | P | Estimate | SE | P | Estimate | SE | P | Estimate | SE | P | |
| Intercept | −0.028 | 0.015 | .063 | −3.244 | 1.737 | .062 | 2.175 | 1.356 | .109 | 1.699 | 0.516 | .001 |
| Age | 0.000 | 0.000 | .199 | −0.050 | 0.019 | .008 | 0.008 | 0.015 | .581 | −0.009 | 0.006 | .112 |
| Female/woman | −0.002 | 0.003 | .488 | −3.244 | 1.737 | .062 | −1.023 | 0.210 | <.001 | −0.242 | 0.080 | .002 |
| Education | – | – | – | 0.407 | 0.051 | <.001 | −0.095 | 0.040 | .016 | −0.031 | 0.015 | .043 |
| Baseline Aβ‐PET | 1.014 | 0.008 | <.001 | – | – | – | – | – | – | – | – | – |
| APOE ε4 | ||||||||||||
| 0 ε4 alleles | – | – | – | – | – | – | – | – | – | – | – | – |
| 1 ε4 allele | −0.003 | 0.003 | .338 | −0.592 | 0.295 | .045 | 0.074 | 0.230 | .748 | −0.007 | 0.088 | .939 |
| 2 ε4 alleles | 0.007 | 0.007 | .328 | −1.012 | 0.761 | .184 | −0.309 | 0.595 | .604 | 0.254 | 0.227 | .262 |
| Pulse pressure | 0.000 | 0.000 | .587 | −0.007 | 0.005 | .154 | 0.000 | 0.004 | .919 | 0.001 | 0.001 | .497 |
| Group | ||||||||||||
| CU/ptau181– (ref) | – | – | – | – | – | – | – | – | – | – | – | – |
| CU/ptau181+ | 0.000 | 0.004 | .948 | −0.611 | 0.362 | .091 | 0.140 | 0.280 | .617 | 0.040 | 0.106 | .705 |
| Obj‐SCD/ptau181– | −0.001 | 0.004 | .744 | −2.045 | 0.349 | <.001 | 0.515 | 0.270 | .057 | 0.170 | 0.102 | .096 |
| Obj‐SCD/ptau181+ | −0.004 | 0.005 | .358 | −3.060 | 0.467 | <.001 | 1.222 | 0.361 | .001 | 0.587 | 0.137 | <.001 |
| Time | 0.005 | 0.002 | .034 | 0.093 | 0.087 | .287 | 0.161 | 0.082 | .049 | 0.009 | 0.030 | .757 |
| Group x Time | ||||||||||||
| CU/ptau181– x Time (ref) | – | – | – | – | – | – | – | – | – | – | – | – |
| CU/ptau181+ x Time | 0.002 | 0.004 | .594 | −0.466 | 0.153 | .002 | 0.160 | 0.144 | .268 | 0.112 | 0.052 | .032 |
| Obj‐SCD/ptau181– x Time | 0.005 | 0.004 | .251 | −0.024 | 0.154 | .874 | 0.162 | 0.144 | .261 | 0.118 | 0.053 | .026 |
| Obj‐SCD/ptau181+ x Time | 0.011 | 0.006 | .050 | −1.131 | 0.206 | <.001 | 0.448 | 0.191 | .020 | 0.266 | 0.070 | <.001 |
Abbreviations: PET, positron emission tomography; PACC, modified Preclinical Alzheimer Cognitive Composite; FAQ, Functional Activities Questionnaire; CDR‐SB, Clinical Dementia Rating–Sum of Boxes; Aβ, amyloid beta; APOE, apolipoprotein E; CU, Cognitively normal; Obj‐SCD, Objectively‐defined subtle cognitive decline.
For Aβ‐PET, after adjusting for covariates, there was no main effect of group on level of Aβ SUVR, and while the overall sample showed increases in amyloid over the 4 years, only the Obj‐SCD/p‐tau181+ group was associated with faster amyloid accumulation relative to the CU/p‐tau181– participants (b = .011, 95% CI: .000 to .022, P = .050, r = .085) over 4 years. On average, being classified as Obj‐SCD and p‐tau181‐positive was associated with a roughly .044‐point increase in Aβ SUVRs above and beyond the Aβ increase in the CU/p‐tau181– group over 4 years. For the PACC, relative to CU/p‐tau181– participants, both the CU/p‐tau181+ (b = –.466, 95% CI: –.766 to –.166, P = .002, r = –.134) and the Obj‐SCD/p‐tau181+ (b = –1.13, 95% CI: –1.535 to –.727, P < .001, r = –.228) had faster decline over 4 years. While the Obj‐SCD/p‐tau181– participants had lower PACC performance than both CU groups at baseline, they did not show faster PACC decline than the CU/p‐tau181– group (P = .874). The Obj‐SCD/p‐tau181+ group had faster decline than both the CU/p‐tau181+ (b = .664, 95% CI: .223 to 1.106, P = .003, r = .126) and Obj‐SCD/p‐tau181– (b = 1.106, 95% CI: .664 to 1.549, P < .001, r = .207) groups. On average, being classified as Obj‐SCD and p‐tau181 positive was associated with a roughly 4.52‐point decline on the PACC over 4 years relative to the CU/p‐tau181– group.
Regarding everyday functioning, after adjusting for covariates, relative to the CU/p‐tau181– group, only the Obj‐SCD/p‐tau181+ group reported worse FAQ scores at baseline. The Obj‐SCD/p‐tau181+ group was associated with greater declines in everyday functioning (ie, higher difficulties scores) relative to the CU/p‐tau181– participants (b = .448, 95% CI: .072 to .823, P = .020, r = .104) such that, on average, being classified as Obj‐SCD and p‐tau181‐positive was associated with a roughly 1.79‐point increase (ie, more difficulty) on the FAQ over 4 years relative to the CU/p‐tau181– group. For the CDR‐SB, relative to the CU/p‐tau181– group, only the Obj‐SCD/p‐tau181+ group had worse functioning at baseline. Longitudinally, relative to CU/p‐tau181– participants, CU/p‐tau181+ (b = .112, 95% CI: .010 to .215, P = .032, r = .096), Obj‐SCD/p‐tau181– (b = .118, 95% CI: .014 to .221, P = .026, r = .098), and Obj‐SCD/p‐tau181+ (b = .266, 95% CI: .128 to .403, P < .001, r = .163) participants all had faster declines in everyday functioning over 4 years. On average, being classified as Obj‐SCD and p‐tau181‐positive was associated with a roughly 1.07‐point increase (ie, more impairment) on the CDR‐SB over 4 years relative to the CU/p‐tau181– group. Notably, the Obj‐SCD/p‐tau181+ group had a faster decline in everyday functioning relative to both the CU/p‐tau181+ group (b = –.153, 95% CI: –.304 to –.003, P = .045, r = .087), and a nonsignificant but similar pattern relative to the Obj‐SCD/p‐tau181– group (b = –.148, 95% CI: –.299 to .003, P = .054, r = –.084).
4. DISCUSSION
Despite no observed differences in baseline plasma p‐tau181 between CU and Obj‐SCD participants, as well as both groups demonstrating lower plasma p‐tau181 levels than the MCI group, the Obj‐SCD group had a faster increase in plasma p‐tau181 over 4 years relative to both the CU and MCI groups. Next, when participants were classified as CU or Obj‐SCD and positive or negative for plasma p‐tau181, results showed that participants with both Obj‐SCD and p‐tau181‐positivity had accelerated amyloid accumulation as well as cognitive and functional decline.
Previous work has shown that increases in plasma p‐tau181 are associated with clinical stage, such that MCI and AD dementia groups demonstrate higher p‐tau181 levels than a CU group regardless of amyloid status, though plasma p‐tau181 has been found at higher concentrations in Aβ+ groups 5 . The current study extends these analyses to a pre‐MCI phase using the Obj‐SCD classification. Interestingly, plasma p‐tau181 levels did not significantly differ at baseline between Obj‐SCD and CU groups, but, consistent with prior work, Aβ+ participants had higher plasma p‐tau181 levels across both groups. These results differ from our recent findings regarding plasma NfL, where the Obj‐SCD participants had higher NfL levels than CU participants and did not differ from MCI participants. 22 Prior work has demonstrated that plasma p‐tau and NfL have similar change points in AD 38 ; however, given the multiple etiologies that could cause increases in NfL, it is possible that NfL may be detecting any pathologic change (both AD and non‐AD) that causes neuroaxonal damage and degeneration in this Obj‐SCD group, 39 while p‐tau181 increases only in the context of AD‐specific pathologic changes. 8
Related to the finding that the Obj‐SCD group had an increase in plasma p‐tau181 over time, it is important to consider that these results do not appear to be driven by Aβ+ Obj‐SCD participants (see Supplemental Table S2). In fact, the Obj‐SCD participants who were considered Aβ– seemed to be driving the faster increase in plasma p‐tau181 over time for the Obj‐SCD group, suggesting that individuals may be on an AD‐trajectory before Aβ biomarkers have reached a threshold. 40 Given that there were no p‐tau181 baseline differences between the CU and Obj‐SCD group, in combination with the current longitudinal findings and previous evidence suggesting that Obj‐SCD is associated with faster amyloid accumulation, 19 it appears as though Obj‐SCD may be a particularly sensitive marker of risk for future biomarker changes. Despite the long‐held assumption that cognitive changes invariably follow biomarker changes along the AD continuum, 41 these current and previous findings suggest that early neuropsychological difficulties or inefficiencies may emerge before or in tandem with measurable changes in plasma p‐tau181 and Aβ PET markers. 19
Prior work contributing to the assumption that cognition follows biomarker changes was likely based on the use of cognitive measures that are too insensitive to capture subtle cognitive changes (eg, global cognitive measures, rating scales). There is now consistent evidence that sensitive neuropsychological measures can capture cognitive difficulties coincident with very early AD biomarker changes and provide added prognostic value for predicting progression to MCI/dementia. 14 , 15 , 42 As posited by Braak and colleagues, 43 it is possible that amyloid and tau pathologic changes, neuronal and synaptic degeneration and loss, and arguably subtle cognitive declines, may all occur within a narrow time sequence. In this context, the differences previously found between patients at different stages of the disease may be caused or influenced by varying sensitivities of the biomarkers and cognitive tests. Applying sensitive neuropsychological markers appears to capture subtle cognitive decline within a similar time sequence with which AD biomarkers such as plasma p‐tau181 and Aβ‐PET begin to change at a faster rate.
Given the potential of both Obj‐SCD and plasma p‐tau181 as methods for improving early detection, we examined whether having both Obj‐SCD and elevated p‐tau181 conferred faster rates of amyloid accumulation, cognitive decline, and functional decline over 4 years. Consistent across all longitudinal outcomes, being classified as both Obj‐SCD and plasma p‐tau181‐positive was associated with a faster worsening of outcomes. Importantly, these findings suggest that there is a prognostic benefit of combining Obj‐SCD and plasma markers, which both have the potential to be early markers of future AD‐related declines. These results are consistent with recent work showing that cognitive measures add value to plasma p‐tau and APOE genotype in AD diagnosis. 24 Further, the accessible and non‐invasive nature of both plasma p‐tau and Obj‐SCD methods may increase our ability to assess biomarker changes and AD trajectories in larger and more representative samples.
The current study, as with all studies using ADNI data, is limited by the sample that is not representative of the general population and is predominately white, highly educated, and overall very healthy. This is a significant limitation that restricts the generalizability of these results, and future work should examine these relationships in more representative and community‐based cohorts, including within racial/ethnic groups that are often underrepresented in aging research. Additionally, given that the process scores used as part of the Obj‐SCD classification were all derived from a verbal memory measure, it is possible that the Obj‐SCD may not adequately capture the earliest cognitive changes associated with non‐amnestic or “atypical” presentations of Alzheimer's disease. Future work is needed to ensure that pre‐MCI/subtle cognitive decline adequately captures the known heterogeneity of Alzheimer's disease variants. 44 Strengths of the current study include the large sample size with neuropsychological testing and plasma p‐tau181 data as well as other AD risk information such as Aβ‐PET. Additionally, the 4 years of longitudinal plasma p‐tau181, Aβ‐PET, cognitive, and everyday functioning data represent a relatively long follow‐up period, allowing us to consider the timing of when plasma p‐tau181 differences emerge across early clinical stages of AD.
The results of this study add support to the potential use of the Obj‐SCD classification in clinical research as a tool to assist with early detection, and may also support use of this classification to identify and recruit research and clinical trial participants at risk for future disease progression. Importantly, the prognostic value of the Obj‐SCD classification appears to be augmented by including plasma p‐tau181 data, which appears to increase the specificity in identifying those who are likely to decline at a faster rate due to AD. Compared to PET or lumbar puncture, both the brief neuropsychological testing needed to classify Obj‐SCD and the blood draw to obtain plasma p‐tau181 are non‐invasive, likely to be much less expensive, and have the potential to be vastly more accessible (ie, community data collection beyond an academic medical center) methods for identifying those at greater risk for biomarker, cognitive, and functional declines.
Data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH‐12‐2‐0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer's Association; Alzheimer's Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol‐Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann‐La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Data used in preparation of this article were obtained from the Alzheimer's Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.usc.edu/wp‐content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf
CONFLICT OF INTEREST
Dr. Bondi receives royalties from Oxford University Press and is a consultant for Novartis and Roche. Dr. Galasko is a consultant for Biogen, vTv Pharmaceuticals, Fujirebio, Esai, General Electric Healthcare. Other authors report no disclosures.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
This work was supported by the U.S. Department of Veterans Affairs Clinical Sciences Research and Development Service (1IK2CX001865, 1I01CX001842, and 1IK2CX001415), NIH/NIA grants (P30 AG062429, R03 AG070435, R01 AG063782, R01 AG049810 and R01 AG054049), and the Alzheimer's Association (AARF‐17‐528918, AARG‐18‐566254, and AARG‐17‐500358).
Thomas KR, Bangen KJ, Edmonds EC, et al. Objective subtle cognitive decline and plasma phosphorylated‐tau181: early markers of Alzheimer's disease‐related declines. Alzheimer's Dement. 2021;13:e12238. 10.1002/dad2.12238
REFERENCES
- 1. Hansson O, Seibyl J, Stomrud E, et al. CSF biomarkers of Alzheimer's disease concord with amyloid‐β PET and predict clinical progression: a study of fully automated immunoassays in BioFINDER and ADNI cohorts. Alzheimers Dement. 2018;14:1470‐1481. 10.1016/j.jalz.2018.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ossenkoppele R, Rabinovici GD, Smith R, et al. Discriminative accuracy of [18F]=flortaucipir positron emission tomography for Alzheimer disease vs other neurodegenerative disorders. JAMA. 2018;320:1151‐1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thijssen EH, Rabinovici GD. Rapid progress toward reliable blood tests for Alzheimer disease. JAMA Neurol. 2021;78:143‐145. [DOI] [PubMed] [Google Scholar]
- 4. Blennow K. A review of fluid biomarkers for Alzheimer's disease: moving from CSF to blood. Neurol Ther. 2017;6:15‐24. 10.1007/s40120-017-0073-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Karikari TK, Benedet AL, Ashton NJ, et al. Diagnostic performance and prediction of clinical progression of plasma phospho‐tau181 in the Alzheimer's Disease Neuroimaging Initiative. Mol Psychiatry. 2021;26:429‐442. 10.1038/s41380-020-00923-z. [DOI] [PubMed] [Google Scholar]
- 6. Mattsson‐Carlgren N, Janelidze S, Palmqvist S, et al. Longitudinal plasma p‐tau217 is increased in early stages of Alzheimer's disease. Brain. 2020;143:3234‐3241. 10.1093/brain/awaa286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moscoso A, Grothe MJ, Ashton NJ, et al. Longitudinal associations of blood phosphorylated tau181 and neurofilament light chain with neurodegeneration in Alzheimer disease. JAMA Neurol. 2021;78:396‐406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thijssen EH, La Joie R, Wolf A, et al. Diagnostic value of plasma phosphorylated tau181 in Alzheimer's disease and frontotemporal lobar degeneration. Nat Med. 2020;26:387‐397. 10.1038/s41591-020-0762-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lantero Rodriguez J, Karikari TK, Suárez‐Calvet M, et al. Plasma p‐tau181 accurately predicts Alzheimer's disease pathology at least 8 years prior to post‐mortem and improves the clinical characterisation of cognitive decline. Acta Neuropathol. 2020;140:267‐278. 10.1007/s00401-020-02195-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Janelidze S, Berron D, Smith R, et al. Associations of plasma phospho‐tau217 levels with tau positron emission tomography in early Alzheimer disease. JAMA Neurol. 2021;78:149‐156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Karikari TK, Pascoal TA, Ashton NJ, et al. Blood phosphorylated tau 181 as a biomarker for Alzheimer's disease: a diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol. 2020;19:422‐433. 10.1016/S1474-4422(20)30071-5. [DOI] [PubMed] [Google Scholar]
- 12. Moscoso A, Grothe MJ, Ashton NJ, et al. Time course of phosphorylated‐tau181 in blood across the Alzheimer's disease spectrum. Brain. 2021;144:325‐339. 10.1093/brain/awaa399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. O'Connor A, Karikari TK, Poole T, et al. Plasma phospho‐tau181 in presymptomatic and symptomatic familial Alzheimer's disease: a longitudinal cohort study [published online ahead of print July 14, 2020]. Mol Psychiatry. 10.1038/s41380-020-0838-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jedynak BM, Lang A, Liu B, et al. A computational neurodegenerative disease progression score: method and results with the Alzheimer's disease Neuroimaging Initiative cohort. Neuroimage. 2012;63:1478‐1486. 10.1016/j.neuroimage.2012.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thomas KR, Eppig J, Edmonds EC, et al. Word‐list intrusion errors predict progression to mild cognitive impairment. Neuropsychology. 2018;32:235‐245. 10.1037/neu0000413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Loewenstein DA, Curiel RE, Duara R, Buschke H. Novel cognitive paradigms for the detection of memory impairment in preclinical Alzheimer's disease. Assessment. 2018;25:348‐359. 10.1177/1073191117691608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Loewenstein DA, Curiel RE, DeKosky S, et al. Utilizing semantic intrusions to identify amyloid positivity in mild cognitive impairment. Neurology. 2018;91:e976‐e984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Thomas KR, Edmonds EC, Eppig J, Salmon DP, Bondi MW, Alzheimer's Disease Neuroimaging Initiative . Using neuropsychological process scores to identify subtle cognitive decline and predict progression to mild cognitive impairment. J Alzheimers Dis. 2018;64:195‐204. 10.3233/JAD-180229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Thomas KR, Bangen KJ, Weigand AJ, et al. Objective subtle cognitive difficulties predict future amyloid accumulation and neurodegeneration. Neurology. 2020;94:e397‐e406. 10.1212/WNL.0000000000008838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thomas KR, Bangen KJ, Weigand AJ, et al. Type 2 diabetes interacts with alzheimer disease risk factors to predict functional decline. Alzheimer Dis Assoc Disord. 2020;34:10‐17. 10.1097/WAD.0000000000000332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thomas KR, Osuna JR, Weigand AJ, et al. Regional hyperperfusion in older adults with objectively‐defined subtle cognitive decline. J Cereb Blood Flow Metab. 2021;41:1001‐1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bangen KJ, Thomas KR, Weigand AJ, et al. Elevated plasma neurofilament light is associated with objectively‐defined subtle cognitive difficulties and predicts future cognitive and functional decline. Alzheimers Dement. 2021. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Heister D, Brewer JB, Magda S, Blennow K, McEvoy LK. Alzheimer's Disease Neuroimaging Initiative . Predicting MCI outcome with clinically available MRI and CSF biomarkers. Neurology. 2011;77:1619‐1628. 10.1212/WNL.0b013e3182343314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Palmqvist S, Tideman P, Cullen N, et al. Prediction of future Alzheimer's disease dementia using plasma phospho‐tau combined with other accessible measures. Nat Med. 2021;27:1034‐1042. 10.1038/s41591-021-01348-z. [DOI] [PubMed] [Google Scholar]
- 25. Petersen RC, Aisen PS, Beckett LA, et al. Alzheimer's Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology. 2010;74:201‐209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thomas KR, Edmonds EC, Eppig JS, et al. MCI‐to‐normal reversion using neuropsychological criteria in the Alzheimer's Disease Neuroimaging Initiative. Alzheimers Dement. 2019;15:1322‐1332. 10.1016/j.jalz.2019.06.4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bondi MW, Edmonds EC, Jak AJ, et al. Neuropsychological criteria for mild cognitive impairment improves diagnostic precision, biomarker associations, and progression rates. J Alzheimers Dis. 2014;42:275‐289. 10.3233/JAD-140276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jak AJ, Bondi MW, Delano‐Wood L, et al. Quantification of five neuropsychological approaches to defining mild cognitive impairment. Am J Geriatr Psychiatry. 2009;17:368‐375. 10.1097/JGP.0b013e31819431d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Karikari TK, Benedet AL, Ashton NJ, et al. Diagnostic performance and prediction of clinical progression of plasma phospho‐tau181 in the Alzheimer's Disease Neuroimaging Initiative. Mol Psychiatry. 2021;26:429‐442. [DOI] [PubMed] [Google Scholar]
- 30. Shen XN, Li JQ, Wang HF, et al. Plasma amyloid, tau, and neurodegeneration biomarker profiles predict Alzheimer's disease pathology and clinical progression in older adults without dementia. Alzheimers Dement. 2020;12:e12104. 10.1002/dad2.12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Landau SM, Harvey D, Madison CM, et al. Associations between cognitive, functional, and FDG‐PET measures of decline in AD and MCI. Neurobiol Aging. 2011;32:1207‐1218. 10.1016/j.neurobiolaging.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Landau SM, Breault C, Joshi AD, et al. Amyloid‐β imaging with Pittsburgh compound B and florbetapir: comparing radiotracers and quantification methods. J Nucl Med. 2013;54:70‐77. 10.2967/jnumed.112.109009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Donohue MC, Sperling RA, Salmon DP, et al. The preclinical Alzheimer cognitive composite: measuring amyloid‐related decline. JAMA Neurol. 2014;71:961‐970. 10.1001/jamaneurol.2014.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pfeffer RI, Kurosaki TT, Harrah CH Jr, Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37:323‐329. 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- 35. O'Bryant SE, Lacritz LH, Hall J, et al. Validation of the new interpretive guidelines for the Clinical Dementia Rating Scale Sum of Boxes Score in the National Alzheimer's Coordinating Center Database. Arch Neurol. 2010;67:746‐749. 10.1001/archneurol.2010.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nation DA, Edland SD, Bondi MW, et al. Pulse pressure is associated with Alzheimer biomarkers in cognitively normal older adults. Neurology. 2013;81:2024‐2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Qiu C, Winblad B, Viitanen M, Fratiglioni L. Pulse pressure and risk of Alzheimer disease in persons aged 75 years and older: a community‐based, longitudinal study. Stroke. 2003;34:594‐599. 10.1161/01.STR.0000060127.96986.F4. [DOI] [PubMed] [Google Scholar]
- 38. Palmqvist S, Insel PS, Stomrud E, et al. Cerebrospinal fluid and plasma biomarker trajectories with increasing amyloid deposition in Alzheimer's disease. EMBO Mol Med. 2019;11:e11170. 10.15252/emmm.201911170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Forgrave LM, Ma M, Best JR, DeMarco ML. The diagnostic performance of neurofilament light chain in CSF and blood for Alzheimer's disease, frontotemporal dementia, and amyotrophic lateral sclerosis: a systematic review and meta‐analysis. Alzheimers Dement. 2019;11:730‐743. 10.1016/j.dadm.2019.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Landau SM, Horng A, Jagust WJ. Alzheimer's Disease Neuroimaging Initiative . Memory decline accompanies subthreshold amyloid accumulation. Neurology. 2018;90:e1452‐60. 10.1212/WNL.0000000000005354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jack CR Jr, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. 2010;9:119‐128. 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Caselli RJ, Langlais BT, Dueck AC, et al. Neuropsychological decline up to 20 years before incident mild cognitive impairment. Alzheimers Dement. 2020;16(3):512‐523. 10.1016/j.jalz.2019.09.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Braak H, Zetterberg H, Del Tredici K, Blennow K. Intraneuronal tau aggregation precedes diffuse plaque deposition, but amyloid‐β changes occur before increases of tau in cerebrospinal fluid. Acta Neuropathol. 2013;126:631‐641. 10.1007/s00401-013-1139-0. [DOI] [PubMed] [Google Scholar]
- 44. Vogel JW, Young AL, Oxtoby NP, et al. Four distinct trajectories of tau deposition identified in Alzheimer's disease. Nat Med. 2021;27:871‐881. 10.1038/s41591-021-01309-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


