Abstract
Objective
To review evidence regarding the use of Health Information Technology (health IT) interventions aimed at improving care for people living with multiple chronic conditions (PLWMCC) in order to identify critical knowledge gaps.
Data Sources
We searched MEDLINE, CINAHL, PsycINFO, EMBASE, Compendex, and IEEE Xplore databases for studies published in English between 2010 and 2020.
Study Design
We identified studies of health IT interventions for PLWMCC across three domains as follows: self‐management support, care coordination, and algorithms to support clinical decision making.
Data Collection/Extraction Methods
Structured search queries were created and validated. Abstracts were reviewed iteratively to refine inclusion and exclusion criteria. The search was supplemented by manually searching the bibliographic sections of the included studies. The search included a forward citation search of studies nested within a clinical trial to identify the clinical trial protocol and published clinical trial results. Data were extracted independently by two reviewers.
Principal Findings
The search yielded 1907 articles; 44 were included. Nine randomized controlled trials (RCTs) and 35 other studies including quasi‐experimental, usability, feasibility, qualitative studies, or development/validation studies of analytic models were included. Five RCTs had positive results, and the remaining four RCTs showed that the interventions had no effect. The studies address individual patient engagement and assess patient‐centered outcomes such as quality of life. Few RCTs assess outcomes such as disability and none assess mortality.
Conclusions
Despite a growing body of literature on health IT interventions or multicomponent interventions including a health IT component for chronic disease management, current evidence for applying health IT solutions to improve care for PLWMCC is limited. The body of literature included in this review provides critical information on the state of the science as well as the many gaps that need to be filled for digital health to fulfill its promise in supporting care delivery that meets the needs of PLWMCC.
Keywords: algorithms, care coordination, caregivers, delivery of health care, health information technology, multiple chronic conditions, self‐management
What is known on this topic
People living with multiple chronic conditions have worse clinical outcomes than those without and often experience fragmented and/or burdensome care.
Health information technology has shown promise in bringing knowledge and information to the point of care to improve care processes and, to some extent, clinical outcomes and quality of life outcomes.
Little is known about effective uses of health information technology to improve these outcomes for people living with multiple chronic conditions.
What this study adds
This study summarizes and defines three domains where health information technology may help people living with multiple chronic conditions—in self‐management, in care coordination, and algorithms to support clinical decision making.
Evidence for the effectiveness of health information technology in these areas is still limited despite promising initial studies.
Research studies specific to the use of health information technology in people living with multiple chronic conditions are needed.
1. INTRODUCTION
In 2018, the Center for Disease Control and Prevention (CDC) estimated that one in four Americans have at least two chronic conditions requiring ongoing medical care. 1 More than two‐thirds of Medicare beneficiaries have multiple chronic conditions (MCCs), with 14% having six or more conditions. 1 , 2 The prevalence of MCCs is higher among women, persons of color, older adults, people using Medicare and/or Medicaid, low‐income individuals, and those living in rural areas. 1 , 2 MCCs are an issue across the life course: growing numbers of children and young adults are also living with MCCs. 3 , 4
1.1. Challenges for people living with multiple chronic conditions
Historically, in the United States, the reimbursement system and health care delivery system have failed to meet the needs of people living with MCC (PLWMCC). 5 PLWMCC, as compared to people with no chronic conditions, experience tremendous burdens in navigating the health system and adhering to recommended care including lifestyle changes and medication management. As the number of conditions increases, the complexity of care increases. A patient with five or more chronic conditions sees an average of 14 physicians in 1 year and uses a larger number of medications, lab tests, and imaging studies than other Medicare patients while suffering more adverse events. 2 , 5 , 6 , 7 , 8 , 9 , 10 PLWMCC use health care services of all types at higher rates; they encounter fragmentation and poor coordination of care leading to higher levels of avoidable resource utilization, including avoidable emergency department (ED) visits and hospitalizations. 11 , 12 PLWMCC have significant health‐related social needs, including financial instability both from medical expenses and time lost from work, social isolation, and higher mood‐related symptoms. 6 , 13 , 14 , 15 Many of these factors impact the likelihood of poor outcomes, for example, exacerbation of illnesses and adverse events, disability, hospitalizations, and mortality; the impact goes beyond simple counts or combinations of MCCs. 2 , 10 , 16 , 17 In comparison to a person with a single chronic condition, PLWMCC face the problem of conflicting guidelines and lack of prioritization of care for each condition. 18 , 19 , 20
1.2. The potential for health IT in multimorbidity
The data, information, and knowledge needs to help people manage their chronic illnesses are high; and previous work has shown that health IT systems may provide higher quality and more engaging care for people with a single chronic condition when compared to usual care for people with a single chronic condition. 21 , 22 However, the ability of health IT tools to meet the needs of PLWMCCs is much less clear. 23 In assessing the potential for health IT, we focus on the health needs of PLWMCC in each of three domains as follows: (1) self‐management support; (2) care coordination and care planning; and (3) algorithms to support clinical decision making. Health IT could be used to prioritize and synthesize recommendations and provide support for people to self‐manage their multiple chronic conditions in a way that applications designed for a single condition cannot. When comparing the needs of PLWMCC to the needs of a person with a single chronic condition, health IT may be particularly useful for exchanging data for care coordination and facilitating multiteam care planning. Finally, algorithmic or AI systems can help provide early warning when health is at risk from multiple interacting chronic conditions or provide decision support for complex situations. Currently, health IT applications are limited by problems such as poor usability, poor workflow integration, fragmentation, lack of interoperability, and the uneven distribution of technology (the so‐called “digital divide”). 24 , 25 However, advances in our ability to exchange data, to use advanced algorithms, to parse complex situations or provide tailored support, and broad changes in policy and uptake using health IT present opportunities to leverage new capabilities to improve care and outcomes for PLWMCC. 26 , 27 , 28
Previous literature reviews have synthesized relevant studies of health IT tools (e.g., for self‐management care coordination, or to guide clinical decision making) and, less frequently, multimorbidity (Smith et al., Waschkau et al. 29 , 30 , 31 , 32 , 33 , 34 ). None of these review articles directly explored how health IT was meeting the needs of PLWMCC. Washchkau et al. reviewed “big data analytics” and “multimorbidity,” finding five articles that clustered diseases and one that proposed—but did not implement—a model for prioritizing clinical decisions. Smith et al. 33 covered interventions in primary care focused on multimorbidity without a focus on health IT. Bright 29 noted that 25% of clinical decision support (CDS) studies addressed multiple conditions, but the lack of integration between CDS for different conditions and timing/prioritization of rules was an ongoing gap for PLWMCCs. Fraccaro et al. 31 evaluated CDS use in PLWMCCs and included 20 articles; the majority were focused on medication use, clinical guidance, or diagnosis. Of these, 10 performed evaluations of the accuracy, timeliness, or perceived performance, but only one reported impact. In this study by Bindoff et al., 35 a CDS system found more problems in medication reconciliation than pharmacists. Finally, care coordination and care planning reviews either did not examine the impact of health IT separately or had limited examples of multimorbidity with their health IT comparisons. 36 , 37 , 38 A self‐management and mobile health IT review focused on the dyad of hypertension and diabetes, finding 11 articles with three reporting improvement in outcomes, all decreases in blood pressure. 30
We sought to review the literature on how health IT tools have been evaluated to address the health needs of PLWMCC in each of three domains as follows: (1) self‐management support; (2) care coordination and care planning; and (3) algorithms and CDS. Our motivation was to understand both what is known and the current gaps in understanding of how these technologies impact the lives of PLWMCC. Specifically, we sought to identify studies where clinical or quality outcomes were evaluated as function of health IT use.
2. METHODS
2.1. Conceptual frameworks
To understand potential domains where health IT use may be beneficial for PLWMCC, we drew upon several frameworks including the Chronic Care Model (CCM) framework. The CCM is a commonly used framework for complex chronic illness care, and CCM‐based interventions have been associated with improved quality and outcomes. 39 , 40 , 41 , 42 Many successful interventions using CCM are interventions that incorporate health IT. 6 , 8 , 15 , 43 Core components of CCM include delivery system design, self‐management support, decision support, and clinical information systems. 44 , 45 However, CCM only includes clinical information systems, which must be broadened to include other forms of health IT—including mobile health—that may impact outcomes for PLWMCC. To refine delivery system design, we reference a care coordination framework focused on identification of needs, management across transitions, and alignment of care plans. 46 , 47 , 48 For outcomes, we reference the Outcomes Measurement Framework to identify relevant outcomes, categorized as survival, clinical response, events of interest, patient‐reported, and resource utilization. 49 Health IT has the potential to capture clinical data and patient‐reported outcomes data efficiently, which allows for a diverse set of outcomes measures in evaluation of health IT interventions. 50 , 51 , 52 , 53 In addition to these widely accepted outcomes, we identified measures of accuracy appropriate for assessment of analytic prediction models, such as area under the curve (AUC). We also identified other measures, such as those of feasibility and usability, which are appropriate for assessment of health IT interventions. The intersection of these conceptual models yielded three domains as follows: (1) self‐management support and patient‐reported outcomes, (2) care coordination and care planning, and (3) analytics and algorithm to support clinical decision making. We define each of these below.
2.1.1. Self‐management and patient‐reported outcomes
Self‐management support includes health IT tools that empower patients to take charge of their own health to improve quality of life and self‐efficacy. 54 Remote patient monitoring tools that can facilitate the interaction between patients and health care providers allow monitoring of adherence to medication and allow remote patient monitoring of physiological data such as blood pressure, weight, cardiac rhythm, and oxygen saturation. 51 , 55 , 56 , 57 Such medical devices can also support more accurate diagnosis and monitoring in the home and can provide a feedback loop and support patients' coping behaviors. 51 , 53 Health IT tools can also help patients articulate their goals, values, and preferences. 50 , 51 , 58 Electronic risk assessments may be programmed to present targeted information on self‐management of risk factors or refer patients to specific online programs that can provide advice and interactive self‐management tools to help manage risk factors. 59 , 60 Personal health records (PHRs) often incorporate electronic risk assessments, patient goals and preferences, and automated systems to trigger a reminder to the patient to perform a routine action or test can support self‐management. 50 , 59
2.1.2. Care coordination and the activity of care planning
Prior to 2010, there were few reports of health IT tools supporting care coordination activities in a real‐world clinical setting. 46 , 61 Important care coordination activities, such as information transfer between health care providers in different settings, establishing accountability, and negotiating responsibility, continue to be limited by interoperability barriers. 62 One particularly important activity, creating a proactive plan of care, requires shared decision making between health care providers, PLWMCC, their families, and other caregivers. In addition to a collaborative approach, care planning must include reliable, open communication between all parties, and prioritization of care based on evidence as well as patient preferences. 63
2.1.3. Using analytics and algorithms to support clinical decision making
Algorithms have many potential uses in PLWMCCs, including identification of risk, prevention, diagnosis, care, and treatment. 64 , 65 Health data processed through algorithms may provide appropriate information and insights for patients and clinicians in personalizing therapy, providing nuanced CDS recommendations in complex scenarios, and mitigating health risks. 58 , 65 This includes simple decision support rules to artificial intelligence (AI), advanced algorithms that can provide real‐time continued learning to adapt to the needs of patients and outcomes over time. 66 Complex and multiple chronic diseases require personalized diagnostics and therapy, which may be supported by AI to improve prediction of poor outcomes, therapy guidance, and prevention of deterioration. 65 Algorithms can identify at‐risk populations and individual patients and match them to appropriate and cost‐effective care coordination interventions. 15 Prediction of increasing risk of PLWMCCs may help reallocate resources and redesign health care to ameliorate risks. 64 , 67 CDS systems can recommend appropriate care, treatments, and best practice for the complex profile of a patient with multiple chronic conditions and facilitate proactive responses that would have profound impact on outcomes. 51 There is an additional benefit of pharmacology CDS with the ability to reduce physician errors. 65
2.2. Search strategy and eligibility criteria
We used a systematic approach based on the Preferred Reporting Items for Systematic reviews and Meta‐Analyses. 68 69 Data sources included MEDLINE, CINAHL, PsycINFO, EMBASE, Compendex, and IEEE Xplore databases. We searched for studies published in English between January 1, 2010 and September 28, 2020 (Appendix S1). The search was supplemented by manually searching the bibliographic sections of the included studies. The search also included a forward citation search of quasi‐experimental studies nested within a clinical trial to identify the clinical trial protocol and subsequently published clinical trial results.
We included studies of health IT interventions and multicomponent interventions including a health IT component. We sought RCTs but also included quasi‐experimental, usability, feasibility, qualitative studies, or development/validation studies of analytic models. The search was supplemented by manually searching the bibliographic sections of the included studies.
Articles were included in the review if they met all of the following criteria: (1) described a health IT tool as a component of a planned or explicit intervention, (2) included people living with two or more chronic conditions, (3) evaluated clinical or quality outcomes as a function of health IT use, and (4) targeted one of three domains:
self‐management support, health IT tools must include functionality for bidirectional communication between PLWMCC and other members of the care team (to support self‐management, patient education, goal‐setting, and other patient‐generated health data, including patient‐reported outcomes)
care coordination between care team members or the activity of care planning
algorithmic or machine learning–based models for predictive analytics, risk calculation, or other clinical decision support.
The full search strategy is contained in Appendix S1.
2.3. Study selection
Titles and abstracts were reviewed for the listed inclusion criteria. A portion of article titles and abstracts was initially reviewed by two reviewers in order to refine inclusion and exclusion criteria, then remaining articles were reviewed by one reviewer. In cases where the reviewer was uncertain if the article should be included, the title and abstract were reviewed by an additional reviewer. Full‐text articles were retrieved for all potentially suitable studies. Data extraction was performed independently by two reviewers and inconsistencies were resolved by consensus.
2.4. Data extraction
Data were extracted independently by two reviewers. Included articles were abstracted for objective, study design, population, health IT intervention or component, health IT user, and outcomes with a standardized data collection form.
3. RESULTS
We identified 1907 articles and selected 44 fulfilling the eligibility criteria (Figure 1). Nine were RCTs, and the remaining 35 articles were quasi‐experimental, usability, feasibility, qualitative studies, or development/validation studies of analytic models.
FIGURE 1.
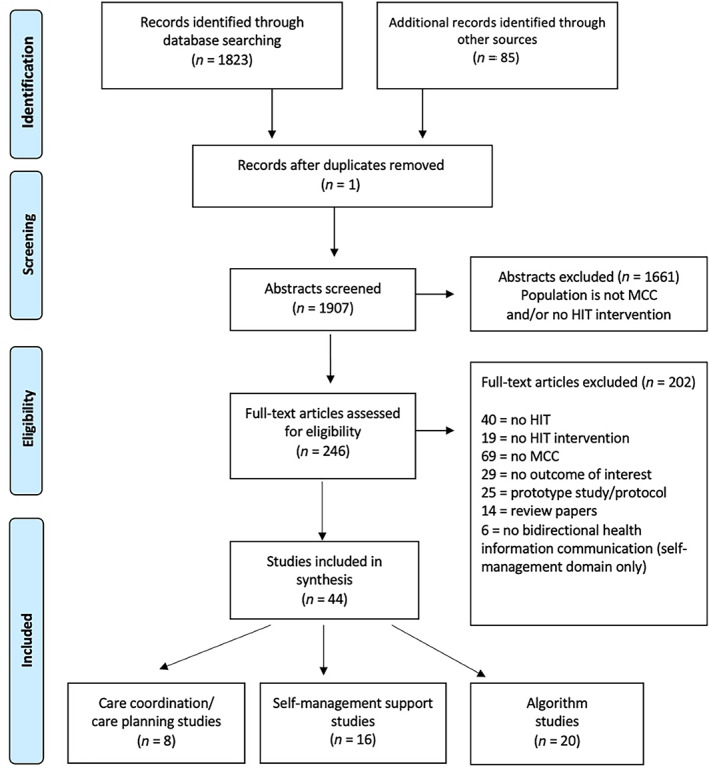
Preferred reporting items for systematic reviews and meta‐analyses (PRISMA) flowchart. MCC, multiple chronic condition; HIT, health information technology [Color figure can be viewed at wileyonlinelibrary.com]
3.1. Self‐management support and patient‐reported outcomes
The review identified 16 studies including seven RCTs that studied self‐management support, quality of life, and health service utilization (Table 1). 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 These health IT interventions were delivered via patient portal, remote patient monitoring technology, patient‐reported outcome collection applications, and telemedicine virtual coaching. 72 , 73 , 78 , 79 , 81 , 82 , 87
TABLE 1.
Self‐management studies
| Year | Author | Title | Objective | Study design | Population | HIT Component | User | Outcomes | Results |
|---|---|---|---|---|---|---|---|---|---|
| RCT | |||||||||
| 2020 | Druss et al. 70 | Randomized trial of a mobile personal health record for behavioral health homes | To evaluate whether a mobile personal health record application improves quality of medical care in behavioral health homes, which provide onsite primary medical care in mental health clinics. | Randomized control trial (RCT) of personal health record for behavioral health homes of patients with serious mental illness and one or more cardiometabolic risk factors across two behavioral health homes assigned to intervention or usual care and followed for 12 months (n = 311) | Patients with serious mental illness and one or more cardiometabolic risk factors across two behavioral health homes | PHR Mobile application: A secure mobile personal health record (mPHR), programmed using Sencha Touch, including key information about diagnoses, medications, and laboratory test values and allowed them to track health goals | Patients |
A chart‐derived composite measure of quality of cardiometabolic and preventive services |
At 1 year follow‐up, participants in the mPHR group sustained high quality of care (70% of indicated services at baseline and at 12‐month follow‐up), in contrast to a decreased in quality for the usual‐care group (71% at baseline and 67% at follow‐up), resulting in a statistically significant (p < 0.05). |
| 2018 | Walker et al. 71 | Telemonitoring in Chronic Obstructive Pulmonary Disease (CHROMED): a randomized clinical trial | To evaluate the efficacy of home monitoring of lung mechanics by the forced oscillation technique and cardiac parameters in older patients with chronic obstructive pulmonary disease (COPD) and comorbidities. | Multicenter RCT of Telemonitoring of Chronic Obstructive Pulmonary Disease in patients with Global Initiative for Chronic Obstructive Lung Disease grades II to IV COPD with a history of exacerbation in the previous year and at least one nonpulmonary comorbidity assigned to intervention or usual care and followed for 9 months (n = 312) | Patients with global initiative for chronic obstructive lung disease grades II–IV COPD (median age, 71 yr [interquartile range, 66–76 yr]; 49.6% grade II, 50.4% grades III–IV), with a history of exacerbation in the previous year and at least one nonpulmonary comorbidity | Telemonitoring: CHROMED monitoring platform comprised a device that measured within‐breath respiratory mechanical impedance. Telemonitoring of physiological variables blood pressure, oxygen saturation, heart rate, and body temperature to reduce the frequency of hospitalization | Patients, physicians | Time to first hospitalization (TTFH) and change in the EuroQoL EQ‐5D utility index score | No group difference found on TTFH, EQ‐5D utility index score, antibiotic prescriptions, hospitalization rate, or questionnaire scores. (p > 0.05) In an exploratory analysis, daily telemonitoring was associated with fewer repeat hospitalizations (−54%; p = 0.017). |
| 2014 | Druss et al. 72 | Randomized trial of an electronic personal health record for patients with serious mental illnesses | To evaluate the effect of an electronic personal health record on the quality of medical care in a community mental health setting. | RCT of electronic personal health record of patients with serious mental illness and at least one chronic condition assigned to intervention or usual care and followed for 1 year (n = 170) | Mental illness + 1 chronic condition | PHR Web‐based application: Patients can access the personal health record data with protected passwords from any computer with an Internet connection. My Health Record is an adaptation of the existing Shared Care Plan: diagnosis, goals and action steps, health indicators, (BP, lipid and BG levels), medication, ALLG, hospital stay, immunization, medical and fam history. Patient reminder of preventive service | Patients, designated health partners (physicians, other providers, and friends and/or family) | Quality of medical care, patient activation, service use, and health‐related quality of life |
Having a personal health record was associated in improved quality of medical care. Quality of preventive services (p < 0.00001) and quality of cardiometabolic services (p < 0.003) Service use: Patient used personal health record a mean of 42.1 in 1 yr., In personal health record group, preventative services 24% increased to 40% (usual group decline from 25% to 18%)., increase in the # of outpatient visits in personal health record group (p < 0.001) |
| 2014 | Gellis et al. 73 | Integrated telehealth care for chronic illness and depression in geriatric home care patients: the integrated telehealth education and activation of mood (I‐TEAM) study | To evaluate an integrated telehealth intervention (integrated telehealth education and activation of mood [I‐TEAM]) to improve chronic illness (congestive heart failure,COPD), and comorbid depression in the home health care setting. | RCT of I‐TEAM in patients with CHF or COPD depression assigned to intervention or usual care and followed for 3 months (n = 102) | CHF or COPD (hospital admission/ED user, 3+ home care per wk.), + depression | Telemonitoring: The telemonitoring device comprised of a small in‐home monitor connected to an agency central station. Daily monitoring of WT, BP, pulse, pulse oxygenation, and temperature data, messaging with primary care provider. Provided chronic illness and depression care | Patients | Depression, health, problem solving, and health utilization (readmission, care, ED visit) at 3, 6, and 12 months | I‐TEAM group had fewer ED visits (p = 0.01), but did not have significantly fewer hospital days at 12 months (p = 0.06). |
| 2013 | Pecina et al. 74 a | Impact of Telemonitoring on older adults health‐related quality of life: The Tele‐ERA study | To assess the effect of a home telemonitoring intervention on patient's health‐related quality of life for PLWMCC. | RCT of telemonitoring for older patients with MCC assigned to intervention or usual care and followed for 1 year (n = 205) | Older adults with MCC and high risk as assessed by a risk assessment score | Telemonitoring, message and video conference: monitoring of biometric data (BP, WT, pulse, temp, pulse oxygenation, peak flow); administering symptom questionaries with goal of early detection of health status decline; all done with the Intel Health Guide. | Patient, nurse, geriatric nurse practitioner (NP), primary care physician (PCP) | QOL: physical and mental score on the short form health questionnaire PCS | Intervention yielded a decrease in PCS scores (−4.3 ± 9.3), compared to the usual care group (−1.2 ± 8.5) during the study (p = 0.03). No difference in the 12‐month PCS scores (p = 0.39) or MCS scores (p = 0.10) between groups |
| 2012 | Logan et al. 75 | Effect of home blood pressure telemonitoring with self‐care support on uncontrolled systolic hypertension in Diabetics | To test the system's effectiveness in a randomized controlled trial in diabetic patients with uncontrolled systolic hypertension. | RCT of telemonitoring for DM patients with uncontrolled HTN assigned to intervention or usual care and followed for 1 year (n = 110) | Adult 30 years and over recruited with DM and uncontrolled HTN | Telemonitoring: Bluetooth‐enabled home BP monitoring device paired with an app on a BlackBerry smartphone, readings trend and applied decision rules, self‐care messages to the patient's phone immediate after each reading, patient call to initiate an automated process to fax a one‐page summary report to provider | Patients, physicians | Systolic BP, target BP control of <130/80 mmHg, anxiety, depression, comfort with BP self‐monitoring changes in 7 days of home BP readings | The intervention (BP device + self‐care support) was associated with decreased in systolic BP by 9.1 ± 15.6 mmHg, (p = 0.003); compared to control group, providing self‐care support did not affect anxiety but worsened depression (p = 0.76 vs. p = 0.032) |
| 2012 | Takahashi et al. 76 a | A randomized controlled trial of telemonitoring in older adults with multiple health issues to prevent hospitalizations and emergency department visits | To determine the effectiveness of home telemonitoring compared with usual care in reducing the combined outcomes of hospitalization and emergency department visits in an at‐risk population 60 years of age or older. | RCT of telemonitoring for high‐risk older adults with MCCs living in assisted care (elderly risk assessment score > 16) assigned to intervention or usual care and followed for 1 year (n = 205) | High‐risk older adults with MCCs living in assisted care, elderly risk assessment score > 16 | Telemonitoring: Intel Health Guide, an FDA‐approved device/monitoring system capable of collecting biometric data (BP, WT, pulse, temp, pulse oxygenation, peak flow); symptom questionaries with goal of early detection of health status decline; message, video conference | Patient, nurse, geriatric NP, PCP | Hospitalization, ED visits over 1 year | Telemonitoring did not result in fewer hospitalizations or ED visits (p = 0.345). Mortality was higher in the telemonitoring 14.7%, versus 3.9% to usual care group (p = 0.008). |
| Non‐RCT | |||||||||
| 2019 | Steele Gray et al. 77 b | Using exploratory trials to identify relevant contexts and mechanisms in complex electronic health interventions: evaluating the electronic patient‐reported outcome tool | To use exploratory trial data to identify relevant context, process, and outcome variables, as well as central versus peripheral mechanisms at paly for the ePRO intervention. | Mixed method survey evaluating patients, providers, and administrators experience with the ePRO intervention assigned pre and post intervention and followed for 4 months (n = 24) | MCC patients |
Mobile and web‐based application: My Goal Tracker—ePRO tool and portal to support goal‐oriented care in primary care = uses goal‐attainment scaling to capture standardized outcome measures across diverse patient groups, standardize goal attainment measures, and address the challenge of writing multiple goal. ePRO also supports health status scales and outcome measures |
Patients, primary care provider, social worker, nursing staff, DM educator | QOL, self‐management, patient experience; provider effectiveness; system usability; goals attainments; person‐centeredness | Quantitative: No statistical difference in change scores between control and intervention arms. Assessment of Quality of Life Scale (p = 0.21) and Patient Assessment of Chronic Illness Care (p = 0.52) Qualitative?: Identify—perceived meaningfulness of the ePRO tool, assign roles and responsibilities to set up appropriate goals, pts remembering their goals, and monitoring if achieved or not was essential to meet outcomes reported in qualitative findings |
| 2019 | Easton et al. 78 | A virtual agent to support individuals living with physical and mental comorbidities: co‐design and acceptability testing | To co‐design the content, functionality, and interface modalities of an autonomous virtual agent to support self‐management for patients with an exemplar long‐term condition (COPD) and then to assess the acceptability and system content | Qualitative study of patients' and health professionals' experience design and development of an autonomous virtual agent with natural language processing capabilities (n = 11) | COPD, mental health, Comorbid long‐term conditions (LTCs) | Artificial intelligence‐based virtual agent: Avachat, a conversational agent is an autonomous virtual agent with natural language processing abilities for mapping a day in the life journey, mood boards, what situations it was advisable and acceptable to depart from the script to alert a provider or caregiver | Patients, clinicians | Content, functionality, and interface modalities of an autonomous virtual agent user acceptance | Patients and clinicians identified four priority scenarios pts like to receive support: (1) at the point of diagnosis—information provision; in the course of acute exacerbation—crisis support; (2) while in low mood—emotional support; (3) general self‐management motivation. Contents desired by patients were behavior change practices, emotional well‐being advice, and peer‐driven support. Based on the scenario testing 10 older adults with comorbidities felt acceptable to have both self‐management support and support for acute exacerbations from an AI‐based virtual agent |
| 2019 | Portz et al. 79 c | Using the technology acceptance model to explore user experience, intent to use, and use behavior of a patient portal among older adults with multiple chronic conditions: descriptive qualitative study | To use the Technology Acceptance Model (TAM) as a framework for qualitatively describing the (user interface) UI and (user experience) UX, intent to use, and use behaviors among older patients with MCC | Qualitative study of focus groups on Technology Acceptance Model (TAM) (n = 24) | Older adults (aged 65 years and over), with MCC, Charlson Comorbidity Index >2 | Web‐based application: My Health Manager is a patient portal for appointment, medical records (view test results, immunization, problem list, care plans), pharmacy (manage and order medication), health resources and self‐management tools, message (email provider), e‐visit and provider chat for non‐emergent questions/visits | Patients, providers | Usability, ease of use | Portal use affected by challenges related to log‐ins, UI design (color and font). Focus groups indicated that portal improved patient‐provider communication, saved time and money, provided appropriate health info. Intend to use functionalities that were valuable to their health management and easy to use |
| 2019 | Portz et al. 80 c | “Call a Teenager… That's What I Do!”—Grandchildren help older adults use new technologies: qualitative study | To explore older adults' experiences with technology support from family members to inform strategies for promoting adoption of new health technologies by older adults | Qualitative study of secondary analysis on family support themes from six focus groups assigned to user or nonuser groups (n = 24) | Older adults (65 year old and over) with MCC, Charlson Comorbidity Index >2 | Web‐portal Patient portal: The functionalities of the portal include appointment, medical records (view test results, immunization, problem list, care plans), pharmacy (manage and medication order), health resources and self‐management tools, message (email provider), e‐visit and provider chat for nonemergent questions/visits | Patients, providers, family members | Usability, training | Grandchildren and adult children are teaching their (grand)parents to use new technology, troubleshoot, and adapt new technologies to older adults; Family members faced difficulty when teaching tech use, they struggle to elucidate simple technology tasks and exasperated by the slow learning of older adults |
| 2018 | Hans et al. 81 b | The provider perspective: investigating the effect of the electronic patient‐reported outcome (ePRO) mobile application and portal on primary care provider workflow | To investigate how the ePRO mobile application and portal system, designed to capture patient‐reported measures to support self‐management, affected primary care provider workflows | Qualitative study of training notes, patient focus groups and provider focus groups, and issue tracker reports followed for 6 weeks (n = 18) | MCC (2+ conditions) | Mobile application and web‐based portal: Electronic patient reported, using a patient centered app and portal system developed by patient and professional collaboration previously outcome (wk./1 set health goals and monitoring protocol) | Patients, providers collaborating | PROMIS: global health scale; pain interference scale; health assessment questionnaire; GAD‐7; PHQ‐9 feasibility and effect of system on provider workflow |
ePRO application encouraged care planning and collaborative conversation on goal‐setting b/t patients and providers. Providers worried about lack of interoperability b/t app and EHR lead to increased documentation; Provider concerned on clinical workflow disruption and increased needs for patients' engagement. High level of provider opposition rather than adapting behavior, regular attempt to shift the app to fit with existing workflow |
| 2018 | Irfan Khan et al. 82 b | mHealth tools for the self‐management of patients with multimorbidity in primary care settings: pilot study to explore user experience | To explore the experience and expectations of patients with multimorbidity and their providers around the use of the ePRO tool in supporting self‐management efforts | Qualitative study of thematic analysis of focus groups followed for 4 weeks (n = 18) | MCC, social complexity | Mobile and web‐based application: ePRO (electronic patient reported outcome) mobile app is linked to the web portal. The platform is capable to support (1) set goals and track self‐management goals, and (2) a hospital discharge function to notify providers of hospital visits. | Patients, primary care provider, social worker, nursing staff, DM educator | Self‐management goals: (1) physical and social, (2) mood and memory, (3) mobility, (4) pain, and (5) WT/diet | From providers: ePRO offered important insights into the broader patient context that help formulate recommendation on self‐management approach and activities to pts; From patients perspectives: the tool advance access to providers in a team‐based primary care setting. But, both patients and providers highlighted: (1) lack more customization of content to better adapt to the complexity and fluidity of self‐management, (2) absence of direct provider engagement through the ePRO tool |
| 2017 | Middlemass et al. 83 | Perceptions on use of home telemonitoring in patients with long term conditions—concordance with the health information technology acceptance model (HITAM): a qualitative collective case study | To examine the usefulness of the HITAM for understanding acceptance of HIT in older people (≥60 years) participating in a RCT for older people with Chronic Obstructive Pulmonary Disease (COPD) and associated heart diseases (CHROMED). | Qualitative collective case study of interviews from a parent study clinical trial in patients and caregivers all assigned to the intervention arm and followed for 9 months (n = 21, n = 8 respectively) | COPD and CHF or ischemic heart disease | Telemonitoring: Telemonitoring devices used by health care professionals to received clinical alerts are the following: (1). Resmon pro©, monitored measure lung function of participants (2). The Wristclinic measured HR, ECG, BP, heart rhythm, RR, pulse oxygenation, temperature (3). A computer monitor for daily responses number of symptom questions relating to their illness. | Patients in their own home, caregivers | User behavior: use intention, beliefs, and attitudes Acceptance of tele‐monitoring using HITAM | HITAM can explain the likelihood that older people with LTCs would use HIT. HIT self‐efficacy depended on good organization factors and informal support, ease of use for older adults. HIT perceived usefulness correlated in seeing trends in health status, early detection of infection and potential to self‐manage. Factors of nonacceptance of HIT included: increased illness anxiety and fear, reinforcement of “Sick‐role”; insufficient support for self‐management due to inadequate feedback to user from clinicians |
| 2016 | Steele Gray et al. 84 b | The electronic patient reported outcome tool: testing usability and feasibility of a mobile app and portal to support care for patients with complex chronic disease and disability in primary care settings | To test the usability and feasibility of adopting the ePRO tool into a single interdisciplinary primary health care practice in Toronto, Canada | Mixed method design of pilot execution, descriptive statistics, content analysis, interviews, and focus groups followed for 4 weeks (n = 17) | Mobile and web portal application: Goal tracker and check out alert are two main features. Patients used Samsung Galaxy II android phones with the ePRO app uploaded to track their goals and report hospital visits using the Hospital discharge. The provider portal enables providers to set up care plans and to track patients ‘goals | Patients, primary care provider, social worker, nursing staff, DM educator | Feasibility, usability | Eight patients completed 210 monitoring protocols, 1300+ questions answered daily; patients and providers noted ePRO easy to use. From patients: it facilitated self‐manage (sense of responsibility over their care), improved patient‐centered care delivery; From providers: ePRO focused conversations on goal setting; However, ePRO did not well suited for provider workflow, monitoring questions were not well aligned with individual patient needs, daily reporting became burdensome and time consuming for patients | |
| 2011 | Pecina et al. 85 a | Telemonitoring increases patient awareness of health and prompts health‐related action: initial evaluation of the Tele‐ERA study | To assessing MCC patients opinions about their telemonitoring experience. | Qualitative and usability study of interviews of patients randomly selected from ongoing Trial (Tele‐ERA) (n = 20) | Telemonitoring: Intel Health Guide uses to monitor daily weight, blood pressure, heart rate, pulse oximetry, peak flow, and glucose level as well as ask questions on self‐reported symptoms | Patients | Usability and usefulness | MCC patients perceived telemonitoring be acceptable and satisfying. elderly patients noted that telemonitoring provided peace of mind; awareness; minimally difficulties, assertive in using the monitor, and helped with clinician communication | |
Abbreviations: ALLG, allergies; BG, blood glucose; BP, blood pressure; CHF, congested heart failure; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; DM, diabetes; ECG, electrocardiogram; ED, emergency department; EHR, electronic health record; ePRO, electronic patient reported outcome; health IT, health information technology; HR, heart rate; HTN, hypertension; MCC, multiple chronic conditions; PCS, Pain Catastrophizing Scale; PHR, personal health record; PLWMCC, people living with multiple chronic conditions; QOL, quality of life; RCT, randomized controlled trial; RR, respiration rate; SUS, system usability score; WT, weight.
Tele‐ERA study, Mayo Clinic Rochester Minnesota.
Health System Performance Research Network‐Bridgepoint electronic Patient‐Reported Outcomes mobile device and portal system in collaboration with QoC Health Inc., Toronto Canada.
My Health Manager, Kaiser Permanente Colorado.
Five of the seven RCTs evaluated telemonitoring interventions with functionality for daily monitoring of weight, blood pressure, pulse, pulse oxygenation, and temperature, along with messaging with the primary care physician. 71 , 73 , 74 , 75 , 76 These telemonitoring interventions overall yielded mixed results. The two positive telemonitoring studies showed effects on systolic blood pressure and ED visits. 73 , 75 One web‐based PHR intervention for patients with serious mental illness and comorbid chronic disease led to improvements in preventive care. 72 Another study of a mobile PHR application demonstrated that a composite measure of quality of preventive care and cardiovascular care stayed stable in the intervention group as opposed to declining in the usual care group. 70 The settings were diverse with one international study involving seven European countries, 71 three US studies, 73 , 74 , 76 and one Canadian study. 75
The non‐RCT studies included qualitative studies of self‐management, PRO, health‐related quality of life using health IT tools. These studies reveal interoperability challenges, major barriers in clinical workflow integration, burden of data review, complexity related to older age, disability, social needs, and mental health, and disruption of collaborative conversations about goal‐setting and forming a person‐centered care plan. 81 , 82 , 84 These barriers inhibit interactive feedback from clinicians and lower patient motivation to stay engaged in self‐management. 82 , 83 Also, PLWMCC are often older adults who rely on family/caregivers for technical support, but family members reported that they lacked confidence to give this training, struggled to explain simple technology tasks, and were frustrated by the slow learning process. 80 These two qualitative studies assessed the usability and acceptance of telemonitoring with regard to MCC self‐management. 83 , 85 They revealed that MCC patients perceived telemonitoring to be useful, acceptable, and satisfying. HIT usefulness was correlated in seeing trends in health status, early detection of infection, and potential to self‐manage. MCC patients perceived telemonitoring be acceptable and satisfying, and some elderly patients noted that telemonitoring provided peace of mind. 85 In the mixed methods evaluation of an ePRO tool, there was no statistical difference in measures of quality of life or self‐management between intervention and control arm. 78
3.2. Care coordination and the activity of care planning
We identified eight studies meeting the inclusion criteria for care coordination or care planning including one RCT (Table 2). 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 The RCT evaluated an intervention that assessed needs and goals of patients and created a proactive plan of care. 88 , 92 The health IT component of the intervention was a computerized note template to improve clinician–patient communication. It was perceived as disruptive to patient‐physician communication, but measures of patient‐centeredness improved. 88 , 92 The trial's primary outcome was quality of life, and there was no significant difference in this outcome at 15 months. 88 A quasi‐experimental observational study evaluated an intervention that assessed needs and goals, supported self‐management goals, and linked patients to community resources. 94 The health IT component was an online health community linked to a shared electronic health record where patients and their caregivers gave invitations to clinicians. The tool increased multidisciplinary care coordination within the patient record. 94 The main quantitative finding was that there was no difference between arms at 12 months post‐implementation in activities of daily living. 94 Four of the included studies were observational studies exploring usability, feasibility, acceptance, and intensity of use for care coordination platforms, which generally include collaborative tools for multiple health care providers. 89 , 90 , 93 , 95 Two of the platforms included chronic disease CDS. 89 , 90 Two of the platforms included a patient‐facing health IT tool. 90 , 94 Broadly, the content of these platforms was found to be valuable, but it did not decrease the complexity or time burden of care coordination and the technology was disruptive. The majority of the published literature on care coordination tools was excluded because the tools have not yet been implemented in clinical settings. 96 , 97 , 98 , 99 , 100
TABLE 2.
Care coordination studies
| Year | Author | Title | Objective | Study design | Population | HIT component | User | Outcome | Results |
|---|---|---|---|---|---|---|---|---|---|
| RCT | |||||||||
| 2018 | Salisbury et al. 88 a | Management of multimorbidity using a patient‐centered care model: a pragmatic cluster‐randomized trial of the 3D approach | Was the patient‐centered, so‐called 3D approach (based on dimensions of health, depression, and drugs) for patients with multimorbidity would improve their health‐related quality of life, which is the ultimate aim of the 3D intervention | Pragmatic cluster‐RCT of 33 GP practices in England and Scotland assigned to intervention or usual care and followed for 15 months (n = 1546) | Age > 18 with at least three chronic conditions | Multicomponent intervention “the 3D approach.” Note template including prompts to ask patients about their most important concerns, their quality of life, and to perform depression screening. The template created a print out of collaborative management plan including names of a specific physician and nurse on the patient's care team | Nurse, pharmacist, provider | Health‐related QOL (EQ‐5D‐5L; illness burden, treatment burden, medication adherence score, and number of medications, and patient‐centered care | The intervention was associated with significant improvements in measures of patient centered care. Adjusted difference in means for patients reporting having a written care plan, health plan, or treatment plan (mean = 1.97 p < 0.001); Patients reporting they almost always discuss the problems most important to them in managing their own health (mean = 1.85 p < 0.001) In the intention‐to‐treat analysis, there was no difference between trial groups in the primary outcome of quality of life (adjusted difference in mean EQ‐5D‐5L 0·00, 95% CI –0·02 to 0·02; p = 0·93) |
| Non‐RCT | |||||||||
| 2019 | Kersting & Welterman 89 | Evaluating the feasibility of a software prototype supporting the management of multimorbid seniors: mixed methods study in general practices | To evaluate the prototypes (which is add‐on for German EHR systems to support longitudinal care management) feasibility from both a technical and users' perspective | Mixed method study of feasibility interviews and questionnaires assigned to general practitioners and practice assistants (n = 18) | Age > 65 years | CDS eCare Plan: information flags (reminders) on age‐ and sex‐specific preventive measures, diagnosis‐specific measures, and/or for predefined patient groups and identify quality deficits by providing dynamic action flags such as critical for uncontrolled BP | General practitioners and practice assistants (German health care system) | Usefulness/usability | The new EHR add on was well accepted and achieved a good usability rating. The users found it easy to install and worked without problems; (78%) were interested in using the software long‐term; The system usability scored SUS 73%–78%; Challenges encountered were mainly installation, and EHR missing interface to extract needed data |
| 2019 | Laleci Erturkmen et al. 90 | A collaborative platform for management of chronic diseases via guideline‐driven individualized care plans | To present a method and corresponding implementation of a semi‐automatic care plan management tool and further report the results of usability studies carried out in four pilot sites by patients and clinicians of a care planning platform “Coordinated Care and Cure Delivery Platform” which helps with care planning for older adults with multimorbidity. | Usability study of product reaction cards and Nielsen walkthrough heuristic evaluation assigned to care team members, patients, and experts (n = 22, n = 26, n = 5, respectively) | Age > 65 years with special emphasis on CDS for type 2 diabetes, renal failure, heart failure, and depression | CDS eCare Plan: risk prediction and stratification; personalized treatment goals and interventions; reconciliation of conflicting treatment options and management of polypharmacy; Patient Empowerment Platform to incorporate patient needs, preferences, and psychosocial aspects of care | Patients and care team members (providers, specialists, nurses, pharmacists, physical therapist, nutritionists, social worker, homecare staff | Usability: QUIS7 questionnaire on learning factors and product reaction cards |
This method was able to address the needs of care plan personalization and implementing clinical care guidelines Feedback on usability: (1) 23% Collaborative, (2) 17% Useful Empowering (3) 14% Complex (4) 20% Time‐consuming for the subgroup of care team members. QUIS7 Learning scores = 5.8–6.17 out of 9 (9 as “easy”) |
| 2019 | Mann et al. 91 | Can Implementation failure or intervention failure explain the result of the 3D multimorbidity trial in general practice: mixed‐methods process evaluation | To examine whether the measured lack of effect on the primary outcome in the 3D trial was due to implementation or intervention failure |
Mixed methods process evaluation Mixed methods study for a process evaluation of the 3D multimorbidity trial assigned to the trial's overall dataset (n = 1546) |
Age > 18 years with at least three chronic conditions | Multicomponent intervention “the 3D approach.” See description of HIT component in 2018 Mann et al. | Nurse, pharmacist, provider | Adoption of the 3D intervention; delivery of 3D reviews to patients; maintenance and reach | Adoption was incomplete, 49% of patients received both reviews, 30% partially reviewed; In completed reviews >90% of components were delivered |
| 2018 | Mann et al. 92 a | A computer template to enhance patient‐centeredness in multimorbidity reviews: a qualitative evaluation in primary care | To evaluate the effect on patient‐centeredness of a novel computer template used in multimorbidity reviews | Observations and interviews about a computerized note template as one component in a multicomponent RCT assigned to clinicians receiving intervention and usual care (n = 37) | Age > 18 years with at least three chronic conditions | Electronic disease template: 3D review template, structures chronic disease management, and data recording. The template prompts to ask patients about their important concerns, quality of life, and to perform depression screening. A report is printed out for collaborative management plan including names of the care team | Nurse, pharmacist, provider | Observations of different activities performed in intervention and control visits, perceptions of patient‐centeredness of visit | Patients' perceptions of the patient centeredness of reviews enhanced and patients appreciated the more complete comprehensive reviews; most clinicians admired identifying patients' agendas. Users stated that the template usage disrupted eye contact and dialog |
| 2016 | de Jong et al. 93 | How professionals share an e‐care plan for the elderly in primary care: evaluating the use of an e‐communication tool by different combinations of professionals | To evaluate the use of a tool, Congredi, for electronic communication by professionals for the care of home‐dwelling elderly patients | Observational study of patient record analysis from the Congredi system assigned to patients and social workers and followed for 42 weeks (n = 448, n = 203, respectively) | Home‐dwelling elderly patients with MCCs in the Hague region of the Netherlands | e‐Communication and coordination tool; Named Congredi: an application for documenting care planning activities; emailing; linking other providers | Nurses, general practitioners, others professionals | Platform utility (number of contributors and number of activities documented) |
A large group of professionals (n = 203, 21%) were active in 448 patient records. Where, three types of actions were registered: care activities (mean = 9.14), emailing (mean = 0.89), and process activities (mean = 0.29). Determined to be usable for improving multidisciplinary communication among professionals. |
| 2014 | Makai et al. 94 | Evaluation of an eHealth intervention in chronic care for frail older people: why adherence is the first target | To investigate the effectiveness of an online health community (OHC) intervention for older people with frailty aimed at facilitating multidisciplinary communication | Observational controlled trial of 17 practices in university primary care network around the city of Nijmegen, the Netherlands, assigned to before and after implementation and followed for 12 months (n = 682) | Frail older patients identified through EASYcare Two‐step Older person Screening | Online health community (ZWIP) which contains a secure messaging system supplemented by a shared electronic health record. Access can be granted to clinicians by patients or their caregivers. | Frail older patients, their caregivers, general practitioners | Katz ADL, Katz 15, SF‐36 (mental health and social limitation), patient and GP rating of care coordination, patient experience | The use of this OHC did not significantly improve patient outcome. 26% of intervention patients used ZWIP at least once per month standardized difference between study groups for ADL 0.21; 95% CI −0.17 to 0.59; p = 0.27; for SF‐36 mental −8.34; 95% CI −17.02 to 0.34; SF‐36 social 0.84; 95% CI −0.78 to 2.45 |
| 2013 | Martinez‐Garcia et al. 95 | Sharing clinical decisions for multimorbidity case management using social network and open‐source tools | To develop a tool for collaborative work among health professionals for multimorbidity patient care | Pilot study of the use and acceptance of the SCP by health care professionals through questionnaire based on the theory of the technology acceptance model assigned to Internal Medicine dept. of a University Hospital in Seville, Spain and two primary care centers and followed for 6 months (n = 16) | Patients with >2 chronic conditions | Web application and social network technologies The Shared Care Platform (SCP) includes: a social network component (the Clinical Wall and enables communication/collaboration b/t health professionals, the future version of SCP will include CDS, patient assessment section, discussion section, conclusion section) | Nurses, primary care providers, internists | Usability | During the pilot 16 records created in Clinical Wall; A total of 10 professionals exchanged 33 messages; 12 of the 16 records (75%) were answered by the targeted health professionals; providers valued the clinical wall with mean scores of 7.87 for intention to use; 7.54 for perceived usefulness; 7.08 for perceived ease of use; 7.74 for subjective norm; 6.85 for facilitating condition; |
Abbreviations: 3D, three‐dimensional; ADL, activities of daily living; health IT, health information technology, CDS, clinical decision support; eCare, electronic care; EHR, electronic health record; EQ‐5D‐5L, health‐related quality of life instrument; PLWMCC, people living with multiple chronic conditions; QOL, quality of life; QUIS7, questionnaire for user interaction satisfaction 7; RCT, randomized controlled trial; SUS, system usability score.
The 3D study: improving whole person care in England and Scotland.
3.3. Algorithms and advanced analytics
Twenty articles met criteria for inclusion and were focused on predicting risk at the individual or population level and tailoring care to mitigate risks and avoid overtreatment (Table 3). One RCT was included; Prabhakaran et al. 101 found that a mobile health (mHealth) application that helped PLWMCC weigh preventive and chronic disease monitoring options was not more successful at controlling diabetes and blood pressure than the addition of a nurse‐driven reminder system. Besides the RCT, other articles focused on prediction of risk and in guiding through complexity of medical management in the context of conflicting clinical guidelines. The prediction articles focused on which PLWMCCs are at‐risk for death, hospitalizations, exacerbations of illness, disability, frailty, and other poor outcomes. Researchers found success in predicting mortality, hospitalizations, disability/frailty, and illness exacerbations or complications but rarely applied these algorithms to change care or reallocate resources. 96 , 102 , 103 , 105 , 106 , 107 , 108 , 110 , 112 , 113 For PLWMCCs, however, adding data beyond conditions was helpful—from personal emergency response to drug‐derived indices to measures of adverse childhood experiences, additional data sources helped increase the accuracy and potential actionability of the metrics. 106 , 111 Researchers tailored algorithms to their purpose by identifying key sets of illnesses to proactively manage in an accountable care organization or predicting mortality to inform goals of care or palliative care planning. 104 , 107 , 108 Many algorithms had only moderate AUC statistics (0.60–0.80), limiting their usefulness in overall prediction, even for segments of the populations. 113
TABLE 3.
Algorithm studies
| Year | Author | Title | Objective | Study design | Population | HIT component | User | Outcome | Results |
|---|---|---|---|---|---|---|---|---|---|
| RCT | |||||||||
| 2018 | Prabhakaran et al. 101 | Effectiveness of an mHealth‐based electronic decision support system for integrated management of chronic conditions in primary care: the mwellcare cluster‐randomized controlled trial | Assess whether mHealth for integrated management of common multiple morbidities improves outcomes | RCT of mHealth‐based electronic decision support system for patients with MCCs assigned to intervention or enhanced usual care and followed for 12 months (n = 3698) | >30 years old with 1+ preselected illness, only 16% had MCC | Electronic clinical decision support | Nurses/nonphysician providers | Change in systolic BP; and A1c at 12‐month follow‐up; | No increased benefit of using the application over enhanced usual care for systolic blood pressure (Δ = −0.98; 95% CI −4.64 to 2.67) or A1c (Δ = 0.11; 95% CI −0.24 to 0.45) |
| Non‐RCT | |||||||||
| Algorithms to predict adverse outcomes | |||||||||
| 2020 | Dovgan et al. 102 | Using machine learning models to predict the initiation of renal replacement therapy among chronic kidney disease patients | Predict the onset of renal disease (at 3, 6 and 12 months) from time of first CKD diagnosis | Case–control analysis to predict 3, 6, 12 month onset of RTT after first CKD diagnosis outcome matched with a control group and followed for 12 months (N = 8492) | Residents in Taiwan database who were diagnosed with CKD before RTT |
Healthcare Forecasting Model: machine learning algorithm |
Systems, and potential clinical practice after evaluating model with large population | Onset of renal replacement therapy (RTT) at 3, 6, 12 months from time of first CKD diagnosis | Forecasting model prediction probabilities were between 0.470–0.505 for 3 months, 0.509–0.513 for 6 months and 0.310–0.555 for 12 months. AUC for predicting RRT within 12 months was 0.77; Sensitivity was 0.50.‐0.62. |
| 2019 | Pajewski et al. 103 | Frailty screening using the electronic health record within a medicare accountable care organization | To create an EHR frailty score to predict mortality, hospitalization, ED visits, falls | Retrospective cohort analysis of development and validation of a frailty index within an EHR using the Charlson Comorbidity Index using a 2‐year lookback period (n = 12,798) | Older adults (65+) with MCCs, N = 12,798 in total Medicare organization | Electronic Frailty Index (eFI): | Electronic health records | Frailty score predicting mortality, hospitalization, ED visits, falls | Mortality, hospitalization, ED visits and falls were all independently predicted (all p < 0.001) Sensitivity 0.83; Specificity 0.52 for mortality; |
| 2018 | Chen et al. 104 | Learning bundled care opportunities from electronic medical records | To combine MCCs into treatment bundles for ACOs, and determine if these can be automatically learned | Validation of clustering of HCCs and workflows for hospitalizations and expert review using 4 months of inpatient data (n = 16,569) | N = 16,569, hospitalized MCCs | Framework to infer health condition collections. | Health systems, health care management routines | Evaluation of framework and validity through experts and literature |
‐Evaluation of framework: bundled groups were detected, resulting in four clusters from EHR (all p < 0.05) ‐Validation through literature and experts was achieved. |
| 2018 | Magnan et al. 105 | Stratifying patients with diabetes into clinically relevant groups by combination of chronic conditions to identify gaps in quality of care | Creating valuable clusters of comorbidities among patients with diabetes | Observational study using patient‐level retrospective EHR data from 2 years for condition class and quality metrics as compared to a control group with no comorbidities and followed for 1 year (n = 29,562) | N = 29,562 MCC with diabetes, seen at eight health systems over 1 year | Clustering algorithm: | Systems, identifying proper interventions for patients | Relationship between combinations of comorbidities and diabetes metrics, and validity |
Accurately predicted probabilities, produced five condition classes for comorbidities. Validity evidenced by: “Those in less severe classes were less likely to achieve diabetes metrics.” |
| 2018 | Op den Buijs et al. 106 | Predictive modeling of 30‐day emergency hospital transport of patients using a personal emergency response system: prognostic retrospective study | To use a personal emergency response system to predict hospitalization | Retrospective cohort analysis of development and validation of a predictive rule based on demographics, self‐entered data, and emergency response using training, validation, and linked cohorts using 2 years of retrospective data (n = 581,675) | Training cohort N = 290,434 adults using a personal emergency system; Validation cohort: N = 289,426; N = 1815 adults receiving homecare using a personal emergency system | Predictive model | Patients; health care providers, Systems |
Emergency transports over 30 days Comparing model to clinical outcomes |
Predicted patients at risk of hospital transport AUC = 0.779 (95% CI 0.774–0.785). Comparison after 1 year showed prediction capability for risk rate of emergencies between high‐ and low‐risk patients |
| 2018 | Satchidanand et al. 107 | Development of a risk tool to support discussions of care for older adults admitted to the ICU with pneumonia | To use a mortality prediction tool to help inform goals of care | Retrospective cohort analysis of development and validation of mortality prediction tool using 2.5 years of retrospective data (n = 1237) | N = 1237 older adults (75+) in hospital (ICU) for pneumonia | Prediction tool | Providers, and hospital palliative care teams | Primary outcome: 30‐day mortality | AUC 0.74 and sensitivity 0.71 for 30‐day mortality. Mortality rate was 14.3% |
| 2017 | Duenk et al. 108 | Development of the ProPal‐COPD tool to identify patients with COPD for proactive palliative care | Develop an index of comorbidities, symptoms and other biomarkers to identify need for palliative care in COPD | Development and validation of COPD and comorbidity score to predict palliative care needs as compared to the PROLONG study findings and the CODEX index and followed for 1 year (n = 174) | Patients with COPD who were hospitalized | Multivariable prediction Pro‐Pal COPD tool | Systems; integration into EHR systems |
Primary outcomes: Mortality within 1 year and development/validation of prediction tool |
Prediction model was internally validated and had good discriminating power (AUC = 0.82); Tool was a stronger predictor of mortality within 1 year than the CODEX index; |
| 2016 | Alemi et al. 109 | The multimorbidity index: a tool for assessing the prognosis of patients from their history of illness | Describing the existing multimorbidity index and implementing it into EHR. | Implementation of a multimorbidity index predicting into EHR with adequate performance as compared to other published prognostic indices | Patients with comorbid conditions | Prediction index for multimorbidity's: | Providers, policy and comparative analyses | Primary outcomes: prediction, accuracy and prognostic ability of the multimorbidity index compared to other indices |
Index outperformed physiologic markers, other prognostic indices, and commercially available measures. Included high AUC across many populations |
| 2016 | Robusto et al. 110 | The drug derived complexity index (DDCI) predicts mortality, unplanned hospitalization and hospital readmissions at the population level | “To develop and validate the drug derived complexity index (DDCI)” | Population based retrospective cohort study of development and validation of drug derived complexity index as compared to a assigned to a random 50% sample of the population (validation cohort) and followed for 1 year (n = 2,000,000) | Adults 40 years and older on civil registry in Italy | Predictive model | Systems, risk adjustment, policy making |
Primary outcomes: ‐1. Mortality rates, hospitalizations and hospital readmissions 2. Compare DDCI to Charlson Comorbidity Index |
“DDCI predicted 1‐year mortality, overall mortality and unplanned hospitalization (accuracy: 0.851, 0.835, and 0.584)” DDCI works best when combined with Charlson Index. |
| 2015 | Hammond et al. 111 | The feasibility of using large‐scale text mining to detect adverse childhood experiences in a VA‐treated population | To assess if adverse childhood experiences can be detected and used to predict outcomes. | Retrospective cohort for adverse childhood experience terms; then relation to comorbidities. Text mining from over 44 million clinical notes in veteran database. | Veterans | Text‐mining machine learning algorithm | Systems | Primary outcomes: feasibility, accuracy of adverse childhood exposures (ACE) to adult illness. | 68–0.92 AUC by term; precision 0.74–0.90; identifies key comorbidity cluster; equity issue. Text mining in large population feasible |
| 2013 | Dong et al. 112 | Development and validation of a pharmacy‐based comorbidity measure in a population‐based automated health care database | To develop the Pharmacy‐Based Disease Indicator (PBDI), and determine if it can predict hospitalizations | Retrospective cohort analysis of development and validation of risk as compared to the Charlson Comorbidity Score and followed for 1 year (n = 1,411,895) | All adults registered in the national health insurance system in Taiwan | Predictive measure | Systems, automated health care databases |
Primary outcomes: hospitalization and outpatient diagnosis at 1 yr Comparison to Charlson Index |
Pharmacy score c‐statistic for subsequent‐year hospitalizations was 0.72 versus Charlson 0.69 |
| 2010 | Crane et al. 113 | Use of an electronic administrative database to identify older community dwelling adults at high‐risk for hospitalization or emergency department visits: the elders risk assessment index | To predict within the elderly population who will be hospitalized in the next year. | Retrospective cohort analysis of Development and validation of hospitalization risk score in EHR followed for 2 years (n = 12,650) | Older adults (>60 years) with MCC | Administrative Index | Systems, electronic health records | Primary Outcomes: total number of ED visits and hospitalizations over 2 years | Primary outcome AUC was 0.68. Patients stratified into highest part of risk group had highest risk factors for ED visits and hospitalizations over 2‐years. |
| 2010 | Vitry et al. 114 | Influence of comorbidities on therapeutic progression of diabetes treatment in Australian veterans: a cohort study | To assess if the number of unrelated comorbidities to diabetes change the treatment timing for diabetes? | Retrospective cohort study of development and validation of model in Australian veterans with diabetes using 8 years of retrospective data (n = 20,134) | Australian Veteran patients with diabetes | Risk regression analyses with adjustments for covariates | Systems, quality measures, clinical guidelines | Primary Outcomes: “Time to addition or switch to another antidiabetic treatment and therapeutic progression.” |
Time to addition of medication or switch to another treatment was significantly associated with comorbidities (subhazard ratio [SHR] 0.87 [95% CI 0.84–0.91], p < 0.001) “Increasing numbers of unrelated conditions decreased the likelihood of therapeutic progression” |
| Systems that use CDS to improve care of persons with MCCs | |||||||||
| 2020 | Winocour et al. 115 | Holistic review of people with diabetes and chronic kidney disease reveals important multimorbidity and unmet clinical need: The ENHIDE diabetes renal telehealth pilot study | Feasibility of collecting and extracting data for patients prior to telehealth consultations. | Feasibility study with 14 practices of a Project ECHO style case conference and followed using 2 years of retrospective data (n = 2356) | Systems that use CDS to improve care of persons with MCCs | Data extraction for virtual consulting | Primary care practices, telehealth‐based care | “Feasibility of data extraction from primary care records.” | Determined feasible to extract the data and present it to the practices; lipid‐lowering changes were recommended in 39% of patients with MCCs. |
| 2019 | Jafarpour et al. 116 | Execution‐time integration of clinical practice guidelines to provide decision support for comorbid conditions | Integrating machine‐encoded clinical practice guideline recommendations into a comorbidity framework | Development, validation, and usability testing of ontology extension to allow comparison between conflicting guideline recommendation as compared to existing temporal computer interpretable guideline integration approaches and state of the art approaches | Adults with MCC with diabetes and CKD | Computer Interpretable Guideline Integration; | Providers | Time to present/usability | Able to show that the system can display the conflict quickly and that many experts felt it was easy to use and useful (75%–93% good or very good). |
| 2019 | Rieckert et al. 117 | Reduction of inappropriate medication in older populations by electronic decision support (the PRIMA‐eDS project): a survey of general practitioners' experiences | To determine experiences and usability of the PRIMA‐eDS system which attempts to reduce inappropriate medications in older adults | Usability and usefulness analysis of PRIMA‐eDS system gathered from surveys delivered to users of the PRIMA‐eDS system during the RCT; as compared to usual care (n = 176) | MCC | Electronic decision support tool | Providers | Quantify findings from a prior qualitative study using PRISMA eDS tool and its usability |
Analysis of the surveys indicated it was useful (69%) and increased awareness (86%). Barriers were time, security, and technical issues |
| 2018 | Bottiger et al. 118 | Development and pilot testing of PHARAO—a decision support system for pharmacological risk assessment in the elderly | To detect and reduce polypharmacy and adverse events in multimorbid elderly using PHARAO tool | Content validation, adjudication, and usability assessment by providers in EHR of PHARAO‐a decision support system ranking drug–drug interactions; followed for 4 months (n = 129) | Older adults with polypharmacy | Clinical decision support system | Providers | Development, usability/validation in a pilot test |
PHARAO system worked and integrated into EHR. Pilot test showed 933 uses in 871 patients, and was ranked as useful and usable by providers |
| 2017 | Abidi 96 | A knowledge‐modeling approach to integrate multiple clinical practice guidelines to provide evidence‐based clinical decision support for managing comorbid conditions | To integrate guidelines to reconcile multiple disease‐specific clinical procedures for people with MCC's using COMET (Comorbidity Ontological Modeling and ExecuTion) |
Usability evaluation of CDS flow that incorporates MCCs. COMET system manifests a knowledge management approach to model, computerize and integrate multiple CPG's to provide evidence‐based recommendations for handling comorbid patients. |
Older adults with MCCs | Web‐accessible clinical decision support system | Providers | Qualitative and quantitative analysis of survey data for COMET | Highly usable and receptive to decision support tools, if it does not impact workflow and is evidence‐based |
| 2017 | Seroussi et al. 119 | Using therapeutic circles to visualize guideline‐based therapeutic recommendations for patients with multiple chronic conditions: a case study with GO‐DSS on hypertension, Type 2 diabetes, and dyslipidemia | To utilize therapeutic circles for MCC's within an existing GO‐DSS clinical decision support tool to present recommendations together | Visualization usability study of GO‐DSS with professionals (n = 12) | Patients with comorbid conditions | Clinical decision support system | Providers | Usability through qualitative assessment | Usability of the system had a mean rating of 91% |
Abbreviations: A1C, glycated hemoglobin; ACO, accountable care organization; AUC, area under the curve; CDS, clinical decision support; CI, confidence interval; CKD, chronic kidney disease; HCC, hierarchical condition categories; HIT, health information technology; COPD, chronic obstructive pulmonary disease; ECHO, extension for community health care outcomes; ED, emergency department; EHR, electronic health record; MCC, multiple chronic conditions; NLP, natural language processing; PLWMCC, people living with multiple chronic conditions; PRIMA‐eDS, polypharmacy in chronic diseases: reduction of inappropriate medication and adverse drug events in elderly populations by electronic decision support; RTT, renal replacement therapy; SBP, systolic blood pressure; VA, veterans' administration.
Algorithms were also studied to help health care professionals weigh risks and benefits of treatments to guide care in clinical decision support tools or risk prediction, finding that balancing these recommendations by including algorithms that assess burden and harm is possible. Abidi, Seroussi et al., and Jafarpour et al. found high subjective usability of CDS tools to help medical practitioners weigh options when recommendations conflict. 96 , 116 , 119 Seroussi et al. used a visual diagram to help prioritize the best treatments, while Abidi developed a flexible ontology and Jafarpour et al. implemented branching CDS to help decision making when conflicting guideline recommendations exist and concluded that focusing on smaller set of illnesses (such as diabetes and chronic kidney disease [CKD]) may help. Winocour et al. used a learning collaborative coupled with CDS to help care teams decide on the best approach for managing complex situations for persons with diabetes and CKD. They found it feasible to extract comprehensive data from primary care and other sources for diabetes consultants to review and develop individualized care plans and then present individualized care plans to general practices during telehealth consultations. The intervention resulted in changes in lipid‐lowering medications among 39% of MCC patients. 115 Vitry et al. 114 developed and validated a risk prediction model that showed that the number of unrelated comorbidities was associated with a longer time to intensification of diabetes treatment. Focusing on the accumulation of treatments may also be beneficial, such as systems that try to reduce polypharmacy or avoid additional prescribing that may be contraindicated due to age or interactions. Several studies in these areas also found good usability of such systems. 117 , 118 , 120
4. DISCUSSION
This evidence synthesis identified evaluations of health IT interventions or multicomponent interventions for PLWMCC; identified the functionality of the health IT component or intervention; and classified whether the technology was intended for PLWMCC or clinicians. We found just nine relatively small clinical trials of health IT solutions that fit our inclusion criteria across the three domains as follows: self‐management, care coordination, and advanced analytics. Mixed results were shown for the effect of telemonitoring. 69 , 78 , 81 , 86 Self‐management interventions including PHRs for patients with mental illness showed promise in two studies. 70 , 72 Health IT platforms that aim to improve care coordination have yet to demonstrate a positive impact on quality of life and ADLs. 88 , 94 In the domain of advanced analytics, we found just one trial of an mHealth application utilizing algorithmic clinical decision support to prioritize care for MCC, which did not show benefit of the intervention. 101 Other evaluations of algorithms and advanced analytics tools assessed feasibility and usability and showed promising results. Algorithms to predict adverse outcomes addressed a broad set of relevant outcomes including falls, the need for renal replacement therapy, ED visits, hospitalizations, and mortality, but predictive power was generally moderate.
Algorithms can generate personalized risk scores or treatment options, which could be used as the starting point of a shared decision making discussion where patient goals, values, and preferences are considered. However, algorithms and AI may perpetuate gaps in data quality and underlying validation issues in their development potentially leading to worsening equity and obfuscating decision making rationales. 121 , 122 , 123 , 124 There is growing concern about the potential of algorithms to exacerbate racial and ethnic disparities. 123 , 125 , 126 Racial and ethnic minorities and low‐income individuals have more chronic conditions and develop them at an earlier age so could be at greater risk of potential harms from algorithms developed to support the care of PLWMCC. Integrating algorithms into patient‐centered workflows to reduce burden and harm from medical treatment options is still in its infancy, but several studies showed promise. Algorithms can be built into health IT and used for PLWMCCs to predict meaningful outcomes, but their performance is only moderate. These tools are limited by the need for more precise data, as well as ethical, privacy, and security concerns. 127 AI, or learning algorithms, was not observed in any implemented systems for PLWMCC. However, issues related to data privacy, transparency of underlying models, and equity must be addressed so that AI can support clinical decision making for PLWMCC.
4.1. Future directions for health IT for PLWMCC
There is opportunity to use health IT to improve the lives of PLWMCC across these three domains. While some promising practices have been assessed, the literature is limited, and much more evidence is needed on the effectiveness of health IT solutions to improve health care delivery and health outcomes of these patients with complex needs. For example, we did not find any trials in a population of PLWMCC where health IT is used for self‐management or care coordination that evaluated the impact of health IT tools on reduction of readmissions, reduction of avoidable readmissions, or mortality. We also need to understand the contribution of health IT solutions to the multicomponent interventions needed to redesign care delivery for PLWMCC. The goal of creating a learning health system has been a priority at the federal level for some time. A learning health system is envisioned as an ecosystem where all stakeholders can contribute, share, and analyze data in order to enact continuous learning cycles where data support operational decision making and lead to improved health outcomes. 128 , 129 Learning health systems can use data generated from health IT tools to learn more about the best ways to care for PLWMCC, given their unique challenges related to complexity and the lack of an evidence base to guide prioritization of care. Additionally, due to the increased complexity of care for PLWMCC, the design of health IT tools should capture the needs of end‐users (patients and clinicians) to maximize usability and clinical usefulness. There is a need for more tools to truly engage patients in self‐management and to amplify the patient voice, especially in the self‐management domain since the focus so far has been on remote patient monitoring by clinicians. We also need more studies that include experiences of care by PLWMCC and whether these tools can help reduce the burden of care on patients and caregivers. Algorithms and machine learning must be employed to help patients or care teams take actions that maximize benefit and minimize harm; to do so, they must be developed, tested, and validated in the context of potential interventions. Grand challenges exist in bringing together data from multiple sources, including the patient—conflicting data must be adjudicated; data must be summarized to enable time‐sensitive decision making; the data must be at the right level of specificity; the data must be prioritized based on risk; and the data must help lead to action. Each of these challenges is magnified in the context of PLWMCCs due to their higher burden of illness and receipt of care from multiple sites and settings, often lacking interoperability, leading to incomplete and hard‐to‐aggregate data to support care.
4.2. Limitations
Our approach was systematic in the definition of topics and selection of articles, but the breadth of this review precludes meta‐analysis. Each of the three domains occupies a different space within evolving care delivery systems and evolving research methods meant to improve health for PLWMCCs; to that end, inclusion criteria captured more intervention studies in the care coordination and self‐management domains than in the algorithm domain where much of the work is still developmental. We did not assess the quality of the included studies, as is standard for scoping reviews.
5. CONCLUSIONS
Continued advances in health information technology offer much promise in delivering solutions to the complex challenges of providing comprehensive, integrated, and longitudinal care for people living with multiple chronic conditions. Although this research is in its infancy, and meta‐analysis is precluded by the use of heterogeneous interventions with heterogeneous outcome measures, this evidence synthesis provides many insights that can be applied in advancing the field. Self‐management support and care coordination are fundamental components of care delivery for PLWMCC where health IT solutions hold promise, and current single disease–focused applications are insufficient and potentially harmful. Increasingly, use of data, predictive analytics, and machine learning are providing tools designed to improve care delivery including for risk stratification and clinical decision support. At the same time, potential harms and limitations of algorithms are increasingly being recognized and need to be considered in the development of algorithms and machine learning applications.
Currently, there are efforts to reform the payment system toward value‐based payment to reward care that improves outcomes rather than volume, including expanded use of a capitated and bundled payments, as well as achieving improved care coordination through accountable care organizations. 130 , 131 , 132 These efforts have the potential to encourage and support innovative and sustainable approaches to improving the health of PLWMCC who are often the target of efforts to improve care quality and efficiency undertaken as part of these efforts. The HITECH and 21st Century Cures Acts aim to improve data interoperability and availability to foster these efforts. While there has been some progress, much work is still needed to achieve the objectives of this legislation. Strategically building upon this work can help us to achieve the ultimate goal of delivering high value care that reduces burden on patients, caregivers, and clinicians while improving health outcomes.
The potential future research agenda is large. The development and use of new technologies, data‐driven tools, and informatics to enhance care coordination and delivery of patient‐centered care for PLWMCCs will require innovative technologies to support care outside of the office, interoperable digital dashboards to support care management, and learning algorithms, or AI, to identify optimal care for different constellations of multimorbidity. Tools must be designed so that they are interoperable, user friendly, integrated into workflow, and rigorously evaluated including with pragmatic trials for their impact on access, quality, and outcomes of care. Evidence is needed on effective implementation of these tools, as well as how to scale and spread them across the health system. PLWMCC currently encounter multiple barriers to getting needed care and consume a large proportion of health care resources. In reality, improving care for this population will require culture change from a disease‐oriented system to one that is patient‐centered, one that is fragmented to one that is integrated, and one that is reactive to one that is proactive. We will need to understand the role of digital health technology in health system redesign and multicomponent interventions aimed at transforming care. The body of literature included in this review provides critical information on the state of the science as well as the many gaps that need to be filled for digital health to fulfill its promise in supporting care delivery that meets the needs of PLWMCCs.
CONFLICT OF INTEREST
None of the authors has any affiliation or financial involvement that conflicts with the material presented in this product.
DISCLAIMER
The authors are solely responsible for this document's contents, findings, and conclusions, which do not necessarily represent the views of AHRQ. Readers should not interpret any statement in this product as an official position of AHRQ or of the US Department of Health and Human Services.
Supporting information
Appendix S1 Supporting information
ACKNOWLEDGMENTS
HF is presently funded as a Postdoctoral Research Fellow in Public and Population Health Informatics at Fairbanks School of Public Health and Regenstrief Institute, supported by the National Library of Medicine of the National Institutes of Health under award number T15LM012502. The authors would like to acknowledge Andrew S. Hamilton, MLS, for literature search and John L. Kilgallon, BA, for assistance with formatting and references.
Samal L, Fu HN, Camara DS, Wang J, Bierman AS, Dorr DA. Health information technology to improve care for people with multiple chronic conditions. Health Serv Res. 2021;56(Suppl. 1):1006‐1036. doi: 10.1111/1475-6773.13860
This work was developed in conjunction with the Agency for Healthcare Research and Quality (AHRQ) and was presented in part at the 2020 AHRQ Research Summit on Transforming Care for People Living with Multiple Chronic Conditions on November 17 and 18, 2020.
Funding information Lipika Samal is funded under R01 DK116898 from the National Institutes of Health (NIH). Lipika Samal also wishes to disclose PI‐initiated grant funding from IBM, unrelated to the current work. David Dorr is funded under U18 HS026849 from the Agency for Healthcare Research and Quality. Arlene Bierman is funded under contract HHSA290201600001B/75Q80119F32009 from the Agency for Healthcare Research and Quality (AHRQ), U.S. Department of Health and Human Services.
Contributor Information
Lipika Samal, Email: lsamal@bwh.harvard.edu.
Helen N. Fu, Email: hnfu@iu.edu.
Djibril S. Camara, Email: Djibril.Camara@ahrq.hhs.gov.
Jing Wang, Email: jwang19@fsu.edu.
Arlene S. Bierman, Email: Arlene.Bierman@ahrq.hhs.gov.
David A. Dorr, Email: dorrd@ohsu.edu.
REFERENCES
- 1. Boersma P, Black L, Ward B. Prevalence of multiple chronic conditions among US adults, 2018. Prev Chronic Dis. 2020;17:200130. 10.5888/pcd17.200130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Agborsangaya CB, Lau D, Lahtinen M, et al. Health‐related quality of life and healthcare utilization in multimorbidity: results of a cross‐sectional survey. Qual Life Res. 2013;22(4):791‐799. 10.1007/s11136-012-0214-7 [DOI] [PubMed] [Google Scholar]
- 3. Adams ML. Differences between younger and older US adults with multiple chronic conditions. Prev Chronic Dis. 2017;14:E76. 10.5888/pcd14.160613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wijlaars LP, Gilbert R, Hardelid P. Chronic conditions in children and young people: learning from administrative data. Arch Dis Child. 2016;101(10):881‐885. 10.1136/archdischild-2016-310716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McCormick WC, Boling PA. Multimorbidity and a comprehensive Medicare care‐coordination benefit. J Am Geriatr Soc. 2005;53(12):2227‐2228. 10.1111/j.1532-5415.2005.00504.x [DOI] [PubMed] [Google Scholar]
- 6. Beverly EA, Wray LA, Chiu CJ, et al. Perceived challenges and priorities in co‐morbidity management of older patients with type 2 diabetes. Diabet Med. 2011;28(7):781‐784. 10.1111/j.1464-5491.2011.03282.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marengoni A, Angleman S, Melis R, et al. Aging with multimorbidity: a systematic review of the literature. Ageing Res Rev. 2011;10(4):430‐439. 10.1016/j.arr.2011.03.003 [DOI] [PubMed] [Google Scholar]
- 8. Onder G, Palmer K, Navickas R, et al. Time to face the challenge of multimorbidity. A European perspective from the joint action on chronic diseases and promoting healthy ageing across the life cycle (JA‐CHRODIS). Eur J Intern Med. 2015;26(3):157‐159. 10.1016/j.ejim.2015.02.020 [DOI] [PubMed] [Google Scholar]
- 9. Sambamoorthi U, Tan X, Deb A. Multiple chronic conditions and healthcare costs among adults. Expert Rev Pharmacoecon Outcomes Res. 2015;15(5):823‐832. 10.1586/14737167.2015.1091730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vogeli C, Shields AE, Lee TA, et al. Multiple chronic conditions: prevalence, health consequences, and implications for quality, care management, and costs. J Gen Intern Med. 2007;22(suppl 3):391‐395. 10.1007/s11606-007-0322-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ahn S, Hussein M, Mahmood A, et al. Emergency department and inpatient utilization among U.S. older adults with multiple chronic conditions: a post‐reform update. BMC Health Serv Res. 2020;20(1):77. 10.1186/s12913-020-4902-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hanlon P, Nicholl BI, Jani BD, et al. Examining patterns of multimorbidity, polypharmacy and risk of adverse drug reactions in chronic obstructive pulmonary disease: a cross‐sectional UKbiobank study. BMJ Open. 2018;8(1):e018404. 10.1136/bmjopen-2017-018404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jowsey T, Jeon YH, Dugdale P, et al. Challenges for co‐morbid chronic illness care and policy in Australia: a qualitative study. Aust New Zealand Health Policy. 2009;6:22. 10.1186/1743-8462-6-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liddy C, Blazkho V, Mill K. Challenges of self‐management when living with multiple chronic conditions: systematic review of the qualitative literature. Can Fam Physician. 2014;60(12):1123‐1133. [PMC free article] [PubMed] [Google Scholar]
- 15. Rudin RS, Gidengil CA, Predmore Z, et al. Identifying and Coordinating Care for Complex Patients: Findings from the Leading Edge of Analytics and Health Information Technology. Rand Health Q. 2017;6(3):2. [PMC free article] [PubMed] [Google Scholar]
- 16. Grembowski D, Schaefer J, Johnson KE, et al. A conceptual model of the role of complexity in the care of patients with multiple chronic conditions. Med Care. 2014;52(suppl 3):S7‐S14. 10.1097/MLR.0000000000000045 [DOI] [PubMed] [Google Scholar]
- 17. Noël PH, Parchman ML, Williams JW Jr, et al. The challenges of multimorbidity from the patient perspective. J Gen Intern Med. 2007;22(Suppl 3):419‐424. 10.1007/s11606-007-0308-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Boyd CM, Darer J, Boult C, et al. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA. 2005;294(6):716‐724. 10.1001/jama.294.6.716 [DOI] [PubMed] [Google Scholar]
- 19. Sinnott C, Mc Hugh S, Browne J, et al. GPs' perspectives on the management of patients with multimorbidity: systematic review and synthesis of qualitative research. BMJ Open. 2013;3(9):e003610. 10.1136/bmjopen-2013-003610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bierman AS, Tinetti ME. Precision medicine to precision care: managing multimorbidity. Lancet. 2016;388(10061):2721‐2723. 10.1016/S0140-6736(16)32232-2 [DOI] [PubMed] [Google Scholar]
- 21. Kebede MM, Zeeb H, Peters M, et al. Effectiveness of digital interventions for improving glycemic control in persons with poorly controlled type 2 diabetes: a systematic review, meta‐analysis, and meta‐regression analysis. Diabetes Technol Ther. 2018;20(11):767‐782. 10.1089/dia.2018.0216 [DOI] [PubMed] [Google Scholar]
- 22. Milani RV, Lavie CJ, Bober RM, et al. Improving hypertension control and patient engagement using digital tools. Am J Med. 2017;130(1):14‐20. 10.1016/j.amjmed.2016.07.029 [DOI] [PubMed] [Google Scholar]
- 23. Dorr D, Bonner LM, Cohen AN, et al. Informatics systems to promote improved care for chronic illness: a literature review. J Am Med Inform Assoc. 2007;14(2):156‐163. 10.1197/jamia.M2255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Perzynski AT, Roach MJ, Shick S, et al. Patient portals and broadband Internet inequality. J Am Med Inform Assoc. 2017;24(5):927‐932. 10.1093/jamia/ocx020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rodriguez JA, Lipsitz SR, Lyles CR, et al. Association between patient portal use and broadband access: a national evaluation. J Gen Intern Med. 2020;35(12):3719‐3720. 10.1007/s11606-020-05633-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dalal AK, Dykes P, Samal L, et al. Potential of an electronic health record‐integrated patient portal for improving care plan concordance during acute care. Appl Clin Inform. 2019;10(3):358‐366. 10.1055/s-0039-1688831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Heitkemper EM, Mamykina L, Travers J, et al. Do health information technology self‐management interventions improve glycemic control in medically underserved adults with diabetes? A systematic review and meta‐analysis. J Am Med Inform Assoc. 2017;24(5):1024‐1035. 10.1093/jamia/ocx025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wagner J, Hall JD, Ross RL, et al. Implementing risk stratification in primary care: challenges and strategies. J Am Board Fam Med. 2019;32(4):585‐595. 10.3122/jabfm.2019.04.180341 [DOI] [PubMed] [Google Scholar]
- 29. Bright TJ, Wong A, Dhurjati R, et al. Effect of clinical decision‐support systems: a systematic review. Ann Intern Med. 2012;157(1):29‐43. 10.7326/0003-4819-157-1-201207030-00450 [DOI] [PubMed] [Google Scholar]
- 30. Choi W, Wang S, Lee Y, et al. A systematic review of mobile health technologies to support self‐management of concurrent diabetes and hypertension. J Am Med Inform Assoc. 2020;27(6):939‐945. 10.1093/jamia/ocaa029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fraccaro P, Arguello Casteleiro M, Ainsworth J, et al. Adoption of clinical decision support in multimorbidity: a systematic review. JMIR Med Inform. 2015;3(1):e4. 10.2196/medinform.3503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jones SS, Rudin RS, Perry T, et al. Health information technology: an updated systematic review with a focus on meaningful use. Ann Intern Med. 2014;160(1):48‐54. 10.7326/M13-1531 [DOI] [PubMed] [Google Scholar]
- 33. Smith SM, Soubhi H, Fortin M, et al. Interventions for improving outcomes in patients with multimorbidity in primary care and community settings. Cochrane Database Syst Rev. 2012;4:CD006560. 10.1002/14651858.CD006560.pub2 [DOI] [PubMed] [Google Scholar]
- 34. Waschkau A, Wilfling D, Steinhauser J. Are big data analytics helpful in caring for multimorbid patients in general practice? A scoping review. BMC Fam Pract. 2019;20(1):37. 10.1186/s12875-019-0928-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bindoff I, Stafford A, Peterson G, et al. The potential for intelligent decision support systems to improve the quality and consistency of medication reviews. J Clin Pharm Ther. 2012;37(4):452‐458. 10.1111/j.1365-2710.2011.01327.x [DOI] [PubMed] [Google Scholar]
- 36. Hersh WR, Totten AM, Eden KB, et al. Outcomes from health information exchange: systematic review and future research needs. JMIR Med Inform. 2015;3(4):e39. 10.2196/medinform.5215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Leniz J, Weil A, Higginson IJ, et al. Electronic palliative care coordination systems (EPaCCS): a systematic review. BMJ Support Palliat Care. 2020;10(1):68‐78. 10.1136/bmjspcare-2018-001689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lion KC, Mangione‐Smith R, Britto MT. Individualized plans of care to improve outcomes among children and adults with chronic illness: a systematic review. Care Manag J. 2014;15(1):11‐25. 10.1891/1521-0987.15.1.11 [DOI] [PubMed] [Google Scholar]
- 39. Asch SM, Baker DW, Keesey JW, et al. Does the collaborative model improve care for chronic heart failure? Med Care. 2005;43(7):667‐675. 10.1097/01.mlr.0000167182.72251.a1 [DOI] [PubMed] [Google Scholar]
- 40. Wagner EH. Chronic disease management: what will it take to improve care for chronic illness? Eff Clin Pract. 1998;1(1):2‐4. [PubMed] [Google Scholar]
- 41. Wagner EH, Austin BT, Von Korff M. Organizing care for patients with chronic illness. Milbank Q. 1996;74(4):511‐544. [PubMed] [Google Scholar]
- 42. Wagner EH, Sandhu N, Newton KM, et al. Effect of improved glycemic control on health care costs and utilization. JAMA. 2001;285(2):182‐189. 10.1001/jama.285.2.182 [DOI] [PubMed] [Google Scholar]
- 43. Jones J, Ashford P, Asher D, et al. Guidelines for the specification, implementation and management of information technology systems in hospital transfusion laboratories. Transfus Med. 2014;24(6):341‐371. 10.1111/tme.12159 [DOI] [PubMed] [Google Scholar]
- 44. Dorr DA, Wilcox A, Burns L, et al. Implementing a multidisease chronic care model in primary care using people and technology. Dis Manag. 2006;9(1):1‐15. 10.1089/dis.2006.9.1 [DOI] [PubMed] [Google Scholar]
- 45. Young AS, Chaney E, Shoai R, et al. Information technology to support improved care for chronic illness. J Gen Intern Med. 2007;22(suppl 3):425‐430. 10.1007/s11606-007-0303-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Care Coordination Measures Atlas Update. Rockville, MD: Agency for Healthcare Research and Quality; 2014. [Google Scholar]
- 47. Blumenthal D, Abrams MK. Tailoring complex care management for high‐need, high‐cost patients. JAMA. 2016;316(16):1657‐1658. 10.1001/jama.2016.12388 [DOI] [PubMed] [Google Scholar]
- 48. O'Malley AS, Rich EC, Sarwar R, et al. How accountable care organizations use population segmentation to care for high‐need, high‐cost patients. Issue Brief (Commonw Fund). 2019;2019:1‐17. [PubMed] [Google Scholar]
- 49. Gliklich RE, Leavy MB, Karl J, et al. A framework for creating standardized outcome measures for patient registries. J Comp Eff Res. 2014;3(5):473‐480. 10.2217/cer.14.38 [DOI] [PubMed] [Google Scholar]
- 50. Singh K, Drouin K, Newmark LP, et al. Many mobile health apps target high‐need, high‐cost populations, but gaps remain. Health Aff. 2016;35(12):2310‐2318. 10.1377/hlthaff.2016.0578 [DOI] [PubMed] [Google Scholar]
- 51. Barbabella F, Melchiorre MG, Quattrini S, et al. European observatory policy briefs. In: Richardson E, van Ginneken E, eds. How can eHealth Improve Care for People with Multimorbidity in Europe?. Copenhagen, Denmark: European Observatory on Health Systems and Policies © NIVEL and TU Berlin; 2017. [PubMed] [Google Scholar]
- 52. Falconer E, Kho D, Docherty JP. Use of technology for care coordination initiatives for patients with mental health issues: a systematic literature review. Neuropsychiatr Dis Treat. 2018;14:2337‐2349. 10.2147/NDT.S172810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lewis J, Ray P, Liaw ST. Recent worldwide developments in eHealth and mHealth to more effectively manage cancer and other chronic diseases – a systematic review. Yearb Med Inform. 2016;25(1):93‐108. 10.15265/IY-2016-020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Canada HCo . Self‐Management Support for Canadians with Chronic Health Conditions: A Focus for Primary Health Care. Toronto, Canada: Health Council of Canada; 2012. [Google Scholar]
- 55. Conway A, Inglis SC, Clark RA. Effective technologies for noninvasive remote monitoring in heart failure. Telemed J E Health. 2014;20(6):531‐538. 10.1089/tmj.2013.0267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jeminiwa R, Hohmann L, Qian J, et al. Impact of eHealth on medication adherence among patients with asthma: a systematic review and meta‐analysis. Respir Med. 2019;149:59‐68. 10.1016/j.rmed.2019.02.011 [DOI] [PubMed] [Google Scholar]
- 57. Shaw T, McGregor D, Brunner M, et al. What is eHealth (6)? Development of a conceptual model for eHealth: qualitative study with key informants. J Med Internet Res. 2017;19(10):e324. 10.2196/jmir.8106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Boers SN, Jongsma KR, Lucivero F, et al. SERIES: eHealth in primary care. Part 2: exploring the ethical implications of its application in primary care practice. Eur J Gen Pract. 2020;26(1):26‐32. 10.1080/13814788.2019.1678958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Carey M, Noble N, Mansfield E, et al. The role of eHealth in optimizing preventive care in the primary care setting. J Med Internet Res. 2015;17(5):e126. 10.2196/jmir.3817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hou C, Carter B, Hewitt J, et al. Do mobile phone applications improve glycemic control (HbA1c) in the self‐management of diabetes? A systematic review, meta‐analysis, and GRADE of 14 randomized trials. Diabetes Care. 2016;39(11):2089‐2095. 10.2337/dc16-0346 [DOI] [PubMed] [Google Scholar]
- 61. Samal L, Dykes PC, Greenberg JO, et al. Care coordination gaps due to lack of interoperability in the United States: a qualitative study and literature review. BMC Health Serv Res. 2016;16:143. 10.1186/s12913-016-1373-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bates DW, Samal L. Interoperability: what is it, how can we make it work for clinicians, and how should we measure it in the future? Health Serv Res. 2018;53(5):3270‐3277. 10.1111/1475-6773.12852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Dorr DA. Managing and coordinating health care: creating collaborative, proactive systems—David A. Dorr. In: Council NR , ed. Frontiers of Engineering: Reports on Leading‐Edge Engineering from the 2009 Symposium. Washington, DC: The National Academies Press; 2010. [Google Scholar]
- 64. Allard JP, Keller H, Jeejeebhoy KN, et al. Decline in nutritional status is associated with prolonged length of stay in hospitalized patients admitted for 7 days or more: a prospective cohort study. Clin Nutr. 2016;35(1):144‐152. 10.1016/j.clnu.2015.01.009 [DOI] [PubMed] [Google Scholar]
- 65. Barrett M, Boyne J, Brandts J, et al. Artificial intelligence supported patient self‐care in chronic heart failure: a paradigm shift from reactive to predictive, preventive and personalised care. EPMA J. 2019;10(4):445‐464. 10.1007/s13167-019-00188-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Labovitz DL, Shafner L, Reyes Gil M, et al. Using artificial intelligence to reduce the risk of nonadherence in patients on anticoagulation therapy. Stroke. 2017;48(5):1416‐1419. 10.1161/STROKEAHA.116.016281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Symum H, Zayas‐Castro JL. Prediction of chronic disease‐related inpatient prolonged length of stay using machine learning algorithms. Healthc Inform Res. 2020;26(1):20‐33. 10.4258/hir.2020.26.1.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA‐ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467‐473. 10.7326/M18-0850 [DOI] [PubMed] [Google Scholar]
- 69. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264‐269, W64. 10.7326/0003-4819-151-4-200908180-00135 [DOI] [PubMed] [Google Scholar]
- 70. Druss BG, Li J, Tapscott S, et al. Randomized trial of a mobile personal health record for behavioral health homes. Psychiatr Serv. 2020;71(8):803‐809. 10.1176/appi.ps.201900381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Walker PP, Pompilio PP, Zanaboni P, et al. Telemonitoring in chronic obstructive pulmonary disease (CHROMED). a randomized clinical trial. Am J Respir Crit Care Med. 2018;198(5):620‐628. 10.1164/rccm.201712-2404OC [DOI] [PubMed] [Google Scholar]
- 72. Druss BG, Ji X, Glick G, et al. Randomized trial of an electronic personal health record for patients with serious mental illnesses. Am J Psychiatry. 2014;171(3):360‐368. 10.1176/appi.ajp.2013.13070913 [DOI] [PubMed] [Google Scholar]
- 73. Gellis ZD, Kenaley BL, Ten Have T. Integrated telehealth care for chronic illness and depression in geriatric home care patients: the integrated Telehealth education and activation of mood (I‐TEAM) study. J Am Geriatr Soc. 2014;62(5):889‐895. 10.1111/jgs.12776 [DOI] [PubMed] [Google Scholar]
- 74. Pecina JL, Hanson GJ, Van Houten H, et al. Impact of telemonitoring on older adults health‐related quality of life: the Tele‐ERA study. Qual Life Res. 2013;22(9):2315‐2321. 10.1007/s11136-013-0361-5 [DOI] [PubMed] [Google Scholar]
- 75. Logan AG, Irvine MJ, McIsaac WJ, et al. Effect of home blood pressure telemonitoring with self‐care support on uncontrolled systolic hypertension in diabetics. Hypertension. 2012;60(1):51‐57. 10.1161/HYPERTENSIONAHA.111.188409 [DOI] [PubMed] [Google Scholar]
- 76. Takahashi PY, Pecina JL, Upatising B, et al. A randomized controlled trial of telemonitoring in older adults with multiple health issues to prevent hospitalizations and emergency department visits. Arch Intern Med. 2012;172(10):773‐779. 10.1001/archinternmed.2012.256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Steele Gray C, Gravesande J, Hans PK, et al. Using exploratory trials to identify relevant contexts and mechanisms in complex electronic health interventions: evaluating the electronic patient‐reported outcome tool. JMIR Form Res. 2019;3(1):e11950. 10.2196/11950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Easton K, Potter S, Bec R, et al. A virtual agent to support individuals living with physical and mental comorbidities: co‐design and acceptability testing. J Med Internet Res. 2019;21(5):e12996. 10.2196/12996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Portz JD, Bayliss EA, Bull S, et al. Using the technology acceptance model to explore user experience, intent to use, and use behavior of a patient portal among older adults with multiple chronic conditions: descriptive qualitative study. J Med Internet Res. 2019;21(4):e11604. 10.2196/11604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Portz JD, Fruhauf C, Bull S, et al. “Call a Teenager… That's What I Do!” – Grandchildren help older adults use new technologies: qualitative study. JMIR Aging 2019;2(1):e13713. 10.2196/13713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Hans PK, Gray CS, Gill A, et al. The provider perspective: investigating the effect of the electronic patient‐reported outcome (ePRO) mobile application and portal on primary care provider workflow. Prim Health Care Res Dev. 2018;19(2):151‐164. 10.1017/S1463423617000573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Irfan Khan A, Gill A, Cott C, et al. mHealth tools for the self‐management of patients with multimorbidity in primary care settings: pilot study to explore user experience. JMIR Mhealth Uhealth. 2018;6(8):e171. 10.2196/mhealth.8593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Middlemass JB, Vos J, Siriwardena AN. Perceptions on use of home telemonitoring in patients with long term conditions – concordance with the health information technology acceptance model: a qualitative collective case study. BMC Med Inform Decis Mak. 2017;17(1):89. 10.1186/s12911-017-0486-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Steele Gray C, Gill A, Irfan Khan A, et al. The electronic patient reported outcome tool: testing usability and feasibility of a mobile app and portal to support care for patients with complex chronic disease and disability in primary care settings. JMIR Mhealth Uhealth. 2016;4(2):e58. 10.2196/mhealth.5331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Pecina JL, Vickers KS, Finnie DM, et al. Telemonitoring increases patient awareness of health and prompts health‐related action: initial evaluation of the TELE‐ERA study. Telemed J E Health. 2011;17(6):461‐466. 10.1089/tmj.2010.0213 [DOI] [PubMed] [Google Scholar]
- 86. Smith RL 2nd, Herbert MA, Dewey TM, et al. Does body mass index affect outcomes for aortic valve replacement surgery for aortic stenosis? Ann Thorac Surg. 2012;93(3):742‐746; discussion 46–7. 10.1016/j.athoracsur.2011.11.027 [DOI] [PubMed] [Google Scholar]
- 87. Powell KR, Deroche C. Predictors and patterns of portal use in patients with multiple chronic conditions. Chronic Illn. 2020;16(4):275‐283. 10.1177/1742395318803663 [DOI] [PubMed] [Google Scholar]
- 88. Salisbury C, Man MS, Bower P, et al. Management of multimorbidity using a patient‐centred care model: a pragmatic cluster‐randomised trial of the 3D approach. Lancet. 2018;392(10141):41‐50. 10.1016/S0140-6736(18)31308-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Kersting C, Weltermann B. Evaluating the feasibility of a software prototype supporting the management of multimorbid seniors: mixed methods study in general practices. JMIR Hum Factors. 2019;6(3):e12695. 10.2196/12695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Laleci Erturkmen GB, Yuksel M, Sarigul B, et al. A collaborative platform for management of chronic diseases via guideline‐driven individualized care plans. Comput Struct Biotechnol J. 2019;17:869‐885. 10.1016/j.csbj.2019.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Mann C, Shaw ARG, Guthrie B, et al. Can implementation failure or intervention failure explain the result of the 3D multimorbidity trial in general practice: mixed‐methods process evaluation. BMJ Open. 2019;9(11):e031438. 10.1136/bmjopen-2019-031438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Mann C, Shaw A, Wye L, et al. A computer template to enhance patient‐centredness in multimorbidity reviews: a qualitative evaluation in primary care. Br J Gen Pract. 2018;68(672):e495‐e504. 10.3399/bjgp18X696353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. de Jong CC, Ros WJG, van Leeuwen M, et al. How professionals share an E‐care plan for the elderly in primary care: evaluating the use of an E‐communication tool by different combinations of professionals. J Med Internet Res. 2016;18(11):e304. 10.2196/jmir.6332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Makai P, Perry M, Robben SH, et al. Evaluation of an eHealth intervention in chronic care for frail older people: why adherence is the first target. J Med Internet Res. 2014;16(6):e156. 10.2196/jmir.3057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Martinez‐Garcia A, Moreno‐Conde A, Jodar‐Sanchez F, et al. Sharing clinical decisions for multimorbidity case management using social network and open‐source tools. J Biomed Inform. 2013;46(6):977‐984. 10.1016/j.jbi.2013.06.007 [DOI] [PubMed] [Google Scholar]
- 96. Abidi S. A knowledge‐modeling approach to integrate multiple clinical practice guidelines to provide evidence‐based clinical decision support for managing comorbid conditions. J Med Syst. 2017;41(12):193. 10.1007/s10916-017-0841-1 [DOI] [PubMed] [Google Scholar]
- 97. Laleci Erturkmen GB, Yuksel M, Sarigul B, et al. Personalised care plan management utilizing guideline‐driven clinical decision support systems. Stud Health Technol Inform. 2018;247:750‐754. [PubMed] [Google Scholar]
- 98. Morales‐Asencio JM, Kaknani‐Uttumchandani S, Cuevas‐Fernandez‐Gallego M, et al. Development of the Andalusian registry of patients receiving community case management, for the follow‐up of people with complex chronic diseases. J Eval Clin Pract. 2015;21(5):861‐872. 10.1111/jep.12392 [DOI] [PubMed] [Google Scholar]
- 99. Riano D, Real F, Lopez‐Vallverdu JA, et al. An ontology‐based personalization of health‐care knowledge to support clinical decisions for chronically ill patients. J Biomed Inform. 2012;45(3):429‐446. 10.1016/j.jbi.2011.12.008 [DOI] [PubMed] [Google Scholar]
- 100. Stead WW, Gregg WM, Jirjis JN. Extending closed‐loop control to the management of chronic disease. Trans Am Clin Climatol Assoc. 2011;122:93‐102. [PMC free article] [PubMed] [Google Scholar]
- 101. Prabhakaran D, Jha D, Prieto‐Merino D, et al. Effectiveness of an mHealth‐based electronic decision support system for integrated management of chronic conditions in primary care: the mWellcare cluster‐randomized controlled trial. Circulation. 2018. 10.1161/CIRCULATIONAHA.118.038192 [DOI] [PubMed] [Google Scholar]
- 102. Dovgan E, Gradisek A, Lustrek M, et al. Using machine learning models to predict the initiation of renal replacement therapy among chronic kidney disease patients. PLoS One. 2020;15(6):e0233976. 10.1371/journal.pone.0233976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Pajewski NM, Lenoir K, Wells BJ, et al. Frailty screening using the electronic health record within a Medicare accountable care organization. J Gerontol A Biol Sci Med Sci. 2019;74(11):1771‐1777. 10.1093/gerona/glz017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Chen Y, Kho AN, Liebovitz D, et al. Learning bundled care opportunities from electronic medical records. J Biomed Inform. 2018;77:1‐10. 10.1016/j.jbi.2017.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Magnan EM, Bolt DM, Greenlee RT, et al. Stratifying patients with diabetes into clinically relevant groups by combination of chronic conditions to identify gaps in quality of care. Health Serv Res. 2018;53(1):450‐468. 10.1111/1475-6773.12607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Op den Buijs J, Simons M, Golas S, et al. Predictive modeling of 30‐day emergency hospital transport of patients using a personal emergency response system: prognostic retrospective study. JMIR Med Inform. 2018;6(4):e49. 10.2196/medinform.9907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Satchidanand N, Servoss TJ, Singh R, et al. Development of a risk tool to support discussions of care for older adults admitted to the ICU with pneumonia. Am J Hosp Palliat Care. 2018;35(9):1201‐1206. 10.1177/1049909118764093 [DOI] [PubMed] [Google Scholar]
- 108. Duenk RG, Verhagen C, Bronkhorst EM, et al. Development of the ProPal‐COPD tool to identify patients with COPD for proactive palliative care. Int J Chron Obstruct Pulmon Dis. 2017;12:2121‐2128. 10.2147/COPD.S140037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Alemi F, Levy CR, Kheirbek RE. The multimorbidity index: a tool for assessing the prognosis of patients from their history of illness. EGEMS. 2016;4(1):1235. 10.13063/2327-9214.1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Robusto F, Lepore V, D'Ettorre A, et al. The drug derived complexity index (DDCI) predicts mortality, unplanned hospitalization and hospital readmissions at the population level. PLoS One. 2016;11(2):e0149203. 10.1371/journal.pone.0149203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Hammond KW, Ben‐Ari AY, Laundry RJ, et al. The feasibility of using large‐scale text mining to detect adverse childhood experiences in a VA‐treated population. J Trauma Stress. 2015;28(6):505‐514. 10.1002/jts.22058 [DOI] [PubMed] [Google Scholar]
- 112. Dong YH, Chang CH, Shau WY, et al. Development and validation of a pharmacy‐based comorbidity measure in a population‐based automated health care database. Pharmacotherapy. 2013;33(2):126‐136. 10.1002/phar.1176 [DOI] [PubMed] [Google Scholar]
- 113. Crane SJ, Tung EE, Hanson GJ, et al. Use of an electronic administrative database to identify older community dwelling adults at high‐risk for hospitalization or emergency department visits: the elders risk assessment index. BMC Health Serv Res. 2010;10:338. 10.1186/1472-6963-10-338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Vitry AI, Roughead EE, Preiss AK, et al. Influence of comorbidities on therapeutic progression of diabetes treatment in Australian veterans: a cohort study. PLoS One. 2010;5(11):e14024. 10.1371/journal.pone.0014024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Winocour PH, Moore‐Haines K, Sullivan K, et al. Holistic review of people with diabetes and chronic kidney disease reveals important multimorbidity and unmet clinical need: the ENHIDE diabetes renal telehealth pilot study. Clin Med. 2020;20(2):133–138. 10.7861/clinmed.2019-0418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Jafarpour B, Raza Abidi S, Van Woensel W, et al. Execution‐time integration of clinical practice guidelines to provide decision support for comorbid conditions. Artif Intell Med. 2019;94:117‐137. 10.1016/j.artmed.2019.02.003 [DOI] [PubMed] [Google Scholar]
- 117. Rieckert A, Teichmann AL, Drewelow E, et al. Reduction of inappropriate medication in older populations by electronic decision support (the PRIMA‐eDS project): a survey of general practitioners' experiences. J Am Med Inform Assoc. 2019;26(11):1323‐1332. 10.1093/jamia/ocz104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Bottiger Y, Laine K, Korhonen T, et al. Development and pilot testing of PHARAO‐a decision support system for pharmacological risk assessment in the elderly. Eur J Clin Pharmacol. 2018;74(3):365‐371. 10.1007/s00228-017-2391-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Seroussi B, Galopin A, Gaouar M, et al. Using therapeutic circles to visualize guideline‐based therapeutic recommendations for patients with multiple chronic conditions: a case study with GO‐DSS on hypertension, type 2 diabetes, and dyslipidemia. Stud Health Technol Inform. 2017;245:1148‐1152. [PubMed] [Google Scholar]
- 120. Crowley EK, Sallevelt B, Huibers CJA, et al. Intervention protocol: OPtimising thERapy to prevent avoidable hospital admission in the multi‐morbid elderly (OPERAM): a structured medication review with support of a computerised decision support system. BMC Health Serv Res. 2020;20(1):220. 10.1186/s12913-020-5056-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Gianfrancesco MA, Tamang S, Yazdany J, et al. Potential biases in machine learning algorithms using electronic health record data. JAMA Intern Med. 2018;178(11):1544‐1547. 10.1001/jamainternmed.2018.3763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Lucivero F, Jongsma KR. A mobile revolution for healthcare? Setting the agenda for bioethics. J Med Ethics. 2018;44(10):685‐689. 10.1136/medethics-2017-104741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Obermeyer Z, Powers B, Vogeli C, et al. Dissecting racial bias in an algorithm used to manage the health of populations. Science. 2019;366(6464):447‐453. 10.1126/science.aax2342 [DOI] [PubMed] [Google Scholar]
- 124. Improving Diagnosis in Health Care. Washington (DC): National Academies Press; 2015. [PubMed] [Google Scholar]
- 125. Benjamin R. Assessing risk, automating racism. Science. 2019;366(6464):421‐422. 10.1126/science.aaz3873 [DOI] [PubMed] [Google Scholar]
- 126. Powe NR. Black kidney function matters: use or misuse of race? JAMA. 2020;324(8):737‐738. 10.1001/jama.2020.13378 [DOI] [PubMed] [Google Scholar]
- 127. Vyas DA, Eisenstein LG, Jones DS. Hidden in plain sight – reconsidering the use of race correction in clinical algorithms. N Engl J Med. 2020;383(9):874‐882. 10.1056/NEJMms2004740 [DOI] [PubMed] [Google Scholar]
- 128. Olsen LA, Aisner D, McGinnis JM, eds. The Learning Healthcare System: Workshop Summary. Washington (DC): National Academies Press (US); 2007. [PubMed] [Google Scholar]
- 129. Montori VM, Hargraves I, McNellis RJ, et al. The care and learn model: a practice and research model for improving healthcare quality and outcomes. J Gen Intern Med. 2019;34(1):154‐158. 10.1007/s11606-018-4737-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Liao JM, Navathe AS, Werner RM. The impact of Medicare's alternative payment models on the value of care. Annu Rev Public Health. 2020;41:551‐565. 10.1146/annurev-publhealth-040119-094327 [DOI] [PubMed] [Google Scholar]
- 131. Williams MD, Asiedu GB, Finnie D, et al. Sustainable care coordination: a qualitative study of primary care provider, administrator, and insurer perspectives. BMC Health Serv Res. 2019;19(1):92. 10.1186/s12913-019-3916-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Zhu JM, Patel V, Shea JA, et al. Hospitals using bundled payment report reducing skilled nursing facility use and improving care integration. Health Aff. 2018;37(8):1282‐1289. 10.1377/hlthaff.2018.0257 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting information


