Abstract
Plant derived cysteine proteinases (CPs) have long been known to possess anthelmintic properties but have attracted renewed attention recently because of the acute need to discover novel methods for controlling helminth infections as a result of increasing drug resistance. However, surprisingly little is known about the stability of these proteins under typical storage and in vivo exposure conditions. We found that CPs in a supernatant preparation from papaya latex (PLS) were stable during the initial refinement process and when stored under low temperatures, but lost activity during dialysis and within 7 days of storage when kept at ambient temperature (18–20 °C). The enzyme activity in PLS was not affected by repeated freeze-thaw cycles and was also stable under typical in vitro assay conditions at 37 °C used for quantifying effects on helminths. Active enzyme activity was still detectable in the colon 3–4 h after oral administration in rodent models.
Keywords: Papaya latex supernatant, Cysteine proteinase, Anthelmintic, Enzyme activity
Papaya latex supernatant; Cysteine proteinase; Anthelmintic; Enzyme activity.
1. Introduction
Extracts of papaya latex, containing cysteine proteinases (CPs), have been shown to have potent anthelmintic properties against intestinal nematodes in vitro, and following oral administration have been reported to significantly reduce nematode parasite egg output and worm burdens in monogastric animals such as rodents (Stepek et al., 2006, 2007a, b), pigs (Satrija et al., 1994), Levecke et al., (2014) humans (Hansson et al., 1986) and even poultry (Mursof and He, 1991), as well multi-gastric animals, ruminants e.g. sheep (Buttle et al., 2011). In re-assessing the anthelmintic properties of papaya latex Stepek et al. (2007b) utilised crude papaya latex but more recently a refined preparation has been developed (papaya latex supernatant, PLS). However, CPs are proteins and if they are to be exploited as novel anthelmintics on a wide scale, a comprehensive understanding of their biological properties is required. Since many proteins are thermo-specific and heat labile (Somero, 1995), information on the stability of CPs during extraction and purification from the plant sources, as well as during storage, is crucial if they are to be employed successfully as anthelmintics and are to have a commercial future.
Because of their commercial value the thermostability profile of papaya latex proteases has been reported previously and the proteins have been found to be heat labile (Chaiwut et al., 2007; Hinkel, 1951; Ortiz et al., 1980), but there are no published data on stability during long-term storage nor during incubation at 37 °C such as might be required for assessment of effects on live parasites in vitro. CPs have been shown to survive stomach acidity and reach the colon with retained activity (Stepek et al., 2007a), where they are known to have a marked effect on Trichuris muris (Stepek et al., 2006) and T. suis (Levecke et al., 2014). However, some studies have reported that papain undergoes irreversible damage when subjected to low pH such as would be experienced during passage through the stomach (Huet et al., 2006). In this paper we have reassessed the stability and viability of active enzyme activity attributable to cysteine proteinases in PLS, after storage at various temperatures over a range of periods of time, and following several cycles of freeze-thawing, as well as during incubation under typical in vitro culture conditions and after administration to mice and rats as used in earlier trials of efficacy.
2. Materials and methods
2.1. Enzyme preparation
The enzymes used in this study were cysteine proteinases [chymopapain, glycyl endopeptidase (papaya proteinase IV), caricain and papain – in order of their abundance (Buttle et al., 1990a) found in Carica papaya latex and extracted as a soluble solution from papaya latex (PLS). PLS was prepared by dissolving 4 kg of Carica papaya spray-dried latex purchased from Enzymase (Brussels, Belgium) in 12 L of water. The preparation was centrifuged at 17,700 x g at 4 °C (Beckman model J2-21 centrifuge Rotor, UK) and the pellet was discarded. To facilitate freeze-drying, the supernatant was concentrated to a third of its original volume by placing it in dialysis tubing with MW cut-off 3,500 (SpectraPor 45 mm diam.) over polyethylene glycol 20,000 at 4 °C. Most of the concentrated PLS was freeze-dried using a SB4 Freeze-drier Chemlab, England while the remaining concentrated stock was aliquoted into individual vials and stored at -80 °C.
2.2. Papaya latex stability during refinement
The stability of CPs during the refinement process was investigated by determining enzyme activity at each step of the purification. The amount of active CPs present in a 10μl volume of assay material was determined at each step of the preparation by an active site titration assay adapted from Zucker et al. (1985). This measured hydrolysis of the synthetic substrate benzoyl-arginyl-p-nitroanilide (BAPNA) (Bachem Ltd, UK) against the CP inhibitor, trans-epoxysuccinyl-L-leucylamido-(4-guanidino) butane (E-64) (Apollo Scientific Ltd, UK) with 4mM L-cysteine as the reducing agent. The substrate is susceptible to the action of chymopapain, caricain and papain, and the inhibitor effectively inactivates all four of the CPs (Zucker et al., 1985; Buttle et al., 1990b).
2.3. Stability of freeze-dried papaya latex
Two batches of freeze-dried PLS preparations were used in this experiment. The first (Batch 1) was kept at ambient temperature (18–20 °C) while the second (Batch 2) was kept at 4 °C. At time points of 1 week, 1 month, 6 months and 1 year, a representative sample was assessed for enzyme activity. The amount of active CPs present in 10μl volume of assay material was assessed by active-site titration as described above.
2.4. Papaya latex stability during in vitro and in vivo experiments
2.4.1. In vitro experiment
PLS from batch 1 kept at ambient temperature was used in this experiment. The stability of PLS during a 2 h in vitro experiment at 37 °C, pH 7.2–7.4, such as might be employed for example to assess its effects on parasitic worms, was investigated. The CP activity present in a 10μl volume of assay material was determined by active-site titration at the start of incubation and after 15 min, 60 min and 120 min.
2.4.2. In vivo experiment
In earlier work CP activity was detectable in the mouse GI tract even 2–3hrs after oral administration of crude papaya latex (Stepek et al., 2007a). We determined whether PLS would show comparable persistence in the intestinal tract. All the procedures implemented in this work complied with the ethical standards of the national guides on the care and use of laboratory animals in the UK [The Animals (Scientific Procedures) Act 1986] and were locally approved by the Animal Welfare and Ethical Review Body of the University of Nottingham. Importantly all work was conducted within a recognized culture of care and compliance to meet the expectations of both the university and the UK Home Office Inspectorate. All animal procedures were carried out under UK Home Office licence numbers 40/2942 and 40/3138. Six C3H Mice were purchased from Harlan, UK. Each mouse was treated with a single dose (240 nmols, 0.2ml) of PLS (from Batch 1) at 0 min. One mouse was killed after each of 10 min, 20 min, 60 min, 90 min, 120 min and 180 min and dissected to remove the entire GI tract, which was then washed in PBS. The GI tract was divided into 4 segments; stomach, upper intestine, lower intestine (the small intestine was divided into 2 equal length portions), and the colon (the caecum was not included). Each of the segments was opened and its contents washed in 5 mls of PBS before being filtered. The enzyme activity of each segment was then determined as above. Naturally occurring trypsin in the mammalian GI tract also cleaves the Bz-Arg-pNA substrate therefore E-64 was used to block PLS activity, the residual enzyme activity being due to trypsin. PLS activity was determined by subtracting trypsin activity from total activity. The concentration of enzyme activity was calculated using a molar extinction coefficient of 8800 M−1cm−1(Mole and Horton, 1973) and expressed in units of enzyme activity per ml. In a separate experiment 6 Wistar rats were purchased from Charles River, UK. Each rat was treated with a single dose of PLS (Batch 1) with 2.40 μmols, 2ml of enzyme at 0 min. 1 rat was killed after each of 10 min, 20 min, 60 min, 90 min, 120 min and 180 min and the enzyme activity in the GI tract was determined as described above for the mouse.
2.4.3. PLS stability during storage
Dialysis was found to produce large reductions in the activity of the PLS so was omitted in these experiments comparing different methods for storage. PLS was prepared as described above (without dialysis) and was aliquoted into individual vials before storage at a mean ambient temperature (18–20 °C), 4 °C, -20 °C and -80 °C. At specific time points of 1 week, 1 month, 6 months and 1 year a representative sample was assessed for enzyme activity. The amount of active CPs present in a 10μl volume of assay material was assessed by active-site titration as described earlier.
2.4.4. Papaya latex stability during freeze-thawing
Frozen PLS (−80 °C) was thawed and the amount of active CP present in a 10μl volume of assay material was assessed by active-site titration as described earlier. The same sample was re-refrozen at -80 °C and left for 28 days before repeating the same freeze-thaw cycle four more times, followed on each occasion by determination of retained activity by active-site titration. Each cycle was performed monthly for five months.
3. Results
3.1. The stability of papaya latex during refinement and storage
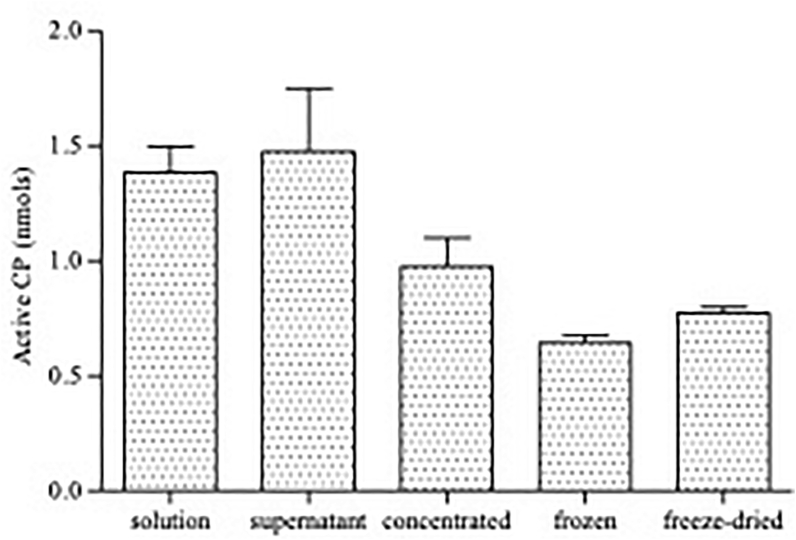
During centrifugation of papaya latex and separation of the supernatant solution, there was no loss of active CP, the crude papaya latex suspension and the supernatant showing comparable enzyme activity, when tested by active site titration. However, during the process of concentration through dialysis which reduced the starting volume by two thirds, there was 34% loss of active CP (Figure 1, comparing 10μl volumes of supernatant with 10μl dialysed supernatant). There was also further loss of active CP (34%) when the concentrated PLS was frozen at -80 °C and thawed for assessment of enzyme activity (Figure 1). During freeze-drying however, the amount of active enzyme activity actually increased by 20.2% relative to the frozen material (Figure 1).
Figure 1.
Amount of CP enzyme activity at different stages of the processing and refinement of papaya latex from the original suspension to the final freeze-dried product. The solution was made by reconstitution of spray dried papaya latex with water at a ratio of 1:3 and was then centrifuged at 17,700 g at 4 °C to obtain the supernatant. Papaya latex supernatant (PLS) was then dialysed against polyethylene glycol to concentrate active CPs. The concentrated PLS was then frozen at-80 °C and frozen PLS was freeze-dried. Each point on the graph represents the amount of active CP present in 10μl. Error bars represent the standard errors of the means.
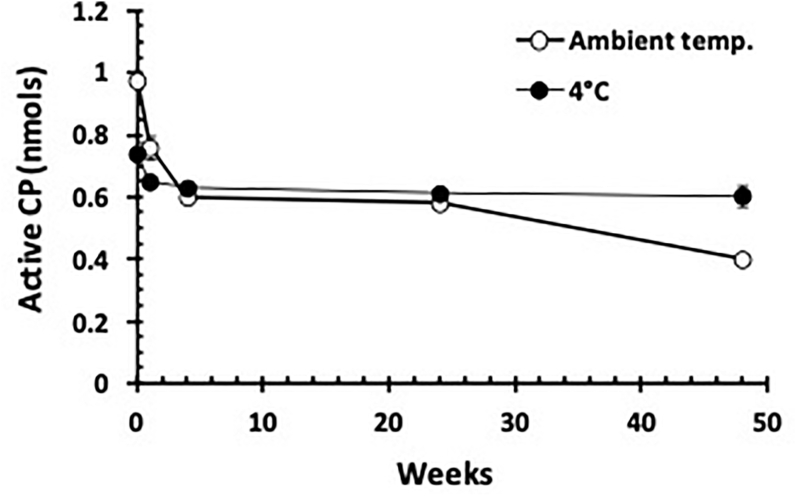
The process of freeze-drying alone caused loss of active enzyme by 10–15% (Figure 2). After one week the amount of active enzyme in the freeze-dried PLS stored at a ambient temperature (18–20 °C) deteriorated further by about 30% from the original level after which it stabilized (Figure 2). After one year of storage at ambient temperature, the amount of active enzymes retained was just 50% of the original level (Figure 2). When freeze-dried PLS was stored at 4 °C however, apart from the initial loss following the freeze-drying process, the amount of active enzymes present was relatively preserved with just 15% loss from the original level for up to one year (Figure 2). In fact there was no loss of active CP even after 2 years of storage at 4 °C (results not shown).
Figure 2.
Amount of CP enzyme activity present in freeze-dried PLS stored at room temperature or 4 °C assayed at different time points. Each point on the graph represents the amount of active CP present in 10μl. Error bars represent the standard errors of the means.
3.2. The thermostability of PLS during in vitro incubation experiments
PLS activity was relatively stable throughout a period of 120 min at 37 °C as used in typical in vitro motility assays diluted in Hank's Balanced Salt Solution, pH 7.2–7.4 (Stepek et al., 2005; Stepek et al., 2006, 2007a, c). There was no obvious loss in the amount of active CP throughout the whole experiment. PLS activity was 0.68 μmol at 0 min, 0.75 μmol at 15 min, 0.77 μmol at 60 min and 0.73 μmol at 120 min.
3.3. The stability of PLS activity in the intestine of treated rodents
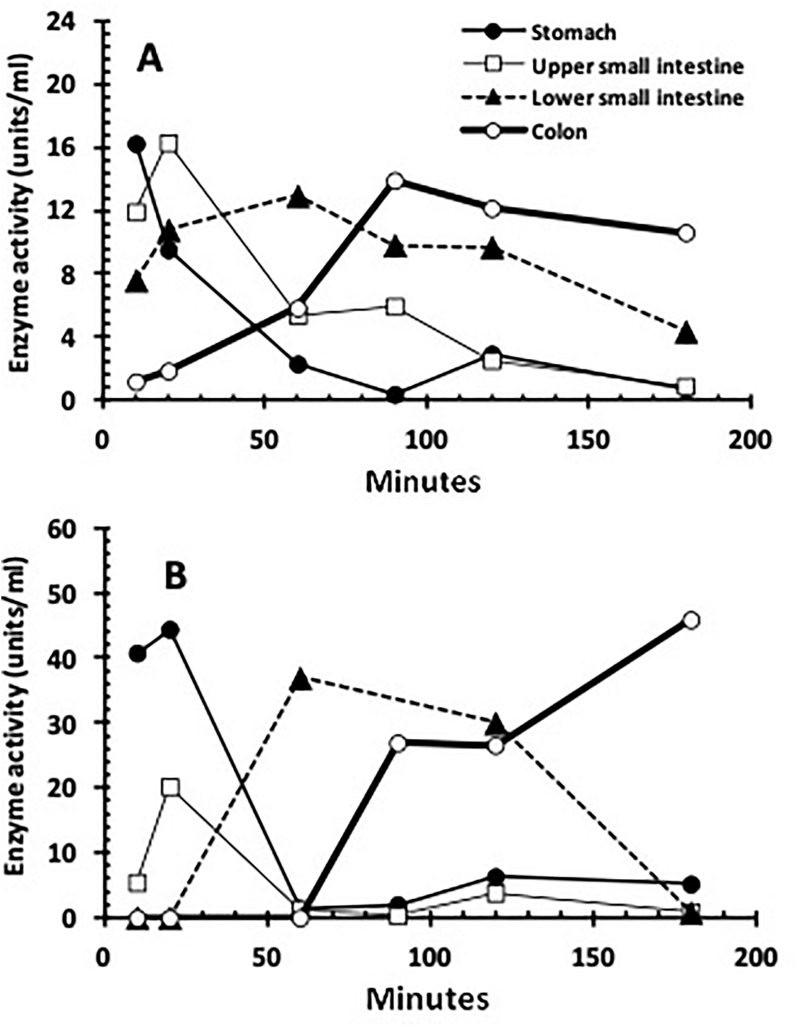
In the mouse GI tract, PLS activity in the stomach and upper small intestine declined rapidly during the first 90 min of treatment, after which only negligible amounts persisted. In contrast, enzyme activity in the lower small intestine and the colon slowly increased during the first 90 min after which it was relatively stable until the end of the experiment at 3 h (Figure 3A). Similarly, in the rat GI tract PLS activity in the stomach and upper small intestine declined rapidly within 60 min, after which only negligible amounts persisted and in contrast, enzyme activity in the colon only rose after 90 min and was well maintained until the end of the experiment at 3 h (Figure 3B).
Figure 3.
(A) The enzyme activity (units/ml) of PLS throughout the GI tract of C3H mice over time. (B) The enzyme activity (units/ml) of PLS throughout the GI tract of Wistar rats over time. In both cases the unit of activity was defined as that which produced 1 nmol min−1 of product. Trend lines were fitted to guide the eye.
3.4. The thermostability of PLS
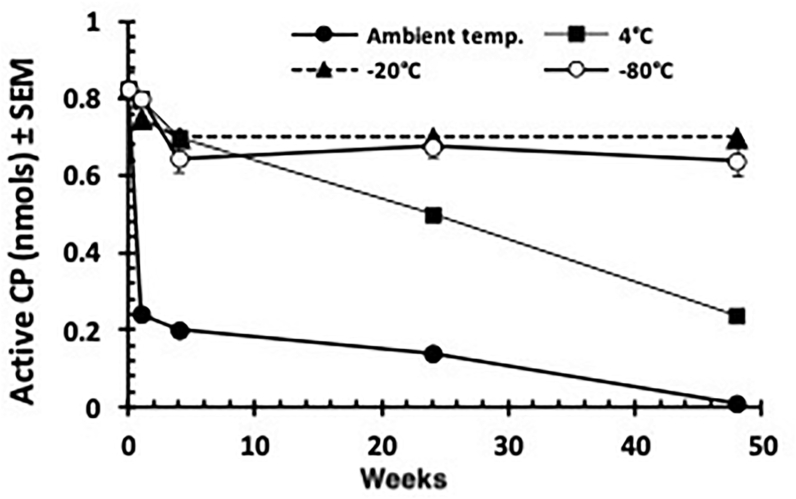
Undialysed PLS was stored at ambient temperature (18–20 °C), at 4 °C, -20 °C and -80 °C. The original amount of active CP in 10μl volume was 0.825 nmols. After one week of storage at ambient temperature this amount declined by around 70% (Figure 4) from its original value, after which a more gradual loss of active enzymes was observed, until after 1 year of storage only about 1% (Figure 4) of the original amount of active CPs remained. When stored in the refrigerator at 4 °C the rate of loss was more gradual with an initial loss of 3% after 1 week, followed by 15% after 1 month and 39% after 6 months. After 1 year of storage at 4 °C, the amount of active CPs was reduced by 71% from its original value. When PLS was frozen at -20 °C the loss was minimal, with an initial loss of only 9%, followed a month later by further loss of 15% and this stabilised with no further loss for up to 1 year of storage. Similarly when PLS was deep frozen at -80 °C the amount of active CP stabilized at around 80% of the original starting value and stayed at this level without further loss for up to 1 year post-processing.
Figure 4.
Amount of CP enzyme activity in PLS stored at different temperatures and assayed at different time points. Each point on the graph represents the amount of active CP present in 10μl. Error bars represent the standard errors of the means.
PLS when prepared in large volume and then frozen (to save space) rather than aliquoted into small amounts for single use will need to undergo repeated freeze-thawing warranting investigation into their activity. When frozen undialysed PLS was subjected to repeated freeze-thaw cycles, apart from the initial loss of active CPs following the first freezing, there was no further loss of active CPs even after five freeze-thaw cycles. The activity before the first cycle of freeze–thawing was 0.825 μmol, SEM ± 0.435 μmol. After the first cycle PLS activity was 0.66 μmol, SEM ± 0.175 μmol. After the second cycle PLS activity was 0.63 μmol (based on a single record). After the third cycle, PLS activity was 0.64 μmol (single value). After the fourth cycle, PLS value was 0.65 μmol, SEM ± 1.68 μmol. After the last fifth cycle, PLS activity was 0.65 μmol (single value).
4. Discussion
In earlier studies investigating the anthelmintic properties of papaya latex, a crude preparation of the latex was used. Here, the preparation of papaya latex was taken a step further by centrifugation and concentration of the soluble fraction which could be administered orally to animals more efficiently. The initial steps used, based on centrifugation, were shown to retain enzyme activity whilst removing the major contaminants present in the latex, particularly the less soluble materials. However, this approach was not entirely new since in fact an earlier version of PLS was shown to be more active than its precursor form by Frankel et al. (1937), although subsequent workers did not exploit this preparation in anthelmintic trials. Concentration by dialysis over ethylene glycol was carried out for logistical reasons (since it is easier to transport and store a small volume of concentrated stock and later dilute to final desired concentrations rather than vice versa) and to facilitate freeze-drying. This process however resulted in a 33.9% loss of active CPs per unit volume as well as considerable reduction in volume, together indicating a significant loss of enzyme activity. On this basis dialysis of PLS is not recommended for the future. The loss of CP activity during purification is quite normal, and is due to a combination of reasons such as irreversible oxidation of the active site thiol (Kimmel et al., 1955), autolysis (the enzyme digesting itself) and/or osmotic loss through defective membrane pores. Because of its commercial value the thermostability profile of papaya latex has been investigated previously and, as typically found with most other proteins (Somero, 1995), heat lability has been reported (Chaiwut et al., 2007; Hinkel, 1951; Ortiz et al., 1980). In contrast there is little in the literature on the stability of the enzyme activity in papaya latex under different storage conditions, knowledge that is critically important if eventually large stocks are to be produced commercially for use in animals and possibly humans. Therefore, a single stock of PLS was produced and stored at different temperatures ranging from ambient temperature (18–20 °C) to -80 °C. It was apparent that when stored at refrigerator temperature (4 °C) PLS underwent a gradual loss of active CPs over the course of one year. Gradual enzyme activity loss (30%–50%) when stored at 4 °C have been reported in Calotropis procera latex (Aworh and Nakai, 1986). When PLS was stored at ambient temperature (18–20 °C) the amount of active CP was drastically reduced by 70% within the first week, after which it gradually fell to almost no detectable CP enzyme activity by one year. By contrast, Calotropic procera latex only suffered 11% enzyme activity loss when stored at ambient temperature of 25% after 6 months (Silveira et al., 2021). This was in contrast to the considerably more stable profile of PLS when stored frozen both at -20 °C and -80 °C. The results are in agreement with Ortiz et al. (1980) who recorded a 20% loss of activity within 24hrs of storage under tropical conditions, but did not investigate for longer periods of storage.
Given the relatively rapid loss of activity in PLS when stored at room temperature, it was surprising, but nevertheless reassuring, to find that PLS stability was maintained at 37 °C over a 2 h period simulating a typical in vitro GI nematode motility assay as utilised by Stepek et al. (2005). It was evident that freeze-drying, freezing (−20 °C) and deep freezing (−80 °C) of PLS resulted in minimal loss of active enzymes amounting to no more than 15–20% over a period of storage of 1 week, after which no further deterioration ensued. This loss of active CP activity can be explained by cold denaturation. It was interesting to note that there was no apparent difference in the stability of PLS stored frozen at -20 °C or -80 °C. This observation is particularly appealing for the prospects of using PLS in the field as domestic freezers are more likely to be available than specialist -80 °C facilities. Unlike heat denaturation, which is clearly correlated with temperature (Somero, 1995), the mechanism of protein inactivation by cold denaturation is multifaceted with damage attributed primarily to crystallisation of water causing physical and chemical changes in the enzymes (Arakawa et al., 2001). One thing to be borne in mind when interpreting all of our stability data is that we are dealing with four distinct CPs in PLS, three of which are active against the substrate being used, and it is known that these CPs do not have identical stability patterns. For instance, papain is irreversibly denatured at a pH of about 4.0 and below, whereas chymopapain remains stable even at pH 2.0, at 4 °C (the Biol Chem Hoppe-Seyler paper cited above). However there have been very few, if any, comparative and exhaustive studies on the overall stability of these enzymes under varying environmental conditions.
With these findings, it might be expected that repeated freeze-thawing would inactivate or at least reduce the amount of active CPs even more dramatically as shown by Cao et al. (2003) who demonstrated the detrimental effects of repeated freeze-thawing on lactate dehydrogenase and catalase. However, contrary to expectations, the PLS was fairly stable throughout freeze-thaw cycles repeated up to five times. This might be due to the fact that PLS is not a singular purified enzyme but rather a partially refined enzyme complex that contains many components other than proteases. Apart from physically protecting the proteases, these excipients may also contribute to the stability of CP at low temperatures and during freezing and thawing. Nevertheless, it is advisable to produce a large stock of PLS for future use and to aliquot this before freezing such that the enzymes be subjected to only one round of thawing before use.
A more practical approach for PLS storage and logistics would be to use lyophilized PLS. Lyophilized or freeze-dried PLS stored in the refrigerator at 4 °C was more stable than when stored at room temperature over a period of one year. The long-term stability of lyophilized proteins has been discussed and the factors affecting it are thought to include moisture and oxidation as suggested by Arakawa et al., (2001) and Roy and Gupta (2004).
It was also important to investigate how PLS would behave in the animal models used in earlier studies to asses anthelmintic properties against intestinal nematodes and cestodes, because to-date stability in vivo has been reported only for oral treatment with crude papaya latex (Stepek et al., 2007a). Reassuringly, the data reported here concur with the findings of Stepek et al. (2007a) in that PLS activity declined by 75% within 60 min in the stomach before steadily rising in the colon and lower part of the small intestine. Similar observations were also made in the rat model confirming that active PLS was still detectable in the colons of both rodents, despite the reported concerns about inactivation of orally administered CPs by stomach acid (Huet et al. (2006).
Finally, the data presented in this paper show that the supernatant extract of papaya latex (PLS), which contains most of the CP enzyme activity, is robust in storage. PLS can be safely stored for use as an anthelmintic for considerable periods of time (>1year) after freezing without major loss of enzyme activity. This is a major asset of this preparation and of potential commercial importance, because anthelmintics often need to be delivered to remote locations for treatment of human populations and livestock. Current work is focusing on even better formulations of papaya latex, exploiting novel matrices for controlled released of active enzyme under conditions that accurately reflect those encountered throughout the mammalian intestinal tract.
Declarations
Author contribution statement
F. A. F. Mansur: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
W. Luoga: Performed the experiments.
J. M. Behnke: Conceived and designed the experiments; Analyzed and interpreted the data, contributed reagents; Materials, analysis tools or data; Wrote the paper.
D. J. Buttle: Analyzed and interpreted the data; Wrote the paper.
I. R. Duce: Analyzed and interpreted the data; Wrote the paper.
M.C. Garnett: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by Leverhulme Trust.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
FM was supported by a postgraduate studentship from the government of Malaysia and WL by the government of Tanzania. We thank Ann Lowe for technical support and advice throughout this work.
References
- Arakawa T., Prestrelski S.J., Kenney W.C., Carpenter J.F. Factors affecting short-term and long-term stabilities of proteins. Adv. Drug Deliv. Rev. 2001;46:307–326. doi: 10.1016/s0169-409x(00)00144-7. [DOI] [PubMed] [Google Scholar]
- Aworh O.C., Nakai S. Extraction of milk clotting enzyme from Sodom apple (Calotropis procera) J. Food Sci. 1986;51:1569–1570. [Google Scholar]
- Buttle D.J., Dando P.M., Coe P.F., Sharp S.L., Shepherd S.T., Barrett A.J. The preparation of fully active chymopapain free of contaminating proteinases. Biol. Chem. Hoppe-Seyler. 1990;371:1083–1088. doi: 10.1515/bchm3.1990.371.2.1083. [DOI] [PubMed] [Google Scholar]
- Buttle D.J., Ritonja A., Dando P.M., Abrahamson M., Shaw E.N., Wikstrom P., Turk V., Barrett A.J. Interactions of papaya proteinase IV with inhibitors. FEBS Lett. 1990;262:58–60. doi: 10.1016/0014-5793(90)80153-a. [DOI] [PubMed] [Google Scholar]
- Buttle D.J., Behnke J.M., Bartley Y., Elsheikha H.M., Bartley D.J., Garnett M.C., Donnan A.A., Jackson F., Lowe A., Duce I.R. Oral dosing with papaya latex is an effective anthelmintic treatment for sheep infected with Haemonchus contortus. Parasites Vectors. 2011;4:36. doi: 10.1186/1756-3305-4-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao E., Chen Y., Cui Z., Foster P.R. Effect of freezing and thawing rates on denaturation of proteins in aqueous solutions. Biotechnol. Bioeng. 2003;82:684–690. doi: 10.1002/bit.10612. [DOI] [PubMed] [Google Scholar]
- Chaiwut P., Nitsawang S., Shank L., Kanasawud P. A comparative study on properties and proteolytic components of papaya peel and latex proteases. Chiang Mai J. Sci. 2007;34:109–118. [Google Scholar]
- Frankel M., Maimin R., Shapiro B. Hydrolytic properties of Carica papaya latex and latex preparations. Biochem. J. 1937;31:1926–1933. doi: 10.1042/bj0311926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson A., Veliz G., Naquira C., Amren M., Arroyo M., Arevalo G. Preclinical and clinical studies with latex from Ficus glabrata HBK, a traditional intestinal anthelminthic in the Amazonian area. J. Ethnopharmacol. 1986;17:105–138. doi: 10.1016/0378-8741(86)90053-x. [DOI] [PubMed] [Google Scholar]
- Hinkel E.T., Jr. Further studies on the effect of drying conditions and of the chemical treatment of papaya latex on the stability of papain. Ann. N. Y. Acad. Sci. 1951;54:263–272. doi: 10.1111/j.1749-6632.1951.tb39920.x. [DOI] [PubMed] [Google Scholar]
- Huet J., Looze Y., Bartik K., Raussens V., Wintjens R., Boussard P. Structural characterization of the papaya cysteine proteinases at low pH. Biochem. Biophys. Res. Commun. 2006;341:620–626. doi: 10.1016/j.bbrc.2005.12.210. [DOI] [PubMed] [Google Scholar]
- Kimmel J.R., Thompson E.O., Smith E.L. Crystalline papain. V. Cysteic acid and cysteic acid peptides from oxidized papain. J. Biol. Chem. 1955;217:151–159. [PubMed] [Google Scholar]
- Levecke B., Buttle D.J., Behnke J.M., Duce I.R., Vercruysse J. Cysteine proteinases from papaya (Carica papaya) in the treatment of experimental Trichuris suis infection in pigs: two randomized controlled trials. Parasites Vectors. 2014;7:255. doi: 10.1186/1756-3305-7-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mole J.E., Horton H.R. Kinetics of papain-catalyzed hydrolysis of -N-benzoyl-L-arginine-p-nitroanilide. Biochemistry. 1973;12:816–822. doi: 10.1021/bi00729a005. [DOI] [PubMed] [Google Scholar]
- Mursof E.P., He S. A potential role of papaya latex as an anthelmintic against patent Ascaridia galli infection in chicken. Hemera Zoa. 1991;74:11–20. [Google Scholar]
- Ortiz A., Madrigal L., Fernandez R., Cooke R.D. The storage and drying characteristics of papaya (Carica-Papaya L) latex. J. Sci. Food Agric. 1980;31:510–514. [Google Scholar]
- Roy I., Gupta M.N. Freeze-drying of proteins: some emerging concerns. Biotechnol. Appl. Biochem. 2004;39:165–177. doi: 10.1042/BA20030133. [DOI] [PubMed] [Google Scholar]
- Satrija F., Nansen P., Bjorn H., Murtini S., He S. Effect of papaya latex against Ascaris suum in naturally infected pigs. J. Helminthol. 1994;68:343–346. doi: 10.1017/s0022149x00001619. [DOI] [PubMed] [Google Scholar]
- Silveira S.R., Coelho R.A., Sousa B.F.E., Oliveira J.S.D., Lopez L.M.I., Lima-Filho J.V.M., Júnior P.A.V.R., de Souza D.P., de Freitas C.D.T., Ramos M.V. Standardized production of a homogeneous latex enzyme source overcoming seasonality and microenvironmental variables. Prep. Biochem. Biotechnol. 2021;51:375–385. doi: 10.1080/10826068.2020.1818258. [DOI] [PubMed] [Google Scholar]
- Somero G.N. Proteins and temperature. Annu. Rev. Physiol. 1995;57:43–68. doi: 10.1146/annurev.ph.57.030195.000355. [DOI] [PubMed] [Google Scholar]
- Stepek G., Buttle D.J., Duce I.R., Lowe A., Behnke J.M. Assessment of the anthelmintic effect of natural plant cysteine proteinases against the gastrointestinal nematode, Heligmosomoides polygyrus, in vitro. Parasitology. 2005;130:203–211. doi: 10.1017/s0031182004006225. [DOI] [PubMed] [Google Scholar]
- Stepek G., Lowe A.E., Buttle D.J., Duce I.R., Behnke J.M. In vitro and in vivo anthelmintic efficacy of plant cysteine proteinases against the rodent gastrointestinal nematode, Trichuris muris. Parasitology. 2006;132:681–689. doi: 10.1017/S003118200500973X. [DOI] [PubMed] [Google Scholar]
- Stepek G., Lowe A.E., Buttle D.J., Duce I.R., Behnke J.M. Anthelmintic action of plant cysteine proteinases against the rodent stomach nematode, Protospirura muricola, in vitro and in vivo. Parasitology. 2007;134:103–112. doi: 10.1017/S0031182006001302. [DOI] [PubMed] [Google Scholar]
- Stepek G., Lowe A.E., Buttle D.J., Duce I.R., Behnke J.M. The anthelmintic efficacy of plant-derived cysteine proteinases against the rodent gastrointestinal nematode, Heligmosomoides polygyrus, in vivo. Parasitology. 2007;134:1409–1419. doi: 10.1017/S0031182007002867. [DOI] [PubMed] [Google Scholar]
- Stepek G., Lowe A.E., Buttle D.J., Duce I.R., Behnke J.M. In vitro anthelmintic effects of cysteine proteinases from plants against intestinal helminths of rodents. J. Helminthol. 2007;81:353–360. doi: 10.1017/S0022149X0786408X. [DOI] [PubMed] [Google Scholar]
- Zucker S., Buttle D.J., Nicklin M.J.H., Barrett A.J. The proteolytic activities of chymopapain, papain, and papaya proteinase III. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 1985;828:196–204. doi: 10.1016/0167-4838(85)90057-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.