Abstract
Aim
This SR aims to assess the effectiveness of pregabalin and gabapentin on pain and disability caused by acute sciatica and the adverse events associated with their clinical use.
Design
Systematic review.
Databases
Electronic databases of Cochrane Central Register of Controlled Trials, MEDLINE, EMBASE, and Clinical Trials.gov were searched from their inception until March 1st of 2021.
Selection criteria
Randomized trials (RCT) with adults > 18 years old with acute sciatica for a minimum of 1 week and a maximum of 1 year (at least moderate pain).
Data treatment
The outcomes were pain, disability and adverse events. Data was summarized using odds ratio and mean difference. GRADE was used to calculate the level of evidence.
Results
Eight RCT involving 747 participants were included. The effect of pregabalin was assessed in 3 RCT and in one three-arm trial (pregabalin vs limaprost vs a combination of limaprost and pregabalin). Two trials assessed the effect of gabapentin compared with placebo and one compared with tramadol. One study assessed the effect of gabapentin vs pregabalin in a crossover head-to-head trial.
A statistically significant improvement on leg pain at 2 weeks and leg pain with movement at 3 and 4 months was found in a RCT comparing gabapentin with placebo. There were no statistically differences on the remaining time periods assessed for leg pain, low back pain and functional disability.
Conclusions
This SR provides clear evidence for lack of effectiveness of pregabalin and gabapentin for sciatica pain management. In view of this, its routine clinical use cannot be supported.
Keywords: Gabapentin, Pregabalin, Systematic review, Sciatica, Adverse events
Abstract
Objetivo
Esta revisión sistemática evalúa la efectividad de pregabalina y gabapentina sobre el dolor y la discapacidad producidas por el dolor agudo causado por ciática, y los eventos adversos asociados al uso clínico.
Diseño
Revisión sistemática.
Bases de datos
Se buscó en Cochrane Central Register of Controlled Trials, MEDLINE, EMBASE, y en Clinical Trials.gov desde su inicio hasta el 1 de marzo del 2021.
Criterios de selección
Ensayos clínicos aleatorizados (ECA) sobre adultos > 18 años con ciática aguda establecida entre una semana como mínimo y un año como máximo (al menos con dolor moderado).
Tratamiento de datos
Los resultados fueron dolor, discapacidad y eventos adversos. Los datos fueron resumidos usando odds ratio y diferencia de medias. Para calcular el nivel de evidencia se empleó GRADE.
Resultados
Se incluyeron 8 ECA con un total de 747 participantes. El efecto de la pregabalina fue evaluado en 3 ECA y en un ensayo de 3 brazos (pregabalina vs. limaprost vs. una combinación de limaprost y pregabalina). Dos ensayos evaluaron el efecto de gabapentina comparado con placebo y uno lo comparó con tramadol. Un estudio evaluó el efecto de gabapentina vs. pregabalina en un ensayo cruzado.
En un ECA se encontró una diferencia estadísticamente significativa en la mejora del dolor de piernas a las 2 semanas y en el dolor de piernas con el movimiento a los 3 y 4 meses, con gabapentina comparado frente a placebo. No hubo diferencias en el resto de los periodos estudiados para el dolor de piernas, dolor en la zona lumbar o en la discapacidad funcional.
Conclusiones
Esta revisión sistemática ofrece evidencia clara de la falta de pruebas sobre la efectividad de pregabalina o gabapentina para el manejo del dolor derivado de la ciática. Por tanto, su uso clínico rutinario no está avalado.
Palabras clave: Gabapentina, Pregabalina, Revisión sistemática, Ciática, Eventos adversos
Introduction
Sciatica refers to a pain caused by compression, irritation or injury of the sciatic nerve, which is characterized by lower back pain that radiates in a dermatomal pattern (posterior leg) extending to the lower leg or even to the feet and toes. The compression of the lumbar nerve root is responsible for most episodes of sciatica1 but foraminal stenosis, piriformis syndrome, obstetrical compression and pelvic floor tumours can also cause it. Sciatica pain usually appears associated with other functional limitations and sensory symptoms such as numbness or tingling.2
In the general population, clinically confirmed sciatica prevalence ranges from 2 to 5% but it may reach up to 43% at working population cohorts.3, 4 The data on sciatica prevalence, however, could vary greatly between studies.
There are a number of inherent and environmental factors associated with the likelihood of sciatica. Poor health status and physical stress, being overweight (BMI > 25) or obese (BMI > 30), smoking, and occupational workload are some of the most common risk factors.5, 6, 7
In general, the prognosis is good, and the clinical course is favourable. In most episodes, pain and disability for activities of daily living resolve within two weeks with conservative treatment. However, symptoms persist up to 30% of cases after one year or longer.8
Pain management is usually based on level I and II analgesics (paracetamol and weak opioids). Other drugs such as muscle relaxants, corticosteroids, anticonvulsants, and antidepressants are also used.9, 10
This study focuses specifically on the use of gabapentin and pregabalin for pain associated with acute sciatica since the evidence on their use is limited.
The Food and Drug Administration (FDA) approved pregabalin for the management of neuropathic pain associated with diabetes mellitus, postherpetic neuralgia, partial-onset seizures and fibromyalgia.11, 12, 13, 14 Gabapentin has the approval of the FDA for seizure therapy and post-herpetic neuralgia.15, 16
Despite the specific indications of gabapentinoids, there is a notable increase in the off-label prescription of, which has raised the concern about the misuse of these drugs since the benefits remain unclear.17, 18, 19 To our knowledge regarding their use on sciatica, pain relief only has been reported in one trial comparing gabapentin with placebo20 and in no one of those investigating pregabalin.21 Therefore, this systematic review aims to assess the efficacy and safety of gabapentin and pregabalin as treatment for acute sciatic pain.
Method
Study registration
This review process was developed under the criteria described on the protocol previously published in PROSPERO (CRD 42018099378).
Eligibility criteria
Study types
Randomized controlled trials (RCTs) at any level of blinding whose purpose was the evaluation of the efficacy and safety of gabapentinoids on acute sciatica in adult patients. Eligible comparisons included placebo, no intervention or any active control group (non-pharmacologic and pharmacologic treatments, e.g. NSAIDs, paracetamol, opioids).
Language of publication and publication status were not exclusion criteria.
Participant types
Male or female adult patients (>18 years) in a current episode of sciatica whose clinical course lasted for a minimum of 1 week and a maximum of 1 year whose baseline leg pain was of moderate intensity (4 points out of 10 measured on a Visual Analogue Scale).
Trials including patients with any of the following conditions were excluded: having cauda equina syndrome, pregnant, planning conception, breastfeeding, spinal malignancy, vertebral fractures or local infection.
Outcome measures
The review aims to check whether these drugs can detect clinically significant differences between groups in self-reported leg pain intensity, disability and serious adverse effects.
Timing and effect measures
Follow-up outcomes were reported as immediate-term (<02 weeks after randomization) short-term (>2 weeks but < 2 months), intermediate-term (>2 months and < 6 months) and long-term (>6 months). When multiple terms were reported within one period, we considered the period closest to 2 weeks, 8 weeks, and 6 months for each follow-up period, respectively.
Search methods
Articles were identified by searches of the following electronic databases: Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (OVID) 1966 to March 2021 Embase (OVID) (1966 to March 2021), US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov.
A specific search strategy was executed for each database (Appendix 1).
Reference lists from systematic reviews and studies related were checked to identify further trials. Also, authors were contacted to get additional data from published trials.
Data collection and analysis
Two review authors independently reviewed titles and abstracts of studies identified in the search to assess which studies might potentially meet the inclusion criteria. Wherever there was a doubt, the full article was acquired for further inspection. Potential studies identified by this process were then obtained, and two authors independently screened them to see if they met the review criteria. A final table was produced in Excel. We solved disagreements through discussion between the reviewers. We planned to explore reporting bias using funnel plots when doing a meta-analysis for 10 or more studies. Meta-analysis was performed using the RevMan 5.3 software to pool outcomes using the random effects model. We estimated Risk Ratios (RR) and mean differences (MD). We used GRADEpro software to calculate the overall level of recommendation of evidence.
Quality of evidence
We rated the quality of evidence through the GRADE recommendations when two or more studies were available for each outcome.
Dealing with missing data
Authors were contacted for unpublished missing data for the purposes of meta-analysis.
Results
Study selection
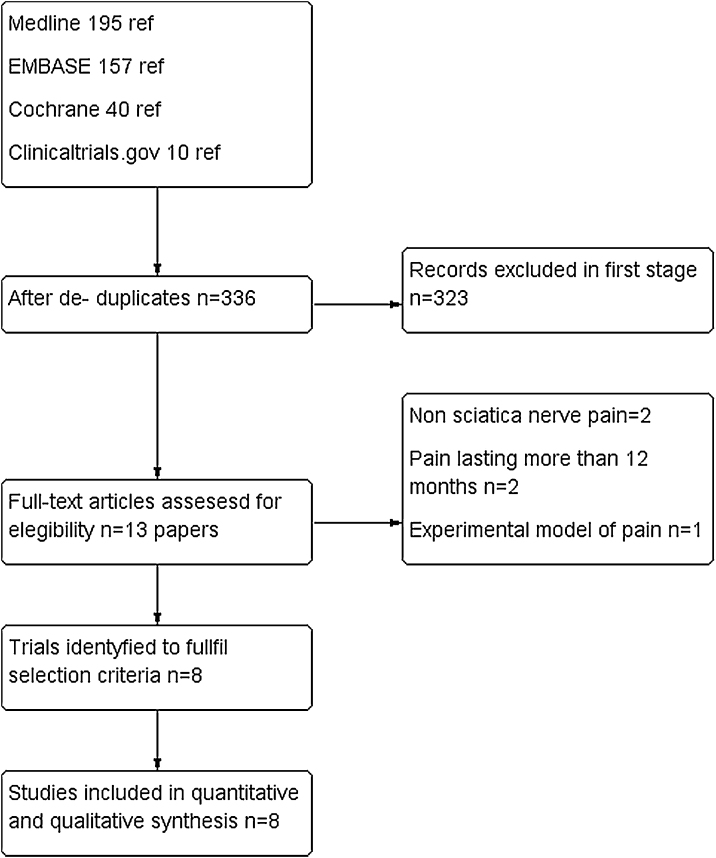
In our literature search, we found 402 papers. After refusing duplicates, 335 were finally assessed. Out of them, 323 were excluded (mainly because they were case reports, observational studies or review articles. For a complete assessment, 13 were selected. Five of them22, 23, 24, 25, 26 were excluded due to the following reasons: non sciatica nerve pain, pain lasting more than 12 months and experimental model of pain. Finally, eight trials were included for quantitative and qualitative synthesis with a total of 747 patients.20, 21, 27, 28, 29, 30, 31, 32 There was a complete agreement between reviewers at full-text screening. (See PRISMA flowchart in Fig. 1).
Figure 1.
PRISMA flow diagram.
Study characteristics
Table 1 shows the main characteristics of the included studies. Four trials tested the effects of pregabalin, three the effects of gabapentin and one assessed the effect of pregabalin versus gabapentin on a head-to-head trial.
Table 1.
Main characteristics of the included studies.
| First author; year of publication | Design | Participant selection criteria | Interventions | Outcomes |
|---|---|---|---|---|
| Baron 2010 | Randomized, double blind, placebo controlled, multicentric, withdrawal trial. | Inclusion criteria: Age: ≥18 years old; pain present ≥3 months prior to the study, stable for ≥4 weeks consistent with a diagnosis of chronic lumbosacral radiculopathy due to spinal stenosis or disc herniation. Mean weekly pain score had to be > 4 out of 10 (ranging from 0 [no pain] to 10 [worst possible pain]) at the end of screening. Exclusion criteria: Lumbosacral radiculopathy neuropathic pain for > 4 years, surgery for lumbosacral radiculopathy in the previous 6 months, more than one previous spinal surgery for L5–S1 pain/radiculopathy, or epidural injection for lumbosacral radiculopathy in the previous 6 weeks. Patients with antiepileptics, nerve blocks, high-potency opioids, and opioid combinations treatment were excluded. |
Intervention (n = 110): 35 days of titrated pregabalin from 150 to 600 mg/day and 7 days of medication taper Control (n = 107): 35 days of placebo and 7 days of medication taper. |
Primary: pain, time to loss of therapeutic response. Secondaries: daily sleep interference, anxiety, depression, patient's perceptions about the efficacy of treatment, sleep problems, pain treatment satisfaction, self-rated physical disability caused by low back pain, quality of life, work productivity and impairment, interventions recommended for treating lumbosacral radiculopathy, adverse events. |
| Kim 2016 | Randomized, double blind, placebo-controlled, double-dummy, three-arm trial. | Inclusion criteria: Age: 20–75 years old, lumbar spinal stenosis diagnosis based on1 or more of the following symptoms: walking intolerance because neurogenic claudication in a 20-min walking trial, a visual analogue scale score of 18 more than 3 for pain/numbness/tingling sensation in the buttocks and lower extremities, and motor weakness along with bladder/bowel dysfunction. Exclusion criteria: Pregnancy current or expected (women who did not agree to use proper contraception), galactose intolerance, serious medical conditions (i.e.: sepsis, cancer), cauda equina syndrome, acute osteoporotic vertebral fractures, walking intolerance caused by ankle, hip or knee joint pain associated with osteoarthritis, a history of epidural steroid injection in the last month, a diagnosis of coronary and/or peripheral arterial occlusive disease within the last 6 months, a diagnosis of avascular necrosis at the hip joints, necrotic ulcerative lesion in the legs, a history of lumbar spine surgery, chronic kidney disease, enrollment in other clinical trials. |
Arm 1 (n = 61): Limaprost 5 mcg t.i.d Arm 2 (n = 60): Pregabalin 75 mg t.i.d Arm 3 (n = 61): Limaprost 5 mcg t.i.d + pregabalin 75 mg t.i.d |
Primary: functional disability for low back pain after treatment. Secondaries: leg pain, health-related quality of life, walking distance in the treadmill test and initial claudication distance; adverse events. |
| Randomized, double blind, placebo-controlled trial. | Inclusion criteria Age: 18–65 years old, pain in dermatomal distribution in either cervical or lumbar region for more than 3 months, at least 2 steroid epidural injections in the past 6 months, presence of motor or sensory neurological signs as weakness, hypo or hyperesthesia and allodynia in the affected dermatomes. Presence of either herniated disc or spinal stenosis or prior spine surgery. Exclusion criteria: Axial pain greater than radicular pain, motor deficits, bowel and/or bladder dysfunction, workmen's compensation or disability issues, depression or the use of anti-depressants, current use of strong narcotics, history of addiction, current or prior gabapentin or pregabalin use, known neuropathy such as diabetic neuropathy or post-herpetic neuralgia, hypersensitivity or angioedema with pregabalin, renal insufficiency, diabetes, CHF, cardiac conduction abnormalities, thrombocytopenia, use of Angiotensin Converting Enzyme Inhibitor, thiazolidinedione or diabetic drugs, pregnancy or breast-feeding. Dropout criteria: Intractable pain requiring additional procedures, worsening neurological signs and symptoms, unacceptable side effects pain requiring additional procedures |
Intervention (n = 20): one week pregabalin 75 mg/12 h followed by pregabalin 150 mg/12 h 2 weeks more. Control (n = 19): placebo |
Primary: pain reliefa Secondaries: functional disability level, patient satisfaction. |
|
| Mathieson 2017 | Randomized, double blind, placebo-controlled trial. | Inclusion criteria: Age: ≥18 years old; a current episode of sciatica present for a minimum of 1 week and a maximum of 1 year, leg pain that had been at least moderate in intensity or had resulted in at least moderate interference with daily activities during the previous week, adequate understanding of English or the availability of interpretation services for the participant to complete the trial. Sciatica was defined as radiating pain into one leg below the knee, accompanied by nerve-root or spinal-nerve involvement as indicated by the presence of at least one of the following clinical features: dermatomal leg pain, myotomal weakness, sensory deficits, or diminished reflex. Exclusion criteria: Known or suspected serious spinal pathology (e.g. cauda equina syndrome, spinal fracture, pregnancy, breastfeeding, planning conception tion (men [with their partners] and women) during the first 8 weeks of the trial; planned spinal surgery or other interventional procedures (e.g., a glucocorticoid injection) for sciatica during the first 8 weeks of the trial; contraindications to pregabalin, under treatment for neuropathic pain (antiepileptic medication, antidepressant medication, or sedative medication and were unable to cease taking such medications), severe depression or suicidal thoughts. |
Intervention (n = 106): pregabalin 75 mg b.i.d titrated up to a maximum of 300 mg b.i.d Control group (n = 101): placebo |
Primary: leg pain intensitya Secondaries: functional disability, back pain severity, global perceived effect, time to recovery, quality of life, work absenteeism (indirect costs), healthcare resource utilization (direct costs. |
| Pirbudak 2015 | Randomized, single-blind, active-controlled trial. | Inclusion criteria: Age: between 20–55 years old; herniated disc-derived acute lumbar radicular pain present for no more than 3 months and a quality of life score over 20% according to the Oswestry Disability Index (ODI; 1–100; <20%: minimal disability, >35%: serious disability), no surgical history, and radiologically confirmed structural pathology without any neurological deficits. Exclusion criteria: bilateral or unilateral multiple root pressure; neurological deficits; history of previous lumbar vertebral surgery; serious cardiac, pulmonary, haematological, gastrointestinal, liver, or kidney disease; glaucoma; urinary retention; statin group drug usage; hormone replacement therapy; and known allergy to drugs used in the study. |
Group 1 (n = 20): tramadol 75 mg short-acting oral form once a day. Group 2 (n = 20): tramadol 75 mg short-acting oral form once a day + 900 mg of gabapentin per day split into 3 doses. Treatment started with a minimal effective dose but planned to increase the doses 2-fold if patients were suffering from pain higher than a VAS score of 3. Both groups were administered a single dose of steroid and local anaesthetic mixture epidurally by the lumbar approach. The injection dose was adjusted to 4 mL from a triamcinolone acetonide (Kenacort ampoule 80 mg, Bristol-Myers Squibb) and 0.25% bupivacaine (10 mg) mixture. |
Analgesia levela, daily activities (walking, sleeping, social activities) objective determination evaluated by the straight leg raising test, leucocyte, erythrocyte sedimentation rate, C-reactive protein blood measurements and serotonin levels in the urine. |
| Robertson 2018 | Randomized, double-blind, double-dummy crossover | Inclusion criteria: Age: ≥18 years old; pain lasting for at least 3 months radiating into 1 leg only to, at, or below the knee level; imaging corroborating a root-level lesion concordant with symptoms and/or signs (determined by the trial clinician); patients who had not used GBP and PGB; sufficient understanding of English (or an available appropriate interpreting service) to complete the study treatments and assessments. Patients with concomitant medications (including analgesics) could be included if the dose was stable 30 days prior to the start of the study. No more than 2 dose modifications were permitted throughout the study. Exclusion criteria: Pregnancy, breastfeeding, women planning conception during the study; history or diagnostic results that suggested an inherited neuropathy or neuropathy attributable to other causes (hypothyroidism, B12 deficiency, connective tissue disease, amyloidosis, toxic exposure); major organ system disease; cardiovascular autonomic neuropathy; baseline postural hypotension of more than 20 mm Hg; specific contraindications to PGB or GBP (allergy to or significant renal impairment); cancer, dementia, severe mental illness, or other condition that would significantly reduce their ability to consent and/or fully undertake the programme; patients with estimated creatinine clearance of less than 60 mL per minute; and, patients unlikely to comply with study procedures (e.g., those with high opiate/opioid tolerance, inconsistent clinic attendances). |
Intervention with gabapentin n = 18): the starting dose was 400 mg once daily for the first week titrated up to a maximum of 800 mg thrice daily, depending on their progress and tolerance at each dose level. Intervention with pregabalin (n = 18): the starting dose was 150 mg once daily for the first week titrated to the participant's optimal dose up to a maximum of 300 mg twice daily, depending on their progress and tolerance at each dose level. The washout period between treatment phases lasted for 1 week. |
Leg pain intensitya; functional disability; adverse event. |
| Yaksi 2007 | Randomized controlled trial | Inclusion criteria: Patients referred with symptoms of neurologic intermittent claudication and diagnosed with lumbar spinal stenosis (LSS) based on radiologic studies. The diagnosis of LSS was confirmed with lumbar computerized tomography and/or lumbar vertebral magnetic resonance imaging). Exclusion criteria: Presence of other pain syndromes in addition to LSS was accepted as an exclusion criteria. |
Intervention (n = 28): starting doses of gabapentin of 900 mg per day. Dosage was increased weekly 300 mg up to the maximum dose of 2400 mg per day, based on the patient's response to the treatment. The daily dose was split into 3 doses for better tolerance. Patients who experienced side effects were prescribed bed rest and increase in oral fluid intake. Control (n = 27): details not stated. Both the control and the treatment groups received physical therapy exercises (lumbar flexion, pelvic traction and strengthening abdominal muscles), lumbosacral corset with steel bracing and pharmacologic treatment with NSAIDs. |
Walking distance (measured on a flat, constant surface); presence or absence of motor and/or sensory deficits (paresthesia, hypoesthesia, hyperesthesia, or dysesthesia of the L4, L5, or S1 dermatomes) and paina. |
| Yildirim 2009 | Randomized, placebo-controlled trial. | Inclusion criteria: Patients with lumbosciatalgia secondary to L5 or S1 radiculopathy. Exclusion criteria: contra-indications to gabapentin treatment, severe depression, severe nephropathy, chronic alcoholism, pregnancy and spinal surgery. In addition, patients who did not tolerate even basal doses of the drug were withdrawn from the study. |
Intervention (n = 25): starting doses of gabapentin of 900 mg per day. Dosage was gradually increased every 3 days up to 3600 mg per day. The daily dose was split into 3 doses. When side effects were observed, the dosage was reduced to tolerable levels. Control (n = 25): placebo three times per day for the two-month trial period. |
Location of pain; severity of pain at rest; limitation of spinal flexion measured by the distance between the finger-tips and the floor; degree of straight leg raising; stretch reflexes; sensory changes; muscle strength. |
b.i.d: twice in a day; t.i.d: three in a day; VAS: visual analogue scale.
Pain measured on a 0–10 numerical pain rating scale.
In particular as regards pregabalin, three studies compared pregabalin versus placebo21, 27, 29 and one compared limaprost versus pregabalin versus an association of both drugs in a three-arm trial.28 Regarding gabapentin trials, one study compared this gabapentinoid with placebo,20 one with no matched intervention,31 and one compared tramadol versus gabapentin plus tramadol.30
Five were double-blinded21, 27, 28, 29, 32; one was single-blinded30; and two were open label.20, 31 All of them were parallel studies except one that was a crossover study.
The pregabalin dosing varied between 150 and 600 mg/day, and for gabapentin, the dosing varied from 300 mg/day up to 3600 mg/day in three divided doses.
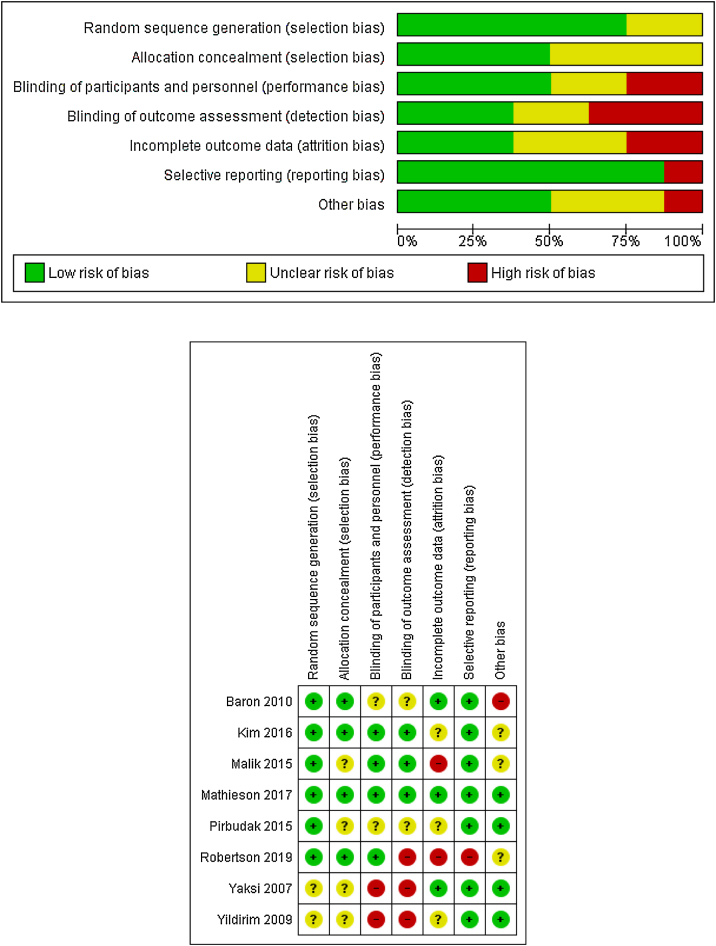
Risk of bias
The potential risk of bias across studies is shown in Fig. 2. Most of the studies have a low or unclear risk of bias regarding randomization sequence generation or allocation concealment. The risk of bias due to blinding of participants and personnel (performance bias) were mainly rated as low or unclear.
Figure 2.
Risk of bias.
Similarly, the risk for blinding of outcome assessment (detection bias) was low or unclear for all but three studies, which were rated as high risk of bias in this item.20, 31, 32
Attrition bias was rated as an unclear risk for most of the trials. Three were rated as low risk of bias,21, 27, 31 and two were rated as high risk of bias.29, 32 According to selective reporting or reporting bias, all but one trial32 included were rated as low risk of bias.
Assessment of other potential sources of bias gave a low risk of bias for four studies.20, 21, 30, 31 Three were rated as unclear risk of bias28, 29, 32 and one was rated as high risk of bias27 due to a run-in period, where placebo respondents and pregabalin non-respondents were eliminated.
Because of the scarce number of studies assessed, no publication bias test (e.g., funnel plot) was performed.
Study outcomes
Pregabalin effect on leg pain and disability
There were no statistically significant differences between pregabalin and placebo in leg pain at 2 weeks, at 8 weeks, nor 26 weeks and 52 weeks, according to mean differences between groups measured with the Visual Analogue Scale (VAS). See Table 2a for all the results.
Table 2a.
Pregabalin effect on leg pain and disability.
| Reference(s) | Outcome | Comparison arm | N Pregabalin/N comparison | MD (CI 95%) |
|---|---|---|---|---|
| Kim 2016 Mathieson 2017 |
Leg pain at 2 weeks | Placebo | 166/158 | −0.19 (−0.67 to 0.29) |
| Kim 2016 Mathieson 2017 |
Leg pain at 8 weeks† | Placebo | 160/154 | −0.03 (−0.54 to 0.48) |
| Mathieson 2017 | Leg pain at 26 weeks | Placebo | 93/91 | −0.10 (−0.94 to 0.74) |
| Mathieson 2017 | Leg pain at 52 weeks | Placebo | 91/87 | 0.40 (−0.45 to 1.25) |
| Mathieson 2017 | Back pain at 2 weeks | Placebo | 101/95 | −0.30 (−1.07 to 0.47) |
| Mathieson 2017 | Back pain at 8 weeks | Placebo | 94/90 | 0.40 (−0.45 to 1.25) |
| Mathieson 2017 | Back pain at 26 weeks | Placebo | 87/88 | −0.30 (−1.22 to 0.62) |
| Mathieson 2017 | Back pain at 52 weeks | Placebo | 83/80 | 0.80 (−0.08 to 1.68) |
| Mathieson 2017 | Disability at 2 weeks | Placebo | 101/96 | −0.80 (−2.52 to 0.92) |
| Kim 2016 Mathieson 2017 |
Disability at 8 weeks† | Placebo | 153/150 | −0.08 (−0.30 to 0.15) |
| Mathieson 2017 | Disability at 26 weeks | Placebo | 85/87 | −1.40 (−3.63 to 0.83) |
| Mathieson 2017 | Disability at 52 weeks | Placebo | 83/79 | 0.80 (−1.48 to 3.08) |
Regarding back pain, no statistically significant differences were found at 2 and 8 weeks, 26 weeks and 52 weeks.
In respect to disability score, measured by self-reported disability scores, the results showed no statistically significant differences between pregabalin and placebo at 2 weeks (one study), 8 weeks (two studies), 26 weeks (one study) or 52 weeks (one study).
Gabapentin effect on leg pain and disability
There was a statistically significant difference in leg pain between gabapentin and placebo at 2 weeks (MD −0.80; CI 95% −1.53 to −0.07) in one study.30
Only one study showed statistically significant differences in the relief of low back and leg pain with the movement at months 3 (MD −1.20; CI 95% −2.36 to −0.04) and 4 (MD −1.80; CI 95% −3.07 to −0.53).31
Regarding disability score measured by self-reported disability scores, no statistically significant difference was found between gabapentin and placebo at 2 weeks. See Table 2b for all the results.
Table 2b.
Gabapentin effect on leg pain and disability.
| Reference(s) | Outcome | Comparison arm | N Gabapentin/N comparison | MD (CI 95%)* |
|---|---|---|---|---|
| Pirbudak 2015 | Leg pain at 2 weeks | Placebo | 20/20 | −0.80 (−1.53 to −0.07) |
| Yaksi 2007 | Low back and leg pain with the movement month 1 | Placebo | 28/27 | −0.50 (−1.44 to 0.44) |
| Yaksi 2007 | Low back and leg pain with the movement month 2 | Placebo | 28/27 | −0.70 (−1.73 to 0.33) |
| Yaksi 2007 | Low back and leg pain with the movement month 3 | Placebo | 28/27 | −1.20 (−2.36 to −0.04) |
| Yaksi 2007 | Low back and leg pain with the movement month 4 | Placebo | 28/27 | −1.80 (−3.07 to −0.53) |
| Pirbudak 2015 | Disability at 2 weeks | Placebo | 20/20 | −1.75 (−7.27 to 3.77) |
MD = mean difference; CI = confidence interval; (†) From meta-analysis.
MD = mean difference; CI = confidence interval; (*) Bold characters denote statistically significant differences.
Gabapentin vs pregabalin on leg pain and disability
Results related to the first period of the crossover trial showed that there was a reduction on leg pain intensity on patients treated with gabapentin at the end of an 8-week treatment period (mean reduction [range], 1.35 [0.5–2.9] vs 1.43 [0.1–4.2]; p = 0.62).
Results were clinically relevant for both gabapentinoid drugs during the first sequence in reducing pain-associated disability. After the 8-week treatment period the mean reduction range was 11.25 [0–30] for gabapentin and 12.4 [2–28]; p = 0.31 for pregabalin.
Adverse events associated with gabapentinoids
Only four studies assessed pregabalin undesirable effects.21, 27, 28, 29 A total of 32 adverse effects were reported which reflected that pregabalin was, in general, tolerated worse than placebo. Dorsalgia (RR 2.10, CI 95% 1.05–4.20), dizziness (RR 3.38; CI 95% 2.26–5.04) and nausea/vomiting (RR 5.22; CI 95% 1.38–19.73) were more common in pregabalin groups. The latter two findings were statistically significant.
Serious AE were similar in the pregabalin group and the placebo group21, 27 as well as in patients with one or more AE.27, 28
The quality of evidence was assessed as high for disability at 8 weeks, serious AE, and dizziness, and as moderate for leg pain at 8 weeks, nausea/vomiting, and somnolence.
Regarding gabapentin trials, only one study evaluated AE.31 Ataxia briefly was reported in two patients of the treatment group. Drowsiness and dizziness were the most frequent side effects mentioned by authors but more detailed data was not available. Serious adverse effects were not reported for gabapentin.
The quality of evidence was assessed as low for nausea/vomiting and very low for somnolence and headache.
The cross-over study comparing the treatment between gabapentinoids reported three adverse events for gabapentin and 21 for pregabalin during the first sequence. However, the available data does not detail the specific adverse events for this period.
Quality of life
Quality of life (QoL) through the SF-12 questionnaire was assessed in one of the pregabalin trials.21 No statistical differences were found at 2, 8, 26 and 52 weeks.
Discussion
Our results suggest that patients with sciatica treated with pregabalin did not get better relief of pain or improvement in their disability after up to 52 weeks of follow-up compared to placebo.
Similarly, gabapentin did not relieve pain or improved disability after 8 weeks of treatment compared to placebo. One study found statistically significant differences at 3–4 months of treatment with gabapentin however it did not reach clinical relevance because it was below two points out of ten in the assessment of the VAS.
In addition, the subjects treated with anticonvulsants experienced a greater number of undesirable effects.
Statistical heterogeneity was low for the outcomes of efficacy and safety (I2 = 0), however there are also some limitations that must be underlined. One on hand, the small number of studies available and the small-sized samples are a restriction to extrapolate the results to larger populations. On the other hand, the short clinical follow-up period on each study. Unfortunately, the maximum time of follow-up was 52 and 8 weeks for pregabalin and gabapentin, respectively. Finally, the crossover trial reported positive results for both gabapentinoids. However, the concomitance with other therapies (nonsteroidal anti-inflammatories, paracetamol, opioids and antiepileptic/anticonvulsant drugs) may affect both efficacy and AE incidence potentially increasing both. Lastly, despite that quality of life measurements were not included in our protocol, we considered pertinent to mention the one study that reported quality of life outcomes due to the relevance of this outcome for the patients.
Levels of evidence were performed for pregabalin treatment in two out of the twelve efficacy outcomes analyzed (leg pain 8 weeks, disability 8 weeks).
Similarly, regarding safety outcomes, levels of evidence were assessed for serious adverse events, dizziness, nausea/vomiting and somnolence.
The reasons to downgrade the evidence for leg pain of 8 weeks, nausea/vomiting and somnolence outcomes were a high risk of bias due to unclear allocation concealment, inconsistency and imprecision.
Our results are consistent with previous systematic reviews33, 34, 35 assessing the effectiveness of anticonvulsants on low and back pain relief. Their findings reflect that these drugs were related to a higher risk for AE and were ineffective both for the treatment of pain associated with acute sciatica and functional disability.
However, some limitations must be noted. First, although we did not formally assess publication bias due to the small number of studies included, it could not be discarded. Studies with non-significant results for AE, leg or back pain may remain unpublished because it is more likely that publication bias could favour the publication of studies with positive results. The review was carried out with a broad search strategy and no restrictions for language or publication date were applied. The search included published and unpublished data. We tried to contact authors in order to reach additional data from trials. Unfortunately, no one responded to our request. Second, the small sample size of the trials included should be considered when extrapolating the results to the overall population both for efficacy and for safety outcomes. Third, regarding the AE, It should be noted that AE were not reported in all trials. Finally, as we mentioned previously, most of the studies have a relatively short follow-up period so it cannot be discarded that AE may occur with long exposure to treatments.
Conclusions
In this review, no evidence has been found to support the use of pregabalin or gabapentin for sciatica pain or low back pain, since the effect is not superior to placebo. In addition, adverse effects of different considerations associated with their use have been reported. In view of this, its routine clinical use cannot be supported.
Authors’ contribution
SGM, PPFR, JIDC, RCS, ELB and VRG were involved in studies selection, data extraction, risk of bias assessment, data analysis and interpretation, drafting of the manuscript and made the final approval of the manuscript. VRG contributed with the study idea and designed the study protocol.
Ethical approval
Not applicable as this article does not contain any studies with human or animal subjects.
Sources of funding
None declared.
Conflict of interest
None of the authors have any conflicts of interest to disclose.
Acknowledgements
We would like to thank Maria del Mar Úbeda Carrillo and Eukene Ansuategui Zengotitabengoa for the support on the literature search.
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.aprim.2021.102144.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Ropper A.H., Zafonte R.D. Sciatica. N Engl J Med. 2015;372:1240–1248. doi: 10.1056/NEJMra1410151. [DOI] [PubMed] [Google Scholar]
- 2.Valat J.P., Genevay S., Marty M., Rozenberg S., Koes B. Sciatica. Best Pract Res Clin Rheumatol. 2010;24:241–252. doi: 10.1016/j.berh.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 3.Konstantinou K., Dunn K.M. Sciatica: review of epidemiological studies and prevalence estimates. Spine. 2008;33:2464–2472. doi: 10.1097/BRS.0b013e318183a4a2. [DOI] [PubMed] [Google Scholar]
- 4.Videman T., Nurminen T., Tola S., Kuorinka I., Vanharanta H., Troup J.D. Low-back pain in nurses and some loading factors of work. Spine. 1984;9:400–404. doi: 10.1097/00007632-198405000-00013. [DOI] [PubMed] [Google Scholar]
- 5.Cook C.E., Taylor J., Wright A., Milosavljevic S., Goode A., Whitford M. Risk factors for first time incidence sciatica: a systematic review. Physiother Res Int J Res Clin Phys Ther. 2014;19:65–78. doi: 10.1002/pri.1572. [DOI] [PubMed] [Google Scholar]
- 6.Shiri R., Lallukka T., Karppinen J., Viikari-Juntura E. Obesity as a risk factor for sciatica: a meta-analysis. Am J Epidemiol. 2014;179:929–937. doi: 10.1093/aje/kwu007. [DOI] [PubMed] [Google Scholar]
- 7.Miranda H., Viikari-Juntura E., Martikainen R., Takala E.-P., Riihimäki H. Individual factors, occupational loading, and physical exercise as predictors of sciatic pain. Spine. 2002;27:1102–1108. doi: 10.1097/00007632-200205150-00017. [DOI] [PubMed] [Google Scholar]
- 8.Koes B.W., van Tulder M.W., Peul W.C. Diagnosis and treatment of sciatica. BMJ. 2007;334:1313–1317. doi: 10.1136/bmj.39223.428495.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pinto R.Z., Verwoerd A.J.H., Koes B.W. Which pain medications are effective for sciatica (radicular leg pain)? BMJ Online. 2017;359 doi: 10.1136/bmj.j4248. (no pagination)(j4248) [DOI] [PubMed] [Google Scholar]
- 10.Jensen R.K., Kongsted A., Kjaer P., Koes B. Diagnosis and treatment of sciatica. BMJ. 2019;367 doi: 10.1136/bmj.l6273. [DOI] [PubMed] [Google Scholar]
- 11.Verma V., Singh N., Singh Jaggi A. Pregabalin in neuropathic pain: evidences and possible mechanisms. Curr Neuropharmacol. 2014;12:44–56. doi: 10.2174/1570159X1201140117162802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bockbrader H.N., Wesche D., Miller R., Chapel S., Janiczek N., Burger P. A comparison of the pharmacokinetics and pharmacodynamics of pregabalin and gabapentin. Clin Pharmacokinet. 2010;49:661–669. doi: 10.2165/11536200-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 13.EBSCO Information Services. Record No. T233001 Pregabalin.
- 14.PubChem. Gabapentin. https://pubchem.ncbi.nlm.nih.gov/compound/3446 [accessed 7.1.20].
- 15.EBSCO Information Services. Record No. T233455 Gabapentin.
- 16.Rose M.A., Kam P.C.A. Gabapentin: pharmacology and its use in pain management. Anaesthesia. 2002;57:451–462. doi: 10.1046/j.0003-2409.2001.02399.x. [DOI] [PubMed] [Google Scholar]
- 17.Goodman C.W., Brett A.S. A clinical overview of off-label use of gabapentinoid drugs. JAMA Intern Med. 2019;179:695–701. doi: 10.1001/jamainternmed.2019.0086. [DOI] [PubMed] [Google Scholar]
- 18.Moore A., Derry S., Wiffen P. Gabapentin for chronic neuropathic pain. JAMA. 2018;319:818–819. doi: 10.1001/jama.2017.21547. [DOI] [PubMed] [Google Scholar]
- 19.Peckham A.M., Evoy K.E., Ochs L., Covvey J.R. Gabapentin for off-label use: evidence-based or cause for concern? Subst Abuse Res Treat. 2018;12 doi: 10.1177/1178221818801311. 1178221818801311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yildirim K., Şışecıoğlu M., Karatay S., Erdal A., Levent A., Uğur M. The effectiveness of gabapentin in patients with chronic radiculopathy. Pain Clin. 2003;15:213–218. doi: 10.1163/156856903767650718. [DOI] [Google Scholar]
- 21.Mathieson S., Maher C.G., McLachlan A.J. Trial of pregabalin for acute and chronic sciatica. N Engl J Med. 2017;376:1111–1120. doi: 10.1056/NEJMoa1614292. [DOI] [PubMed] [Google Scholar]
- 22.Baron R., Martin-Mola E., Müller M., Dubois C., Falke D., Steigerwald I. Effectiveness and safety of tapentadol prolonged release (PR) versus a combination of tapentadol PR and pregabalin for the management of severe, chronic low back pain with a neuropathic component: a randomized, double-blind, phase 3b study. Pain Pract. 2015;15:455–470. doi: 10.1111/papr.12200. [DOI] [PubMed] [Google Scholar]
- 23.McCleane G.J. Gabapentin reduces chronic benign nociceptive pain: a double-blind, placebo-controlled cross-over study. Pain Clin. 2000;12:81–85. doi: 10.1163/156856900750229825. [DOI] [Google Scholar]
- 24.McCleane G.J. Does gabapentin have an analgesic effect on background, movement and referred pain? A randomised, double-blind, placebo controlled study. Pain Clin. 2001;13:103–107. doi: 10.1163/156856901753420945. [DOI] [Google Scholar]
- 25.Markman J.D., Frazer M.E., Rast S.A. Double-blind randomized controlled crossover trial of pregabalin for neurogenic claudication. Neurology. 2015;84:265–272. doi: 10.1212/WN.L.0000000000001168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sumracki N.M., Hutchinson M.R., Gentgall M., Briggs N., Williams D.B., Rolan P. The effects of pregabalin and the glial attenuator minocycline on the response to intradermal capsaicin in patients with unilateral sciatica. PLoS ONE. 2012;7:e38525. doi: 10.1371/journal.pone.0038525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baron R., Freynhagen R., Tölle T.R. The efficacy and safety of pregabalin in the treatment of neuropathic pain associated with chronic lumbosacral radiculopathy. Pain. 2010;150:420–427. doi: 10.1016/j.pain.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 28.Kim Y., Shaffer K.M., Carver C.S., Cannady R.S. Quality of life of family caregivers 8 years after a relative's cancer diagnosis: follow-up of the National Quality of Life Survey for Caregivers. Psychooncology. 2016;25:266–274. doi: 10.1002/pon.3843. [DOI] [PubMed] [Google Scholar]
- 29.Malik K.M., Nelson M.A., Avram J., Lee Robak M.S., Benzon T.H. Efficacy of pregabalin in the treatment of radicular pain: results of a controlled trial. Anesthesiol Pain Med. 2015;5 doi: 10.5812/aapm.28110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pirbudak L., çIçEk H., IşIk M., Zer Y. The effect of tramadol and tramadol + gabapentin combination in patients with lumbar disc herniation after epidural steroid injection. Turk J Med Sci. 2015;45:1214–1219. doi: 10.3906/sag-1310-2. [DOI] [PubMed] [Google Scholar]
- 31.Yaksi A., Özgönenel L., Özgönenel B. The efficiency of gabapentin therapy in patients with lumbar spinal stenosis. Spine. 2007;32:939–942. doi: 10.1097/01.brs.0000261029.29170.e6. [DOI] [PubMed] [Google Scholar]
- 32.Robertson K., Marshman L.A.G., Plummer D., Downs E. Effect of gabapentin vs pregabalin on pain intensity in adults with chronic sciatica. JAMA Neurol. 2019;76:28–34. doi: 10.1001/jamaneurol.2018.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Enke O., New H.A., New C.H. Anticonvulsants in the treatment of low back pain and lumbar radicular pain: a systematic review and meta-analysis. CMAJ. 2018;190:E786–E793. doi: 10.1503/cmaj.171333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Onakpoya I.J., Thomas E.T., Lee J.J., Goldacre B., Heneghan C.J. Benefits and harms of pregabalin in the management of neuropathic pain: a rapid review and meta-analysis of randomised clinical trials. BMJ Open. 2019;9:e023600. doi: 10.1136/bmjopen-2018-023600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shanthanna H., Gilron I., Rajarathinam M. Benefits and safety of gabapentinoids in chronic low back pain: a systematic review and meta-analysis of randomized controlled trials. PLOS Med. 2017;14:e1002369. doi: 10.1371/journal.pmed.1002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.