Key Points
Question
What are the efficacy and safety of omecamtiv mecarbil for the treatment of patients with severe heart failure (HF)?
Finding
In this post hoc analysis of data from 8232 patients with symptomatic HF enrolled in the Global Approach to Lowering Adverse Cardiac Outcomes Through Improving Contractility in Heart Failure (GALACTIC-HF) randomized clinical trial, patients with severe HF benefited from treatment with omecamtiv mecarbil for the primary end point of time to first HF event or death from cardiovascular causes, whereas patients without severe HF experienced no significant treatment benefit. Omecamtiv mecarbil therapy was well tolerated with regard to changes in blood pressure, kidney function, and potassium level, even among patients with severe HF.
Meaning
The findings of this post hoc analysis support a potential role of omecamtiv mecarbil in the treatment of patients with severe HF.
Abstract
Importance
Heart failure with reduced ejection fraction is a progressive clinical syndrome, and many patients’ condition worsen over time despite treatment. Patients with more severe disease are often intolerant of available medical therapies.
Objective
To evaluate the efficacy and safety of omecamtiv mecarbil for the treatment of patients with severe heart failure (HF) enrolled in the Global Approach to Lowering Adverse Cardiac Outcomes Through Improving Contractility in Heart Failure (GALACTIC-HF) randomized clinical trial.
Design, Setting, and Participants
The GALACTIC-HF study was a global double-blind, placebo-controlled phase 3 randomized clinical trial that was conducted at multiple centers between January 2017 and August 2020. A total of 8232 patients with symptomatic HF (defined as New York Heart Association symptom class II-IV) and left ventricular ejection fraction of 35% or less were randomized to receive omecamtiv mecarbil or placebo and followed up for a median of 21.8 months (range, 15.4-28.6 months). The current post hoc analysis evaluated the efficacy and safety of omecamtiv mecarbil therapy among patients classified as having severe HF compared with patients without severe HF. Severe HF was defined as the presence of all of the following criteria: New York Heart Association symptom class III to IV, left ventricular ejection fraction of 30% or less, and hospitalization for HF within the previous 6 months.
Interventions
Participants were randomized at a 1:1 ratio to receive either omecamtiv mecarbil or placebo.
Main Outcomes and Measures
The primary end point was time to first HF event or cardiovascular (CV) death. Secondary end points included time to CV death and safety and tolerability.
Results
Among 8232 patients enrolled in the GALACTIC-HF clinical trial, 2258 patients (27.4%; mean [SD] age, 64.5 [11.6] years; 1781 men [78.9%]) met the specified criteria for severe HF. Of those, 1106 patients were randomized to the omecamtiv mecarbil group and 1152 to the placebo group. Patients with severe HF who received omecamtiv mecarbil experienced a significant treatment benefit for the primary end point (hazard ratio [HR], 0.80; 95% CI, 0.71-0.90), whereas patients without severe HF had no significant treatment benefit (HR, 0.99; 95% CI, 0.91-1.08; P = .005 for interaction). For CV death, the results were similar (HR for patients with vs without severe HF: 0.88 [95% CI, 0.75-1.03] vs 1.10 [95% CI, 0.97-1.25]; P = .03 for interaction). Omecamtiv mecarbil therapy was well tolerated in patients with severe HF, with no significant changes in blood pressure, kidney function, or potassium level compared with placebo.
Conclusions and Relevance
In this post hoc analysis of data from the GALACTIC-HF clinical trial, omecamtiv mecarbil therapy may have provided a clinically meaningful reduction in the composite end point of time to first HF event or CV death among patients with severe HF. These data support a potential role of omecamtiv mecarbil therapy among patients for whom current treatment options are limited.
Trial Registration
ClinicalTrials.gov Identifier: NCT02929329
This post hoc analysis of data from the Global Approach to Lowering Adverse Cardiac Outcomes Through Improving Contractility in Heart Failure (GALACTIC-HF) randomized clinical trial assesses the safety and efficacy of omecamtiv mecarbil for the treatment of patients with severe heart failure.
Introduction
Despite significant improvements in prognosis with contemporary medical therapy, heart failure (HF) with reduced ejection fraction (HFrEF) remains a progressive clinical syndrome, and many patients experience worsening over time despite receiving optimal guideline-based treatment. The nomenclature to describe such patients is varied and includes advanced HF, severe HF, refractory HF, or stage D HF. We used the term severe HF in the current analysis to distinguish the population of interest from patients with advanced HF, a term traditionally used for patients who require heart transplant or mechanical cardiac support (a population that was specifically excluded from the Global Approach to Lowering Adverse Cardiac Outcomes Through Improving Contractility in Heart Failure [GALACTIC-HF] study).1,2,3,4 Regardless of the term used, these patients have a high burden of symptoms, recurrent HF hospitalizations, and high mortality and account for a large proportion of the total costs of HF care.5 As HF progresses, many patients become progressively intolerant of neurohormonal blockade with β-blockers or renin-angiotensin-aldosterone system modulators because of hypotension or kidney dysfunction, limiting their options for medical therapy.6 Selected patients with severe HF may be candidates for other therapies, such as cardiac transplant or mechanical cardiac support, but these therapies are costly and highly invasive and have limited availability. Intravenous inotropic therapy can be used for palliation of symptoms among selected patients but may be associated with increased mortality.7,8 Thus, there is a clear unmet need for effective and safe long-term medical therapies for the treatment of patients with more severe stages of HF.
Omecamtiv mecarbil is a direct activator of cardiac myosin that increases systolic ejection time and stroke volume, improves ventricular remodeling, and decreases natriuretic peptide concentrations in patients with HFrEF.9,10,11 In the GALACTIC-HF randomized clinical trial of patients with HFrEF, treatment with omecamtiv mecarbil improved the primary end point of time to first HF event or death from cardiovascular (CV) causes compared with placebo.12 In the current post hoc analysis, we analyzed the efficacy and safety of omecamtiv mecarbil for the treatment of patients with severe HF who were enrolled in the GALACTIC-HF study.
Methods
The study design, baseline participant characteristics, and primary results of the GALACTIC-HF clinical trial have been previously published.12,13,14 In brief, GALACTIC-HF was a global double-blind, placebo-controlled phase 3 randomized clinical trial conducted at multiple centers between January 2017 and August 2020. The study evaluated treatment with omecamtiv mecarbil compared with placebo among 8232 patients with symptomatic HF (defined as New York Heart Association [NYHA] symptom class II-IV) and left ventricular ejection fraction (LVEF) of 35% or less who were randomized at a 1:1 ratio. Enrolled patients were currently hospitalized for HF (inpatients) or had made an urgent visit to the emergency department or been hospitalized for HF within 1 year before screening (outpatients). All patients had elevated natriuretic peptides, defined as N-terminal pro–B-type natriuretic peptide levels of 400 pg/mL or greater (≥1200 pg/mL for patients with atrial fibrillation), or B-type natriuretic peptide levels of 125 pg/mL or greater (≥375 pg/mL for patients with atrial fibrillation). A history of optimized medical therapy was required for enrollment. The primary end point of the GALACTIC-HF study was a composite of time to first HF event or death from CV causes. Patients were followed up for a median of 21.8 months (range, 15.4-28.6 months). The study protocol was approved by the relevant local ethics committees, and all participants provided written informed consent. The GALACTIC-HF study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline for randomized clinical trials.
Definition of Severe Heart Failure
For the current analysis, we defined severe HF based on the published criteria from the 2018 Heart Failure Association of the European Society of Cardiology advanced HF position statement.2 Although numerous criteria for severe HF have been proposed by different professional societies, we selected this definition as the most quantitative, making it the easiest to apply to a clinical trial population. Based on the Heart Failure Association of the European Society of Cardiology criteria, patients were required to have all of the following: (1) NYHA symptom class III to IV, (2) LVEF of 30% or less, (3) 2 or more hospitalizations for HF within the previous 12 months, and (4) evidence of severe functional impairment measured by cardiopulmonary exercise testing or a 6-minute walk test. For the current analysis, we modified the hospitalization criteria to 1 HF hospitalization within the previous 6 months (including those hospitalized at the time of study enrollment) because we did not have data on the number of previous HF hospitalizations before study enrollment (a Venn diagram of groups defined by severe HF criteria is available in the eFigure in the Supplement). Patients who had an estimated glomerular filtration rate lower than 20 mL/min/1.73 m2 or who were receiving dialysis at screening were excluded. We did not consider measures of functional capacity, such as cardiopulmonary exercise testing, in our classification because we did not collect these data in the GALACTIC-HF study.
Statistical Analysis
Baseline characteristics for patients with vs without severe HF were evaluated using summary statistics. Outcomes for patients with and without severe HF were compared using Cox proportional hazards models and Kaplan-Meier curves. Interaction terms were used to assess whether omecamtiv mecarbil had a differential effect on outcome by severe HF status. Absolute event rates were described using rate per 100 patient-years. As a sensitivity analysis, we assessed the event rates and treatment effect of omecamtiv mecarbil for patients by specific HF severity criteria met as well as the total number of criteria met. For quality-of-life data as assessed by the Kansas City Cardiomyopathy Questionnaire total symptom score, we used linear regression analysis adjusted for baseline scores to compare the treatment effect of omecamtiv mecarbil with placebo. Safety and tolerability data for patients with vs without severe HF were summarized using descriptive statistics. All analyses were conducted using Stata software, version 16 (StataCorp), with P ≤ .05 considered statistically significant.
Results
Among 8232 patients enrolled in the GALACTIC-HF clinical trial, 2258 patients (27.4%; mean [SD] age, 64.5 [11.6] years; 477 women [21.1%] and 1781 men [78.9%]) met the specified criteria for severe HF. Of those, 1106 patients were randomized to the omecamtiv mecarbil group and 1152 to the placebo group. Baseline characteristics stratified by severe HF status and treatment group are shown in Table 1 and eTable 1 in the Supplement. Patients with vs without severe HF (n = 5974) had markers indicating more severe disease, including lower LVEF (mean [SD], 23.4% [5.2%] vs 27.8% [6.2%], respectively), higher NYHA symptom class (eg, class IV: 173 patients [7.7%] vs 75 patients [1.3%]), higher N-terminal pro–B-type natriuretic peptide concentrations (mean, 2804 pg/mL [95% CI, 1450-5795 pg/mL] vs 1768 pg/mL [95% CI, 878-3521 pg/mL]), lower systolic blood pressure (mean [SD], 113.8 [15.0] mm Hg vs 117.5 [15.4] mm Hg), worse kidney function (estimated glomerular filtration rate: mean, 55.1 mL/min/1.73 m2 [95% CI, 41.8-69.9 mL/min/1.73 m2] vs 60.0 mL/min/1.73 m2 [95% CI, 45.4-75.5 mL/min/1.73 m2]), and worse quality of life as assessed by the Kansas City Cardiomyopathy Questionnaire total symptom score (mean, 56.2 points [95% CI, 36.5-77.1 points] vs 74.0 points [95% CI, 54.2-90.6 points]). Patients with vs without severe HF were less likely to be receiving renin-angiotensin-aldosterone system modulators (1873 patients [82.9%] vs 5286 patients [88.5%], respectively) and β-blockers (2093 patients [92.7%] vs 5670 patients [94.9%]) but more likely to be receiving cardiac resynchronization therapy (372 patients [16.5%] vs 786 patients [13.2%]) or have an implantable cardioverter-defibrillator (807 patients [35.7%] vs 1807 patients [30.2%]) at baseline. Patients with severe HF were at significantly higher risk of HF events and CV death, with event rates for patients in the placebo group approximately twice those of patients without severe HF for the primary end point of time to first HF event or CV death (42.6 events per 100 patient-years vs 21.3 events per 100 patient-years, respectively), CV death (17.3 events per 100 patient-years vs 8.5 events per 100 patient-years), and all-cause death (21.7 events per 100 patient-years vs 11.9 events per 100 patient-years).
Table 1. Baseline Patient Characteristics by Severe Heart Failure Classification and Treatment Group.
| Characteristic | No. (%) | |||
|---|---|---|---|---|
| Severe heart failure | No severe heart failure | |||
| Omecamtiv mecarbil (n = 1106) | Placebo (n = 1152) | Omecamtiv mecarbil (n = 3014) | Placebo (n = 2960) | |
| Demographic | ||||
| Age, mean (SD), y | 64.4 (11.7) | 64.5 (11.6) | 64.6 (11.2) | 64.5 (11.3) |
| Sex | ||||
| Female | 253 (22.9) | 224 (19.4) | 622 (20.6) | 650 (22.0) |
| Male | 853 (77.1) | 928 (80.6) | 2392 (79.4) | 2310 (78.0) |
| Race | ||||
| Asian | 69 (6.2) | 84 (7.3) | 286 (9.5) | 271 (9.2) |
| Black | 89 (8.0) | 88 (7.6) | 196 (6.5) | 189 (6.4) |
| White | 881 (79.7) | 918 (79.7) | 2315 (76.8) | 2283 (77.1) |
| Othera | 67 (6.1) | 62 (5.4) | 217 (7.2) | 217 (7.3) |
| Geographic region | ||||
| Asia | 66 (6.0) | 75 (6.5) | 269 (8.9) | 260 (8.8) |
| Australasia, South Africa, and western Europe | 279 (25.2) | 270 (23.4) | 682 (22.6) | 690 (23.3) |
| Canada and US | 213 (19.3) | 221 (19.2) | 480 (15.9) | 472 (15.9) |
| Eastern Europe and Russia | 389 (35.2) | 421 (36.5) | 955 (31.7) | 916 (30.9) |
| Latin America | 159 (14.4) | 165 (14.3) | 628 (20.8) | 622 (21.0) |
| Inpatient randomization setting | 440 (39.8) | 497 (43.1) | 604 (20.0) | 543 (18.3) |
| Clinical | ||||
| Atrial fibrillation or flutter at screening | 352 (31.8) | 365 (31.7) | 794 (26.3) | 734 (24.8) |
| History of hypertension | 782 (70.7) | 791 (68.7) | 2128 (70.6) | 2083 (70.4) |
| Type 2 diabetes | 462 (41.8) | 492 (42.7) | 1190 (39.5) | 1165 (39.4) |
| History of stroke | 116 (10.5) | 124 (10.8) | 261 (8.7) | 253 (8.5) |
| Ischemic heart failure | 576 (52.1) | 637 (55.3) | 161 (5.3) | 1585 (53.5) |
| History of myocardial infarction | 443 (40.1) | 517 (44.9) | 1250 (41.5) | 1225 (41.4) |
| LVEF, mean (SD), % | 23.3 (5.3) | 23.5 (5.2) | 27.8 (6.2) | 27.7 (6.3) |
| NYHA class | ||||
| II | 0 | 0 | 2195 (72.8) | 2173 (73.4) |
| III | 1021 (92.3) | 1064 (92.4) | 780 (25.9) | 751 (25.4) |
| IV | 85 (7.7) | 88 (7.6) | 39 (1.3) | 36 (1.2) |
| KCCQ total symptom score, mean (95% CI) | 55.2 (36.5-75.0) | 58.3 (37.5-77.1) | 74.0 (54.2-89.6) | 74.0 (54.2-91.7) |
| Outpatient, mean (95% CI) | 61.5 (41.7-81.2) | 65.6 (46.9-85.4) | 77.1 (58.3-91.7) | 77.1 (58.3-92.2) |
| Inpatient, mean (95% CI) | 46.9 (29.2-68.8) | 47.9 (28.1-65.6) | 59.4 (38.5-77.1) | 56.2 (35.4-74.0) |
| SBP, mean (SD), mm Hg | 114.0 (15.3) | 113.5 (14.7) | 117.1 (15.4) | 117.9 (15.4) |
| Heart rate, mean (SD), beats/min | 74.5 (12.7) | 74.1 (12.3) | 71.7 (12.0) | 71.6 (11.9) |
| NT-proBNP, mean (95% CI), pg/mL | 2758 (1480-5838) | 2834 (1416-5732) | 1753 (864-3479) | 1795 (893-3540) |
| Cardiac troponin I, mean (95% CI), ng/L | 34 (18-64) | 34 (18-64) | 25 (11-47) | 25 (11-47) |
| eGFR, mean (95% CI), mL/min/1.73 m2 | 54.5 (40.9-69.1) | 55.5 (42.5-70.5) | 60.3 (46.1-75.7) | 59.6 (44.8-75.0) |
| Heart failure therapies | ||||
| ACE inhibitor, ARB, or ARN inhibitor | 926 (83.7) | 947 (82.2) | 2657 (88.2) | 2629 (88.8) |
| ARN inhibitor | 218 (19.7) | 229 (19.9) | 601 (19.9) | 553 (18.7) |
| β-blocker | 1019 (92.1) | 1074 (93.2) | 2861 (94.9) | 2809 (94.9) |
| MRA | 869 (78.6) | 899 (78.0) | 2330 (77.3) | 2299 (77.7) |
| SGLT-2 inhibitor | 23 (2.1) | 27 (2.3) | 81 (2.7) | 87 (2.9) |
| Ivabradine | 92 (8.3) | 96 (8.3) | 163 (5.4) | 182 (6.1) |
| Digitalis glycoside | 204 (18.4) | 232 (20.1) | 483 (16.0) | 466 (15.7) |
| Cardiac resynchronization | 183 (16.5) | 189 (16.4) | 409 (13.6) | 377 (12.7) |
| Implantable cardioverter-defibrillator | 402 (36.3) | 405 (35.2) | 924 (30.7) | 883 (29.8) |
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; ARN, angiotensin receptor neprilysin; eGFR, estimated glomerular filtration rate; KCCQ, Kansas City Cardiomyopathy Questionnaire; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NT-proBNP, N-terminal pro–B-type natriuretic peptide; NYHA, New York Heart Association; SBP, systolic blood pressure; SGLT2, sodium-glucose cotransporter-2.
Specific races and ethnicities included in this category were not reported or available.
Efficacy and Safety of Omecamtiv Mecarbil
Patients classified as having severe HF experienced a greater treatment benefit from omecamtiv mecarbil than those without severe HF. For the primary end point, patients with severe HF had a 20% risk reduction (hazard ratio [HR], 0.80; 95% CI, 0.71-0.90), whereas patients without severe HF had no significant risk reduction (HR, 0.99; 95% CI, 0.91-1.08, P for HF severity by treatment interaction = .005). These results among patients with vs without severe HF were similar for CV death (HR, 0.88 [95% CI, 0.75-1.03] vs 1.10 [95% CI, 0.97-1.25]; P for HF severity by treatment interaction = .03). Kaplan-Meier curves comparing patients with and without severe HF for each of these end points are shown in Figure 1.
Figure 1. Kaplan-Meier Curves.
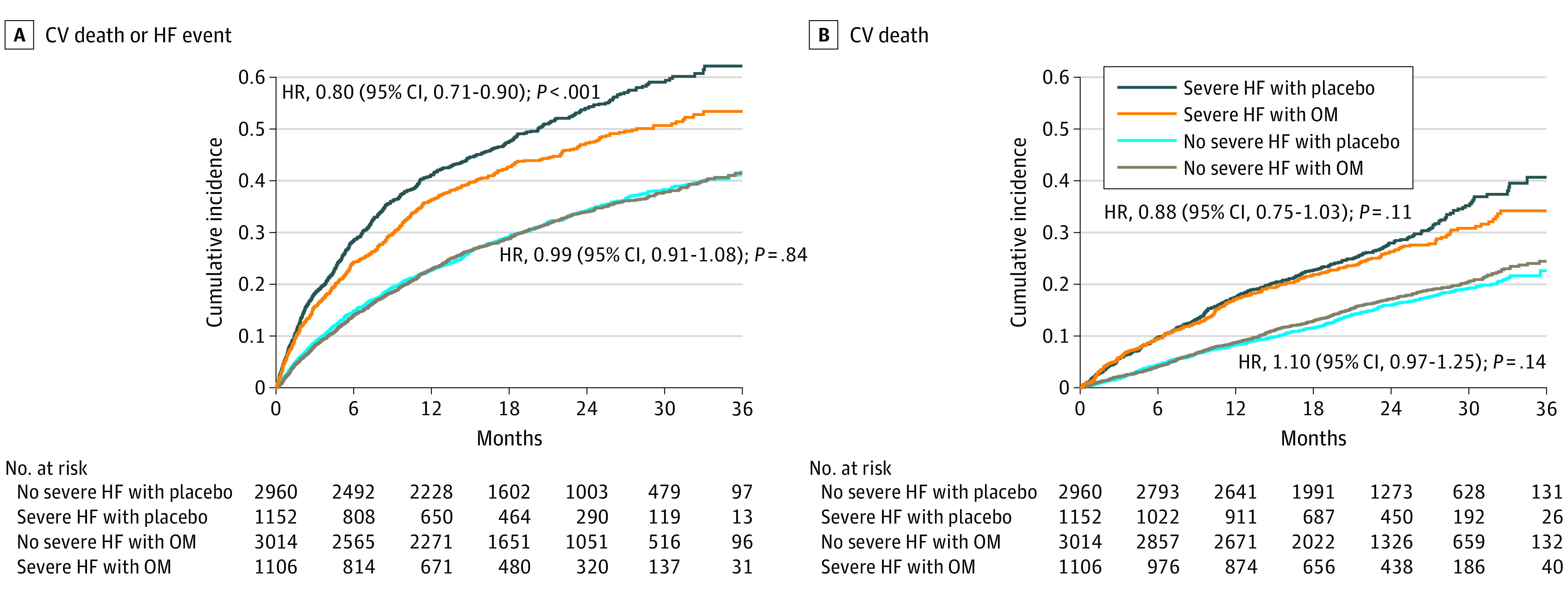
CV indicates cardiovascular; HF, heart failure; HR, hazard ratio; and OM, omecamtiv mecarbil.
In an additional sensitivity analysis, we further assessed the event rate and treatment effect of omecamtiv mecarbil based on which and how many severe HF criteria were met (Figure 2). The observed benefits of omecamtiv mecarbil therapy were greatest in patients who met all 3 severe HF criteria, who were also the group with the highest overall risk. The combination of a 20% relative risk reduction in the primary end point in the context of high baseline risk translated to an absolute risk reduction of 8.3 events per 100 patient-years (number needed to treat = 12; 34.3 events per 100 patient-years in the omecamtiv mecarbil group vs 42.6 events per 100 patient-years in the placebo group; P < .001). These results were broadly consistent across a variety of other secondary outcomes from the GALACTIC-HF clinical trial (Table 2). For the Kansas City Cardiomyopathy Questionnaire, we did not identify a differential effect on the total symptom score by severe HF status (severe HF: 1.1-point increase among inpatients and 1.7-point decrease among outpatients; nonsevere HF: 3.3-point increase among inpatients and 0.2-point decrease among outpatients; P = .09).
Figure 2. Event Rates for Primary End Point by Treatment Assignment and Heart Failure Severity Criteria Met.
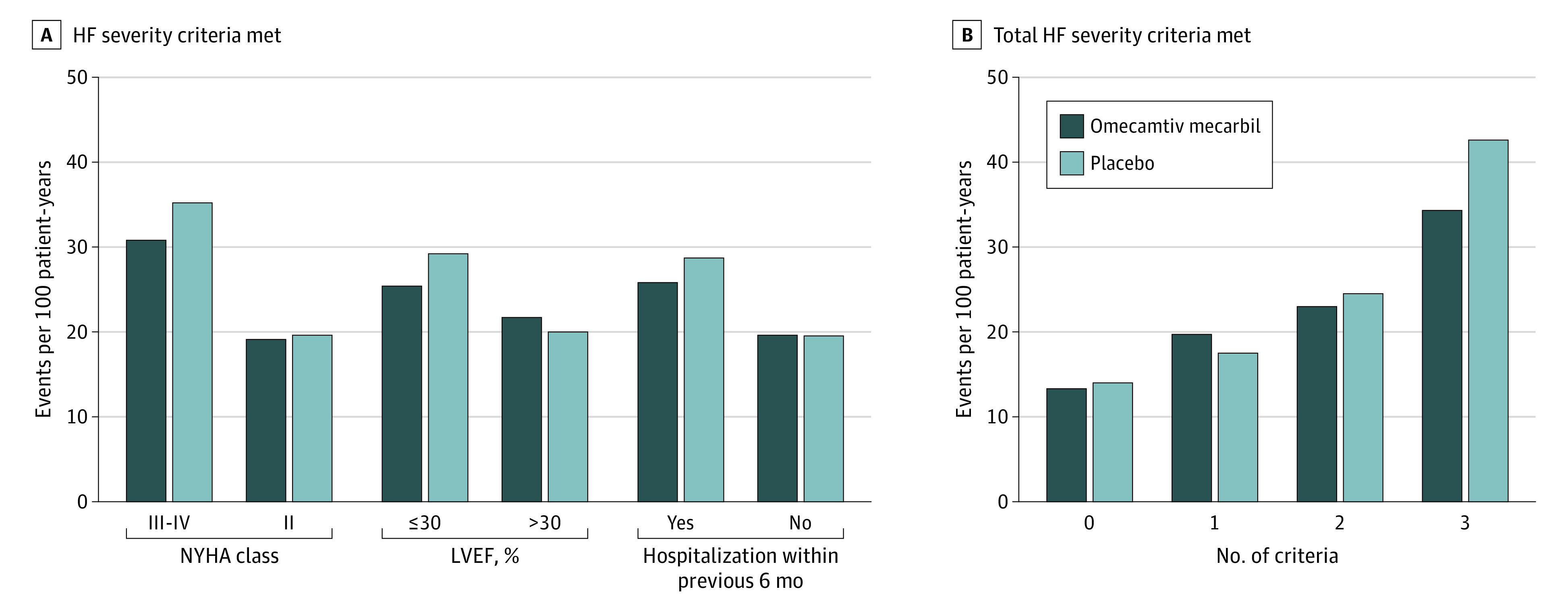
A, A total of 3864 patients were in NYHA class III to IV, 4368 patients were in NYHA class II, 5842 patients had LVEF of 30% or less, 2390 patients had LVEF greater than 30%, 6308 patients were hospitalized within the previous 6 months, and 1924 patients were not hospitalized within the previous 6 months. B, A total of 424 patients met 0 criteria, 1860 patients met 1 criterion, 3690 patients met 2 criteria, and 2258 patients met 3 criteria. HF indicates heart failure; LVEF, left ventricular ejection fraction; and NYHA, New York Heart Association.
Table 2. Event Rates by Treatment Group and Severe Heart Failure Classification.
| Outcome | Omecamtiv mecarbil | Placebo | HR (95% CI) | ARR per 100 patient-years | P value | ||
|---|---|---|---|---|---|---|---|
| No./total No. (%) | Events per 100 patient-years | No./total No. (%) | Events per 100 patient-years | ||||
| Severe heart failure | |||||||
| Primary end point (time to first HF event or CV death) | 510/1106 (46.1) | 34.3 | 611/1152 (53.0) | 42.6 | 0.80 (0.71-0.90) | 8.3 | <.001 |
| CV death as first primary event | 107/1106 (9.7) | 7.2 | 142/1152 (12.3) | 9.9 | NA | NA | NA |
| HF hospitalization as first primary event | 385/1106 (34.8) | 25.9 | 441/1152 (38.3) | 30.8 | NA | NA | NA |
| Urgent outpatient visit as first primary event | 18/1106 (1.6) | 1.2 | 28/1152 (2.4) | 2.0 | NA | NA | NA |
| CV death | 288/1106 (26.0) | 15.5 | 332/1152 (28.8) | 17.3 | 0.88 (0.75-1.03) | 1.8 | .11 |
| HF hospitalization | 396/1106 (35.8) | 26.4 | 455/1152 (39.5) | 31.3 | 0.84 (0.74-0.97) | 4.8 | .02 |
| All-cause death | 375/1106 (33.9) | 20.2 | 416/1152 (36.1) | 21.7 | 0.92 (0.80-1.06) | 1.5 | .24 |
| No severe heart failure | |||||||
| Primary end point (time to first HF event or CV death) | 1013/3014 (33.6) | 21.1 | 996/2960 (33.6) | 21.3 | 0.99 (0.91-1.08) | 0.2 | .84 |
| CV death as first primary event | 239/3014 (7.9) | 5.0 | 229/2960 (7.7) | 4.9 | NA | NA | NA |
| HF hospitalization as first primary event | 722/3014 (24.0) | 15.0 | 692/2960 (23.4) | 14.8 | NA | NA | NA |
| Urgent outpatient visit as first primary event | 52/3014 (1.7) | 1.1 | 75/2960 (2.5) | 1.6 | NA | NA | NA |
| CV death | 520/3014 (17.3) | 9.3 | 466/2960 (15.7) | 8.5 | 1.10 (0.97-1.25) | –0.8 | .14 |
| HF hospitalization | 746/3014 (24.8) | 15.4 | 724/2960 (24.5) | 15.3 | 1.01 (0.91-1.12) | –0.1 | .88 |
| All-cause death | 692/3014 (23.0) | 12.4 | 649/2960 (21.9) | 11.9 | 1.05 (0.94-1.17) | –0.6 | .37 |
Abbreviations: ARR, absolute risk reduction; CV, cardiovascular; HF, heart failure; HR, hazard ratio; NA, not applicable.
Safety data for omecamtiv mecarbil vs placebo by severe HF category are summarized in Table 3. Patients with severe HF were more likely to have treatment-emergent serious adverse events than patients without severe HF (1532 of 2251 patients [68.1%] vs 3276 of 5960 patients [55.0%], respectively), but these events were similar between patients with severe HF who received omecamtiv mecarbil (742 of 1103 patients [67.3%]) vs placebo (790 of 1148 patients [68.8%]; P = .43). There were no significant differences in adverse events associated with ventricular tachyarrhythmia between patients with severe HF who received omecamtiv mecarbil (80 of 1103 patients [7.3%]) vs placebo (86 of 1148 patients [7.5%]; P = .89). Patients with severe HF who received omecamtiv mecarbil vs placebo experienced a greater number of myocardial infarctions (42 of 1103 patients [3.8%] vs 29 of 1148 patients [2.5%], respectively; P = .08) but fewer index strokes (18 of 1103 patients [1.6%] vs 31 of 1148 patients [2.7%]; P = .08). In the severe HF group, myocardial infarctions were more common among patients with HF of ischemic origin (37 of 576 patients [6.4%] in the omecamtiv mecarbil group vs 20 of 637 patients [3.1%] in the placebo group) vs patients with HF of nonischemic origin (5 of 530 patients [0.9%] in the omecamtiv mecarbil group vs 9 of 515 patients [1.7%] in the placebo group).
Table 3. Safety by Treatment Group and Severe Heart Failure Classification.
| Event | No. (%) | RR (95% CI) | P value | |
|---|---|---|---|---|
| Omecamtiv mecarbil | Placebo | |||
| Severe heart failure | ||||
| Total patients, No. | 1103 | 1148 | NA | NA |
| Any treatment-emergent SAE | 742 (67.3) | 790 (68.8) | 0.98 (0.92-1.03) | .43 |
| AE associated with ventricular tachyarrhythmia | 80 (7.3) | 86 (7.5) | 0.98 (0.73-1.31) | .89 |
| Positively adjudicated MI | 42 (3.8) | 29 (2.5) | 1.51 (0.95-2.40) | .08 |
| First stroke | 18 (1.6) | 31 (2.7) | 0.60 (0.34-1.07) | .08 |
| No severe heart failure | ||||
| Total patients, No. | 3007 | 2953 | NA | NA |
| Any treatment-emergent SAE | 1631 (54.2) | 1645 (55.7) | 0.97 (0.93-1.02) | .26 |
| AE associated with ventricular tachyarrhythmia | 210 (7.0) | 218 (7.4) | 0.95 (0.79-1.14) | .58 |
| Positively adjudicated MI | 80 (2.7) | 89 (3.0) | 0.88 (0.66-1.19) | .41 |
| First stroke | 58 (1.9) | 81 (2.7) | 0.70 (0.50-0.98) | .04 |
Abbreviations: AE, adverse event; MI, myocardial infarction; NA, not applicable; RR, relative risk; SAE, serious adverse event.
Data on tolerability and changes in biomarkers are shown in eTable 2 in the Supplement. Consistent with the results of the overall clinical trial,12 treatment with omecamtiv mecarbil among patients with severe HF was not associated with changes in systolic blood pressure (difference in change from week 0 to week 24, 0.6 mm Hg; 95% CI, −0.7 to 2.0 mm Hg; P = .35), worsening of kidney function (difference in change in creatinine level from week 0 to week 24, −0.01 mg/dL; 95% CI, −0.04 to 0.02; P = .53), or worsening of potassium levels (difference in change from week 0 to week 24, −0.03 mmol/L; 95% CI, −0.08 to 0.02; P = .27) compared with placebo. The heart rate among those who received omecamtiv mecarbil vs placebo was slightly lower (difference in change from week 0 to week 24, −1.9 beats/min; 95% CI, −2.9 to −0.8 beats/min; P < .001). Among those with severe HF, omecamtiv mecarbil therapy was associated with a significant decrease in N-terminal pro–B-type natriuretic peptide concentration (from 2758 pg/mL [95% CI, 1480-5838 pg/mL] at week 0 to 1837 pg/mL [95% CI, 856-4043 pg/mL] at week 24; P = .002) and a small increase in circulating cardiac troponin I levels (median difference in change from week 0 to week 24, 5 ng/L; 95% CI, 3-7 ng/L; P < .001) (eTable 2 in the Supplement).
Discussion
In the current post hoc analysis of data from the GALACTIC-HF clinical trial, we found that treatment with omecamtiv mecarbil provided a clinically important improvement in outcomes among patients who met an accepted definition of severe HF. Despite substantial improvements in medical therapy for HFrEF, patients with severe HF continue to experience a high burden of symptoms, frequent HF hospitalizations, and high mortality. As HF worsens, the economic costs of care increase substantially, and patients with severe HF account for a disproportionate share of HF costs.15 Given that patients with severe HF have higher baseline risk, the 20% relative risk reduction found in this trial translated into a significant absolute risk reduction of 8.3 events per 100 patient-years (number needed to treat = 12) for the primary end point of time to first HF event or CV death.
As HF progresses, the pathologic manifestations of severely impaired systolic function and low cardiac output, including hypotension and progressive kidney insufficiency, often begin to predominate. These features progressively limit the ability to tolerate guideline-recommended HF therapies, such as β-blockers, renin-angiotensin-aldosterone system modulators, or mineralocorticoid receptor antagonists,16 creating a mismatch between patient risk and intensity of medical therapy.17 Omecamtiv mecarbil differs from other HF therapies because it directly targets systolic performance rather than modulating associated neurohormonal perturbations. Unlike other HFrEF treatments, omecamtiv mecarbil does not lower blood pressure, affect kidney function, or alter potassium homeostasis, allowing its use in patients with cardiorenal limitations that prevent the use of other HF therapies. Among patients classified as having severe HF in the GALACTIC-HF study, no significant difference was found in systolic blood pressure, serum creatinine, or serum potassium levels at 24 weeks between those who received omecamtiv mecarbil vs placebo. Consistent with previous studies,9,10,11,12 patients with severe HF who were randomized to receive omecamtiv mecarbil experienced a modest increase in cardiac troponin I levels (between-group median difference of 5 ng/L from baseline to 24 weeks). In the population with severe HF, a nonsignificant imbalance in the number of myocardial infarctions (3.8% for omecamtiv mecarbil vs 2.5% for placebo; P = .08) also occurred. These findings should be considered in the context of a clinically important improvement in the primary end point as well as favorable point estimates for CV death and all-cause death among those who received omecamtiv mecarbil therapy. Overall, these data support both the efficacy and tolerability of omecamtiv mecarbil in a patient population that may be difficult to treat effectively with other HF drugs.
Terms and definitions for severe HF differ widely in the literature and describe various overlapping populations with different levels of severity. Previous data from the GALACTIC-HF clinical trial have demonstrated a clear relationship between LVEF and the treatment effect of omecamtiv mecarbil, but LVEF is only 1 potential marker of HF severity.18 Patients with true end-stage HF who may require mechanical support, cardiac transplant, or hospice care (referred to as patients with stage D disease in the American College of Cardiology/American Heart Association guidelines3) represent a small proportion of the HF population (approximately 2% in an unselected community cohort19) and were not the focus of the current analysis. Patients requiring intravenous inotropic therapy or mechanical ventilatory or circulatory support were excluded from the GALACTIC-HF clinical trial. An increasing population of ambulatory patients with HF have substantial symptoms, severely impaired cardiac performance, and frequent hospitalizations but do not yet require advanced HF therapies, such as mechanical support or cardiac transplant. These patients, who represented 27.4% of the GALACTIC-HF population, were straightforward to identify based on 3 readily available parameters (NYHA symptom class III-IV, LVEF ≤30%, and HF hospitalization within the previous 6 months). Patients who did not meet all 3 of these criteria had lower absolute risk and lower relative and absolute benefit from omecamtiv mecarbil therapy compared with placebo.
Limitations
This study has limitations. The population with severe HF that is the focus of the current analysis was defined post hoc and, as such, is subject to the limitations of such analyses. To enhance validity, we used the framework of a published scientific statement from the Heart Failure Association of the European Society of Cardiology to define severe HF and modified the definition to accommodate limitations of the source data.2 Although these data are focused on a subgroup of the overall GALACTIC-HF population and therefore subject to the known limitations of statistical power associated with subgroup analyses, the severe HF subgroup included 2258 patients, making the sample substantially larger than those of other studies that have assessed medical therapy in this population.20,21,22,23 Although the GALACTIC-HF study enrolled patients with relatively severe kidney impairment, patients who had an estimated glomerular filtration rate lower than 20 mL/min/1.73 m2 or were receiving dialysis at screening were excluded.
Conclusions
Among patients with severe HF defined by NYHA symptom class, LVEF, and recent HF hospitalization, omecamtiv mecarbil therapy may have provided a clinically meaningful reduction in the composite end point of time to first HF event or CV death. These data may support the possible role of omecamtiv mecarbil therapy in patients for whom current treatment options are limited.
eTable 1. Baseline Characteristics by Severe Heart Failure Status
eTable 2. Tolerability by Treatment Group and Severe Heart Failure Status
eFigure. Venn Diagram of Groups Defined by Specific Severe Heart Failure Criteria
References
- 1.Fang JC, Ewald GA, Allen LA, et al. ; Heart Failure Society of America Guidelines Committee . Advanced (stage D) heart failure: a statement from the Heart Failure Society of America Guidelines Committee. J Card Fail. 2015;21(6):519-534. doi: 10.1016/j.cardfail.2015.04.013 [DOI] [PubMed] [Google Scholar]
- 2.Crespo-Leiro MG, Metra M, Lund LH, et al. Advanced heart failure: a position statement of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2018;20(11):1505-1535. doi: 10.1002/ejhf.1236 [DOI] [PubMed] [Google Scholar]
- 3.Yancy CW, Jessup M, Bozkurt B, et al. ; Writing Committee Members; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines . 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128(16):e240-e327. doi: 10.1161/CIR.0b013e31829e8776 [DOI] [PubMed] [Google Scholar]
- 4.Bozkurt B, Coats AJ, Tsutsui H, et al. Universal definition and classification of heart failure: a report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure. J Card Fail. Published online March 1, 2021. doi: 10.1016/j.cardfail.2021.01.02233663906 [DOI] [Google Scholar]
- 5.Metra M, Ponikowski P, Dickstein K, et al. ; Heart Failure Association of the European Society of Cardiology . Advanced chronic heart failure: a position statement from the Study Group on Advanced Heart Failure of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2007;9(6-7):684-694. doi: 10.1016/j.ejheart.2007.04.003 [DOI] [PubMed] [Google Scholar]
- 6.Allen LA, Stevenson LW, Grady KL, et al. ; American Heart Association; Council on Quality of Care and Outcomes Research; Council on Cardiovascular Nursing; Council on Clinical Cardiology; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Surgery and Anesthesia . Decision making in advanced heart failure: a scientific statement from the American Heart Association. Circulation. 2012;125(15):1928-1952. doi: 10.1161/CIR.0b013e31824f2173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allen LA, Fonarow GC, Grau-Sepulveda MV, et al. ; American Heart Association’s Get With the Guidelines Heart Failure Investigators . Hospital variation in intravenous inotrope use for patients hospitalized with heart failure: insights from Get With the Guidelines. Circ Heart Fail. 2014;7(2):251-260. doi: 10.1161/CIRCHEARTFAILURE.113.000761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nizamic T, Murad MH, Allen LA, et al. Ambulatory inotrope infusions in advanced heart failure: a systematic review and meta-analysis. JACC Heart Fail. 2018;6(9):757-767. doi: 10.1016/j.jchf.2018.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teerlink JR, Clarke CP, Saikali KG, et al. Dose-dependent augmentation of cardiac systolic function with the selective cardiac myosin activator, omecamtiv mecarbil: a first-in-man study. Lancet. 2011;378(9792):667-675. doi: 10.1016/S0140-6736(11)61219-1 [DOI] [PubMed] [Google Scholar]
- 10.Cleland JGF, Teerlink JR, Senior R, et al. The effects of the cardiac myosin activator, omecamtiv mecarbil, on cardiac function in systolic heart failure: a double-blind, placebo-controlled, crossover, dose-ranging phase 2 trial. Lancet. 2011;378(9792):676-683. doi: 10.1016/S0140-6736(11)61126-4 [DOI] [PubMed] [Google Scholar]
- 11.Teerlink JR, Felker GM, McMurray JJV, et al. ; COSMIC-HF Investigators . Chronic oral study of myosin activation to increase contractility in heart failure (COSMIC-HF): a phase 2, pharmacokinetic, randomised, placebo-controlled trial. Lancet. 2016;388(10062):2895-2903. doi: 10.1016/S0140-6736(16)32049-9 [DOI] [PubMed] [Google Scholar]
- 12.Teerlink JR, Diaz R, Felker GM, et al. Cardiac myosin activation with omecamtiv mecarbil in systolic heart failure. N Engl J Med. 2021;384(2):105-116. doi: 10.1056/NEJMoa2025797 [DOI] [PubMed] [Google Scholar]
- 13.Teerlink JR, Diaz R, Felker GM, et al. ; GALACTIC-HF Investigators . Omecamtiv mecarbil in chronic heart failure with reduced ejection fraction: GALACTIC-HF baseline characteristics and comparison with contemporary clinical trials. Eur J Heart Fail. 2020;22(11):2160-2171. doi: 10.1002/ejhf.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teerlink JR, Diaz R, Felker GM, et al. Omecamtiv mecarbil in chronic heart failure with reduced ejection fraction: rationale and design of GALACTIC-HF. JACC Heart Fail. 2020;8(4):329-340. doi: 10.1016/j.jchf.2019.12.001 [DOI] [PubMed] [Google Scholar]
- 15.Unroe KT, Greiner MA, Hernandez AF, et al. Resource use in the last 6 months of life among Medicare beneficiaries with heart failure, 2000-2007. Arch Intern Med. 2011;171(3):196-203. doi: 10.1001/archinternmed.2010.371 [DOI] [PubMed] [Google Scholar]
- 16.Stewart GC, Kittleson MM, Patel PC, et al. INTERMACS (Interagency Registry for Mechanically Assisted Circulatory Support) profiling identifies ambulatory patients at high risk on medical therapy after hospitalizations for heart failure. Circ Heart Fail. 2016;9(11):e003032. doi: 10.1161/CIRCHEARTFAILURE.116.003032 [DOI] [PubMed] [Google Scholar]
- 17.Lee DS, Tu JV, Juurlink DN, et al. Risk-treatment mismatch in the pharmacotherapy of heart failure. JAMA. 2005;294(10):1240-1247. doi: 10.1001/jama.294.10.1240 [DOI] [PubMed] [Google Scholar]
- 18.Teerlink JR, Diaz R, Felker GM, et al. Effect of ejection fraction on clinical outcomes in patients treated with omecamtiv mecarbil in GALACTIC-HF. J Am Coll Cardiol. 2021;78(2):97-108. doi: 10.1016/j.jacc.2021.04.065 [DOI] [PubMed] [Google Scholar]
- 19.Ammar KA, Jacobsen SJ, Mahoney DW, et al. Prevalence and prognostic significance of heart failure stages: application of the American College of Cardiology/American Heart Association heart failure staging criteria in the community. Circulation. 2007;115(12):1563-1570. doi: 10.1161/CIRCULATIONAHA.106.666818 [DOI] [PubMed] [Google Scholar]
- 20.Mann DL, Greene SJ, Givertz MM, et al. ; LIFE Investigators . Sacubitril/valsartan in advanced heart failure with reduced ejection fraction: rationale and design of the LIFE trial. JACC Heart Fail. 2020;8(10):789-799. doi: 10.1016/j.jchf.2020.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Packer M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med. 1996;334(21):1349-1355. doi: 10.1056/NEJM199605233342101 [DOI] [PubMed] [Google Scholar]
- 22.Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341(10):709-717. doi: 10.1056/NEJM199909023411001 [DOI] [PubMed] [Google Scholar]
- 23.Metra M, Eichhorn E, Abraham WT, et al. ; ESSENTIAL Investigators . Effects of low-dose oral enoximone administration on mortality, morbidity, and exercise capacity in patients with advanced heart failure: the randomized, double-blind, placebo-controlled, parallel group ESSENTIAL trials. Eur Heart J. 2009;30(24):3015-3026. doi: 10.1093/eurheartj/ehp338 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Baseline Characteristics by Severe Heart Failure Status
eTable 2. Tolerability by Treatment Group and Severe Heart Failure Status
eFigure. Venn Diagram of Groups Defined by Specific Severe Heart Failure Criteria


