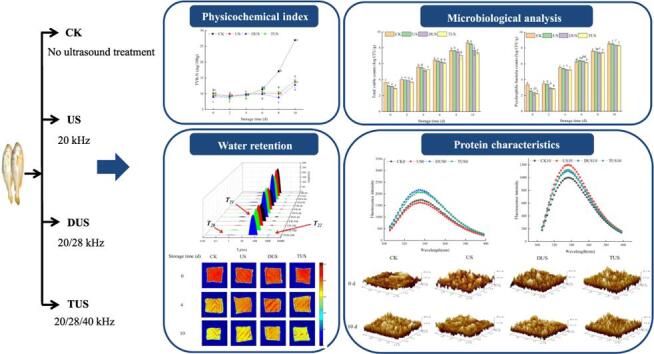
Graphical abstract
Keywords: Multi-frequency ultrasound, Large yellow croaker, Surface decontamination, Structural characteristics
Highlights
-
•
Multi-frequency ultrasound retarded the growth of microorganisms.
-
•
Increase in the number of ultrasonic frequencies enhanced the bacteriostatic effect.
-
•
DUS treated samples retained better structural characteristics.
-
•
AFM observation was adopted to study the degradation of MPs of refrigerated fish.
Abstract
The effects of multi-frequency ultrasound on surface decontamination and structural characteristics of large yellow croaker (Pseudosciaena crocea) during refrigerated storage were evaluated. The results of total viable counts (TVCs) and psychrophilic bacteria counts (PBCs) demonstrated that multi-frequency ultrasound retarded the growth of microorganisms. The bacteriostatic effect was positively correlated with the increase of ultrasound frequencies. However, compared with triple-frequency ultrasound (TUS, 20/28/40 kHz) treatment, dual-frequency ultrasound (DUS, 20/28 kHz) treatment had higher water-holding capacity (WHC) and immobilized water content, better texture characteristics, lower pH and total volatile basic nitrogen (TVB-N). Through the results of myofibrillar fragmentation index (MFI), intrinsic fluorescence intensity (IFI) and atomic force microscope (AFM), multi-frequency ultrasound could effectively stabilize the myofibrillar protein structure of refrigerated large yellow croaker, which could maintain better texture characteristics. The effects of DUS were the most significant. Therefore, multi-frequency ultrasound treatment could inhibit the growth of microorganisms and improve the structural characteristics of large yellow croaker during refrigerated storage.
1. Introduction
Large yellow croaker (Pseudosciaena crocea) is a kind of marine fish with important commercial value, which widely cultivated in Southeast China due to its high nutritional value and delicious taste [1]. However, large yellow croaker is readily affected by enzymes, microorganisms and oxidative processes during storage, which causes muscle tissues corrupted. Microbial activity is the main cause during post-mortem storage, which produces a large amount of metabolites and spoilage odor [2]. Nowadays, the demand of consumers for food safety is increasing, while maintaining its good sensory properties has led the food industry to develop potential preservation technologies continuously [3]. Controlling corruption and delaying quality deterioration are still the major challenges for aquatic products industry.
In recent years, the application of non-thermal technologies in food industry is getting more and more attention, such as pulsed electric fields (PEF), ultra-high hydrostatic pressure (UHP) and ultrasound (US). At present, the application of US to protein and sterilization has received extensive attention. US can cause cavitation bubbles, high temperature, high pressure, etc., which may destroy the structure of microorganisms, change the structure and functional properties of proteins, thereby improving the quality of aquatic products [4], [5]. In addition, US treatment is a “green” technology that reduces energy usage, shorter processing time and no chemical additives, making consumers more at ease [6]. Most of the current researches on US in aquatic products only use single-frequency ultrasound treatment. Antunes-Rohling et al. [7] reported that US treatment (2.9 W/kg) could greatly make the quality of thawed cod fillets better. Pedrós-Garrido et al.[3] also found that the US with high intensity (30 kHz, 51.41 W/L) treatment for 45 min could significantly reduce microbiological counts and improve the quality of salmon (S. salar), mackerel (S. scombrus), cod (G. morhua) and hake (M. merluccius) fillets. He et al. [4] reported that the number of Escherichia coli O157:H7 was decreased 0.76–3.52 log CFU/mL with US treatment (64, 191, 372, and 573 W/cm2, 20 kHz) for 27 min. However, Huang et al. [8] found that dual-frequency ultrasound had a wider scope of energy dissipation compared with single-frequency ultrasound. Ma et al. [9] also demonstrated that multi-frequency ultrasound treatment significantly improved the freezing rate and quality of frozen large yellow croaker.
Based on the above mentioned research results, there was little research on the effects of multi-frequency ultrasound on the quality changes of aquatic products during storage. Therefore, the aim of this study was to investigate the effects of multi-frequency ultrasound on surface decontamination and structural characteristics of refrigerated large yellow croaker.
2. Materials and methods
2.1. Preparation of samples
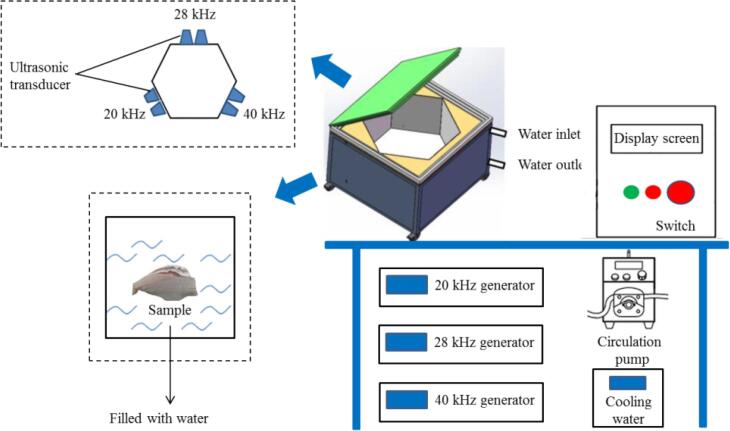
Forty fresh large yellow croakers (weight 500.0 ± 25.0 g) were purchased from the local market (Shanghai, China) and transported to the laboratory alive immediately. Then samples were randomly divided into four groups: (1) samples immersed in sterile distilled water (CK, n = 10); (2) samples treated with 20 kHz single-frequency ultrasound (US, n = 10); (3) samples treated with 20 and 28 kHz dual-frequency ultrasound (DUS, n = 10); (4) samples treated with 20, 28 and 40 kHz triple-frequency ultrasound (TUS, n = 10). All samples were immersed for 10 min, dried, put into PE sterile bags and stored at 4 °C. The conditions of ultrasound treatment were referred to the results of previous research [9]. The multi-frequency ultrasound equipment (Fig. 1) was designed by Shandong Xiecheng Ultrasound Equipment Co., Ltd., (Qingdao, China). Moreover, the ultrasound system consists of a hexahedral ultrasound processing system (side length: 60 cm) and three ultrasound transducers.
Fig. 1.
Schematic diagrams of the multi-frequency ultrasound device.
2.2. Microbiological analysis
Referring to the method of Lan et al. [10], total viable counts (TVCs) and psychrophilic bacteria counts (PBCs) were determined. The bacteria of TVCs was aerobically cultured by using plate count agar (Qingdao Haibo Biotechnology Co., Ltd., China) at 30 ± 2 °C for 72 h; The psychrophilic bacteria was aerobically cultured by using plate count agar at 4 °C for 10 days;.
2.3. Physicochemical indexes
2.3.1. pH value
According to the method of Lan et al [10], a pH meter (Mettler toledo, Shanghai, China) was used to measure the pH value.
2.3.2. Total volatile basic nitrogen (TVB-N)
The TVB-N of samples was determined by using a Kjeldahl (FOSS, Denmark), according to the method of Lan et al [10].
2.3.3. Texture profile analysis (TPA)
TPA was performed using a TA. XT Plus texture analyser (Stable Micro Systems., Ltd, UK), which used a P/5 probe. The test speed was 1 m/s and the sample deformation was 50%. Each sample was tested 6 times.
2.4. Water retention
2.4.1. Water-holding capacity (WHC)
According to the method of Feng et al. [11], the WHC was measured. The weight of the sample before centrifugation was recorded as m1, and the weight after centrifugation was recorded as m2. The WHC was obtained by formula 1:
| (1) |
2.4.2. Low-field nuclear magnetic resonance (LF-NMR) and magnetic resonance imaging (MRI)
LF-NMR and MRI were measured by using a LF-NMR analyzer (Shanghai Niumag Electronic Technology Co., Ltd., Shanghai, China), according to the method of Lan et al. [12].
2.5. Protein characteristics
2.5.1. Preparation of myofibrillar proteins (MPs)
For the extraction of MPs, refer to the method of Yang et al. [13] with slightly modifications. Buffer configuration: Buffer A was 20 mM phosphate buffer containing 100 mM NaCl, 1 mM EDTA, pH 7.0; Buffer B was 25 mM phosphate buffer containing 0.6 mM NaCl, pH 7.0. 2.0 g samples plus 20 mL buffer A, ice water bath homogenizated at 12000 × g (30 s/time) twice and centrifuged at 12000 × g for 15 min. After repeating the above steps, add 15 mL buffer B to the precipitate, mix well and filter with double gauze. The filtrate is myofibrillar protein solution.
2.5.2. Myofibrillar fragmentation index (MFI)
Referring to the method of Zou et al. [14] with slight modifications, MFI was measured. The concentration of MPs was diluted to 0.5 mg/mL and then measured at 540 nm for 5 times by using a microplate reader (BioTek, US). The MFI was obtained by formula (2):
| (2) |
2.5.3. Intrinsic fluorescence intensity (IFI)
The IFI of MPs was collected by the JASCO FP-8300 fluorescence photometer. The MPs solution was diluted to 0.1% (w/v) by using phosphate buffer B. The analysis conditions: excitation wavelength of 290 nm, emission wavelength of 300 ∼ 400 nm, slit width of 5 nm and voltage of 700 mV.
2.5.4. Atomic force microscope (AFM) for MPs
The surface morphology of the MPs was analyzed using an AFM (Bruker Co., Santa Barbara, CA) by the method of Hu et al. [15]. For observing the MPs particles, MPs suspensions (20 µL, 10 µg/mL) were deposited onto the mica and dried in air at room temperature for 12 h. The analysis conditions: the scan area was 2.0 × 2.0 μm2, the scan frequency was 0.998 Hz and the height images in 512 × 512 pixels. The AFM images were analyzed by the software of Nanoscope Analysis 1.8 (Bruker Co., Santa Barbara, CA).
2.6. Statistical analysis
All data were analyzed by SPSS 19.0 (IBM Corporation, USA) and Origin 8.5(Pro) (Origin Lab Corporation, USA). All measurements were parallel tested in triplicate.
3. Results and discussion
3.1. Microbiological analysis
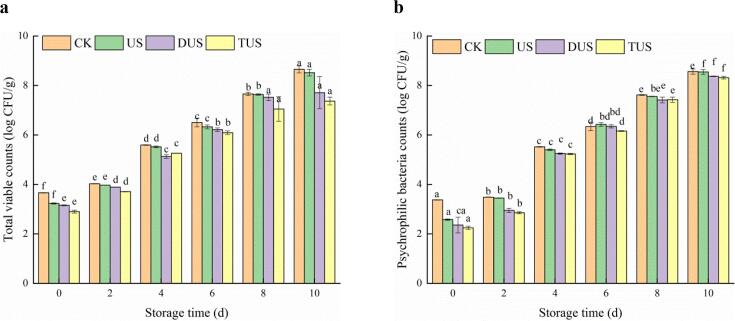
Figure 2 showed the changes of TVCs and PBCs of refrigerated large yellow croaker. Compared with CK group, the ultrasound treatment significantly (P < 0.05) inhibited microbial growth (Fig. 4a). Joyce et al. [16] found that the cavitation of ultrasound could produce the ultra-high pressure that made the microbial cells broken down, thereby restrained the growth and reproduction of microorganisms. Although the TVCs of all groups outnumbered the limit of 7.0 log CFU/g on day 8, the TVCs of TUS group were still lower than those of other groups significantly (P < 0.05). This might be due to multi-frequency ultrasound could strengthen cavitation enhancement, which led to a stronger antimicrobial effect compared with single-frequency ultrasound [17]. Zhou et al [18] observed the inactivation rate higher in the dual-frequency ultrasound than the triple-frequency ultrasound. Ultrasound treatment could reduce the TVCs of fresh samples by up to 0.76 log CFU/g on day 0 (CK: 3.66 log CFU/g, TUS: 2.90 log CFU/g). Nguyen Huu et al.[19] also found that the 40 kHz ultrasound treatment within 30 and 45 min did not significantly reduce the number of E. coli O157:H7 and L. innocua. This limitation has made ultrasound treatment to be used along with other methods so as to attain the killing effects of microorganisms. The growth pattern for PBCs (Fig. 4b) was similar to that of TVCs, and the PBCs increased in all samples during refrigerated storage.
Fig. 2.
Changes of total viable counts (TVCs) (a), psychrophilic bacteria counts (PBCs) (b) in large yellow croaker with different treatments during refrigerated storage. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
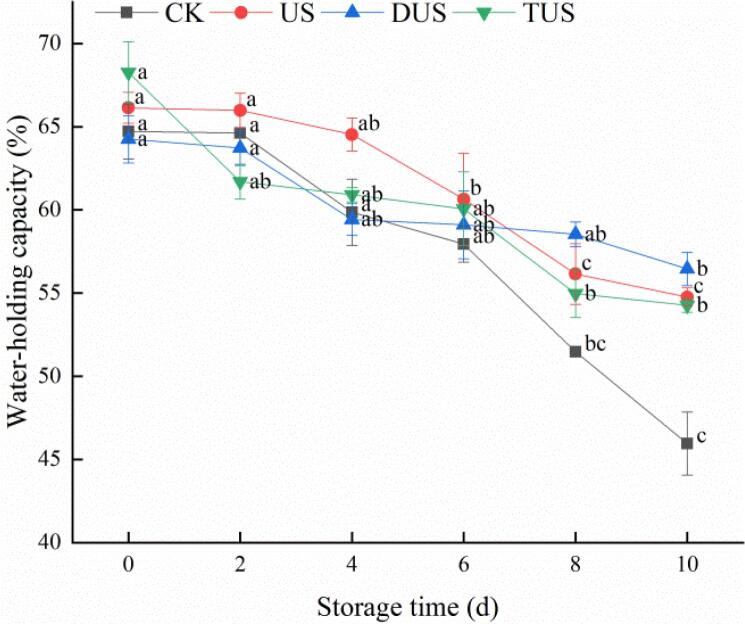
Fig. 4.
Changes of water-holding capacity (WHC) in large yellow croaker with different treatments during refrigerated storage. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.2. Physicochemical indexes
3.2.1. pH value
Samples treated with ultrasound showed lower pH values than that of CK group at the end of storage (Table 1). However, the pH values of samples that treated with ultrasound were higher than that of CK group at the beginning of storage. This might be due to the destruction of tissue and cell structure by ultrasound, which changed the protein conformation of samples, causing the acidic groups to be buried, leading to the rise of pH value [20]. Ultrasound treatment could also promote the diffusion of ions from the cell structure to the cytoplasm, resulting in the change of the position of ionic functional groups and further improve the pH value [21]. Moreover, the pH value of each group decreased within 2 d, increasing from 2 to 10 d. There were some possible reasons. On the one hand, glycolysis would produce acidic substances during the death of fish, which led to the decrease of pH value [22]. On the other hand, with the extension of storage time, the microorganisms and endogenous enzymes would decompose muscle protein to produce alkaline substances, which led to the increase of pH value [23].
Table 1.
Changes of pH value in large yellow croaker with different treatments during refrigerated storage.
| Group | Storage time (d) |
|||||
|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 8 | 10 | |
| CK | 7.36 ± 0.02Bab | 7.07 ± 0.04Ac | 7.27 ± 0.06ABbc | 7.35 ± 0.01Aab | 7.31 ± 0.02Aabc | 7.53 ± 0.01Aa |
| US | 7.61 ± 0.01Aa | 6.94 ± 0.02Aab | 7.15 ± 0.01Bbc | 7.32 ± 0.02Ac | 7.27 ± 0.02Ac | 7.45 ± 0.04Ad |
| DUS | 7.49 ± 0.01ABa | 6.94 ± 0.01Ab | 7.31 ± 0.02Ab | 7.32 ± 0.02Ab | 7.26 ± 0.02Ab | 7.33 ± 0.02Ac |
| TUS | 7.51 ± 0.01ABa | 7.01 ± 0.03Aa | 7.18 ± 0.04ABa | 7.41 ± 0.03Ab | 7.19 ± 0.01Ab | 7.39 ± 0.03Ac |
Values are means ± standard deviation. The different capital letters on the same row within different treatment represented significant differences (P < 0.05). The different lowercase letter on the same column within different storage time represented significant differences (P < 0.05).
3.2.2. TVB-N
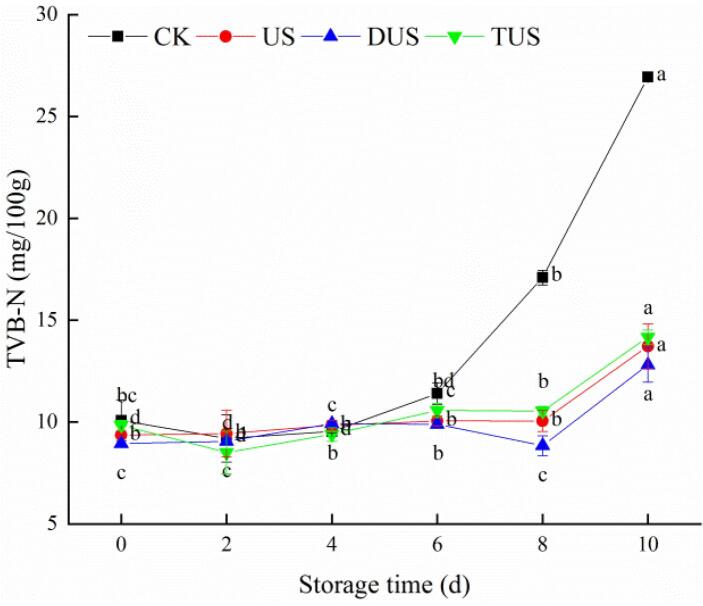
The TVB-N values of all groups showed lower than the acceptance limit (GB/T 18108–2019). The initial TVB-N value of the CK group was 10.07 mg/100 g (Fig. 3). Before the 6th day, the TVB-N values of all groups rose extremely slowly. On day 10, TVB-N value of the CK group increased rapidly to 26.93 mg/100 g, which closed to the corruption limit. Meanwhile, TVB-N values of US, DUS and TUS groups were 13.72 mg/100 g, 12.81 mg/100 g and 14.17 mg/100 g, which showed that ultrasound treatment could notably (P < 0.05) restrain the formation of TVB-N of refrigerated large yellow croaker. The drastically increase of TVB-N might be owing to the microorganisms degraded nitrogen-containing macromolecules into volatile small molecule compounds, which usually occurs in the late period of storage [24]. Wang et al. [25] also found that 20/40 kHz ultrasound thawing could better protect the muscle structure and inhibit the reproduction of microorganisms, thereby reducing the TVB-N value.
Fig. 3.
Changes of total volatile basic nitrogen (TVB-N) in large yellow croaker with different treatments during refrigerated storage. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.2.3. TPA
It can be seen from Table 2 that different ultrasound frequencies had important impacts on the changes in muscle texture of large yellow croakers.. The values of hardness, springiness, resilience, and chewiness of samples treated with ultrasound appeared no significant difference (P > 0.05). TPA values of all samples decreased greatly with the extension of storage time. The texture softening in fillets during storage was mainly attributed to protein deterioration by the action of endogenous cathepsins and exogenous proteases [26]. In contrast, after 10 days of storage, the textural values of the DUS group were lowest. It might be because multi-frequency ultrasound could produce more cavitation effect, which formed more cavitation nuclei [25]. The results showed that DUS pretreatment exhibited a notable improvement in maintaining textural quality of refrigerated large yellow croaker.
Table 2.
Changes of TPA in large yellow croaker with different treatments during refrigerated storage.
| TPA | Group | Storage time (d) |
|||||
|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 8 | 10 | ||
| Hardness/g | CK | 1965.54 ± 0.39Ba | 1707.07 ± 0.97Aab | 1418.77 ± 1.03Ab | 1246.28 ± 1.23Bb | 1202.51 ± 1.27Ab | 1139.87 ± 2.77Ab |
| US | 2262.45 ± 1.86ABa | 1817.72 ± 0.68Aab | 1746.58 ± 1.22Aab | 1446.17 ± 1.11ABb | 1445.07 ± 0.37Ab | 1175.22 ± 1.27Ab | |
| DUS | 2023.18 ± 1.85Ba | 1748.02 ± 1.05Aab | 1636.59 ± 0.9Aab | 1633.83 ± 0.6ABab | 1460.81 ± 1.58Ab | 1204.74 ± 0.47Ab | |
| TUS | 2379.92 ± 0.68Aa | 1887.79 ± 1.35Ab | 1632.93 ± 1.38Abc | 1558.00 ± 0.94Abc | 1329.01 ± 1.15Ac | 1173.47 ± 1.37Ad | |
| Springiness/% | CK | 49.87 ± 0.97Aa | 48.97 ± 0.66Aa | 47.68 ± 0.43Aa | 46.84 ± 1.16Aab | 42.86 ± 1.38Abc | 39.35 ± 1.41Bc |
| US | 50.59 ± 1.08Aa | 48.69 ± 1.55Aab | 45.17 ± 0.41Aab | 44.79 ± 0.67Aab | 43.74 ± 1.16Aab | 38.04 ± 0.72Bb | |
| DUS | 52.32 ± 0.58Aa | 47.80 ± 0.5Aab | 46.48 ± 1.69Aab | 45.71 ± 1.53Aab | 44.78 ± 0.91Aab | 43.85 ± 1.37Ab | |
| TUS | 49.16 ± 0.94Aa | 48.87 ± 1.51Aa | 48.11 ± 0.93Aa | 46.15 ± 1.60Aa | 42.44 ± 0.71Aa | 40.73 ± 0.21Bba | |
| Resilience/% | CK | 17.17 ± 0.19Aa | 15.16 ± 0.28Bb | 14.32 ± 0.43Ab | 13.48 ± 1.56Ab | 13.43 ± 0.02Ab | 10.40 ± 1.73Bc |
| US | 16.73 ± 1.62Aa | 16.51 ± 1.00ABa | 14.86 ± 0.08Aab | 14.59 ± 0.57ABab | 13.4 ± 0.23Aab | 12.70 ± 1.24ABb | |
| DUS | 18.83 ± 0.76Aa | 17.92 ± 0.41Aa | 15.53 ± 1.55Aa | 15.41 ± 0.12ABa | 14.43 ± 2.29Aa | 13.23 ± 6.03Aa | |
| TUS | 18.85 ± 0.44Aa | 17.13 ± 1.10Aab | 16.81 ± 2.22Aab | 15.13 ± 0.56Abc | 14.06 ± 0.58Abc | 12.73 ± 2.31ABc | |
| Chewiness | CK | 360.01 ± 1.27Aa | 317.73 ± 0.21Aab | 295.19 ± 2.41Aab | 252.06 ± 0.72Abc | 218.9 ± 0.61Abc | 163.35 ± 1.87ABc |
| US | 352.22 ± 0.75Aa | 306.45 ± 1.32Aab | 248.49 ± 0.66Aab | 228.46 ± 1.53Aab | 227.68 ± 0.88Aab | 174.29 ± 1.87ABb | |
| DUS | 372.05 ± 0.48Aa | 326.89 ± 0.89Aa | 298.41 ± 1.03Aa | 243.01 ± 0.15Aa | 234.24 ± 1.36Aa | 221.71 ± 0.33Aa | |
| TUS | 372.84 ± 1.67Aa | 342.77 ± 0.13Aa | 311.39 ± 0.88Aa | 229.5 ± 1.42Ab | 185.75 ± 0.64Ab | 116.64 ± 1.42Bc | |
Values are means ± standard deviation. The different capital letters on the same row within different treatment represented significant differences (P < 0.05). The different lowercase letter on the same column within different storage time represented significant differences (P < 0.05).
3.3. Water retention
3.3.1. WHC
The changes of WHC in large yellow croaker during storage were shown in Fig. 4. All groups showed the same downward trend, while the CK groups exhibited a greater water loss than other groups. In CK, US, DUS and TUS groups, the initial WHC of large yellow croaker were 64.72%, 66.14%, 64.25%, and 68.27%, and then it decreased to 45.95%, 54.76%, 56.45%, and 54.27% on day 10, respectively. From the results, ultrasound treatment reduced water loss of samples, which might be due to a suitable ultrasound power loosen the pores between muscle fibers and increase water retention in muscle [27]. Although the WHC of US, DUS and TUS groups had no significant difference (P > 0.05) on day 10, the DUS group had minimal water loss of samples. The increase of WHC might be attributed to the high values of TPA [28].
3.3.2. LF-NMR and MRI
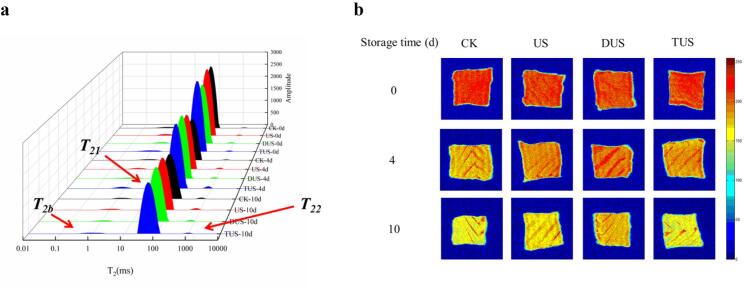
The freshness of fish could be assessed by LF-NMR [29]. The three peaks correspond to three relaxation components, called T21 (<10 ms, bound water), T22 (20–400 ms, immobilized water) and T23 (>1000 ms, free water) (Fig. 5a). The pT21, pT22 and pT23 equivalent to the areas of T21, T22 and T23 [12].
Fig. 5.
Changes of transverse relaxation time (T2) (a) and magnetic resonance imaging (MRI) (b) in large yellow croaker with different treatments during refrigerated storage. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
As shown in Fig. 5a and Table 3, All samples showed only slightly changes in pT21, which might because highly organized myofibril structure allowed the water to be entrapped [30]. During refrigerated storage, the pT22 gradually decreased while pT23 increased for all samples. Among the three types of water, pT22 always accounted for the largest proportion. The CK group had lowest immobilized water (from 97.02% on day 0 to 94.92% on day 10) than those of other groups. However, no significant difference was exhibited in the immobilized water amounts of ultrasound treated samples. Compared with US and TUS groups, the free water content of the DUS group was relatively low, which indicated that the DUS could retain more bounded water and improve the freshness of fish. The results of LF-NMR were consistent with WHC.
Table 3.
Changes of water distribution in large yellow croaker with different treatments during refrigerated storage.
| pT2i | Storage time (d) | CK | US | DUS | TUS |
|---|---|---|---|---|---|
| pT21/% | 0 | 2.42 | 2.48 | 2.21 | 2.24 |
| 4 | 2.45 | 2.20 | 2.64 | 2.35 | |
| 10 | 2.42 | 2.46 | 2.46 | 2.50 | |
| pT22/% | 0 | 97.02 | 96.90 | 96.81 | 96.94 |
| 4 | 96.31 | 96.18 | 96.46 | 96.44 | |
| 10 | 94.92 | 95.75 | 96.14 | 95.96 | |
| pT23/% | 0 | 0.56 | 0.62 | 0.98 | 0.82 |
| 4 | 1.24 | 1.62 | 0.90 | 1.21 | |
| 10 | 2.66 | 1.79 | 1.40 | 1.54 | |
Values are means ± standard deviation. The different capital letters on the same row within different treatment represented significant differences (P < 0.05). The different lowercase letter on the same column within different storage time represented significant differences (P < 0.05).
At the same time, MRI is used to comprehend water migration in fish during storage, as an assistive method [29]. Areas with high proton density are represented by red and areas with low proton density are represented by blue [31]. As can be seen from Fig. 5b, the brightness of the samples in all groups gradually changed to yellow from day 0 to day 10. However, compared with DUS group, the samples of CK, US and TUS groups became yellower on day 10, which demonstrated that DUS treatment could reduce water migration and keep more water in fish. Moreover, the results were same with the changes of LF-NMR.
3.4. Protein characteristics
3.4.1. MFI
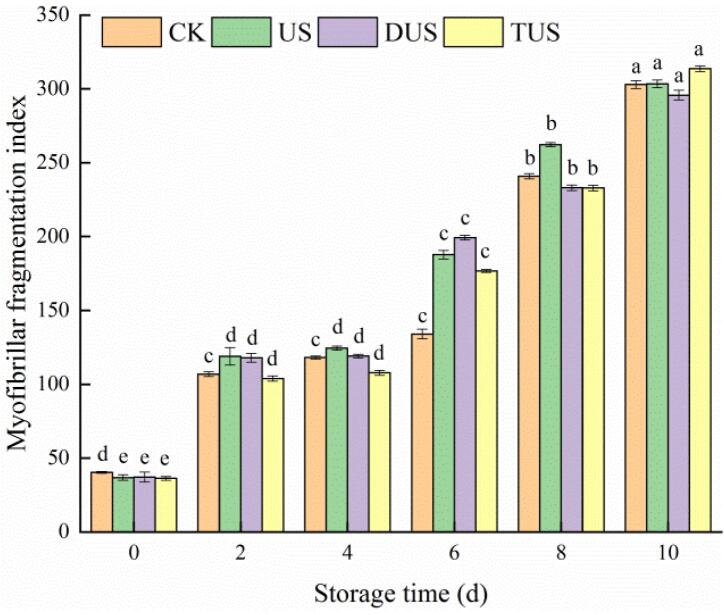
MFI reflects the integrity of MPs. The larger MFI value may be related to the intense rupture of MPs into segments or near the Z-disk in I band [14]. It can be seen from Fig. 6 that the MFI values of all samples increased from 0 d to 10 d, which might be due to the activation of calpain in muscle after the death of fish, which caused the degradation of Z-disk-related MPs resulting in the breakage of muscle fiber [32]. In the early storage period, the MFI values of US and DUS groups were higher than that of the CK group. This might be attributed to the cavitation effect of ultrasound, which destroyed the MPs and connective tissues of fillets, so the MFI value increased [20]. In the late storage period, the MFI value of CK group increased rapidly, which may be due to the proliferation of microorganisms and the increase of protein degradation. The results were in accordance with the results of TVCs, PBCs and TVB-N.
Fig. 6.
Changes of myofibrillar fragmentation index (MFI) in large yellow croaker with different treatments during refrigerated storage. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.4.2. IFI
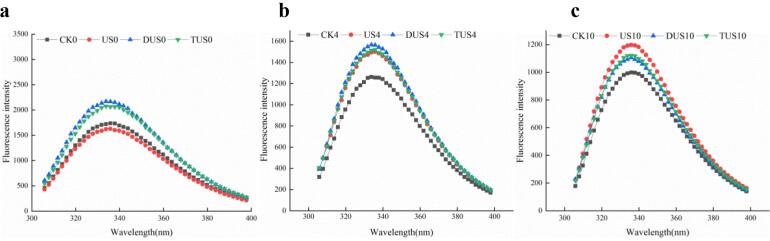
Intrinsic fluorescence intensity (IFI) is often used for indicating the change of protein conformation [33]. It could be seen from Fig. 7a, fresh samples showed the highest fluorescence intensity at 335 nm, indicating that its protein structure was intact. With the increase of storage time, the intrinsic fluorescence intensity of MPs in the CK group dropped sharply (Fig. 7). This might be due to the gradual unfolding of the MPs in large yellow croaker, which made the fluorescent substances such as Tryptophan (Trp) residues exposed to a polar environment, thereby fluorescence quenching [20]. However, ultrasound treatment could delay the decrease of fluorescence intensity in samples (especially DUS group), which indicated that ultrasound treatment could protect the structure of MPs or MPs aggregation [34]. The fluorescence intensity of US group was the highest at the end of storage. It might be due to the increased degree of protein aggregation, which led to the relocation of Trp residues to the inside of the protein molecule, thereby intrinsic fluorescence intensity was strengthened accordingly [35].
Fig. 7.
Changes of intrinsic fluorescence for myofibrillar proteins (MPs) in large yellow croaker with different treatments during refrigerated storage (a: 0 d; b: 4 d; c: 10 d). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.4.3. AFM
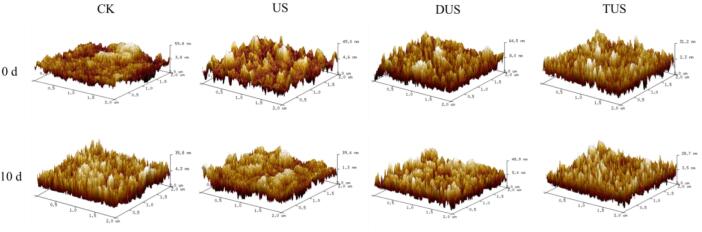
AFM revealed the degradation pathway of MPs and the effects of ultrasound treatment. AFM can conduct more detailed information on biological macromolecule particles [36]. On day 0, compared with CK group, MPs in ultrasound treatment groups collapsed to a smaller number of particles and the particles of MPs were more evenly distributed (Fig. 8). This might be because the acoustic cavitation of ultrasound waves could produce strong physical forces, including shear force, shock waves and turbulence, which could effectively break protein particles and reduce their particle size [37]. In addition, with the extension of storage time, MPs continued to be degraded. However, the MPs particles in ultrasound treatment groups partially aggregated into a polymer form after 10 d storage. Zou et al [14] reported that the effect of ultrasound would lead to the self-assembly of amphiphilic molecules. Among them, the MPs of DUS group had a more regular structure and less agglomeration. However, the MPs of US group had excessive aggregation, which was consistent with the results of intrinsic fluorescence intensity. Wang et al. [38] also reported the similar results.
Fig. 8.
Changes of surface morphology of myofibrillar proteins (MPs) in large yellow croaker with different treatments during refrigerated storage. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.5. Correlation analysis
The relationship between microbial indicators, physicochemical indexes, WHC and MFI affected with different treatments were shown in Table 4. The TVC, PBC had a very significant (P < 0.01) positive correlation with TVB-N and MFI. However, TVC, PBC had a very significant (P < 0.01) negative correlation with WHC and TPA indicators (hardness, springiness, resilience and chewiness). Besides, the MFI had a very significant (P < 0.01) negative correlation with WHC and TPA indicators. However, there was no correlation between the pH and other indexes. In summary, the rapid growth of microorganisms could degrade the protein of fish, which made the texture characteristics and water holding capacity worse, and finally led to the deterioration of fish.
Table 4.
Correlation analysis between microbial indicators, physicochemical indexes, WHC and MFI in large yellow croaker with different treatments during refrigerated storage.
| TVC | PBC | pH | TVB-N | Hardness | Springiness | Resilience | Chewiness | WHC | MFI | |
|---|---|---|---|---|---|---|---|---|---|---|
| TVC | 1 | |||||||||
| PBC | 0.990** | 1 | ||||||||
| pH | 0.20 | 0.22 | 1 | |||||||
| TVB-N | 0.603** | 0.569** | 0.39 | 1 | ||||||
| Hardness | −0.915** | −0.912** | 0.00 | −0.554** | 1 | |||||
| Springiness | −0.918** | −0.910** | −0.17 | −0.662** | 0.829** | 1 | ||||
| Resilience | −0.917** | −0.910** | −0.21 | −0.699** | 0.891** | 0.866** | 1 | |||
| Chewiness | −0.923** | −0.939** | −0.16 | −0.580** | 0.876** | 0.935** | 0.889** | 1 | ||
| WHC | −0.896** | −0.875** | −0.24 | −0.792** | 0.875** | 0.847** | 0.869** | 0.827** | 1 | |
| MFI | 0.942** | 0.939** | 0.10 | 0.590** | −0.865** | −0.932** | −0.863** | −0.938** | −0.858** | 1 |
Note: *, significantly correlated (P < 0.05); **, very significantly correlated (P < 0.01).
4. Conclusions
The multi-frequency ultrasound was demonstrated that it greatly reduced the number of microorganisms of large yellow croaker during refrigerated storage. The effect became more obvious with the increase of ultrasound frequencies. Among them, the samples treated by DUS with frequency of 20/28 kHz delayed the increase of pH and TVB-N, retained better texture characteristics, maintained higher WHC and immobilized water content. Through the results analysis of MFI, IFI and AFM, it deeply demonstrated that multi-frequency ultrasound could make MPs expand moderately and more uniform. At the same time, it could maintain a more stable protein structure at the end of storage, so as to maintain a better texture of fish. In conclusion, multi-frequency ultrasound treatment is a promising auxiliary method for improving the quality of aquatic products during refrigerated storage.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
The study was financially supported by National Key R&D Program of China (2019YFD0901602), China Agriculture Research System (CARS-47-G26), Ability promotion project of Shanghai Municipal Science and Technology Commission Engineering Center (19DZ2284000).
Author contributions
Weiqing LAN and Xinyu ZHAO designed the experiment, finished the study, collected test data and drafted the original manuscript. Yuting ZHAI finished the study. Xinyu ZHAO reviewed the data interpretation and edited the manuscript. Jing XIE was responsible for project administration.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ultsonch.2021.105787.
Contributor Information
Weiqing Lan, Email: wqlan@shou.edu.cn.
Jing Xie, Email: jxie@shou.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Mu H., Li J., Pan X., Liu J., Chen J., Pan Y., Zhang W., Mai K. Alterations in fatty acid composition and volatile compounds in muscle of large yellow croaker Larimichthys crocea fed different dietary lipid sources. Aquacult. Rep. 2021;20:100688. doi: 10.1016/j.aqrep.2021.100688. [DOI] [Google Scholar]
- 2.Sun X., Hong H., Jia S., Liu Y., Luo Y. Effects of phytic acid and lysozyme on microbial composition and quality of grass carp (Ctenopharyngodon idellus) fillets stored at 4 °C. Food Microbiol. 2020;86:103313. doi: 10.1016/j.fm.2019.103313. [DOI] [PubMed] [Google Scholar]
- 3.S. Pedrós-Garrido, S. Condón-Abanto, J.A. Beltrán, J.G. Lyng, N.P. Brunton, D. Bolton, P. Whyte, Assessment of high intensity ultrasound for surface decontamination of salmon (S. salar), mackerel (S. scombrus), cod (G. morhua) and hake (M. merluccius) fillets, and its impact on fish quality, Innov. Food Sci. Emerg. Technol. 41(2017) 64-70.https://doi.org/10.1016/j.ifset.2017.02.006.
- 4.He Q., Liu D., Ashokkumar M., Ye X., Jin T.Z., Guo M. Antibacterial mechanism of ultrasound against Escherichia coli: Alterations in membrane microstructures and properties. Ultrason. Sonochem. 2021;73 doi: 10.1016/j.ultsonch.2021.105509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pan J., Lian H., Jia H., Li S., Hao R., Wang Y., Zhang X., Dong X. Ultrasound treatment modified the functional mode of gallic acid on properties of fish myofibrillar protein. Food Chem. 2020;320:126637. doi: 10.1016/j.foodchem.2020.126637. [DOI] [PubMed] [Google Scholar]
- 6.Ma W., Wang J., Xu X., Qin L., Wu C., Du M. Ultrasound treatment improved the physicochemical characteristics of cod protein and enhanced the stability of oil-in-water emulsion. Food Res. Int. 2019;121:247–256. doi: 10.1016/j.foodres.2019.03.024. [DOI] [PubMed] [Google Scholar]
- 7.Antunes-Rohling A., Astráin-Redín L., Calanche-Morales J.B., Marquina P., Beltrán J.A., Raso J., Cebrián G., Álvarez I. Eco-innovative possibilities for improving the quality of thawed cod fillets using high-power ultrasound. Food Control. 2021;121:107606. doi: 10.1016/j.foodcont.2020.107606. [DOI] [Google Scholar]
- 8.Ma H., Huang L., Peng L., Wang Z., Yang Q. Pretreatment of garlic powder using sweep frequency ultrasound and single frequency countercurrent ultrasound: optimization and comparison for ACE inhibitory activities. Ultrason. Sonochem. 2015;23:109–115. doi: 10.1016/j.ultsonch.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 9.Ma X., Mei J., Xie J. Effects of multi-frequency ultrasound on the freezing rates, quality properties and structural characteristics of cultured large yellow croaker (Larimichthys crocea) Ultrason. Sonochem. 2021;76:105657. doi: 10.1016/j.ultsonch.2021.105657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lan W., Sun Y., Zhang N., Xie J. Effects of ε-polylysine and rosemary extract on quality attributes and microbial communities in vacuum-packaged large yellow croaker (Pseudosciaena crocea) during ice storage. Food Sci. Biotechnol. 2021;30:465–474. doi: 10.1007/s10068-021-00880-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng H., Lan W., Sun X., Xie J. Effects of slightly acidic electrolyzed water pretreatment combined with biopreservatives on the shelf life of refrigerated obscure pufferfish (Takifugu obscurus) J. Food. Sci. 2021;86:484–494. doi: 10.1111/1750-3841.15596. [DOI] [PubMed] [Google Scholar]
- 12.W. Lan, J. Liu, M. Wang, J. Xie, Effects of apple polyphenols and chitosan-based coatings on quality and shelf life of large yellow croaker (Pseudosciaena crocea) as determined by low field nuclear magnetic resonance and fluorescence spectroscopy, J. Food Safety, 41 (2021) e12887.https://doi.org/10.1111/jfs.12887.
- 13.Yang K., Zhou Y., Guo J., Feng X., Wang X., Wang L., Ma J., Sun W. Low frequency magnetic field plus high pH promote the quality of pork myofibrillar protein gel: a novel study combined with low field NMR and Raman spectroscopy. Food Chem. 2020;326 doi: 10.1016/j.foodchem.2020.126896. [DOI] [PubMed] [Google Scholar]
- 14.Zou Y., Zhang K., Bian H., Zhang M., Sun C., Xu W., Wang D. Rapid tenderizing of goose breast muscle meat based on actomyosin dissociation by low frequency ultrasonication. Process Biochem. 2017;65 doi: 10.1016/j.procbio.2017.11.010. [DOI] [Google Scholar]
- 15.Hu Y., Zhang L., Yi Y., Solangi I., Zan L., Zhu J. Effects of sodium hexametaphosphate, sodium tripolyphosphate and sodium pyrophosphate on the ultrastructure of beef myofibrillar proteins investigated with atomic force microscopy. Food Chem. 2021;338 doi: 10.1016/j.foodchem.2020.128146. [DOI] [PubMed] [Google Scholar]
- 16.Joyce E., Al-Hashimi A., Mason T.J. Assessing the effect of different ultrasonic frequencies on bacterial viability using flow cytometry. J. Appl. Microbiol. 2011;110:862–870. doi: 10.1111/j.1365-2672.2011.04923.x. [DOI] [PubMed] [Google Scholar]
- 17.Alenyorege E.A., Ma H., Ayim I., Zhou C., Wu P., Hong C., Osae R. Effect of multi-frequency ultrasound surface washing treatments on Escherichia coli inactivation and some quality characteristics of non-heading Chinese cabbage. J. Food Process. Preserv. 2018;42(10):e13747. doi: 10.1111/jfpp.v42.1010.1111/jfpp.13747. [DOI] [Google Scholar]
- 18.Taiye Mustapha A., Zhou C., Amanor-Atiemoh R., Owusu-Fordjour M., Wahia H., Abiola Fakayode O., Ma H. Kinetic modeling of inactivation of natural microbiota and Escherichia coli on cherry tomato treated with fixed multi-frequency sonication. Ultrason. Sonochem. 2020;64:105035. doi: 10.1016/j.ultsonch.2020.105035. [DOI] [PubMed] [Google Scholar]
- 19.C. Nguyen Huu, R. Rai, X. Yang, R.V. Tikekar, N. Nitin, Synergistic inactivation of bacteria based on a combination of low frequency, low-intensity ultrasound and a food grade antioxidant, Ultrason. Sonochem. 74 (2021) 105567.10.1016/j.ultsonch.2021.105567. [DOI] [PMC free article] [PubMed]
- 20.D. Zhou, W. Lan, M.O. Yaxian, J. Mei, H. Feng, J. Xie, Effects of ultrasound pretreatment on the changes of quality and protein characteristics in Japanese sea bass (Lateolabrax japonicas) during refrigerated storage, Food Fermentation Ind. 46(17) (2020) 204-211.10.13995/j.cnki.11-1802/ts.024252.
- 21.Zhang J., Zhang Y., Zou Y., Zhang W. Effects of ultrasound-assisted cooking on quality characteristics of spiced beef during cold storage. LWT. 2021;136:110359. doi: 10.1016/j.lwt.2020.110359. [DOI] [Google Scholar]
- 22.Huang H., Sun W., Xiong G., Shi L., Jiao C., Wu W., Li X., Qiao Y., Liao L., Ding A., Wang L. Effects of HVEF treatment on microbial communities and physicochemical properties of catfish fillets during chilled storage. LWT. 2020;131:109667. doi: 10.1016/j.lwt.2020.109667. [DOI] [Google Scholar]
- 23.Wu H., Zhao Y., Du Y., Miao S., Liu J., Li Y., Caiyin Q., Qiao J. Quantitative proteomics of Lactococcus lactis F44 under cross-stress of low pH and lactate. J. Dairy Sci. 2018;101(8):6872–6884. doi: 10.3168/jds.2018-14594. [DOI] [PubMed] [Google Scholar]
- 24.Zhuang S., Li Y., Jia S., Hong H., Liu Y., Luo Y. Effects of pomegranate peel extract on quality and microbiota composition of bighead carp (Aristichthys nobilis) fillets during chilled storage. Food Microbiol. 2019;82:445–454. doi: 10.1016/j.fm.2019.03.019. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y.-Y., Yan J.-K., Rashid M.T., Ding Y., Chikari F., Huang S., Ma H. Dual-frequency sequential ultrasound thawing for improving the quality of quick-frozen small yellow croaker and its possible mechanisms. Innovative Food Sci. Emerg. Technol. 2021;68:102614. doi: 10.1016/j.ifset.2021.102614. [DOI] [Google Scholar]
- 26.Zarandona I., López-Caballero M.E., Montero M.P., Guerrero P., de la Caba K., Gómez-Guillén M.C. Horse mackerel (Trachurus trachurus) fillets biopreservation by using gallic acid and chitosan coatings. Food Control. 2021;120:107511. doi: 10.1016/j.foodcont.2020.107511. [DOI] [Google Scholar]
- 27.Q. Sun, B. Kong, S. Liu, O. Zheng, C. Zhang, Ultrasound-assisted thawing accelerates the thawing of common carp (Cyprinus carpio) and improves its muscle quality, LWT 141 (2021) 111080.https://doi.org/10.1016/j.lwt.2021.111080.
- 28.Li D., Zhao H., Muhammad A.I., Song L., Guo M., Liu D. The comparison of ultrasound-assisted thawing, air thawing and water immersion thawing on the quality of slow/fast freezing bighead carp (Aristichthys nobilis) fillets. Food Chem. 2020;320:126614. doi: 10.1016/j.foodchem.2020.126614. [DOI] [PubMed] [Google Scholar]
- 29.Li P., Zhou Q., Chu Y., Lan W., Mei J., Xie J. Xie, Effects of chitosan and sodium alginate active coatings containing ε-polysine on qualities of cultured pufferfish (Takifugu obscurus) during cold storage. Int. J. Biol. Macromol. 2020;160:418–428. doi: 10.1016/j.ijbiomac.2020.05.092. [DOI] [PubMed] [Google Scholar]
- 30.N. Qin, Influence of lightly salting and sugaring on the quality and water distribution of grass carp (Ctenopharyngodon idellus) during super-chilled storage, J. Food Eng. 215 (2017) 104-112-2017 v.2215.10.1016/j.jfoodeng.2017.07.011.
- 31.Wang X.-Y., Xie J. Evaluation of water dynamics and protein changes in bigeye tuna (Thunnus obesus) during cold storage. LWT. 2019;108:289–296. doi: 10.3390/molecules24173119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.D'Alessandro A., Marrocco C., Rinalducci S., Mirasole C., Failla S., Zolla L. Chianina beef tenderness investigated through integrated Omics. J. Proteomics. 2012;75(14):4381–4398. doi: 10.1016/j.jprot.2012.03.052. [DOI] [PubMed] [Google Scholar]
- 33.Pan J., Lian H., Jia H., Hao R., Wang Y., Ju H., Li S., Dong X. Dose affected the role of gallic acid on mediating gelling properties of oxidatively stressed Japanese seerfish myofibrillar protein. LWT. 2020;118:108849. doi: 10.1016/j.lwt.2019.108849. [DOI] [Google Scholar]
- 34.Dong Z.Y., Li M.Y., Tian G., Zhang T.H., Ren H., Quek S.Y. Effects of ultrasonic pretreatment on the structure and functionality of chicken bone protein prepared by enzymatic method. Food Chem. 2019;299:125103. doi: 10.1016/j.foodchem.2019.125103. [DOI] [PubMed] [Google Scholar]
- 35.Shi J., Lei Y., Shen H., Hong H., Yu X., Zhu B., Luo Y. Effect of glazing and rosemary (Rosmarinus officinalis) extract on preservation of mud shrimp (Solenocera melantho) during frozen storage. Food Chem. 2019;272:604–612. doi: 10.1016/j.foodchem.2018.08.056. [DOI] [PubMed] [Google Scholar]
- 36.Huang L., Ding X., Dai C., Ma H. Changes in the structure and dissociation of soybean protein isolate induced by ultrasound-assisted acid pretreatment. Food Chem. 2017;232 doi: 10.1016/j.foodchem.2017.04.077. [DOI] [PubMed] [Google Scholar]
- 37.Chang H., Shi Y., Wang H., Huang X., Ruiping L.I., Bao Z., Zongcai T.U. Effect of ultrasonic treatment on physico-chemical properties of myofibrillar protein from grass carp. Food Sci. 2015;36:56–60. doi: 10.7506/spkx1002-6630-201505011. [DOI] [Google Scholar]
- 38.Wang Y.-Y., Rashid M., Ma H. Effect of multi-frequency ultrasound thawing on the structure and rheological properties of myofibrillar proteins from small yellow croaker. Ultrason. Sonochem. 2020;70 doi: 10.1016/j.ultsonch.2020.105352. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.