Highlights
-
•
IL-6 has been associated with poorer facial emotion recognition.
-
•
fMRI was performed during a faces task and IL-6 measured from blood samples.
-
•
IL-6 predicted increased neural response during facial emotion recognition.
Abbreviations: ACC, anterior cingulate cortex; ART, Artefact Detection Tools; CAMI, Centre for Advanced Medical Imaging; DMN, default mode network; ELISA, enzyme-linked immunosorbent assay; ERT, emotion recognition task; FFA, fusiform face area; FWE, family-wise error; IL-6, interleukin 6; IQ, intelligence quotient; LLP, left lateral parietal; mPFC, medial prefrontal cortex; SPM, Statistical Parametric Mapping; STS, superior temporal sulcus; SZ, schizophrenia
Keywords: IL-6, Facial emotion recognition, fMRI, Schizophrenia
Abstract
Background
Deficits in facial emotion recognition are a core feature of schizophrenia and predictive of functional outcome. Higher plasma levels of the cytokine interleukin 6 (IL-6) have recently been associated with poorer facial emotion recognition in individuals with schizophrenia and healthy participants, but the neural mechanisms affected remain poorly understood.
Methods
Forty-nine individuals with schizophrenia or schizoaffective disorder and 158 healthy participants were imaged using functional magnetic resonance imaging during a dynamic facial emotion recognition task. Plasma IL-6 was measured from blood samples taken outside the scanner. Multiple regression was used in statistical parametric mapping software to test whether higher plasma IL-6 predicted increased neural response during task performance.
Results
Higher plasma IL-6 predicted increased bilateral medial prefrontal response during neutral face processing compared to angry face processing in the total sample (N = 207, tmax = 5.67) and increased left insula response during angry face processing compared to neutral face processing (N = 207, tmax = 4.40) (p < 0.05, family-wise error corrected across the whole brain at the cluster level).
Conclusions
These findings suggest that higher peripheral IL-6 levels predict altered neural response within brain regions involved in social cognition and emotion during facial emotion recognition. This is consistent with recent neuroimaging research on IL-6 and suggesting a possible neural mechanism by which this cytokine might affect facial emotion recognition accuracy.
1. Introduction
Schizophrenia (SZ) is a debilitating neuropsychiatric disorder that encompasses positive and negative symptoms, significant neuropsychological impairments, and poor social and occupational functioning. Among the range of cognitive deficits presented, deficits in social cognition (i.e., the mental operations underlying social behaviour) strongly predict disability, as measured in terms of both social and occupational functioning, both in early and chronic illness (Cowman et al., 2021, Fett et al., 2011). Social cognitive deficits measured in SZ typically include emotion recognition, theory of mind, and attributional style, with impaired recognition of facial expressions and facial emotion processing from faces amongst the most frequently reported findings (Behere, 2015).
Visual processing of dynamic faces (e.g., using the dynamic face processing task (Grosbras and Paus, 2006) is consistently associated with activation of many cortical regions, which have since been parsed into a “a core system”, including the fusiform face area (FFA), posterior superior temporal sulcus (STS), and occipital face area, and an “extended system” involving other cortical regions implicated in the emotional processing of faces (Haxby et al., 2000, Pitcher et al., 2014). Of these, deficits in processing of emotional content from faces in SZ have been consistently associated with altered activation of the anterior cingulate cortex (ACC) (e.g., during processing of negative emotion and social threat stimuli (Habel et al., 2010, Mothersill et al., 2014) and the medial prefrontal cortex (mPFC) (e.g., during the processing of angry faces (Mothersill et al., 2014). One interpretation of these findings is that they reflect more general alterations in functioning of the default mode network (DMN) - an interconnected network of brain regions important for social cognitive processes (Li et al., 2014), including facial emotion recognition (Shi et al., 2015, Spies et al., 2017). Typically, the DMN is associated with increased activation at rest and deactivation during a cognitive task (Anticevic et al., 2012). Disruption to the DMN is consistently reported in SZ in terms of increased activation/weaker suppression of this network during cognitive tasks (Fornito et al., 2012, Zhou et al., 2016).
While the biological causes for altered neural activation during social cognitive task performance are unclear, one hypothesis is that these neural effects are mediated by dysregulation of the immune system (Aruldass et al., 2021, Eisenberger et al., 2009, Nusslock et al., 2019, Müller, 2013, Müller, 2014, Müller et al., 2015). Like other neuropsychiatric disorders (e.g., depression), elevated pro-inflammatory states have been observed in SZ (Müller, 2013, Müller, 2014, Müller et al., 2015), suggesting an association between immune dysregulation and SZ psychopathology (Feng et al., 2020). Of these immune markers, altered levels of the pro-inflammatory cytokine interleukin 6 (IL-6) has been consistently reported in schizophrenia, with a reduction in IL-6 levels associated with successful treatment with the antipsychotic risperidone (Feng et al., 2020). As evidence that these changes in immune signalling pre-exist illness onset, longitudinal data show that elevated serum IL-6 in childhood predicted increased illness risk in adulthood (Upthegrove and Khandaker, 2020), suggest a potential causal role for IL-6 in schizophrenia pathophysiology that is further supported by mendelian randomization studies of IL-6 (Upthegrove and Khandaker, 2020).
Recent social cognitive studies from our group have found associations with immune function both at the level of genetic variation and with IL-6 cytokine levels. Studies of genetic variation within individual SZ-associated genes (e.g., C4A; (Khandaker et al., 2017) and biological pathways (e.g., Complement) have been associated with variation in cognitive function (Donohoe et al., 2018, Holland et al., 2019, Mondelli et al., 2020, Sekar et al., 2016); we have shown that these genetic variants also predicted altered middle temporal gyrus response in healthy controls using a dynamic face processing task (Donohoe et al., 2018). In recent studies of childhood trauma and emotion recognition, we further found evidence that the effects of childhood neglect on behavioural measures of emotion recognition were mediated via activity of the default mode network (Dauvermann et al., 2021), and subsequently, that these mediating effects were in turn mediated by IL-6 (King et al., in press).
1.1. Aims and hypothesis
The purpose of this study was to investigate the role of increased levels of plasma IL-6 levels and activation during a face processing task. Identifying brain regions associated with immune dysfunction during a faces task is important for better understanding the neurobiological basis of social cognitive function in healthy individuals and in disorders characterised by deficits in social cognition. We used the dynamic face processing task designed by Grosbras and Paus (Grosbras and Paus, 2006) to examine neural activation during passive viewing of dynamic angry and neutral faces in patients and healthy controls. Based on the literature reviewed above for both SZ and healthy participants, we formulated the following hypotheses: Firstly, given our recent observation that higher plasma IL-6 levels were associated with reduced behavioural performance on facial emotion recognition, we hypothesised that increased plasma IL-6 levels would predict altered neural response during a facial emotion recognition task. Following on from our recent studies in the same sample, on childhood trauma and emotion recognition highlighted above (Dauvermann et al., 2021, King et al., in press), we secondly hypothesised that the observed effects would be comparable i.e., across patients and controls, thus representing more a general neurobiological process rather than a pathology specific effect.
2. Material and methods
2.1. Participants
Two hundred and thirty-one participants took part in the study overall. Fifty-three individuals with schizophrenia (SZ) or schizoaffective disorder were recruited across Galway and Dublin through community mental health services. All patients (SZ and schizoaffective disorder) had a diagnosis confirmed by the Structured Clinical Interview for Diagnostic Statistical Manual-IV. All participants were aged between 18 and 65, and no participants had a documented history of neurological disorder, comorbid axis I mental health disorder, intelligence quotient (IQ) <70, previous head injury associated with a loss of consciousness of more than 60 s, evidence of substance use disorder in the previous month or reported pregnancy.
One hundred and seventy-eight healthy control participants were recruited using media advertising from Galway and Dublin. In addition to the exclusion criteria outlined above, healthy controls also met the criteria of not having a substance use disorder in the previous six months, first-degree relative with a psychotic disorder, or substance abuse in the previous six months. Twelve healthy controls were excluded due to excessive signal dropout in the functional MRI images, and five healthy controls were excluded due to missing logfiles, and/or problems with the timing of stimuli when the Paus Emotion Recognition Task (ERT) was run. This resulted in a final sample of 53 patients and 161 healthy controls with fMRI facial emotion recognition data. Within this sample, 49 patients and 158 controls had plasma IL-6 data. Within the final patient sample with plasma IL-6 data, 35 patients had a diagnosis of schizophrenia, and 14 patients had a diagnosis of schizoaffective disorder.
All participants provided written informed consent in accordance with the local Ethics Committees of Galway University Hospital, National University of Ireland Galway, and St. James’s Hospital.
2.2. Paus emotion recognition test
During functional MRI, participants completed the Paus Emotion Recognition Test (Grosbras and Paus, 2006), which we have previously used to examine emotion recognition in SZ (Mothersill et al., 2014) as well as the relationship between variation in the expression of the immune-related complement component 4A and face processing in healthy volunteers (Donohoe et al., 2018). In this cognitive task, participants watched a series of 2–5 s black-and-white videos of people displaying neutral or angry facial expressions. An additional control condition involved watching expanding and contracting concentric circles. In this block-design task, there were 19 blocks, each of which lasted 18 s and included 4 to 7 videos: nine blocks of circles, five blocks of neutral faces and five blocks of angry faces. Participants also completed a post-scan face recognition task outside the scanner to examine attention to the task.
2.3. Plasma isolation and analysis
Plasma Isolation & Analysis was carried out as described in (King et al., in press) but in brief: Blood samples were taken at approximately the same time of day (9.30 am) from each participant in a 6 ml EDTA tube (BD367873). The sample was centrifuged at 1200g for 10 min at ambient temperature. Following centrifugation, plasma was aspirated and stored in 1.5 ml Eppendorf tubes at −80 °C until further analysis. Basal plasma levels of IL-6 were measured using a quantikine high sensitivity enzyme-linked immunosorbent assay (ELISA) (Bio-Techne Catalog Number HS600C) which has an assay sensitivity of 0.09 pg/mL and range of 0.156–10 pg/mL and read at 450 nm.
2.4. Neuroimaging data acquisition
Neuroimaging was carried out on a 3 Tesla Philips Achieva MR system (Philips Medical Systems, Best, The Netherlands) which was equipped with a gradient strength of 80 mT/m and a slew rate of 200 T/m/s using a 32-channel head coil. Neuroimaging was carried out at the Centre for Advanced Medical Imaging (CAMI), St. James’s Hospital, Dublin, Ireland.
High resolution T1-weighted images were obtained as described previously (Dauvermann et al., 2021). Functional MRI data were acquired during the Faces task using a SE-EPI sequence with a dynamic scan time of 2 s, with: FOV = 240 × 240 × 131 mm, REC voxel MPS (mm) = 3 × 3 × 3.2, 37 slices with interslice gap = 0.349999905 mm, TR/TE = 2000/28 ms, and flip angle = 90°. For the Faces task, 174 volumes were acquired, taking 5 min and 55.9 s.
2.5. Neuroimaging data analysis
MRI data were processed using Statistical Parametric Mapping software (SPM12, v7771, https://www.fil.ion.ucl.ac.uk/spm/software/spm12/) (Ashburner et al., 2014) and MATLAB R2019b 64-bit (v9.7.0.1296695), running on a Dell Optiplex 5040 PC running Microsoft Windows 7. Functional MRI spatial pre-processing included the following steps:
-
1.
Realignment to reduce the confounding effects of motion and re-slicing applied to the mean functional image (Friston et al., 1996).
-
2.
Co-registration between the re-sliced mean functional image and the T1 image.
-
3.
Estimation of the normalisation parameters between the T1 image and the standard SPM12 template and application of these parameters to the functional images, resampled with a voxel size of 2 × 2 × 2 mm3 (Ashburner and Friston, 2005).
-
4.
Smoothing of the normalised functional images with an 8 mm full-width half maximum Gaussian kernel (Friston et al., 1995, Friston et al., 1995).
Artefact detection was performed on pre-processed data using the Artefact Detection Tools (ART) toolbox (https://www.nitrc.org/projects/artifact_detect/). Pre-processed images that showed variations in global mean intensity greater than 3 standard deviations, and/or composite motion greater than 1 mm were considered outliers and entered as covariates in the first-level model for each participant (Whitfield-Gabrieli, S., personal correspondence).
Statistical analysis of functional MRI data was performed using the general linear model (Friston et al., 1994) and using the following contrasts:
-
1.
All faces (neutral and angry) versus the non-face control condition (circles)
-
2.
Neutral faces versus angry faces
-
3.
Angry faces versus neutral faces
Contrast maps generated by these analyses were entered into random effects analysis to examine (a) differences in neural response between cases and controls (independent samples t-test) and (b) whether higher plasma IL-6 predicted increased neural response (multiple regression). Before random effects analysis was carried out, differences in demographic variables between cases and controls and correlations between plasma IL-6 and demographic variables were examined in IBM SPSS Statistics Version 26.
BMI was only available for 205 of the 214 participants– thus, mean BMI for the whole sample (25.97) was inputted in place of the nine missing BMI values so that BMI could be included as a covariate in the independent samples t-test comparing neural response between cases and controls. Similarly, plasma IL-6 data was only available for 207 of the 214 participants– thus, mean plasma IL-6 for the whole sample (1.9964) was inputted in place of the seven missing plasma IL-6 values so that plasma IL-6 could be included as a covariate in the independent samples t-test comparing neural response between cases and controls. Finally, given that BMI data was only available for 198 of the 207 participants with plasma IL-6 data, the mean BMI for the whole sample with plasma IL-6 data (25.87) was inputted in place of the nine missing BMI values so that BMI could be included as a covariate in multiple regression analysis examining plasma IL-6. The following covariates were included in second-level analyses for all contrasts:
Cases versus controls (N = 214): Age, BMI, plasma IL-6
Plasma IL-6 (total sample, N = 207): ART outliers, Faces post-scan correct performance, BMI, diagnosis
Plasma IL-6 (SZ group, N = 49): Faces post-scan correct performance
For second-level analyses, statistical significance was set at an initial threshold of p < 0.001, uncorrected at the whole brain level, and clusters were considered statistically significant at the level of p < 0.05, family-wise error (FWE) corrected for multiple comparisons across the whole brain at the cluster level. Probable anatomical locations for cluster peaks were identified using the SPM Anatomy Toolbox Version 2.2b (Eickhoff et al., 2005, Eickhoff et al., 2006, Eickhoff et al., 2007). Sensitivity power analysis conducted in G*Power 3.1.9.7 suggests that a multiple regression analysis with a total sample of 207 participants and 6 predictors would have 80% power to detect medium to large effects (Cohen’s f2 = 0.13 or higher) of IL-6 at a p = 0.001 level (i.e., the initial statistical threshold used in the fMRI analysis) (Faul et al., 2007, Faul et al., 2009, Cohen, 1988). This allows us to detect effects of plasma IL-6 on neural activation of similar magnitude or smaller than those reported previously (Eisenberger et al., 2009).
Finally, t-values associated with significant effects of plasma IL-6 on neural activation were converted to Cohen’s d using the following effect size calculator to provide a measure of effect size: https://lbecker.uccs.edu/.
3. Results
3.1. Demographic information
Demographic information for patient and healthy participants is presented in Table 1. This table presents the gender ratio, as well as the mean and standard deviation of age, number of ART outliers, Faces post-scan correct performance, BMI, and plasma IL-6 for the total sample, patient sample, and healthy control sample. For the patient sample, chlorpromazine equivalent score and PANSS scores are also provided. For each variable, statistics indicating the relationship between that variable and plasma IL-6 and associated p-values are provided. There was a significant association between plasma IL-6 and ART outliers, Faces post-scan correct performance, and BMI in the total sample, and a significant association between plasma IL-6 and Faces post-scan correct performance, PANSS negative total score, PANSS general total score, and PANSS total, in the patient sample. Mean Faces post-scan correct performance scores (number of correct responses out of 5) were close to ceiling as indicated by mean scores above 4 out of 5 in the total sample, patient sample, and healthy control sample.
Table 1.
Demographic information.
| Total sample (N = 214) |
Schizophrenia (N = 53) |
Healthy controls (N = 161) |
|||||
|---|---|---|---|---|---|---|---|
| Mean (S.D.a) | Relationship with plasma IL-6b (p value) | Statistic comparing schizophrenia and healthy controls (p value) | Mean (S.D. a) | Relationship with plasma IL-6b (p value) | Mean (S.D. a) | Relationship with plasma IL-6 b (p value) | |
| Age | 37.61 (12.163) | r = 0.112 (0.108) | t = 3.497 (0.001) | 42.55 (11.289) | r = 0.237 (0.101) | 35.98 (12.032) | r = -0.112 (0.161) |
| Gender (M:F) | 135:79 | t = 1.485 (0.139) | Chi-Square = 1.369 (0.242) | 37:16 | t = 0.900 (0.373) | 98:63 | t = 1.136 (0.258) |
| ART outliers | 6.7336 (13.81192) | r = 0.224 (0.001) | t = 1.851 (0.070) | 11.6226 (25.33258) | r = 0.220 (0.130) | 5.1242 (5.90525) | r = -0.011 (0.889) |
| Faces post-scan correct performance | 4.2523 (1.03546) | r = -0.140 (0.045) | t = 1.128 (0.260) | 4.1132 (1.13782) | r = -0.289 (0.044) | 4.2981 (0.99903) | r = 0.046 (0.568) |
| BMIc | 25.97 (4.702) | r = 0.179 (0.012) | t = 7.483 (<0.001) | 29.71 (4.795) | r = 0.138 (0.351) | 24.70 (3.942) | r = 0.032 (0.698) |
| Plasma IL-6b in pg/mld | 1.9964 (3.44161) | r = 1 | t = 2.122 (0.039) | 3.4596 (6.25757) | r = 1 | 1.5427 (1.64147) | r = 1 |
| Chlorpromazine equivalent score in mg/daye | – | – | – | 1023.22 (2194.449) | r = 0.005 (0.977) | – | – |
| PANSS positive total scoref | – | – | – | 8.75 (2.331) | r = 0.184 (0.216) | – | – |
| PANSS negative total scoref | – | – | – | 9.71 (3.823) | r = 0.297 (0.043) | – | – |
| PANSS general total scoref | – | – | – | 20.47 (4.076) | r = 0.291 (0.047) | – | – |
| PANSS totalf | – | – | – | 38.79 (8.185) | r = 0.329 (0.023) | – | – |
S.D. = standard deviation.
IL-6 = interleukin 6.
BMI = body mass index; BMI available for 52 of 53 participants in the schizophrenia group and 153 of 161 healthy controls.
Plasma IL-6 available for 49 of 53 participants in the schizophrenia group and 158 of 161 healthy controls.
Total chlorpromazine equivalent score available for 46 of 53 participants in the schizophrenia group.
PANSS = Positive and Negative Syndrome Scale Score; PANSS positive, negative and general scores available for 51 of 53 participants in the schizophrenia group; PANSS total score available for 52 of 53 participants in the schizophrenia group.
Overall, the patient group was significantly older than the healthy participant (on average 7 years) group and had a higher BMI (on average 5.01 points higher). As expected, the patient group also had significantly higher levels of plasma IL-6 compared to the healthy participants (on average 1.9169 pg/ml higher).
3.2. Neural response during facial emotion recognition in patients compared to controls
No significant differences in neural response were observed between patients and controls for any of the contrasts examined. This contrasts with previous studies we have conducted using this task, in which increased mPFC response was observed in patients compared to controls during the faces condition compared to non-faces.
3.3. Plasma IL-6 and neural response during facial emotion recognition
In relation to IL-6, there were three sets of analyses carried out in the full sample to test for increased activation during processing of: (1) All faces versus non-faces, (2) neutral versus angry faces and (3) angry versus neutral faces. These contrasts were then re-analysed separately in the patient and control samples to identify any group specific findings.
3.3.1. Plasma IL-6 and neural response during face processing (all faces versus non-faces) in the total sample (N = 207)
Higher plasma IL-6 was not associated with increased neural response during processing of faces versus non-faces at p < 0.05, FWE-corrected for multiple comparisons across the whole brain at the cluster level.
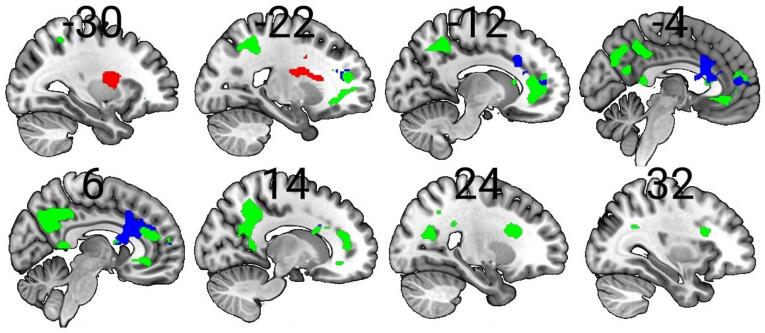
3.3.2. Plasma IL-6 and neural response during neutral face processing compared to angry face processing (total sample, N = 207)
Higher plasma IL-6 predicted increased neural response during neutral face processing compared to angry face processing in several cortical areas including the mPFC, precuneus, and left superior parietal lobule (p < 0.05 FWE-corrected across the whole brain at the cluster level) (Table 2 and Fig. 1). These associations are not the same in patients and healthy controls when groups are examined separately. However, plasma IL-6 predicted increased neural response during neutral face processing compared to angry face processing in patients in an mPFC cluster overlapping with the cluster observed in the pooled sample (see Section 3.3.4 below), indicating that increased neural response in this cluster may be driven by effects in the patient group.
Table 2.
Higher plasma IL-6 predicted increased neural response during face processing.
| Sample | Contrast | Cluster | Cluster size | t-value | Cohen’s d | Z-value | x | y | z | Cluster peak |
|---|---|---|---|---|---|---|---|---|---|---|
| Total sample | Neutral versus angry | 1 | 2475 | 5.67 | 0.80 | 5.46 | 26 | 18 | 24 | Not found in any probability map |
| 5.64 | 0.80 | 5.43 | 0 | 60 | 12 | Superior Medial Gyrus | ||||
| 5.33 | 0.75 | 5.14 | −12 | 40 | 0 | Not found in any probability map | ||||
| 2 | 2862 | 5.34 | 0.75 | 5.15 | −18 | −50 | 46 | Not found in any probability map | ||
| 4.88 | 0.69 | 4.74 | 8 | −56 | 40 | Precuneus | ||||
| 4.58 | 0.65 | 4.46 | −14 | −62 | 46 | Superior Parietal Lobule | ||||
| 3 | 430 | 4.73 | 0.67 | 4.60 | 40 | −60 | 42 | Angular Gyrus | ||
| 4.00 | 0.57 | 3.92 | 38 | −54 | 28 | Not found in any probability map | ||||
| 3.33 | 0.47 | 3.28 | 44 | −46 | 26 | Angular Gyrus | ||||
| Total sample | Angry versus neutral | 1 | 588 | 4.40 | 0.62 | 4.29 | −32 | −2 | 14 | Insula Lobe |
| 4.06 | 0.57 | 3.98 | −32 | 4 | 8 | Insula Lobe | ||||
| 3.92 | 0.55 | 3.84 | −44 | 4 | 16 | Rolandic Operculum | ||||
| Schizophrenia group | Neutral versus angry | 1 | 1424 | 6.86 | 2.02 | 5.67 | 4 | 18 | 26 | Anterior Cingulate Cortex |
| 6.35 | 1.87 | 5.36 | −6 | 24 | 16 | Not found in any probability map | ||||
| 5.14 | 1.52 | 4.54 | −4 | 50 | 10 | Anterior Cingulate Cortex | ||||
Fig. 1.
Higher plasma interleukin 6 (IL-6) predicted increased neural response during face processing. Green = increased neural response during neutral face processing compared to angry face processing in the total sample; red = increased neural response during angry face processing compared to neutral face processing in the total sample; blue = increased neural response during neutral face processing compared to angry face processing in the schizophrenia group; p < 0.05, family-wise error-corrected for multiple comparisons across the whole brain at the cluster level; clusters were rendered on the ‘ch256′ brain template using MRIcroGL version 1.2.20200331 (https://www.mccauslandcenter.sc.edu/mricrogl/). Additional editing of the Figure (e.g., changing the size/resolution) performed using MS Paint and Paint.NET v4.2.16. Minus coordinates indicate sagittal slices in the left-hemisphere. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.3.3. Plasma IL-6 and neural response during angry face processing compared to neutral face processing (total sample, N = 207)
Higher plasma IL-6 predicted increased neural response during angry face processing compared to neutral face processing in the left insula (p < 0.05 FWE-corrected across the whole brain at the cluster level) (Table 2 and Fig. 1).
3.3.4. Plasma IL-6 and neural response when SZ and healthy control groups were examined separately
When SZ and healthy control groups were examined separately, higher plasma IL-6 additionally predicted increased neural response during neutral face processing compared to angry face processing in the bilateral ACC in the SZ group (p < 0.05 FWE-corrected across the whole brain at the cluster level) (Table 2 and Fig. 1). This cluster overlaps with a cluster observed in the pooled sample during the same contrast (see Section 3.3.2). No other significant relationships were observed, and none of the other associations reported at the pooled level were found at the group level.
4. Discussion
4.1. Summary of main findings
The primary objective of this study was to test the hypothesis that higher plasma IL-6 predicts increased neural response during face processing in a pooled sample of both healthy participants and patients with schizophrenia. Firstly, plasma IL-6 levels were significantly higher in patients compared to controls. During the ‘neutral’ face processing condition, higher plasma IL-6 predicted increased neural response in several cortical areas including the mPFC, precuneus and left lateral parietal cortex in the total sample. During the ‘angry’ face processing condition, higher plasma IL-6 predicted increased insula response compared to neutral face processing in the total sample. The secondary objective of this study was to assess the role of higher IL-6 and altered neural response in patients and controls separately. In patients, and during ‘neutral’ face processing, higher plasma IL-6 levels also predicted increased neural response in an overlapping region of the mPFC, but none of the other effects seen in the total pooled sample were seen in patient or healthy control subgroups.
To our knowledge, this is the first study that investigated associations between plasma IL-6 and neural response during a dynamic facial emotion recognition task. Dynamic videos of faces may be more ecologically valid, and have been associated with increased neural response, compared to static faces (Sato et al., 2004). To our knowledge this is the first study to examine these associations in individuals with schizophrenia, for whom illness risk has been associated with higher IL-6 levels. Our results suggest that, across the entire sample, when processing dynamic facial displays of emotion, higher IL-6 plasma levels correspond to greater activation. This is observed in multiple regions typically associated with the DMN (mPFC, precuneus and lateral parietal cortex) when processing neutral facial expressions that are potentially more ambiguous in terms of emotional expression and observed in the insula when facial expressions of anger are being processed.
4.2. IL-6 and social cognition
4.2.1. Higher IL-6 is associated with increased activation of regions encompassing the DMN while viewing neutral faces
Associations between increased IL-6 plasma levels and increased activation within regions of the DMN during emotional processing of neutral faces is consistent with recent research by us and others (Bradley et al., 2019, Eisenberger, 2012). For example, Aruldass and colleagues (Aruldass et al., 2021) reported a relationship between higher plasma IL-6 and reduced functional connectivity within the DMN and the insula, in 72 individuals with major depressive disorder.
We have recently reported (in the same sample as reported on here (N = 311) (King et al., in press), that higher plasma IL-6 was associated with decreased resting state DMN connectivity (i.e., precuneus – left lateral parietal (LLP) cortex). We further found that higher IL-6 and decreased resting DMN connectivity together sequentially mediated the relationship between measures of early life stress and lower emotion recognition performance on a behavioural task. In the present study, during processing of emotionally neutral stimuli, variation in IL-6 was again associated with activation changes in the same regions implicated in our DMN connectivity study. We have previously speculated that increased neural activation during neutral faces might indicate misperception of social threat from neutral faces (Mothersill et al., 2014). Whether because of this, or because emotionally neutral faces are just harder to interpret, and hence require representation by a wider network of activity, the overlap with regions identified in the resting DMN findings is noteworthy, particularly as these regions were not selected for a priori as regions of interest. Specifically, it suggests that this network may be particularly sensitive to inflammatory immune response, at least as measured by IL-6, and in a manner relevant to processing of socially relevant stimuli.
4.2.2. Higher IL-6 is associated with increased activation of the insula while viewing angry faces
Associations between increased IL-6 plasma levels and increased activation within the insular cortex during the emotional processing of angry faces is consistent with other studies in this area. At a cortical level, the insular cortex has consistently been associated with various aspects of emotion regulation and social interaction, including anger and social threat (Emmerling et al., 2016). Eisenberger and colleagues (Eisenberger et al., 2009) examined whether neural response to social threat was associated with increases in plasma IL-6 after endotoxin injection in a sample of 20 healthy volunteers using the Cyberball task, in which participants are socially excluded from a ball-tossing game. Increases in plasma IL-6 were associated with increased activity within several regions, including the insula. In a similar fMRI study by Slavich and colleagues in 31 healthy participants, increases in plasma IL-6 during a social stress test were associated with increased insula response during the Cyberball task, although only at marginal levels (Slavich et al., 2010). Finally, in a study by Muscatell and colleagues, endotoxin-induced increases in plasma IL-6 correlated with increased neural response across several areas including the insula during an fMRI task in which participants received negative evaluative feedback, in 55 healthy participants (Muscatell et al., 2016).
In contrast, findings from previous studies on plasma IL-6 and neural activation during facial emotion recognition itself are more mixed, with three studies reporting no statistically significant associations (Dutcher et al., 2021, Harrison et al., 2009, Muscatell et al., 2016). However, differences in findings from these studies may reflect their use of static facial stimuli. As noted, dynamic videos of facial expressions may be more ecologically valid given that temporal cues are an important aspect of facial emotion recognition, and previous research by Sato and colleagues has shown that neural response is greater for dynamic faces compared to static faces (Sato et al., 2004).
Combining the results of previous studies with the present, larger scale, study, one interpretation of these findings is that IL-6 is particularly relevant to processing of socially relevant cues such as social threat, when represented as either expressions of social exclusion, negative evaluative feedback, or dynamic videos of angry faces. On the other hand, IL-6 is not only associated with processing of representations of anger and threat by others; two studies in older healthy cohorts report evidence that IL-6 was also associated with the subjective experience of anger (Puterman et al., 2014, Wrosch et al., 2018). Considering these findings, the association between higher IL-6 levels and higher insular activation during angry processing observed here may reflect the association between IL and 6 and stress reported more broadly in the literature.
4.3. Limitations & future research
It was surprising that no significant differences in neural response were observed between patients and controls during this task, with patients showing comparable neural response to the faces compared to controls. We previously reported that patients show increased mPFC response compared to controls during face processing compared to non-faces (Mothersill et al., 2014). One possible reason for this difference could be differences in the demographics and clinical characteristics of the patient group. For example, in our previous study, there were 20 males to 5 females, compared to 37 males to 16 females in the current study. Similarly, the mean chlorpromazine equivalent in mg/day in our previous study was 377.52 compared to 1023.22 in the current study. Another reason may be sample size. Our previous study included 46 participants (25 patients and 21 controls), while our current study included 214 participants (53 patients and 161 controls). As such, the smaller sample size in our previous study may have led to an overestimation of the true differences in neural response during this task in patients compared to controls, which might be smaller in magnitude. Additional study of the association between IL-6 and cortical activation during face processing and in patients and controls will no doubt clarify the extent of patient and control differences, especially in relation to both antipsychotic dosage and symptom severity. The high CPZ levels and low PANSS scores in this patient sample reflects a clinically stable outpatient group and may further explain the non-significant differences between groups. Further work is also currently being conducted by our lab to investigate patient specific effects in relation to these findings, such as antipsychotic dosage, illness duration and symptom severity.
Given that effects of plasma IL-6 on neural activation were observed in an mPFC cluster in the patient group overlapping with a cluster observed in the pooled sample, effects of IL-6 on this cluster in the pooled sample may be driven by effects in the patient group. And given that no significant associations were observed in the healthy control group, this suggests the association in this cluster observed in the pooled sample may be specific to an illness-related process. However, many of the effects of plasma IL-6 on neural activation observed in our pooled sample were not observed when patients and healthy controls were examined separately. Although our pooled sample was sufficiently powered to detect medium to large effects of plasma IL-6 on neural activation, patient and healthy control sub-groups were smaller and may have been underpowered to detect as wide a range of effects. Thus, future studies should examine effects of plasma IL-6 in larger samples of patients and healthy controls to better understand group-specific effects and whether any associations observed in our pooled sample are specific to an illness-related process.
Finally, peripheral immune responses such as plasma IL-6 levels are thought to affect the brain through a variety of mechanisms including signalling from the vagus nerve, cytokine transporters across the blood brain barrier, and production of pro-inflammatory cytokines within the brain by microglial cells (Dantzer et al., 2008). Although our results suggest multiple effects of peripheral IL-6 circulation on cortical response to faces, further cellular studies will be needed to examine the specific mechanisms by which these immune signals from the body ultimately affect cortical response to faces.
4.4. Conclusions
In conclusion, the primary objective of this study was to test the hypothesis that higher plasma IL-6 would predict increased neural response during facial emotion recognition in a sample of SZ and healthy participants. Using functional MRI analysis, we found that higher plasma IL-6 predicted increased neural response of the mPFC, precuneus, and lateral parietal cortex during neutral face processing compared to angry face processing, and increased insula response during angry face processing compared to neutral face processing. These findings, observed across patients and healthy participants, suggests that inflammatory processes (at least as measured by IL-6) are associated with changes in cortical activity during processing of socially relevant information, and may at least partly explain the association with social cognition. Future research examining the mechanisms underpinning this association between peripheral IL-6 and brain activation (such as altered microglial function and changes in synaptic plasticity) will be important to better understand the cellular basis of these relationships.
CRediT authorship contribution statement
David Mothersill: Conceptualization, Software, Formal analysis, Investigation, Data curation, Writing – original draft, Writing - review & editing, Supervision, Project administration. Sinead King: Conceptualization, Data curation, Writing – original draft, Writing - review & editing, Supervision, Project administration. Laurena Holleran: Investigation, Data curation, Supervision, Project administration. Maria Dauvermann: Investigation, Data curation, Supervision, Project administration. Saahithh Patlola: Investigation, Data curation. Karolina Rokita: Investigation, Data curation. Ross McManus: Conceptualization, Resources, Supervision. Marcus Keynon: Investigation, Data curation. Colm McDonald: Conceptualization, Resources, Supervision. Brian Hallahan: Conceptualization, Resources, Supervision. Aiden Corvin: Conceptualization, Resources, Supervision. Derek Morris: Conceptualization, Resources, Supervision. John Kelly: Conceptualization, Resources, Supervision. Declan McKernan: Conceptualization, Resources, Supervision. Gary Donohoe: Conceptualization, Resources, Writing – original draft, Writing - review & editing, Supervision, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We sincerely thank all participants who took part in the study. We would also like to thank Michael Gill, Laura Costello, Caroline Cullen, Niamh Daly Ryan, Laura McHugh, Ainé McNicholas, Marta Grzywacz, Cathal Ó Curraoin, and Catherine O’Donoghue for their assistance with the project. This work was funded by grants to GD from the European Research Council (ERC-2015-STG-677467) and Science Foundation Ireland (SFI-16/ERCS/3787).
References
- Anticevic A., Cole M.W., Murray J.D., Corlett P.R., Wang X.-J., Krystal J.H. The role of default network deactivation in cognition and disease. Trends Cognit. Sci. 2012;16(12):584–592. doi: 10.1016/j.tics.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aruldass AR, Kitzbichler MG, Morgan SE, Lim S, Lynall M-E, Turner L, et al. (2021): Dysconnectivity of a brain functional network was associated with blood inflammatory markers in depression. medRxiv. doi: https://doi.org/10.1101/2021.04.02.21254853. [DOI] [PubMed]
- Ashburner J, Barnes G, Chen C-C, Daunizeau J, Flandin G, Friston K, et al. (2014): SPM12 manual. Wellcome Trust Centre for Neuroimaging, London, UK.
- Ashburner J., Friston K.J. Unified segmentation. NeuroImage. 2005;26(3):839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Behere RishikeshV. Facial emotion recognition deficits: The new face of schizophrenia. Ind. J. Psychiatry. 2015;57(3):229. doi: 10.4103/0019-5545.166641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley K.A., Stern E.R., Alonso C.M., Xie H., Kim-Schulze S., Gabbay V. Relationships between neural activation during a reward task and peripheral cytokine levels in youth with diverse psychiatric symptoms. Brain Behav. Immun. 2019;80:374–383. doi: 10.1016/j.bbi.2019.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Lawrence Erlbaum Associates; Hillsdale (NJ): 1988. Statistical Power Analysis for the Behavioral Sciences; pp. 18–74. [Google Scholar]
- Cowman M, Holleran L, Lonergan E, O’Connor K, Birchwood M, Donohoe G (2021): Cognitive Predictors of Social and Occupational Functioning in Early Psychosis: A Systematic Review and Meta-analysis of Cross-Sectional and Longitudinal Data. Schizophrenia Bulletin. Online ahead of print, doi: 10.1093/schbul/sbab033. [DOI] [PMC free article] [PubMed]
- Dantzer R., O'Connor J.C., Freund G.G., Johnson R.W., Kelley K.W. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 2008;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauvermann M, Mothersill D, Rokita KI, King S, Holleran L, Kane R, et al. (2021): Changes in Default-Mode Network Associated With Childhood Trauma in Schizophrenia. Schizophrenia Bulletin. Online ahead of print, doi: 10.1093/schbul/sbab025. [DOI] [PMC free article] [PubMed]
- Donohoe G., Holland J., Mothersill D., McCarthy-Jones S., Cosgrove D., Harold D., Richards A., Mantripragada K., Owen M.J., O'Donovan M.C., Gill M., Corvin A., Morris D.W. Genetically predicted complement component 4A expression: effects on memory function and middle temporal lobe activation. Psychol. Med. 2018;48(10):1608–1615. doi: 10.1017/S0033291717002987. [DOI] [PubMed] [Google Scholar]
- Dutcher J.M., Boyle C.C., Eisenberger N.I., Cole S.W., Bower J.E. Neural responses to threat and reward and changes in inflammation following a mindfulness intervention. Psychoneuroendocrinology. 2021;125:105114. doi: 10.1016/j.psyneuen.2020.105114. [DOI] [PubMed] [Google Scholar]
- Eickhoff S.B., Stephan K.E., Mohlberg H., Grefkes C., Fink G.R., Amunts K., Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage. 2005;25(4):1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Eickhoff S.B., Heim S., Zilles K., Amunts K. Testing anatomically specified hypotheses in functional imaging using cytoarchitectonic maps. NeuroImage. 2006;32(2):570–582. doi: 10.1016/j.neuroimage.2006.04.204. [DOI] [PubMed] [Google Scholar]
- Eickhoff S.B., Paus T., Caspers S., Grosbras M.-H., Evans A.C., Zilles K., Amunts K. Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. NeuroImage. 2007;36(3):511–521. doi: 10.1016/j.neuroimage.2007.03.060. [DOI] [PubMed] [Google Scholar]
- Eisenberger N.I. The neural bases of social pain: evidence for shared representations with physical pain. Psychosom. Med. 2012;74:126. doi: 10.1097/PSY.0b013e3182464dd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger N.I., Inagaki T.K., Rameson L.T., Mashal N.M., Irwin M.R. An fMRI study of cytokine-induced depressed mood and social pain: the role of sex differences. NeuroImage. 2009;47(3):881–890. doi: 10.1016/j.neuroimage.2009.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmerling F., Schuhmann T., Lobbestael J., Arntz A., Brugman S., Sack A.T. The role of the insular cortex in retaliation. PloS One. 2016;11 doi: 10.1371/journal.pone.0152000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul F., Erdfelder E., Lang A.-G., Buchner A. G* Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods. 2007;39(2):175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Faul F., Erdfelder E., Buchner A., Lang A.-G. Statistical power analyses using G* Power 3.1: tests for correlation and regression analyses. Behav. Res. Methods. 2009;41(4):1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- Feng T., Tripathi A., Pillai A. Inflammatory pathways in psychiatric disorders: the case of schizophrenia and depression. Curr. Behav. Neurosci. Rep. 2020:1–11. doi: 10.1007/s40473-020-00207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fett A.-K., Viechtbauer W., Dominguez M.-D.-G., Penn D.L., van Os J., Krabbendam L. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: a meta-analysis. Neurosci. Biobehav. Rev. 2011;35(3):573–588. doi: 10.1016/j.neubiorev.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Fornito A., Zalesky A., Pantelis C., Bullmore E.T. Schizophrenia, neuroimaging and connectomics. NeuroImage. 2012;62(4):2296–2314. doi: 10.1016/j.neuroimage.2011.12.090. [DOI] [PubMed] [Google Scholar]
- Friston K.J., Holmes A.P., Worsley K.J., Poline J.-P., Frith C.D., Frackowiak R.S.J. Statistical parametric maps in functional imaging: a general linear approach. Hum. Brain Mapp. 1994;2(4):189–210. [Google Scholar]
- Friston K.J., Frith C.D., Frackowiak R.S.J., Turner R. Characterizing dynamic brain responses with fMRI: a multivariate approach. NeuroImage. 1995;2(2):166–172. doi: 10.1006/nimg.1995.1019. [DOI] [PubMed] [Google Scholar]
- Friston K.J., Holmes A.P., Poline J.-B., Grasby P.J., Williams S.C.R., Frackowiak R.S.J., Turner R. Analysis of fMRI time-series revisited. NeuroImage. 1995;2(1):45–53. doi: 10.1006/nimg.1995.1007. [DOI] [PubMed] [Google Scholar]
- Friston K.J., Williams S., Howard R., Frackowiak R.S.J., Turner R. Movement-related effects in fMRI time-series. Magn. Reson. Med. 1996;35(3):346–355. doi: 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- Grosbras M.-H., Paus T. Brain networks involved in viewing angry hands or faces. Cereb. Cortex. 2006;16:1087–1096. doi: 10.1093/cercor/bhj050. [DOI] [PubMed] [Google Scholar]
- Habel U., Chechko N., Pauly K., Koch K., Backes V., Seiferth N., Shah N.J., Stöcker T., Schneider F., Kellermann T. Neural correlates of emotion recognition in schizophrenia. Schizophr. Res. 2010;122(1-3):113–123. doi: 10.1016/j.schres.2010.06.009. [DOI] [PubMed] [Google Scholar]
- Harrison N.A., Brydon L., Walker C., Gray M.A., Steptoe A., Critchley H.D. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biol. Psychiatry. 2009;66(5):407–414. doi: 10.1016/j.biopsych.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby J.V., Hoffman E.A., Gobbini M.I. The distributed human neural system for face perception. Trends Cognit. Sci. 2000;4:223–233. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- Holland J.F., Cosgrove D., Whitton L., Harold D., Corvin A., Gill M., Mothersill D.O., Morris D.W., Donohoe G. Beyond C4: Analysis of the complement gene pathway shows enrichment for IQ in patients with psychotic disorders and healthy controls. Genes Brain Behav. 2019;18(8) doi: 10.1111/gbb.v18.810.1111/gbb.12602. [DOI] [PubMed] [Google Scholar]
- Khandaker G.M., Dantzer R., Jones P.B. Immunopsychiatry: important facts. Psychol. Med. 2017;47(13):2229–2237. doi: 10.1017/S0033291717000745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King S, Holleran L, Mothersill D, Patlola S, Rokita K, McManus R, et al. (Article in press): Early Life Adversity, Functional Connectivity And Cognitive Performance In Schizophrenia: The Mediating Role Of IL-6 Brain, Behavior, and Immunity. [DOI] [PubMed]
- Li W., Mai X., Liu C. The default mode network and social understanding of others: what do brain connectivity studies tell us. Front. Hum. Neurosci. 2014;8:74. doi: 10.3389/fnhum.2014.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondelli V., Di Forti M., Morgan B.P., Murray R.M., Pariante C.M., Dazzan P. Baseline high levels of complement component 4 predict worse clinical outcome at 1-year follow-up in first-episode psychosis. Brain Behav. Immun. 2020;88:913–915. doi: 10.1016/j.bbi.2020.01.014. [DOI] [PubMed] [Google Scholar]
- Mothersill O., Morris D.W., Kelly S., Rose E.J., Bokde A., Reilly R., Gill M., Corvin A.P., Donohoe G. Altered medial prefrontal activity during dynamic face processing in schizophrenia spectrum patients. Schizophr. Res. 2014;157(1-3):225–230. doi: 10.1016/j.schres.2014.05.023. [DOI] [PubMed] [Google Scholar]
- Müller N. The role of anti-inflammatory treatment in psychiatric disorders. Psychiatria Danubina. 2013;25 [PubMed] [Google Scholar]
- Müller N. Immunology of schizophrenia. NeuroImmunoModulation. 2014;21(2-3):109–116. doi: 10.1159/000356538. [DOI] [PubMed] [Google Scholar]
- Müller N., Weidinger E., Leitner B., Schwarz M.J. The role of inflammation in schizophrenia. Front. Neurosci. 2015;9:372. doi: 10.3389/fnins.2015.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscatell K.A., Eisenberger N.I., Dutcher J.M., Cole S.W., Bower J.E. Links between inflammation, amygdala reactivity, and social support in breast cancer survivors. Brain Behav. Immun. 2016;53:34–38. doi: 10.1016/j.bbi.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscatell K.A., Moieni M., Inagaki T.K., Dutcher J.M., Jevtic I., Breen E.C., Irwin M.R., Eisenberger N.I. Exposure to an inflammatory challenge enhances neural sensitivity to negative and positive social feedback. Brain Behav. Immun. 2016;57:21–29. doi: 10.1016/j.bbi.2016.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R., Brody G.H., Armstrong C.C., Carroll A.L., Sweet L.H., Yu T., Barton A.W., Hallowell E.S., Chen E., Higgins J.P., Parrish T.B., Wang L., Miller G.E. Higher peripheral inflammatory signaling associated with lower resting-state functional brain connectivity in emotion regulation and central executive networks. Biol. Psychiatry. 2019;86(2):153–162. doi: 10.1016/j.biopsych.2019.03.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher D., Duchaine B., Walsh V. Combined TMS and fMRI reveal dissociable cortical pathways for dynamic and static face perception. Curr. Biol. 2014;24(17):2066–2070. doi: 10.1016/j.cub.2014.07.060. [DOI] [PubMed] [Google Scholar]
- Puterman E., Epel E.S., O’Donovan A., Prather A.A., Aschbacher K., Dhabhar F.S. Anger is associated with increased IL-6 stress reactivity in women, but only among those low in social support. Int. J. Behav. Med. 2014;21(6):936–945. doi: 10.1007/s12529-013-9368-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato W., Kochiyama T., Yoshikawa S., Naito E., Matsumura M. Enhanced neural activity in response to dynamic facial expressions of emotion: an fMRI study. Cognit. Brain Res. 2004;20(1):81–91. doi: 10.1016/j.cogbrainres.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Sekar A., Bialas A.R., de Rivera H., Davis A., Hammond T.R., Kamitaki N., Tooley K., Presumey J., Baum M., Van Doren V., Genovese G., Rose S.A., Handsaker R.E., Daly M.J., Carroll M.C., Stevens B., McCarroll S.A. Schizophrenia risk from complex variation of complement component 4. Nature. 2016;530(7589):177–183. doi: 10.1038/nature16549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H., Wang X., Yi J., Zhu X., Zhang X., Yang J. Default mode network alterations during implicit emotional faces processing in first-episode, treatment-naive major depression patients. Front. Psychol. 2015;6:1198. doi: 10.3389/fpsyg.2015.01198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich G.M., Way B.M., Eisenberger N.I., Taylor S.E. Neural sensitivity to social rejection is associated with inflammatory responses to social stress. Proc. Natl. Acad. Sci. U.S.A. 2010;107(33):14817–14822. doi: 10.1073/pnas.1009164107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spies M., Kraus C., Geissberger N., Auer B., Klöbl M., Tik M. Default mode network deactivation during emotion processing predicts early antidepressant response. Transl Psychiatry. 2017;7 doi: 10.1038/tp.2016.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upthegrove R., Khandaker G.M. Cytokines, oxidative stress and cellular markers of inflammation in Schizophrenia. Curr. Top. Behav. Neurosci. 2020;44:49–66. doi: 10.1007/7854_2018_88. [DOI] [PubMed] [Google Scholar]
- Wrosch C., Barlow M.A., Kunzmann U. Age-related changes in older adults' anger and sadness: The role of perceived control. Psychol. Aging. 2018;33(2):350–360. doi: 10.1037/pag0000229. [DOI] [PubMed] [Google Scholar]
- Zhou L.i., Pu W., Wang J., Liu H., Wu G., Liu C., Mwansisya T.E., Tao H., Chen X., Huang X., Lv D., Xue Z., Shan B., Liu Z. Inefficient DMN suppression in schizophrenia patients with impaired cognitive function but not patients with preserved cognitive function. Sci. Rep. 2016;6(1) doi: 10.1038/srep21657. [DOI] [PMC free article] [PubMed] [Google Scholar]