Abstract
Previous meta-analyses have identified moderate deficits in executive function (EF) in children born low birth weight (birth weight<2500 g; LBW). The current study tests the joint contribution of LBW and parenting quality on trajectories of executive function in 1121 preschoolers (50 % boys). We estimated latent growth curve models to represent linear change in EF from 3 to 5 years of age, and tested the impact of LBW, parenting, and their interaction, on the estimated trajectory parameters. Although LBW was related to lower EF ability at all three time points (Cohen’s d=0.43–0.55), LBW children who experienced high levels of sensitive parenting in toddlerhood exhibited faster rates of improvement in EF, and were virtually indistinguishable from their normal birth weight peers by age 5. On the other hand, LBW children who experienced below average levels of sensitive parenting showed lasting deficits in EF ability. These findings suggest that sensitive parenting may buffer LBW children from lasting deficits in EF. Implications of these findings for future interventions are discussed.
Keywords: Low birth weight, Executive function, Parenting, Latent growth curve models
In 2012, 8 % of all babies born in the United States were low birth weight (LBW), defined as a birth weight of less than 2500 g (Martin et al. 2013). Although medical advances within Neonatal Intensive Care Units (NICU) have been associated with dramatic decreases in mortality for LBW infants (Fanaroff et al. 2007), infants that do survive experience subtle developmental difficulties in both behavior and cognition more often than do infants born at a normal birth weight (NBW; e.g., Aylward et al. 1989; Bhutta et al. 2002).
Though early research suggested that LBW and preterm infants differ temperamentally from their NBW counterparts (Goldberg 1978), more recent research indicates that these differences may not be reflected in current LBW survivors (Voegtline et al. 2010). Considering more extreme behavioral differences, LBW children may be at a greater risk for internalizing and externalizing disorders, as well as ADHD, compared to NBW infants (pooled RR=2.64; Bhutta et al. 2002). Additional studies have replicated a threefold increase in risk for ADHD among children born LBW, controlling for potential genetic and environmental confounds (e.g., Mick et al. 2002). In addition to their increased risk for psychopathology, LBW children tend to exhibit poorer cognitive abilities compared to NBW peers. Early meta-analyses on cognitive outcomes for LBW children have found evidence for large deficits in intelligence (IQ) and developmental quotient (DQ) scores (Cohen’s d=0.8; Aylward et al. 1989) as well as in academic achievement (RR=3.7; de Rodrigues et al. 2006). More recently, research has shifted towards considering specific domains of cognition that are impacted in LBW children. In this vein, the current study focuses specifically on executive function (EF) in LBW and NBW children.
Low Birth Weight and Executive Function
Executive functioning (EF) refers to the set of higher-order cognitive processes necessary for purposeful, goal-oriented behavior. The three most commonly studied components of EF include working memory, inhibitory control, and cognitive flexibility. Although research on adolescent and adult samples supports a multidimensional conceptualization of EF, with factors representing the three distinct components listed above (e.g., Miyake et al. 2000), empirical evidence from younger children suggests that EF is best represented as a unidimensional construct (e.g., Wiebe et al. 2008). Regardless of if and how differentiation in EF occurs across development, considerable research points to the preschool years as a period of rapid growth in EF ability (for a review, see Garon et al. 2008). Further, individual differences in EF that arise during this period have implications for child functioning across multiple domains. While better executive function in the preschool years predicts enhanced theory of mind (Müller et al. 2012) and academic achievement (Biederman et al. 2004), deficits in preschool EF are associated with higher rates of externalizing behaviors (Schoemaker et al. 2013) and ADHD symptomatology (Pauli-Pott and Becker 2011). Therefore, assessing the impact of LBWon EF during the preschool years is of both empirical and practical interest, as it may partially explain why LBW children are at heightened risk for psychopathology.
In order to address the magnitude of EF deficits in individuals born LBW, Aarnoudse-Moens et al. (2009) conducted a meta-analysis on 12 studies that assessed either verbal fluency, working memory, and/or cognitive flexibility. They found LBW to be associated with consistent deficits in EF, with effect sizes ranging from 0.36 (in working memory) to 0.49 (in cognitive flexibility). However, most of the studies included in this meta-analysis were conducted on small samples of LBW children and NBW controls, limiting the extent to which these results may generalize to larger populations. In addition, all of the studies tested EF in children aged 7 and older. Therefore, this meta-analysis cannot inform our understanding of early EF deficits in LBW populations. To address this gap, a limited number of more recent studies have examined EF deficits specifically in preschoolers (Baron et al. 2012; Woodward et al. 2011). However, these studies have been conducted with either very preterm or extremely low birth weight samples.
One such study by Woodward et al. (2011) found that very preterm children (gestational age<32 weeks) underperformed on various measures of EF at age 4, compared to full term children (Cohen’s d=0.54). Further, these impairments were related to mild to moderate white matter abnormalities in preterm children. It is worth noting that the majority of the preterm sample was also LBW, while the full term children were not. An additional study assessed the relationship between birth weight and EF in 3 year olds, focusing primarily on children born extremely low birth weight (ELBW; birth weight≤1000 g; Baron et al. 2012). This study found that ELBW preschoolers performed significantly worse on tasks of working memory and inhibitory control, compared to NBW peers. These deficits remained even after controlling for age at testing and maternal educational level. Therefore, multiple studies suggest that deficits in EF may emerge as early as the preschool years for children born either very premature or well below the range of normal weight. We expect that these relationships will extend to a larger, diverse sample of preschoolers born LBW. However, because the few existing studies on preschoolers have assessed EF at one time point, we do not yet know how LBW influences trajectories of EF development. The present study addresses these gaps in the literature through use of a longitudinal design that includes repeated measures of EF across the preschool years.
Parenting Buffers LBW Outcomes
In light of the known behavioral and cognitive risks for individuals born LBW, intervention studies have long been proposed in the hopes of preventing long-term problems. A review of early intervention efforts concluded that the most successful programs focused on increasing parents’ knowledge of LBW infants’ developmental needs, as well as training parents to read their infants’ cues and derive satisfaction from parent–child interactions (Patteson and Barnard 1990). Building upon these foundational studies, more recent randomized interventions in the United States (Ramey et al. 1992) and Jamaica (Walker et al. 2004) have bolstered support for parenting as an effective intervention strategy. LBW preschoolers whose families participated in these interventions had higher IQ and DQ scores, as well as fewer parent-rated behavioral problems (Brooks-Gunn et al. 1993; Walker et al. 2004), compared to LBW children in the control groups. Though these gains were partially attenuated over time, intervention effects persisted at 6- and 8-years for measures such as IQ (McCarton et al. 1997; Walker et al. 2010).
Though existing intervention studies suggest that high quality, responsive parenting promotes better outcomes for LBW children, all of the studies mentioned above reported on general cognitive outcomes, not EF. However, research studies outside of intervention contexts suggest that sensitive parenting is critically important for promoting the development of EF within normally-developing children (for a review, see Fay-Stammbach et al. 2014).
Parenting and Executive Function
A growing body of literature suggests that variation within the normative range of observed parenting behaviors is predictive of children’s EF. Four studies have examined links between parenting in toddlerhood and EF in preschool (Blair et al. 2014; Hammond et al. 2012; Hughes and Ensor 2009; Towe-Goodman et al. 2014). Both Hughes and Ensor (2009) and Hammond et al. (2012) found that higher levels of maternal scaffolding, measured when children were ages 2 and 3, predicted better EF at age 4. In addition, two studies utilizing the same data as the current study (The Family Life Project; FLP), found that a composite measure of maternal sensitivity, coded from parent–child interactions in toddlerhood, predicted children’s EF ability. Specifically, higher parental sensitivity and responsiveness at 36 months predicted better EF at 60 months, controlling for initial levels of EF at 36 months (Blair et al. 2014). Fathers’ parenting matters too: a new study by Towe-Goodman et al. (2014) suggests that fathers’ sensitive parenting at 24 months is positively associated with children’s EF at 36–60 months, making an independent contribution above and beyond mothers’ parenting.
Three additional studies have extended the observation of parenting into earlier periods of development, to test the relation between parenting quality in infancy and preschool EF (Bernier et al. 2010, 2012; Blair et al. 2011). In one set of studies, Bernier et al. (2010, 2012) found that maternal sensitivity, autonomy-support, and mind-mindedness at 12 and 18 months predicted child EF at 18, 26, and 36 months. Within FLP data, parental sensitivity and intrusiveness at 6, 15, and 24 months has been shown to predict child EF at 36 months, in the expected directions (Blair et al. 2011).
Taken together, this body of literature suggests that parenting quality is important for children’s development of EF. However, the studies reviewed here either excluded LBW children, or collapsed across birth weight groups. Thus, the extent to which LBW children may differentially benefit from sensitive parenting is not yet known. The studies reviewed here also fail to address the key issue of timing of parenting effects. Thus far, parenting in infancy and toddlerhood have been studied largely independently, limiting our understanding of how both earlier and later parenting jointly predict the development of EF. Arguably, both time periods should be important, albeit for different reasons.
During the first 2 years of life, the infant brain reaches 90 % of its eventual size, and dramatic increases in both myelination and synaptic pruning underlie the emergence of foundational cognitive abilities (Nelson et al. 2006). Moreover, neural systems are particularly susceptible to experiential input during this period of rapid growth and plasticity (for a review, see Singer 1995), suggesting that feedback from responsive caregivers in infancy may be critical to ensure normative early brain development. However, experiences with caregivers from ages 2 to 3 may also be important to consider, as these years demarcate the beginning of a period of rapid improvement in EF (Garon et al. 2008). Caregiving experienced at a time when EF skills are first emerging may allow children to successfully practice and build upon their nascent abilities within a supportive context. Given the evidence suggesting that both early and late parenting matter for children’s development of EF, the current study makes use of repeated observations of parenting across both infancy and toddlerhood. By accounting for potential continuity and change in parenting across both periods, we hope to shed light on the timing of parental input that matters most for LBW children’s development of EF.
The Current Study
As reviewed above, there are well-established, yet isolated, literatures examining the impact of LBW status and parenting quality on children’s development of EF. The current study bridges these two disparate literatures to test whether LBW status predicts differing trajectories of EF across the preschool years, and whether the magnitude of these differences is moderated by parental sensitivity and intrusiveness. By incorporating repeated assessment of EF at three time points (36, 48, 60 months), we expand upon previous research that has compared LBWand NBW individuals’ EF performance at a single time point. Additionally, although previous research has examined the role of parenting in infancy and toddlerhood separately, we are the first to examine both influences within the same model. Thus, we hope this study will enhance understanding about the specificity of LBW-related cognitive deficits, as well as the extent to which environmental factors (i.e., sensitive parenting) may buffer children from early adversity.
Method
Participants
The Family Life Project (FLP) was designed to study families living in two of the four rural geographical regions of high child poverty. Three counties in eastern North Carolina (NC) and three counties in central Pennsylvania (PA) were chosen to represent the Black South and Appalachia, respectively. Complex sampling methods yielded a representative sample of 1292 families recruited at the time that they gave birth to a child. Low-income families were oversampled in both states, and African American families were oversampled in NC. African American families were not oversampled in PA because the target counties were >95 % Caucasian. Detailed recruitment and sampling procedures are documented elsewhere (Burchinal et al. 2008).
Out of the 5471 women who gave birth during the recruitment period, 72 % were eligible for the study. FLP investigators excluded families who did not live in the selected counties, spoke a primary language other than English in the home, or intended to move out of the area in the next 3 years. Out of the eligible families, 68 % consented to participate, and out of these, 58 % were invited to participate. The final sample was made up of 1292 families who completed the 2 month home visit, at which point they were officially enrolled in the study. The current analyses are based upon a sample of 1121 children (50 % boys) who participated in at least one assessment of EF (at 36, 48, and/or 60 months). Our sample does not differ from the total FLP sample in terms of poverty status at recruitment, child gender, or primary caregiver’s race or education, (ps>0.05).
Procedures
The current study analyzes data collected from home visits when children were approximately 2, 6, 15, 24, 36, 48, and 60 months of age. Two home visits were conducted at the 6, 24, and 36 month time points, and one visit was conducted at the 2, 15, 48, and 60 month time points. Each home visit lasted approximately two hours, and consisted of a variety of parent (e.g., questionnaires, interviews), child (e.g., EF), and dyadic (e.g., free play) tasks that were videotaped for later coding by trained research assistants. All study protocols were approved by the necessary institutional review boards, and appropriate consent and assent were obtained from all research participants.
At 2 months, mothers completed demographic and pregnancy history questionnaires, including a self-report of their child’s birth weight in pounds and ounces. From this measure, we calculated birth weight in grams for use in determining LBW status. At 6, 15, 24, and 36 months, infants and their primary caregivers engaged in a 10-min semi-structured play activity which was later coded for parental warm sensitivity and harsh intrusiveness. At 36, 48, and 60 months, children spent between 30 and 45 min completing up to six EF tasks. Full details of task administration and the creation of longitudinally scalable scores appear elsewhere (Willoughby et al. 2012).
Measures
Executive Function
Each EF task was presented in an open spiral bound flip-book format, with pages measuring 8×14 in. Each page presented stimuli to the child on one page and scripted instructions for the research assistant on the other. For each task, children first had to pass a set of training trials, assessing their comprehension of task constructs and procedures, before continuing on to the test trials. The battery of EF tasks included two measures of working memory, three measures of inhibitory control, and one measure of attention shifting. The psychometric properties of this task battery, including its partial strong invariance over time, has been previously established (Willoughby et al. 2012). Because each task in the battery has been explained in detail elsewhere (see Willoughby et al. 2010), we provide abbreviated descriptions here.
Working Memory Span (WMS; Working Memory)
In the Working Memory Span, based upon principles described by Engle, Kane and collaborators (e.g., Kane and Engle 2003), children are shown a picture of a house with an animal and a colored circle inside. The child must name and hold in mind both pieces of information. Next, they are shown an empty house and asked to remember the animal or color that was previously in the house. This task requires working memory because children must activate one piece of information (i.e., animal name) while overcoming interference occurring from the other (i.e., color name). The task increases in difficulty such that children must remember information from up to three houses at a time.
Pick the Picture Game (PTP; Working Memory)
The Pick-the-Picture task is a self-ordered pointing task (Cragg and Nation 2007; Petrides and Milner 1982). Children are instructed to touch each picture one time, so that every picture “gets a turn.” The task requires working memory because children must remember which picture(s) they have already touched in each set. Because the PTP task was determined during pilot testing to be too difficult for many 3-year-olds, it was only administered at the 48 and 60 month assessments.
Silly Sounds Stroop (SSS; Inhibitory Control)
The Silly Sounds Stroop task is based upon the Day/Night Stroop task designed by Gerstadt et al. (1994). In this task, children are instructed to point to the dog when they hear a “meow” and to point to the cat when they hear a “woof.” Thus, children are required to inhibit the sounds normally associated with cats and dogs in order to successfully complete the task.
Spatial Conflict (SC; Inhibitory Control)
The Spatial Conflict task is a Simon task similar to that used by Gerardi-Caulton (2000). Whereas children respond to the initial set of items by touching a response card in the same position as the stimuli (e.g., the stimuli is presented on the left side of the test booklet and the correct response requires that the child touch the left side of his/her response card), the test items require a contra-lateral response (e.g., the stimuli is presented on the left side of the test booklet and the correct response requires that the child touch the right side of his/her response card; spatial location is no longer informative). At 36 months, task stimuli were boats and cars; at 48 and 60 months, task stimuli were arrows.
Animal Go/No-Go (GNG; Inhibitory Control)
The Animal Go/No-Go task is a standard go no-go task (e.g., Durston et al. 2002). Children are instructed to click a button every time they see an animal, but not when that animal is a pig. The task includes varying numbers of go trials prior to each no-go trial, in standard order: 1-go, 3-go, 3-go, 5-go, 1-go, 1-go, and 3-go trials. No-go trials require inhibitory control.
Something’s The Same Game (STS; Attention Shifting)
The Something’s The Same task is derived from the Flexible Item Selection Task (Jacques and Zelazo 2001). In this task, children are first shown two pictures that match on one dimension (e.g., shape). Next, children are shown the same two pictures, and a new third picture. The third picture is similar to one of the first two pictures along a different dimension (e.g., color). Children must choose which one of the two original pictures is the same as the new picture. This task requires the child to shift the focus of his/her attention from the first dimension of similarity to a second dimension of similarity.
Task Scoring
Item response theory (IRT) models were used to create expected a-posteriori (EAP) scores for each task at each assessment. This scoring method enhanced our precision of measurement by taking into account the difficulty and discrimination properties of items within each task, as opposed to a simple mean score approach that weighs each item equally. EAP scores were free of measurement error and were scaled on a z-score metric, where a value of 0 represented the average task performance at the 48 month assessment (Willoughby et al. 2012). Positive and negative values for EAPs refer to above and below average scores, relative to age 4. We took the mean EAP score across all completed tasks at a given assessment as a measure of child’s EF ability at that time point. Given the low communality of individual EF tasks, this approach was preferable to estimating second-order latent growth models, and is consistent with our recent work (Towe-Goodman et al. 2014; Willoughby et al. 2014).
Maternal Parenting Quality
Mother–child interactions were coded from video recordings of structured play activities. At 6 and 15 months, the dyad engaged in a free play activity. Parents were given a standardized set of toys (e.g., stacking rings, shape sorter) and instructed to play with their child as they normally would if they had some free time during the day. At 24 and 36 months, the dyads engaged in a puzzle task, in which experimenters provided a set of developmentally-appropriate puzzles of increasing difficulty for children to complete, and mothers were instructed to provide any assistance that they thought was necessary. These interactions were coded for the constructs of Sensitivity, Intrusiveness, Detachment, Stimulation of Cognitive Development, Positive Regard, Negative Regard and Animation, in line with the coding scheme developed by Cox and Crnic (2002) and based on the coding used in the NICHD Study of Early Child Care and Youth Development (NICHD SECCYD; NICHD ECCRN 1999). Global ratings of parents’ behaviors were made on a 1–5 scale at 6 and 15 months, and a 1–7 scale at 24 and 36 months, with values ranging from not at all characteristic to highly characteristic. Based on exploratory factor analysis conducted with oblique rotation (i.e., Promax), which suggested two underlying factors, we created composite scores of warm sensitivity and harsh intrusiveness by taking the mean across multiple parenting scales. Warm sensitivity was comprised of scores from the Sensitivity, Detachment (reverse coded), Stimulation of Cognitive Development, Positive Regard, and Animation scales, while harsh intrusiveness was comprised of scores from the Intrusiveness and Negative Regard scales. We used the composite scores of warm sensitivity and harsh intrusiveness in our analyses, as these scores capture broadly both positive and negative dimensions of parenting. This composite score approach has been widely used in previous analyses with FLP data (e.g., Blair et al. 2011; Towe-Goodman et al. 2014).
All coders were trained and certified by one master coder. In addition, reliability checks were completed on approximately 30 % of tapes to ensure intraclass correlations (ICC) between all pairs of coders exceeded 0.80 (ICC=0.80–0.98 for all subscales and composite scores). To test the impact of timing of parental input, we collapsed warm sensitivity and harsh intrusiveness scores across 6–15 and 24–36 months to represent the periods of infancy and toddlerhood.
Income-to-Needs Ratio
Income-to-needs ratio was retained as a covariate in all analyses, given its known association with EF in the preschool years. At the 6, 15, 24, and 36 month home visits, mothers self-reported their total household income from all sources. Aggregated household income was divided by the U.S. poverty threshold for the year (adjusted for family size and household composition) to create an income-to-needs ratio for each family at each time point. The average income-to-needs ratio across 6 to 36 months was retained as a single indicator.
Analytic Strategy
The data from this study were analyzed in three steps. First, we estimated latent growth curve (LGC) models to represent linear changes in EF from 3 to 5 years of age. Then, we tested the unique impact of LBW status and parenting quality on the estimated trajectory parameters (intercept and slope). Finally, we tested the interaction of LBW status and parenting quality in order to determine whether sensitive parenting may buffer LBW individuals from deficits in EF. All descriptive statistics were completed using SAS version 9.3 while LGC modeling was conducted in MPLUS version 4.3 (Muthén and Muthén 2007). Unweighted scores were used in all descriptive analyses, while LGC models included population weight and stratification variables. Household income-to-needs ratio was included as a covariate in all LGC models. Because of the presence of missing data in both predictor and outcome variables, we utilized a direct maximum likelihood (DML) estimator for all analyses. This technique is considered appropriate when data is missing at random (MAR) and allowed us to take advantage of all available data, without the need to impute missing values.
Results
Descriptive Statistics
Table 1 contains unweighted descriptive statistics for all predictor and outcome variables. Infants weighing≤2500 g were classified as LBW. Consistent with national averages (Martin et al. 2013), this classification resulted in a LBW sample of 7.7 % (N=86). For both LBW and NBW groups, mean EF scores increased steadily between 36 and 60 months, and there was considerable variation in scores at each age. However, LBW children performed worse than their NBW counterparts on the battery of EF tasks at each time point, showing deficits of moderate effect size (Cohen’s d=0.43–0.55; see Table 1). LBW was negatively associated with sensitive parenting and positively associated with intrusive parenting in both infancy and toddlerhood (for sensitivity, Cohen’s d=0.32 and 0.32; for intrusiveness, Cohen’s d=0.18 and 0.38, respectively). As shown in Table 2, sensitive parenting in infancy and toddlerhood was in turn associated with higher EF scores at all three assessments (at 36 months, r(955)=0.26 and r(967)=0.29; at 48 months, r(992)=0.34 and r(991)=0.39; at 60 months, r(1021)=0.33 and r(1010)=0.35, respectively). Similarly, intrusive parenting in infancy and toddlerhood was associated with lower EF scores (at 36 months, r(955)=−0.23 and r(967)=−0.31; at 48 months, r(992)=−0.23 and r(991)=−0.41; at 60 months, r(1021)=−0.19 and r(1010)=−0.33, respectively). These results were consistent with the expected relationships among LBW, parenting quality, and EF, allowing us to next turn to LGC models to test the joint impact of LBW status and parenting on trajectories of EF growth.
Table 1.
Unweighted descriptive statistics by birth weight group
| Total sample (N=1121) | LBW (N=86) | NBW (N=1035) | d | ||||
|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | ||
| Demographic variables | |||||||
| Birth weight | 3282 | 577.5 | 2079 | 473.1 | 3383 | 460.2 | 2.26 |
| Income-to-needs ratio | 1.85 | 1.52 | 1.39 | 1.04 | 1.90 | 1.56 | 0.34 |
| Parenting variables | |||||||
| Sensitive parenting (6–15 mo) | 2.84 | 0.73 | 2.63 | 0.76 | 2.86 | 0.72 | 0.32 |
| Sensitive parenting (24–36 mo) | 2.88 | 0.69 | 2.68 | 0.63 | 2.90 | 0.69 | 0.32 |
| Intrusive parenting (6–15 mo) | 2.34 | 0.62 | 2.44 | 0.61 | 2.33 | 0.62 | 0.18 |
| Intrusive parenting (24–36 mo) | 2.35 | 0.74 | 2.61 | 0.66 | 2.33 | 0.74 | 0.38 |
| Executive function ability | |||||||
| EF at 36 mo | −0.54 | 0.54 | −0.75 | 0.50 | −0.52 | 0.54 | 0.43 |
| EF at 48 mo | −0.13 | 0.51 | −0.29 | 0.49 | −0.01 | 0.51 | 0.55 |
| EF at 60 mo | 0.29 | 0.48 | 0.08 | 0.54 | 0.31 | 0.47 | 0.48 |
The LBW group was comprised of children who weighed≤2500 g at birth. The NBW group was comprised of children who weighed>2500 g at birth. Means and standard deviations are unweighted
EF executive function, mo months
Table 2.
Unweighted correlations for predictor and outcome variables
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
|---|---|---|---|---|---|---|---|---|---|
| 1. Low birth weight | 1.0 | ||||||||
| 2. Income-to-needs ratio | −0.09** | 1.0 | |||||||
| 3. Sensitive parenting (6–15 mo) | −0.08** | 0.43*** | 1.0 | ||||||
| 4. Sensitive parenting (24–36 mo) | −0.09** | 0.44*** | 0.70*** | 1.0 | |||||
| 5. Intrusive parenting (6–15 mo) | 0.05 | −0.32*** | −0.29*** | −0.34*** | 1.0 | ||||
| 6. Intrusive parenting (24–36 mo) | 0.10*** | −0.33*** | −0.38*** | −0.55*** | 0.48*** | 1.0 | |||
| 7. EF at 36 mo | −0.11*** | 0.23*** | 0.26*** | 0.29*** | −0.23*** | −0.31*** | 1.0 | ||
| 8. EF at 48 mo | −0.10** | 0.28*** | 0.34*** | 0.39*** | −0.23*** | −0.41*** | 0.37*** | 1.0 | |
| 9. EF at 60 mo | −0.13*** | 0.26*** | 0.33*** | 0.35*** | −0.19*** | −0.33*** | 0.32*** | 0.59*** | 1.0 |
| n | 1116 | 1113 | 1099 | 1085 | 1099 | 1085 | 973 | 1009 | 1038 |
Sample sizes for correlations vary from 889 to 1116, given variation among participants’ task completion EF executive function, mo months
p<0.10,
p<0.05,
p<0.01,
p<0.001
Latent Growth Curve Modeling
Unconditional Model
We first estimated an unconditional LGC model for our repeated measures of EF ability. The model was parameterized such that the intercept term represented EF at the 48 month assessment. With three repeated measures, only a linear functional form was considered. The model fit the data well, χ2 (1)=1.2, p=0.28, CFI=1.0, RMSEA (90 % confidence interval)=0.01 (0.00–0.08). The means and variances of the intercept (μInt=−0.05, p<0.001; ϕInt=0.12, p<0.001) and slope factors (μSlope=0.41, p<0.001; ϕSlope=0.04, p<0.001) were significant, indicating variability in both levels of EF at 48 months as well as rates of change in EF from 36 to 60 months. The intercept and slope terms were modestly correlated (ϕInt, Slope=0.27, p=0.002); children with higher EF scores at 48 months tended to exhibit faster rates of growth in EF from 36 to 60 months.
Conditional Models
Next, three conditional LGCs were estimated to test study hypotheses. In the first model, the EF intercept and slope terms were regressed on sensitive and intrusive dimensions of parenting from infancy and toddlerhood, LBW status, and demographic covariates. Model coefficients are summarized in Table 3. Household income-to-needs ratio was positively associated with child EF at 48 months (β=0.10, p=0.01). Controlling for covariates, there was a trend for children with LBW to exhibit lower levels of EF at age 48 months (β=−0.06, p=0.06). Sensitive parenting in both infancy (β=0.20, p<0.001) and toddlerhood (β=0.12, p=0.02 were associated with higher levels of EF at age 48 months. In addition, intrusive parenting in toddlerhood (β=−0.29, p<0.001) was associated with lower levels of EF at 48 months. In contrast, none of the predictors explained individual differences in the rate of change of EF.
Table 3.
Summary of standardized regression coefficients
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| I | S | I | S | I | S | |
| Income-to-needs ratio | 0.10* (0.04) | −0.01 (0.06) | 0.10* (0.04) | −0.01 (0.06) | 0.10* (0.04) | −0.01 (0.06) |
| Low birth weight (LBW) | −0.06+ (0.03) | −0.04 (0.06) | −0.04 (0.03) | 0.01 (0.07) | −0.06+ (0.03) | 0.01 (0.06) |
| Sensitive parenting (6–15 mo) | 0.20*** (0.05) | 0.06 (0.08) | 0.20*** (0.05) | 0.04 (0.08) | 0.20*** (0.05) | 0.07 (0.08) |
| Sensitive parenting (24–36 mo) | 0.12* (0.05) | −0.14 (0.09) | 0.10+ (0.06) | −0.16+ (0.09) | 0.12* (0.05) | −0.17* (0.09) |
| Intrusive parenting (6–15 mo) | −0.05 (0.04) | 0.03 (0.06) | −0.06 (0.04) | 0.02 (0.06) | −0.05 (0.04) | 0.03 (0.06) |
| Intrusive parenting (24–36 mo) | −0.29*** (0.05) | −0.01 (0.08) | −0.29*** (0.05) | −0.02 (0.08) | −0.29*** (0.05) | −0.01 (0.08) |
| LBW × sensitive parenting (6–15 mo) | −0.02 (0.04) | 0.07 (0.09) | ||||
| LBW × sensitive parenting (24–36 mo) | 0.05 (0.05) | 0.14 (0.10) | 0.16** (0.06) | |||
| LBW × intrusive parenting (6–15 mo) | 0.04 (0.04) | 0.05 (0.06) | ||||
| LBW × intrusive parenting (24–36 mo) | −0.03 (0.06) | 0.03 (0.08) | ||||
| Model R2 | 0.355 | 0.015 | 0.356 | 0.039 | 0.355 | 0.034 |
Tabled values are standardized parameter estimates with standard errors in parentheses. Model 1 represents main effects only. Model 2 shows all main effects and possible interactions between LBW and parenting variables. Model 3 retains all main effects, but is trimmed of all interaction terms p>0.15 I intercept (EF at 48 months), S slope, mo months
p<0.10,
p<0.05,
p<0.01,
p<0.001
The second conditional model added four interaction terms (i.e., each measure of parenting × LBW) as additional predictors. While no single interaction term was a significant predictor of either EF intercept or slope in the presence of all other interaction terms, the interaction between LBW and sensitive parenting in toddlerhood approached significance (β=0.14, p=0.15). Therefore, for our third and final model, we trimmed all interaction terms p>0.15. This final model, depicted graphically in Fig. 1, fit the data well, χ2 (9)=17.4, p=0.04, CFI=0.99, RMSEA (90 % confidence interval)=0.03 (0.01–0.05). Moreover, the prediction of EF slope from the LBW×sensitive parenting (toddlerhood) interaction term was statistically significant (β=0.16, p=0.004). Figure 1 provides a path diagram of this final model.
Fig. 1.
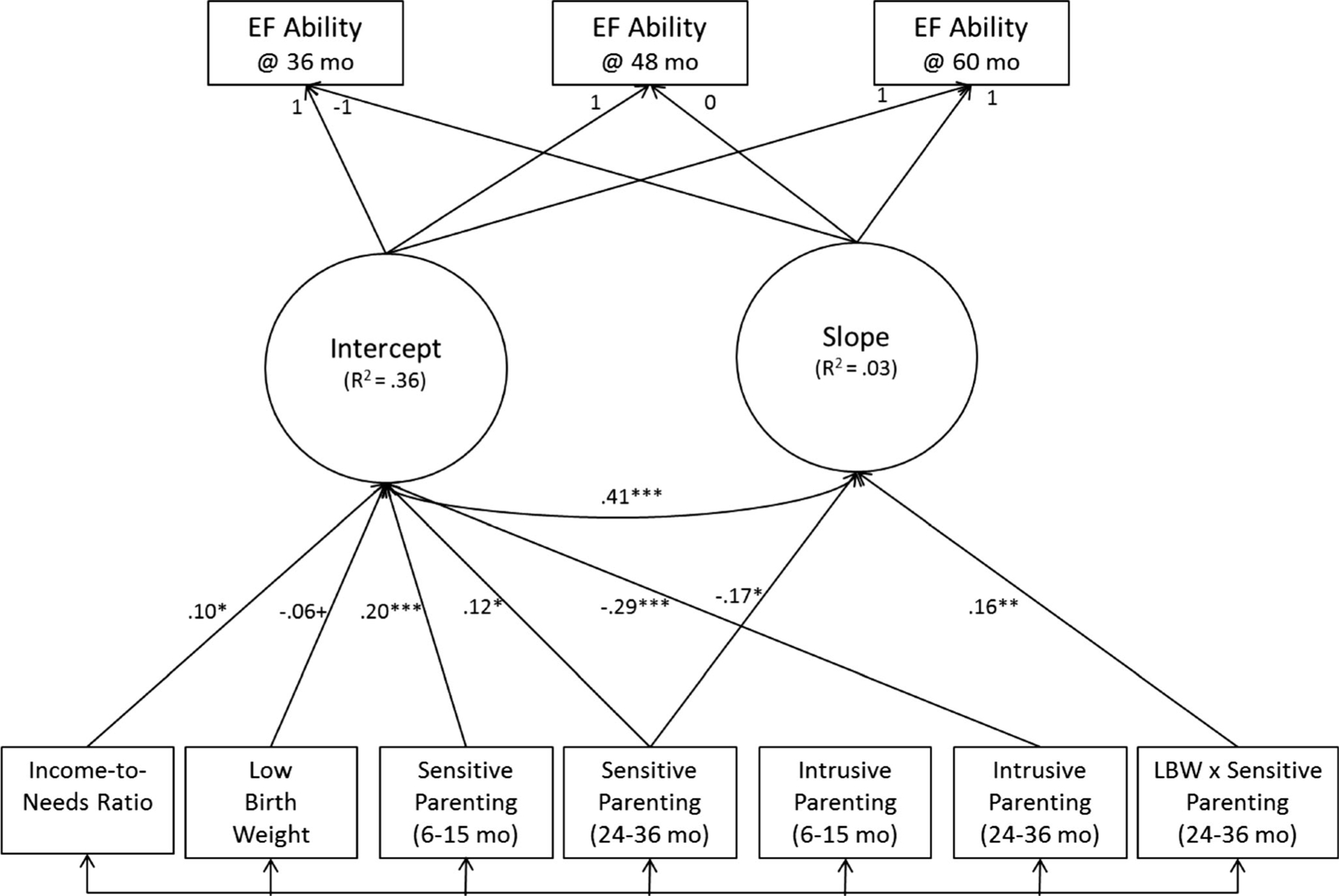
EF executive function, mo months, LBW low birth weight. Parameter estimates from final structural equation model (standardized estimates). The factor loadings are fixed, while the mean and variance of the latent intercept and slope factors are freely estimated. Residual variances and non-significant paths (p>0.10) are omitted for presentation purposes. +p<0.10, *p<0.05, **p<0.01, ***p<0.001
In order to better characterize the significant interaction, we plotted simple trajectories of EF as a function of LBW status and low (observed 25th percentile) and high (observed 75th percentile) parental sensitivity scores (see Fig. 2). From the figure, four points are worth mentioning. First, all children exhibited significant linear increases in EF across the preschool period (i.e., LBW/low sensitivity b=0.38, LBW/high sensitivity b=0.48, NBW/low sensitivity b=0.39, NBW/high sensitivity b=0.44, all ps<0.05). Second, children born LBW (solid lines) tended to have lower initial levels of EF relative to NBW children (dotted lines). Third, LBW children who experienced above average levels of sensitive parenting exhibited faster rates of change in EF from 36 to 60 months than LBW children who experienced below average sensitivity.
Fig. 2.
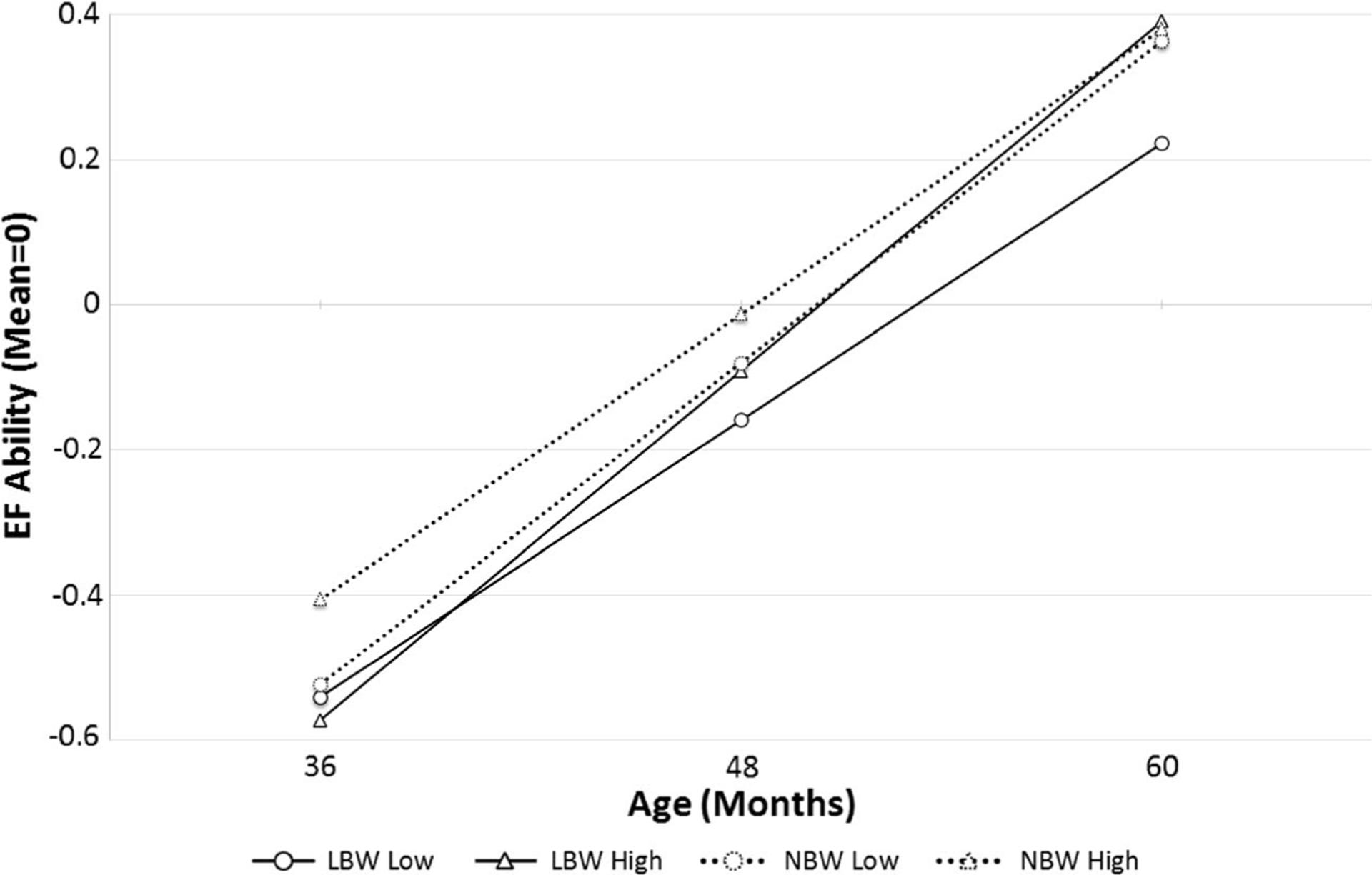
EF executive function, LBW low birth weight, NBW normal birth weight. Executive function trajectories as predicted by birth weight and sensitive parenting in toddlerhood, holding all other variables constant at mean levels. The conditional levels of parenting reflect the 25th (low) and 75th percentile (high) of observed scores
Finally, these LBW children who experienced above average sensitive caregiving were indistinguishable from both NBW groups at the 60 month assessment. LBW children who experienced below average sensitive parenting, on the other hand, lagged behind the other three groups at the final EF assessment. The interaction between LBW and parenting quality therefore suggests that highly sensitive caregiving in toddlerhood protects LBW children from lasting deficits in EF by promoting significant “catch-up” growth across the preschool period.
Discussion
The main goal of the current study was to compare trajectories of EF in preschool children who differed both on birth weight status (LBW vs. NBW) and parenting quality (high vs. low levels of sensitive and intrusive parenting in infancy and toddlerhood). In doing so, we found that sensitive parenting experienced in toddlerhood effectively protected LBW children from enduring deficits in EF. Although LBW was associated with lower levels of EF at 36 and 48 months, experiences with highly sensitive caregivers promoted “catch-up” growth in this at-risk group. LBW children who experienced above average parenting sensitivity were indistinguishable from NBW controls by the 60 month assessment, while children whose caregivers were below average showed enduring deficits in EF. These findings are consistent with previous research showing associations between prenatal risk, parenting, and executive function.
Previous studies have found LBW, ELBW, and extremely preterm children to be at an increased risk for deficits in all three domains of EF (Aarnoudse-Moens et al. 2009; Woodward et al. 2011; Baron et al. 2012), with effect sizes ranging from 0.36 to 0.54. In the current study, we found differences in LBW children’s overall EF ability with similar effect sizes (d=0.43–0.55). This consistency among studies, despite different definitions of prenatal risk, ages at testing, and methods of assessing EF, suggests that LBW-related deficits appear as early as the preschool years and persist into later childhood and young adulthood.
Several mechanisms may underlie the impact of LBW on EF, including neurological and environmental processes. MRI studies have shown that the brains of LBW infants are structurally different at birth, showing less complex surface structure as well as reduced white and gray matter volume (for a review, see Jobe 2010). Moreover, multiple studies suggest that the extent of cerebral abnormality in LBW and preterm children is predictive of cognitive outcomes, including IQ, perceptual-motor skills, and executive function (Clark and Woodward 2010; Taylor et al. 2011; Lowe et al. 2011; Woodward et al. 2011). Thus, deficits in brain volume and function may partially underlie the cognitive impairments observed in LBW children. In addition to neurological differences between LBW and NBW infants, environmental exposures after birth may also explain deficits in cognitive performance across the preschool years. Given the propensity of LBW children to display symptoms of psychopathology (e.g., Bhutta et al. 2002), parenting these at-risk children may be more difficult. Consistent with this viewpoint, parents of LBW children in this study tended to be rated lower on sensitivity and higher on intrusiveness. However, because this was not the main focus of the present investigation, further research is needed in order to understand whether LBW children evoke more negative parenting, and how this reciprocal process may relate to cognitive outcomes.
In addition to LBW-related deficits in EF, we found several main effects of parenting on levels of EF at 48 months. In particular, higher levels of sensitive parenting in infancy (6–15 months) and toddlerhood (24–36 months) predicted better child EF at 48 months, regardless of birth weight group. This finding is consistent with previous studies conducted across diverse samples of preschoolers, including previous work utilizing FLP data (e.g., Blair et al. 2011, 2014; Bernier et al. 2010, 2012). The enduring importance of sensitive parenting suggests that it may play a role both in promoting children’s foundational brain development, as well as supporting their later attempts to practice cognitive control. On the other hand, harsh, intrusive parenting experienced in toddlerhood predicted poorer child EF at 48 months. This finding, which suggests that intrusiveness in toddlerhood is especially harmful for children’s developing cognitive control, is consistent with prior research, as well as developmental theory.
A new study by Cuevas et al. (2014) found that the inverse relationship between negative parenting and child EF becomes increasingly expressed across early childhood, perhaps due to the increasing parenting challenges presented by the toddler years and beyond. Further, parental reliance on intrusive control may have particularly harmful and long-lasting consequences in toddlerhood, because it interferes with children’s beginning attempts to establish autonomy in the context of the parent–child dyad. Previous empirical and theoretical work supports the notion that certain patterns of behaviors emerge specifically within the toddler–parent dyad, that are both markedly different from patterns of interaction that existed in infancy, and that have important implications for children’s later risk for psychopathology (e.g., Kochanska and Kim 2012).
The particular importance of parenting during the toddler years is highlighted again by the significant conditional effect we found in our model. LBW children who experienced highly sensitive parenting in toddlerhood exhibited markedly faster rates of growth in EF from 36 to 60 months, compared to LBW children who experienced less sensitive parenting, Thus, although LBW was marginally associated with lower levels of EF at ages 3 and 4, experiences with warm, sensitive caregiving promoted significant “catch-up” growth by age 5. These findings provide support for a buffering hypothesis, whereby the impact of early adversity may be attenuated by later experiences with positive, supportive parenting. The role of maternal sensitivity in buffering LBW children from enduring cognitive deficits is supported by at least one other study of which we are aware (Jaekel et al. 2014). In this study, LBW and VLBW children who experienced highly sensitive parenting at age 6 performed as well as their NBW peers on tests of mathematics, reading, and spelling at age 8. Without the positive influence of sensitive parenting, however, these at-risk children showed deficits in all three measures of academic achievement. These results confirm the notion that LBW children may be especially reliant on appropriate input from caregivers in order to develop cognitive competence. It may be the case that sensitive caregiving helps to normalize the structural brain abnormalities seen in LBW and preterm infants at birth (Milgrom et al. 2010). Another possibility is that LBW children require additional practice harnessing cognitive control as compared to NBW peers; success in practicing these capacities may be more likely to occur within sensitive parent–child dyads. Further work is needed to address the plausibility and potential synergism of these mechanisms in explaining growth in EF across the preschool years.
Regardless of underlying mechanisms, our current findings suggest that sensitive parenting in toddlerhood may be especially important for LBW children’s orderly development of EF. However, previous interventions conducted with LBW children have focused on training responsive parenting in infancy (e.g., Ramey et al. 1992). While early intervention may be important for promoting the development of foundational cognitive abilities, follow-up training in toddlerhood may also be necessary to help parents foster the emergence of higher-order skills, including EF. The creation of empirically-based intervention programs is especially important given the high rates of LBW children who are later diagnosed with ADHD and other behavioral disorders (e.g., Mick et al. 2002). Given that early deficits in executive function predict higher risk of ADHD and externalizing disorder (Pauli-Pott and Becker 2011; Schoemaker et al. 2013), we hope that encouraging parents to support their LBW children’s developing EF may also result in attenuated rates of psychopathology in this at-risk group. Thus, parenting-based interventions for LBW children have the potential to benefit multiple domains of child functioning, especially if these interventions focus on increasing parental sensitivity across infancy and toddlerhood.
We have confidence in our findings given several strengths in our research design, including our use of a large, diverse sample of children, as well as detailed and repeated assessment of our observed variables. Parenting was qualitatively coded from videotaped observations, and our final composite scores captured the multiple dimensions of parental sensitivity and intrusiveness. In addition, our measurement of EF ability utilized multiple tasks representing components of working memory, inhibitory control, and set shifting. We then used EAP scores and LGC models to represent trajectories of overall EF ability, unencumbered by task specific and/or measurement error.
Despite these strengths, we acknowledge certain limitations to the conclusions we can draw from the current investigation. Although we found evidence for LBW-related deficits in EF at all three assessments, with effect sizes similar to those found in previous studies, these deficits were no longer significant in our LGC models once we accounted for household poverty (see Model 1 in Table 3). Because low socioeconomic status (SES) is related to higher risk of LBW delivery (e.g., Starfield et al. 1991) and to deficits in EF (e.g., Blair et al. 2011), any study examining LBW as a unique predictor of EF should necessarily control for income. However, much of the previous research linking LBW to deficits in EF has failed to include income-to-needs as a covariate (e.g., Baron et al. 2012), relying instead on maternal education as a proxy for SES. Thus, further work is needed to clarify whether LBW is a unique risk factor for deficits in EF, above and beyond associated demographic conditions. It also remains to be seen whether the magnitude of EF deficits varies as a function of more nuanced birth weight categories (e.g., very low birth weight; VLBW; birth weight<1500 g), a level of specificity that was not possible in the current sample given the small percentage of LBW children. Future research should combine a stratified sampling design with strategic oversampling of families at risk for LBW in order to optimize statistical power and better address these questions.
It is difficult to make causal claims about the impact of parental sensitivity on LBW children’s development of EF, given the data presented here. For one, the role of maternal EF was not assessed in the current investigation, though it may contribute directly and indirectly to child EF. Beyond the impact of shared genetics, parental EF may contribute to the regulation of family environments, as well as parenting practices, in ways that are relevant for children’s development of cognitive control (Deater-Deckard 2014). Further, because parenting was observed during different tasks in infancy and toddlerhood (i.e., a free play versus a puzzle task), the differing effects found for parenting during these two time periods may be partially attributable to the unique parenting demands imposed by each activity. Specifically, a puzzle task may be more likely to elicit the use of EF than would a free play task; parental sensitivity or intrusion that coincides with children’s attempt at harnessing EF ability may therefore have a greater impact on their future development of cognitive control. Additional research is needed to test whether the context of observed parenting behaviors moderates the relationship between parental sensitivity and children’s EF. Finally, given the potential for stability in parents’ tendencies towards sensitivity and intrusion across their children’s early years of life, it may be difficult to draw conclusions about the importance of parenting in any one period of development. However, the importance of timing of parenting behaviors should not be overlooked, as identifying critical periods of environmental input may help inform future empirically-based interventions for LBW children.
In sum, the current study is the first to assess the relationship between LBW status and trajectories of EF ability in the preschool years. Our findings suggest that warm, sensitive parenting may normalize the development of EF in LBW preschoolers. Though children born LBW had lower EF ability at 36 and 48 months, experiences with highly sensitive parents in toddlerhood predicted subsequent “catch-up” growth, and EF ability at 60 months that was indistinguishable from NBW controls.
Acknowledgments
The Family Life Project (FLP) Phase I Key Investigators include: Lynne Vernon-Feagans, The University of North Carolina; Martha Cox, The University of North Carolina; Clancy Blair, The Pennsylvania State University; Peg Burchinal, The University of North Carolina; Linda Burton, Duke University; Keith Crnic, The Arizona State University; Ann Crouter, The Pennsylvania State University; Patricia Garrett-Peters, The University of North Carolina; Mark Greenberg, The Pennsylvania State University; Stephanie Lanza, The Pennsylvania State University; Roger Mills-Koonce, The University of North Carolina; Emily Werner, The Pennsylvania State University and Michael Willoughby, The University of North Carolina.
The Family Life Project has been supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (P01HD39667) with co-funding from the National Institute on Drug Abuse.
Footnotes
Conflict of Interest The authors declare that they have no conflicts of interest.
References
- Aarnoudse-Moens CSH, Weisglas-Kuperus N, van Goudoever JB, & Oosterlaan J (2009). Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics, 124, 717–728. [DOI] [PubMed] [Google Scholar]
- Aylward GP, Pfeiffer SI, Wright A, & Verhulst SJ (1989). Outcome studies of low birth weight infants published in the last decade: a meta-analysis. The Journal of Pediatrics, 115, 515–520. [DOI] [PubMed] [Google Scholar]
- Baron IS, Kerns KA, Müller U, Ahronovich MD, & Litman FR (2012). Executive functions in extremely low birth weight and late-preterm preschoolers: effects on working memory and response inhibition. Child Neuropsychology, 18, 586–599. [DOI] [PubMed] [Google Scholar]
- Bernier A, Carlson SM, & Whipple N (2010). From external regulation to self-regulation: early parenting precursors of young children’s executive functioning. Child Development, 81, 326–339. [DOI] [PubMed] [Google Scholar]
- Bernier A, Carlson SM, Deschênes M, & Matte-Gagné C (2012). Social factors in the development of early executive functioning: a closer look at the caregiving environment. Developmental Science, 15, 12–24. [DOI] [PubMed] [Google Scholar]
- Bhutta AT, Cleves MA, Casey PH, Cradock MM, & Anand KJS (2002). Cognitive and behavioral outcomes of school-aged children who were born preterm: a meta-analysis. JAMA, 288, 728–737. [DOI] [PubMed] [Google Scholar]
- Biederman J, Monuteaux MC, Doyle AE, Seidman LJ, Wilens TE, Ferrero F, … & Faraone SV (2004). Impact of executive function deficits and ADHD on academic outcomes in children. Journal of Consulting and Clinical Psychology, 72, 757–766. [DOI] [PubMed] [Google Scholar]
- Blair C, Granger DA, Willoughby M, Mills‐Koonce R, Cox M,Greenberg MT, … & The Family Life Project Investigators (2011). Salivary cortisol mediates effects of poverty and parenting on executive functions in early childhood. Child Development, 82, 1970–1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C, Raver C, Berry DJ, & The Family Life Project Investigators. (2014). Two approaches to estimating the effect of parenting on the development of executive function in early childhood. Developmental Psychology, 50, 554–565. doi: 10.1037/a0033647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks-Gunn J, Klebanov PK, Liaw FR, & Spiker D (1993). Enhancing the development of low-birthweight, premature infants: changes in cognition and behavior over the first three years. Child Development, 64, 736–753. [DOI] [PubMed] [Google Scholar]
- Burchinal M, Vernon-Feagans L, Cox M, & The Family Life Project Investigators. (2008). Cumulative social risk, parenting, and infant development in rural low-income communities. Parenting: Science and Practice, 8, 41–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark CC, & Woodward LJ (2010). Neonatal cerebral abnormalities and later verbal and visuospatial working memory abilities of children born very preterm. Developmental Neuropsychology, 35, 622–642. doi: 10.1080/87565641.2010.508669. [DOI] [PubMed] [Google Scholar]
- Cox M, & Crnic K (2002). Qualitative ratings for parent–child interaction at 3–12 months of age. Unpublished manuscript, University of North Carolina at Chapel Hill. [Google Scholar]
- Cragg L, & Nation K (2007). Self-ordered pointing as a test of working memory in typically developing children. Memory, 15, 526–535. [DOI] [PubMed] [Google Scholar]
- Cuevas K, Deater-Deckard K, Kim-Spoon J, Watson AJ, Morasch KC, & Bell MA (2014). What’s mom got to do with it? Contributions of maternal executive function and caregiving to the development of executive function across early childhood. Developmental Science, 17, 224–238. doi: 10.1111/desc.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rodrigues MC, Mello RR, & Fonseca SC (2006). Learning difficulties in schoolchildren born with very low birth weight. Jornal de Pediatria, 82, 6–14. [DOI] [PubMed] [Google Scholar]
- Deater-Deckard K (2014). Family matters: intergenerational and interpersonal processes of executive function and attentive behavior. Current Directions in Psychological Science, 23, 230–236. doi: 10.1177/0963721414531597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durston S, Thomas KM, Yang YH, Ulug AM, Zimmerman RD, & Casey BJ (2002). A neural basis for the development of inhibitory control. Developmental Science, 5, F9–F16. [Google Scholar]
- Fanaroff AA, Stoll BJ, Wright LL, Carlo WA, Ehrenkranz RA, Stark AR, Poole WK, & NICHD Neonatal Research Network. (2007). Trends in neonatal morbidity and mortality for very low birthweight infants. American Journal of Obstetrics and Gynecology, 196, 147.e1–147.e8. [DOI] [PubMed] [Google Scholar]
- Fay-Stammbach T, Hawes DJ, & Meredith P (2014). Parenting influences on executive function in early childhood: a review. Child Development Perspectives, 8, 258–264. [Google Scholar]
- Garon N, Bryson SE, & Smith IM (2008). Executive function in preschoolers: a review using an integrative framework. Psychological Bulletin, 134, 31–60. [DOI] [PubMed] [Google Scholar]
- Gerardi-Caulton G (2000). Sensitivity to spatial conflict and the development of self-regulation in children 24–36 months of age. Developmental Science, 3, 397–404. [Google Scholar]
- Gerstadt C, Hong Y, & Diamond A (1994). The relationship between cognition and action: performance of children 3 1/2–7 years old on a Stroop-like day-night test. Cognition, 53, 129–153. [DOI] [PubMed] [Google Scholar]
- Goldberg S (1978). Prematurity: effects on parent-infant interaction. Journal of Pediatric Psychology, 3, 137–144. [Google Scholar]
- Hammond SI, Carpendale JM, Bibok MB, Müller U, & Liebermann-Finestone DP (2012). The effects of parental scaffolding on preschoolers’ executive function. Developmental Psychology, 48, 271–281. doi: 10.1037/00025519. [DOI] [PubMed] [Google Scholar]
- Hughes C, & Ensor R (2009). How do families help or hinder the emergence of early executive function? New Directions for Child and Adolescent Development, 123, 35–50. [DOI] [PubMed] [Google Scholar]
- Jacques S, & Zelazo PD (2001). The Flexible Item Selection Task (FIST): a measure of executive function in preschoolers. Developmental Neuropsychology, 20, 573–591. [DOI] [PubMed] [Google Scholar]
- Jaekel J, Pluess M, Belsky J, & Wolke D (2014). Effects of maternal sensitivity on low birth weight children’s academic achievement: a test of differential susceptibility versus diathesis stress. Journal of Child Psychology and Psychiatry. doi: 10.1111/jcpp.12331. [DOI] [PubMed] [Google Scholar]
- Jobe AH (2010). “Miracle” extremely low birth weight neonates: examples of developmental plasticity. Obstetrics & Gynecology, 116, 1184–1190. [DOI] [PubMed] [Google Scholar]
- Kane MJ, & Engle RW (2003). Working-memory capacity and the control of attention: the contributions of goal neglect, response competition, and task set to Stroop interference. Journal of Experimental Psychology: General, 132, 47–70. [DOI] [PubMed] [Google Scholar]
- Kochanska G, & Kim S (2012). Toward a new understanding of legacy of early attachments for future antisocial trajectories: evidence from two longitudinal studies. Development and Psychopathology, 24, 783–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe J, Duvall SW, MacLean PC, Caprihan A, Ohls R, Qualls C, & Phillips J (2011). Comparison of structural magnetic resonance imaging and development in toddlers born very low birth weight and full-term. Journal of Child Neurology, 26, 586–592. [DOI] [PubMed] [Google Scholar]
- Martin JA, Hamilton BE, Osterman MJ, Curtin SC, & Matthews TJ (2013). Births: Final data for 2012. National Vital Statistics Report, 62. [PubMed] [Google Scholar]
- McCarton CM, Brooks-Gunn J, Wallace IF, Bauer CR, Bennett FC, Bernbaum JC, … & Meinen CL, (1997). Results at age 8 years of early intervention for low-birth-weight premature infants: The Infant Health and Development Program. JAMA, 277, 126–132. [PubMed] [Google Scholar]
- Mick E, Biederman J, Prince J, Fischer MJ, & Faraone SV (2002). Impact of low birth weight on attention-deficit hyperactivity disorder. Journal of Developmental & Behavioral Pediatrics, 23, 16–22. [DOI] [PubMed] [Google Scholar]
- Milgrom J, Newnham C, Anderson PJ, Doyle LW, Gemmill AW, Lee K, … & Inder T, (2010). Early sensitivity training for parents of preterm infants: impact on the developing brain. Pediatric Research, 67, 330–335. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, & Howerter A (2000). The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: a latent variable analysis. Cognitive Psychology, 41, 49–100. [DOI] [PubMed] [Google Scholar]
- Müller U, Liebermann-Finestone DP, Carpendale JM, Hammond SI, & Bibok MB (2012). Knowing minds, controlling actions: the developmental relations between theory of mind and executive function from 2 to 4 years of age. Journal of Experimental Child Psychology, 111, 331–348. doi: 10.1016/j.jecp.2011.08.014. [DOI] [PubMed] [Google Scholar]
- Muthén LK, & Muthén BO (2007). Mplus user’s guide (6th ed.). Los Angeles: Muthén & Muthén. [Google Scholar]
- Nelson CA, Thomas KM, & de Haan M (2006). Neural bases of cognitive development. In Kuhn D, Siegler RS, Damon W, & Lerner RM (Eds.), Handbook of child psychology: Vol. 2, Cognition, perception, and language (6th ed., pp. 3–57). Hoboken: Wiley. [Google Scholar]
- NICHD Early Child Care Research Network. (1999). Child care and mother-child interaction in the first three years of life. Developmental Psychology, 35, 1399–1413. [PubMed] [Google Scholar]
- Patteson DM, & Barnard KE (1990). Parenting of low birth weight infants: a review of issues and interventions. Infant Mental Health Journal, 11, 37–56. [Google Scholar]
- Pauli-Pott U, & Becker K (2011). Neuropsychological basic deficits in preschoolers at risk for ADHD: a meta-analysis. Clinical Psychology Review, 31, 626–637. [DOI] [PubMed] [Google Scholar]
- Petrides M, & Milner B (1982). Deficits on subject-ordered tasks after frontal- and temporal-lobe lesions in man. Neuropsychologia, 20, 249–262. [DOI] [PubMed] [Google Scholar]
- Ramey CT, Bryant DM, Wasik BH, Sparling JJ, Fendt KH, & La Vange LM (1992). Infant Health and Development Program for low birth weight, premature infants: program elements, family participation, and child intelligence. Pediatrics, 89, 454–465. [PubMed] [Google Scholar]
- Schoemaker K, Mulder H, Deković M, & Matthys W (2013). Executive functions in preschool children with externalizing behavior problems: a meta-analysis. Journal of Abnormal Child Psychology, 41, 457–471. doi: 10.1007/s10802-012-9684-x. [DOI] [PubMed] [Google Scholar]
- Singer W (1995). Development and plasticity of cortical processing architectures. Science, 270, 758–764. [DOI] [PubMed] [Google Scholar]
- Starfield B, Shapiro S, Weiss J, Liang KY, Ra K, Paige D, & Wang X (1991). Race, family income, and low birth weight. American Journal of Epidemiology, 134, 1167–1174. [DOI] [PubMed] [Google Scholar]
- Taylor H, Filipek PA, Juranek J, Bangert B, Minich N, & Hack M (2011). Brain volumes in adolescents with very low birth weight: effects on brain structure and associations with neuropsychological outcomes. Developmental Neuropsychology, 36, 96–117. doi: 10.1080/87565641.2011.540544. [DOI] [PubMed] [Google Scholar]
- Towe-Goodman N, Willoughby MT, Blair CB, Gustaffson H, Mills-Koonce WR, Cox MJ, & The Family Life Project Investigators. (2014). Fathers’ sensitive parenting and the development of early executive functioning. Journal of Family Psychology, 28, 867–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voegtline KM, Stifter CA, & The Family Life Project Investigators. (2010). Late-preterm birth, maternal symptomatology, and infant negativity. Infant Behavior and Development, 33, 545–554. doi: 10.1016/j.inhbeh.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker SP, Chang SM, Powell CA, & Grantham-McGregor SM (2004). Psychosocial intervention improves the development of term low-birth-weight infants. The Journal of Nutrition, 134, 1417–1423. [DOI] [PubMed] [Google Scholar]
- Walker SP, Chang SM, Younger N, & Grantham-McGregor SM (2010). The effect of psychosocial stimulation on cognition and behaviour at 6 years in a cohort of term, low-birthweight Jamaican children. Developmental Medicine & Child Neurology, 52, 148–154. [DOI] [PubMed] [Google Scholar]
- Wiebe SA, Espy KA, & Charak D (2008). Using confirmatory factor analysis to understand executive control in preschool children: I. Latent structure. Developmental Psychology, 44, 575. [DOI] [PubMed] [Google Scholar]
- Willoughby MT, Blair CB, Wirth RJ, Greenberg M, & The Family Life Project Investigators. (2010). The measurement of executive function at age 3: psychometric properties and criterion validity of a new battery of tasks. Psychological Assessment, 22, 306–317. [DOI] [PubMed] [Google Scholar]
- Willoughby MT, Wirth RJ, Blair CB, & The Family Life Project Investigators. (2012). Executive function in early childhood: longitudinal measurement invariance and developmental change. Psychological Assessment, 24, 418–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby MT, Holochwost SJ, Blanton ZE, & Blair CB (2014). Executive functions: formative versus reflective measurement. Measurement: Interdisciplinary Research and Perspectives, 12, 69–95. [Google Scholar]
- Woodward LJ, Clark CC, Pritchard VE, Anderson PJ, & Inder TE (2011). Neonatal white matter abnormalities predict global executive function impairment in children born very preterm. Developmental Neuropsychology, 36, 22–41. [DOI] [PubMed] [Google Scholar]


