Abstract
Background:
Despite significant interest in diet by the MS community, research on this topic is limited; there are no published studies evaluating associations between diet and neuroimaging in MS.
Methods:
We utilized baseline data from the RADIEMS cohort of early MS (diagnosed <5.0 years, n=180). Participants underwent brain MRIs to derive normalized total gray and thalamic volumes, T2 lesion volume, and white matter microstructural integrity of normal appearing white matter (NAWM). Participants completed food frequency questionnaires (FFQ) from which we calculated adherence scores to pre-specified dietary patterns including the Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) diet. We evaluated intake of the following pre-specified dietary components: fruits, vegetables, legumes, nuts, whole grains, dairy, fried foods, processed meats, and fat intake. We used multivariable-adjusted linear regression to evaluate MRI metrics versus dietary measures.
Results:
MIND diet score was associated with thalamic volume; individuals in the highest quartile of MIND diet scores had greater thalamic volumes versus those in the lowest quartile (Q4 vs. Q1: 1.03mL; 95%CI: 0.26mL, 1.79mL; p<0.01). For individual food/nutrients, higher intakes of full-fat dairy were associated with lower T2 lesion volumes (Q4 vs. Q1: −0.93mL; 95%CI: −1.51mL, −0.35ml; p<0.01). Higher intakes of marine omega-3 fatty acids were associated with greater NAWM microstructural integrity (Q4 vs. Q1: 0.40; 95%CI: 0.03, 0.76; p=0.04). Other foods/nutrients were not associated with MRI outcomes.
Conclusions:
In this first study focused on neuroimaging and diet in MS, we note significant associations in a cross-sectional early MS cohort. Longitudinal follow-up of imaging/clinical outcomes will provide additional insights.
Keywords: multiple sclerosis, diet, MRI, thalamus, Mediterranean
1. Introduction
Persons with MS frequently seek guidance from their neurologists (and the internet) about dietary changes that may attenuate disease and disability progression;1 however, empirical evidence to inform specific clinical recommendations is limited. Case-control studies suggest links between overall dietary patterns2 or particular factors (e.g. fish intake3) and incident MS, though not all studies have been positive. Higher saturated fat intake and lower vegetable intake have been linked to higher clinical relapse rates in pediatric MS,4 and survey research in large adult cohorts of established MS patients suggests links between healthy dietary habits and lower severity of patient-reported symptoms and disability.5 To date, however, no neuroimaging research has investigated relationships between dietary patterns and MRI outcomes relevant to MS, such as T2 lesion volume and thalamic volume.
The Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) diet was developed as a hybrid between the DASH and Mediterranean dietary patterns in an attempt to focus on foods specifically relevant to neurodegenerative disorders based on best available evidence.6 Scoring is based on the number of servings of 10 “brain healthy” food groups (green leafy vegetables, other vegetables, nuts, berries, beans, whole grains, seafood, poultry, olive oil, wine) and five “unhealthy” food groups (red meat, butter/margarine, cheese, pastries/sweets, fried/fast food). Higher adherence to the MIND dietary pattern has been linked to lower incidence of Alzheimer’s disease,7 better cognitive outcomes in older adults,6 and reduced incidence/progression of parkinsonism in older adults.8 Notably, adherence to a MIND diet has been linked to lower risk for cognitive decline and dementia in older adults than DASH and Mediterranean diets.7,9–11 While aging and MS represent distinct pathophysiological entities, there are shared features, and aging research lends credibility to the concept that dietary factors may impact brain health. Here we investigated associations between diet and multimodal MRI measures of T2 lesion volume, normalized total gray matter and thalamic volumes, and microstructural integrity of normal-appearing white matter (NAWM) in the Reserve against Disability in Early MS (RADIEMS) cohort of patients with early MS (diagnosed ≤5.0 years).12 Building upon a brain maintenance model of reserve in MS,13 we investigated whether diet is associated with greater brain integrity early in disease, which may serve as cerebral reserve against future disability.
2. Methods
2.1. Study Population:
The Institutional Review Boards at Mount Sinai and Columbia approved the study and all participants provided written informed consent. Reserve against Disability in Early Multiple Sclerosis (RADIEMS) is a longitudinal cohort study of risk and protective factors for disability in early MS (≤5.0 years since MS diagnosis).12 RADIEMS enrolled 185 persons with relapsing-remitting MS (n=165) or clinically isolated syndrome (n=20)14 between October 2016 and December 2017; 180 (97.3%) had complete data and are included in current analyses: aged 34.4±7.6 years, 119 (66.1%) women, 36 (20.0%) Black / African-American, 40 (22.2%) Hispanic/Latino, 2.2±1.4 years since diagnosis.
2.2. Dietary Data:
Participants completed a detailed food frequency questionnaire (FFQ, Harvard University 2007 paper form) to derive dietary pattern scores and estimate consumption of other key nutrients of interest (e.g., omega 3 fatty acids). Participants were assigned MIND diet scores based on levels of reported consumption of the 15 MIND diet components (Table 2) using published criteria.6, 7 For reference we also derived the Dietary Approach to Stop Hypertension (DASH) and alternative Mediterranean overall diet quality (aMed)15, 16, which are other established overall diet quality scores that are associated with chronic disease risk in non-MS populations. The DASH and aMed scores generally emphasize intake of similar foods groups as the MIND diet (e.g. fruits and vegetables and whole grains), but differ slightly in individual constituent foods. For example, the DASH diet promotes intake of fruits, vegetables, nuts and legumes, low fat dairy and whole grains, while minimizing intake of sodium, red and processed meats and sugar sweetened beverages). The aMed diet score emphasizes intake of fruits, vegetables, legumes, nuts, whole grains, fish, unsaturated fats and moderate alcohol intake. Higher scores for each of the dietary quality indices denote higher quality diets.
Table 2.
Demographic and Clinical Characteristics of RADIEMS Cohort of People with Early MS
| Overall cohort |
Approximate Quartile of MIND Diet | ||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | ||
| N | 180 | 49 | 52 | 39 | 40 |
| Mean (SD)* | 8.46 (1.45) | 6.49 (1.03) | 8.29 (0.25) | 9.28 (0.25) | 10.68 (0.81) |
| Median (range)* | 8.5 (4.00–12.00) | 7 (6–7) | 8.5 (8–8.5) | 9.5 (9–9.5) | 10.5 (10–11) |
| Age (years), mean (SD) | 34.3 (7.6) | 31.6 (7.7) | 35.9 (7.1) | 36.2 (7.4) | 34.30 (7.4) |
| Male sex, n (%) | 61 (33.4) | 23 (46.9) | 41 (19.2) | 28 (28.2) | 23 (42.5) |
| White, n (%) | 130 (72.2) | 29 (59.2) | 38 (73.1) | 28 (71.8) | 35 (87.5) |
| Hispanic/Latino ethnicity, n (%) | 40 (22.2) | 13 (26.5) | 11 (21.2) | 8 (20.5) | 8 (20.0) |
| SES Z-score, mean (SD) | 0.0 (1.0) | -0.3 (1.1) | 0.2 (0.9) | 0.0 (1.0) | 0.0 (0.9) |
| BMI (kg/m2), mean (SD) | 26.8 (6.4 | 29.2 (8.2) | 26.6 (5.3) | 26.6 (6.0) | 24.5 (4.5) |
| Current smoker, n (%) | 12 (6.7) | 3 (6.1) | 5 (9.6) | 4 (10.3) | 0 (0.0) |
| Composite physical activity (times per week), mean (SD) | 6.0 (4.4) | 5.3 (4.5) | 5.8 (4.4) | 6.0 (5.0) | 6.9 (3.7) |
| Disease duration (years), mean (SD) | 2.2 (1.4) | 2.1 (1.47) | 2.3 (1.4) | 2.4 (1.5) | 1.8 (1.5) |
| CIS, n (%) | 19 (10.6) | 5 (10.2) | 5 (9.6) | 4 (10.3) | 5 (12.5) |
| High efficacy disease modifying therapy, n (%) |
39 (21.7) | 13 (26.5) | 9 (17.3) | 6 (15.4) | 11 (27.5) |
| EDSS, median (IQR) | 1.0 (0.0–1.5) | 1.0 (0.0–1.5) | 1.0 (0.0–1.5) | 1.0 (0.0–1.5) | 1.0 (0.8–1.6) |
| BDI-FS, median (IQR) | 2.00 (1.00–4.00) | 2 (1–6) | 2 (1–4) | 2 (0.5–3.5) | 1 (0–3) |
| Key MRI variables, mean (SD) * | |||||
| Thalamic Volume | 21.1 (1.7) | 20.6 (1.9) | 21.2 (1.7) | 21.6 (1.5) | 21.4 (1.7) |
| Lesion volume | 0.4 (1.4) | 0.8 (1.5) | 0.3 (1.4 | 0.3 (1.3) | 0.4 (1.4) |
| NAWM FA MD | 0.0 (0.9) | −0.3 (1.0) | 0.2 (0.9) | 0.0 (0.9) | 0.1 (0.9) |
| Gray matter volume* | 804.6 (47.8) | 806.7 (54.6) |
799.5 (38.4) |
808.8 (46.1) | 804.3 (52.5) |
Units for volumetric measures are presented as mL. T2 lesion volume was log-transformed to improve normality; NAWM FA MD: Normal appearing white matter fractional anisotropy, mean diffusivity.
2.3. Relevant Covariates:
Analyses controlled for important covariates to isolate contributions of diet to neuroimaging outcomes. Covariates included age (continuous), sex, socioeconomic status (SES), race (Causasian, African-American, other), ethnicity (Hispanic or Latino vs. non-Hispanic/Latino), body mass index (BMI, continuous), physical activity (quartiles), calorie intake, disease duration, and disease modifying therapy class (none, low [injectable/oral], high [infusion]). SES was coded as the mean rank across (a) maternal education, (b) median household income of current zip code, and (c) literacy assessed with the Wechsler Test of Adult Reading. Physical activity was coded as quartiles of mean frequency (daily, several times per week, several times per month, several times per year, ≤ once per year) of seven activities: (a) run, jog, elliptical, (b) swim for exercise, (c) high intensity sports (e.g., soccer), (d) walking for exercise or low intensity activities (e.g., golf), (e) toning and stretching, (f) biking, (g) high intensity exercise class (e.g., kickboxing).
2.4. Neuroimaging Outcomes:
Neuroimaging outcomes were derived from high resolution 3.0 Tesla brain MRIs (Siemens Skyra,16 channel coil). T2 lesion volume (T2LV) was derived from 3D T2-weighted images using a local thresholding segmentation technique (Jim v6.0, Xinapse Systems; TR=3200 ms, TE=566.0 ms, FOV=256 mm, 224 slices, voxel size=0.9 × 0.9 × 0.9 mm). T2LV was log-transformed. Normalized volumes of total gray matter and thalamus were measured with SIENAX and FIRST (parts of FSL17) using lesion-filled 3D Tl-weighted images (TR=2400 ms, TE= 2.0 ms, flip angle=8°, FOV=256 mm, 176 contiguous slices, voxel size=1.0 × 1.0 × 1.0 mm), and applying the volume-scaling factor to adjust for intracranial volume.
2.5. Microstructural integrity of normal appearing white matter (NAWM FA MD)
was estimated from diffusion-weighted images corrected for distortions caused by motion, eddy current, and field inhomogeneity using FMRIB’s Diffusion Toolbox18 within FSL 5.0.7 (TR=4100 ms, TE=91.0 ms; FOV=220 mm, flip angle=85°, 75 slices with 2.0 mm thickness, multi-band acceleration factor=3.0, 60 diffusion encoding directions with b = 1200 seconds/mm2 and one b=0 s/mm2). To correct the field inhomogeneity and improve the signal to noise ratio, data were acquired twice with alternating phase-encoding directions (left/right, right/left). Diffusion tensor was estimated in each voxel using linear regression. Means of Fractional Anisotropy (FA) and Mean Diffusivity (MD) were calculated across NAWM after masking out T2 lesions, and these values were combined into one NAWM metric (MD reverse coded; higher values indicate better microstructural integrity).
2.6. Statistical Methodology
Initial analyses evaluated the association between quartiles of MIND diet quality with patient characteristics and specific food and nutrient dietary components. Our primary analysis assessed differences in quantitative MRI outcomes across quartiles of MIND diet quality scores using linear regression models. As noted above, all models were adjusted for age, sex, race, ethnicity, BMI, physical activity, SES, total calorie intake, disease duration, and disease modifying therapy class. Since RADIEMS cohort members’ disability scores are relatively low and consistent across the cohort (EDSS median=1.0, IQR 0.0-1.5), we did not include this variable in multivariable models. Partial Spearman correlations examined associations between MRI outcomes and continuous MIND diet scores after adjusting all variables for aforementioned covariates using the approach outlined by Liu et al.19
Secondary analyses used the same approach to explore links between MRI outcomes and MIND diet components as well as a priori individual dietary factors of interest (e.g. food and nutrient groups), adjusting for the same covariates. Key food groups included fruits, vegetables, legumes, nuts, whole grains, dairy (divided into full-fat and low-fat), and processed meats. Based on prior work suggesting potential particular importance of fatty acids in MS,20 we examined associations between MRI metrics and saturated fatty acids, monounsaturated fatty acids, and polyunsaturated fatty acids (PUFAs), as well as subsets of n-3 fatty acids including alpha linolenic acid (ALA) and those from marine sources (e.g. eicosapentaenoic acid [EPA] + docosahexaenoic acid [DHA]).
For comparison, we also assessed the association between other overall dietary indices (e.g. DASH, aMed scores) and MRI outcomes using multivariable-adjusted linear models.
3. Results
3.1. Dietary Characteristics of Sample
MIND diet scores averaged 8.5 (standard deviation [SD]: 1.45) and ranged from 4 to 12. Demographic and disease characteristics across quartiles of the MIND diet score are outlined in Table 2 and Table 3. Participants with higher MIND diet scores tended to be slightly older, be white and non-Hispanic, non-smokers, be less overweight, and more physically active than those with lower MIND diet scores.
Table 3.
Dietary characteristics of RADIEMS Cohort of People with Early MS
| Overall cohort | Approximate Quartile of MIND Diet | ||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | ||
| N | 180 | 49 | 52 | 39 | 40 |
| Key Dietary Variables, mean (SD) | |||||
| DASH score | 24.1 (5.8) | 19.9 (5.4) | 22.9 (3.5) | 25.7 (4.7) | 29.4 (5.0) |
| Vegetables, servings/day | 4.3 (3.2) | 2.3 (1.4) | 3.9 (2.5) | 4.5 (1.8) | 6.8 (4.7) |
| Fruits, servings/day | 1.7 (1.4) | 1.0 (0.9) | 1.3 (1.1) | 1.9 (1.0) | 2.6 (1.9) |
| Whole grains, servings/day | 0.8 (0.8) | 0.6 (0.6) | 0.6 (0.6) | 0.9 (1.0) | 1.1 (0.9) |
| Nuts, servings/day | 0.4 (0.7) | 0.2 (0.5) | 0.3 (0.4) | 0.4 (0.4) | 0.8 (1.1) |
| Low fat dairy, servings/day | 1.2 (1.1) | 1.2 (0.7) | 1.3 (1.1) | 1.3 (1.1) | 1.1 (1.5) |
| Full fat dairy, servings/day | 0.7 (1.0) | 0.5 (0.5) | 0.9 (1.2) | 0.9 (1.1) | 0.6 (1.0) |
| Red and processed meats, servings/day | 0.8 (0.7) | 0.9 (0.8) | 0.8 (0.6) | 0.8 (0.8) | 0.5 (0.6) |
| Sweets/desserts, servings/day | 0.7 (0.8) | 0.8 (1.1) | 0.7 (0.8) | 0.6 (0.4) | 0.5 (0.4) |
| Saturated fat, grams/day | 24.1 (5.0) | 26.5 (4.2) | 24.7 (4.6) | 23.9 (4.5) | 20.4 (5.0) |
| Polyunsaturated fat intake, grams/day | 2.2 (0.9) | 1.9 (0.4) | 2.1 (0.6) | 2.2 (0.7) | 2.6 (1.6) |
| Omega-3 fatty acid intake, grams/day | 0.4 (0.4) | 0.3 (0.3) | 0.5 (0.4) | 0.4 (0.3) | 0.6 (0.5) |
3.2. Primary Analysis
MIND diet scores did not appear to vary appreciably across MS clinical characteristics. In multivariable models adjusted for age, sex, race, ethnicity, SES, BMI, physical activity, smoking status, DMT use and years of diagnosis, individuals in the top quartile of MIND diet scores had significantly higher thalamic volumes relative to those in the bottom quartile (Table 4; adjusted mean difference for Q4 vs. Q1: 1.03mL; 95% CI: 0.26mL to 1.79mL; p for linear trend across quartiles=0.002). Consistent results were observed in analyses using multivariable-adjusted splines (Figure 1A) and correlations; MIND diet scores were significantly positively correlated with higher thalamic volumes (Figure 1B; r=0.22; 95% CI: 0.07 to 0.35; p=0.004). We observed a potential trend between MIND diet scores and other MRI outcomes of interest; however, these differences did not attain statistical significance. In additional analyses, we also evaluated the association between MIND diet quartiles and thalamic volume also adjusting for lesion volume. Individuals in the top quartile of MIND diet scores had 0.98mL higher thalamic volumes relative to those in the bottom quartiles (mean difference: 0.98mL; 95% CI: 0.25mL to 1.71mL; p=0.009). We also did not observe evidence of effect modification by BMI status for any of the analyses assessing MIND diet scores and MRI outcomes (all p>0.05).
Table 4. Primary Analyses:
Association between MIND Diet Score and MRI measures in RADIEMS Cohort of People with Early MS
| Thalamic Volume* | Lesion volume* | NAWM FA MD* | Gray matter volume* | |||
|---|---|---|---|---|---|---|
| Diet quality score |
Quartile | Mean (SD) | Beta** (95% CI) | Beta** (95% CI) | Beta** (95% CI) | Beta** (95% CI) |
| MIND | Q1 | 6.47 (1.03) | 0.00 [ref] | 0.00 [ref] | 0.00 [ref] | 0.00 [ref] |
| Q2 | 8.29 (0.25) | 0.62 (-0.08, 1.33) | -0.32 (-0.87, 0.23) | 0.52 (0.15, 0.89) | 4.46 (-12.66, 21.58) | |
| Q3 | 9.28 (0.25) | 1.16 (0.41, 1.91) | -0.29 (-0.88, 0.30) | 0.36 (-0.04, 0.76) | 14.75 (-3.41, 32.90) | |
| Q4 | 10.68 (0.81) | 1.03 (0.26, 1.79) | -0.11 (-0.72, 0.49) | 0.40 (-0.00, 0.80) | 7.17 (-11.43, 25.78) | |
| P for linear trend across quartiles | 0.002 | 0.65 | 0.08 | 0.24 |
Units for volumetric measures are presented as mL. T2 lesion volume was log-transformed to improve normality; NAWM FA MD: Normal appearing white matter fractional anisotropy, mean diffusivity.
Adjusted for age, sex, race, ethnicity, socio-economic status, physical activity, BMI, smoking status, disease duration, disease modifying therapy use and class, and total energy intake.
Figure 1.
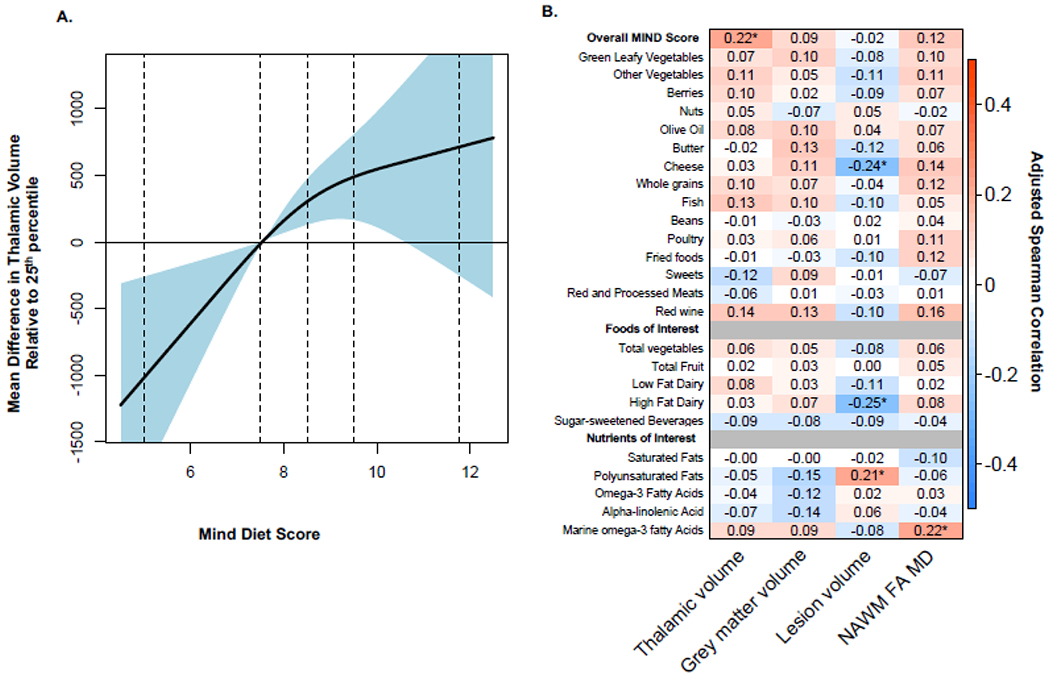
Overall MIND Diet Score, Components, Foods and Nutrients and MRI Outcomes
A. The association between overall MIND diet score and thalamic volume using restricted cubic splines. Plotted values denote the mean difference in thalamic volume for an increase or decrease in MIND diet score relative to the 25th percentile (scores of 7.5). Curves are additionally adjusted for age, sex, race, ethnicity, socio-economic status, physical activity, BMI, smoking status, disease duration, disease modifying therapy use and class, and total energy intake. Vertical dotted lines denote the 2.5th, 25th, 50th, 75th and 97.5th percentile of MIND diet scores. B. Adjusted Spearman correlations between overall MIND diet score, components, foods, and nutrients of interest and MRI outcomes. Spearman correlations are adjusted for age, sex, race, ethnicity, socio-economic status, physical activity, BMI, smoking status, disease duration, disease modifying therapy use and class, and total energy intake. The “*” denotes p-values <0.05.
3.3. Secondary Analyses
Secondary analyses assessed links between MRI outcomes and individual MIND diet components (as the MIND score was associated with thalamic volumes in our primary analyses). Only higher intakes of other vegetables (not including green-leafy vegetables) were marginally associated with higher thalamic volumes (Table 4; Figure 1B).
3.3.1. Analyses of pre-specified individual food groups.
Higher intakes of full-fat dairy products were associated with lower T2 lesion volumes; individuals in the top quartile had an adjusted log mean difference of −0.92 (Table 4; 95% CI: −1.51 to −0.33; p for trend=0.002) in lesion volume relative to those in the bottom quartile. Similar results were obtained when using multivariable-adjusted correlations (r=−0.25, 95% CI: −0.40 to −0.08; p=0.003; Figure 1B). Other pre-specified foods of interest were not associated with MRI outcomes in continuous or categorical models.
3.3.2. Analyses of individual fatty acids:
Higher intakes of marine n-3 fatty acids were marginally associated with higher white matter integrity (NAWM FA MD); individuals in the top quartile had an adjusted mean difference of 0.40 (95% CI: 0.03 to 0.76; p for trend=0.04) NAWM FA MD relative to those in the bottom quartile. Similar results were obtained when using multivariable-adjusted correlations (r=0.22, 95% CI: 0.05 to 0.37; p=0.02). Unexpectedly, higher intakes of polyunsaturated fats were associated with higher lesion volumes both in both continuous and in analyses using quartiles (Table 5; Figure 1B). Other types of dietary fat were not associated with MRI outcomes in continuous or categorical models.
Table 5.
Secondary analyses: Individual dietary components and adjusted mean difference* in MRI measures
| Thalamic Volume | Lesion volume* | NAWM FA MD** | Gray matter volume | |||
|---|---|---|---|---|---|---|
| Quartile | Mean (SD) |
Beta** (95% CI) | Beta** (95% CI) | Beta** (95% CI) | Beta** (95% CI) | |
| Vegetables, servings per day | Q1 | 1.38 (0.42) |
0.00 [ref] | 0.00 [ref] | 0.00 [ref] | 0.00 [ref] |
| Q2 | 2.71 (0.48) |
-177.32 (-943.15, 588.51) | -0.08 (-0.65, 0.50) | 0.05 (-0.34, 0.45) | -4.83 (-22.74, 13.07) | |
| Q3 | 4.42 (0.55) |
232.49 (-558.74, 1023.73) | -0.41 (-1.01, 0.18) | -0.00 (-0.41, 0.40) | 4.89 (-13.60, 23.39) | |
| Q4 | 8.47 (3.55) |
274.12 (-607.32, 1155.56) | -0.44 (-1.10, 0.22) | 0.23 (-0.22, 0.68) | 7.48 (-13.13, 28.09) | |
| P for Trend |
0.33 | 0.13 | 0.33 | 0.27 | ||
| Other vegetables, servings per day | Q1 | 0.87 (0.29) |
0.00 [ref] | 0.00 [ref] | 0.00 [ref] | 0.00 [ref] |
| Q2 | 1.80 (0.31) |
660.17 (-112.11, 1432.46) | -0.20 (-0.78, 0.38) | 0.24 (-0.15, 0.64) | 9.03 (-9.11, 27.17) | |
| Q3 | 2.98 (0.40) |
886.53 (96.19, 1676.87) | -0.64 (-1.23, 0.05) | 0.28 (-0.12, 0.69) | 16.04 (-2.52, 34.61) | |
| Q4 | 5.82 (2.34) |
758.64 (-106.66, 1623.94) | -0.65 (-1.30, 0.00) | 0.53 (0.09, 0.97) | 18.96 (-1.37, 39.28) | |
| P for Trend |
0.17 | 0.03 | 0.03 | 0.07 | ||
| Fruits, servings per day | Q1 | 0.41 (0.17) |
0.00 [ref] | 0.00 [ref] | 0.00 [ref] | 0.00 [ref] |
| Q2 | 0.94 (0.19) |
-442.97 (-1169.31, 283.37) | 0.20 (-0.34, 0.75) | -0.01 (-0.38, 0.36) | -1.41 (-18.47, 15.66) | |
| Q3 | 1.77 (0.28) |
-272.02 (-1027.84, 483.80) | 0.39 (-0.18, 0.96) | -0.02 (-0.41, 0.36) | -3.93 (-21.69, 13.83) | |
| Q4 | 3.57 (1.37) |
175.66 (-628.54, 979.86) | -0.02 (-0.63, 0.58) | 0.18 (-0.24, 0.59) | 7.65 (-11.25, 26.54) | |
| P for Trend |
0.42 | 0.89 | 0.39 | 0.41 | ||
| Whole grains, servings per day | Q1 | 0.13 (0.08) |
0.00 [ref] | 0.00 [ref] | 0.00 [ref] | 0.00 [ref] |
| Q2 | 0.40 (0.10) |
539.74 (-179.70, 1259.19) | -0.19 (-0.73, 0.35) | 0.06 (-0.31, 0.42) | 4.80 (-12.03, 21.62) | |
| Q3 | 0.83 (0.15) |
629.98 (-117.79, 1377.74) | -0.64 (-1.20, -0.08) | 0.46 (0.08, 0.84) | 17.92 (0.43, 35.40) | |
| Q4 | 1.88 (0.77) |
720.17 (-69.05, 1509.38) | -0.30 (-0.89, 0.30) | 0.26 (-0.14, 0.66) | 6.39 (-12.06, 24.85) | |
| P for Trend |
0.12 | 0.29 | 0.13 | 0.44 | ||
| Low fat dairy, Servings per day | Q1 | 0.02 (0.04) |
0.00 [ref] | 0.00 [ref] | 0.00 [ref] | 0.00 [ref] |
| Q2 | 0.25 (0.10) |
224.28 (-476.66, 925.22) | -0.26 (-0.78, 0.27) | 0.17 (-0.19, 0.53) | 3.27 (-13.11, 19.65) | |
| Q3 | 0.74 (0.18) |
110.91 (-581.14, 802.96) | -0.41 (-0.93, 0.10) | 0.08 (-0.28, 0.43) | 7.01 (-9.16, 23.18) | |
| Q4 | 2.16 (1.16) |
199.86 (-544.10, 943.83) | -0.34 (-0.90, 0.21) | 0.07 (-0.31, 0.45) | -0.89 (-18.27, 16.50) | |
| P for Trend |
0.72 | 0.24 | 0.91 | 0.94 | ||
| Full fat dairy, servings per day | Q1 | 0.14 (0.15) |
0.00 [ref] | 0.00 [ref] | 0.00 [ref] | 0.00 [ref] |
| Q2 | 0.73 (0.19) |
303.61 (-455.57, 1062.79) | -0.38 (-0.94, 0.17) | 0.14 (-0.25, 0.53) | 12.42 (-5.32, 30.16) | |
| Q3 | 1.37 (0.19) |
18.76 (-733.30, 770.82) | -0.62 (-1.17,-0.07) | -0.01 (-0.40, 0.37) | 2.47 (-15.11, 20.04) | |
| Q4 | 2.70 (1.09) |
-207.42 (-997.66, 582.82) | -0.93 (-1.51, -0.35) | 0.18 (-0.23, 0.58) | 2.36 (-16.11, 20.83) | |
| P for Trend |
0.40 | 0.002 | 0.53 | 0.82 | ||
| Nuts, servings per day | Q1 | 0.03 (0.04) |
0.00 [ref] | 0.00 [ref] | 0.00 [ref] | 0.00 [ref] |
| Q2 | 0.20 (0.04) |
419.50 (-289.47, 1128.47) | -0.00 (-0.54, 0.53) | -0.06 (-0.42, 0.30) | 8.91 (-7.66, 25.47) | |
| Q3 | 0.35 (0.07) |
-42.50 (-802.00, 716.99) | 0.19 (-0.39, 0.76) | 0.04 (-0.36, 0.43) | 0.40 (-17.35, 18.14) | |
| Q4 | 1.29 (1.09) |
262.17 (-524.37, 1048.71) | 0.32 (-0.27, 0.91) | -0.22 (-0.62, 0.19) | -4.71 (-23.09, 13.67) | |
| P for Trend |
0.66 | 0.25 | 0.26 | 0.4 | ||
| Red and processed meats, servings per day | Q1 | 0.15 (0.11) |
0.00 [ref] | 0.00 [ref] | 0.00 [ref] | 0.00 [ref] |
| Q2 | 0.48 (0.08) |
0.33 (-0.39, 1.04) | -0.01 (-0.56, 0.53) | 0.07 (-0.30, 0.44) | 5.28 (-11.65, 22.20) | |
| Q3 | 0.78 (0.10) |
-0.21 (-0.93, 0.51) | -0.24 (-0.78, 0.31) | 0.13 (-0.25, 0.50) | 5.19 (-11.85, 22.23) | |
| Q4 | 1.62 (0.76) |
-0.14 (-0.88, 0.61) | -0.25 (-0.82, 0.32) | 0.14 (-0.25, 0.53) | -0.38 (-18.09, 17.33) | |
| P for Trend |
0.46 | 0.31 | 0.45 | 0.91 | ||
| Sweets/ desserts, servings per day | Q1 | 0.10 (0.08) |
0.00 [ref] | 0.00 [ref] | 0.00 [ref] | 0.00 [ref] |
| Q2 | 0.34 (0.07) |
-35.37 (-752.25, 681.52) | -0.20 (-0.74, 0.34) | 0.12 (-0.24, 0.49) | 12.80 (-3.97, 29.58) | |
| Q3 | 0.62 (0.12) |
257.43 (-469.25, 984.12) | -0.06 (-0.61, 0.49) | 0.12 (-0.25, 0.49) | 9.71 (-7.29, 26.71) | |
| Q4 | 1.57 (1.04) |
-416.88 (-1190.90, 357.13) | -0.01 (-0.60, 0.58) | -0.11 (-0.51, 0.29) | 14.94 (-3.17, 33.05) | |
| P for Trend |
0.27 | 0.81 | 0.40 | 0.21 |
T2 lesion volume was log-transformed to improve normality; NAWM FA MD: Normal appearing white matter fractional anisotropy, mean diffusivity.
Adjusted for age, sex, race, ethnicity, socio-economic status, physical activity, BMI, smoking status, disease duration, disease modifying therapy use and class, and total energy intake.
3.3.3. Additional sensitivity analyses:
We also assessed the association between other dietary quality scores including the DASH and aMED scores with the MIND diet score as well as MRI outcomes. DASH and aMED scores were moderately correlated with MIND diet scores (for DASH: Spearman’s r: 0.62, p<0.001; for aMed: 0.52; p<0.001), and patient characteristics were distributed similarly across quartiles of DASH and aMed scores (data not shown). Neither the DASH nor the aMed overall diet quality scores were associated with MRI outcomes considered, (data not shown).
4. Discussion
If we conceptualize MS as both an autoimmune and neurodegenerative disease, then theoretically any agent able to promote immune tolerance, provide neuroprotection, or encourage remyelination and repair can act as a disease-modifier. An increasing body of preclinical research suggests that diet has the potential to impact MS with regard to each of these mechanisms through direct effects of dietary metabolites and/or indirectly through effects on gut microbiota composition and metabolite production.21
Given the importance of neurodegeneration in MS and links between MIND and other neurodegenerative diseases, we hypothesized the MIND dietary pattern might be linked to brain volumes in MS, though we anticipated it might be difficult to identify a relationship in such a young cohort with very short disease duration. However, interestingly we identified an association between MIND score and thalamic volume in this early MS cohort. Thalamic atrophy has been demonstrated as a significant and consistent measure of neurodegeneration throughout MS disease course.22 Importantly for our findings in the RADIEMS cohort with early disease, thalamic atrophy occurs very early in RRMS,23 and has also been noted among patients with clinically isolated syndrome (CIS), radiologically isolated syndrome (RIS), and even in pediatric MS. Because it is associated with disability progression over time,24 it has been proposed as a clinical trial outcome in MS.25 The fact that thalamic volume loss is among the most ubiquitous and early-occurring gray matter changes in MS may explain the specific link to thalamic volume but not overall gray matter volume in our early cohort. Longitudinal follow up of this cohort will help determine if MIND adherence is associated with the rate of decline in thalamic volume over time, and whether links to diet expand to other gray matter structures.
Coming down a level to specific food groups, we did not identify significant relationships between most individual groups and the MRI measures we studied in our cohort. However, we did note a significant relationship between higher intake of dairy, driven by full-fat dairy intake, and lower T2LV. The preclinical literature, while limited, might have led to the expectation of the opposite relationship. Children and adults with MS have heightened immunologic responses to milk antigens.26 The milk protein butyrophilin has been implicated as potentially harmful due to antigenic mimicry/overlap with myelin oligodendrocyte glycoprotein in animal models and MS patients.27 In addition, research utilizing the Nurses’ Health Study cohorts demonstrated increased risk for MS among women with high whole milk intake during adolescence.28 However, none of these studies explored the potential clinical impact among patients with established MS. Two studies utilizing self-reported data from persons with MS noted conflicting results regarding dairy intake. Research utilizing the HOLISM (Health Outcomes in a Sample of people with MS) study of 2047 persons with MS found that those who reported not consuming dairy were less likely to report recent disease activity and reported higher health-related quality of life compared to those who did report consuming dairy.29 In contrast, and consistent with the relationship we noted in our cohort, a study utilizing NARCOMS (North American Research Committee on MS) registry data from nearly 7000 participants noted a significantly reduced odds ratio for severe disability among those in the top vs. bottom quintile for dairy intake.30 The potential underlying mechanism for our observation is unclear. Of note, dairy intake and vitamin D level were not related in our cohort and therefore vitamin D level does not explain the observed relationship between dairy and T2 lesion volume. One possibility to explore, though it would not necessarily explain the full-fat association, is that this relationship is mediated through the encouragement of increased abundance of Lactobacillus species in the gut, which have been suggested to be of potential benefit in MS.29 This observation will need to be further studied longitudinally in MS patients and mechanistic work carried out to further investigate this hypothesis.
While we did not perform an exhaustive evaluation for associations between MRI metrics and micronutrients, we did evaluate relationships with fatty acids based on a priori hypotheses regarding their potential importance in MS. In particular, there is a significant body of preclinical and epidemiologic literature, as well as limited work in patients with established MS, studying the role of polyunsaturated fatty acid (PUFA) intake. Preclinical work suggests multiple hypothetical mechanisms in MS including immunomodulatory actions of benefit in experimental autoimmune encephalomyelitis,31 as well as protection from demyelination/promotion of remyelination in a toxic demyelination animal model.32 Epidemiologic studies of incident MS have suggested a potential protective effect of PUFA intake, though they have not all agreed on which underlying component is important. Follow up of the Nurses’ Health cohort suggested the largely plant-based alpha-linolenic acid,33 while several other studies have suggested the importance of marine-derived sources,20 including findings independent from vitamin D intake.3 In our cohort we detected significant relationships between marine-derived omega-3 fatty acid intake and microstructural injury in the normal appearing white matter, demonstrated by differences in mean diffusivity and fractional anisotropy. Abnormalities in normal-appearing white matter in patients with early MS have been suggested to reflect early neuroaxonal injury that seems to be at least partly independent from lesional demyelinating pathology.34 The mechanism by which marine-derived omega-3 fatty acids may be protective in this respect is unclear. However, our observation here in this cohort of young patients with MS interestingly parallels previous work demonstrating a significant protective effect of PUFAs on cognition in older adults which is mediated by white matter microstructural integrity.35
Our study has several limitations. We used a questionnaire-based assessment of diet and it is possible that measurement error in the estimation of adherence scores or foods/nutrients of interest could have impacted the results. However, we expect this to be non-differential as each person with MS completed the questionnaire under similar circumstances. Next, we have conducted this research in a sample of participants with early MS; our results may not be applicable to cohorts of those with longer disease duration or progressive phenotypes. We also did not include a comparable cohort of people without MS; it’s possible that the observed associations may be similar in healthy individuals and not specific to MS. Since diet is highly modifiable, an association between diet and thalamic volume would still be notable, regardless of whether it is specific to MS. However, previous research of diet and quantitative MRI measures in a much older population (mean age = 80.1 years) did not observe an association between similar healthy diet scores and thalamic volume despite links to overall gray matter volume.36 As noted above, the specific link between MIND diet and thalamic volume in the RADIEMS cohort may be because disease-related thalamic atrophy is among the first gray matter changes in MS.
Lastly, our design was cross-sectional. While cross-sectional studies are helpful regarding initial impressions and generation of hypotheses for future study, the potential impact of the identified factors here will need to be addressed through longitudinal imaging and clinical follow up and further mechanistic work including more detailed imaging measures will need to be completed before conclusions can be drawn. Ideally, the advice we provide regarding lifestyle modifications to people living with MS should be based on clinical trials. However, this first study linking diet with MRI metrics in MS provides valuable guidance regarding future directions on this critically important topic to the MS community.
Table 1.
Components of Diet Quality Scores Considered
| MIND | |
|---|---|
|
| |
| Green leafy vegetables (+) | |
| Other vegetables (+) | |
| Berries (+) | |
| Nuts (+) | |
| Olive oil (+) | |
| Whole grain (+) | |
| Fish (+) | |
| Score Components | Poultry (+) |
| Beans (+) | |
| Fried foods (-) | |
| Butter (-) | |
| Cheese (-) | |
| Sweets (-) | |
| Red and processed meats (-) | |
| Red wine (1 glass / day) | |
| Range | 0 – 15 |
Table 6:
Types of dietary fat and adjusted mean difference* in MRI measures
| Thalamic Volume | Lesion volume | NAWM FA MD** | Gray matter volume** | |||
|---|---|---|---|---|---|---|
| Quartile | Mean (SD) | Beta* (95% CI) | Beta* (95% CI) | Beta* (95% CI) | Beta* (95% CI) | |
| Saturated fat, grams per day | Q1 | 18.22 (3.12) | −63.44 (−797.03, 670.15) | 0.19 (−0.36, 0.74) | −0.28 (−0.65, 0.10) | 0.22 (−16.83, 17.28) |
| Q2 | 22.52 (0.75) | −73.99 (−829.75, 681.77) | 0.13 (−0.43, 0.70) | −0.35 (−0.73, 0.04) | −10.92 (−28.49, 6.65) | |
| Q3 | 25.23 (0.93) | −101.62 (−852.52, 649.28) |
−0.03 (−0.59, 0.53) | −0.20 (−0.58, 0.18) | −0.88 (−18.34, 16.58) | |
| Q4 | 30.31 (3.55) | −63.44 (−797.03, 670.15) | 0.19 (−0.36, 0.74) | −0.28 (−0.65, 0.10) | 0.22 (−16.83, 17.28) | |
| P for Trend | 0.80 | 0.85 | 0.34 | 0.73 | ||
| Polyunsaturated fat, grams per day | Q1 | 12.31 (1.41) | 0.00 [ref] | 0.00 [ref] | 0.00 [ref] | |
| Q2 | 15.14 (0.59) | 169.95 (−602.92, 942.81) | 0.16 (−0.41, 0.73) | −0.06 (−0.45, 0.34) | 0.02 (−17.96, 18.01) | |
| Q3 | 16.70 (0.42) | −76.57 (−824.34, 671.20) | 0.62 (0.07, 1.18) | −0.33 (−0.70, 0.05) | −3.24 (−20.65, 14.16) | |
| Q4 | 21.62 (7.25) | 216.54 (−527.66, 960.74) | 0.54 (−0.01, 1.09) | 0.01 (−0.37, 0.39) | −12.10 (−29.42, 5.22) | |
| P for Trend | 0.70 | 0.02 | 0.80 | 0.14 | ||
| Omega-3 polyunsaturated fats, milligrams per day | Q1 | 1.44 (0.20) | 0.00 [ref] | 0.00 [ref] | 0.00 [ref] | |
| Q2 | 1.84 (0.09) | 116.72 (−632.19, 865.63) | 0.33 (−0.23, 0.89) | −0.07 (−0.45, 0.32) | 1.44 (−16.05, 18.94) | |
| Q3 | 2.18 (0.10) | −68.73 (−832.11, 694.65) | 0.29 (−0.29, 0.86) | −0.00 (−0.39, 0.39) | −4.34 (−22.17, 13.50) | |
| Q4 | 3.24 (1.26) | 311.12 (−441.73, 1063.97) | 0.07 (−0.50, 0.63) | 0.14 (−0.25, 0.52) | −9.22 (−26.80, 8.37) | |
| P for Trend | 0.44 | 0.92 | 0.37 | 0.21 | ||
| Alpha-linolenic acid, milligrams per day | Q1 | 1.13 (0.26) | 0.00 [ref] | 0.00 [ref] | 0.00 [ref] | |
| Q2 | 1.49 (0.07) | −227.17 (−991.68, 537.34) | 0.12 (−0.45, 0.70) | 0.11 (−0.29, 0.50) | 2.66 (−15.21, 20.53) | |
| Q3 | 1.73 (0.07) | 150.07 (−637.52, 937.66) | 0.11 (−0.48, 0.70) | 0.11 (−0.30, 0.52) | −0.97 (−19.38, 17.44) | |
| Q4 | 2.59 (1.19) | −388.39 (−1127.09, 350.32) | 0.45 (−0.11, 1.00) | −0.06 (−0.44, 0.32) | −11.01 (−28.28, 6.26) | |
| P for Trend | 0.39 | 0.11 | 0.65 | 0.15 | ||
| Marine n-3 fatty acids, milligrams per day | Q1 | 0.08 (0.08) | 0.00 [ref] | 0.00 [ref] | 0.00 [ref] | |
| Q2 | 0.25 (0.03) | 302.55 (−416.76, 1021.87) | −0.09 (−0.63, 0.45) | 0.15 (−0.21, 0.51) | 5.35 (−11.37, 22.07) | |
| Q3 | 0.42 (0.08) | 560.17 (−159.92, 1280.26) | −0.23 (−0.77, 0.32) | 0.49 (0.12, 0.85) | 17.47 (0.73, 34.21) | |
| Q4 | 1.00 (0.29) | 371.14 (−350.09, 1092.37) | −0.45 (−0.99, 0.09) | 0.40 (0.03, 0.76) | 3.50 (−13.27, 20.26) | |
| P for Trend | 0.42 | 0.09 | 0.04 | 0.86 |
Adjusted for age, sex, race, ethnicity, socio-economic status, physical activity, BMI, smoking status, disease duration, disease modifying therapy use and class, and total energy intake.
Highlights.
The MIND (Mediterranean-Dietary Approach to Stop Hypertension Intervention for Neurodegenerative Delay) dietary pattern is associated with outcomes in cognitive aging and neurodegenerative disease
MIND diet score is associated with thalamic volume in this cohort of people with early MS
Intake of marine-derived omega 3 fatty acids is associated with integrity of normal appearing white matter in this cohort of people with early MS
Acknowledgements:
The authors would like to thank all of the Corinne Goldsmith Dickinson Center for MS clinicians and research coordinators (Sam Horng, Oluwasheyi Ayeni, Gretchen Mathewson, Aliza Ben-Zacharia, Christina Lewis, Gabrielle Pelle) and Columbia MS Center who participated in recruitment and data collection for the RADIEMS cohort. Most importantly, the authors would like to thank all of those living with MS who volunteered their time and effort to participate.
Funding acknowledgements:
This study was funded by NIH R01 HD082176, to Dr. Sumowski
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
Dr. Katz Sand reports no disclosures
Dr. Fitzgerald reports no disclosures
Dr. Gu reports no disclosures
Dr. Brandstadter reports no disclosures
Dr. Riley reports consulting or advisory work with Biogen Idec, Celgene, Genentech/Roche, Genzyme, TG Therapeutics
Dr. Leavitt reports consulting work with Healios, LLC, and is co-founder of eSupport Health, PBC
Dr. Krieger reports consulting or advisory work with Acorda, Bayer, Biogen, Celgene, EMD Serono, Genentech, Genzyme, Mallinckrodt, MedDay, Novartis, Teva, and TG Therapeutics, and non-promotional speaking with Biogen
Dr. Miller reports consulting or advisory work with Accordant Health Services (Caremark), Adamas, Biogen Idec, Celgene, Corrona, EMD Serono, Genzyme/Sanofi, Mallinckrodt, Mapi-Pharma, Novartis, Roche/Genentech and research support from Biogen Idec, Genzyme/Sanofi, Mallinckrodt, Novartis, Roche/Genentech, MedDay.
Dr. Lublin reports funding for research from Novartis Pharmaceuticals Corp, Teva Neuroscience, Actelion, Transparency Life Sciences, NMSS, and Sanofi and consulting agreements/advisory boards/DSMB fees from Bayer HealthCare, Biogen, EMD Serono, Novartis, Teva; Actelion; Sanofi/Genzyme; Acorda; Roche/Genentech; MedImmune; Receptos/Celgene; Forward Pharma; TG Therapeutics; Abbvie; Regeneron; Medday; Atara Biotherapeutics; Polpharma; Mapi Pharma; Innate Immunotherapeutics; Apitope; and Orion Biotechnology.
Dr. Klineova reports advisory board work with Genentech and Biogen Idec and has given non-promotional lectures with Biogen Idec.
Dr. Fabian reports no disclosures.
Dr. Sumowski reports consulting or advisory work with Biogen Idec and Sanofi Genzyme
References
- 1.Motl RW, Mowry EM, Ehde DM, et al. Wellness and multiple sclerosis: The National MS Society establishes a Wellness Research Working Group and research priorities. Mult Scler 2018; 24: 262–267. DOI: 10.1177/1352458516687404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Black LJ, Rowley C, Sherriff J, et al. A healthy dietary pattern associates with a lower risk of a first clinical diagnosis of central nervous system demyelination. Mult Scler 2019; 25: 1514–1525. DOI: 10.1177/1352458518793524. [DOI] [PubMed] [Google Scholar]
- 3.Langer-Gould A Fresh fish consumption is associated with a lower risk of multiple sclerosis independent of serum 25OHD levels. In: ECTRIMS; Paris, 2017. [Google Scholar]
- 4.Azary S, Schreiner T, Graves J, et al. Contribution of dietary intake to relapse rate in early paediatric multiple sclerosis. J Neurol Neurosurg Psychiatry 2018; 89: 28–33. DOI: 10.1136/jnnp-2017-315936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fitzgerald KC. Diet quality is associated with disability and symptom severity in multiple sclerosis. Neurology; 90: e1–e11. DOI: 10.1212/WNL.0000000000004768. [DOI] [PubMed] [Google Scholar]
- 6.Morris MC, Tangney CC, Wang Y, et al. MIND diet slows cognitive decline with aging. Alzheimers Dement 2015; 11: 1015–1022. DOI: 10.1016/j.jalz.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morris MC, Tangney CC, Wang Y, et al. MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimers Dement 2015; 11: 1007–1014. DOI: 10.1016/j.jalz.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agarwal P, Wang Y, Buchman AS, et al. MIND Diet Associated with Reduced Incidence and Delayed Progression of ParkinsonismA in Old Age. The journal of nutrition, health & aging 2018; 22: 1211–1215. 2018/12/01. DOI: 10.1007/s12603-018-1094-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van den Brink AC, Brouwer-Brolsma EM, Berendsen AAM, et al. The Mediterranean, Dietary Approaches to Stop Hypertension (DASH), and Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) Diets Are Associated with Less Cognitive Decline and a Lower Risk of Alzheimer’s Disease-A Review. Advances in nutrition 2019. DOI: 10.1093/advances/nmz054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munoz-Garcia MI, Toledo E, Razquin C, et al. “A priori” Dietary Patterns and Cognitive Function in the SUN Project. Neuroepidemiology 2019: 1–13. DOI: 10.1159/000502608. [DOI] [PubMed] [Google Scholar]
- 11.Hosking DE, Eramudugolla R, Cherbuin N, et al. MIND not Mediterranean diet related to 12-year incidence of cognitive impairment in an Australian longitudinal cohort study. Alzheimer’s & dementia : the journal of the Alzheimer’s Association 2019; 15: 581–589. DOI: 10.1016/j.jalz.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 12.Brandstadter R, Ayeni O, Krieger SC, et al. Detection of subtle gait disturbance and future fall risk in early multiple sclerosis. Neurology 2020; 94: e1395–e1406. DOI: 10.1212/WNL.0000000000008938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brandstadter R. Beyond rehabilitation: A prevention model of reserve and brain maintenance in multiple sclerosis. Multiple sclerosis; 25: 1372–1378. DOI: 10.1177/1352458519856847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2018; 17: 162–173. DOI: 10.1016/S1474-4422(17)30470-2. [DOI] [PubMed] [Google Scholar]
- 15.Fung TT, Hu FB, Wu K, et al. The Mediterranean and Dietary Approaches to Stop Hypertension (DASH) diets and colorectal cancer. Am J Clin Nutr 2010; 92: 1429–1435. DOI: 10.3945/ajcn.2010.29242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fung TT, Chiuve SE, McCullough ML, et al. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Archives of internal medicine 2008; 168: 713–720. 2008/04/17. DOI: 10.1001/archinte.168.7.713. [DOI] [PubMed] [Google Scholar]
- 17.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004; 23 Suppl 1: S208–219. DOI: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 18.Behrens TE, Woolrich MW, Jenkinson M, et al. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med 2003; 50: 1077–1088. DOI: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- 19.Liu Q, Li C, Wanga V, et al. Covariate-adjusted Spearman’s rank correlation with probability-scale residuals. Biometrics 2018; 74: 595–605. DOI: 10.1111/biom.12812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoare S, Lithander F, van der Mei I, et al. Higher intake of omega-3 polyunsaturated fatty acids is associated with a decreased risk of a first clinical diagnosis of central nervous system demyelination: Results from the Ausimmune Study. Mult Scler 2016; 22: 884–892. DOI: 10.1177/1352458515604380. [DOI] [PubMed] [Google Scholar]
- 21.Katz Sand I. The Role of Diet in Multiple Sclerosis: Mechanistic Connections and Current Evidence. Current nutrition reports 2018; 7: 150–160. DOI: 10.1007/s13668-018-0236-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Azevedo CJ, Cen SY, Khadka S, et al. Thalamic atrophy in multiple sclerosis: A magnetic resonance imaging marker of neurodegeneration throughout disease. Ann Neurol 2018; 83: 223–234. DOI: 10.1002/ana.25150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eshaghi A, Marinescu RV, Young AL, et al. Progression of regional grey matter atrophy in multiple sclerosis. Brain : a journal of neurology 2018; 141: 1665–1677. 2018/05/10. DOI: 10.1093/brain/awy088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rocca MA, Mesaros S, Pagani E, et al. Thalamic damage and long-term progression of disability in multiple sclerosis. Radiology 2010; 257: 463–469. DOI: 10.1148/radiol.10100326. [DOI] [PubMed] [Google Scholar]
- 25.Schoonheim MM and Ciccarelli O. The value of including thalamic atrophy as a clinical trial endpoint in multiple sclerosis. Neurology 2018; 90: 677–678. DOI: 10.1212/WNL.0000000000005279. [DOI] [PubMed] [Google Scholar]
- 26.Banwell B, Bar-Or A, Cheung R, et al. Abnormal T-cell reactivities in childhood inflammatory demyelinating disease and type 1 diabetes. Annals of Neurology 2008; 63: 98–111. DOI: 10.1002/ana.21244 [doi]. [DOI] [PubMed] [Google Scholar]
- 27.Guggenmos J, Schubart AS, Ogg S, et al. Antibody cross-reactivity between myelin oligodendrocyte glycoprotein and the milk protein butyrophilin in multiple sclerosis. Journal of immunology (Baltimore, Md: 1950) 2004; 172: 661–668. 2003/12/23. DOI: 10.4049/jimmunol.172.1.661. [DOI] [PubMed] [Google Scholar]
- 28.Munger KL, Chitnis T, Frazier AL, et al. Dietary intake of vitamin D during adolescence and risk of multiple sclerosis. Journal of neurology 2011; 258: 479–485. DOI: 10.1007/s00415-010-5783-1 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tankou SK, Regev K, Healy BC, et al. A probiotic modulates the microbiome and immunity in multiple sclerosis. Ann Neurol 2018; 83: 1147–1161. DOI: 10.1002/ana.25244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fitzgerald KC, Tyry T, Salter A, et al. Diet quality is associated with disability and symptom severity in multiple sclerosis. Neurology 2018; 90: e1–e11. DOI: 10.1212/WNL.0000000000004768. [DOI] [PubMed] [Google Scholar]
- 31.Unoda K, Doi Y, Nakajima H, et al. Eicosapentaenoic acid (EPA) induces peroxisome proliferator-activated receptors and ameliorates experimental autoimmune encephalomyelitis. Journal of neuroimmunology 2013; 256: 7–12. DOI: 10.1016/j.jneuroim.2012.12.003 [doi]. [DOI] [PubMed] [Google Scholar]
- 32.Torkildsen O, Brunborg LA, Thorsen F, et al. Effects of dietary intervention on MRI activity, de-and remyelination in the cuprizone model for demyelination. Experimental neurology 2009; 215: 160–166. DOI: 10.1016/j.expneurol.2008.09.026 [doi]. [DOI] [PubMed] [Google Scholar]
- 33.Bjornevik K, Chitnis T, Ascherio A, et al. Polyunsaturated fatty acids and the risk of multiple sclerosis. Mult Scler 2017; 23: 1830–1838. DOI: 10.1177/1352458517691150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Granberg T, Fan Q, Treaba CA, et al. In vivo characterization of cortical and white matter neuroaxonal pathology in early multiple sclerosis. Brain : a journal of neurology 2017; 140: 2912–2926. 2017/10/21. DOI: 10.1093/brain/awx247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gu Y, Vorburger RS, Gazes Y, et al. White matter integrity as a mediator in the relationship between dietary nutrients and cognition in the elderly. Annals of Neurology 2016; 79: 1014–1025. DOI: 10.1002/ana.24674 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gu Y, Brickman AM, Stern Y, et al. Mediterranean diet and brain structure in a multiethnic elderly cohort. Neurology 2015; 85: 1744–1751. DOI: 10.1212/WNL.0000000000002121. [DOI] [PMC free article] [PubMed] [Google Scholar]


