Abstract
Cancer chemotherapeutics face several challenges, including uncontrollable drug release, off-target toxic effects, and poor bioavailability. Recently, supramolecular nanovesicles, such as calix[n]arenes (CXs), cyclodextrins (CDs), cucurbiturils (CBs), and pillar[n]arenes (PRs), have attracted attention as potential smart nanocarriers for chemotherapeutics because of their exceptional cavities that can achieve high encapsulation capacity and accommodate both hydrophilic and hydrophobic drugs. In addition, they can be functionalized with different stimuli-responsive groups, which facilitate controlled drug release. Supramolecular nanovesicles, loaded with drugs and decorated with stimuli-responsive targeting moieties, are designed by either host–guest complexation or self-assembly of amphiphilic cavitands. Pillar[n]arenes, in particular, are novel supramolecular host molecules that have recently been employed in cancer targeted drug delivery because of their symmetric pillar-shaped structure, simplicity of functionalization, and biocompatibility. This review summarizes state-of-the-art strategies for developing single or multiple stimuli-responsive pillar[n]arene nanovesicles for effective cancer treatment.
1. Targeted Delivery of Chemotherapeutics Using Supramolecular Approaches
Targeted delivery of chemotherapeutics to tumors is essential to minimize side effects on normal cells. Although cytotoxicity of the chemotherapeutic drugs affects both the tumor and normal tissues, most of the targeting strategies rely on the fact that rapidly proliferating neoplastic cells are more sensitive to anticancer drugs than normal ones.1,2 This warrants the search for innovative chemotherapeutics delivery platforms that can selectively target neoplastic cells.
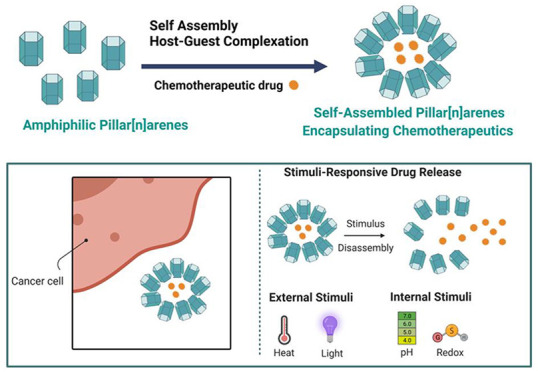
Lately, amphiphilic supramolecular approaches, including cyclodextrins (CDs), calix[n]arenes (CXs), cucurbiturils (CBs), and pillar[n]arenes (PRs), have attracted attention as possible smart nanocarriers that can escort chemotherapeutic agents selectively to tumor tissues. The supramolecular systems can encapsulate high concentrations of hydrophilic and hydrophobic drugs and be decorated with different stimuli-responsive functional groups. Moreover, encapsulating drugs into supramolecular nanovesicles overcomes most of the challenges associated with chemotherapeutic drugs, such as improving water-solubility, bioavailability, stability, and minimizing resistance. The spontaneous self-assembly of supramolecules to form responsive nanovesicles could be achieved via weak and reversible interactions such as electrostatic forces, π–π-stacking bonding, van der Waals forces, ion–dipole interactions, noncovalent bonding, and/or hydrophobic interactions.1−3
Several amphiphilic supramolecules can be employed to design based on self-assembly methods. These assemblies are held together by weak and reversible interactions imparting them with specific responsiveness. They have promising applications in cancer therapy because they (1) are soluble in different solvents, (2) can be easily functionalized with different hydrophilic functional groups, which improve their water solubility and physicochemical properties, (3) can increase the stability of the drugs within the blood circulation, (4) can host various guest molecules, in their electron-rich cavities, through complexation, and (5) can minimize effective therapeutic doses and toxic effects via selective targeting and controlled sustained release of drugs.1−6
Many studies reported using amphiphilic supramolecules for effective and selective delivery of different anticancer drugs to their specific sites of action.1−6 Markowitz et al. (1989) developed the first amphiphilic supramolecular nanovesicles, with sizes ranging 0.5–1 μm, through injecting p-sulfonato-calix[6]arene tetrahydrofuran solution into an aqueous phase. Further studies have been conducted to construct various amphiphilic supramolecular nanovesicles to escort chemotherapeutic drugs selectively to cancer cells.1 This review summarizes state-of-the-art strategies for developing single or multiple stimuli-responsive pillar[n]arene nanovesicles for effective cancer treatment.
2. Pillar[n]arenes (PRs)
PRs are a novel class of supramolecular host molecules that were first synthesized by Ogoshi et al. in 2008.7 Currently, PRs are key players in supramolecular chemistry, and many intelligent, targeted delivery systems are formulated based on them. PRs are composed of 1,4-dimethoxybenzene units linked together by methylene bridges at the para position (2,5 positions), imparting them with a unique symmetrical and rigid pillar-shaped structure (Figure 1A).1,8 The unique structure of PRs makes them superior to other supramolecular host molecules and affords them many unique features such as high selectivity to different guest molecules, solubility in various types of solvents, and the ability to accommodate electron-deficient and neutral guest molecules due to their electron-rich cavities.8 Moreover, PRs are biocompatible and can be easily functionalized with different functional hydrophilic groups, enhancing their water solubility. According to the groups used to functionalize PRs, three types of amphiphilic PRs (cationic, anionic, or neutral) could be synthesized for multiple drug delivery and molecular recognition applications.8 In addition, few PRs were reported to possess biological activities. For instance, cationic PRs were reported to induce cell apoptosis via inhibiting tyrosine–protein phosphorylation.9 Three approaches are involved in the synthesis of PRs; (1) the condensation of para-formaldehyde and 1,4-dialkoxybenzene in the presence of Lewis acid catalyst, (2) the condensation of 1,4-dialkoxy-2,5-bis(alkoxymethyl)benzene in the presence of toluenesulfonic acid catalyst, or (3) the cyclooligomerization of either 2,5-dialkoxybenzyl bromides or 2,5-dialkoxybenzyl alcohols in the presence of Lewis acid catalyst (Figure 1B).
Figure 1.
(A) Chemical structure of pillar[n]arenes. (B) Different approaches for the synthesis of pillar[n]arenes. Reprinted with permission from ref (8). Copyright 2012 American Chemical Society.
In particular, pillar[5]arene and pillar[6]arene are widely used in drug delivery because of their water solubility and the ease of their surface functionalization with numerous functional groups resulting in improved optical response, solubility, crystallinity, and selective targeting to cancer cells.1,6
2.1. Pillar[n]arene (PR) Based Stimuli-Responsive Nanovesicles for Targeted Delivery of Cancer Drugs
The tumor microenvironment has unique features that differ from those of healthy cells. These include acidic pH, high levels of hydrogen peroxide and glutathione (GSH), and overexpressed receptors and proteins, which could be exploited to disrupt the amphiphilic pillar[n] arene nanovesicles and consequent release of their drug loads selectively inside cancer cells without off-target toxic effects on healthy ones.10
Stimuli-responsive pillar[n]arenes can increase the loading capacity of chemotherapeutic agents, enhance the cytotoxicity and targeting to different cancer cells (rapid release into cancer cells in response to different stimuli) followed by high internalization and endocytosis, and reduce toxicity to normal cells.6 Therefore, smart drug delivery supramolecular systems can be designed to target the tumor cells where they release their cargo in response to either intracellular stimuli (such as pH variation, overexpression of specific biomolecules, and redox reactions) or external stimuli such as exposure to light.
2.1.1. pH-Responsive PR Nanosystems
The pH in tumor tissues (pH of 5.4) and late endosomes and lysosomes (pH of 4.5–5.5) is lower than that in the physiological microenvironments of healthy tissues and blood circulation (pH of 7.4).11,12 In the tumor acidic microenvironment, partial protonation of the amino, carboxylate, and phosphate groups of the amphiphilic PRs and/or the cleavage of some chemical bonds which are unstable under acidic conditions such as hydrazide bonds may occur. This weakens the interaction between PRs and the guest molecules leading to the selective release of chemotherapeutics into tumors.11,12 Some recent studies have reported the design and application of pH-responsive PRs as potential host molecules for many chemotherapeutic agents, for effective targeted cancer therapy.
Li et al. designed a pH-responsive system based on the host–guest encapsulation of oxaliplatin by the pH-responsive carboxylatopillar[6]arene (CPA6) host molecule.11 The pH sensitivity of the developed system was confirmed by computing the association constants of the CPA6/oxaliplatin host–guest system. At physiological pH, the association constant (Ka = 1.02 × 104 ± 0.05 M–1) was 24 times higher than that at pH 5.4 (Ka = 4.21 × 102 ± 0.06 M–1). The biocompatibility of the CPA6 carrier was investigated at two different concentrations (160 and 320 mM) using normal cell lines (293T cells), and the cell viability was 93% ± 6.8 and 89.8% ± 2.6, respectively. The ability of oxaliplatin to prevent the regrowth of cancer cells was assessed in mice xenografted with sarcoma. A 70% enhancement in the tumor inhibitory activity was noticed in the CPA6/oxaliplatin host–guest complex compared to free oxaliplatin.11
Another study reported the use of CPA6 as a pH-responsive amphiphilic supramolecular system for the dual delivery of oxaliplatin and doxorubicin (DOX).12 This was achieved by forming a host–guest complex between CPA6 and oxaliplatin with association constants of 1.16 × 104 ± 0.03 M–1 and 1.73 × 103 ± 0.15 M–1 at pH 7.4 and pH 5.0, respectively. Then, the designed complex was self-assembled in an aqueous medium forming hollow nanovesicles that can accommodate DOX in their hydrophilic hollow cavities after the simple addition of the drug and stirring. The designed nanovesicles had an average diameter of 122 nm, a zeta potential of −27.3 mV, and encapsulation efficiencies of 83.8% and 25.8% for oxaliplatin and DOX, respectively. Release studies showed slower release rates at physiological medium than at acidic pH due to the disassembly of the CPA6/oxaliplatin complex and high release rates of both oxaliplatin and DOX. Only 7% of encapsulated DOX was released at pH 7.4, which was increased to 80% at pH 5.0 within 24 h. Similarly, 6% of complexed oxaliplatin was released at pH 7.4 compared to 70% at pH 5.0. This demonstrates the ability of the designed supramolecular nanovesicles to act as a pH-stimulus codelivery system. Moreover, the DOX-loaded CPA6/oxaliplatin nanovesicles exhibited an enhanced in vitro synergistic cytotoxic effect against the human liver hepatocellular carcinoma (HepG-2) cell line, and the combination index was found to be 0.61 (two drugs are considered synergistic when the combination index is < 1.0). In addition, the designed nanovesicles were found to be safer than either free oxaliplatin or free DOX, where negligible body weight loss was observed in HepG-2 tumor xenograft bearing nude mice.12
Lan et al. designed a pH-responsive supramolecular system based on pillar[5]arene functionalized with acetal group (Ac-PA5) conjugated with folic acid (a targeting group) for the targeted delivery of paclitaxel (PTX), a hydrophobic anticancer drug.13 In this study, the Ac-PA5 was formulated into nanoparticles by dissolution in an organic solvent, followed by emulsification in poly(vinyl alcohol) polymer using probe sonication. The designed nanoparticles had a size of about 198 nm, a polydispersity index (PDI) of 0.095, a zeta potential of −18 mV, and pronounced stability in blood serum and buffered solutions. Paclitaxel was loaded in the nanoparticles via a single emulsion method, and the loading percentage was found to be 9.1%. Release studies showed slow release rates under physiological conditions, which remarkably accelerated at pH of 4.0 and 5.0. The blank Ac-PA5 nanoparticles were biocompatible and nontoxic when assessed against normal human (MRC-5) and ovarian cancer (HeLa) cell lines at concentrations of 200 and 100 μg/mL, respectively. Surface conjugation with folic acid was conducted via host–guest interactions by adding pyridinium-conjugated folic acid during the emulsification step. Folic acid is a perfect targeting candidate for cancer cells with folate receptor (FR) overexpression, such as breast cancer cells (MCF-7). Therefore, the nanoparticles decorated with folic acid are effectively internalized by cancer cells through binding to the overexpressed FRs. The efficiency of the targeted delivery of the prepared nanoparticles functionalized with folic acid was investigated by the MTT assay against MCF-7 cancer cell lines. The cancer cell lines were incubated with the supramolecular system at various concentrations ranging from 0 to 1000 μg/L for 24 h. A 5.1-fold increase in cytotoxicity in the case of folic acid-functionalized nanoparticles (IC50 value of 33 μg/L) compared to free paclitaxel and nontargeting nanoparticles (IC50 values of 200 and 202 μg/L, respectively) was observed.13
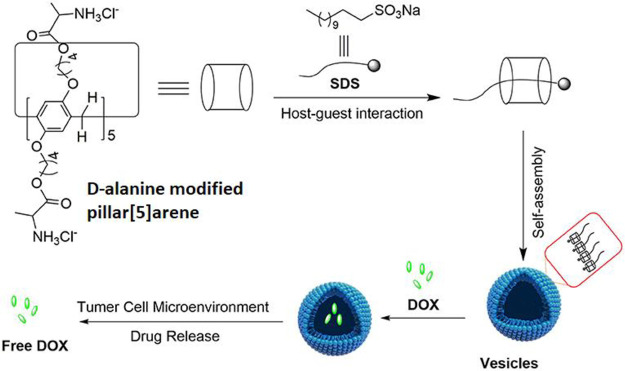
Liu et al. designed supramolecular nanovesicles based on cationic pillar[5]arenes (PR5) with pH-responsive effect for targeted delivery of doxorubicin (DOX).14 The cationic PR5, composed of d-alanine functionalized water-soluble PR5, was formulated into nanovesicles encapsulating DOX via the host–guest inclusion complexation with sodium dodecyl sulfonate (SDS) in a ratio 1:3 followed by self-assembly in an aqueous medium. The designed spherical nanovesicles loaded with DOX had a size of 220 nm, a zeta potential of 30.8 mV, and an encapsulation efficiency of 75%. Release studies showed that the percent cumulative release of DOX at pH 4.8 was 72% within 13 h. In comparison, the percent cumulative release of DOX under physiological conditions was 37% at 13 h, indicating the ability of the nanovesicles to release their cargo at acidic pH. Furthermore, the DOX loaded cationic PR5 nanovesicles exhibited significant cytotoxicity (IC50 values of 0.77 μM and 1.09 μM against HepG2 and T24 cancer cell lines, respectively) compared to free DOX (IC50 values of 3.43 μM and 1.76 μM against HepG2 and T24 cancer cell lines, respectively) (Figure 2).14
Figure 2.
Schematic illustration of supramolecular-based pillar[5]arenes nanovesicles for effective cancer therapy. DOX: doxorubicin; SDS: sodium dodecyl sulfonate. Reprinted with permission from ref (14). Copyright 2021 Liu, Zhou, He, Duan, and Huang. CC BY License.
Guo et al. designed a hydrazide-pillar[5]arene (HPA5)/bisdemethoxycurcumin (BDMC) host–guest inclusion complex with 1:1 binding stoichiometry and association constant of 1.6 × 104 ± 0.3 M–1.15 This complex was further self-assembled in water/ethanol solution to form supramolecular nanofibers. BDMC, one of the curcuminoid compounds, has anticancer activity and more stability than curcumin. The designed supramolecular nanofibers had a diameter of 450 nm, a length of 8 μm, and a width of 300 nm. The percent cumulative release of BDMC was only 5% at pH 7.4 within 24 h. In contrast, the release of BDMC at a pH of 5.1 dramatically increased to 86% within 12 h. This supports the ability of the designed supramolecular system to release BDMC selectively in the tumor acidic microenvironment. The prepared nanofibers exhibited low cytotoxicity against 293T normal cells (IC50 values of 85.2 μg/mL) and hepatocellular carcinoma HepG2 cells (IC50 values of 32.4 μg/mL).15
Sun et al. fabricated dual acid-responsive supramolecular nanovesicles, with faster release rates in the tumor acidic microenvironment, for the targeted intracellular delivery of DOX.16 The dual acid-responsive nanovesicles were constructed via the host–guest interactions between water-soluble PR5 and 2,4,8,10-tetraoxaspiro[5.5]undecane, a cleavable linker that improves the acid responsiveness of the system, forming supramolecular amphiphiles. The fabricated supramolecular amphiphiles were further self-assembled forming hollow supramolecular nanovesicles that encapsulated DOX in the interior hydrophobic region by passive loading. The constructed nanovesicles had a size of about 200 nm, a zeta potential of −31 mV, and a DOX loading content of 79%. The developed nanovesicles exhibited high stability at pH 7.4. On the contrary, 37% and 91% of the loaded DOX were released at pH 6.0 (extracellular pH) and pH 5.0 (endolysosomal pH), respectively, within 1 h. When evaluated against mouse embryonic fibroblast (NIH 3T3) normal cells, the dual pH-responsive nanovesicles exhibited cell viability >90%. The DOX-loaded supramolecular nanovesicles improved the anticancer activity of DOX against human breast adenocarcinoma (MCF-7), human primary glioblastoma cell line (U87MG), and HepG2 cancer cell lines.16
2.1.2. Photothermal Responsive PR Nanosystems
Photothermal therapy (PTT) is a local noninvasive strategy that displayed potential in combating cancer. It depends on utilizing molecules that absorb near-infrared (NIR) light irradiation generating local thermal energy that can selectively eradicate tumor cells in the vicinity. The engineering of supramolecular nanosystems that involve the integration of PTT with chemotherapy (chemophotothermal therapy) has been reported to improve cancer treatment effectiveness while minimizing the off-target systemic toxic effects.17−19
Wu et al. engineered an effective chemophotothermal supramolecular nanohybrid system composed of polypyrrole (PPy) and UiO-66 metal–organic framework (MOF) nanoparticles (PU NPs) fortified with water-soluble pillar[6]arene-based pseudorotaxanes (WP6) nanovalves and decorated with folic acid–polyethylenimine (PEI–Fa) (PPy/UiO-66/WP6/PEI–Fa) loaded with 5-fluorouracil (5-FU).17 The PPy NPs served as a biocompatible photothermal agent when irradiated with light at 808 nm, and the PEI-Fa served as a cancer-targeting moiety. The fabricated nanohybrid system had a spherical morphology, an average diameter of 150 nm, a MOF thickness of about 10 nm, and photothermal conversion efficiency of 38.7%. The designed 5-FU loaded PPy/UiO-66/WP6/PEI–Fa exhibited a sustained release behavior within 4 days and a high 5-FU encapsulation capacity (0.6 μmol/mg), owing to the presence of nanovalves which hindered the drug loss. Furthermore, the ability of the nanohybrid supramolecular system to selectively target tumor cells was evaluated against human cervical cancer cells (HeLa cells) using NIR irradiation of 808 nm (2.0 W/cm2) for 10 min. A remarkable reduction in the cell viability (about 20%) of 5-FU loaded PPy/UiO-66/WP6/PEI–Fa compared to free 5-FU (about 60%) was observed. This is attributed to the synergistic effect caused by the integration of both the chemotherapeutic (5-FU) and photothermal (PPy/UiO-66/WP6/PEI–Fa) moieties.17
Another study reported the design of an intelligent supramolecular nanoplatform that combined chemophotothermal therapy and selective targeting to treat human liver hepatocellular carcinoma efficiently.18 The nanoplatform is fabricated using carboxylatopillar[5]arene (CPA5) capped with CuS nanoparticles (CuS/CP NPs) followed by surface decoration with galactose (G), a hepatic tumor-targeting moiety, via host–guest interaction forming CuS/CPG. DOX loading was facilitated by the electrostatic interactions between the negatively charged CPA5 and the positively charged DOX generating CuS/CPG/DOX with a loading capacity of 48%. CuS was used as a photothermal agent that exhibited outstanding cytotoxicity toward HepG2 cancer cells after irradiating with NIR light of 808 nm. The designed CuS/CPG/DOX was biocompatible to normal cells but showed remarkable cytotoxicity against HepG2 cancer cells (cell viability of 25%) after exposure to NIR laser (808 nm) at a light dose of 2.0 W/cm2 for 3 min (Figure 3).18
Figure 3.
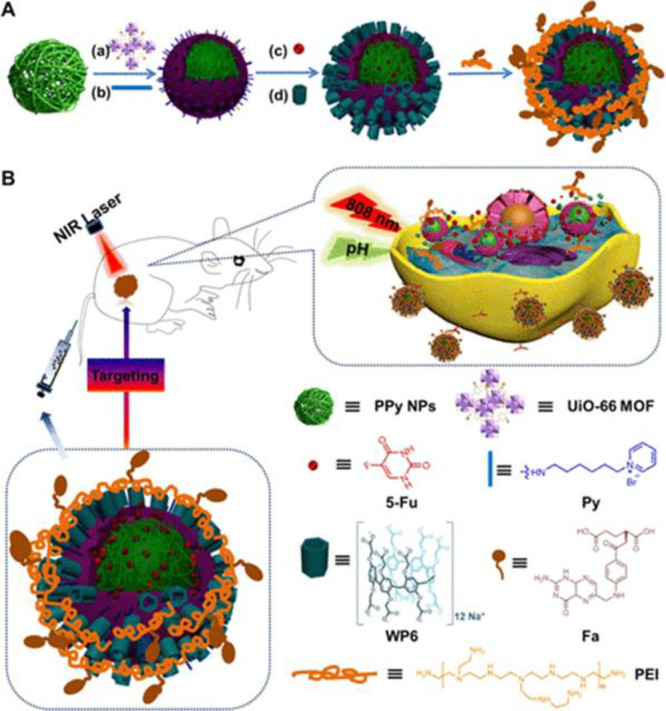
Schematic illustration of (A) the fabrication of nanohybrid supramolecular system comprised of polypyrrole/UiO-66 metal–organic framework nanoparticles fortified with water-soluble pillar[6]arene-based pseudorotaxanes nanovalves conjugated with folic acid-polyethylenimine loaded with 5 fluorouracil, and (B) its application as a potential chemophotothermal nanoplatforms in the treatment of cervical cancer. Fa: folic acid; MOF: metal–organic framework; PEI: polyethylenimine; PPy: polypyrrole; WP6: pillar[6]arene-based pseudorotaxanes. Reprinted from ref (18). Copyright 2018 American Chemical Society.
Chen et al. constructed DOX-loaded amphiphilic supramolecular nanovesicles with chemophotothermal effect for the effective treatment of colorectal cancer.19 In this regard, a host–guest complexion was formed between water-soluble pillar[5]arene (PR5) and aniline tetramer (TANI) with 1:1 binding stoichiometry and association constant of 3.7 × 103 ± 1.3 M–1. This complex was further self-assembled, forming supramolecular nanovesicles encapsulating DOX. The fabricated nanovesicles had an average diameter of 105.8 nm, a zeta potential of −56 mV, and a DOX encapsulation efficiency of 91.7%. Moreover, the constructed supramolecular vesicles had high cytocompatibility (cell viability >80%) when tested on L02 normal cells. In contrast, the prepared nanovesicles killed more than 50% of colorectal (CT26) cancer cells after exposure to an 808 nm NIR laser for 10 min. In addition, in vivo studies using CT26 tumor-bearing mice showed the complete eradication of the tumor after the IV administration of the designed nanovesicles and exposure to 808 or 1064 nm NIR laser at a light dose of 2.3 W/cm2.19
2.1.3. Redox Responsive PR Nanosystems
Redox responsive supramolecular nanoplatforms represent a promising targeting strategy based on the high intracellular reductive glutathione (GSH) concentrations in some tumor tissues (1–10 mM) as compared to GSH levels in extracellular tissues such as plasma (1–10 μM).20
Cui et al. engineered a GSH-responsive amphiphilic supramolecular nanovesicle for the targeted delivery of DOX. The nanovesicles were formed by the self-assembly of PEG-decorated pillar[5]arene-based pseudo[1]rotaxane (PPR) in an aqueous medium in the presence of DOX forming DPPR. The weak disulfide bonds, embedded within the hydrophobic cavities of PPR, are cleaved upon exposure to high levels of GSH inside lung cancer (A549) cells leading to the disassembly of the nanovesicles, which release their cargo selectively in the tumor tissue.20 The designed nanovesicles had a spherical morphology, a size of 490 nm, a critical micelle concentration of 4.4 × 10–2 mg/mL, and a DOX loading capacity of 21.3%. Release studies were conducted in the presence of various concentrations of GSH with negligible amounts of DOX released at 0 mM GSH. On the contrary, about 70% and 90% of DOX were released at 5 and 10 mM GSH due to the cleavage of disulfide bonds. The designed nanovesicles exhibited a remarkable in vitro cytotoxicity against A549 cancer cells (cell viability of 40%).20
Another study reported constructing a GSH-responsive supramolecular nanovesicle to enhance the internalization and anticancer activity of camptothecin (CPT), a DNA topoisomerase I inhibitor.21 It was designed via the self-assembly of pillar[5]arene (PR5) and a CPT-conjugated pyridinium salt via a disulfide bond (PR5/CPT-ss-Py). The host–guest self-assembled complex is generated via the hydrophobic and π–π stacking interactions between the electron-rich PR5 and electron-deficient CPT-conjugated pyridinium salt. The designed supramolecular system had a 1:1 binding stoichiometry, an association constant of 2.0 × 104 ± 0.3 M–1, a petal-shaped structure, an average diameter of 130 nm, and was stable under physiological conditions. Release studies showed CPT release of 9%, 50%, and 96% (within 24 h) in the presence of 0, 1.0, or 10.0 mM GSH (which cleaved the disulfide bond between CPT and Py), respectively. The PR5/CPT-ss-Py system displayed a significant in vitro cytotoxicity against HeLa cancer cells (IC50 of 423 ng/mL). Moreover, confocal laser scanning microscopy confirmed the internalization and endocytosis of the nanovesicles by the HeLa cells.21
2.1.4. Multiple Stimuli-Responsive PR Nanosystems
The multiple stimuli-responsive targeted deliveries of chemotherapeutic agents from amphiphilic supramolecular systems represent a promising approach for enhancing the anticancer activity and minimizing off-target toxic effects.22
Jiang et al. designed an innovative carboxylated pillar[6]arene (PR6) based supramolecular nanovesicles to deliver DOX selectively to cancer cells in response to five stimuli (glutathione, pH, carbon dioxide, zinc ions, and hexanediamine).22 The nanovesicles are engineered by the coassembly of carboxylated pillar-[6]arene (CPA6) in the presence of disulfide-linked benzimidazolium amphiphiles and DOX in an aqueous medium. The disulfide linkages of the benzimidazolium amphiphiles are cleaved in the presence of high GSH inside cancer cells. The acidic cancer microenvironment and carbon dioxide mediated the protonation of CPA6, leading to the dissociation of the nanovesicles and drug release. Zinc ions chelate the carboxylate groups of the CPA6, weakening the electrostatic interactions between benzimidazolium moieties and CPA6, leading to the disassembly of the nanovesicles. Hexanediamine, an analogue of biogenic amines that are overexpressed in tumorous tissues, could competitively replace the drug from the cavity of CPA6, resulting in the disruption of the nanovesicles as well as the targeted delivery of chemotherapeutic drugs.21 The designed supramolecular amphiphiles had a size of 216 nm, a zeta potential of −52 mV, and a DOX encapsulation efficiency of about 18%. Cytotoxicity studies suggested the selective release of DOX from the supramolecular system in the cancer cells. Flow cytometry showed that the apoptosis induced by the DOX-loaded supramolecular nanovesicles was higher in the HepG2 cancer cells than in human normal liver cells (LO2).22
Another study reported the fabrication of a dual (GSH and spermine) stimuli-responsive supramolecular nanosystem based on disulfide bridge-linked bispillar[5]arene (bisPA5) encapsulating paclitaxel (PTX) anticancer drug using microemulsion technique.23 The designed nanovesicles had a spherical shape, a size of about 180 nm, shelf stability of 14 days, and PTX encapsulation efficiency of 84.5%. Since GSH and spermine are typically overexpressed in specific cancer cells (such as lung, prostate, and breast cancers), release studies were conducted in the presence of different concentrations of GSH and spermine. The highest PTX release rate (65%) was observed at the highest concentrations of GSH (10 mM) and spermine (1 mM). The designed PTX-loaded nanovesicles displayed a 10-fold increase in cytotoxicity against A549 cancer cells compared to free PTX (IC50 values of 22.23 and 2.76 nM, respectively). In the meantime, the prepared nanovesicles showed cytocompatibility when tested against L02 normal cells.23
Finally, a recent study reported the construction of a supramolecular photosensitizer system formed of oxygen–copper–zeolitic imidazolate frameworks-8 (Cu/ZIF-8) nanohybrid decorated with carboxylated pillar[6]arene (WP6)/methylene blue (MB) host–guest complex-forming OCZWM.24 In this regard, oxygen was adsorbed on the surface of synthesized Cu/ZIF-8. Then, ZIF-8 shells were homogeneously developed around the Cu/ZIF-8 forming OCZ. Finally, the WP6/MB host–guest complex (with a 1:1 binding stoichiometry) was conjugated to the surface of OCZ via coordination, forming OCZWM. The presence of oxygen and Cu2+ in the nanohybrid is vital to induce the Fenton reaction inside the tumor microenvironment. The acidic tumor microenvironment triggers the release of Cu2+ inside the tumor cells, which involves a redox reaction with the overexpressed GSH forming Cu+. The generated Cu+ ions react with oxygen, producing reactive oxygen species (ROS) and hydroxyl free radicals (·OH), which cause irreversible oxidation of the cellular membrane resulting in cancer cell death (Fenton reaction). Furthermore, the MB photosensitizer is photoactivated by light irradiation at a wavelength of ∼630 nm and then reacted with oxygen, generating ROS (Figure 4). The designed nanohybrid supramolecular system had a hexagonal morphology, a diameter range of 190–290 nm, and a MB loading capacity of about 8%. The internalization of OCZWM by HepG2 cancer cells and the controlled release of MB were confirmed by flow cytometry and confocal laser scanning microscopy. This work highlights the significance of combining photodynamic therapy and stimuli-responsive nanosystems in cancer treatment.24
Figure 4.
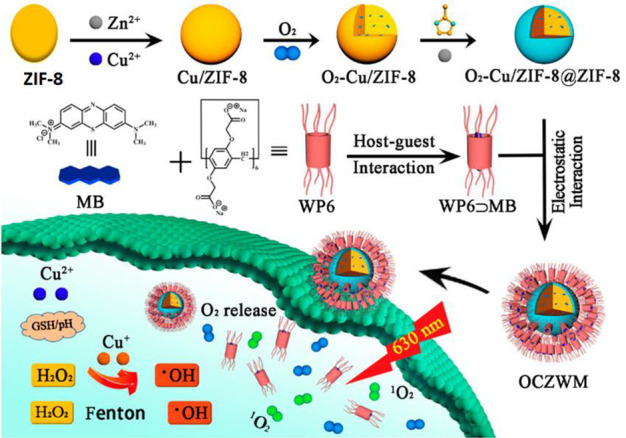
Design of supramolecular photosensitizer system formed of copper-zeolitic imidazolate frameworks-8 (Cu/ZIF-8) nanohybrid decorated with carboxylated pillar[6]arene (WP6)/methylene blue (MB) host–guest complex. GSH: glutathione; OCZWM: oxygen–copper-zeolitic imidazolate frameworks-8 (Cu/ZIF-8) nanohybrid decorated with carboxylated pillar[6]arene (WP6)/methylene blue (MB) host–guest complex. Reprinted from ref (24). Copyright 2021 The Authors. CC BY license.
3. Conclusions
The unique structural features of pillar[n]arenes make them superior to other supramolecular host molecules. Pillar[n]arenes have a symmetrical pillar-shaped structure, can easily be synthesized and functionalized with various functional groups, and are biocompatible and soluble in different solvents. Additionally, they can accommodate electron-deficient and neutral guest molecules due to their electron-rich cavities. Several pillar[n]arenes-based supramolecular systems functionalized with various stimuli-responsive moieties have been designed and proposed as promising nanoplatforms for targeted delivery of cancer chemotherapeutics. The major types of stimuli utilized in engineering stimuli-responsive pillar[n]arene-based systems include pH, photothermal response, redox, and multiple stimuli responses. Reported studies have shown that stimuli-responsive pillar[n]arene-based systems offer several advantages such as high loading capacity of chemotherapeutics, enhanced the cytotoxicity and drug targeting against different cancer cells, rapid drug release into tumors in response to various stimuli, and high internalization and endocytosis by cancer cells and consequently low toxicity to healthy cells (Table 1). However, large-sized pillar[n]arenes (n = 7–10) are still challenging to be synthesized and are unstable. Future studies should address this challenge and provide insight into the biodegradation of pillar[n]arene-based nanoplatforms, their immunological responses, pharmacokinetics, and biodistribution.
Table 1. Characteristics of pH-Responsive Pillar[n]arenes Used for Targeted Delivery of Anticancer Drugs.
| Pillar[n]arene Design | Chemotherapeutic agent | Stimulus | Targeted cancer cells | Advantage | References |
|---|---|---|---|---|---|
| Carboxylatopillar[6]arene (CPA6) | Oxaliplatin | pH | HepG-2, MCF-7, and A549 cancer Cells | -Improved drug stability | (11) |
| -Enhanced tumor inhibitory activity (by 70%). | |||||
| Carboxylatopillar[6]arene (CPA6) | Oxaliplatin and doxorubicin (DOX) | pH | HepG-2 | -High encapsulation efficiency. | (12) |
| -Enhanced synergistic cytotoxic effect. | |||||
| -Biocompatibility. | |||||
| Acetal pillar[5]arene | Paclitaxel (PTX) | pH | MCF-7 | -Enhanced cytotoxicity (by 5.1- fold) and targeting ability. | (13) |
| -Biocompatibility. | |||||
| d-alanine functionalized pillar[5]arenes | DOX | pH | HepG2 and T24 | -High encapsulation efficiency. | (14) |
| -Enhanced anticancer activity and targeting ability. | |||||
| Hydrazide-pillar[5]arene (HPA5) | Bisdemethoxycurcumin (BDMC) | pH | HepG2 | Enhanced selectivity and anticancer effect. | (15) |
| Pillar[5]arene | DOX | pH | MCF-7, U87MG, and HepG2 | -High loading capacity. | (16) |
| -Cytocompatibility. | |||||
| -Enhanced anticancer activity. | |||||
| Pillar[6]arene-based pseudorotaxanes | 5-fluorouracil (5-FU) | Light (NIR irradiation of 808 nm (2.0 W/cm2) for 10 min | HeLa | -Sustained release behavior. | (17) |
| -High encapsulation capacity. | |||||
| -Improved cytotoxicity. | |||||
| Carboxylatopillar[5]arene (CPA5) capped with CuS nanoparticles | DOX | Light (NIR laser of 808 nm (at a power of 2.0 W/cm2) for 3 min | HepG-2 | -Reduced toxicity to normal cells and enhanced anticancer activity. | (18) |
| Pillar[5]arene | DOX | Light (NIR laser of 808 or 1064 nm (at a power of 2.3 W/cm2) for 10 min | CT26 | -High encapsulation efficiency. | (19) |
| -Enhanced anticancer activity and targeting capacity. | |||||
| PEG-decorated pillar[5]arene-based pseudo[1]rotaxane (PPR) | DOX | Redox | A549 | -High loading capacity. | (20) |
| -Rapid release into cancer cells. | |||||
| -Improved cytotoxicity. | |||||
| Pillar[5]arene | Camptothecin (CPT) | Redox | HeLa | - Rapid release into cancer cells. | (21) |
| - High internalization and endocytosis by the cancer cells. | |||||
| -Improved cytotoxicity. | |||||
| Carboxylated pillar[6]arene (PR6) | DOX | Five stimuli (glutathione, pH, carbon dioxide, zinc ions, and hexanediamine) | HepG2 | -Enhanced apoptosis. | (22) |
| -On-demand release of DOX from the supramolecular system into the cancer cells in response to different stimuli. | |||||
| Disulfide bridge-linked bispillar[5]arene (bisPA5) | PTX | GSH and spermine | A549 | -Significant enhancement (10-fold increase) in the cytotoxicity. | (23) |
| -Selective release of drug in response to the overexpressed GSH and spermine inside lung cancer cells. | |||||
| Carboxylated pillar[6]arene | Methylene blue photosensitizer | pH | HepG2 | High internalization by HepG2 cancer cells. | (24) |
| Photodynamic therapy (at an excitation wavelength of ∼630 nm) | Controlled release. |
Acknowledgments
This work has been funded by a grant from the American University in Cairo to H.M.E-S.A.
Biographies
Dr. Hassan Azzazy is a tenured Distinguished University Professor and Chairman of the department of Chemistry at the American University in Cairo. Before joining AUC, Dr. Azzazy was a postdoctoral fellow and assistant professor at University of Maryland School of Medicine, Baltimore, MD. He holds board certifications in Clinical Chemistry and Molecular Diagnostics from the American Board of Clinical Chemistry, Washington, DC. He is certified as a Specialist in Chemistry by the American Society for Clinical Pathology in Chicago (USA) and is a fellow of the Royal Society of Chemistry (UK). He has over 30 years of biomedical research experience with a focus on developing novel diagnostics for detection of infectious agents and cancer markers, optical chemosensors for detection of environmental toxins, biodegradable nanofibrous wound dressings, and targeted drug delivery using nanocarriers. Dr. Azzazy has received many awards including State Prize in Laboratory Medicine (Egypt), Global Innovator Award (Texas Christian University), Arab Innovation and Entrepreneurship Award (UAE), Shoman Award in Medical Sciences (Jordan), Life Achievement Award (American Society for Clinical Pathology), and State Merit Prize in Advanced Technological Sciences (Egypt). Dr. Azzazy is a strong advocate of entrepreneurship and has cofounded two biomedical startup companies.
Dr. Sherif Ashraf Fahmy obtained his B. Pharm with honors from the Faculty of Pharmacy, Cairo University, and his MSc (2015) and PhD (2020) in Chemistry from The American University in Cairo (AUC). His PhD was conducted cojointly with the Department of Pharmaceutics & Biopharmaceutics, Faculty of Pharmacy, Philipps-Universität Marburg, Germany. He has been selected as a Fulbright Scholar at Ohio University in 2019, Athens, USA. Also, Dr. Fahmy received the prestigious and very competitive fellowship offered by the Al-Alfi Foundation for PhD students. Dr. Fahmy has received several other awards and recognitions, including the Royal Society of Chemistry (RSC, UK) Travel Grant for Ph.D. students & Early Career Scientists, 2019, and was selected People’s Choice (Winner) of the international Three Minute Thesis Competition, 2019. He has also spent time as a Visiting Scholar at the Department of Pharmaceutics and Biopharmaceutics, Faculty of Pharmacy, Philipps University of Marburg, Germany, in 2018. His research interests revolve around the fields of Targeted Drug Nanocarrier Drug Delivery; Chemotherapeutic Drugs and Cancer Therapy; Polymeric Nanoparticles; Green Chemistry; and Liposomes and Supramolecular Chemistry. Dr. Fahmy is a member of the Royal Society of Chemistry (MRSC) and the American Chemical Society (ACS). Currently, Dr. Fahmy is a Full-time Assistant Professor of Chemistry, School of Pharmacy, Hertfordshire University-Egypt campus. Also, he is an adjunct Assistant Professor at the Chemistry Department, The American University in Cairo.
Ms. Asmaa Ramzy obtained her BSc of Pharmacy from the German University in Cairo. Currently, she is pursuing her MSc degree in Pharmaceutical Sciences at the same university. Also, Asmaa is a Research Assistant at the Chemistry Department, The American University in Cairo.
Ms. Basma M. Saleh obtained her BSc of Pharmacy from the School of Pharmacy, Cairo University. Currently, she is pursuing her Master’s degree at the American University in Cairo. Also, Basma was a Research Assistant at the Chemistry Department, The American University in Cairo.
The authors declare no competing financial interest.
References
- Fahmy S. A.; Brüßler J.; Alawak M.; El-Sayed M. M. H.; Bakowsky U.; Shoeib T. Chemotherapy Based on Supramolecular Chemistry: A Promising Strategy in Cancer Therapy. Pharmaceutics 2019, 11, 292. 10.3390/pharmaceutics11060292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahmy S. A.; Ponte F.; Abd El-Rahman M. K.; Russo N.; Sicilia E.; Shoeib T. Investigation of the host-guest complexation between 4-sulfocalix[4]arene and nedaplatin for potential use in drug delivery. Spectrochim. Acta, Part A 2018, 193, 528–536. 10.1016/j.saa.2017.12.070. [DOI] [PubMed] [Google Scholar]
- Fahmy S. A.; Ponte F.; Sicilia E.; El-Said Azzazy H. M. Experimental and Computational Investigations of Carboplatin Supramolecular Complexes. ACS Omega 2020, 5, 31456–31466. 10.1021/acsomega.0c05168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahmy S. A.; Ponte F.; Fawzy I. M.; Sicilia E.; Bakowsky U.; Azzazy H.-S. Host-Guest Complexation of Oxaliplatin and Para-Sulfonatocalix[n]Arenes for Potential Use in Cancer Therapy. Molecules 2020, 25, 5926. 10.3390/molecules25245926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahmy S. A.; Issa M. Y.; Saleh B. M.; Meselhy M. R.; Azzazy H. M. E.-S. Peganum Harmala Alkaloids Self-Assembled Supramolecular Nanocapsules with Enhanced Antioxidant and Cytotoxic Activities. ACS Omega 2021, 6 (18), 11954–11963. 10.1021/acsomega.1c00455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua Y.; Chen L.; Hou C.; Liu S.; Pei Z.; Lu Y. Supramolecular Vesicles Based on Amphiphilic Pillar[n]arenes for Smart Nano-Drug Delivery. Int. J. Nanomed. 2020, 10 (15), 5873–5899. 10.2147/IJN.S255637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogoshi T.; Kanai S.; Fujinami S.; Yamagishi T. A.; Nakamoto Y. Parabridged symmetrical pillar[5]arenes: their lewis acid catalyzed synthesis and host-guest property. J. Am. Chem. Soc. 2008, 130, 5022–5023. 10.1021/ja711260m. [DOI] [PubMed] [Google Scholar]
- Xue M.; Yang Y.; Chi X.; Zhang Z.; Huang F. Pillararenes, A New Class of Macrocycles for Supramolecular Chemistry. Acc. Chem. Res. 2012, 45, 1294–1308. 10.1021/ar2003418. [DOI] [PubMed] [Google Scholar]
- Li C.-P.; Lu Y.-X.; Zi C.-T.; Zhao Y.-T.; Zhao H.; Zhang Y.-P. Cationic Pillar[6]arene Induces Cell Apoptosis by Inhibiting Protein Tyrosine Phosphorylation Via Host-Guest Recognition. Int. J. Mol. Sci. 2020, 21, 4979. 10.3390/ijms21144979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.; Jin M.; Chen Z.; Hu X.; Pu L.; Pei Z.; Pei Y. Tumor microenvironment responsive supramolecular glyco-nanovesicles based on diselenium-bridged pillar[5]arene dimer for targeted chemotherapy. Chem. Commun. 2020, 56, 10642–10645. 10.1039/D0CC04149A. [DOI] [PubMed] [Google Scholar]
- Li B.; Meng Z.; Li Q.; Huang X.; Kang Z.; Dong H.; Chen J.; Sun J.; Dong Y.; Li J.; Jia X.; Sessler J. L.; Meng Q.; Li C. A pH responsive complexation-based drug delivery system for oxaliplatin. Chem. Sci. 2017, 8, 4458–4464. 10.1039/C7SC01438D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.; Zhang Y.; Meng Z.; Guo L.; Yuan X.; Zhang Y.; Chai Y.; Sessler J. L.; Meng Q.; Li C. Supramolecular combination chemotherapy: a pH-responsive co-encapsulation drug delivery system. Chem. Sci. 2020, 11, 6275–6282. 10.1039/D0SC01756F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan S.; Liu Y.; Shi K.; Ma D. Acetal-Functionalized Pillar[5]arene: A pH-Responsive and Versatile Nanomaterial for the Delivery of Chemotherapeutic Agents. ACS Appl. Bio Mater. 2020, 3, 2325–2333. 10.1021/acsabm.0c00086. [DOI] [PubMed] [Google Scholar]
- Liu L.; Zhou Q.; He Q.; Duan W.; Huang Y. A pH-Responsive Supramolecular Drug Delivery System Constructed by Cationic Pillar[5]arene for Enhancing Antitumor Activity. Front. Chem. 2021, 9, 661143. 10.3389/fchem.2021.661143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F.; Xia T.; Xiao P.; Wang Q.; Deng Z.; Zhang W.; Diao G. A supramolecular complex of hydrazide-pillar[5]arene and bisdemethoxycurcumin with potential anti-cancer activity. Bioorg. Chem. 2021, 110, 104764. 10.1016/j.bioorg.2021.104764. [DOI] [PubMed] [Google Scholar]
- Sun G.; He Z.; Hao M.; Zuo M.; Xu Z.; Hu X.; Zhu J.; Wang L. Dual acid-responsive bola-type supramolecular vesicles for efficient intracellular anticancer drug delivery. J. Mater. Chem. B 2019, 7, 3944–3949. 10.1039/C9TB00555B. [DOI] [Google Scholar]
- Wu M. X.; Yan H. J.; Gao J.; Cheng Y.; Yang J.; Wu J. R.; Gong B. J.; Zhang H. Y.; Yang Y. W. Multifunctional Supramolecular Materials Constructed from Polypyrrole@UiO-66 Nanohybrids and Pillararene Nanovalves for Targeted Chemophotothermal Therapy. ACS Appl. Mater. Interfaces 2018, 10, 34655–34663. 10.1021/acsami.8b13758. [DOI] [PubMed] [Google Scholar]
- Li Q. L.; Sun Y.; Ren L.; Wang X.; Wang C.; Li L.; Yang Y. W.; Yu X.; Yu J. Supramolecular Nanosystem Based on Pillararene-Capped CuS Nanoparticles for Targeted Chemo-Photothermal Therapy. ACS Appl. Mater. Interfaces 2018, 10 (35), 29314–29324. 10.1021/acsami.8b09330. [DOI] [PubMed] [Google Scholar]
- Chen X.; Wang Z.; Sun X.; Han y.; Huang Y.; Xi J.; Bian X.; Han J.; Guo R. Photothermal supramolecular vesicles coassembled from pillar[5]arene and aniline tetramer for tumor eradication in NIR-I and NIR-II biowindows. Chem. Eng. J. 2021, 403, 126423. 10.1016/j.cej.2020.126423. [DOI] [Google Scholar]
- Cui Y. H.; Deng R.; Li Z.; Du X.; Jia Q.; Wang X.; Wang C.; Meguellati K.; Yang Y. Pillar[5]arene pseudo[1]rotaxane-based redox responsive supramolecular vesicles for controlled drug release. Mater. Chem. Front. 2019, 3, 1427. 10.1039/C9QM00237E. [DOI] [Google Scholar]
- Wu D.; Li Y.; Shen J.; Tong Z.; Hu Q.; Li L.; Yu G. Supramolecular chemotherapeutic drug constructed from pillararene-based supramolecular amphiphile. Chem. Commun. 2018, 54 (59), 8198–8201. 10.1039/C8CC04334E. [DOI] [PubMed] [Google Scholar]
- Jiang L.; Huang X.; Chen D.; Yan H.; Li X.; Du X. Supramolecular Vesicles Coassembled from Disulfide-Linked Benzimidazolium Amphiphiles and Carboxylate-Substituted Pillar-[6]arenes that Are Responsive to Five Stimuli. Angew. Chem., Int. Ed. 2017, 56, 2655. 10.1002/anie.201611973. [DOI] [PubMed] [Google Scholar]
- Cheng Q.; Teng K. X.; Ding Y. F.; Yue L.; Yang Q. Z.; Wang R. Dual stimuli-responsive bispillar[5]arene-based nanoparticles for precisely selective drug delivery in cancer cells. Chem. Commun. 2019, 55, 2340–2343. 10.1039/C8CC09432B. [DOI] [PubMed] [Google Scholar]
- Hu C.; Yu Y.; Chao S.; Zhu H.; Pei Y.; Chen L.; Pei Z. A Supramolecular Photosensitizer System Based on Nano-Cu/ZIF-8 Capped with Water-Soluble Pillar[6]arene and Methylene Blue Host-Guest Complexations. Molecules 2021, 26, 3878. 10.3390/molecules26133878. [DOI] [PMC free article] [PubMed] [Google Scholar]