Abstract
A national surveillance conducted in Colombia between 1994 and 1996 identified serotype 5 Streptococcus pneumoniae as the second most frequent cause of invasive disease in children younger than 5 years of age. All 43 serotype 5 isolates collected during this period were shown to be susceptible to penicillin, erythromycin, cefotaxime, and vancomycin, but most (38 of 43, or 88%) were highly resistant to chloramphenicol. In order to clarify a possible genetic relatedness among these isolates, additional microbiological and molecular characterizations were performed. Most (40 of 43, or 93%) of the isolates were found to be resistant to tetracycline. Pulsed-field gel electrophoresis (PFGE) patterns of chromosomal DNAs revealed that all the 43 isolates were closely related and that 38 of the 43 isolates were representatives of a “Colombian clone” of S. pneumoniae isolates which were recovered throughout the 3-year surveillance period from patients in 13 hospitals located in five Colombian cities. Isolates belonging to this Colombian clone were resistant to chloramphenicol and tetracycline, hybridized with the cat and tetM DNA probes in the same 340-kb SmaI fragment, and had identical PFGE patterns after both SmaI and ApaI digestions.
Streptococcus pneumoniae is the leading bacterial cause of childhood pneumonia in the developing world (10). It is estimated that more than 1 million children die each year from pneumococcal pneumonia; approximately one-half of them are less than 1 year old (15). The high incidence of pneumococcal infections and the increasing emergence of drug-resistant isolates are the major reasons for the establishment of surveillance programs.
Since 1994, an epidemiological surveillance study has been conducted in six Latin American countries in order to identify S. pneumoniae causing invasive disease in children less than 5 years old (10, 21). The study is part of an initiative (Regional System of Vaccines) by the Pan American Health Organization (PAHO). The study has already provided important information on the distribution of invasive pneumococcal serotypes among children less than 5 years old, on antibiotic susceptibility patterns (21), and on the genetic relationships among the isolates with diminished susceptibility to penicillin (36). In particular, the work conducted with 324 Colombian pneumococcal isolates showed that the most invasive serotypes in children were, in decreasing order of frequency, 14, 5, 23F, 1, and 6B (5). Diminished susceptibility to penicillin was found in 12% of the isolates and was associated with serotypes 23F (53.8%), 14 (25.6%), 6B (7.7%), 9V (5.1%), 19F (5.1%), and 34 (2.6%) (5). Pulsed-field gel electrophoresis (PFGE) analysis of these isolates identified multidrug-resistant epidemic international clones associated with serotypes 23F and 14, among others (6). Interestingly, all 43 of the serotype 5 isolates collected during this period and representing the second most prevalent group of pneumococci causing invasive disease in Colombian children were susceptible to penicillin.
The aim of the follow-up study described here was to characterize the Colombian serotype 5 isolates for epidemiological and genetic relatedness. The study was carried out at the Instituto de Tecnologia Química e Biológica as part of a collaborative project between the Instituto Nacional de Salud in Colombia and the Center for Molecular Epidemiology in Portugal (35).
Bacterial isolates.
Forty-three Colombian S. pneumoniae serotype 5 invasive isolates were recovered between March 1994 and December 1996 from children less than 5 years old. The isolates were selected on the basis of the uniform criteria established in the PAHO epidemiological surveillance study conducted in six Latin American countries, including Colombia (5). Most of the S. pneumoniae isolates were collected in hospitals located in the three most developed cities in the country, based on the infrastructure of their health services: 14 isolates were recovered from 13 hospitals located in Bogotá, 10 isolates were from 3 hospitals in Medellín, and 15 isolates were from 3 hospitals in Cali. Two provincial towns, Manizales and Pereira, yielded two isolates each. The pneumococcal isolates were recovered from 40 patients, 33 with pneumonia and 7 with meningitis. All isolates were from sterile sites: blood (27 isolates), pleural fluid (9 isolates), and cerebrospinal fluid (7 isolates). From three patients diagnosed with pneumonia, S. pneumoniae was isolated from both blood and pleural fluid.
Eight other serotype 5 isolates were also included in the study for comparison: three isolates from Argentina and four isolates from Brazil were resistant only to trimethoprim-sulfamethoxazole (TMP-SMZ); the single isolate from the United States was susceptible to all antibacterial agents tested.
Antimicrobial susceptibility testing.
MICs of penicillin G, ceftriaxone, and TMP-SMZ were determined by the broth microdilution method with cation-adjusted Mueller-Hinton broth (BBL Microbiology Systems, Cockeysville, Md.) containing 3% lysed horse blood (26). Mueller-Hinton agar (Difco Laboratories, Detroit, Mich.) containing 5% sheep blood was used to determine MICs of chloramphenicol and erythromycin by the E test (20) and to determine sensitivity to tetracycline and vancomycin by the Kirby-Bauer disk diffusion method (26). MICs and inhibition zones were interpreted according to National Committee for Clinical Laboratory Standards guidelines (26). S. pneumoniae ATCC 49619 was used as the control strain.
PFGE.
Chromosomal DNA was prepared according to a published procedure (33). Chromosomal DNA was digested with 20 U of SmaI or 20 U of ApaI (New England Biolabs, Beverly, Mass.) and PFGE was performed with a CHEF DR-II apparatus (Bio-Rad, Birmingham, United Kingdom) for 23 h. The assay parameters were as follows: initial pulse, 5 s; final pulse, 35 s; voltage, 6 V/cm; and temperature, 13°C. Standard methodologies were used for staining and photographing the gels (30). Strain R6 of S. pneumoniae and a PFGE lambda marker (New England Biolabs) were used as molecular weight standards. The macrorestriction profiles were analyzed by visual inspection of the patterns by use of the criteria of Tenover et al. (34): isolates showing six or fewer fragment differences were considered subtypes of a major pattern.
Southern hybridization with chloramphenicol and tetracycline probes.
Gels were transferred to Hybond N+ membranes (Amersham International, Little Chalfont, United Kingdom) by use of a vacuum blotter (VaguGene XL; Pharmacia, Uppsala, Sweden) as previously described (8). A 338-bp conserved region internal to the cat genes belonging to both catpC194 and catpC221 classes and a 1,080-bp region internal to the tetM gene were prepared as previously described (24, 25, 27). For probe labeling and hybridization, an enhanced chemiluminescence nonradioactive labeling kit (RPN3001; Amersham, Birmingham, United Kingdom) was used according to the manufacturer’s instructions.
Table 1 summarizes the relevant characteristics of the Colombian isolates, including isolation date, geographic origin, antibiotic susceptibility patterns, PFGE patterns, and results of hybridization of SmaI-digested DNA from PFGE gels with DNA probes. An earlier study (5) had already established that of the 43 serotype 5 invasive pneumococcal isolates, all were susceptible to penicillin, erythromycin, cefotaxime, and vancomycin; the majority (88%) showed resistance to chloramphenicol. Chloramphenicol is one of the antimicrobial agents of choice for the treatment of invasive diseases in children in Colombia (5). Additional susceptibility testing demonstrated that most of the 43 isolates, including the 38 chloramphenicol-resistant isolates, were also resistant to tetracycline, and a variable proportion were resistant to TMP-SMZ. Three of the serotype 5 isolates (CLB 32, CLB 33, and CLB 35) were susceptible to all antimicrobial agents tested, and two isolates (CLB 34 and CLB 43) were resistant only to tetracycline and not to chloramphenicol. Differences in resistance to TMP-SMZ among the serotype 5 isolates listed in Table 1 may reflect differences in local antibiotic use. A single Ile 100-to-Leu mutation in dihydrofolate reductase is known to be sufficient for trimethoprim resistance in S. pneumoniae (1), and evidence for the rapid emergence of resistance upon the introduction of TMP-SMZ into clinical use has been described (17).
TABLE 1.
Properties of serotype 5 invasive isolates of S. pneumoniae from Colombia
| Straina | Isolation date (mo/day/yr) | Hospital code | City or town | Susceptibility tob:
|
SmaI PFGE pattern | Fragment (kb) hybridizing to the following probec:
|
ApaI PFGE pattern | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PEN | CHL | TET | ERY | TMP-SMZ | CTX | VAN | cat | tetM | ||||||
| CLB 1 | 03/25/94 | 1 | Bogotá | S | R | R | S | I | S | S | X1 | 340 | 340 | Y1 |
| CLB 31 | 01/24/96 | 2 | Bogotá | S | R | R | S | I | S | S | X1 | 340 | 340 | Y1 |
| CLB 43 | 12/03/96 | 2 | Bogotá | S | S | R | S | I | S | S | X4 | NH | 340 | Y4 |
| CLB 9 | 10/23/94 | 3 | Bogotá | S | R | R | S | R | S | S | X1 | 340 | 340 | Y1 |
| CLB 16 | 04/05/94 | 3 | Bogotá | S | R | R | S | I | S | S | X1 | 340 | 340 | Y1 |
| CLB 17 | 04/25/95 | 3 | Bogotá | S | R | R | S | S | S | S | X1 | 340 | 340 | Y1 |
| CLB 2 | 04/30/94 | 4 | Bogotá | S | R | R | S | I | S | S | X1 | 340 | 340 | Y1 |
| CLB 11 | 12/01/94 | 4 | Bogotá | S | R | R | S | S | S | S | X1 | 340 | 340 | Y1 |
| CLB 26 | 08/28/95 | 4 | Bogotá | S | R | R | S | S | S | S | X1 | 340 | 340 | Y1 |
| CLB 4 | 07/02/94 | 5 | Bogotá | S | R | R | S | R | S | S | X1 | 340 | 340 | Y1 |
| CLB 5 | 07/02/94 | 5 | Bogotá | S | R | R | S | R | S | S | X1 | 340 | 340 | Y1 |
| CLB 20 | 06/03/94 | 5 | Bogotá | S | R | R | S | R | S | S | X1 | 340 | 340 | Y1 |
| CLB 15 | 03/23/95 | 5 | Bogotá | S | R | R | S | I | S | S | X1 | 340 | 340 | Y1 |
| CLB 40 | 05/16/96 | 5 | Bogotá | S | R | R | S | R | S | S | X1 | 340 | 340 | Y1 |
| CLB 10 | 11/10/94 | 6 | Medellín | S | R | R | S | S | S | S | X1 | 340 | 340 | Y1 |
| CLB 34 | 06/22/95 | 6 | Medellín | S | S | R | S | S | S | S | X1 | NH | 340 | Y1 |
| CLB 22 | 06/08/94 | 7 | Medellín | S | R | R | S | S | S | S | X1 | 340 | 340 | Y1 |
| CLB 25 | 08/10/94 | 7 | Medellín | S | R | R | S | S | S | S | X1 | 340 | 340 | Y1 |
| CLB 28 | 11/18/95 | 7 | Medellín | S | R | R | S | S | S | S | X1 | 340 | 340 | Y1 |
| CLB 12 | 12/15/94 | 7 | Medellín | S | R | R | S | S | S | S | X1 | 340 | 340 | Y1 |
| CLB 14 | 02/23/95 | 7 | Medellín | S | R | R | S | I | S | S | X1 | 340 | 340 | Y1 |
| CLB 13 | 12/05/94 | 8 | Medellín | S | R | R | S | I | S | S | X1 | 340 | 340 | Y1 |
| CLB 33 | 12/18/94 | 8 | Medellín | S | S | S | S | I | S | S | X3 | NH | NH | Y3 |
| CLB 27 | 11/29/95 | 8 | Medellín | S | R | R | S | S | S | S | X1 | 340 | 340 | Y1 |
| CLB 7 | 08/24/94 | 9 | Cali | S | R | R | S | I | S | S | X1 | 340 | 340 | Y1 |
| CLB 8 | 08/24/94 | 9 | Cali | S | R | R | S | I | S | S | X1 | 340 | 340 | Y1 |
| CLB 19 | 05/13/95 | 9 | Cali | S | R | R | S | S | S | S | X1 | 340 | 340 | Y1 |
| CLB 21 | 06/06/95 | 9 | Cali | S | R | R | S | I | S | S | X1 | 340 | 340 | Y1 |
| CLB 24 | 08/08/95 | 9 | Cali | S | R | R | S | I | S | S | X1 | 340 | 340 | Y1 |
| CLB 35 | 05/07/95 | 9 | Cali | S | S | S | S | S | S | S | X2 | NH | NH | Y2 |
| CLB 29 | 12/19/95 | 9 | Cali | S | R | R | S | S | S | S | X1 | 340 | 340 | Y1 |
| CLB 30 | 12/24/95 | 9 | Cali | S | R | R | S | S | S | S | X1 | 340 | 340 | Y1 |
| CLB 37 | 04/16/96 | 9 | Cali | S | R | R | S | I | S | S | X1 | 340 | 340 | Y1 |
| CLB 38 | 04/16/96 | 9 | Cali | S | R | R | S | I | S | S | X1 | 340 | 340 | Y1 |
| CLB 39 | 07/05/96 | 9 | Cali | S | R | R | S | I | S | S | X1 | 340 | 340 | Y1 |
| CLB 3 | 06/20/94 | 10 | Cali | S | R | R | S | S | S | S | X1 | 340 | 340 | Y1 |
| CLB 6 | 08/16/94 | 10 | Cali | S | R | R | S | S | S | S | X1 | 340 | 340 | Y1 |
| CLB 32 | 09/22/94 | 10 | Cali | S | S | S | S | S | S | S | X2 | NH | NH | Y2 |
| CLB 23 | 06/16/95 | 11 | Cali | S | R | R | S | S | S | S | X1 | 340 | 340 | Y1 |
| CLB 18 | 04/27/95 | 12 | Manizales | S | R | R | S | I | S | S | X1 | 340 | 340 | Y1 |
| CLB 44 | 11/20/96 | 12 | Manizales | S | R | R | S | I | S | S | X1 | 340 | 340 | Y1 |
| CLB 41 | 06/27/96 | 13 | Pereira | S | R | R | S | I | S | S | X1 | 340 | 340 | Y1 |
| CLB 42 | 11/14/96 | 13 | Pereira | S | R | R | S | I | S | S | X1 | 340 | 340 | Y1 |
CLB 4 and CLB 5, CLB 7 and CLB 8, and CLB 37 and CLB 38 are duplicate isolates recovered from blood and pleural fluid from the same patient.
PEN, penicillin; CHL, chloramphenicol; TET, tetracycline; ERY, erythromycin; CTX, cefotaxime; VAN, vancomycin. S, susceptible; R, resistant; I, intermediate. Except for tetracycline resistance, all susceptibility data were obtained from the laboratory records of the Instituto Nacional de Salud in Colombia (3).
After SmaI restriction. NH, no hybridization.
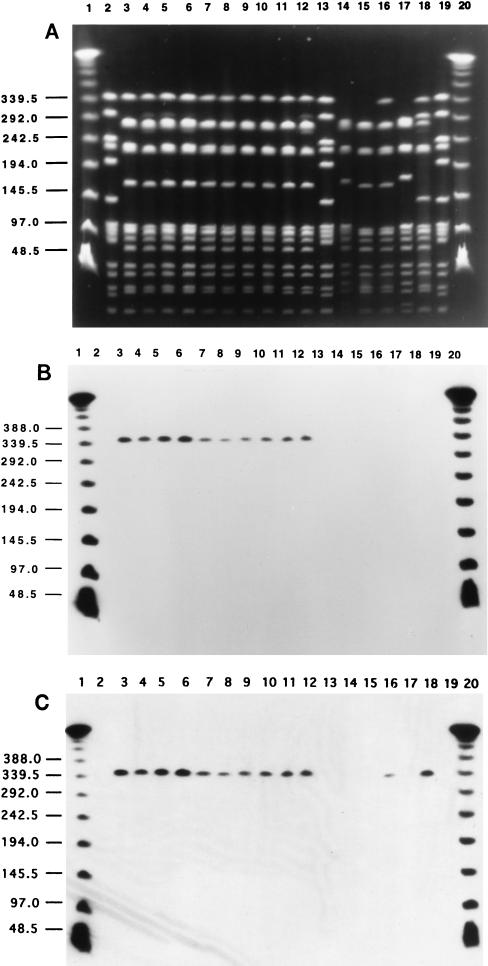
All 38 serotype 5 strains showing resistance to both tetracycline and chloramphenicol represented a “Colombian clone”: a homogeneous group of S. pneumoniae isolates which were susceptible to penicillin and erythromycin, had a common PFGE pattern (X1) after SmaI restriction (Fig. 1A), and hybridized with the tetM and cat DNA probes in a common 340-kb DNA fragment (Fig. 1B and C). A conjugative transposon, Tn5253, containing the cat and tetM genes has been described for S. pneumoniae and may explain the colocalization of the two genes in the same SmaI fragment (2). The same PFGE pattern (X1) was also identified in isolate CLB 34, which was resistant only to tetracycline and not to chloramphenicol (lane 16 in Fig. 1A). The 38 tetracycline- and chloramphenicol-resistant isolates and isolate CLB 34 also had a common PFGE pattern after restriction of their chromosomal DNAs with the endonuclease ApaI (data not shown). Table 1 shows that the serotype 5 isolates classified as the Colombian clone were widely dispersed in Colombia: they were recovered in 13 hospitals located in five cities and were prevalent throughout the surveillance period from early 1994 until late 1996.
FIG. 1.
PFGE illustrative of the clonal dissemination of the serotype 5 S. pneumoniae invasive isolates in Colombia. The restriction enzyme used was SmaI. (A) PFGE subtypes X1 to X4. Lanes 1 and 20 contain a lambda ladder; lanes 2, 13, and 19 contain reference strain R6 used as a molecular weight marker. Numbers at left show molecular sizes in kilobases. Lane 3, CLB 12 (X1); lane 4, CLB 30 (X1); lane 5, CLB 29 (X1); lane 6, CLB 2 (X1); lane 7, CLB 4 (X1); lane 8, CLB 5 (X1); lane 9, CLB 37 (X1); lane 10, CLB 38 (X1); lane 11, CLB 7 (X1); lane 12, CLB 8 (X1); lane 14, CLB 32 (X2); lane 15, CLB 33 (X3); lane 16, CLB 34 (X1); lane 17, CLB 35 (X2); lane 18, CLB 43 (X4). (B) Hybridization of the same gel with the cat probe. Chloramphenicol-resistant isolates are in lanes 3 to 12; chloramphenicol-susceptible isolates are in lanes 14 to 18. (C) Hybridization of the same gel with the tetM probe. Tetracycline-resistant isolates are in lanes 3 to 12, 16, and 18; tetracycline-susceptible isolates are in lanes 14, 15, and 17.
Because of the genetic homogeneity of the 38 isolates representing the Colombian clone, a group of additional serotype 5 S. pneumoniae isolates with different antibiotic susceptibility profiles and different geographic origins were also examined by PFGE after digestion of the chromosomal DNAs with SmaI and ApaI. This group of serotype 5 pneumococci included four Colombian isolates (CLB 32, CLB 33, CLB 35, and CLB 43), which are listed in Table 1, four isolates from Brazil, three isolates from Argentina, and one isolate from the United States. Three of the four Colombian isolates (CLB 32, CLB 33, and CLB 35) were susceptible to both chloramphenicol and tetracycline, while isolate CLB 43 was resistant to tetracycline only (Table 1). The four Brazilian and three Argentinian isolates were susceptible to both chloramphenicol and tetracycline but were resistant to TMP-SMZ; the U.S. isolate was susceptible to all antimicrobial agents tested.
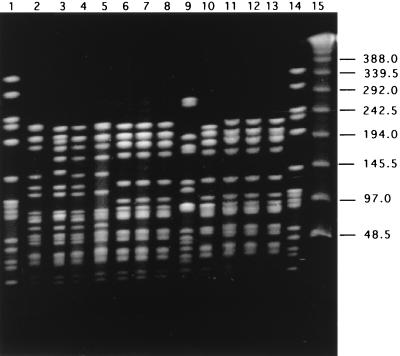
A comparison of the PFGE patterns of these additional serotype 5 isolates to the uniform PFGE pattern characteristic of the Colombian clone is shown for SmaI digestion in Fig. 1 (for Colombian isolates only) and for ApaI digestion in Fig. 2 (for serotype 5 isolates from several countries). After ApaI digestion, all 38 isolates of the Colombian clone had a common PFGE pattern (Y1), which is illustrated by a single representative isolate in lane 2 of Fig. 2. CLB 35 (and CLB 32; data not shown) in lane 3 of Fig. 2 (PFGE pattern Y2), CLB 33 in lane 4 (PFGE pattern Y3), and CLB 43 in lane 5 (PFGE pattern Y4) differed from the Colombian clone in three, one, and two DNA bands, respectively. The three Argentinian isolates (lanes 6, 7, and 8 of Fig. 2) and three of the four Brazilian isolates (lanes 11, 12, and 13) had an identical and unique PFGE pattern which differed in five bands from that of the Colombian clone. The last of the four Brazilian serotype 5 isolates (ST 204 in lane 10 of Fig. 2) differed from the Argentinian-Brazilian group in one band only (and differed from the Colombian clone in six bands). The single type 5 isolate from the United States had a PFGE pattern unrelated to those of any of the other serotype 5 isolates (the number of DNA band differences was greater than 12).
FIG. 2.
PFGE after ApaI restriction of serotype 5 S. pneumoniae invasive isolates from Colombia (CLB), Argentina (ARG), the United States (PP), and Brazil (ST). Lane 2, CLB 34; lane 3, CLB 35; lane 4, CLB 33; lane 5, CLB 34; lane 6, ARG 30; lane 7, ARG 314; lane 8, ARG 364; lane 9, PP 20; lane 10, ST 204; lane 11, ST 206; lane 12, ST 207; lane 13, ST 331. Lane 15 contains a lambda ladder; lanes 1 and 14 contain reference strain R6 used as a molecular weight marker. Numbers at right show molecular sizes in kilobases.
Serotype 5 S. pneumoniae was common in the United States and Europe in the 1930s (16), but since then, its frequency of isolation has declined, leading to its virtual disappearance (14). In Spain, serotype 5 pneumococci ranked 6th in order of frequency between 1979 and 1989 but only 13th in 1990 to 1996 (12, 13). Antibiotic resistance in isolates of this serotype was seen most often for tetracycline, sometimes associated with resistance to chloramphenicol or general susceptibility to all antimicrobial agents. Penicillin resistance has been rare among serotype 5 isolates (for a review, see references 13 and 22).
In contrast to the findings in Europe, the United States, and Canada, serotype 5 (and serotype 1) remained the most frequent pneumococcal serotype in Africa (3, 7, 9) and India (19) in the 1980s and 1990s, and the continued importance of serotypes 5 and 1 in other developing countries, including countries in South America, was also documented in recent studies (31, 32). In particular, serotype 5 S. pneumoniae isolates were found to be the second most frequent causative agents of invasive disease in children in Argentina (28, 29), Chile (23), and Uruguay (18) and were ranked third in Brazil (4) and ninth in Mexico (11). These observations led to the suggestion that serotype 5 should be included in a future protein conjugate vaccine (21, 32).
In conclusion, the observations described demonstrate that the great majority of serotype 5 pneumococcal isolates currently in circulation in Colombia represent a unique Colombian clone, with most isolates expressing resistance to tetracycline and chloramphenicol but remaining susceptible to penicillin. Since these isolates were all from sterile sites and also included closely related serotype 5 bacteria susceptible to all antimicrobial agents tested, this S. pneumoniae lineage appears to carry all virulence factors necessary to invade the human host. It is important to determine the degree of dispersion of this clone throughout Latin America and in other countries where this serotype is frequently isolated.
Acknowledgments
Support for this work was provided by the CEM/NET initiative (CEM/NET Project 31 from IBET, contract PRAXIS XXI-2/2.1/BIO/1154/95, and contract PECS/C/SAU/145/95 from JNICT) and by a grant from Fundação Calouste Gulbenkian awarded to H. de Lencastre. R. Sá-Leão was supported by grant BD/4259/96 from PRAXIS XXI from Fundação para a Ciência e Tecnologia. The work of M. Tamayo in Portugal was supported by Fundação Calouste Gulbenkian, Lisbon, Portugal; Support for M. Tamayo in Colombia came from PAHO, the Canadian Agency for International Development, and the Instituto Nacional de Salud, Santa Fe de Bogotá, Colombia.
We acknowledge Clara Inês Agudelo and María Victoria Ovalle from the Instituto Nacional de Salud and Alejandra Corso from the Instituto Nacional de Enfermedades Infecciosas, Buenos Aires, Argentina, for stimulating discussions. We express our gratitude to Alexander Tomasz of The Rockefeller University, New York, N.Y., for help in the interpretation of data and writing of the manuscript. Idalina Bonfim from Instituto de Tecnologia Química e Biológica, Oeiras, Portugal, provided technical assistance in some of the antibiotic susceptibility testing.
REFERENCES
- 1.Adrian P V, Klugman K P. Mutations in the dihydrofolate reductase gene of trimethoprim-resistant isolates of Streptococcus pneumoniae. Antimicrob Agents Chemother. 1997;41:2406–2413. doi: 10.1128/aac.41.11.2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ayoubi P, Kilic A O, Vijayakumar M N. Tn5253, the pneumococcal omega (cat tet) BM6001 element, is a composite structure of two conjugative transposons, Tn5251 and Tn5252. J Bacteriol. 1991;173:1617–1622. doi: 10.1128/jb.173.5.1617-1622.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bogaerts J, Lepage P, Taelman H, Rouvroy D, Batungwanayo J, Kestelyn P, Hitimana D G, Van de Perre P, Vandepitte J, Verbist L, Verhaegen J. Antimicrobial susceptibility and serotype distribution of Streptococcus pneumoniae from Rwanda, 1984–1990. J Infect. 1993;27:157–168. doi: 10.1016/0163-4453(93)94728-t. [DOI] [PubMed] [Google Scholar]
- 4.Brandileone M C, Vieira V S, Casagrande S T, Zanella R C, Guerra M L, Bokermann S, de Moraes J C, Baldacci E R, Chamone C B, Oliveira M A, de Matos D G, Arruda T M, Coelho M F, D’Avila S M, dos Santos A R, di Fabio J L the Pneumococcal Study Group in Brazil for the SIREVA Project. Prevalence of serotypes and antimicrobial resistance of Streptococcus pneumoniae strains isolated from Brazilian children with invasive infections. Microb Drug Resist. 1997;3:141–146. doi: 10.1089/mdr.1997.3.141. [DOI] [PubMed] [Google Scholar]
- 5.Castañeda E, Leal A L, Castillo O, de la Hoz F, Vela M C, Arango M, Trujillo H, Levy A, Gama M E, Calle M, Valencia M L, Parra W, Agudelo N, Mejía G I, Jaramillo S, Montoya F, Porras H, Sánchez A, Saa D, di Fabio J L, Homma A the Pneumococcal Study Group in Colombia. Distribution of capsular types and antimicrobial susceptibility of invasive isolates of Streptococcus pneumoniae in Colombian children. Microb Drug Resist. 1997;3:147–152. doi: 10.1089/mdr.1997.3.147. [DOI] [PubMed] [Google Scholar]
- 6.Castañeda E, Penuela I, Vela M C, Tomasz A the Colombian Pneumococcal Study Group. Penicillin-resistant Streptococcus pneumoniae in Colombia: presence of international epidemic clones. Microb Drug Resist. 1998;4:233–239. doi: 10.1089/mdr.1998.4.233. [DOI] [PubMed] [Google Scholar]
- 7.Dagan R, Engelhard D, Piccard E the Israeli Pediatric Bacteremia and Meningitis Group. Epidemiology of invasive childhood pneumococcal infections in Israel. JAMA. 1992;268:3328–3332. [PubMed] [Google Scholar]
- 8.De Lencastre H, Couto I, Santos I, Melo-Cristino J, Torres-Pereira A, Tomasz A. Methicillin-resistant Staphylococcus aureus disease in a Portuguese hospital: characterization of clonal types by a combination of DNA typing methods. Eur J Clin Microbiol Infect Dis. 1994;13:64–73. doi: 10.1007/BF02026129. [DOI] [PubMed] [Google Scholar]
- 9.Denis F A, Greewood B D, Rey J I, Prince-David M, Mboup S, Lloyd-Evans N, Williams K, Benbachir I, El Ndaghri N, Hansman D, Omanga V, Krubwa K, Duchassin M, Perrin J. Etude multicentrique des sérotypes de pneumocoques en Afrique. Bull Org Mondiale de la Santé. 1983;61:517–524. [PMC free article] [PubMed] [Google Scholar]
- 10.Di Fabio J L, Homma A, de Quadros C. Pan American Health Organization Epidemiological Surveillance Network for Streptococcus pneumoniae. Microb Drug Resist. 1997;3:131–133. doi: 10.1089/mdr.1997.3.131. [DOI] [PubMed] [Google Scholar]
- 11.Echaniz-Aviles G, Velazquez-Meza M E, Carnalla-Barajas M N, Soto-Noguerón A, Solórzano-Santos F, Pérez Miravete A, Gatica-Marquina R, di Fabio J L. Antimicrobial susceptibilities and capsular types of invasive Streptococcus pneumoniae isolated in children in Mexico City. Microb Drug Resist. 1997;3:153–157. doi: 10.1089/mdr.1997.3.153. [DOI] [PubMed] [Google Scholar]
- 12.Fenoll A, Bourgon M, Muñoz R, Vicioso D, Casal J. Serotype distribution and antimicrobial resistance of Streptococcus pneumoniae isolates causing systemic infections in Spain, 1979–1989. Rev Infect Dis. 1991;13:56–60. doi: 10.1093/clinids/13.1.56. [DOI] [PubMed] [Google Scholar]
- 13.Fenoll A, Jado I, Vicioso D, Perez A, Casal J. Evolution of Streptococcus pneumoniae serotypes and antibiotic resistance in Spain: update (1990 to 1996) J Clin Microbiol. 1998;36:3447–3454. doi: 10.1128/jcm.36.12.3447-3454.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finland M, Barnes M W. Changes in occurrence of capsular serotypes of Streptococcus pneumoniae at Boston City Hospital during selected years between 1935 and 1974. J Clin Microbiol. 1977;5:154–166. doi: 10.1128/jcm.5.2.154-166.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garenne M M, Ronsmaus C, Campbell H. The magnitude of mortality from acute respiratory infections in children under 5 years in developing countries. World Health Stat Q. 1992;45:180–191. [PubMed] [Google Scholar]
- 16.Heffron R. Pneumonia with special reference to pneumococcus lobar pneumonia. 1st ed. Oxford, United Kingdom: The Commonwealth Fund, Oxford University Press; 1939. [Google Scholar]
- 17.Henderson F W, Gilligan P H, Wait K, Goff D A. Nasopharyngeal carriage of antibiotic-resistant pneumococci by children in group day care. J Infect Dis. 1988;157:256–263. doi: 10.1093/infdis/157.2.256. [DOI] [PubMed] [Google Scholar]
- 18.Hortal M, Algorta G, Bianchi I, Borthagaray G, Cestau I, Camou T, Castro M, de los Santos M, Diez R, Dell’Acqua L, Galiana A, Giordano A, Giordano P, Lopez-Ghemi G, Milanese N, Mogdasy C, Palacio R, Pedreira W, Pisano A, Pivel L. Capsular type distribution and susceptibility to antibiotics of Streptococcus pneumoniae clinical strains isolated from Uruguayan children with systemic infections. Microb Drug Resist. 1997;3:159–163. doi: 10.1089/mdr.1997.3.159. [DOI] [PubMed] [Google Scholar]
- 19.John T J, Pai R, Lalitha M K, Jesudason M V, Brahmadathan K N, Sridharan G, Steinhoff M C. Prevalence of pneumococcal serotypes in invasive diseases in southern India. Indian J Med Res. 1996;104:205–207. [PubMed] [Google Scholar]
- 20.Jorgensen J H, Howell A W, Maher L A. Antimicrobial susceptibility testing of Haemophilus influenzae and Streptococcus pneumoniae by using the E test. J Clin Microbiol. 1991;29:109–114. doi: 10.1128/jcm.29.1.109-114.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kertesz D A, di Fabio J L, Brandileone M C C, Castañeda E, Echániz-Aviles G, Heitmann I, Homma A, Hortal M, Lovgren M, Ruvinsky R O, Talbot J A, Weekes J, Spika J S the PAHO Pneumococcal Surveillance Study Group. Invasive Streptococcus pneumoniae infection in Latin American children: results of the Pan American Health Organization Surveillance study. Clin Infect Dis. 1998;26:1355–1361. doi: 10.1086/516350. [DOI] [PubMed] [Google Scholar]
- 22.Klugman K P. Pneumococcal resistance to antibiotics. Clin Microbiol Rev. 1990;3:171–196. doi: 10.1128/cmr.3.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levine M M, Lagos R, Levine O S, Heitmann I, Enriquez N, Pinto M E, Alvarez A M, Wu E, Mayorga C, Reyes A. Epidemiology of invasive pneumococcal infections in infants and young children in metropolitan Santiago, Chile, a newly industrializing country. Pediatr Infect Dis J. 1998;17:287–293. doi: 10.1097/00006454-199804000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Marchese A, Ramirez M, Schito G C, Tomasz A. Molecular epidemiology of penicillin-resistant Streptococcus pneumoniae isolates recovered in Italy from 1993 to 1996. J Clin Microbiol. 1998;36:2944–2949. doi: 10.1128/jcm.36.10.2944-2949.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McDougal L K, Facklam R, Reeves M, Hunter S, Swenson J M, Hill B H, Tenover F C. Analysis of multiply antimicrobial-resistant isolates of Streptococcus pneumoniae from the United States. Antimicrob Agents Chemother. 1992;36:2176–2184. doi: 10.1128/aac.36.10.2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial susceptibility testing; 8th informational supplement. NCCLS publication M100-S58. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1998. [Google Scholar]
- 27.Nesin M, Ramirez M, Tomasz A. Capsular transformation of a multidrug-resistant Streptococcus pneumoniae in vivo. J Infect Dis. 1998;177:707–713. doi: 10.1086/514242. [DOI] [PubMed] [Google Scholar]
- 28.Rossi A, Ruvinsky R, Regueira M, Corso A, Pace J, Gentile A, di Fabio J L the Streptococcus pneumoniae Working Group. Distribution of capsular types and penicillin resistance of strains of Streptococcus pneumoniae causing systemic infections in Argentinean children under 5 years of age. Microb Drug Resist. 1997;3:135–140. doi: 10.1089/mdr.1997.3.135. [DOI] [PubMed] [Google Scholar]
- 29.Rossi A, Corso A, Pace J, Regueira M, Tomasz A. Penicillin-resistant Streptococcus pneumoniae in Argentina: frequent occurrence of an internationally spread serotype 14 clone. Microb Drug Resist. 1998;4:225–231. doi: 10.1089/mdr.1998.4.225. [DOI] [PubMed] [Google Scholar]
- 30.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N. Y.: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 31.Scott J A, Hall A J, Dagan R, Dixon J M, Eykyn S J, Fenoll A, Hortal M, Jette L P, Jorgensen J H, Lamothe F, Latorre C, Macfarlane J T, Shlaes D M, Smart L E, Taunay A. Serogroup-specific epidemiology of Streptococcus pneumoniae: associations with age, sex, and geography in 7,000 episodes of invasive disease. Clin Infect Dis. 1996;22:973–981. doi: 10.1093/clinids/22.6.973. [DOI] [PubMed] [Google Scholar]
- 32.Sniadack D H, Schwartz B, Lipman H, Bogaerts J, Butler J C, Dagan R, Echaniz-Aviles G, Lloyd-Evans N, Fenoll A, Girgis N I, Henrichsen J, Klugman K, Lehmann D, Takala A K, Vandepitte J, Gove S, Breiman R F. Potential interventions for the prevention of childhood pneumonia: geographic and temporal differences in serotype and serogroup distribution of sterile site pneumococcal isolates from children—implications for vaccine strategies. Pediatr Infect Dis J. 1995;14:503–510. [PubMed] [Google Scholar]
- 33.Soares S, Kristinsson K G, Musser J M, Tomasz A. Evidence for the introduction of a multiresistant clone of serotype 6B Streptococcus pneumoniae from Spain to Iceland in the late 1980s. J Infect Dis. 1993;168:158–163. doi: 10.1093/infdis/168.1.158. [DOI] [PubMed] [Google Scholar]
- 34.Tenover F, Arbeit R D, Goering R V, Mickelsen P A, Murray B E, Persing D H, Swaminathan B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tomasz A, de Lencastre H. Molecular microbiology and epidemiology: coexistence or alliance? In: Wenzel R P, editor. Prevention and control of nosocomial infections. Baltimore, Md: Williams & Wilkins; 1997. pp. 309–321. [Google Scholar]
- 36.Tomasz A, Corso A, Severina E P, Echaniz-Aviles G, Brandileone M C, Camou T, Castaneda E, Figueroa O, Rossi A, di Fabio J L and Members of the PAHO/Rockefeller University Workshop: Molecular epidemiologic characterization of penicillin-resistant Streptococcus pneumoniae invasive pediatric isolates recovered in six Latin-American countries: an overview. Microb Drug Resist. 1998;4:195–207. doi: 10.1089/mdr.1998.4.195. [DOI] [PubMed] [Google Scholar]