Abstract
A 43-year-old male presented to the emergency department with acute left testicular pain. Physical exam showed a tender left testicle and epididymis with mild swelling. Doppler and contrast enhanced ultrasound revealed a heterogeneous, avascular lesion with hyper vascularized surrounding. Follow-up contrast enhanced ultrasound performed a few days later showed persistence of the sparsely vascularized lesion with more hypoechoic echo structure.
Despite the tumor markers being negative, a necrotic tumor could not be ruled out and a left orchiectomy was performed. Pathology report described an extensive segmental testicular infarction with no evidence of malignant tissue.
We present the ultrasound and pathology findings, differential diagnostic pearls and clinical perspective of segmental testicular infarction.
Keywords: Testicular ultrasound, Conservative management, Contrast Enhanced Ultrasound, Acute scrotum, Testicular abscess, Burned-out testicular tumor
Introduction
Segmental testicular infarction (STI) is an uncommon condition that presents with acute or, less frequently, with recurrent scrotal pain. It is more likely to occur in patients between the age of 30 and 40. [1] Physical examination can show swelling and tenderness of the testicle but can also be completely normal. Few cases have been reported in the literature and little is known about the etiopathology of STI. Small case series report that most cases are idiopathic [1] although acute epididymorchitis, bell-clapper deformity, [2] intimal fibroplasia [3] and haematological and systemic disorders such as sickle cell disease, [4] polycythaemia and vasculitis [5,6] have been identified as probable causes. Diagnosis is suspected when a Doppler ultrasound reveals a hypoechoic testicular lesion with absence of inner vascularity. [7] Despite testicular tumour markers being negative, these radiologic findings cannot exclude testicular tumour with intratumoral necrosis as a differential diagnosis. Definite diagnosis can be made by histological examination of the orchiectomy specimen; therefore, some experts recommend surgery. [6] With the increase of clinical and radiological awareness, conservative management is considered more frequently. [1]
Case report
A 43-year-old male with a previous medical history of anxiety disorder and liver steatosis presented to the emergency department with left testicular pain that had started 24 hours beforehand and had intensified prior to consultation. No other symptoms were reported. Physical examination showed a painful and tender left testicle and epididymis with mild swelling; no testicular or epididymal masses were palpated. The blood test showed mild leukocytosis with neutrophilia. A scrotal Doppler ultrasound was requested at the emergency department to rule out testicular torsion or orchiepididymitis.
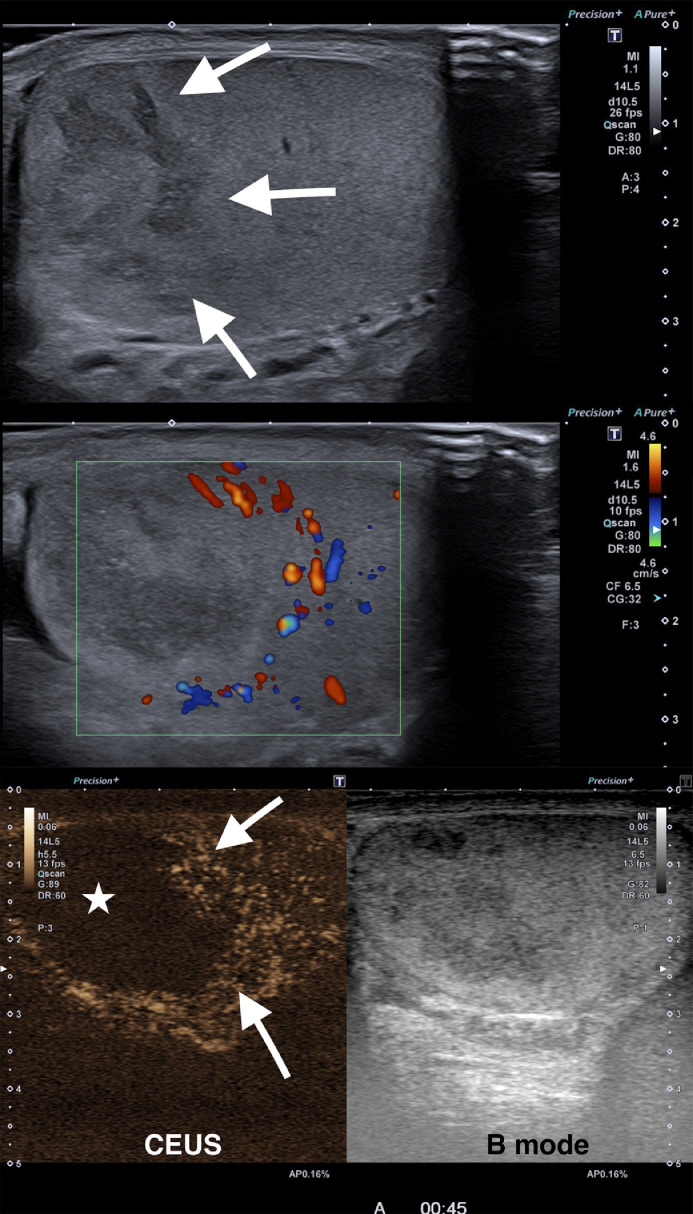
The ultrasound showed a normal right testicle and a mildly hypoechoic, heterogeneous ill-defined lesion in the upper pole of the left testicle. On colour Doppler the lesion showed no inner vascularity, however, a peripheral area with increased Doppler signal could be depicted. The rest of the testicle was slightly hyperaemic on colour Doppler but showed normal echo structure. The epididymis and the rest of the scrotal ultrasound were normal. After intravenous ultrasound contrast material administration, the lesion showed total avascularity without internal viable solid tissue. A peripheral hyper enhancing rim confirmed the presence of hyper vascular adjacent parenchyma (Fig. 1). (see Video, Supplemental Digital Content 1)
Fig. 1.
A. Longitudinal B mode image of the left testicle with high frequency (14 MHz) linear ultrasound array shows a heterogeneous ill-defined mass in the upper pole (arrows). B. Color Doppler shows no inner vascularity and a peripheral hyper vascularized rim. C. Contrast enhanced ultrasound image captured 45 seconds after the administration of 1.2 ml of microbubble contrast agent (SonoVue©, Bracco, Milan, Italy) shows total lack of enhancement of the lesion (asterisk), a hyper enhancing peripheral rim (arrows) and normal parenchymal enhancement of the lower testicular pole
The rest of the left testicle and the epididymis were normal; therefore the diagnosis of an infarction or abscess was uncertain and the possibility of a necrotic testicular tumour could not be ruled out completely. As a consequence, a strict imaging follow-up was recommended. Antibiotic treatment was started and the patient was initially managed conservatively.
Further tests reported negative tumour markers, including Lactate dehydrogenase, Alfa-1 fetoprotein and beta-HCG. A thoracoabdominal CT scan showed neither signs of any underlying systemic disease nor evidence of a disseminated malignancy.
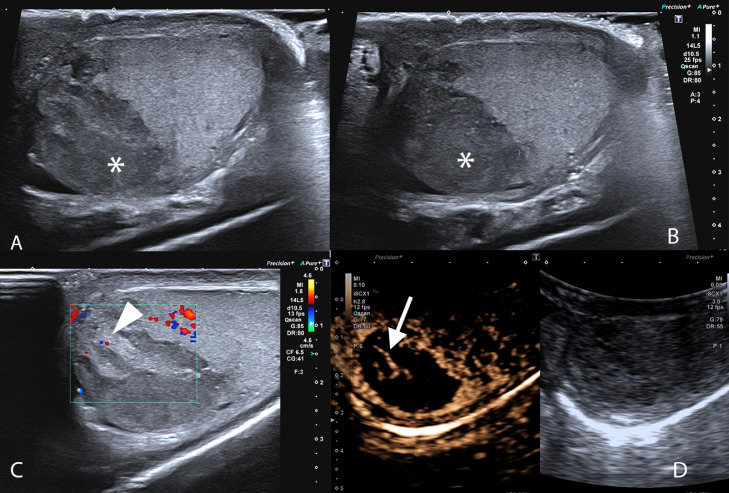
A follow-up ultrasound was performed 5 days later (Fig. 2). The lesion persisted without any reduction in size, but showed more hypoechoic and heterogeneous echo structure and remained mainly without inner Doppler signal. A minute, slightly hyperechoic inner area could be depicted showing Doppler signal. A repeated CEUS showed minimal vascularization of the heterogeneous central areas and an otherwise completely avascular lesion. No inflammatory changes were detected in the rest of the testicle and epididymis.
Fig. 2.
Follow-up ultrasound performed 5 days after the first examination. A-B. B mode image of the left testicle performed with a linear array high frequency transducer. The lesion remained stable in size but showed diffusely hypoechoic (asterisk) with inner isoechoic structures presenting mild color Doppler signal (C - arrowhead). On CEUS (D) (2.4ml SonoVue© performed with a curved array ultrasound transducer (8 MHz)) the lesion remained hypo vascular with central linear enhancing structures (arrow). Peripheral hyper enhancing rim persisted unchanged
As the possibility of the lesion being a testicular tumour with extensive necrosis could not be ruled out, left inguinal radical orchiectomy was performed.
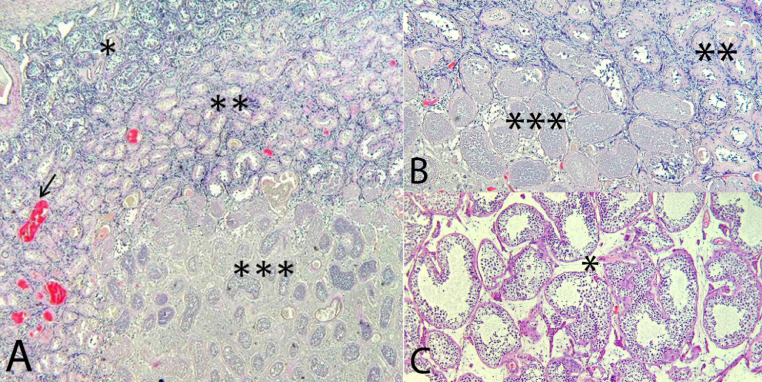
At gross examination a brownish nodular lesion was observed in the upper pole of the testis, measuring 1.7 cm. The rest of the parenchyma and adnexal structures did not show any change. A total inclusion of the testis was performed. Microscopic studies showed a well-defined area of ischemic necrosis surrounded by a thin rim of sclerotic spermatic tubules and congestive vessels in the stroma without significant inflammatory infiltration either in the interstitium or in the vessels (Fig. 3). The germinal cells of the tubules surrounding the infarcted area were normal. In order to rule out the presence of any germ cell neoplasia, PLAP and c-kit immunostains were performed, both with a negative result.
Fig. 3.
Hematoxylin and eosin 4x (A), 10x (B), 20x (C)
*Normal parenchyma
**Transition area with sclerotic tubules and congestive vessels (arrow)
***Parenchyma with ischemic necrosis (infarction)
Discussion
While testicular torsion with total infarction of the testis is a relatively common and well-known clinical scenario, STI is a very uncommon pathology. The clinical presentation is characterized by acute scrotal pain usually without any concomitant symptoms and therefore it is clinically indistinguishable from other causes of acute scrotum.
Its aetiology is mainly idiopathic, but in some cases an association can be discovered with an underlying systemic disease, e.g.. vasculitis, hypercoagulability, or a recent history of trauma or testicular torsion. [4,5,6] An interesting finding of the published literature is that STI is found more frequently on the left side without a clear explanation. [7] Anatomic factors, such as venous drainage differences between the right and left testis are likely to play a role in its pathomechanism as it is a well-known factor in varicocele development. In the present case, the upper third of the left testicle was affected. It is also described in small case series that the middle or upper third is involved more frequently, explained by disproportional intratesticular vascular irrigation. [7]
The diagnosis of STI can be suspected by imaging studies with negative tumour marker results; however, the differential diagnosis usually includes testicular tumour or abscess, depending on the clinical setting. Colour Doppler ultrasound is the imaging technique of choice for the evaluation of painful scrotum.
According to Bilagi et al. [8] a typical STI appears as a solitary solid wedge-shaped or round area in the testis formed by ischemic testicular lobules. However, the grey-scale ultrasound appearance depends on the time elapsed between the onset of symptoms and the moment of the US. Within the first 24 hours it can be either barely visible or almost isoechoic to the normal testicular parenchyma and during the next few days the lesion becomes more hypoechoic and heterogeneous. [7]
On colour Doppler US performed during the first 24 hours the ischemic area shows significantly diminished or no vascularity due to the presence of viable and non-viable parenchyma. At the early stage intervening vascular structures might be depicted between the avascular parenchymal lobules, corresponding to centripetal arteries. [9] After the first 24 hours a peripheral hyper emic rim can be seen on colour Doppler US, which probably corresponds to inflammatory changes, granulation tissue and compressed parenchymal vessels. [7] In our case there were no signs of inflammation or granulation tissue in the periphery of the lesion on microscopic examination, however, congestive vessels and sclerotic spermatic tubules could be depicted.
Even though Duplex US is very sensitive in the evaluation of vascular anomalies, [10] the increasingly available contrast enhanced ultrasound (CEUS) is highly useful in the characterization of testicular lesions. [7] Intravenously administered ultrasound contrast agents are purely intravascular inert gas bubbles encapsulated by a protein, lipid or phospholipid shell with a short in-vivo lifetime that allows a continuous evaluation of a lesion. [11] In focal infarction from the very acute phase there is a diffuse hypo enhancement of the avascular parts of the lesion which is surrounded by a discrete hyperattenuating rim, corresponding to the previously described hyperemic tissue. CEUS is more sensitive in the detection of preserved viable parenchyma and shows earlier and more objective vascular changes of the whole testicle than Doppler US. Furthermore, CEUS is especially useful for the evaluation of small testicular tumours, as these can present a falsely avascular pattern on Doppler US. [7]
If CEUS is not available, a contrast enhanced testicular MRI could be a good alternative. However, if CEUS was previously performed, the MRI usually adds little to the diagnostic workup as dynamic contrast enhanced MRI findings are mainly superimposable to those found on CEUS. [10] On MRI a testicular infarction shows low signal intensity on T1 and T2 weighted images and presents diffuse hypo enhancement with a hyper vascular rim, similar to CEUS images. In some cases, on T1 weighted images haemorrhagic hyperintense foci can be depicted. Fernández-Pérez et al. [10] described retraction of the tunica albuginea in chronic or advanced cases, a sign that should be taken into account on follow-up imaging.
The list of differential diagnosis includes testicular abscess, since it can have similar US and contrast enhancement characteristics. Usually other accompanying scrotal changes (heterogeneous hyper enhancing viable parenchyma, hyper vascularized and thickened epididymis) along with septic laboratory parameters suggest a complicated orchiepididymitis. Furthermore, it can be challenging to differentiate from a less vascularized or necrotic testicular tumour if the lesion is rounded and the vascularity is not completely absent on colour Doppler examination. [12] STI is formed by ischemic testicular lobules; depicting a wedge-shaped or lobular morphology on imaging studies is fundamental to suspect the diagnosis. Generally, a testicular neoplasm presents as a solid hyper vascular nodular lesion on colour Doppler and shows intense contrast enhancement. However, a mainly necrotic or scarcely vascularized focal tumour can mimic a segmental infarct in its early phase with viable parenchymal indentation.
The burned-out testicular tumour (BOTT) is described as a spontaneously and completely regressed germ cell tumour, which usually presents at the stage of metastases. It is a rare clinical presentation that is found in approximately 10% of patients initially diagnosed of primary retroperitoneal germinal cell tumour. [13] The physiopathology of BOTT is still unknown but an immune-mediated response has been proposed, or -due to the high metabolic rate of the tumour - outgrowing of the blood supply could also explain the absence of remaining viable tumour. [14,15] ultrasound findings of a burned-out testicular tumour may show loss of testicular homogeneity, non-specific hypovascular, hypoechoic areas within the parenchyma, focal areas of calcification, microlithiasis and testicular atrophy. [16] CEUS may show reduced enhancement of the entire testis and lack of vascularization of the ill-defined lesion. [17] Few cases have been described presenting with testicular mass without metastasis. [18] This possibility made the differential diagnosis with STI challenging in our patient. Histologic analysis of burned-out testicular tumours shows mainly a central scar formation surrounded by inflammation, presence of siderophages and coarse tubular calcifications with peripheral intratubular germ cell neoplasia.
In our case the histological findings excluded the burned-out tumour because there was no central scar but only an infarcted area with ghost tubules containing shadows of identifiable normal germ cells. Moreover, there were no significant inflammatory infiltrate, calcifications and intratubular germ cell neoplasia within the lesion or in the surrounding tissue.
In cases with low clinical and radiological tumour suspicion, in addition to negative testicular tumour markers, a close follow-up has been proposed as a safe option. [1,8] After no signs of resolution on a strict follow-up exam and/or persistent diagnostic doubt, partial or total orchiectomy is usually performed and the final diagnosis of the benign disease is based on histopathology.
Conclusion
Awareness of STI in the correct clinical scenario with negative tumour markers, typical US and CEUS findings may allow a conservative management avoiding orchiectomy. If the possibility of a necrotic tumour cannot be ruled out on follow-up imaging, orchiectomy is usually performed.
Acknowledgement
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Footnotes
Competing Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Patient consent: Written consent was obtained from the patient for publishing the case report.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.radcr.2021.09.021.
Appendix. Supplementary materials
References
- 1.Madaan S, Joniau S, Klockaerts K, DeWever L., Lerut E. Segmental testicular infarction: conservative management is feasible and safe. Eur Urol. 2008;53(2):441–445. doi: 10.1016/j.eururo.2007.03.061. [DOI] [PubMed] [Google Scholar]
- 2.Dogra V, Ledwidge ME, Winter TC, Lee F.T., Jr Bell-clapper deformity. Am J Roentgenol. 2003;180:1176–1177. doi: 10.2214/ajr.180.4.1801176. [DOI] [PubMed] [Google Scholar]
- 3.Brehmer-Andersson E, Andersson L, Johansson J. Haemorrhagic infarctions of testis due to intimal fibroplasia of spermatic artery. Urology. 1985;25:379–382. doi: 10.1016/0090-4295(85)90493-5. [DOI] [PubMed] [Google Scholar]
- 4.Gofrit ON, Rund D, Shapiro A, Pappo O., Landau E.H., Pode D. Segmental testicular infarction due to sickle cell disease. J Urol. 1998;160:835–836. doi: 10.1016/S0022-5347(01)62803-9. [DOI] [PubMed] [Google Scholar]
- 5.Baer HM, Gerber WL, Kendall AR, Locke J.L., Putong P.B. Segmental infarct of the testis due to hypersensitivity angiitis. J Urol. 1989;142:125–127. doi: 10.1016/s0022-5347(17)38682-2. [DOI] [PubMed] [Google Scholar]
- 6.Braeckman P, Joniau S, Oyen R, Croes R., Van Poppel H. Polyarteritis nodosa mimicking a testis tumour: a case report and review of the literature. Cancer Imag. 2002;2:96–98. [Google Scholar]
- 7.Bertolotto M, Derchi LE, Sidhu PS, Serafini G., Valentino M., Grenier N. Acute segmental testicular infarction at contrast-enhanced ultrasound: early features and changes during follow-up. Am J Roentgenol. 2011;196(4):834–841. doi: 10.2214/AJR.10.4821. [DOI] [PubMed] [Google Scholar]
- 8.Bilagi P, Sriprasad S, Clarke JL, Sellars M.E., Muir G.H., Sidhu P.S. Clinical and ultrasound features of segmental testicular infarction: six-year experience from a single centre. Eur Radiol. 2007;17(11):2810–2818. doi: 10.1007/s00330-007-0674-2. [DOI] [PubMed] [Google Scholar]
- 9.Shiraj S, Ramani N, Wojtowycz AR. Segmental testicular infarction, an underdiagnosed entity: case report with histopathologic correlation and review of the diagnostic features. Case Rep Radiol. 2016;2016 doi: 10.1155/2016/8741632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernández-Pérez GC, Tardaguila FM, Velasco M, Rivas C., Dos Santos J., Cambronero J. Radiologic findings of segmental testicular infarction. Am J Roentgenol. 2005;184:1587–1593. doi: 10.2214/ajr.184.5.01841587. [DOI] [PubMed] [Google Scholar]
- 11.Huang DY, Yusuf G, Daneshi M, Husainy M.A., Ramnarine R., Sellars M.E.K. Contrast-enhanced US–guided interventions: improving success rate and avoiding complications using US contrast agents. RadioGraphics. 2017;37(2):652–664. doi: 10.1148/rg.2017160123. [DOI] [PubMed] [Google Scholar]
- 12.Aquino M, Nghiem H, Jafri SZ, Schwartz J., Malhotra R., Amin M. Segmental testicular infarction: sonographic findings and pathologic correlation. J Ultrasound Med. 2013;32(2):365–372. doi: 10.7863/jum.2013.32.2.365. [DOI] [PubMed] [Google Scholar]
- 13.Kühn MW, Weissbach L. Localization, incidence, diagnosis and treatment of extratesticular germ cell tumors. Urol Int. 1985;40:166–172. doi: 10.1159/000281074. [DOI] [PubMed] [Google Scholar]
- 14.de Souza P, So CW, Batura D, Gayed W., Vrentzou E. Burned-out testicular germ cell tumour presenting as acute inferior vena cava syndrome. BMJ Case Rep. 2020;13(11) doi: 10.1136/bcr-2020-237481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El-Sharkawy MS, Al-Jibali AS. Burned-out metastatic testicular tumor: choriocarcinoma. Int J Health Sci (Qassim) 2017;11(2):81–82. [PMC free article] [PubMed] [Google Scholar]
- 16.Astigueta JC, Abad-Licham MA, Agreda FM, Leiva B.A., De la Cruz J.L. Spontaneous testicular tumor regression: case report and historical review. Ecancermedicalscience. 2018;12:888. doi: 10.3332/ecancer.2018.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rocher L, Glas L, Bellin MF, Ferlicot S., Izard V., Benoit G. Burned-out testis tumors in asymptomatic infertile men: multiparametric sonography and MRI findings. J Ultrasound Med. 2017;36(4):821–831. doi: 10.7863/ultra.15.08037. [DOI] [PubMed] [Google Scholar]
- 18.Balzer BL, Ulbright TM. Spontaneous regression of testicular germ cell tumors: an analysis of 42 cases. Am J Surg Pathol. 2006;30(7):858–865. doi: 10.1097/01.pas.0000209831.24230.56. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.