Abstract
Conjoined twin is an extremely rare condition and needs a thorough knowledge of anatomy and a multidisciplinary approach is essential to successfully separate, the twins. Thoracopagus are the twins attached by chest and umbilicus and are the commonest among all the varieties but carries a poor survival rate. We describe our approach and experience of management of thoracopagus twins who were separated at eighty-three day of life and are alive and well after 4 years of follow up. The most important decisive parameter for successful separation is the extent of sharing of organs between twins but the role of a motivated multidisciplinary team is also indispensable.
KEYWORDS: Conjoined twins, the survival of thoracopagus twins, thoraco-omphalopagus twins, thoracopagus twins
INTRODUCTION
Conjoined twins being one of the rarest congenital entities, has always been a fascinating topic for clinicians and society. The reported incidence of conjoined twins is 1:50,000 and is three times commoner in females.[1,2,3,4] They have poor compatibility with life, as most of the infants die either in utero (28%) or soon after birth (54%) and only 18% survive for more than 24 h.[2,4] Thoracopagus twins usually share hearts and have complex cardiac anomalies, which jeopardize the success of separation surgery. Nevertheless, detailed preoperative evaluation of the anatomy of shared organs, multidisciplinary team approach, and rehearsals lead to a favorable outcome of operable cases. We report a rare case of the successful separation of thoracopagus twins and discuss the challenges of the procedure.
CASE REPORT
A pair of male conjoined twins was referred to us at the age of 2 months, attached ventrally, and weighing 5.4 kg together [Figure 1a]. On examination, they were fused from the sternum to the upper abdomen with a small omphalocele (Thoracopagus). Anal orifices were separate and they were passing stools at different times, which suggested a separate colon. The twins were delivered by emergency cesarean section of a 22-year-old second gravida mother. The children cried immediately at birth and weighed 4.6 kg together at birth. Antenatal ultrasound in the second trimester was suggestive of a twin pregnancy and conjoined twinning was not anticipated.
Figure 1.
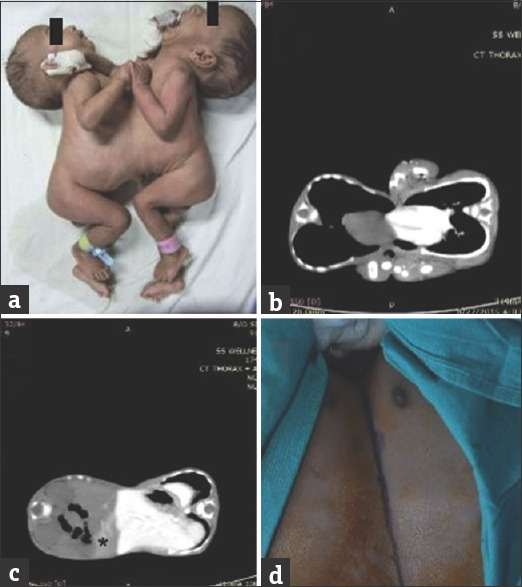
(a) Thoracopagus twins at presentation (b) Two separate hearts in computed tomography (c) liver shared by parenchymal bridge with minimum transfer of dye (*) from one twin to other (d) marking of incision
On auscultation, there were no murmurs, and heart sounds were not synchronous. On plain X-ray, the heart shadows were separated. They had different heart rates and electrocardiogram had normal and different QRS complexes.
Computed tomography of the abdomen and thorax revealed that the twins shared a common pericardium [Figure 1b] and fused livers [Figure 1c]. Both the livers had separate hilum and hepatic veins draining into separate vena cava. Each infant had a separate and normal stomach, duodenum, biliary system, kidneys, ureters, and bladders. There were separate aorta and vena cava in each twin.
The separation surgery was planned, after 2 weeks and two separate surgical and anesthesia teams were made. The collective weight was 6.2 kg at the time of surgery. Proper identification of babies was assured by color coding, which was applied on all the four limbs (1st baby: green, 2nd baby: red). Routine anesthetic and emergency drugs were prepared according to the bodyweight of babies (assuming 50% sharing of total weight). Blood and blood products were made available in the Operation Theatre (OT) before incision. Anesthesia workstations, monitoring lines, and infusion pumps with tubing were also color-coded. Anesthesia, surgery, and nursing teams were separately color-coded. Two intravenous (IV) lines were secured in hand (24G) and one peripherally inserted central catheter in lower limb was secured in both babies, respectively. Central venous catheter could not be inserted because of their complex head position. IV atropine (0.06 mg) was given to the first baby and response was observed in both babies to evaluate for the presence of cross circulation (it was minimal).
Both the babies had hemoglobin of 8.5 g/dl and twin 2 had slightly acidotic serum pH. There was difficulty intubation and the second baby was held above the first in to facilitate intubation in the first baby.
The bridging skin was marked [Figure 1d] and skin, subcutaneous tissue, peritoneal bridge, and the costal arch was divided. The bridge of liver parenchyma was encircled and divided using an ultrasonic dissector [Figure 2a]. The peritoneal cavities were well separated, except at the site of the common liver. The two sets of the small and large intestine were found separated in each twin. The two hearts were enclosed in the single pericardium, which was separated by dividing the pericardium [Figure 2b]. The pericardium was repaired by composite mesh. The division of posterior skin completed the separation of twins [Figure 2c].
Figure 2.
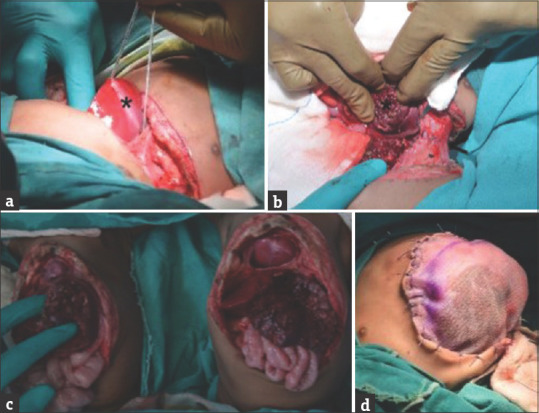
(a) Parenchymal bridge of liver encircled by feeding tube (b) separated liver (*) (c) completely separated twins (d) abdominal closure by polypropylene mesh
The intestines were positioned back into the abdominal cavity and single-layer closure was done. The second twin developed hypoxia, hypotension, and bradycardia on closure and thus, the sutures were removed and the abdomen was closed with polypropylene mesh [Figure 2d]. This reduced the intra-abdominal pressure and improved the circulatory parameters. The surgery lasted nearly 8 h.
The intraoperative blood loss during the separation of liver parenchyma led to severe hypotension which was managed by massive blood transfusion (also replacing plasma and platelets), and vasopressors. The estimation of blood loss from individual baby was very difficult. Blood loss was assessed by measuring surgical sponge and calculating drain output from the surgical area, although accurate assessment was not possible. In the absence of central venous pressure monitoring, the intravascular volume assessment was done according to the vital parameters, hourly arterial blood gas, and urine output monitoring. Due to excessive IV fluids and massive blood transfusion, patients developed metabolic acidosis (pH 7.21 and 7.23), hyperkalemia (potassium 5.68 and 5.8 mEq l/dl), and hyperglycemia (glucose 320 and 350 mg/dl, respectively), which were successfully managed.
Postoperatively, twin 1 required inotropes for 3 days, ventilation for 6 days, and full feeds were established after 8 days. He developed sepsis for which the antibiotics were given for 28 days. Twin 2 was ventilated for 3 days, inotropes were continued for 3 days while the skin flap was closed on the 6th postoperative day and full oral feeds were started in 12 days. Both the babies were discharged after 45 days of hospitalization when it was ensured that the children were gaining adequate weight under the care of the parents. At the time of reporting this case, both the children are 4 years old and thriving well [Figure 3]. Twin 2 has a ventral hernia and is planned for repair.
Figure 3.
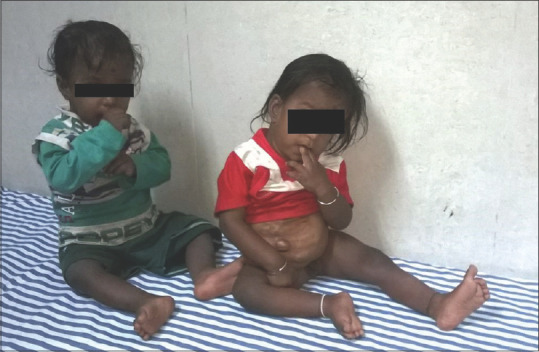
Separated twins 3 years after surgery
DISCUSSION
Conjoined twins are always monozygotic, monochorionic, monoamniotic, and thus belong to the same sex. They occur during embryogenesis, either due to the failure of separation of embryonic discs at 15–17 days or because of the fusion of two embryonic discs.[2,5] There are no genetic or environmental predisposing factors associated with the occurrence of conjoined twins.[6]
Conjoined twins are classified according to the most prominent part of fusion added with the suffix “pagus” which is a Greek term meaning fixed.[7] Thoracopagus twins are ones who are, attached by their chest, upper abdomen wall, have an exomphalos, and share organs such as the heart (75%), pericardium (90%), diaphragm, liver (100%), bile ducts (25%), and upper small intestine (50%). They are the most common accounting for almost 40% of all varieties and have the highest mortality rates of 51%.[1,8,9,10]
The separation of conjoined twins is a challenging process and requires a multidisciplinary team approach. The overall success rate of separation is around 65%.[1,2,3,4] Thoracopagus twins are associated with the highest mortality rates owing to complex cardiac anomalies.[8,9,10]
A detailed evaluation of cardiac, hepatopancreatobiliary anatomy, and gastrointestinal anatomy is required for proper preoperative planning.
Anesthesia is a major challenge in separation surgery and two separate color-coded teams for each twin should be designated. To expedite and avoid hypovolemic shock the procedure there should be two separate color-coded surgical teams which we also followed and should perform the reconstruction separately.[3]
The optimal time for surgical separation is 3 months as in our case; however, it may extend up to 3 years.[1-4,8] This interval allows completion of exhaustive work-up to assess the anatomy of sharing organs for accurate preoperative planning and rehearsal. The overall survival rate of emergency separation for all varieties of conjoined twins is around 30% while that of elective separation reaches up to 80% in different series.[1,2,3,4] The high incidence of complex cardiac anomalies is responsible for the dismal prognosis of thoracopagus twins.
The liver is shared in almost all the sets of thoracopagus twins. For the feasibility of separation, each liver should have separate hepatic veins draining into its vena cava. The livers may have vessels crossing from one baby to the other which needs to be separately ligated.[11] Otherwise, the hepatic veins of one twin may traverse the liver to enter into the heart of the other twin, in whom the twin without hepatic veins is sacrificed and the heart is auto-transplanted to the twin with hepatic veins.[3]
Despite extensive workup, it is not possible to define biliary anatomy, which is best identified during surgery. The closure of the defect is always a concern in thoracopagus twins due to its large size. Although a tissue expander may help in closure by increasing tissue for reconstruction most of the authors have discontinued it due to frequent complications like wound infection and skin necrosis in up to 60% of cases, and most of the authors prefer closure with the help of prolene mesh or skin grafts.[2-4,12]
CONCLUSION
Conjoined twins are one of the rarest congenital entity and bear a poor survival rate. Although thoracopagus twins are the commonest variety, very few successful separation surgeries are reported until now in literature. The success of separation surgeries in thoracopagus twins is dependent on the extent of shared organs between twins. A shared heart and single set of hepatic veins preclude the survival of both the twins. A small proportion of such twins who have separate hearts and common pericardium have better survival rates and with exhaustive preoperative investigations and rehearsals, successful separation can be achieved.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient (s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initial s will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Spitz L, Kiely EM. Experience in the management of conjoined twins. Br J Surg. 2002;89:1188–92. doi: 10.1046/j.1365-2168.2002.02193.x. [DOI] [PubMed] [Google Scholar]
- 2.Rode H, Fieggen AG, Brown RA, Cywes S, Davies MR, Hewitson JP, et al. Four decades of conjoined twins at Red Cross Children's Hospital--lessons learned. S Afr Med J. 2006;96:931–40. [PubMed] [Google Scholar]
- 3.O'Neill JA, Jr, Holcomb GW, 3rd, Schnaufer L, Templeton JM, Jr, Bishop HC, Ross AJ, 3rd, et al. Surgical experience with thirteen conjoined twins. Ann Surg. 1988;208:299–312. doi: 10.1097/00000658-198809000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tannuri AC, Batatinha JA, Velhote MC, Tannuri U. Conjoined twins: Twenty years' experience at a reference center in Brazil. Clinics (Sao Paulo) 2013;68:371–7. doi: 10.6061/clinics/2013(03)OA14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spencer R. Theoretical and analytical embryology of conjoined twins: Part 1: Embryogenesis. Clin Anat. 2000;13:36–53. doi: 10.1002/(SICI)1098-2353(2000)13:1<36::AID-CA5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 6.Mutchinick OM, Luna-Muñoz L, Amar E, Bakker MK, Clementi M, Cocchi G, et al. Conjoined twins: A worldwide collaborative epidemiological study of the International Clearinghouse for Birth Defects Surveillance and Research. Am J Med Genet C Semin Med Genet. 2011;157C:274–87. doi: 10.1002/ajmg.c.30321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spencer R. Anatomic description of conjoined twins: A plea for standardized terminology. J Pediatr Surg. 1996;31:941–4. doi: 10.1016/s0022-3468(96)90417-0. [DOI] [PubMed] [Google Scholar]
- 8.Spitz L. Conjoined twins. Prenat Diagn. 2005;25:814–9. doi: 10.1002/pd.1268. [DOI] [PubMed] [Google Scholar]
- 9.Singh M, Jacob R, Naik V, Baines D. Separation of thoraco-omphalopagus twins in a rural secondary hospital: Perioperative management. Indian J Anaesth. 2012;56:442–7. doi: 10.4103/0019-5049.103957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lalwani J, Dubey K, Shah P. Anaesthesia for the separation of conjoined twins. Indian J Anaesth. 2011;55:177–80. doi: 10.4103/0019-5049.79902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ray AK, Mukherjee NN, Mukherjee G, Patra R, Ghosh DK. Separation of thoraco-omphalopagus Siamese twin. Indian J Pediatr. 2004;71:755–7. doi: 10.1007/BF02730669. [DOI] [PubMed] [Google Scholar]
- 12.Al Rabeeah A. Conjoined twins--past, present, and future. J Pediatr Surg. 2006;41:1000–4.v. doi: 10.1016/j.jpedsurg.2005.12.045. [DOI] [PubMed] [Google Scholar]


