Abstract
Introduction
The pathogenesis of vascular cognitive impairment (VCI) is not fully understood. GPR39, an orphan G‐protein coupled receptor, is implicated in neurological disorders but its role in VCI is unknown.
Methods
We performed GPR39 immunohistochemical analysis in post mortem brain samples from mild cognitive impairment (MCI) and control subjects. DNA was analyzed for GPR39 single nucleotide polymorphisms (SNPs), and correlated with white matter hyperintensity (WMH) burden on pre mortem magnetic resonance imaging.
Results
GPR39 is expressed in aged human dorsolateral prefrontal cortex, localized to microglia and peri‐capillary cells resembling pericytes. GPR39–capillary colocalization, and density of GPR39‐expressing microglia was increased in aged brains compared to young. SNP distribution was equivalent between groups; however, homozygous SNP carriers were present only in the MCI group, and had higher WMH volume than wild‐type or heterozygous SNP carriers.
Discussion
GPR39 may play a role in aging‐related VCI, and may serve as a therapeutic target and biomarker for the risk of developing VCI.
Keywords: GPR39, microglia, mild cognitive impairment, polymorphism, vascular cognitive impairment, white matter hyperintensity
1. BACKGROUND
Aging is the strongest risk factor for Alzheimer's disease (AD) and related dementia. 1 With people increasingly surviving into their 80s, 90s, and beyond, the number of people living with dementia is projected to triple by 2050. 1 Historically, vascular cognitive impairment (VCI), the second most common cause of dementia after AD, and AD were considered distinct entities. 2 There is a growing appreciation, however, of the overlap and similarities between AD‐ and VCI‐related dementias. 3 Indeed, many patients with AD have vascular pathology, 3 and brains from VCI patients also exhibit Alzheimer's pathology. Furthermore, both entities often occur in the same individual and exacerbate each other. For example, AD patients with ischemic lesions have poorer cognitive function than those with no vascular lesions, 4 highlighting the importance of studying cerebrovascular mechanisms underlying VCI.
While the pathogenesis of VCI is not fully understood, small vessel disease (SVD) is a key underlying factor. 5 Ischemic lesions resulting from SVD appear on T2‐weighted magnetic resonance imaging (MRI) scans as hyperintensities primarily localized in the cerebral white matter, termed white matter hyperintensities (WMH). In a prospective study, we have shown that acceleration of WMH burden is an early, presymptomatic pathological change predictive of conversion to the clinical diagnosis of mild cognitive impairment (MCI). 6 Furthermore, we have also confirmed that autopsy brains from deceased patients with dementia and histopathological evidence of SVD had larger WMH volume on pre mortem MRI. 7
The current study was designed to investigate the involvement of the orphan G‐protein coupled receptor GPR39 in neurovascular injury underlying VCI. GPR39 has been implicated in a variety of neurological disorders, 8 but its involvement in VCI has not been investigated. GPR39 binds zinc, which plays an important role in neurotransmission, and is implicated in the pathology of AD. 9 Zinc is necessary for formation of the toxic forms of amyloid beta, which in turn attenuates zinc neuronal signaling, 9 , 10 identifying GPR39 as a potential novel therapeutic target in neurological disorders including AD. GPR39 has also been implicated in a range of pathological processes other than amyloid pathology, 9 and has been proposed to play a role in other neurological conditions, including depression and seizures. GPR39 has also been implicated in vascular pathology, including vascular inflammation and calcification. 11 , 12 The expression and cellular localization of GPR39 in the human brain is not known, and its potential link to VCI has not been investigated. Therefore, we set out to characterize GPR39 expression in the human brain and its potential association with VCI.
HIGHLIGHTS
There is increased density of GPR39‐expressing microglia in aged post mortem human brains.
Homozygous GPR39 single nucleotide polymorphism carriers have higher white matter hyperintensity burden.
GPR39 may play a role in aging‐related vascular cognitive impairment.
GPR39 may serve as a biomarker and a therapeutic target for mild cognitive impairment.
RESEARCH IN CONTEXT
Systematic review: The authors reviewed the literature using databases (e.g., PubMed, Google) and meeting abstracts and presentations. Animal studies have examined GPR39 in various neurological disorders, including Alzheimer's disease, and in vascular pathology; however, none have linked GPR39 to vascular cognitive impairment (VCI) in humans.
Interpretation: Our findings suggest that GPR39 may play a role in VCI by regulating inflammation and capillary function in the aging human brain, and that GPR39 polymorphisms may serve as a biomarker, and a therapeutic target for VCI.
Future directions: These findings demonstrate that GPR39 single nucleotide polymorphisms (SNPs) are associated with white matter hyperintensity (WMH) burden in the aged human brain. Future larger scale genome‐wide association studies are required to confirm the association between GPR39 SNPs and VCI, and to explore the role and therapeutic targeting of GPR39 in VCI.
To that end, we identified post mortem brain samples collected from former participants of the Oregon Brain Aging Study (OBAS) with a clinical diagnosis of MCI. As control, we used brains from age‐ and sex‐matched subjects with no cognitive deficit, as well as young adults. We conducted two studies. We first performed immunohistochemical analysis to localize GPR39 and determine its correlation with VCI. In the second study, we determined if known GPR39 single nucleotide polymorphisms (SNPs) correlate with WMH burden on pre mortem MRI.
2. METHODS
2.1. Study subjects
Thirty‐five brain and 78 DNA samples were obtained from the Layton Aging and Alzheimer's Disease Research Center Brain Bank Repository at Oregon Health & Science University (OHSU). Distribution of samples between the two cohorts and demographic data are presented in Table 1.
TABLE 1.
Demographic data of subjects for immunohistochemistry and SNP/WMH analysis
| No. | M/F ratio [n, (%)] | Mean age (St Dev) | P value (age) | Post mortem interval [hr, (range)] | |
|---|---|---|---|---|---|
| Immunohistochemistry | |||||
| Young adult | 5 | 4/1 (80%/20%) | 31.40 (5.90) | 108.1 (10.5–363) | |
| Control | 16 | 4/12 (25%/75%) | 93.56 (3.95) | 13.87 (2.5–96) | |
| 0.06 | |||||
| VCI | 14 | 4/10 (28.57%/71.43%) | 96.71 (4.92) | Control vs. VCI | 11.39 (1.75–24) |
| SNP/WMH | |||||
| Control | 37 | 14/23 (37.84%/62.16%)) | 89.30 (10.33) | N/A | |
| VCI | 41 | 17/24 (41.46%/58.54%) | 89.23 (4.24) | 0.99 | N/A |
Abbreviations: SNP, single nucleotide polymorphism; VCI, vascular cognitive impairment; WMH, white matter hyperintensity.
For immunohistochemical assessment, post mortem tissues were selected based on a clinical diagnosis of MCI before death, or in the case of control subjects, on absence of cognitive impairment. The collection, storage, and distribution of information and autopsy tissue were in accordance with guidelines established by the Layton Center Research Repository in compliance with Federal Regulations and Oregon Health & Science University policies.
For measurement of WMH volume and genetic analysis, subjects were selected from the OBAS 13 based on availability of pre mortem 3T MRI measurements, genomic DNA, and clinicopathologic diagnosis indicative of MCI. WMH volume measurements and Clinical Dementia Ratings (CDR) were quantified as previously described. 14
2.2. Immunohistochemistry
Formalin‐fixed dorsolateral prefrontal cortex (dlPFC) sections were immunolabeled with antibodies against GPR39 (anti‐GPR39, extracellular domain, antibodies online ABIN1048812), CD68 (anti‐CD68, abcam ab955), collagen IV (anti‐collagen IV, COL‐94, ThermoFisher Scientific MA1‐22148), NeuN (anti‐NeuN, clone A60, Millipore Sigma MAB377), glial fibrillary acidic protein (GFAP; anti‐GFAP, clone2E8, abcam ab68428), and smooth muscle actin (SMA; anti‐actin, α‐smooth muscle, Sigma A2547). Antigen retrieval was carried out by pretreatment with citrate buffer, pH 6 in a steamer for 30 minutes followed by 3% hydrogen peroxide to reduce endogenous peroxidase activity. For both CD68 and collagen IV ImmPRESS HRP anti‐mouse IgG (peroxidase) polymer detection kit was used (Vector, MP‐7402) and subsequently the reaction visualized using diaminobenzidine. For GPR39 ImmPRESS‐AP anti‐rabbit immunoglobulin G (IgG; alkaline phosphatase) polymer detection kit was used (Vector, MP‐5401) and subsequently the reaction was visualized by Vector blue alkaline phosphatase (Blue AP) substrate kit (Vector, SK‐5300). For negative controls the primary antibody was replaced by species‐appropriate serum.
2.3. Image acquisition and analysis
Images were acquired on a Zeiss AxioScan.Z1 Slide Scanner. Resulting czi images were converted into tiff files after which gray and white tissue brain regions were delineated and separated into two different images using Adobe Photoshop; gray and white regions were separated as mutually exclusive regions and files. Images were broken down by tiling each image into an array of 8 × 8 tiff files (64/ image) using freeware Irfanview. Resultant files were processed using FIJI/ImageJ. Density of each marker was quantified by converting labeled areas into a binary mask, within which particles were filtered (5 to 150 µm in diameter), counted, and divided by total tissue area. Co‐localization was determined by quantifying overlapping regions of interest of each of the markers and expressed as a percentage of either CD68 or collagen IV. Image acquisition and analysis were carried out in a blinded manner.
2.4. Genotyping
DNA was extracted from patients as previously described. 7 Two previously reported SNPs linked to vascular disease, rs13420028 and rs10188442 15 were investigated. The genotype of each subject for SNP status (homozygous wild‐type [WT], heterozygous, or homozygous for each SNP) for the gene that encodes for GPR39 was determined by allelic discrimination using TAQMAN technology (Invitrogen). SNP assays were purchased from ThermoFisher for rs10188442 and rs13420028. Quantitative polymerase chain reaction (qPCR) assays were performed on the QuantStudio Real‐time PCR System (Life Technologies) and data collected using Applied Biosystems QuantStudio 12K Flex Software. DNA quality assessment and qPCR assays were performed in the OHSU Gene Profiling Shared Resource, according to manufacturer's instructions.
2.5. Statistics
For immunohistochemical data, one‐way analysis of variance (ANOVA) with Tukey's multiple comparisons test was used, with significance threshold set at P < 0.05 using Prism 8 (GraphPad). Data are presented as mean ± standard error of the mean (SEM). For SNP analysis, Fisher's exact test was used, analysis was carried out using Stata Statistical Software, Release 16 (StataCorp LLC).
2.6. Study approval
Consent for research purposes specifically was obtained from next of kin at time of autopsy.
3. RESULTS
There were no significant differences in mean age and post mortem interval between the aged MCI and control subjects for either the immunohistochemical or the genotype and WMH analysis (Table 1).
3.1. Immunohistochemical localization and expression of GPR39 in the young and aged human dlPFC
We first assessed whether GPR39 protein is expressed in the dlPFC of the human brain, its cellular localization and distribution, and whether expression levels are altered in aging and in individuals with VCI. We found that GPR39 is robustly expressed throughout the dlPFC. We observe a non‐significant trend for increased GPR39 in the aged dlPFC, with overall density of GPR39 immunoreactivity being similar in aged control and VCI, both in gray and white matter (Figure 1). In terms of distribution, we observed two populations of GPR39‐positive cells, one in close proximity to blood vessels, and the other within the tissue parenchyma.
FIGURE 1.
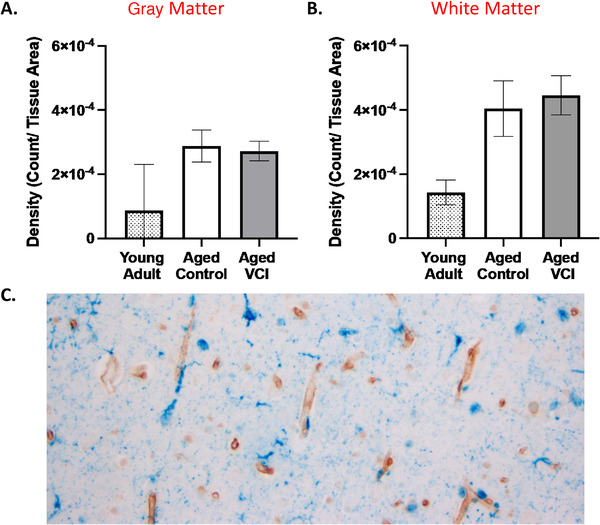
GPR39 expression in dorsolateral prefrontal cortex (dlPFC) of young and aged control and aged vascular cognitive impairment (VCI) human brains. Quantification of GPR39 expression density in both gray (A), and white matter (B), of young adult control and aged control and VCI human brains. C, Representative image of GPR39 immunolabeling (blue) of human aged dlPFC. Data represent mean ± standard error of the mean (n = 5–15/group)
To investigate the cellular identity of GPR39‐expressing cells in the human dlPFC, we performed double‐labelling of brain sections for GPR39 and collagen IV, which labels the basal lamina of blood vessels (Figure 2). We observed that many, but not all of GPR39‐positive cells are intimately associated with capillaries, with some cells colocalizing with capillaries (arrows), while others are in close proximity (peri‐capillary; arrowheads). Based on morphology and close association with capillaries, this cell population is consistent with pericytes. We used multiple antibodies against two pericyte markers, human platelet‐derived growth factor receptor beta (PDGFRβ) and human neuron‐glial antigen 2 (NG2). However, none of these antibodies tested worked on our human formalin‐fixed tissue. We were therefore unable to immunohistochemically confirm the identity of these GPR39‐positive peri‐capillary cells. These GPR39‐positive cells were predominantly associated with capillaries (Figure 2C‐D), and rarely seen in larger blood vessels (not shown), further supporting pericyte‐localized GPR39 expression. Upon quantification, we observe a trend for decreased collagen IV density in the aged groups compared to the young adult control samples. Furthermore, we observe a striking increase in co‐localization of GPR39 with collagen IV in the aged dlPFC, compared to young where colocalization is largely absent (Figures 2A‐B). In gray matter, co‐localization is increased in both aged groups compared to young adults, increasing from 1 ± 0.02% co‐localization in young adult to 32.21 ± 5.19% in aged control and 21.55 ± 3.14% in aged VCI (P < 0.05, one‐way ANOVA with Tukey's multiple comparisons test), with no significant difference between aged control and VCI. In white matter co‐localization was increased in the aged control dlPFC compared to young adult (P < 0.05, one‐way ANOVA with Tukey's multiple comparisons test); again, there was no significant difference between aged control and aged VCI.
FIGURE 2.
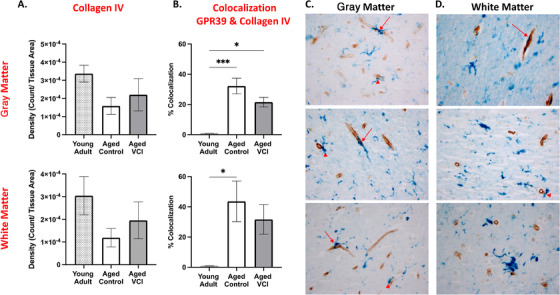
Peri‐capillary localization of GPR39 in aged human dorsolateral prefrontal cortex (dlPFC). A, Quantification of collagen IV expression density and (B), colocalization of GPR39 with collagen IV, in both gray (top) and white (bottom) matter of young and aged control and aged vascular cognitive impairment (VCI) human dlPFC. Representative images of collagen IV (brown) and GPR39 (blue) labeling in human dlPFC gray (C), and white matter (D), arrows indicate colocalization of GPR39 with collagen IV, arrowheads indicate close proximity of GPR39 with collagen IV. * P < 0.05, ** P < s0.001, one‐way analysis of variance, data represent mean ± standard error of the mean (n = 5–15/group)
We then investigated the cellular identity of the second population of GPR39‐positive cells within the parenchyma. Morphologically these cells resemble microglia. We therefore performed double‐labeling for GPR39 and the microglia/macrophage marker CD68 (Figure 3). The density of CD68‐positive cells as well as cells positive for both GPR39 and CD68 was higher in white matter than gray matter (Figure 3C‐D). We found that CD68 density was unaltered by age or dementia status (Figure 3A‐B). While there were no significant differences in density of CD68‐GPR39 co‐localization between groups in gray matter, we observed a significant increase in the co‐localization density in the aged VCI white matter compared to both young adult and age‐matched control, increasing from 8.633 × 10–6 ± 4.68 × 10–5 in young adult and 2.49 × 10–5 ± 0.57 × 10–5 in aged control to 6.16 × 10–5 ± 1.34 × 10–5 count/tissue area in aged VCI dlPFC (P < 0.05, one‐way ANOVA with Tukey's multiple comparisons test; Figure 3B). Overall, we observed high levels of colocalization of GPR39 with CD68, with 60% to 85% of CD68‐positive area also positive for GPR39 in both aged groups (Figure S1 in supporting information). Finally, we performed double immunolabeling of GPR39 with NeuN (Figure S2 in supporting information), SMA, and GFAP, where no co‐localization was observed (not shown), indicating that GPR39 is not expressed by neurons, smooth muscle cells, or astrocytes, respectively, in the human dlPFC.
FIGURE 3.
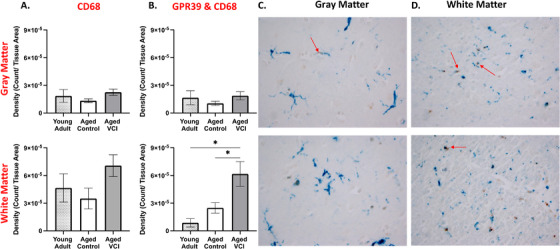
Microglial localization of GPR39 in aged human dorsolateral prefrontal cortex (dlPFC). A, Quantification of CD68 expression density and (B), density of colocalized GPR39 with CD68, in both gray (top) and white (bottom) matter of young and aged control and aged vascular cognitive impairment (VCI) human dlPFC. Representative images of CD68 (brown) and GPR39 (blue) labeling in human gray (C), and white matter (D), arrows indicate colocalization of GPR39 with CD68. * P < 0.05, one‐way analysis of variance, data represent mean ± standard error of the mean (n = 5–15/group)
3.2. GPR39 polymorphisms in control and MCI subjects and correlation with WMH burden
We next sought to determine whether polymorphisms in the gene encoding for GPR39 are associated with MCI diagnosis. We determined the presence of two SNPs 15 and WT GPR39 in MCI and control subjects. In agreement with previous studies, 15 we observed that the two SNPs were in high linkage disequilibrium with each other (SNPs were separated by a small distance genetically), allowing us to group genotypes into WT, homozygous SNP (both SNPs are homozygous), or heterozygous SNP (both SNPs are heterozygous). We found that in our cohort, there was no statistically significant difference between control and MCI groups in distribution of genotypes (Fisher's exact test, P = 0.102). Importantly, however, out of a total of 78 subjects, only 5 carried the homozygous SNP; these were exclusively in the MCI group (Figure 4A).
FIGURE 4.
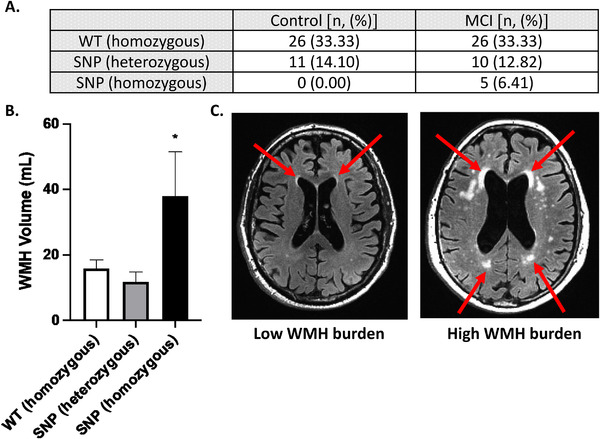
GPR39 single nucleotide polymorphisms in control and vascular cognitive impairment (VCI) subjects. A, Number of subjects carrying one of two GPR39 polymorphisms, or the wild‐type GPR39 gene, in control and VCI groups. Fisher's exact test determined lack of significance in genotypes between control and VCI subjects (P = 0 .102). B, Quantification of white matter hyperintensity (WMH) volumes by GPR39 genotype, regardless of clinical status. Significance was determined by one‐way analysis of variance with Tukey's multiple comparisons test, * indicates P < 0.05 compared to both other groups. C, Representative 3T magnetic resonance imaging scans of subjects with low (left) and high (right) WMH burden. Arrows indicate areas of WMH
We then determined if GPR39 SNPs are specifically linked to WMH burden, which we previously demonstrated to be higher in VCI. 7 We found that carriers of the homozygous SNPs, which are only present in the MCI group, had significantly higher WMH volume compared to either WT or heterozygous SNP carriers (Figure 4B). WMH volume was 15.87 ± 2.59 mL and 11.90 ± 2.91 mL in WT and heterozygous SNP carriers, respectively, compared to 38.03 ± 13.46 mL in the homozygous SNP carriers (P < 0.05, one‐way ANOVA with Tukey's multiple comparisons test).
4. DISCUSSION
This is the first study of GPR39 in the human brain, and the first to implicate GPR39 in VCI. We made three novel observations: (1) GPR39 is abundantly expressed throughout the human dlPFC; (2) GPR39 is expressed in two cell populations in the aged human brain: CD68‐positive microglia/macrophages and CD68‐negative cells in close proximity to capillaries, likely pericytes; (3) homozygous GPR39 SNPs correlate with WMH burden and are found exclusively in our study in individuals with cognitive impairment. The findings implicate GPR39 SNPs as a risk factor for VCI, and suggest that GPR39 may play a role in the pathogenesis of VCI and serve as a therapeutic target.
GPR39 is an orphan G‐protein coupled receptor that belongs to the ghrelin receptor subfamily, which in addition to ghrelin includes motilin, neurotensin, and neuromedin U (NMU) receptors. 16 The natural ligand for GPR39 remains unknown. Obestatin, a ghrelin‐associated peptide derived from posttranslational processing of the prepro‐ghrelin gene, was once proposed to be the GPR39 endogenous agonist, but this conclusion has been refuted. 17 Like ghrelin and NMU receptors, GPR39 is constitutively active. 18 Zinc ion (Zn2+) has also been shown to activate GPR39 receptor, 19 and to modulate receptor activation. 20 GPR39 has been implicated in a variety of functions and disease conditions. 21 Because GPR39 is in the same family as ghrelin (the “hunger hormone”), studies initially investigated its role in insulin secretion, type 2 diabetes, food intake, and weight control. 21 , 22 , 23 , 24 More recent studies have investigated its role in a range of brain disorders, including epilepsy, 25 alcohol use disorders, 26 anxiety and depression, 27 and protection from neuronal cell death. 28 Of interest to the current study, GPR39 has been implicated in cognitive impairment associated with AD. 9
Regional expression of GPR39 transcript and protein have been reported in both the mouse and human brain, 16 with expression detected in multiple brain regions, including the amygdala, hippocampus, and frontal cortex. 29 , 30 , 31 , 32 Most of these studies, however, have been conducted using northern or western blot analysis of tissue homogenates, which precluded the identification of the specific cell type expressing GPR39. The few studies that used in situ hybridization or immunohistochemistry to localize GPR39 in the mouse brain have reported expression in neurons. 33 , 34 Our study in human dlPFC does not find neuronal expression, but rather expression in microglia and peri‐capillary cells. Furthermore, we find that perivascular expression is increased in both the aged and aged MCI dlPFC, compared to young, whereas microglial expression is increased in MCI dlPFC compared to both aged and young control.
A recent study demonstrated that CD68 immunoreactivity is increased in human brains corresponding to higher level of white matter lesions. 35 Based on this and other observations, Waller et al. concluded that microglial activation is a prominent feature of age‐associated white matter lesions. 35 As the resident brain immune cell, microglia may be increased in number or activated by oxidative stress associated with SVD, which contributes to the pathogenesis of dementia. 36 Our finding that a high percentage of microglia in the aging brain express GPR39, and that the density of GPR39‐positive cells is increased in the white matter of MCI dlPFC implicates GPR39 in the neuroinflammation underlying aging‐related cognitive decline. In a model of chronic cerebral hypoperfusion, a zinc complex has been shown to ameliorate cognitive impairments and reduce microglial activation, 37 raising the possibility that this effect may be mediated by GPR39. Although the GPR39‐expressing CD68 cells in our study appear morphologically microglial, the possibility that these cells are blood‐derived macrophages cannot be discounted. CD68 labels both microglia and macrophages, although combinations of CD68 with other markers attempt to distinguish the two cell types, a definitive identification is difficult, particularly because the phenotype of these cells is age‐ and function‐specific. 38 For example, TMEM119 may be used in combination with CD68; however, its expression is not stable throughout the lifespan or activation state of the microglia, 39 while the traditional microglial marker, Iba1, does not appear to be expressed in all microglial populations. 35 Combinations of markers and their expression levels (TMEM119, CD45, Iba1, CD68) may help distinguish between the cell types; 39 such quantitative analysis of immunolabeling intensity with multiple markers is challenging on human tissue.
Perivascular expression of GPR39 in our study suggests a role in pericyte function. Brain peri‐capillary pericytes, lodged between capillary endothelium and astrocytic end feet, form part of the neurovascular unit. Analogous to smooth muscle cells in arteries and arterioles, they are contractile and control blood flow at the capillary level. 40 , 41 , 42 , 43 It is therefore unsurprising that experimentally, loss of pericytes reduces brain capillary perfusion, leading to chronic perfusion stress, hypoxia, and progressive neurodegeneration, including white matter abnormalities suggestive of SVD. 44 , 45 In humans, alterations in pericyte function contribute to impaired neurovascular coupling with aging, believed to contribute to SVD and subsequent cognitive decline. 46 , 47 , 48 We demonstrate a robust increase in perivascular expression of GPR39 in aged human dlPFC, both control and MCI, compared to young adult, in cells morphologically consistent with pericytes, with expression located around small, but not large, vessels. We also observe a trend toward decreased capillary density in the aged dlPFC, in both control and MCI brain, consistent with previous findings in other brain regions. 49 Although we did not observe differences in expression levels between MCI and age‐matched control subjects, this does not discount that receptor function may be altered between the two groups. The function of GPR39 in these cells, and whether it may contribute to age‐related vascular alterations, remains to be determined.
Our study demonstrates for the first time that homozygous SNPs in the gene encoding for GPR39 are associated with increased WMH burden. We have previously demonstrated that acceleration of WMH burden, a common indicator of cerebral SVD in the elderly, occurs prior to symptomatic MCI. 6 Although genotyping for the SNPs did not distinguish between MCI and control groups, subjects homozygous for both SNPs fell exclusively into the MCI group; it is possible that with increased sample size (there were only 5 homozygous out of 78 subjects), we may observe a correlation between these SNPs and the clinical diagnosis. In our cohort we observed linkage disequilibrium in every subject, an observation also made by Slavin et al., 15 indicating that these two particular SNPs are close to each other. If validated in a larger study, these SNPs can therefore be used as biomarkers to identify individuals at higher risk of developing WMH in later life and adopting disease‐modifying strategies prior to WMH acceleration and dementia onset. Although GPR39 SNPs have not been implicated in cognitive impairment, previous studies implicated variants in GPR39 in multiple conditions including hypertension, nerve injury 50 , and pediatric obesity. 51
In summary, we report that GPR39 is expressed in the dlPFC of the aged human brain in microglia/macrophages and peri‐capillary cells resembling pericytes: the colocalization of GPR39 with capillaries is increased in the aged dlPFC compared to young adult, and the density of GPR39‐positive microglia is higher in dlPFC white matter in VCI versus age‐matched and young adult control brain. We also demonstrate that homozygous GPR39 SNPs are associated with increased WMH volume. These findings suggest that GPR39 may play a role in regulating inflammation and capillary function in the aging brain, and also identify homozygous SNPs as a potential biomarker for development of SVD and MCI.
CONFLICTS OF INTEREST
NJA is co‐inventor of GPR39‐targeting compounds that have been licensed to a company with a commercial interest in the results; in the past 36 months, NJA co‐founded, then sold shares in, a company (Vasocardea) that was formed to develop GPR39‐targeting drugs and diagnostic probes. He is also founder of another company (NeuvaRx), formed to develop GPR39‐targeting drugs and diagnostic probes for central nervous system disorders. This potential conflict of interest has been reviewed and managed by OHSU. NJA has received royalties for a co‐invention ([18F]FNDP for PET Imaging of Soluble Epoxide Hydrolase), licensed to Precision Molecular. NJA has also received compensation from National Institutes of Health for grant reviews, and honoraria from academic universities for invited lectures. NJA has one patent issued (WO2017192854A1: Radiofluorinated FNDP for PET imaging of soluble epoxide hydrolase [sEH]), and two other provisional patents are pending (Use of GPR39 probes and ligands for the diagnosis and treatment of cardiovascular disease, and biomarkers for detection of coronary artery disease and its management). KG has received consultant fees from OHSU for coding tools. Other authors have declared that no conflict of interest exists.
Supporting information
Supplementary information
Supplementary information
ACKNOWLEDGMENTS
We would like to acknowledge expert technical assistance by staff in the OHSU Advanced Light Microscopy Core, supported by grant P30NS061800 and OHSU Biostatistics & Design Program (partially supported by the Oregon Clinical and Translational Research Institute [UL1TR002369]) for data analysis expertise. DNA quality assessment and qPCR assays were performed in the OHSU Gene Profiling Shared Resource, which receives support from the OHSU Knight Cancer Institute NCI Cancer Center Support Grant P30CA069533. We thank the Oregon Alzheimer's Disease Research Center (NIA P30AG066518, P30AG008017) and the Department of Veterans Affairs. In the past 36 months the following authors have received support outside this project: CMD, Oregon Partnership for Alzheimer's Research; JWN, NIH 1K01DK121737; LCS, NIH/NIA R01 AG056712; NJA, NINDS R01 NS108501. This work was supported by NIH grant to NJA 1RF1AG058273‐01.
Davis CM, Bah TM, Zhang WH, et al. GPR39 localization in the aging human brain and correlation of expression and polymorphism with vascular cognitive impairment. Alzheimer's Dement. 2021;7:e12214. 10.1002/trc2.12214
REFERENCES
- 1. 2020 Alzheimer's disease facts and figures. Alzheimers Dement, 2020. [DOI] [PubMed] [Google Scholar]
- 2. Iadecola C. The overlap between neurodegenerative and vascular factors in the pathogenesis of dementia. Acta Neuropathol. 2010;120(3):287‐296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Iadecola C. Vascular and metabolic factors in Alzheimer's disease and related dementias: introduction. Cell Mol Neurobiol. 2016;36(2):151‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer's disease. The Nun Study. JAMA. 1997;277(10):813‐817. [PubMed] [Google Scholar]
- 5. Bowler JV. Modern concept of vascular cognitive impairment. Br Med Bull. 2007;83:291‐305. [DOI] [PubMed] [Google Scholar]
- 6. Silbert LC, Dodge HH, Perkins LG, et al. Trajectory of white matter hyperintensity burden preceding mild cognitive impairment. Neurology. 2012;79(8):741‐747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nelson JW, Young JM, Borkar RN, et al. Role of soluble epoxide hydrolase in age‐related vascular cognitive decline. Prostaglandins Other Lipid Mediat. 2014;113‐115:30‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Doboszewska U, Wlaź P, Nowak G, Radziwoń‐Zaleska M, Cui R, Młyniec K. Zinc in the monoaminergic theory of depression: its relationship to neural plasticity. Neural Plast. 2017;2017:3682752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rychlik M, Mlyniec K. Zinc‐mediated neurotransmission in Alzheimer's disease: a potential role of the GPR39 in dementia. Curr Neuropharmacol. 2020;18(1):2‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Abramovitch‐Dahan C, Asraf H, Bogdanovic M, Sekler I, Bush AI, Hershfinkel M. Amyloid beta attenuates metabotropic zinc sensing receptor, mZnR/GPR39, dependent Ca(2+) , ERK1/2 and Clusterin signaling in neurons. J Neurochem. 2016;139(2):221‐233. [DOI] [PubMed] [Google Scholar]
- 11. Xu Y, Wang M, Xie Y, et al. Activation of GPR39 with the agonist TC‐G 1008 ameliorates ox‐LDL‐induced attachment of monocytes to endothelial cells. Eur J Pharmacol. 2019;858:172451. [DOI] [PubMed] [Google Scholar]
- 12. Voelkl J, Tuffaha R, Luong TTD, et al. Zinc inhibits phosphate‐induced vascular calcification through TNFAIP3‐mediated suppression of NF‐κB. J Am Soc Nephrol. 2018;29(6):1636‐1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Green MS, Kaye JA, Ball MJ. The Oregon brain aging study: neuropathology accompanying healthy aging in the oldest old. Neurology. 2000;54(1):105‐113. [DOI] [PubMed] [Google Scholar]
- 14. Silbert LC, Nelson C, Howieson DB, Moore MM, Kaye JA. Impact of white matter hyperintensity volume progression on rate of cognitive and motor decline. Neurology. 2008;71(2):108‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Slavin TP, Feng T, Schnell A, Zhu X, Elston RC. Two‐marker association tests yield new disease associations for coronary artery disease and hypertension. Hum Genet. 2011;130(6):725‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McKee KK, Tan CP, Palyha OC, et al. Cloning and characterization of two human G protein‐coupled receptor genes (GPR38 and GPR39) related to the growth hormone secretagogue and neurotensin receptors. Genomics. 1997;46(3):426‐434. [DOI] [PubMed] [Google Scholar]
- 17. Dong XY, He JM, Tang SQ, Li HY, Jiang QY, Zou XT. Is GPR39 the natural receptor of obestatin?. Peptides. 2009;30(2):431‐438. [DOI] [PubMed] [Google Scholar]
- 18. Holst B, Holliday ND, Bach A, Elling CE, Cox HM, Schwartz TW. Common structural basis for constitutive activity of the ghrelin receptor family. J Biol Chem. 2004;279(51):53806‐53817. [DOI] [PubMed] [Google Scholar]
- 19. Holst B, Egerod KL, Schild E, et al. GPR39 signaling is stimulated by zinc ions but not by obestatin. Endocrinology. 2007;148(1):13‐20. [DOI] [PubMed] [Google Scholar]
- 20. Sato S, Huang X‐P, Kroeze WK, Roth BL. Discovery and characterization of novel GPR39 agonists allosterically modulated by zinc. Mol Pharmacol. 2016;90(6):726‐737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hershfinkel M. The zinc sensing receptor, ZnR/GPR39, in health and disease. Int J Mol Sci. 2018;19(2):439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tremblay F, Richard AM, Will S, et al. Disruption of G protein‐coupled receptor 39 impairs insulin secretion in vivo. Endocrinology. 2009;150(6):2586‐2595. [DOI] [PubMed] [Google Scholar]
- 23. Depoortere I. GI functions of GPR39: novel biology. Curr Opin Pharmacol. 2012;12(6):647‐652. [DOI] [PubMed] [Google Scholar]
- 24. Fjellström O, Larsson N, Yasuda S, et al. Novel Zn2+ modulated GPR39 receptor agonists do not drive acute insulin secretion in rodents. PLoS One. 2015;10(12):e0145849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Doboszewska U, Młyniec K, Wlaź A, Poleszak E, Nowak G, Wlaź P. Zinc signaling and epilepsy. Pharmacol Ther. 2019;193:156‐177. [DOI] [PubMed] [Google Scholar]
- 26. Cuzon Carlson VC, Ford MM, Carlson TL, et al. Modulation of Gpr39, a G‐protein coupled receptor associated with alcohol use in non‐human primates, curbs ethanol intake in mice. Neuropsychopharmacology. 2019;44(6):1103‐1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Młyniec K, Budziszewska B, Holst B, Ostachowicz B, Nowak G. GPR39 (zinc receptor) knockout mice exhibit depression‐like behavior and CREB/BDNF down‐regulation in the hippocampus. Int J Neuropsychopharmacol. 2014;18(3):pyu002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mo F, Tang Y, Du P, et al. GPR39 protects against corticosterone‐induced neuronal injury in hippocampal cells through the CREB‐BDNF signaling pathway. J Affect Disord. 2020;272:474‐484. [DOI] [PubMed] [Google Scholar]
- 29. Jackson VR, Nothacker HP, Civelli O. GPR39 receptor expression in the mouse brain. Neuroreport. 2006;17(8):813‐816. [DOI] [PubMed] [Google Scholar]
- 30. Młyniec K, Budziszewska B, Reczyński W, Sowa‐Kućma M, Nowak G. The role of the GPR39 receptor in zinc deficient‐animal model of depression. Behav Brain Res. 2013;238:30‐35. [DOI] [PubMed] [Google Scholar]
- 31. Mlyniec K, Nowak G. GPR39 up‐regulation after selective antidepressants. Neurochem Int. 2013;62(7):936‐939. [DOI] [PubMed] [Google Scholar]
- 32. Młyniec K, Doboszewska U, Szewczyk B, et al. The involvement of the GPR39‐Zn(2+)‐sensing receptor in the pathophysiology of depression. Studies in rodent models and suicide victims. Neuropharmacology. 2014;79:290‐297. [DOI] [PubMed] [Google Scholar]
- 33. Ehrlich AT, Maroteaux G, Robe A, et al. Expression map of 78 brain‐expressed mouse orphan GPCRs provides a translational resource for neuropsychiatric research. Commun Biol. 2018;1:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Besser L, Chorin E, Sekler I, et al. Synaptically released zinc triggers metabotropic signaling via a zinc‐sensing receptor in the hippocampus. J Neurosci. 2009;29(9):2890‐2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Waller R, Baxter L, Fillingham DJ, et al. Iba‐1‐/CD68+ microglia are a prominent feature of age‐associated deep subcortical white matter lesions. PLoS One. 2019;14(1):e0210888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hort J, Vališ M, Kuča K, Angelucci F. Vascular cognitive impairment: information from animal models on the pathogenic mechanisms of cognitive deficits. Int J Mol Sci. 2019;20(10):2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Feng L, Gao J, Wang Y, Cheong YK, Ren G, Yang Z. Etidronate‐zinc complex ameliorated cognitive and synaptic plasticity impairments in 2‐vessel occlusion model rats by reducing neuroinflammation. Neuroscience. 2018;390:206‐217. [DOI] [PubMed] [Google Scholar]
- 38. Grassivaro F, Menon R, Acquaviva M, et al. Convergence between microglia and peripheral macrophages phenotype during development and neuroinflammation. J Neurosci. 2020;40(4):784‐795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Koizumi T, Kerkhofs D, Mizuno T, Steinbusch HWM, Foulquier S. Vessel‐Associated immune cells in cerebrovascular diseases: from perivascular macrophages to vessel‐associated microglia. Front Neurosci. 2019;13:1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Alarcon‐Martinez L, Yilmaz‐Ozcan S, Yemisci M, et al. Capillary pericytes express alpha‐smooth muscle actin, which requires prevention of filamentous‐actin depolymerization for detection. Elife. 2018;7:e34861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Peppiatt CM, Howarth C, Mobbs P, Attwell D. Bidirectional control of CNS capillary diameter by pericytes. Nature. 2006;443(7112):700‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hall CN, Reynell C, Gesslein B, et al. Capillary pericytes regulate cerebral blood flow in health and disease. Nature. 2014;508(7494):55‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hamilton NB, Attwell D, Hall CN. Pericyte‐mediated regulation of capillary diameter: a component of neurovascular coupling in health and disease. Front Neuroenergetics. 2010;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bell RD, Winkler EA, Sagare AP, et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68(3):409‐427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Montagne A, Nikolakopoulou AM, Zhao Z, et al. Pericyte degeneration causes white matter dysfunction in the mouse central nervous system. Nat Med. 2018;24(3):326‐337. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46. De Silva TM, Faraci FM. Contributions of aging to cerebral small vessel disease. Annu Rev Physiol. 2020;82:275‐295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Iadecola C. The pathobiology of vascular dementia. Neuron. 2013;80(4):844‐866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Moretti R, Caruso P. Small vessel disease‐related dementia: an invalid neurovascular coupling?. Int J Mol Sci. 2020;21(3):1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Brown WR, Thore CR. Review: cerebral microvascular pathology in ageing and neurodegeneration. Neuropathol Appl Neurobiol. 2011;37(1):56‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dershem R, Metpally RPR, Jeffreys K, et al. Rare‐variant pathogenicity triage and inclusion of synonymous variants improves analysis of disease associations of orphan G protein‐coupled receptors. J Biol Chem. 2019;294(48):18109‐18121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Selvanayagam T, Walker S, Gazzellone MJ, et al. Genome‐wide copy number variation analysis identifies novel candidate loci associated with pediatric obesity. Eur J Hum Genet. 2018;26(11):1588‐1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information
Supplementary information


