Abstract
Introduction
The impact of the COVID‐19 pandemic on the global use of anti‐dementia medication is unknown. We aimed to determine the changes of anti‐dementia medication use in Europe (EU) and North America (NA) during the pandemic.
Methods
This is a cross‐sectional study using sales data of anti‐dementia medications in 2019 and 2020 from 34 EU and NA countries. The monthly uses of anti‐dementia medications from January through June in 2020 were compared to the corresponding months in 2019 for each country.
Results
In the pre‐pandemic period of January to March 2020, 70 out of 102 (3 months x 34 countries) measurements (68.6%) of monthly sales volume showed an increase. In contrast, 76.5% and 85.3% countries showed reduced sales in April and May 2020, respectively.
Discussion
These findings indicate changes in use of anti‐dementia medications during the pandemic. The delivery of pharmaceutical care for dementia patients may be heavily disrupted in certain countries.
Keywords: anti‐dementia medications, COVID‐19, dementia, global health
1. BACKGROUND
The coronavirus disease 2019 (COVID‐19) pandemic was expected to have a tremendous impact on patients with dementia. 1 The severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection and dementia share common risk factors such as old age and co‐morbidities, and patients living with dementia are particularly vulnerable to the infection. 2 , 3 The lockdown measures could have additional adverse disruptions to the diagnosis and delivery of care for dementia, and result in further strains on physical and mental well‐being of the patients.
Anti‐dementia medications are those used to control the progression of dementia. 4 Four agents are licensed for the pharmaceutical management of dementia, namely donepezil, memantine, galantamine, and rivastigmine. Given that family and social support to patients with dementia could have been heavily disrupted by the pandemic and lockdown measures, 5 pharmaceutical care with anti‐dementia medications becomes increasingly valuable in relieving the dementia burden. However, it may also be more difficult to initiate or maintain anti‐dementia treatment during the lockdowns in patients with dementia due to reduced access to dementia clinics. In this study, we used country‐level pharmaceutical sales data in European (EU) and North American (NA) countries to evaluate impact of the COVID‐19 pandemic and national lockdown measures on anti‐dementia medication use. We hypothesized that the use of anti‐dementia medications would be reduced during the pandemic.
2. METHODS
We conducted a cross‐sectional study nested within the sales data from the IQVIA‐Multinational Integrated Data Analysis System (MIDAS) database. The IQVIA‐MIDAS database provides long‐term sales data from a large number of countries and has been validated against external data sources. 6 The database has been used for epidemiological studies. 7 , 8 The database contains monthly pharmacy sales data for individual products in retail and hospital pharmacies collected from wholesalers in different countries. In countries with limited raw data, projections have been applied in IQVIA‐MIDAS to represent 100% of the total market sales volume based on knowledge of market share.
Country‐level data on the monthly sales of the four anti‐dementia medications were analyzed from January to June 2020 and the same months in 2019. January to March 2020 was defined as pre‐pandemic period and April to June was defined as the pandemic period, as most EU and NA countries instigated lockdown measures in late March. The sales data were measured in the defined daily dose (DDD) using the World Health Organization Anatomical Therapeutic Chemical classification system. After standardizing to the population of each country from the United Nations Population Division, 9 we estimated the use rates of the medications in the unit of DDD per 1000 inhabitants per day (DDDTID). We calculated the percentage changes in the monthly sales volumes of 2020 compared to the same months in 2019 for each country. There were six monthly comparisons from 34 countries, and in total 204 measurements (102 before and 102 during pandemic). All data were analyzed using SAS version 9.4 (SAS Institute).
3. RESULTS
There were 34 countries (32 EU countries and 2 NA countries) with data on use of anti‐dementia medications available. During the pre‐pandemic period of January to March 2020, there were 102 measurements (3 months from each country), 70 out of 102 measurements (68.6%) of monthly sales volume showed an increase in 2020 compared to the same month in 2019. In March 2020, the month starting lockdown, 28 out of the 34 countries (82.3%) had an increase in consumption volume. The percentage change in March 2020 ranged from –29.2% in Denmark to +59.0% in Belarus (Figure 1A).
FIGURE 1.
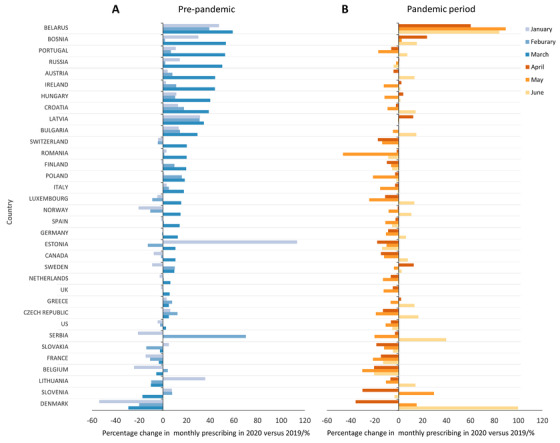
Percentage changes in the use of anti‐dementia medications from January to June 2020 in 34 European Union/North American countries, compared to the corresponding months in 2019, (A) pre‐pandemic period (January–March); (B) pandemic period (April–June)
During April to June 2020 after lockdown was initiated, 69 out of the 102 measurements (67.7%) of monthly anti‐dementia medication use showed a decrease in 2020 compared to the same months in 2019. The sales of anti‐dementia medication started to increase in June (20 out of the 34 countries, 58.8%; Figure 1B).
RESEARCH IN CONTEXT
Systematic review: The authors reviewed the literature using PubMed sources. Although a few publications have highlighted the challenges in the care and management of patients with dementia during the COVID‐19 pandemic, data on the use of anti‐dementia medications during the pandemic is lacking. These relevant citations are appropriately mentioned.
Interpretation: Our findings showed changes in the use pattern of anti‐dementia medications during the pandemic. The general rise in the sales of anti‐dementia medications during the prepandemic period in EU and NA countries might reflect stockpiling of medications, while the reduction within the pandemic period may be due to reduced access to health‐care facilities. The delivery of pharmaceutical care for dementia patients may be heavily disrupted in certain countries.
Future directions: Further studies are warranted to investigate factors contributing to the changes in each country and support future delivery of pharmaceutical care to patients with dementia during the continuing COVID‐19 pandemic.
4. DISCUSSION
In this study, we presented the monthly changes in anti‐dementia medication sales over 6 months before and during the COVID‐19 pandemic, compared to the corresponding months in 2019, in 34 EU and NA countries. Overall, we observed increased sales during the pre‐pandemic period, with the most noticeable increase in March. During the pandemic period, there was reduced use in April and May, while an increase was observed in June.
There was a clear difference in the use patterns for anti‐dementia medications 3 months before and during the pandemic. Previous studies have reported a general trend increasing anti‐dementia prescriptions in EU and NA countries in recent years. 10 , 11 In our study, we found 82.3% countries had increased sales volume in March 2020, which may reflect preparation for the upcoming lockdown by health‐care providers on top of the general increased prescribing trend. However, the significant drops in use after lockdown may demonstrate the impact of the COVID‐19 crisis. Apart from the health‐care sector's preparation for the COVID‐19 crisis, the outbreak of the pandemic and introduction of the lockdown measures may have induced a changed demand for pharmaceutical products for the management of chronic disorders. The demand for prescription medicines increased after the emergence of infected cases but before the lockdown may be due to preparation by health‐care providers and stockpiling of medications by patients, while the lockdown policies in most of the countries during April and May reduced patient–prescriber contact, resulting in a reduced access to prescriptions. 12 In addition, the global supply shortage of pharmaceutical products, induced by decreased production capacity, ruptured supply chains, and earlier stockpiling practices, may have further contributed to the decline in use volumes in April and May. 13
There was a marked inter‐country difference in the use of anti‐dementia medications before and during the pandemic period. While most countries had increased sales in March followed by a decline in April and May in 2020, some countries did not follow this pattern. For example, in Romania, a high rate of decline in anti‐dementia medication sales was observed in May (–46.5%), compared to relatively small increases during the 3‐month pre‐lockdown period (+3.0% in January, +1.2% in February, and 20.0% in March). This suggests there might be an unmet need for medication for patients with dementia. Similar results were also observed in other countries, for example, Luxembourg and the United Kingdom. On the contrary, the highly increased sales volume in March in certain other countries was not coupled with a reduction during the post‐lockdown period, for example, Russia and Austria, suggesting a lack of regulatory policies to control the stockpiling practice. 14
This study has limitations. First, we could not analyze patient factors associated with the use of anti‐dementia medications during the pandemic period as we do not have patient‐level data in the database. Second, we are not able to establish a causal relationship between the observed changes and the COVID‐19 outbreak. Other interventions or factors occurring during the study period could have affected our results. However, no previous studies have been conducted to provide data on the global use of anti‐dementia medications during the COVID‐19 pandemic.
In conclusion, anti‐dementia medication use changed drastically in EU and NA countries during the COVID‐19 pandemic period from January to June 2020, compared to the corresponding months in 2019. A common pattern of an increased use in March followed by a decline in April and May was observed in the majority of countries. The general rise in the sales of anti‐dementia medications during the pre‐pandemic period in EU and NA countries might reflect stockpiling of medications, while the reduction within the pandemic period may be due to reduced access to health‐care facilities. Variation in the changes across countries existed. Appropriate policy to deliver dementia care under ongoing population‐wide lockdown measures is urgently needed for some countries.
CONFLICTS OF INTEREST
JFH is supported by grants from the Wellcome Trust, University College London Hospitals NIHR Biomedical Research Centre and the NIHR North Thames Applied Research Collaboration. DO is supported by the National Institute for Health Research (NIHR) Biomedical Research Centre (BRC) at University College London Hospitals (UCLH). DO is also supported by the National Institute for Health Research ARC North Thames. KKCM is supported by the CW Maplethorpe Fellowship; has received grants from National Institute of Health Research, European Commission Horizon 2020 Framework; personal fee from IQVIA Ltd; not related to the current study. EWC has received honorarium from the Hospital Authority, research grants from Narcotics Division of the Security Bureau of HKSAR, National Health and Medical Research Council (NHMRC, Australia), National Natural Science Foundation of China (NSFC), Research Fund Secretariat of the Food and Health Bureau (HMRF, HKSAR), Research Grants Council (RGC, HKSAR), Wellcome Trust; Amgen, AstraZeneca, Bayer, Bristol‐Myers Squibb, Janssen, Pfizer, RGA and Takeda outside the submitted work. ICKW has received research funding outside the submitted work from Amgen, Bristol‐Myers Squibb, Pfizer, Janssen, Bayer, GSK, Novartis, the Hong Kong Research Grants Council, and the Hong Kong Health and Medical Research Fund, National Institute for Health Research in England, European Commission, National Health and Medical Research Council in Australia but none is associated with current study. He also received speaker fees from Janssen and Medice in the previous 3 years.
AUTHOR CONTRIBUTIONS
Chengsheng Ju conducted the data analysis, interpreted the data, and wrote the original draft under the supervision of Li Wei. Chengsheng Ju, Wallis C.Y. Lau and Li Wei conceptualized the study, interpreted the data, and critically reviewed and commented on all drafts. Ian C.K. Wong and Li Wei provided resources and acquired the data. Joseph F. Hayes, David Osborn, Kenneth K.C. Man, Esther W. Chan, and Ian C.K. Wong interpreted the data and critically reviewed and commented on all drafts.
ACKNOWLEDGMENT
The collaboration between UCL and HKU is supported by the Laboratory of Data Discovery for Health Limited (D24H), Hong Kong Science Park, New Territories, Hong Kong.
Ju C, Lau WCY, Hayes JF, et al. Impact of the COVID‐19 pandemic on use of anti‐dementia medications in 34 European and North American countries. Alzheimer's Dement. 2021;7:e12206. 10.1002/trc2.12206
Funding information
None
REFERENCES
- 1. Mok VCT, Pendlebury S, Wong A, et al. Tackling challenges in care of Alzheimer's disease and other dementias amid the COVID‐19 pandemic, now and in the future. Alzheimers Dement. 2020;16(11):1571‐1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Verity R, Okell LC, Dorigatti I, et al. Estimates of the severity of coronavirus disease 2019: a model‐based analysis. Lancet Infect Dis. 2020;20(6):669‐677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Petrazzuoli F, Vinker S, Palmqvist S, et al. Unburdening dementia ‐ a basic social process grounded theory based on a primary care physician survey from 25 countries. Scand J Prim Health Care. 2020;38(3):253‐264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dening KH, Lloyd‐Williams M. Minimising long‐term effect of COVID‐19 in dementia care. Lancet. 2020;396(10256):957‐958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. ACTS: IQVIA Quality assurance 2019 IQVIA . Available from: https://www.iqvia.com/library/publications/acts-2019-33rd-edition-quality-assurance-report-of-iqvia 2021. [Google Scholar]
- 7. Hsia Y, Sharland M, Jackson C, et al. Consumption of oral antibiotic formulations for young children according to the WHO Access, Watch, Reserve (AWaRe) antibiotic groups: an analysis of sales data from 70 middle‐income and high‐income countries. Lancet Infect Dis. 2019;19(1):67‐75. [DOI] [PubMed] [Google Scholar]
- 8. Jackson C, Hsia Y, Bielicki JA, et al. Estimating global trends in total and childhood antibiotic consumption, 2011‐2015. BMJ Glob Health. 2019;4(1): e001241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. World Population Prospectives: United Nations Population Divisions: United Nations Population Divisions . Available from: https://www.un.org/development/desa/pd/accessed 2020. [Google Scholar]
- 10. Donegan K, Fox N, Black N, et al. Trends in diagnosis and treatment for people with dementia in the UK from 2005 to 2015: a longitudinal retrospective cohort study. Lancet Public Health. 2017;2(3):e149‐e56. [DOI] [PubMed] [Google Scholar]
- 11. Hernandez I, Zhang Y. Pharmaceutical use and spending trend in Medicare beneficiaries with dementia, from 2006 to 2012. Gerontol Geriatr Med. 2017;3:2333721417704946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ayati N, Saiyarsarai P, Nikfar S. Short and long term impacts of COVID‐19 on the pharmaceutical sector. Daru. 2020;28(2):799‐805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. European Commission : Guidelines on the optimal and rational supply of medicines to avoid shortages during the COVID‐19 outbreak. European Commission, 2020. [Google Scholar]
- 14. Romano S, Galante H, Figueira D, et al. Time‐trend analysis of medicine sales and shortages during COVID‐19 outbreak: data from community pharmacies. Res Social Adm Pharm. 2021;17(1):1876‐1881. [DOI] [PMC free article] [PubMed] [Google Scholar]


