Abstract
Dry eye (DE), especially severe DE (SDE), can cause ocular surface defects and reduce the patient's quality of life. Several clinical studies have shown that 0.1% cyclosporin A cationic emulsion (CsA CE) could decrease corneal damage. However, no experimental study has reported the effect of 0.1% CsA CE on SDE. The present study aimed to compare the efficacy of 0.1% CsA CE with that of 0.05% CsA emulsion for ocular surface damage and inflammation in the cases of murine DE with different severities. Following exposure to desiccating stress and subcutaneous injection of scopolamine for 5 days, C57BL/6 female mice were divided into SDE and non-SDE (NSDE) groups based on corneal fluorescein staining scores (CFSs). Mice from both groups were topically treated with 0.05% CsA emulsion or 0.1% CsA CE for 10 days. The results demonstrated that 0.1% CsA CE-treated mice in the SDE and NSDE groups exhibited significant improvements in all the clinical and experimental parameters. Furthermore, the CFS of 0.1% CsA CE-treated mice in the SDE group was lower compared with that of the 0.05% CsA-treated mice. In addition, in the SDE group, 0.1% CsA CE-treated mice had significantly lower levels of nuclear factor-κB activation, inflammatory infiltrations and apoptosis on the ocular surface, and they also exhibited higher conjunctival goblet cell density compared with the 0.05% CsA-treated mice. In summary, these findings indicated that 0.1% CsA CE was more effective than topical 0.05% CsA emulsion at improving corneal epithelial injury and decreasing the levels of inflammatory cytokines and T cells in mice with SDE.
Keywords: severe dry eye, cyclosporin A, cationic emulsion, inflammation, ocular surface damage
Introduction
Dry eye (DE) is a multifactorial disease of the tear film and ocular surface that is characterized by multiple symptoms, including discomfort, visual disturbance and tear film instability, which may potentially damage the ocular surface (1). DE is accompanied by increased osmolarity of the tear film and inflammation of the ocular surface (2,3). Long-term progression of inflammation at the ocular surface has the potential to aggravate symptoms and signs, resulting in severe DE (SDE). SDE is associated with an increased risk of infection, vision loss and ocular surface epithelial defects (4). Numerous patients with SDE report ocular pain, which may reduce their quality of life due to ocular surface damage (5,6).
At present, topical 0.05% cyclosporin A (CsA; Restasis®; Allergan), an anionic oil in water emulsion possessing anti-inflammatory properties, has demonstrated marked efficacy for DE treatment (7). Following treatment with 0.05% CsA emulsion, patients with DE exhibited ameliorated symptoms and improvements in other indicators, including improvements in the ocular surface disease index (OSDI), Schirmer values and corneal fluorescein staining scores (CFS) (8-10). However, in patients with severe inflammatory forms of DE, such as graft-versus-host disease (GVHD) or Sjögren syndrome (SS), the use of 0.05% CsA emulsion twice daily was found to have limitations in controlling ocular surface inflammations (11).
In previous clinical studies published over the last decade, marked improvements have been observed for subjective symptoms (i.e., based on the OSDI, Schirmer test, and CFS) in patients with severe keratoconjunctivitis, including GVHD and SS, following treatment with 0.1% CsA cationic emulsion (CsA CE; iKervis®; Santen Pharmaceutical Co., Ltd.) compared with 0.05% CsA emulsion (12-14). Although clinical studies have demonstrated that 0.1% CsA CE leads to an improvement in symptoms and indicators in patients following treatment for longer or shorter periods of time, to the best of our knowledge, no study has investigated the effect of 0.1% CsA CE topical application on ocular surface inflammation and damage in experimental DE (EDE). The aim of the present study was therefore to investigate the therapeutic effects of topical 0.1% CsA CE treatment on tear film parameters [tear volume and tear film break-up time (BUT)], ocular surface damage (via evaluating CFS) and inflammatory properties (i.e., the levels of inflammatory cytokines and T cells) in a murine model of EDE with different severities and to compare these effects with those of topical 0.05% CsA emulsion treatment.
Materials and methods
Design of the mouse model and in vivo experiments
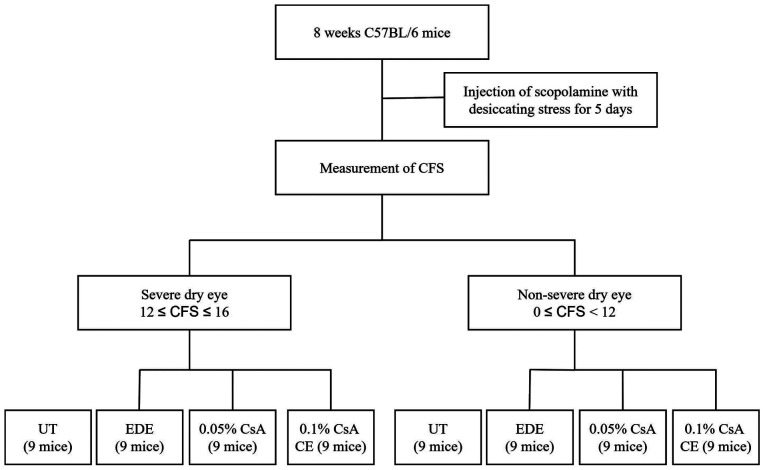
The research protocol was approved by the Chonnam National University School Research Institutional Animal Care and Use Committee (approval no. CNU IACUC-H-2018-73). EDE was induced by desiccating stress (exposure to an air draft all day; 30% ambient humidity) and subcutaneous injection of scopolamine (0.5 mg, 0.2 ml; MilliporeSigma) three times a day (at 9 a.m., 1:30 p.m. and 6 p.m.) as previously described (15,16). In the present study, 8-week-old female C57BL/6 mice (weight, 16.0±2 g) were used, and EDE was induced by desiccating stress (exposure to an air draft all day and 30% ambient humidity) and subcutaneous injection of scopolamine (0.5 mg, 0.2 ml; MilliporeSigma) three times a day (at 9 a.m., 1:30 p.m. and 6 p.m.) as previously described (15,16). A total of 54 EDE-induced mice were divided into two groups based on the CFSs: i) The SDE group (12≤CFS≤16); and ii) the NSDE group (0≤CFS<12; Fig. 1). In addition, 27 EDE-induced mice from each group were separated into three subgroups of 9 mice according to topical treatment as follows: i) EDE group, where mice were exposed to desiccating stress and received no eye drops; ii) the 0.05% CsA group, where EDE mice were treated with 2 µl 0.05% CsA emulsion twice daily (Restasis®; Allergan); iii) the 0.1% CsA CE group, where EDE mice were treated with 2 µl 0.1% CsA CE once daily (iKervis®; Santen). The mice not exposed to desiccating stress were used as untreated (UT) controls.
Figure 1.
Study design for the SDE and NSDE groups. The mice were divided into the SDE and NSDE groups based on CFSs, after 5 days of EDE induction. Euthanasia was performed at day 15 after initiation of the treatment, and corneal and conjunctival tissues were collected for analysis. SDE, severe dry eye; NSDE, non-severe dry eye; CFS, corneal fluorescein staining; CsA, cyclosporin A; CE, cationic emulsion; UT, untreated; EDE, experimental dry eye.
The mice in all treatment groups, except for the UT group, received scopolamine injections. Nine animals in each subgroup were used for clinical and experimental analysis and no animal was found dead before euthanasia. Tear film parameters (tear volume and tear film BUT) and CFSs were evaluated after 5 and 10 days of CsA application. After assessment of the clinical parameters, mice were deeply anesthetized with 3% sevoflurane and intraperitoneal injection of sodium pentobarbital (50 mg/kg). Transcardial perfusion was subsequently performed with 4% paraformaldehyde in phosphate buffer (pH 7.4) for euthanasia. Animals were euthanized by experienced experts and when needed, medications and supplies were available, minimizing pain and stress for the animals. Euthanasia was performed in accordance with the AVMA Animal Euthanasia Guidelines: 2020 Edition (https://www.avma.org). Following euthanasia, animal death was confirmation by the absence of cardiovascular and respiratory movements. Western blotting, multiplex immunobead assay and flow cytometric analysis, histological analysis and TUNEL staining were performed at day 15 after initiation of the treatment. During these experiments, animals were treated in accordance with ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, and animal movement, food and water intake were not restricted (https://www.arvo.org/About/policies/statement-for-the-use-of-animals-in-ophthalmic-and-vision-research/#three). Experiments involving EDE induction and CsA application lasted 15 consecutive days and the experiment was repeated three times. In addition, all clinical and laboratory analyzes were performed after each experiment. As all experiments were repeated 3 times, a total of 216 mice were used within the present study.
Evaluation of tear film parameters and corneal epithelial damage
Tear volume was measured using phenol red impregnated cotton threads (Zone-Quick™; Oasis Medical, Inc.), and the threads were placed in the lateral canthus for 20 sec as previously described (17,18). The length of the wet red thread was measured in mm under a photomicroscope (light microscope; magnification, x1; SMZ 1500; Nikon Corporation).
After allowing 1 µl of 1% sodium fluorescein to fall into the inferior conjunctival sac for 20 sec, the ocular surface was washed with PBS and the tear film BUT (in sec) was recorded using slit lamp biomicroscopy (BQ-900; Haag-Streit Diagnostics) under cobalt blue light. The cornea was distributed into four parts, which were scored separately. The CFSs were calculated according to a 4 point scale and added together to obtain a final score (range, 0-16) as previously described (19).
Western blotting
The expression of the nuclear factor (NF)-κB p65 protein was determined using western blotting. Proteins were extracted from the conjunctival tissues (4 eyes per group) using lysis buffer (RIPA buffer; GeneAll Biotechnology Co., Ltd.) supplemented with a protease inhibitor cocktail (cat. no. 11836153001; Roche Diagnostics GmbH) on ice, and lysates were centrifuged at 25,200 x g for 10 min at 4˚C, as previously described (20). Proteins (20 µg) were separated by 12% SDS-PAGE and were transferred onto PVDF membranes. Membranes were washed with TBST-Tween-20 [TBST; 10 mM Tris-HCl (pH 7.6), 150 mM NaCl and 0.05% Tween-20] and blocked with 5% skimmed milk in TBST for 1 h at room temperature. Membranes were incubated for 2 h at room temperature with the following primary antibodies: Rabbit polyclonal antibody against NF-κB p65 (cat. no. ab16502; Abcam; diluted by 1:1,000), rabbit anti-phosphorylated NF-κB p65 (cat. no. ab76302; Abcam; diluted by 1:500) and anti-β-actin (cat. no. ab8227; Abcam; diluted by 1:1,000). The membranes were washed three times with 1X TBST buffer for 5 min and incubated with secondary antibodies goat anti-rabbit IgG H&L (cat. no. ab205718; Abcam; diluted by 1:5,000, 1:5,000, and 1:10,000 for visualization of NF-κB p65, phosphorylated NF-κB p65 and β-actin antibodies, respectively) diluted in 1X TBST for 60 min at room temperature. After incubation, membranes were washed five times with TBST for 5 min. Enhanced chemiluminescence system (ECL Blotting Analysis System; Cytiva) was used to detect the signal on the membrane. The data were analyzed via densitometry (Alliance MINI HD9; UVItec Ltd.) and normalized to the expression of the internal control β-actin.
Multiplex immunobead assay
The levels of tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), interleukin (IL)-6, IL-17 and IL-21 in mice conjunctiva (six eyes per group) were evaluated using MILLIPLEX MAP Mouse Cytokine/Chemokine Magnetic Bead Panel - Immunology Multiplex Assay kit (all from Milliplex®; MilliporeSigma; cat. no. MCYTOMAG-70K) and the Luminex 200 detection method (Luminex Corporation) as previously described (16). The conjunctival tissues (10 mg) were collected, pooled and lysed in TissueLyser lysis buffer (Qiagen, Inc.) containing protease inhibitors (cat. no. 11836153001; Roche Diagnostics GmbH) for 30 min on ice. The extracts were subsequently centrifuged at 14,000 x g for 15 min at 4˚C. After centrifugation, the samples were added to a 96-well plate (25 µl/well) and incubated overnight at 4˚C in the dark with 25 µl 1X beads coupled to mouse cytokine/chemokine-specific antibodies. Serial dilutions of each cytokine/chemokine were also performed on the same plate to generate a standard curve. The following day, the beads were washed and mixed with 25 µl 1X biotinylated secondary cytokine/chemokine antibody mixture for 1 h at room temperature, followed by a wash and subsequent incubation with 25 µl streptavidin-phycoerythrin for 30 min at room temperature (both steps performed in the dark). After a final wash, the beads were resuspended in 150 µl sheath fluid assay buffer. The reactions were detected after addition of streptavidin-phycoerythrin using an analysis system (xPONENT; Luminex Corporation). The concentrations of cytokines in the tissues were calculated using standard curves of known concentrations of recombinant mouse cytokines.
Flow cytometric analysis
The percentages of CD4+ IFN-γ+ T cells and CD4+ IL-17+ T cells in mice cornea and conjunctiva (6 eyes per group) were evaluated using flow cytometric analysis as previously described (21). Tissues from each group were surgically removed and immersed in PBS. Subsequently, samples were torn apart with scissors and incubated with 0.5 mg/ml collagenase type D (Roche Applied Science) under agitation at 37˚C for 45 min. The samples were disrupted by grinding using a syringe plunger and subsequently passed through a cell strainer with a pore size of 100 µm. Cells were then centrifuged for 7 min at 450 x g at 4˚C. Subsequently, samples were resuspended in PBS containing 1% BSA, then 2 µl of fluorescein-conjugated anti-CD4 antibody (0.5 mg/ml; cat. no. 553651; BD Biosciences), phycoerythrin-conjugated anti-IFN-γ-antibody (0.5 mg/ml; cat. no. 554412; BD Biosciences) and phycoerythrin-conjugated anti-IL-17 antibody (0.5 mg/ml; cat. no. 561020; BD Biosciences) were added for an incubation at 4˚C for 30 min. Phycoerythrin-conjugated rat IgG isotype (BD Biosciences) was used as the control. The percentage of CD4+ IFN-γ+ and CD4+ IL-17+ T cells were evaluated using a FACSCalibur cytometer with CellQuest software (version 5.2.1; BD Biosciences).
Histological analysis
Mice eye and adnexa were surgically excised, fixed in 4% paraformaldehyde overnight at 4˚C and embedded in paraffin. Sections (thickness, 6 µm) were stained with Periodic Acid-Schiff reagent (cat. no. 395B-1 KT; MilliporeSigma; Merck KGaA) for 15 min at room temperature, and those obtained from four animals in each group were subsequently examined and imaged using a light microscope (magnification, x10; Olympus Corporation) equipped with a digital camera. Goblet cell density in the superior and inferior conjunctiva was measured in three sections from each eye using Image-Pro version 10.0.5 (Medial Cybernetics, Inc.) and was expressed as the number of goblet cells per 100 µm.
TUNEL staining
A TUNEL assay was used to detect the 3'hydroxyl ends of the fragmented DNA as an early event in the apoptotic cascade and to identify apoptotic cells. Mice eye and adnexa were surgically excised, fixed in 4% paraformaldehyde overnight at 4˚C and embedded in paraffin. After deparaffinization and washing, the samples were rehydrated by sequential immersion. Graded ethanol washes (100, 95, 85, 70 and 50%) were performed at room temperature for 3 min each. After rehydration, the tissues were immersed in a 4% methanol-free formaldehyde solution in PBS for 15 min at room temperature to fix the tissue. The slides were then incubated in 20 µg/ml proteinase K for 10 min at room temperature, before being rinsed with PBS for 5 min. The samples were subsequently incubated in terminal deoxynucleotidyl transferase, recombinant, enzyme containing equilibration buffer and nucleotide mix for 60 min at 37˚C in the dark. The reaction was terminated by adding 2X saline-sodium citrate buffer for 15 min. The samples were washed three times with PBS for 5 min and stained with VECTASHIELD® and DAPI. Staining was evaluated using the DeadEnd™ Fluorometric TUNEL System (Promega Corporation) according to the manufacturer's instructions. The images were observed on a Leica TCS SP5 AOBS laser scanning confocal microscope (Zeiss GmbH) under an LSM 800 10x (N.A. 0.4) oil objective. Cell images were obtained separately with the following fluorescence excitation and emission settings: Excitation wavelengths at 405 and 488 nm and emission wavelengths between 424-472 and 502-550 nm for TUNEL assay and DAPI staining, respectively. TUNEL positive cells and nuclear staining of cells with DAPI in the cornea were viewed under a fluorescent microscope (magnification, x20).
Statistical analyses
SPSS software (version 18.0; SPSS, Inc.) was used for all statistical analyses. Data were presented as the means ± standard deviation. The normal distribution of the data was verified using Kolmogorov-Smirnov test. Statistical differences for tear volume, tear film BUT and CSS among the groups were determined using one-way ANOVA tests followed by Dunnett's post hoc tests (sphericity assumptions were evaluated with a Mauchly's test, and in the case of violation, the data were adjusted with an Epsilon Greenhouse-Geisser statistic). A Kruskal-Wallis test followed by a Dunn's multiple comparisons post-hoc test was used to compare the expression levels of NF-κB, the cytokine levels and the goblet cell density and apoptotic cell density data derived from the flow cytometric analysis experiments between the groups. P<0.05 was considered to indicate a statistically significant difference.
Results
Tear film parameters on the ocular surface
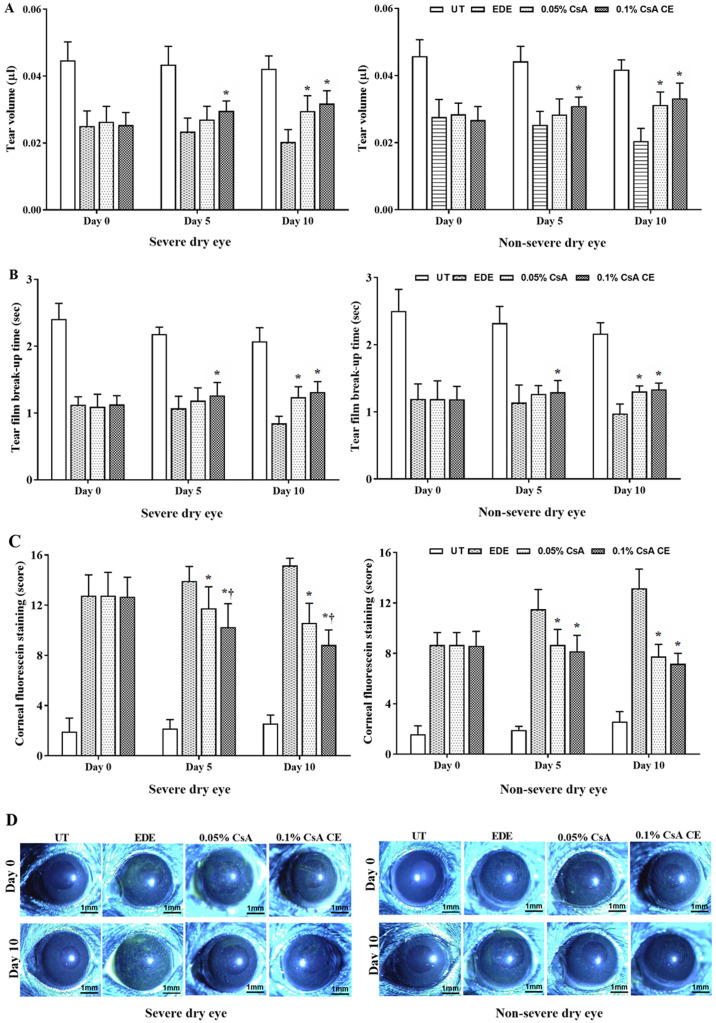
Mean tear volumes of the SDE group at 5 and 10 days were 0.043±0.005 µl and 0.042±0.004 µl in UT mice, 0.023±0.004 µl and 0.02±0.004 µl in EDE mice, 0.027±0.004 µl and 0.03±0.005 µl in 0.05% CsA-treated mice, and 0.03±0.003 µl and 0.032±0.004 µl in 0.1% CsA CE-treated mice, respectively. In addition, mean tear volumes of the NSDE group at 5 and 10 days were 0.044±0.004 µl and 0.042±0.003 µl in UT mice, 0.025±0.004 µl and 0.020±0.004 µl in EDE mice, 0.028±0.005 µl and 0.031±0.004 µl in 0.05% CsA-treated mice, and 0.031±0.003 µl and 0.033±0.005 µl in 0.1% CsA CE-treated mice, respectively (Fig. 2A). Mice treated with 0.1% CsA CE in the SDE and NSDE groups exhibited a significant increase in tear volume compared with EDE mice at 5 and 10 days, whereas 0.05% CsA-treated mice in both groups had an improvement in tear volume at 10 days (all P<0.05). No significant differences were observed between 0.05% CsA and 0.1% CsA CE-treated mice from the two groups.
Figure 2.
Analysis of clinical parameters. (A) Mean tear volume, (B) tear film break-up time, (C) corneal fluorescein staining scores and (D) representative images of corneal fluorescein staining in UT, EDE, 0.05% CsA emulsion-treated and 0.1% CsA CE-treated mice of the severe dry eye and non-severe dry eye groups at 0 and 10 days. *P<0.05 vs. EDE΄ †P<0.05 vs. 0.05% CsA. UT, untreated control; EDE, experimental dry eye; CsA, cyclosporin A; CE, cationic emulsion.
The mean tear film BUTs in the SDE group at 5 and 10 days were 2.18±0.10 and 2.07±0.21 sec in UT mice, 1.07±0.18 and 0.85±0.10 sec in EDE mice, 1.18±0.19 and 1.24±0.16 sec in 0.05% CsA-treated mice, and 1.26±0.19 and 1.32±0.15 sec in 0.1% CsA CE-treated mice, respectively. Tear film BUTs in the NSDE group at 5 and 10 days were 2.32±0.25 and 2.16±0.16 sec in the UT mice, 1.14±0.26 and 0.97±0.14 sec in the EDE mice, 1.27±0.12 and 1.30±0.08 sec in the 0.05% CsA mice, and 1.29±0.18 and 1.34±0.09 sec in 0.1% CsA CE-treated mice, respectively (Fig. 2B). Mice treated with 0.1% CsA CE in the NSDE and SDE groups had a significantly higher tear film BUT compared with EDE treated mice at 5 and 10 days (all P<0.05); however, no significant differences were identified with 0.05% CsA-treated mice. The 0.05% CsA-treated mice in the two groups did present with an increased tear film BUT compared with EDE mice at 10 days (all P<0.05).
Ocular surface damages
The mean CFSs in the SDE group for UT, EDE, 0.05% CsA and 0.1% CsA CE-treated mice at days 5 and 10 were 2.17±0.72 and 2.58±0.67, 13.92±1.17 and 15.17±0.58, 11.75±1.71 and 10.58±1.56, and 10.25±1.87 and 8.83±1.19, respectively. Mean CFSs in the NSDE group at 5 and 10 days were 1.92±0.29 and 2.58±0.79 (UT mice), 11.50±1.57 and 13.17±1.53 (EDE mice), 8.67±1.23 and 7.75±0.97 (0.05% CsA-treated mice), and 8.17±1.27 and 7.17±0.84 (0.1% CsA CE-treated mice), respectively (Fig. 2C and D). Mice treated with 0.1% CsA CE and 0.05% CsA in both groups exhibited a significantly decreased CFS compared with EDE mice at 5 and 10 days (all P<0.05). In addition, in the SDE group, 0.1% CsA CE-treated mice had a significantly lower CFS compared with 0.05% CsA-treated mice at 5 and 10 days (both P<0.05).
Expression of NF-κB in mice conjunctiva
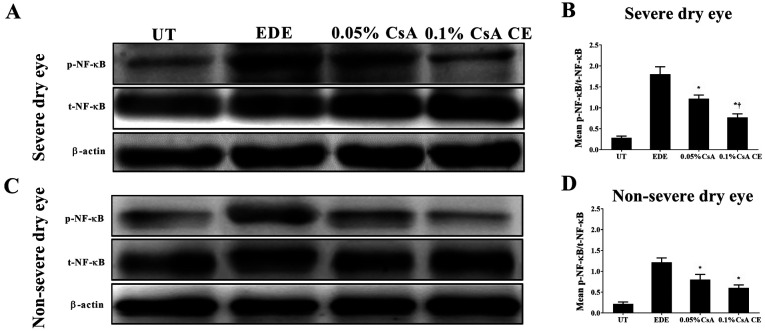
To investigate the involvement of NF-κB activation in the conjunctiva, the expression of total NF-κB p65 and phosphorylated-NF-κB p65 was evaluated in conjunctival tissues (Fig. 3). CsA-treated mice in the SDE and NSED groups showed a decreased expression of NF-κB in the conjunctiva. Furthermore, in the SDE group, mice treated with 0.1% CsA CE had a lower NF-κB expression compared with those treated with 0.05% CsA (all P<0.05).
Figure 3.
Western blotting analysis for p-NF-κB/t-NF-κB expression in UT, EDE, 0.05% CsA emulsion-treated and 0.1% CsA CE-treated mice of the (A) severe dry eye and (C) non-severe dry eye groups at 10 days. (B and D) Relative protein expression of NF-κB in the conjunctiva. *P<0.05 vs. EDE; †P<0.05 vs. 0.05% CsA. UT, untreated control; EDE, experimental dry eye; CsA, cyclosporin A; CE, cationic emulsion; NF-κB, nuclear factor-κB; p, phosphorylated; t, total.
Inflammatory cytokine levels in conjunctival tissues
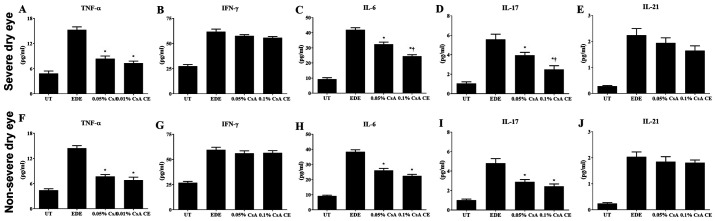
Significantly decreased levels of TNF-α, IL-6 and IL-17 were observed in the conjunctiva of 0.1% CsA CE and 0.05% CsA-treated mice compared with EDE mice (all P<0.05). In the SDE group, mice treated with 0.1% CsA CE exhibited significantly lower IL-6 and IL-17 levels compared with 0.05% CsA-treated mice (both P<0.05; Fig. 4A-J).
Figure 4.
Multiplex immunobead assay for inflammatory levels. Levels of TNF-α, IFN-γ, IL-6, IL-17, and IL-21 in the conjunctiva in UT, EDE, 0.05% CsA emulsion and 0.1% CsA CE-treated mice from the (A-E) severe dry eye and (F-J) non-severe dry eye groups at day 10. *P<0.05 vs. EDE; †P<0.05 vs. 0.05% CsA. UT, untreated control; EDE, experimental dry eye; CsA, cyclosporin A; CE, cationic emulsion; TNF-α, tumor necrosis factor-α; IFN, interferon-γ; IL, interleukin.
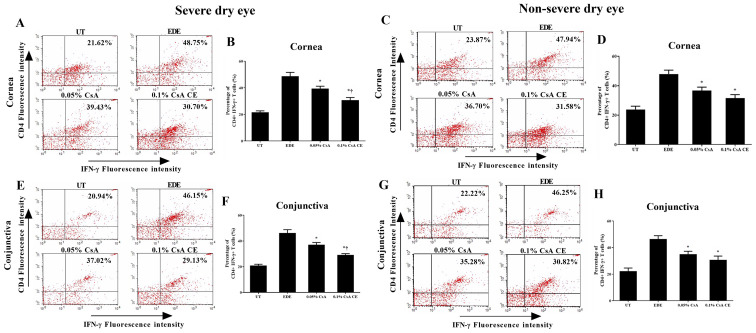
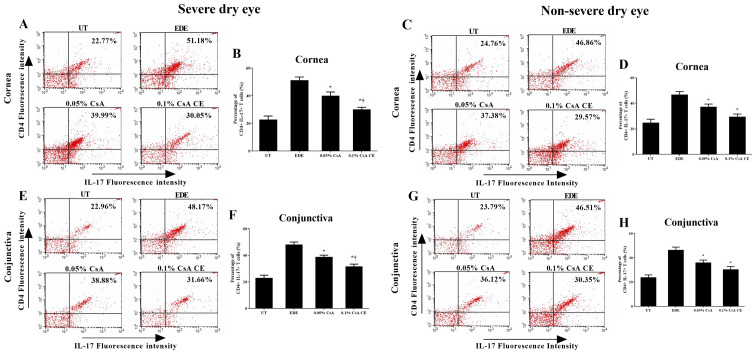
Flow cytometric analysis
The percentage of CD4+ IFN-γ+ and CD4+ IL-17+ T cells in the cornea and conjunctiva of the SDE and NSDE groups from the UT, EDE, 0.05% CsA, and 0.1% CsA CE mice treatment groups are presented in Figs. 5 and 6. Mice treated with 0.1% CsA CE and 0.05% CsA in both groups showed a decreased percentage of CD4+ IFN-γ+ and CD4+ IL-17+ T cells at 10 days (all P<0.05). In addition, in the SDE group, 0.1% CsA CE-treated mice had significantly lower percentages of CD4+ IFN-γ+ and CD4+ IL-17+ T cells compared with 0.05% CsA-treated mice (all P<0.05).
Figure 5.
Flow cytometry analysis for CD4+ T cells. Percentage of CD4+ IFN-γ+ T cells in the (A-D) cornea and (E-H) conjunctiva in UT, EDE, 0.05% CsA emulsion-treated and 0.1% CsA CE-treated mice from the severe dry eye and non-severe dry eye groups at day 10. *P<0.05 vs. EDE; †P<0.05 vs. 0.05% CsA. UT, untreated control; EDE, experimental dry eye; CsA, cyclosporin A; CE, cationic emulsion; IFN, interferon-γ.
Figure 6.
Flow cytometric analysis for CD4+ T cells. Percentage of CD4+ IL-17+ T cells in the (A-D) cornea and (E-H) conjunctiva in UT, EDE, 0.05% CsA emulsion-treated and 0.1% CsA CE-treated mice from the severe dry eye and non-severe dry eye groups at day 10. *P<0.05 vs. EDE; †P<0.05 vs. 0.05% CsA. UT, untreated control; EDE, experimental dry eye; CsA, cyclosporin A; CE, cationic emulsion; IL-17, interleukin-17.
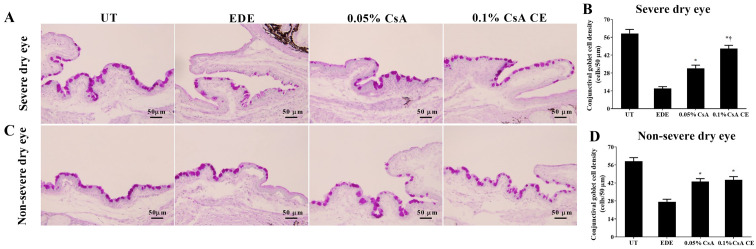
Conjunctival goblet cell density
Mean goblet cell densities in the SDE group for UT, EDE, 0.05% CsA and 0.1% CsA CE-treated mice were 59.00±7.85 cells/50 µm, 15.83±3.60 cells/50 µm, 31.67±6.12 cells/50 µm and 47.17±6.24 cells/50 µm, respectively (Fig. 7A and B). Mean goblet cell densities in the NSDE group were 58.83±6.94 cells/50 µm, 27.33±4.89 cells/50 µm, 43.00±5.69 cells/50 µm and 44.33±6.35 cells/50 µm for the UT, EDE, 0.05% CsA and 0.1% CsA CE mice treatment groups, respectively (Fig. 7C and D). Mice treated with CsA CE in the SDE and NSDE groups showed a significantly increased density of conjunctival goblet cells compared with EDE mice (all P<0.05). Furthermore, in the SDE group, mice treated with 0.1% CsA CE exhibited significantly higher conjunctival goblet cell densities compared with those treated with 0.05% CsA (P<0.05).
Figure 7.
Periodic Acid-Schiff staining for conjunctival goblet cell densities. (A and C) Representative specimens of conjunctiva and (B and D) mean conjunctival goblet cell densities in UT, EDE, 0.05% CsA emulsion-treated and 0.1% CsA CE-treated mice from the severe dry eye and non-severe dry eye groups at day 10. Scale bar, 50 µm. *P<0.05 vs. EDE; †P<0.05 vs. 0.05% CsA. UT, untreated control; EDE, experimental dry eye; CsA, cyclosporin A; CE, cationic emulsion.
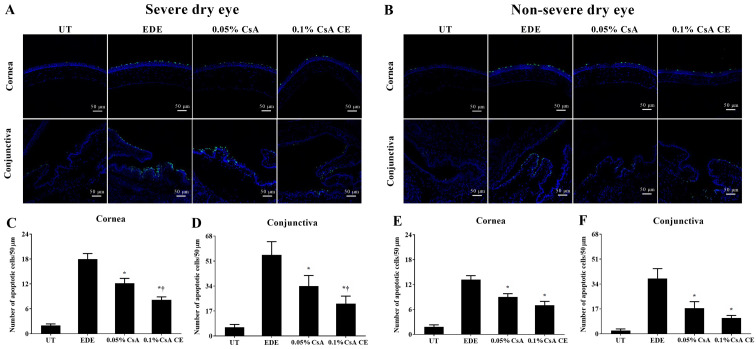
TUNEL staining
Mean apoptotic cell counts in the SDE group for UT, EDE, 0.05% CsA, and 0.1% CsA CE-treated mice were 2.00±0.89 cells/50 µm, 18.00±3.23 cells/50 µm, 12.17±2.86 cells/50 µm and 8.17±1.72 cells/50 µm in the cornea, and 5.00±2.04 cells/50 µm, 54.83±8.59 cells/50 µm, 34.00±7.16 cells/50 µm and 22.00±5.14 cells/50 µm in the conjunctiva, respectively (Fig. 8A, C and D). Apoptotic cell counts in the NSDE group were 1.83±1.17 cells/50 µm and 2.33±1.03 cells/50 µm (UT group), 13.17±2.40 cells/50 µm and 37.83±6.68 cells/50 µm (EDE group), 9.00±2.00 cells/50 µm and 17.50±4.46 cells/50 µm (0.05% CsA-treated group), and 7.00±2.37 cells/50 µm and 10.83±1.72 cells/50 µm (0.1% CsA CE-treated group) in the cornea and conjunctiva, respectively (Fig. 8B E and F). Mice treated with CsA in the SDE and NSDE groups showed a significantly decreased numbers of apoptotic cells in the corneal and conjunctival tissues compared with those in the EDE group. Furthermore, in the SDE group, mice treated with 0.1% CsA CE showed lower numbers of apoptotic cells in the cornea and conjunctiva compared with those treated with 0.05% CsA (all P<0.05).
Figure 8.
TUNEL staining for apoptosis on the ocular surface. (A and B) Representative pictures of cornea and conjunctiva in the severe dry eye group and in the non-severe dry eye group. Mean numbers of TUNEL-positive cells in the (C and E) cornea and (D and F) conjunctiva of UT, EDE, 0.05% CsA emulsion-treated and 0.1% CsA CE-treated mice from the severe dry eye and non-severe dry eye groups at day 10. *P<0.05 vs. EDE; †P<0.05 vs. 0.05% CsA. UT, untreated control; EDE, experimental dry eye; CsA, cyclosporin A; CE, cationic emulsion.
Discussion
Inflammation is a major pathogenic mechanism underlying DE, ultimately resulting in apoptotic cell death. The infiltration of T cells and proinflammatory cytokines at the ocular surface is known to initiate a cascade of events that leads to the progression of DE indicators and symptoms (22,23). In the absence of adequate treatment methods, the ocular surface is gradually damaged that would eventually lead to SDE, which has a negative impact on the patient's quality of life. The CsA emulsion, which serves as an important agent for DE treatment, can inhibit the activation of CD4+ T cells by blocking IL-2 production thereby decreasing apoptosis on the ocular surface (24).
In the present study, to evaluate the change in tear film and corneal epithelial damage following CsA emulsion treatment, tear film BUT and CFSs were evaluated in mice with EDE of different severities. Mice treated with 0.05% CsA emulsion and 0.1% CsA CE exhibited significantly improved tear volumes, tear film BUTs and CFSs compared with EDE mice in the SDE and NSDE groups. Interestingly, in SDE mice, topical application of 0.1% CsA CE led to a significantly lower CFS on the ocular surface compared with that of 0.05% CsA emulsion. The significant differences between groups in terms of therapeutic effect on SDE could be explained by the cationic property of CsA CE as well as its concentration. This innovative formulation increases the retention time of the nanodroplets on the ocular surface, improving therefore the drug delivery by interacting electrostatically with the negatively charged components of the tear film (25). A previous study reported that CsA CE is well tolerated and effectively improves the signs and symptoms in patients with moderate-to-severe DE to SDE over 6 months, in particular in patients with severe disease who are at risk of irreversible corneal damage (14). Furthermore, previous clinical studies demonstrated that 0.1% CsA CE improves the CFS, CFS OSDI response and conjunctival expression of human leukocyte antigen DR in SDE cases with keratitis and SS (12-14). Consistently with these previous reports, the results from the present study demonstrated that the application of 0.05% CsA emulsion and 0.1% CsA CE both led to improvements in the clinical parameters in EDE mice. In addition, the efficacy of 0.1% CsA CE was more notable than that of the 0.05% CsA emulsion in SDE mice.
To investigate the inflammatory changes and cell death on the ocular surface, the expression levels of NF-κB and of the inflammatory cytokines TNF-α, IL-6 and IL-17 were evaluated, and the extent of apoptotic cell death on the ocular surface was also explored. In addition, the percentage of CD4+ T cells and conjunctival goblet cell density were also measured. The results demonstrated that mice treated with 0.05% and 0.1% CsA CE presented with lower expression of NF-κB and decreased levels of inflammation on the ocular surface. In addition, CsA-treated mice had a reduced number of apoptotic cells on the ocular surface and an increased density of conjunctival goblet cells compared with non-treated groups. Furthermore, in the SDE group, mice treated with 0.1% CsA CE showed improved results in terms of the reduction in the number of apoptosis cells and the increased density of conjunctival goblet cells compared with mice treated with 0.05% CsA. The CE may be able to enhance film hydration, lubrication and stability, as the aqueous medium of the emulsion droplets may allow rehydration, and the oily phase replenishes the lipid layer (25-27). In addition, the cationic vehicle of CsA CE itself has been shown to possess anti-inflammatory effects (28,29). Chronic inflammation in the epithelium may promote angiogenesis, invasion and metastasis, and cytokines induce a strong inflammatory response. Furthermore, the TNF/TNF receptor system plays an important role in the induction of apoptotic machinery and inflammation (30,31). Previous studies have reported that blocking inflammatory cytokines can delay the progression of diseases, such as rheumatoid arthritis, multiple sclerosis and inflammatory bowel diseases (30,32,33). In the present study, the levels of inflammatory cytokines, including TNF-α, were significantly decreased in the SDE group following treatment with 0.1% CsA CE. These findings suggested that 0.1% CsA CE may be effective at reducing inflammation and apoptosis on the ocular surface by downregulating the activation of NF-κB and cytokines in SDE compared with 0.05% CsA emulsion.
Based on the results of the present and previous studies, it can be inferred that both higher CsA concentrations and cationic carriers contributed to the increased therapeutic effect of 0.1% CsA CE in SDE. However, further studies are required to provide a more detailed comparative analysis of these two parameters. In addition, since DE is a chronic disease, it would be helpful to analyze the long-term therapeutic effects of 0.1% CsA CE, and to detect CsA concentration in blood in further experiments.
In summary, the present study demonstrated that topical 0.1% CsA CE therapy could improve tear film parameters and ameliorate ocular surface injury and inflammation in SDE and NSDE. In addition, 0.1% CsA CE was more effective in improving corneal epithelial injury and T cell-mediated inflammation in SDE than topical 0.05% CsA emulsion.
Acknowledgements
Not applicable.
Funding Statement
Funding: This study was supported by the Technology Innovation Program (grant no. 20009481) through the Ministry of Trade, Industry & Energy (MOTIE), Korea, by the Korea Health Technology R&D project (grant no. HR20C0021050020) through the Korea Health Industry Development Institute (KHIDI) funded by the Ministry of Health and Welfare, Korea, and by the Chonnam National University Hospital Biomedical Research Institute (grant no. BCRI20072).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
KCY designed the experiments and revised the manuscript. RJ, YL and LL performed the experiments. RJ, HJY and JK analyzed and interpreted the data. RJ, YL and HJY drafted the manuscript. KCY and RJ confirm the authenticity of all the raw data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The research protocol used in the present study was approved by the Chonnam National University Medical School Research Institutional Animal Care and Use Committee (approval no. CNU IACUC-H-2018-73). Maintenance of animals and in vivo experiments were performed in accordance with the Association for Research in Vision and Ophthalmology statement for the Use of Animals in Ophthalmic and Vision Research.
Patient consent for publication
Not applicable.
Competing interests
The authors declare they have no competing interests.
References
- 1.Wolffsohn JS, Arita R, Chalmers R, Djalilian A, Dogru M, Dumbleton K, Gupta PK, Karpecki P, Lazreg S, Pult H, et al. TFOS DEWS II Diagnostic methodology report (2017) Ocul Surf. 2017;15:539–574. doi: 10.1016/j.jtos.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Li Y, Cui L, Lee HS, Kang YS, Choi W, Yoon KC. Comparison of 0.3% hypotonic and isotonic sodium hyaluronate eye drops in the treatment of experimental dry eye. Curr Eye Res. 2017;42:1108–1114. doi: 10.1080/02713683.2017.1297462. [DOI] [PubMed] [Google Scholar]
- 3.Yoon KC, De Paiva CS, Qi H, Chen Z, Farley WJ, Li DQ, Pflugfelder SC. Expression of Th-1 chemokines and chemokine receptors on the ocular surface of C57BL/6 mice: Effects of desiccating stress. Invest Ophthalmol Vis Sci. 2007;48:2561–2569. doi: 10.1167/iovs.07-0002. [DOI] [PubMed] [Google Scholar]
- 4.Pflugfelder SC. Integrating restasis into the management of dry eye. Int Ophthalmol Clin. 2006;46:101–103. doi: 10.1097/01.iio.0000212137.85298.98. [DOI] [PubMed] [Google Scholar]
- 5.Stapleton F, Alves M, Bunya VY, Jalbert I, Lekhanont K, Malet F, Na KS, Schaumberg D, Uchino M, Vehof J, et al. TFOS DEWS II Epidemiology report (2017) Ocul Surf. 2017;15:334–365. doi: 10.1016/j.jtos.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Baudouin C, Creuzot-Garcher C, Hoang-Xuan T, Rigeade MC, Brouquet Y, Bassols A, Guillemin I, Benmedjahed K, Arnould B. Severe impairment of health-related quality of life in patients suffering from ocular surface diseases. J Fr Ophtalmol. 2008;31:369–378. doi: 10.1016/s0181-5512(08)71431-1. [DOI] [PubMed] [Google Scholar]
- 7.Perry HD, Donnenfeld ED. Topical 0.05% cyclosporin in the treatment of dry eye. Expert Opin Pharmacother. 2004;5:2099–2107. doi: 10.1517/14656566.5.10.2099. [DOI] [PubMed] [Google Scholar]
- 8.Schultz C. Safety and efficacy of cyclosporine in the treatment of chronic dry eye. Ophthalmol Eye Dis. 2014;6:37–42. doi: 10.4137/OED.S16067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deveney T, Asbell PA. Patient and physician perspectives on the use of cyclosporine ophthalmic emulsion 0.05% for the management of chronic dry eye. Clin Ophthalmol. 2018;12:569–576. doi: 10.2147/OPTH.S115098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim HS, Kim TI, Kim JH, Yoon KC, Hyon JY, Shin KU, Choi CY. Evaluation of clinical efficacy and safety of a novel cyclosporin A nanoemulsion in the treatment of dry eye syndrome. J Ocul Pharmacol Ther. 2017;33:530–538. doi: 10.1089/jop.2016.0164. [DOI] [PubMed] [Google Scholar]
- 11.Wirta DL, Torkildsen GL, Moreira HR, Lonsdale JD, Ciolino JB, Jentsch G, Beckert M, Ousler GW, Steven P, Krösser S. A clinical phase II study to assess efficacy, safety, and tolerability of waterfree cyclosporine formulation for treatment of dry eye disease. Ophthalmology. 2019;126:792–800. doi: 10.1016/j.ophtha.2019.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leonardi A, Van Setten G, Amrane M, Ismail D, Garrigue JS, Figueiredo FC, Baudouin C. Efficacy and safety of 0.1% cyclosporine A cationic emulsion in the treatment of severe dry eye disease: A multicenter randomized trial. Eur J Ophthalmol. 2016;26:287–296. doi: 10.5301/ejo.5000779. [DOI] [PubMed] [Google Scholar]
- 13.Eroglu YI. A comparative review of Haute Autorité de Santé and National Institute for Health and Care Excellence health technology assessments of Ikervis® to treat severe keratitis in adult patients with dry eye disease which has not improved despite treatment with tear substitutes. J Mark Access Health Policy. 2017;5(1336043) doi: 10.1080/20016689.2017.1336043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baudouin C, Figueiredo FC, Messmer EM, Ismail D, Amrane M, Garrigue JS, Bonini S, Leonardi A, Baudouin C. A randomized study of the efficacy and safety of 0.1% cyclosporine A cationic emulsion in treatment of moderate to severe dry eye. Eur J Ophthalmol. 2017;27:520–530. doi: 10.5301/EJO.5000952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Oliveira RC, Wilson SE. Practical guidance for the use of cyclosporine ophthalmic solutions in the management of dry eye disease. Clin Ophthalmol. 2019;13:1115–1122. doi: 10.2147/OPTH.S184412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoon KC, De Paiva CS, Qi H, Chen Z, Farley WJ, Li DQ, Stern ME, Pflugfelder SC. Desiccating environmental stress exacerbates autoimmune lacrimal keratoconjunctivitis in non-obese diabetic mice. J Autoimmun. 2008;30:212–221. doi: 10.1016/j.jaut.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoon KC, Ahn KY, Choi W, Li Z, Choi JS, Lee SH, Park SH. Tear production and ocular surface changes in experimental dry eye after elimination of desiccating stress. Invest Ophthalmol Vis Sci. 2011;52:7267–7273. doi: 10.1167/iovs.11-7231. [DOI] [PubMed] [Google Scholar]
- 18.Villareal AL, Farley W, Pflugfelder SC. Effect of topical ophthalmic epinastine and olopatadine on tear volume in mice. Eye Contact Lens. 2006;32:272–276. doi: 10.1097/01.icl.0000224360.10319.b1. [DOI] [PubMed] [Google Scholar]
- 19.Pauly A, Brignole-Baudouin F, Labbé A, Liang H, Warnet JM, Baudouin C. New tools for the evaluation of toxic ocular surface changes in the rat. Invest Ophthalmol Vis Sci. 2007;48:5473–5483. doi: 10.1167/iovs.06-0728. [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Jin R, Li L, Yoon HJ, Choi JH, Park JH, Liu Z, Li W, Li Z, Yoon KC. Expression and role of nucleotide-binding oligomerization domain 2 (NOD2) in the ocular surface of murine dry eye. Invest Ophthalmol Vis Sci. 2019;60:2641–2649. doi: 10.1167/iovs.19-27144. [DOI] [PubMed] [Google Scholar]
- 21.Yoon KC, Park CS, You IC, Choi HJ, Lee KH, Im SK, Park HY, Pflugfelder SC. Expression of CXCL9, -10, -11, and CXCR3 in the tear film and ocular surface of patients with dry eye syndrome. Invest Ophthalmol Vis Sci. 2010;51:643–650. doi: 10.1167/iovs.09-3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007;5:75–92. doi: 10.1016/s1542-0124(12)70081-2. The definition and classification of dry eye disease. [DOI] [PubMed] [Google Scholar]
- 23.Baudouin C, Aragona P, Messmer EM, Tomlinson A, Calonge M, Boboridis KG, Akova YA, Geerling G, Labetoulle M, Rolando M. Role of hyperosmolarity in the pathogenesis and management of dry eye disease: Proceedings of the OCEAN group meeting. Ocul Surf. 2013;11:246–258. doi: 10.1016/j.jtos.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 24.Ambroziak AM, Szaflik J, Szaflik JP, Ambroziak M, Witkiewicz J, Skopiński P. Immunomodulation on the ocular surface: A review. Cent Eur J Immunol. 2016;41:195–208. doi: 10.5114/ceji.2016.60995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lallemand F, Daull P, Benita S, Buggage R, Garrigue JS. Successfully improving ocular drug delivery using the cationic nanoemulsion, novasorb. J Drug Deliv. 2012;2012(604204) doi: 10.1155/2012/604204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rabinovich YI, Vakarelski IU, Brown SC, Singh PK, Moudgil BM. Mechanical and thermodynamic properties of surfactant aggregates at the solid-liquid interface. J Colloid Interface Sci. 2004;270:29–36. doi: 10.1016/j.jcis.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 27.Royle L, Matthews E, Corfield A, Berry M, Rudd PM, Dwek RA, Carrington SD. Glycan structures of ocular surface mucins in man, rabbit and dog display species differences. . 2008;25:763–773. doi: 10.1007/s10719-008-9136-6. [DOI] [PubMed] [Google Scholar]
- 28.Daull P, Guenin S, Hamon de Almeida V, Garrigue JS. Anti-inflammatory activity of CKC-containing cationic emulsion eye drop vehicles. Mol Vis. 2018;24:459–470. [PMC free article] [PubMed] [Google Scholar]
- 29.Hwang SB, Park JH, Kang SS, Kang DH, Lee JH, Oh SJ, Lee JY, Kim JY, Tchah H. Protective effects of cyclosporine A emulsion versus cyclosporine A cationic emulsion against desiccation stress in human corneal epithelial cells. Cornea. 2020;39:508–513. doi: 10.1097/ICO.0000000000002244. [DOI] [PubMed] [Google Scholar]
- 30.Jurisic V. Multiomic analysis of cytokines in immuno-oncology. Expert Rev Proteomics. 2020;17:663–674. doi: 10.1080/14789450.2020.1845654. [DOI] [PubMed] [Google Scholar]
- 31.Jurisic V, Terzic T, Colic S, Jurisic M. The concentration of TNF-alpha correlate with number of inflammatory cells and degree of vascularization in radicular cysts. Oral Dis. 2008;14:600–605. doi: 10.1111/j.1601-0825.2007.01426.x. [DOI] [PubMed] [Google Scholar]
- 32.Yang S, Wang J, Brand DD, Zheng SG. Role of TNF-TNF Receptor 2 signal in regulatory T cells and its therapeutic implications. Front Immunol. 2018;9(784) doi: 10.3389/fimmu.2018.00784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pegoretti V, Baron W, Laman JD, Eisel UL. Selective modulation of TNF-TNFRs signaling: Insights for multiple sclerosis treatment. Front Immunol. 2018;9(925) doi: 10.3389/fimmu.2018.00925. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.