Abstract

The development of an efficient photocatalyst with superior activity under visible light has been regarded as a significant strategy for pollutant degradation and environmental remediation. Herein, a series of WO3/Ag2CO3 mixed photocatalysts with different proportions were prepared by a simple mixing method and characterized by XRD, SEM, TEM, XPS, and DRS techniques. The photocatalytic performance of the WO3/Ag2CO3 mixed photocatalyst was investigated by the degradation of rhodamine B (RhB) under visible light irradiation (λ > 400 nm). The photocatalytic efficiency of the mixed WO3/Ag2CO3 photocatalyst was rapidly increased with the proportion of Ag2CO3 up to 5%. The degradation percentage of RhB by WO3/Ag2CO3–5% reached 99.7% within 8 min. The pseudo-first-order reaction rate constant of WO3/Ag2CO3–5% (0.9591 min–1) was 118- and 14-fold higher than those of WO3 (0.0081 min–1) and Ag2CO3 (0.0663 min–1). The catalytic activities of the mixed photocatalysts are not only higher than those of the WO3 and Ag2CO3 but also higher than that of the WO3/Ag2CO3 composite prepared by the precipitation method. The activity enhancement may be because of the easier separation of photogenerated electron–hole pairs. The photocatalytic mechanism was investigated by free radical capture performance and fluorescence measurement. It was found that light-induced holes (h+) was the major active species and superoxide radicals (·O2–) also played a certain role in photocatalytic degradation of RhB.
1. Introduction
With rapid industrialization and urban growth, human beings are in an era of wealth and prosperity. However, the consequent energy shortage and environment pollution have been becoming worldwide problems.1−3 Lots of organic dyes in the industrial wastewater discharged by enterprises can lead to a series of problems such as eutrophication and carcinogenesis of water bodies.4−7 Degradation of toxic and harmful organic pollutants with a semiconductor-mediated photocatalyst is of great significance for solving environmental pollution.8−11 Nevertheless, the wide band gap and low quantum efficiency are still the ″bottlenecks″ for semiconductor photocatalysts to meet the practical application requirements.12 Therefore, it is an urgent need to develop renewable, efficient, and wide light-responsive photocatalysts for pollutant degradation and environmental remediation.1,13,14
Metal oxide semiconductors such as ZnO,15 TiO2,16 Cu2O,17 SnO2,18 and Fe2O319 have been receiving much attention for the photocatalytic degradation of various kinds of pollutants. Tungsten oxide (WO3) has also been widely concerned since 1976 when the first paper on the photocatalysis of WO3 was published.20 As a semiconductor with a band gap of 2.5–2.8 eV,21 WO3 has been widely used in the fields of photochromic,22 electrochromic,23 and photocatalytic applications.24,25 WO3 is one of the most effective and versatile photocatalysts for light corrosion resistance and stable in acidic and oxidizing environments.26 However, due to its relatively low visible light absorption ability and rapid photoelectron–hole pair recombination, the photocatalytic efficiency of WO3 for pollutant degradation is greatly limited.25,27 Silver carbonate (Ag2CO3) is a p-type semiconductor, which has a narrow band gap and easy preparation with good photoactivity under visible light.28−33 Nevertheless, Ag2CO3 possesses the defect of poor stability and rapid recombination of electron–hole pairs, which makes it not able to meet the need of real application as a photocatalyst.
Doping one semiconductor photocatalyst with other suitable metals, metal oxides, or semiconductor salts can enhance the efficiency of the photocatalyst and also endow the catalyst to be active in the wide light region.34−37 Furthermore, in most cases, the photocatalytic activity of a composite material is better than its simple mixed counterpart.38−41 Yuan and coworkers prepared a Ag2CO3/Ag/WO3 composite photocatalyst by a precipitation–light irradiation method and found that the photocatalytic activity of the composite was higher than those of the Ag2CO3 and WO3 for the degradation of organic pollutants.42 Gao and coworkers fabricated a polyhedron-like WO3/Ag2CO3 p-n junction photocatalyst with enhanced photocatalytic activity by a deposition–precipitation method.4
In this work, we report for the first time that the photocatalytic activity of WO3 is highly enhanced by simply mixing with a small amount of Ag2CO3. The proportion of Ag2CO3 on the photocatalytic efficiency of the mixed WO3/Ag2CO3 photocatalyst was systematically investigated by the degradation of RhB under visible light irradiation. The results demonstrated that the photocatalytic efficiency of the mixed WO3/Ag2CO3 photocatalyst was higher than those of both WO3 and Ag2CO3. More interestingly, the photocatalytic activity of the mixed WO3/Ag2CO3 photocatalyst was even higher than that of the WO3/Ag2CO3 composite prepared by a deposition method. The enhanced photocatalytic activity of mixed WO3/Ag2CO3 might be attributed to the surface synergy between WO3 and Ag2CO3. This study may shed new light on the preparation of effective photocatalysts by a simple mixing method.
2. Results and Discussion
2.1. Characterization
Figure 1 shows the XRD patterns of WO3, Ag2CO3, and WO3/Ag2CO3 mixed samples. The WO3 sample shows a series of diffraction peaks at 2θ values of 23.08° (002), 23.52° (020), 24.28° (200), 26.58° (120), 28.60° (112), 33.30°(022), 33.68°(−202), 34.10° (202), 41.86° (222), 47.18° (004), 48.32° (040), 49.76° (140), 50.48° (−114), 53.36° (024), and 55.82° (142), which correspond well with monoclinic phase WO3 (JCPDS No. 43-1035).43 For the Ag2CO3 sample, the diffraction peaks at 2θ values of 18.48° (020), 20.38° (110), 32.52° (−101), 33.60° (−130), 36.94° (200), 37.60° (040), 39.54° (031), 41.6° (220), 44.30° (131), 47.08° (230), 51.36° (150), and 56.08° (231) are in good agreement with monoclinic phase Ag2CO3 (JCPDS No. 26-0399).33,44 The XRD characteristic peaks of WO3 in the mixed WO3/Ag2CO3 samples are obvious, while the peaks of Ag2CO3 are very weak. One possible reason may be that the content of Ag2CO3 in the mixed samples is less than that of WO3. Another reason may be due to the low crystallinity of Ag2CO3, which can be seen from the diffraction peaks of the pure Ag2CO3 sample.
Figure 1.
XRD patterns of WO3, Ag2CO3 and mixed WO3/Ag2CO3 with different Ag2CO3 contents.
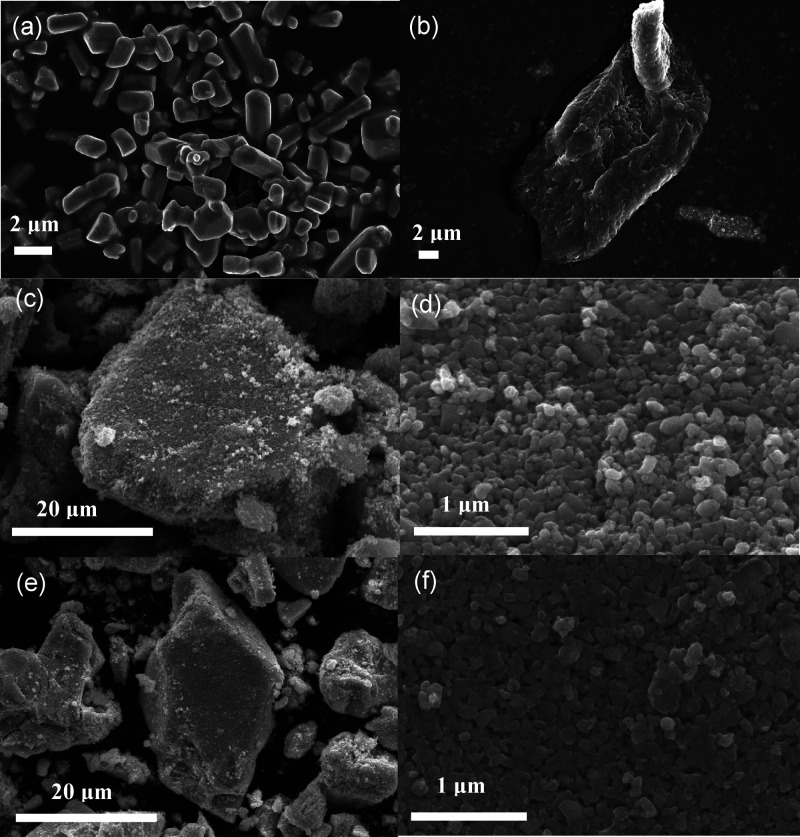
SEM images of WO3, Ag2CO3, and mixed WO3/Ag2CO3 with different mass ratios are shown in Figure 2. The Ag2CO3 is short rod-shaped (Figure 2a) with an average particle size of about 2–5 μm, while WO3 looks like a block morphology that is composed of scale-like particles (Figure 2b). After compositing with Ag2CO3, the surface of the WO3 was decorated with a lot of short rod-like small particles (Figure 2c–f). The length of these rod-like particles was significantly shorter than that of the Ag2CO3 alone (Figure 2a), which may be caused by mechanical mixing. These results indicated that Ag2CO3 was successfully decorated on the surface of the WO3.
Figure 2.
SEM images of (a) Ag2CO3; (b) WO3; (c, d) WO3/Ag2CO3–5%; and (e, f) WO3/Ag2CO3–20%.
TEM and HRTEM were performed to further explore the morphology and facet information of the mixed WO3/Ag2CO3 photocatalyst. It can be seen that the WO3 and Ag2CO3 particles with a diameter in the range of about 50–200 nm were adhered with each other in the mixed WO3/Ag2CO3–5% (Figure 3a,b). The HREM images of WO3/Ag2CO3–5% are displayed in Figure 3c,d. The lattice spacings of 0.23 and 0.266 nm correspond to the (031)45 and (−130)12 facets of Ag2CO3 (JCPDS No. 26-0399), respectively. Meanwhile, the 0.308 and 0.366 nm spacing fringes correspond to the (112)4 and (200)46 facets of the WO3 phase (JCPDS No. 43-1035). The lattice fringes of 0.204 nm may be ascribed to the (200)11 facet of metal Ag crystals (JCPDS No. 65-2871), the metal Ag should be produced from the decomposition of Ag2CO3. These results indicated that heterojunction structures were formed between the interfaces of WO3 and Ag2CO3, which can facilitate the separation of photoexcited electron–hole pairs and enhance the photocatalytic efficiency of the photocatalyst.
Figure 3.

TEM (a, b) and HRTEM (c, d) images of the mixed WO3/Ag2CO3–5%.
The composition and element distribution of the WO3/Ag2CO3–5% were examined by energy-dispersive spectroscopy (EDS). The results showed that tungsten (W), oxygen (O), silver (Ag), and carbon (C) were all detected (Figure 4a) but not uniformly distributed, especially for Ag and C elements (Figure 4b–f). The non-uniform distribution of Ag and C elements might be due to the prepared sample, since the sample was prepared by a simple mixed method and the content of Ag2CO3 was just 5%. Therefore, the non-uniform dispersion of Ag2CO3 in the mixed sample has a significant impact on the element mapping of Ag and C elements.
Figure 4.
EDS spectrum and element mapping of WO3/Ag2CO3–5%. (a) EDS spectrum, (b) SEM image of the mapping area, (c–f) elemental mapping of W (c), O (d), Ag (e), and C elements (f).
X-ray photoelectron spectroscopy (XPS) was employed to examine the chemical composition and surface element states of WO3/Ag2CO3–5%. XPS element detection results showed that Ag, W, O, and C elements were present in the WO3/Ag2CO3–5% (Figure 5a). High-resolution XPS spectra of the Ag, W, O, and C were obtained by using XPS peak 4.1 program fitting according to the Lorentzian–Gaussian model, which facilitated the determination of chemical valence states of different elements. As shown in Figure 5b, the two peaks at about 367.8 and 373.8 in the Ag 3d XPS spectrum can be assigned to the binding energies of Ag 3d5/2 and Ag 3d3/2, corresponding to the Ag+ of Ag2CO3.4,42,47−49 The XPS spectrum of W 4f was shown in Figure 5c; the binding energies of W 4f7/2 and W 4f5/2 of W6+ can be observed at 35.7 and 37.8 eV,24 respectively. In the O 1s XPS spectrum (Figure 5d), the peak at 530.4 eV belonged to the lattice oxygen in WO3 and Ag2CO3, but the fitting peak at 531.1 eV was related to the C–O in Ag2CO3.50 In the C 1s spectrum (Figure 5e), the peak at 284.9 eV can be assigned to the amorphous species,42,51 whereas the peak at 288.3 eV belongs to the C peak in Ag2CO3.52,53
Figure 5.
High-resolution XPS spectra of WO3/Ag2CO3–5%: (a) full spectrum; (b) Ag 3d; (c) W 4f; (d) O 1s; (e) C 1s.
The ultraviolet–visible diffuse reflectance spectra (UV–vis DRS) of WO3, Ag2CO3, and WO3/Ag2CO3–5% were measured on a UV–vis spectrophotometer. All samples exhibit strong absorption in the ultraviolet and part of the visible region (200–470 nm) and weak absorption in the visible region (470–800 nm) (Figure 6a). By using the tangent line method,54 the absorption band edge of WO3 is positioned at 476 nm, and the corresponding band gap (Eg) is 2.605 eV. As for the Ag2CO3, the absorption edge is estimated at about 520 nm, and the corresponding Eg value is 2.385 eV.
Figure 6.
UV–vis DRS spectra WO3, Ag2CO3, and WO3/Ag2CO3–5% (a) and (αhv)1/2vs energy (hv) plots for calculating the band gap of WO3 and Ag2CO3 (b).
The band gaps of WO3 and Ag2CO3 were further calculated according to the Tauc formula:52,55,56
| 1 |
where α and v represent the light absorption coefficient and light frequency, while A and Eg represent a constant and band gap energy, and h is the Plank constant, respectively. The n is an integer, the value of which depends on where the transition is direct (n = 1) or indirect (n = 4).12,57 Since both WO342,57 and Ag2CO342,58,59 are indirect semiconductors, n = 4 is used to determine the Eg values by plotting (αhv)1/2vs hv.42 After calculating the experimental data based on the tangent line method,60 the band gaps of WO3 and Ag2CO3 are 2.61 and 2.36 eV, respectively (Figure 6b). The Eg values of WO3 and Ag2CO3 that are calculated above are in the range of relevant reports.8,21,61−63
The potential values of the valence band (VB) and conduction band (CB) of the semiconductor can be theoretically calculated by using the electronegativity of Mulliken and the band gap of the semiconductor.
| 2 |
| 3 |
In the above formula, EVB and ECB represent the edge potentials of VB top and CB bottom. X is the geometric mean value of Mulliken electronegativity of the constituent atoms in the semiconductor. According to relevant literature reports, the X values for WO3 and Ag2CO3 are 6.594,64 and 6.02 eV,52,65,66 respectively. Ee, usually 4.5 eV, is the free electron energy on the hydrogen scale.64,67 As a result, the EVB of WO3 and Ag2CO3 are calculated as 3.40 and 2.70 eV vs NHE,52,63 respectively. Meanwhile, the ECB of WO3 and Ag2CO3 are 0.79 eV and 0.34 eV vs NHE. These results agreed well with the reported values.48,68,69
2.2. Photocatalytic Performance
The photocatalytic activity of the mixed WO3/Ag2CO3 was explored by the degradation of RhB under visible light irradiation (λ > 400 nm). The original solution of RhB showed a strong absorption peak at 554 nm (Figure 7).8,70 When WO3/Ag2CO3–5% was used for the photodegradation of RhB, the absorption band of RhB solution obviously blue-shifted at 4 min, and the absorbance value at 554 nm decreased rapidly and almost approached zero after 6 min of visible light irradiation (Figure 7a). According to the previous publications, the photodegradation of RhB by WO3/Ag2CO3–5% was accompanied by the decomposition of N-deethylation and conjugated chromophores.42,71 It also indicated that WO3/Ag2CO3–5% has high photocatalytic activity toward RhB degradation. For comparison, pure Ag2CO3 and WO3 were also used as photocatalysts to degrade RhB. The peak intensity of RhB at 554 nm decreased at a slow rate in the presence of Ag2CO3, and only about 50% was degraded within 8 min under visible light illumination (Figure 7b). When WO3 was used as a catalyst, RhB was almost not degraded within 8 min (Figure S1). To better observe the photocatalytic degradation effect of RhB by WO3, we conducted a prolonged photocatalytic reaction (120 min) on RhB degradation. The RhB dye was slowly degraded under long time light irradiation (Figure 7c) in the presence of WO3, as indicated by the absorbance decrease at 554 nm. In order to show the degradation effects of WO3, Ag2CO3 and WO3/Ag2CO3–5% on RhB more intuitively, the kinetic diagrams were analyzed by plotting C/C0vs illumination time (t). It can be clearly observed from Figure 7d that the degradation effect of WO3/Ag2CO3–5% toward RhB is obviously higher than those of WO3 and Ag2CO3. The above results indicated that the photocatalytic activity of WO3 was greatly improved by mixing with Ag2CO3.
Figure 7.
UV–vis spectra of RhB solution under visible light irradiation (λ > 400 nm) with (a) WO3/Ag2CO3–5%, (b) Ag2CO3, and (c) WO3 photocatalysts. (d) Kinetics plots.
In order to more comprehensively uncover the effect of Ag2CO3 content on the photocatalytic performance of the WO3/Ag2CO3 mixed photocatalyst, a series of mixed WO3/Ag2CO3 samples with different Ag2CO3 proportions were prepared and their photocatalytic degradation properties were investigated. As shown in Figure 8a,b, RhB was hardly photodegraded in the absence of a catalyst, which indicated that the photodegradation of RhB could be ignored without a catalyst. The WO3 showed very poor photocatalytic activity for the degradation of RhB. The combination of WO3 with Ag2CO3 greatly improved the photocatalytic activity of WO3 for RhB degradation. The photocatalytic activity of mixed WO3/Ag2CO3 increased significantly at first and then remained unchanged with the increase of Ag2CO3, which indicated that there is a synergistic effect between WO3 and Ag2CO3. Interestingly, the photocatalytic performance of the WO3/Ag2CO3 mixed photocatalyst was similar when the content of Ag2CO3 accounts for 5–20% of the total mass of WO3/Ag2CO3, which may be related to the fact that the short rod-shaped Ag2CO3 has covered the bulk WO3 surface and the synergistic effect has reached the maximum. RhB was almost completely degraded with a degradation rate of 99.7% within about 6 min in the presence of WO3/Ag2CO3–5%, which further confirmed that WO3/Ag2CO3 was a highly efficient photocatalyst for the degradation of RhB.
Figure 8.
Comparison of photocatalytic performance of WO3, Ag2CO3, and mixed WO3/Ag2CO3 in degradation of RhB under visible light (λ > 400 nm). (a) Kinetic diagram; (b) conversion percentage; (c) linear kinetic fitting plot; and (d) pseudo-first order rate constant.
To further illustrate the photocatalytic ability of the mixed catalysts toward RhB, we performed the diagram of ln(C/C0) versus irradiation time (Figure 8c) based on the equation of ln(C/C0) = −kt (k denotes the pseudo-first order rate constant),72 assuming that the photodegradation of RhB obeys pseudo-first order kinetics (dC/dt = kC).73 The calculated pseudo-first order rate constants from Figure 8c are compared and displayed in Figure 8d. It was found that the rate constant of WO3/Ag2CO3–5% (0.9591 min–1) was 118-fold higher than that of WO3 (0.0081 min–1) and 14-fold higher than that of the Ag2CO3 (0.0663 min–1). It can be also seen from Figure 8d that the rate constant of WO3/Ag2CO3–5% was the highest among all samples, showing that WO3/Ag2CO3–5% possesses the best photocatalytic performance toward RhB degradation.
It has been often reported that the photocatalytic activity of a composite photocatalyst was usually better than its simply mixed counterpart.38−41 In order to compare the photocatalytic activity of our mixed WO3/Ag2CO3 photocatalyst with that of the WO3/Ag2CO3 composite, we also prepared WO3/Ag2CO3 composite photocatalysts by a deposition–precipitation method and performed photocatalytic degradation experiment. All the WO3/Ag2CO3 composite photocatalysts prepared in this work displayed improved photocatalytic activity compared to pure WO3 and Ag2CO3 (Figure S2), which is in accordance with general observations that the catalytic activity of a composite photocatalyst is often higher than its constituent photocatalyst. However, the photocatalytic activity of the composite photocatalysts was lower than that of its mixed counterparts in this study (Figure 9), which is contrary to general reports.38−41 The result is very interesting, although the reason for this discrepancy is unclear at present, and we will perform further studies to reveal the mechanism in the future.
Figure 9.
Comparison of the pseudo-first order reaction rate constants of the WO3/Ag2CO3 mixed photocatalyst with that of the WO3/Ag2CO3 composite photocatalyst.
To understand the photocatalytic degradation process of RhB catalyzed by the WO3/Ag2CO3 mixed photocatalyst, the intermediate products of RhB degradation in the presence of WO3/Ag2CO3–5% were further explored by MS analysis (Figure 10a,b). Before light irradiation, the sample mainly showed a peak at an m/z of 443.2 that belongs to the original RhB (Figure 10a).74 Eight different m/z peaks (475.3, 443.2, 415.2, 387.2, 362.3, 359.3, 318.3, and 274.3) were detected after degradation with irradiation (Figure 10b). Based on the measured m/z results and previously reported works, the corresponding mass spectra and chemical structures of the possible intermediate products are listed in Table S1. A possible degradation process of RhB is illustrated in Figure 10c. There are four ethyl groups and one carboxyl group in the RhB molecule. It can be clearly observed that N-deethylation is the primary step in the degradation process of RhB, and a large number of N-deethylation intermediates can be found in the intermediates. Upon light irradiation, the N-deethylation intermediates were generated, and the mass peaks at m/z values of 415.2, 387.2, and 359.3 were identified as N,N,N′-tri-ethylated rhodamine, N,N′-diethylated rhodamine, and N-ethylated rhodamine molecules.74 The active radicals in the aqueous solution generated by WO3/Ag2CO3–5% attack the N-deethylation intermediates, thus producing several primary oxidation products.8,75 When the four ethyl groups in the RhB molecule are degraded, decarboxylating and ring opening may be occurred gradually under the attacking of the active radicals in the solution, resulting in the formation of intermediates with a smaller molecular weight, and the solution becomes colorless gradually.76 Finally, the substances with a smaller molecular weight were decomposed into H2O and CO2.75,77,78 Besides deethylation, hydroxylation may also be involved in the photodegradation of RhB, since two possible hydroxylated intermediates with m/z values of 475.3 and 349.2 were observed in the mass spectroscopy (Figure 10b and Table S1). It was also noted that the peak at the m/z of 443.2 disappeared after 6 min of light irradiation (Figure S3), indicating the complete structure destruction of the original RhB molecule, in line with the UV–visible spectral change.
Figure 10.
Mass spectra of RhB (a) before irradiation and (b) after irradiation for 2 min; (c) schematic diagram of possible intermediates for the photocatalytic degradation of RhB by WO3/Ag2CO3–5%.
The stability of a photocatalyst is considered as a very important factor in practical applications. In this study, the stability of the photocatalyst was evaluated with the photocatalytic degradation of RhB by using WO3/Ag2CO3–5%. The photocatalytic activity of WO3/Ag2CO3–5% on RhB degradation was significantly reduced after the first cycle, and the photocatalytic degradation efficiency was as low as 20% (Figure S4a), which indicated that the stability of WO3/Ag2CO3–5% was poor. Therefore, how to improve the stability of the prepared WO3/Ag2CO3–5% has become the goal of further research.
Since the photocatalytic degradation rates of WO3/Ag2CO3–5% and WO3/Ag2CO3–20% are similar within 8 min, the cyclic photocatalytic degradation of RhB by WO3/Ag2CO3–20% was also studied. The photocatalytic stability of WO3/Ag2CO3–20% was also not good (Figure S4b). However, we found that the cyclability of WO3/Ag2CO3–20% was better than that of WO3/Ag2CO3–5%. Based on the above observation, we put forward the idea that the stability may be related to the percentage of Ag2CO3 in the mixed WO3/Ag2CO3. When the percentage of Ag2CO3 in the mixed WO3/Ag2CO3 is higher, the loss percentage of Ag2CO3 is relatively small during the photoreaction process. Therefore, we speculated that improving the stability of Ag2CO3 may be the key factor to maintaining the photocatalytic activity of the WO3/Ag2CO3 photocatalyst.
XRD and XPS measurements were conducted for the WO3/Ag2CO3–5% after photodegrading RhB. There are two XRD peaks at 2θ = 38.1 and 44.2° that appeared after use (Figure S5), which can be assigned to the (111) and (200) facets42,45,79,80 of the cubic phase of metallic Ag (JCPDS No. 65-2871). The Ag 3d XPS spectrum of WO3/Ag2CO3–5% after use is shown in Figure S6. The two peaks at about 368 and 374 eV can be deconvoluted into two groups, one group at binding energies of 367.7 and 373.7 eV can be assigned to the Ag 3d5/2 and Ag 3d3/2 of Ag+ of the Ag2CO3,42,47,49 and the peak location is the same as the Ag 3d XPS of WO3/Ag2CO3–5% before use, whereas the other group at 368.2 and 374.3 eV can be attributed to metallic silver (Ag0) according to previous reports.42,47 These results suggested that part of Ag+ in Ag2CO3 were reduced to metallic Ag under light illumination in the photocatalytic reaction.
It has been reported previously that the addition of AgNO3 in the photocatalytic reaction system can inhibit the photocorrosion of Ag2CO3.45,80 Therefore, the addition of AgNO3 may also inhibit the photoinactivation of WO3/Ag2CO3 mixed photocatalyst. With this idea in mind, we performed the photocatalytic reaction in the presence AgNO3. Figure 11a shows the effect of AgNO3 concentration on the photocatalytic activity of WO3/Ag2CO3–5%. The photocatalytic activity of the WO3/Ag2CO3–5% was almost not affected when the concentration of AgNO3 was 0.002 or 0.0035 M, while the catalytic activity was slightly decreased with the increase of AgNO3 up to 0.005 M. Therefore, 0.002 M AgNO3 was used as the stabilizer in the further study considering both catalytic efficiency and economic benefit.
Figure 11.
Photocatalytic degradation RhB catalyzed by WO3/Ag2CO3–5% under visible light irradiation (λ > 400 nm). (a) Comparison of photocatalytic performance with different AgNO3 concentrations; (b) cyclic kinetics curve with 0.002 M AgNO3.
Figure 11b shows the cycle experiment of photocatalytic degradation of RhB by WO3/Ag2CO3–5% with 0.002 M AgNO3 as stabilizer. After five cycles repeated use, about 85% degradation efficiency was maintained, indicating the strong inhibition ability of AgNO3 to the photoinactivation of WO3/Ag2CO3–5%. These results demonstrated that AgNO3 can be used as a stabilizer for improving the stability of the mixed WO3/Ag2CO3 photocatalyst.
As we all know, Ag2CO3 is a slightly soluble substance with a solubility product constant (Ksp) of 8.46 × 10–12.81 According to the relationship of solubility and Ksp, the solubility of Ag2CO3 is calculated to be 1.284 × 10–4 mol·L–1 and the solubility of Ag+ can reach 2.57 × 10–4 mol·L–1 (see the Supporting Information for calculation). The Ag+ dissolved in the solution is easily reduced to Ag, promoting the dissolution of Ag2CO3, thus reducing the stability of the Ag2CO3 photocatalyst. Based on the movement principle of the precipitation–dissolution equilibrium, the solubility of Ag2CO3 will be decreased with the increase of Ag+ concentration. Therefore, the addition of AgNO3 into the photocatalytic reaction system can inevitably increase the stability of Ag2CO3. In addition, it is also well known that Ag2CO3 is a light-sensitive compound; the Ag+ ions on the surface of Ag2CO3 particles is easily decomposed to metallic Ag under light conditions, resulting in the decrease of the photocatalytic stability of Ag2CO3.45,80 Thus, the addition of AgNO3 to the photocatalytic reaction system can also prevent Ag2CO3 from photodecomposition under light irradiation. To sum up, the main function of AgNO3 on the inhibition of Ag2CO3 photoinactivation lies not only in reducing the solubility of Ag2CO3 in water but also in inhibiting the photodecomposition of Ag2CO3 under light irradiation.
2.3. Photocatalytic Mechanism
The interface charge separation efficiency of photogenerated electrons (e–) and holes (h+) has been reported as an important factor in determining photocatalytic performance.82 The transfer rate of the interface charge in the photocatalyst was studied by the electrochemical impedance spectroscopy (EIS) technique.4,24,67Figure 12a shows the Nyquist plots of EIS for WO3, WO3/Ag2CO3–1.5%, and WO3/Ag2CO3–5% mixed samples. It can be clearly observed that the arc radius of the electrode modified with WO3/Ag2CO3–5% is smaller than that of WO3/Ag2CO3–1.5%, and much smaller than pure WO3. These results indicated that the charge transfer resistance is smaller and the photogenerated electron–hole pair separation and interface charge transfer are more effective in WO3/Ag2CO3–5%. Then, photoelectrochemical properties were studied by measuring the transient photocurrent in a three-electrode cell. As shown in Figure 12b, under light illumination (λ > 400 nm), the photocurrent intensity of WO3/Ag2CO3–5% is about 1.7 and 5 times as high as that of WO3/Ag2CO3–1.5% and pure WO3, respectively. This result indicated that the separation rate of photogenerated electron–hole pairs was increased after the combination of WO3 with Ag2CO3, and the WO3/Ag2CO3–5% showed the highest separation efficiency of photocharges. The changes of the size of the arc radius and the photocurrent intensity are consistent with that of photocatalytic activity. Based on the above experimental results, it can be concluded that the enhanced photocatalytic activity of WO3/Ag2CO3–5% is attributed to the higher separation efficiency of photoinduced charges and the lower charge transfer resistance.
Figure 12.
Nyquist plots of EIS (a) and transient photocurrent response (b) of WO3, WO3/Ag2CO3–1.5%, and WO3/Ag2CO3–5% mixed samples.
The active species of the photocatalytic process were detected by a trapping experiment. In this study, 1,4-benzoquinone (BQ)8,42 and isopropanol (IPA)4,61 were used as scavenging agents for superoxide radical (·O2–) and hydroxyl radical (·OH), respectively. Ammonium oxalate (AO),47 triethanolamine (TEOA),4 and disodium ethylenediaminetetraacetate (EDTA-2Na)44,61 were used for scavenging of hole (h+). It can be seen that the degradation efficiency of RhB was significantly reduced no matter adding AO, TEOA, or EDTA-2Na (Figure 13a,b), which showed that h+ was the main active species in the reaction process. The degradation rate decreased to a certain degree with the addition of BQ, indicating that ·O2– has a certain effect for RhB degradation. When IPA was added as the scavenger of ·OH, the degradation efficiency of RhB was only slightly decreased, indicating the minor effect of ·OH in the photodegradation of RhB.
Figure 13.
Active species capture of the WO3/Ag2CO3–5% photocatalytic degradation of RhB under visible light (λ > 400 nm). (a) Kinetic curve; (b) conversion percentage.
The role of ·OH in the photocatalytic process were further explored with fluorescence technology by detecting ·OH in the solution in the presence of WO3/Ag2CO3–5%. Terephthalic acid (TA) can react with ·OH to generate hydroxyl terephthalic acid (TA-OH), which can display a fluorescence peak at about 425 nm under the excitation wavelength of 315 nm.47,83 For comparison, ·OH generated by nano-TiO2 under UV–visible light was also conducted as a positive control. It can be seen clearly that the fluorescence absorption peak at 425 nm increased with time under light illumination in the presence of TiO2 (Figure 14a), indicating the formation of TA-OH.83 However, no obvious fluorescence spectral change was observed when WO3/Ag2CO3–5% was used as a photocatalyst under visible light irradiation, which indicated that TA-OH was not produced in this process (Figure 14b). These results demonstrated that ·OH was scarcely produced in the presence of WO3/Ag2CO3–5% during the catalytic reaction.
Figure 14.
Fluorescence spectra of 0.5 mM alkaline terephthalic acid solution (λexc = 315 nm) in the presence of (a) TiO2 under UV–visible light irradiation and (b) WO3/Ag2CO3–5% under visible light irradiation (λ > 400 nm).
On the basis of the above experimental results and the energy band calculation, a probable catalytic mechanism for RhB photodegradation by mixed WO3/Ag2CO3 was proposed (Figure 15). According previous literature studies, WO3 is an n-type semiconductor84 and Ag2CO3 is a p-type semiconductor,28 and the Fermi level of WO3 is higher than that of Ag2CO3.4 After WO3 and Ag2CO3 contacted and compactly coupled with each other, electrons would diffuse from WO3 with a high Fermi level to Ag2CO3 with a low Fermi level, and consequently, positive charge centers were formed at the interface region of WO3 and negative charge centers at the interface of Ag2CO3. The built-in electric field formed in the interface of WO3 and Ag2CO3 can prevent the continuous diffusion of electrons from WO3 to Ag2CO3 and finally a thermal equilibrium state between WO3 and Ag2CO3 can be established.69 Simultaneously, the Fermi level of WO3 (n-type semiconductor) moved down with the Fermi level of Ag2CO3 (p-type semiconductor) moving up (Figure 15a). As a result, the CB band of Ag2CO3 might be more negative than the potential of O2/·O2– (−0.33 V), and the VB of Ag2CO3 more negative than the potential of H2O/·OH (+2.38 V). As is shown in Figure 15b, both WO3 and Ag2CO3 can be photoexcited upon visible light (λ > 400 nm) irradiation to generate electrons and holes (eqs 4 and 5) since the band gap of WO3 and Ag2CO3 are 2.61 and 2.36 eV, respectively (Figure 15a). The photoinduced holes on the VB of WO3 with high potential can degrade RhB directly (eq 6), move to the VB of Ag2CO3 (eq 7), or react with surface adsorbed H2O, forming ·OH radicals to oxidize RhB (eqs 8 and 9). The photoexcited electrons in the CB band of Ag2CO3 could be captured by dissolved O2 generating ·O2– to oxidized RhB (eqs 10 and 11), while the photoinduced holes in the VB of Ag2CO3 could not oxidized H2O to ·OH since the VB energy of Ag2CO3 was lower than the potential of H2O/·OH.
Figure 15.

Diagrams of the energy band (a) and photoexcited electron–hole separation (b) for RhB degradation in the presence of the WO3/Ag2CO3 photocatalyst under visible light irradiation (λ > 400 nm).
In addition, it is also possible that RhB itself could be photoexcited under visible light irradiation and stimulated to an excited state (RhB*). Then, the electrons in the lowest unoccupied molecular orbital (LUMO) of RhB are injected to the CB of Ag2CO34 or captured by O2, generating superoxide radicals ·O2–.85 In addition, the photoexcited electrons in the CB of WO3 may partially move to the highest occupied molecular orbital (HOMO) of RhB so that stabilize the photogenerated holes in the VB of WO3.85
| 4 |
| 5 |
| 6 |
| 7 |
| 8 |
| 9 |
| 10 |
| 11 |
3. Conclusions
A series of mixed WO3/Ag2CO3 with different mass ratios were successfully prepared by a simple mechanical mixing method. In the presence of a proper proportion of mixed WO3/Ag2CO3, RhB was completely degraded within 8 min under visible light irradiation (λ > 400 nm), which was better than both WO3 and Ag2CO3. When the mass percentage of Ag2CO3 ranged from 5 to 20% of WO3/Ag2CO3, the degradation rate of RhB was more than 99.5% within 8 min, which can be attributed to the following: (1) massive WO3 provides a large number of active sites for Ag2CO3; (2) type II double transfer mechanism greatly promotes the separation of the electron hole pairs. The degradation rate of RhB by mixed WO3/Ag2CO3 can still maintain 85.6% after five cycles with the addition of 0.002 M AgNO3 as a stabilizer. The results of the free radical capture indicated that the main active substances were ·O2– and h+, while ·OH was almost not produced in the degradation of RhB. The present work may provide a strategy to prepare efficient visible light photocatalysts.
4. Experimental Section
4.1. Materials
Tungsten trioxide (WO3) was purchased from Adamas Reagent Co., Ltd.; silver nitrate (AgNO3) and rhodamine B (RhB) were obtained from Chengdu Kelong Chemical Reagent Factory; and sodium bicarbonate (NaHCO3) was purchased from Tianjin Komiou Chemical Reagent Co., Ltd. Other chemical reagents were of analytic grade and used without further purification.
4.2. Preparation of Ag2CO3
First, NaHCO3 solution was prepared by dissolving 0.42 g NaHCO3 in 50 mL deionized water. Then, the NaHCO3 solution was dropwise added into 50 mL of 0.1 M AgNO3 under magnetic stirring. After stirring in the dark for 30 min, the Ag2CO3 precipitate was collected by centrifugation and repeatedly washed three times with deionized water before drying in an oven at 60 °C for 6 h.
4.3. Preparation of the Mixed WO3/Ag2CO3 Photocatalyst
Briefly, a certain amount of WO3 and Ag2CO3 was weighed in a 5 mL centrifuge tube and stirred evenly to prepare a series of mixed catalysts with different mass ratios, in which the mass of Ag2CO3 accounted for 1, 1.25, 1.5, 2, 2.5, 5, 7.5, 10, and 20% of the total mass. The mixed WO3/Ag2CO3 were labeled as WO3/Ag2CO3–x% (x% is the mass percentage of Ag2CO3 in the mixture).
4.4. Preparation of WO3/Ag2CO3 Composite Photocatalyst
The WO3/Ag2CO3 composite was prepared by a precipitation method. Briefly, 0.621 g WO3 was dispersed in 20 mL deionized water by ultrasonication for 30 min, and then 0.1 M AgNO3 (5 mL) solution was added to the above dispersion under magnetic stirring. After stirring for 30 min, 0.1 M NaHCO3 (5 mL) was added dropwise into the above solution and further stirred for 2 h. Finally, the WO3/Ag2CO3 composite was collected by centrifugation, washed with deionized water, and dried in an oven at 60 °C for 6 h. This sample was labeled as WO3/Ag2CO3-p–10%. Other WO3/Ag2CO3 composites with different Ag2CO3 content were prepared with the same synthetic steps by varying the proportion of Ag2CO3 to WO3. The composites were labeled as WO3/Ag2CO3-p–x% (x% is the mass percentage of Ag2CO3 in the composite).
4.5. Characterizations
The phase information, morphology, X-ray photoelectron spectroscopy (XPS) analysis, and UV–visible reflectance spectroscopy (DRS) of the composite were measured by using a RigakuDmax/Ultima IV X-ray diffractometer, Hitachi S4800 microscope, Thermo ESCALAB 250XI XPS spectrometer, and Shimadzu UV-3600 spectrophotometer.
4.6. Photocatalytic Evaluation
The photocatalytic properties of the resulting photocatalysts were investigated by the degradation of RhB. The light source was provided with a 70 W metal halide lamp, and a 400 nm ultraviolet cutoff filter was applied to cut off UV light (λ < 400 nm). Typically, 50 mg of the catalyst was dispersed in 50 mL of 10 mg/L RhB solution. Before exposure to visible light, the mixture was stirred for 30 min under darkness to establish an adsorption–desorption equilibrium (Figure S7). About 3 mL of aliquot of the suspension was taken out within a certain irradiation interval. Before the absorption measurement, the mixed WO3/Ag2CO3 catalyst was removed by using a membrane filter (0.45 μm), and then the absorbance values for the RhB solution was measured with a UV–vis spectrophotometer (Agilent Technologies, Cary 8454) or a visible spectrophotometer (723 N, Shanghai Jingke Scientific Instrument Co., Ltd., China). The detection wavelength was selected at 554 nm, the maximum absorption wavelength of RhB in the visible region.
The photocatalytic degradation intermediates of RhB were analyzed by a mass spectroscope (Agent Technologies 6120 Quadrupole).
4.7. Photoelectrochemical Measurement
Electrochemical impedance spectroscopy (EIS) and photocurrent were measured by using a typical three-electrode system in a CHI 760E electrochemical workstation. In the measurement process, Pt wire and Ag/AgCl were used as the counter electrode and the reference electrode, respectively, and the photocatalyst was covered on the ITO conductive glass as the working electrode. In addition, 0.2 M Na2SO4 was used as the electrolyte. EIS was measured at a potential of 1.5 V with a frequency of 0.1–105 Hz and an amplitude of 5 mV. The photocurrent was measured under a bias voltage of 0.5 V, and a 300 W xenon lamp with an ultraviolet light cut-off filter (λ > 400 nm) was used as the light source.
4.8. Active Species Identification
The active species trapping experiment was basically the same as that of the degradation experiment except that specific scavenging agents were added. A total of 10 mM ammonium oxalate (AO), 5 mM ethylenediaminetetraacetic acid disodium (EDTA-2Na), and 1 mM triethanolamine (TEOA) were used as scavengers for the photoinduced holes (h+. A total of 10 mM isopropanol (IPA) and 0.2 mM benzoquinone (BQ) were selected as scavenging agents for the hydroxyl radical (·OH) and superoxide anion radical (·O2–).
Under the excitation wavelength of 315 nm, terephthalic acid (TA) was used as the fluorescent probe, and the content of ·OH was determined on a G9800A Carry eclipse fluorescence spectrophotometer.73 In addition, the RhB solution was replaced by basic terephthalic acid, and the fluorescence experiment was performed with the replaced solution. The concentration of the terephthalic acid was 0.5 mM in 1.5 mM NaOH; the sampling time after the reaction was the same as the photocatalytic degradation experiment; and the fluorescence spectrum was measured after filtering with a filter (0.45 μm).
Acknowledgments
This work was supported by the Opening Project of Key Laboratory of Green Chemistry of Sichuan Institutes of Higher Education (LZJ2002) and the Open Project of Chemical Synthesis and Pollution Control Key Laboratory of Sichuan Province (CSPC2016-3-2).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c03694.
Calculation of the solubility of Ag2CO3; chemical structure of the intermediate products of RhB degradation; UV–vis spectral changes of RhB solution under visible light irradiation (λ > 400 nm) with pure WO3; data of photocatalytic degradation of RhB with WO3/Ag2CO3 composite photocatalysts prepared by the deposition–precipitation method; mass spectra of RhB degradation products by the WO3/Ag2CO3–5% mixed photocatalyst; photochemical stability study of WO3/Ag2CO3 mixed samples; XRD comparison of the WO3/Ag2CO3–5% mixed sample before and after photocatalysis; Ag 3d XPS spectrum of the WO3/Ag2CO3–5% mixed sample after photocatalysis; and the adsorption of RhB dye onto the photocatalysts (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Liu S.; Jin X. Preparation of novel Bi4O5I2/Ag3PO4 with enhanced visible-light photocatalytic activities. Chem. Phys. 2020, 530, 110625. 10.1016/j.chemphys.2019.110625. [DOI] [Google Scholar]
- Gao D.; Li Z.; Wang H.; Liang H. An overview of phthalate acid ester pollution in China over the last decade: Environmental occurrence and human exposure. Sci. Total Environ. 2018, 645, 1400–1409. 10.1016/j.scitotenv.2018.07.093. [DOI] [PubMed] [Google Scholar]
- Liu S.; Wang X.; Guo G.; Yan Z. Status and environmental management of soil mercury pollution in China: A review. J. Environ. Manage. 2021, 277, 111442. 10.1016/j.jenvman.2020.111442. [DOI] [PubMed] [Google Scholar]
- Gao M.; You L.; Guo L.; Li T. Fabrication of a novel polyhedron-like WO3/Ag2CO3 p-n junction photocatalyst with highly enhanced photocatalytic activity. J. Photochem. Photobiol., A 2019, 374, 206–217. 10.1016/j.jphotochem.2019.01.022. [DOI] [Google Scholar]
- Chen C.; Ma W.; Zhao J. Semiconductor-mediated photodegradation of pollutants under visible-light irradiation. Chem. Soc. Rev. 2010, 39, 4206–4219. 10.1039/b921692h. [DOI] [PubMed] [Google Scholar]
- Cui W.; An W.; Liu L.; Hu J.; Liang Y. Novel Cu2O quantum dots coupled flower-like BiOBr for enhanced photocatalytic degradation of organic contaminant. J. Hazard. Mater. 2014, 280, 417–427. 10.1016/j.jhazmat.2014.08.032. [DOI] [PubMed] [Google Scholar]
- Guan S.; Li R.; Sun X.; Xian T.; Yang H. Construction of novel ternary Au/LaFeO3/Cu2O composite photocatalysts for RhB degradation via photo-Fenton catalysis. Mater. Technol. 2021, 36, 603–615. 10.1080/10667857.2020.1782062. [DOI] [Google Scholar]
- Chen J.; Xiao X.; Wang Y.; Ye Z. Ag nanoparticles decorated WO3/g-C3N4 2D/2D heterostructure with enhanced photocatalytic activity for organic pollutants degradation. Appl. Surf. Sci. 2019, 467-468, 1000–1010. 10.1016/j.apsusc.2018.10.236. [DOI] [Google Scholar]
- Kar P.; Shukla K.; Jain P.; Sathiyan G.; Gupta R. K. Semiconductor based photocatalysts for detoxification of emerging pharmaceutical pollutants from aquatic systems: A critical review. Nano Mater. Sci. 2021, 25–46. 10.1016/j.nanoms.2020.11.001. [DOI] [Google Scholar]
- Sharma S.; Dutta V.; Singh P.; Raizada P.; Rahmani-Sani A.; Hosseini-Bandegharaei A.; Thakur V. K. Carbon quantum dot supported semiconductor photocatalysts for efficient degradation of organic pollutants in water: A review. J. Cleaner Prod. 2019, 228, 755–769. 10.1016/j.jclepro.2019.04.292. [DOI] [Google Scholar]
- Cheng T.; Gao H.; Li R.; Wang S.; Yi Z.; Yang H. Flexoelectricity-induced enhancement in carrier separation and photocatalytic activity of a photocatalyst. Appl. Surf. Sci. 2021, 566, 150669. 10.1016/j.apsusc.2021.150669. [DOI] [Google Scholar]
- Fu S.; Yuan W.; Yan Y.; Liu H.; Shi X.; Zhao F.; Zhou J. Highly efficient visible-light photoactivity of Z-scheme MoS2/Ag2CO3 photocatalysts for organic pollutants degradation and bacterial inactivation. J. Environ. Manage. 2019, 252, 109654. 10.1016/j.jenvman.2019.109654. [DOI] [PubMed] [Google Scholar]
- Mohammed A. M.; Mohtar S. S.; Aziz F.; Mhamad S. A.; Aziz M. Review of various strategies to boost the photocatalytic activity of the cuprous oxide-based photocatalyst. J. Environ. Chem. Eng. 2021, 9, 105138. 10.1016/j.jece.2021.105138. [DOI] [Google Scholar]
- Peleyeju M. G.; Viljoen E. L. WO3-based catalysts for photocatalytic and photoelectrocatalytic removal of organic pollutants from water – A review. J. Water Process Eng. 2021, 40, 101930. 10.1016/j.jwpe.2021.101930. [DOI] [Google Scholar]
- Huang N.; Shu J.; Wang Z.; Chen M.; Ren C.; Zhang W. One-step pyrolytic synthesis of ZnO nanorods with enhanced photocatalytic activity and high photostability under visible light and UV light irradiation. J. Alloys Compd. 2015, 648, 919–929. 10.1016/j.jallcom.2015.07.039. [DOI] [Google Scholar]
- Liao T.; Sun Z.; Dou S. X. Theoretically Manipulating Quantum Dots on Two-Dimensional TiO2 Monolayer for Effective Visible Light Absorption. ACS Appl. Mater. Interfaces 2017, 9, 8255–8262. 10.1021/acsami.6b15741. [DOI] [PubMed] [Google Scholar]
- Miller E. B.; Zahran E. M.; Knecht M. R.; Bachas L. G. Metal oxide semiconductor nanomaterial for reductive debromination: Visible light degradation of polybrominated diphenyl ethers by Cu2O@Pd nanostructures. Appl. Catal., B 2017, 213, 147–154. 10.1016/j.apcatb.2017.05.020. [DOI] [Google Scholar]
- Kar A.; Olszówka J.; Sain S.; Sloman S.-R. I.; Montes O.; Fernández A.; Pradhan S. K.; Wheatley A. E. H. Morphological effects on the photocatalytic properties of SnO2 nanostructures. J. Alloys Compd. 2019, 810, 151718. 10.1016/j.jallcom.2019.151718. [DOI] [Google Scholar]
- Wang C.; Huang Z. Controlled synthesis alpha-Fe2O3 nanostructures for efficient photocatalysis. Mater. Lett. 2016, 164, 194–197. 10.1016/j.matlet.2015.10.152. [DOI] [Google Scholar]
- Butler M. A.; Nasby R. D.; Quinn R. K. Tungsten trioxide as an electrode for photoelectrolysis of water. Solid State Commun. 1976, 19, 1011–1014. 10.1016/0038-1098(76)90642-6. [DOI] [Google Scholar]
- Wang L.; Liu J.; Wang Y.; Zhang X.; Duan D.; Fan C.; Wang Y. Insight into the enhanced photocatalytic performance of Ag3PO4 modified metastable hexagonal WO3. Colloids Surf., A 2018, 541, 145–153. 10.1016/j.colsurfa.2018.01.021. [DOI] [Google Scholar]
- Radhika T.; Ramalingam R. J.; Aneesha V.; Albaqami M. D. Synthesis and characterization of Photochromic inkless coating based on WO3-Titania nanocomposite under sun light and solar simulation condition. Optik 2021, 228, 166145. 10.1016/j.ijleo.2020.166145. [DOI] [Google Scholar]
- Pooyodying P.; Ok J.-W.; Son Y.-H.; Sung Y.-M. Electrical and optical properties of electrochromic device with WO3: Mo film prepared by RF magnetron Co-Sputtering. Opt. Mater. 2021, 112, 110766. 10.1016/j.optmat.2020.110766. [DOI] [Google Scholar]
- Zhang J.; Fu X.; Hao H.; Gan W. Facile synthesis 3D flower-like Ag@WO3 nanostructures and applications in solar-light photocatalysis. J. Alloys Compd. 2018, 757, 134–141. 10.1016/j.jallcom.2018.05.068. [DOI] [Google Scholar]
- Hua Z.; Tian C.; Qiu Z.; Li Y.; Tian X.; Wang M.; Li E. An investigation on NO2 sensing mechanism and shielding behavior of WO3 nanosheets. Sens. Actuators, B 2018, 259, 250–257. 10.1016/j.snb.2017.12.016. [DOI] [Google Scholar]
- Gondal M. A.; Dastageer M. A.; Khalil A. Synthesis of nano-WO3 and its catalytic activity for enhanced antimicrobial process for water purification using laser induced photo-catalysis. Catal. Commun. 2009, 11, 214–219. 10.1016/j.catcom.2009.10.011. [DOI] [Google Scholar]
- AbuMousa R. A.; Baig U.; Gondal M. A.; Dastageer M. A.; AlSalhi M. S.; Moftah B.; Yahya Alqahtani F.; Akhter S.; Sfouq Aleanizy F. Investigation of the survival viability of cervical cancer cells (HeLa) under visible light induced photo-catalysis with facile synthesized WO3/ZnO nanocomposite. Saudi J. Biol. Sci. 2020, 27, 1743–1752. 10.1016/j.sjbs.2020.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fa W.; Wang P.; Yue B.; Yang F.; Li D.; Zheng Z. Ag3PO4/Ag2CO3 p–n heterojunction composites with enhanced photocatalytic activity under visible light. Chin. J. Catal. 2015, 36, 2186–2193. 10.1016/S1872-2067(15)61004-X. [DOI] [Google Scholar]
- Sánchez-Cid P.; Jaramillo-Páez C.; Navío J. A.; Martín-Gómez A. N.; Hidalgo M. C. Coupling of Ag2CO3 to an optimized ZnO photocatalyst: Advantages vs. disadvantages. J. Photochem. Photobiol., A 2019, 369, 119–132. 10.1016/j.jphotochem.2018.10.024. [DOI] [Google Scholar]
- Tian J.; Wu Z.; Liu Z.; Yu C.; Yang K.; Zhu L.; Huang W.; Zhou Y. Low-cost and efficient visible-light-driven CaMg(CO3)2@Ag2CO3 microspheres fabricated via an ion exchange route. Chin. J. Catal. 2017, 38, 1899–1908. 10.1016/S1872-2067(17)62924-3. [DOI] [Google Scholar]
- Xie J.; Fang C.; Zou J.; Lu H.; Tian C.; Han C.; Zhao D. In situ ultrasonic formation of AgBr/Ag2CO3 nanosheets composite with enhanced visible-driven photocatalytic performance. Mater. Lett. 2016, 170, 62–66. 10.1016/j.matlet.2016.02.002. [DOI] [Google Scholar]
- Zhao X.; Su Y.; Qi X.; Han X. A Facile Method To Prepare Novel Ag2O/Ag2CO3 Three-Dimensional Hollow Hierarchical Structures and Their Water Purification Function. ACS Sustainable Chem. Eng. 2017, 5, 6148–6158. 10.1021/acssuschemeng.7b01040. [DOI] [Google Scholar]
- An W.; Sun K.; Hu J.; Cui W.; Liu L. The Z-scheme Ag2CO3@g-C3N4 core-shell structure for increased photoinduced charge separation and stable photocatalytic degradation. Appl. Surf. Sci. 2020, 504, 144345. 10.1016/j.apsusc.2019.144345. [DOI] [Google Scholar]
- Zhang Y. H.; Liu M. M.; Chen J. L.; Fang S. M.; Zhou P. P. Recent advances in Cu2O-based composites for photocatalysis: a review. Dalton Trans. 2021, 50, 4091–4111. 10.1039/D0DT04434B. [DOI] [PubMed] [Google Scholar]
- Sudha D.; Sivakumar P. Review on the photocatalytic activity of various composite catalysts. Chem. Eng. Process. 2015, 97, 112–133. 10.1016/j.cep.2015.08.006. [DOI] [Google Scholar]
- Devi L. G.; Kavitha R. A review on plasmonic metal-TiO2 composite for generation, trapping, storing and dynamic vectorial transfer of photogenerated electrons across the Schottky junction in a photocatalytic system. Appl. Surf. Sci. 2016, 360, 601–622. 10.1016/j.apsusc.2015.11.016. [DOI] [Google Scholar]
- Awfa D.; Ateia M.; Fujii M.; Johnson M. S.; Yoshimura C. Photodegradation of pharmaceuticals and personal care products in water treatment using carbonaceous-TiO2 composites: A critical review of recent literature. Water Res. 2018, 142, 26–45. 10.1016/j.watres.2018.05.036. [DOI] [PubMed] [Google Scholar]
- Yang M.; Hu S.; Li F.; Fan Z.; Wang F.; Liu D.; Gui J. The influence of preparation method on the photocatalytic performance of g-C3N4/WO3 composite photocatalyst. Ceram. Int. 2014, 40, 11963–11969. 10.1016/j.ceramint.2014.04.033. [DOI] [Google Scholar]
- Pan J.; Guo F.; Sun H.; Shi Y.; Shi W. Nanodiamonds anchored on porous ZnSnO3 cubes as an efficient composite photocatalyst with improved visible-light photocatalytic degradation of tetracycline. Sep. Purif. Technol. 2021, 263, 118398. 10.1016/j.seppur.2021.118398. [DOI] [Google Scholar]
- Yao X.; Liu X. One-pot synthesis of ternary Ag2CO3/Ag/AgCl photocatalyst in natural geothermal water with enhanced photocatalytic activity under visible light irradiation. J. Hazard. Mater. 2014, 280, 260–268. 10.1016/j.jhazmat.2014.07.079. [DOI] [PubMed] [Google Scholar]
- Zhang N.; Zhang X.; Gan C.; Zhang J.; Liu Y.; Zhou M.; Zhang C.; Fang Y. Heterostructural Ag3PO4/UiO-66 composite for highly efficient visible-light photocatalysts with long-term stability. J. Photochem. Photobiol., A 2019, 376, 305–315. 10.1016/j.jphotochem.2019.03.025. [DOI] [Google Scholar]
- Yuan X.; Jiang L.; Chen X.; Leng L.; Wang H.; Wu Z.; Xiong T.; Liang J.; Zeng G. Highly efficient visible-light-induced photoactivity of Z-scheme Ag2CO3/Ag/WO3 photocatalysts for organic pollutant degradation. Environ. Sci.: Nano 2017, 4, 2175–2185. 10.1039/C7EN00713B. [DOI] [Google Scholar]
- Yan M.; Wu Y.; Zhu F.; Hua Y.; Shi W. The fabrication of a novel Ag3VO4/WO3 heterojunction with enhanced visible light efficiency in the photocatalytic degradation of TC. Phys. Chem. Chem. Phys. 2016, 18, 3308–3315. 10.1039/C5CP05599G. [DOI] [PubMed] [Google Scholar]
- Nyankson E.; Agyei-Tuffour B.; Annan E.; Yaya A.; Mensah B.; Onwona-Agyeman B.; Amedalor R.; Kwaku-Frimpong B.; Efavi J. K. Ag2CO3-halloysite nanotubes composite with enhanced removal efficiency for water soluble dyes. Heliyon 2019, 5, e01969 10.1016/j.heliyon.2019.e01969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X.; Li R.; Wang Y.; Wu K.; Chang S.; Tang H. Solvent-induced controllable synthesis of recyclable Ag2CO3 catalysts with enhanced visible light photocatalytic activity. Ceram. Int. 2016, 42, 13411–13420. 10.1016/j.ceramint.2016.05.119. [DOI] [Google Scholar]
- Han Y.; Liu Y.; Su C.; Chen X.; Zeng M.; Hu N.; Su Y.; Zhou Z.; Wei H.; Yang Z. Sonochemical synthesis of hierarchical WO3 flower-like spheres for highly efficient triethylamine detection. Sens. Actuators, B 2020, 306, 127536. 10.1016/j.snb.2019.127536. [DOI] [Google Scholar]
- Cheng Y.; He L.; Xia G.; Ren C.; Wang Z. Nanostructured g-C3N4/AgI composites assembled by AgI nanoparticles-decorated g-C3N4 nanosheets for effective and mild photooxidation reaction. New J. Chem. 2019, 43, 14841–14852. 10.1039/C9NJ02725D. [DOI] [Google Scholar]
- Li X.; Liu D.; Zhu B.; Wang J.; Lang J. Facile preparation of ZnO/Ag2CO3 heterostructured nanorod arrays with improved photocatalytic activity. J. Phys. Chem. Solids 2019, 125, 96–102. 10.1016/j.jpcs.2018.10.017. [DOI] [Google Scholar]
- Zong S.; Cheng C.; Shi J.; Huang Z.; Hu Y.; Yang H.; Guo L. Molten Ag2SO4-based Ion-Exchange Preparation of Ag0.5La0.5TiO3 for Photocatalytic O2 Evolution. Chem. – Asian J. 2017, 12, 882–889. 10.1002/asia.201700101. [DOI] [PubMed] [Google Scholar]
- Reheman A.; Kadeer K.; Okitsu K.; Halidan M.; Tursun Y.; Dilinuer T.; Abulikemu A. Facile photo-ultrasonic assisted reduction for preparation of rGO/Ag2CO3 nanocomposites with enhanced photocatalytic oxidation activity for tetracycline. Ultrason. Sonochem. 2019, 51, 166–177. 10.1016/j.ultsonch.2018.10.030. [DOI] [PubMed] [Google Scholar]
- Yu X.; Gao L.; Huang J.; Li W.; Liu G.; Li Z.; Liu J.; Hu P. Construction of hybrid Ag2CO3/AgVO3 nanowires with enhanced visible light photocatalytic activity. Mater. Res. Bull. 2018, 101, 246–252. 10.1016/j.materresbull.2018.01.023. [DOI] [Google Scholar]
- Guo R.; Qi X.; Zhang X.; Zhang H.; Cheng X. Synthesis of Ag2CO3/α-Fe2O3 heterojunction and it high visible light driven photocatalytic activity for elimination of organic pollutants. Sep. Purif. Technol. 2019, 211, 504–513. 10.1016/j.seppur.2018.10.011. [DOI] [Google Scholar]
- Tang H.; Chang S.; Tang G.; Liang W. AgBr and g-C3N4 co-modified Ag2CO3 photocatalyst: A novel multi-heterostructured photocatalyst with enhanced photocatalytic activity. Appl. Surf. Sci. 2017, 391, 440–448. 10.1016/j.apsusc.2016.07.021. [DOI] [Google Scholar]
- Zhang Y.; Shi J.; Huang Z.; Guan X.; Zong S.; Cheng C.; Zheng B.; Guo L. Synchronous construction of CoS2 in-situ loading and S doping for g-C3N4: Enhanced photocatalytic H2-evolution activity and mechanism insight. Chem. Eng. J. 2020, 401, 126135. 10.1016/j.cej.2020.126135. [DOI] [Google Scholar]
- Hasija V.; Raizada P.; Sudhaik A.; Singh P.; Thakur V. K.; Khan A. A. P. Fabrication of Ag/AgI/WO3 heterojunction anchored P and S co-doped graphitic carbon nitride as a dual Z scheme photocatalyst for efficient dye degradation. Solid State Sci. 2020, 100, 106095. 10.1016/j.solidstatesciences.2019.106095. [DOI] [Google Scholar]
- Cheng C.; Mao L.; Shi J.; Xue F.; Zong S.; Zheng B.; Guo L. NiCo2O4 nanosheets as a novel oxygen-evolution-reaction cocatalyst in situ bonded on the g-C3N4 photocatalyst for excellent overall water splitting. J. Mater. Chem. A 2021, 9, 12299–12306. 10.1039/D1TA00241D. [DOI] [Google Scholar]
- Butler M. A. Photoelectrolysis and physical properties of the semiconducting electrode WO3. J. Appl. Phys. 1977, 48, 1914–1920. 10.1063/1.323948. [DOI] [Google Scholar]
- Li T.; Hu X.; Liu C.; Tang C.; Wang X.; Luo S. Efficient photocatalytic degradation of organic dyes and reaction mechanism with Ag2CO3/Bi2O2CO3 photocatalyst under visible light irradiation. J. Mol. Catal. A: Chem. 2016, 425, 124–135. 10.1016/j.molcata.2016.10.001. [DOI] [Google Scholar]
- Xu C.; Liu Y.; Huang B.; Li H.; Qin X.; Zhang X.; Dai Y. Preparation, characterization, and photocatalytic properties of silver carbonate. Appl. Surf. Sci. 2011, 257, 8732–8736. 10.1016/j.apsusc.2011.05.060. [DOI] [Google Scholar]
- Han Y.; Wei M.; Qu S.; Zhong M.; Han L.; Yang H.; Liu Y.; Su B.; Lei Z. Ag@AgCl quantum dots embedded on Sn3O4 nanosheets towards synergistic 3D flower-like heterostructured microspheres for efficient visible-light photocatalysis. Ceram. Int. 2020, 46, 24060–24070. 10.1016/j.ceramint.2020.06.184. [DOI] [Google Scholar]
- Xiao Y.; He Z.; Wang R.; Tao X.; Li B. Synthesis of WO3 nanofibers decorated with BiOCl nanosheets for photocatalytic degradation of organic pollutants under visible light. Colloids Surf., A 2019, 580, 123752. 10.1016/j.colsurfa.2019.123752. [DOI] [Google Scholar]
- Xu H.; Zhu J.; Song Y.; Zhu T.; Zhao W.; Song Y.; Da Z.; Liu C.; Li H. Fabrication of AgX-loaded Ag2CO3 (X = Cl, I) composites and their efficient visible-light-driven photocatalytic activity. J. Alloys Compd. 2015, 622, 347–357. 10.1016/j.jallcom.2014.09.148. [DOI] [Google Scholar]
- Mehraj O.; Mir N. A.; Pirzada B. M.; Sabir S.; Muneer M. In-situ anion exchange synthesis of AgBr/Ag2CO3 hybrids with enhanced visible light photocatalytic activity and improved stability. J. Mol. Catal. A: Chem. 2014, 395, 16–24. 10.1016/j.molcata.2014.07.027. [DOI] [Google Scholar]
- Chang F.; Zheng J.; Wu F.; Wang X.; Deng B. Binary composites WO3/g-C3N4 in porous morphology: Facile construction, characterization, and reinforced visible light photocatalytic activity. Colloids Surf., A 2019, 563, 11–21. 10.1016/j.colsurfa.2018.11.058. [DOI] [Google Scholar]
- Tonda S.; Kumar S.; Shanker V. In situ growth strategy for highly efficient Ag2CO3/g-C3N4 hetero/nanojunctions with enhanced photocatalytic activity under sunlight irradiation. J. Environ. Chem. Eng. 2015, 3, 852–861. 10.1016/j.jece.2015.03.021. [DOI] [Google Scholar]
- Yu C.; Wei L.; Zhou W.; Chen J.; Fan Q.; Liu H. Enhancement of the visible light activity and stability of Ag2CO3 by formation of AgI/Ag2CO3 heterojunction. Appl. Surf. Sci. 2014, 319, 312–318. 10.1016/j.apsusc.2014.05.158. [DOI] [Google Scholar]
- Yu C.; Chen F.; Liu Z.; Yang K.; Ji H.; Li D.; Xie W.; Li S. Facile synthesis of a robust visible-light-driven AgCl/WO3 composite microrod photocatalyst. J. Alloys Compd. 2019, 809, 151844. 10.1016/j.jallcom.2019.151844. [DOI] [Google Scholar]
- Jia J.; Jiang C.; Zhang X.; Li P.; Xiong J.; Zhang Z.; Wu T.; Wang Y. Urea-modified carbon quantum dots as electron mediator decorated g-C3N4/WO3 with enhanced visible-light photocatalytic activity and mechanism insight. Appl. Surf. Sci. 2019, 495, 143524. 10.1016/j.apsusc.2019.07.266. [DOI] [Google Scholar]
- Yang H. A short review on heterojunction photocatalysts: Carrier transfer behavior and photocatalytic mechanisms. Mater. Res. Bull. 2021, 142, 111406. 10.1016/j.materresbull.2021.111406. [DOI] [Google Scholar]
- Wang Y.; Lu N.; Luo M.; Fan L.; Zhao K.; Qu J.; Guan J.; Yuan X. Enhancement mechanism of fiddlehead-shaped TiO2-BiVO4 type II heterojunction in SPEC towards RhB degradation and detoxification. Appl. Surf. Sci. 2019, 463, 234–243. 10.1016/j.apsusc.2018.08.239. [DOI] [Google Scholar]
- Gu S.; Chen Y.; Yuan X.; Wang H.; Chen X.; Liu Y.; Jiang Q.; Wu Z.; Zeng G. Facile synthesis of CeO2 nanoparticle sensitized CdS nanorod photocatalyst with improved visible-light photocatalytic degradation of rhodamine B. RSC Adv. 2015, 5, 79556–79564. 10.1039/C5RA16114B. [DOI] [Google Scholar]
- McEvoy J. G.; Cui W.; Zhang Z. Synthesis and characterization of Ag/AgCl-activated carbon composites for enhanced visible light photocatalysis. Appl. Catal., B 2014, 144, 702–712. 10.1016/j.apcatb.2013.07.062. [DOI] [Google Scholar]
- Huang H.; Huang N.; Wang Z.; Xia G.; Chen M.; He L.; Tong Z.; Ren C. Room-temperature synthesis of carnation-like ZnO@AgI hierarchical nanostructures assembled by AgI nanoparticles-decorated ZnO nanosheets with enhanced visible light photocatalytic activity. J. Colloid Interface Sci. 2017, 502, 77–88. 10.1016/j.jcis.2017.04.080. [DOI] [PubMed] [Google Scholar]
- Wu T.; Liu G.; Zhao J.; Hidaka H.; Serpone N. Photoassisted degradation of dye pollutants. V. Self-photosensitized oxidative transformation of rhodamine B under visible light irradiation in aqueous TiO2 dispersions. J. Phys. Chem. B 1998, 102, 5845–5851. 10.1021/jp980922c. [DOI] [Google Scholar]
- Li J.; Li Y.; Zhang G.; Huang H.; Wu X. One-Dimensional/Two-Dimensional Core-Shell-Structured Bi2O4/BiO2-x Heterojunction for Highly Efficient Broad Spectrum Light-Driven Photocatalysis: Faster Interfacial Charge Transfer and Enhanced Molecular Oxygen Activation Mechanism. ACS Appl. Mater. Interfaces 2019, 11, 7112–7122. 10.1021/acsami.8b21693. [DOI] [PubMed] [Google Scholar]
- Bera K. K.; Chakraborty M.; Mondal M.; Banik S.; Bhattacharya S. K. Synthesis of α-β Bi2O3 heterojunction photocatalyst and evaluation of reaction mechanism for degradation of RhB dye under natural sunlight. Ceram. Int. 2020, 46, 7667–7680. 10.1016/j.ceramint.2019.11.269. [DOI] [Google Scholar]
- Liu Y.; Guo H.; Zhang Y.; Cheng X.; Zhou P.; Zhang G.; Wang J.; Tang P.; Ke T.; Li W. Heterogeneous activation of persulfate for Rhodamine B degradation with 3D flower sphere-like BiOI/Fe3O4 microspheres under visible light irradiation. Sep. Purif. Technol. 2018, 192, 88–98. 10.1016/j.seppur.2017.09.045. [DOI] [Google Scholar]
- Liu Y.; Guo H.; Zhang Y.; Tang W.; Cheng X.; Liu H. Activation of peroxymonosulfate by BiVO4 under visible light for degradation of Rhodamine B. Chem. Phys. Lett. 2016, 653, 101–107. 10.1016/j.cplett.2016.04.069. [DOI] [Google Scholar]
- Shu J.; Wang Z.; Xia G.; Zheng Y.; Yang L.; Zhang W. One-pot synthesis of AgCl@Ag hybrid photocatalyst with high photocatalytic activity and photostability under visible light and sunlight irradiation. Chem. Eng. J. 2014, 252, 374–381. 10.1016/j.cej.2014.05.040. [DOI] [Google Scholar]
- Dai G.; Yu J.; Liu G. A New Approach for Photocorrosion Inhibition of Ag2CO3 Photocatalyst with Highly Visible-Light-Responsive Reactivity. J. Phys. Chem. C 2012, 116, 15519–15524. 10.1021/jp305669f. [DOI] [Google Scholar]
- James G. S., Lange’s Handbook of Chemistry ,16th ed, New York: McGraw-Hill Companies Inc., 2005; Table 1.71. [Google Scholar]
- Yu J.; Sun D.; Wang T.; Li F. Fabrication of Ag@AgCl/ZnO submicron wire film catalyst on glass substrate with excellent visible light photocatalytic activity and reusability. Chem. Eng. J. 2018, 334, 225–236. 10.1016/j.cej.2017.10.003. [DOI] [Google Scholar]
- Ishibashi K.-i.; Fujishima A.; Watanabe T.; Hashimoto K. Quantum yields of active oxidative species formed on TiO2 photocatalyst. J. Photochem. Photobiol., A 2000, 134, 139–142. 10.1016/S1010-6030(00)00264-1. [DOI] [Google Scholar]
- Gui Y.; Yang L.; Tian K.; Zhang H.; Fang S. P-type Co3O4 nanoarrays decorated on the surface of n-type flower-like WO3 nanosheets for high-performance gas sensing. Sens. Actuators, B 2019, 288, 104–112. 10.1016/j.snb.2019.02.101. [DOI] [Google Scholar]
- Guang L.; Hui W.; Xuejun Z. Effect of drying temperatures on structural performance and photocatalytic activity of BiOCl synthesized by a soft chemical method. J. Solid State Chem. 2016, 239, 259–264. 10.1016/j.jssc.2016.04.039. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.