Abstract
Background
World Trade Center (WTC)‐exposed responders may be eligible to receive no‐cost medical monitoring and treatment for certified conditions, including cancer. The survival of responders with cancer has not previously been investigated.
Methods
This study compared the estimated relative survival of WTC‐exposed responders who developed cancer while enrolled in two WTC medical monitoring and treatment programs in New York City (WTC‐MMTP responders) and WTC‐exposed responders not enrolled (WTC‐non‐MMTP responders) to non‐responders from New York State (NYS‐non‐responders), all restricted to the 11‐southernmost NYS counties, where most responders resided. Parametric survival models estimated cancer‐specific and all‐cause mortality. Follow‐up ended at death or on December 31, 2016.
Results
From January 1, 2005 to December 31, 2016, there were 2,037 cancer cases and 303 deaths (248 cancer‐related deaths) among WTC‐MMTP responders, 564 cancer cases, and 143 deaths (106 cancer‐related deaths) among WTC‐non‐MMTP responders, and 574,075 cancer cases and 224,040 deaths (158,645 cancer‐related deaths) among the NYS‐non‐responder population. Comparing WTC‐MMTP responders with NYS‐non‐responders, the cancer‐specific mortality hazard ratio (HR) was 0.72 (95% confidence interval [CI] = 0.64–0.82), and all‐cause mortality HR was 0.64 (95% CI = 0.58–0.72). The cancer‐specific HR was 0.94 (95% CI = 0.78–1.14), and all‐cause mortality HR was 0.93 (95% CI = 0.79–1.10) comparing WTC‐non‐MMTP responders to the NYS‐non‐responder population.
Conclusions
WTC‐MMTP responders had lower mortality compared with NYS‐non‐responders, after controlling for demographic factors and temporal trends. There may be survival benefits from no‐out‐of‐pocket‐cost medical care which could have important implications for healthcare policy, however, other occupational and socioeconomic factors could have contributed to some of the observed survival advantage.
Keywords: cancer, medical monitoring and treatment, mortality, rescue/recovery work, World Trade Center
1. INTRODUCTION
Cancer survival rates have steadily improved over the past decades. Factors responsible for survival increases include declines in occurrences of cancer types with poor survival relative to the total cancer burden (e.g., decreased incidence of lung cancer among men), 1 improved early detection (e.g., stool testing and colonoscopy for colorectal cancer and low‐dose CT scan for lung cancer), 2 advances in cancer therapy (e.g., targeted immunotherapy and estrogen‐agonists for breast cancer), 1 and enhanced access to effective therapies (e.g., increased referral to comprehensive cancer centers and expanded health insurance coverage). 3 Nevertheless, changes in survival rates should be interpreted with caution since they may be subject to bias. For example, screening programs may lead to overdiagnosis of cancers with better survival. 4 Furthermore, misclassified causes of death affect cause‐specific survival and mortality rates. These rates may also be affected by determinants of cancer risk and survival, like tobacco smoking. Although some cancer screening programs have demonstrated lower cancer‐specific mortality, few have been able to demonstrate lower all‐cause mortality. 5 , 6 , 7 , 8 Despite limitations, survival analyses are useful to compare different populations of cancer patients, particularly when effective treatments are available.
Eligible first responders to the World Trade Center (WTC) disaster in New York City (NYC) on September 11, 2001, have access to annual monitoring and treatment programs (MMTP) funded by the National Institute of Occupational Safety and Health (NIOSH) that provide screenings, diagnostic procedures, and treatments for certified cancers at no‐cost to the patient. 9 Regular monitoring and screening may lead to downstaging for certain cancers. Although cancer incidence among WTC‐responders exposed to a broad spectrum of environmental carcinogens has been investigated, 10 , 11 , 12 , 13 , 14 , 15 , 16 survival has not been examined and is particularly important as we approach the 20th anniversary of the WTC disaster. The aim of this study is to examine survival among those diagnosed with cancer within the nationally funded New York‐based MMTP.
2. METHODS AND MATERIALS
2.1. Overview of the Combined WTC Rescue/Recovery Cohort
The population for this prospective cohort study consisted of WTC‐exposed responders. Responders primarily included firefighters, emergency medical service providers, police, construction and communication workers, volunteers, and cleanup workers. 11 , 17 We created a combined analytic data set from three WTC‐exposed responder cohorts: The Fire Department of the city of New York (FDNY) (N = 16,221), 18 the General Responder Cohort (GRC) (N = 33,427) 19 and the World Trade Center Health Registry (WTCHR) (N = 29,372). 20 Each of the study cohort data centers submitted a data file to the New York State Cancer Registry (NYSCR) of their members which included all available personal identifiers. Responders enrolled in more than one cohort were identified and duplicate records were consolidated. 17 The following criteria were used to reassign enrollment classification: Responders enrolled in either FDNY or GRC cohorts, regardless of enrollment in the WTCHR, were classified as “WTC‐MMTP responders” (n = 49,346); remaining WTC‐exposed responders not enrolled in an MMTP were classified as “WTC‐non‐MMTP responders” (n = 19,756). WTC‐MMTP responders have access to no‐cost annual medical monitoring examinations, diagnostic procedures and, since October 2012, screening and treatment for numerous cancers; WTC‐non‐MMTP responders do not have this access. 9 , 11 Greater description of the Combined WTC Rescue/Recovery Cohort (Combined Cohort) is described elsewhere. 17
2.2. NYS‐non‐responder population
The NYSCR provided individual‐level cancer and mortality data during the same period for cancers diagnosed among residents of the 11‐southernmost NYS counties. We excluded WTC‐responders who were included in the Combined Cohort from this comparison population. The NYS population is used as a common comparison group for both subgroups of the Combined Cohort.
2.3. Cancer Registry linkage and mortality case ascertainment
Cancer outcomes were assessed by matching the Combined Cohort (n = 69,102) to data from the NYSCR. Records of cancers diagnosed among NYS residents, between January 1, 2002, and December 31, 2015, were returned for analysis, together with mortality data including date of last contact (i.e., date of death for persons known to be deceased) and cause of death. We also obtained demographics including sex, race/ethnicity, age, county of residence at diagnosis, and clinical information such as cancer site, histology, stage, and time of diagnosis (month and year) for cancer cases.
2.4. Analysis population
Our source population included adults (i.e., 18 years or older on September 11, 2001) diagnosed with cancer in the Combined Cohort (WTC‐MMTP and WTC‐non‐MMTP responders) and non‐responders from NYS. Analyses were restricted to only first primary malignant tumors (including in situ bladder cancer) (Figures 1). To account for potential self‐selection bias, participants who enrolled into one of the WTC‐responder cohorts after their cancer diagnosis were excluded. Participants whose death date was in the same month and year as their cancer diagnosis (e.g., diagnosed via autopsy/death certificate) were removed from analyses. Since some WTC‐exposed responders may have died from cancer before being eligible for entry into the study, we excluded cases diagnosed before January 1, 2005 (71% of the cohort was already established by this date). Finally we excluded participants who did not reside in the 11‐southernmost counties of NYS (Bronx, Kings, Nassau, New York, Orange, Putnam, Queens, Richmond, Rockland, Suffolk, and Westchester) as most responders lived in these areas, yielding similar proximity to large cancer centers and environmental exposures.
Figure 1.
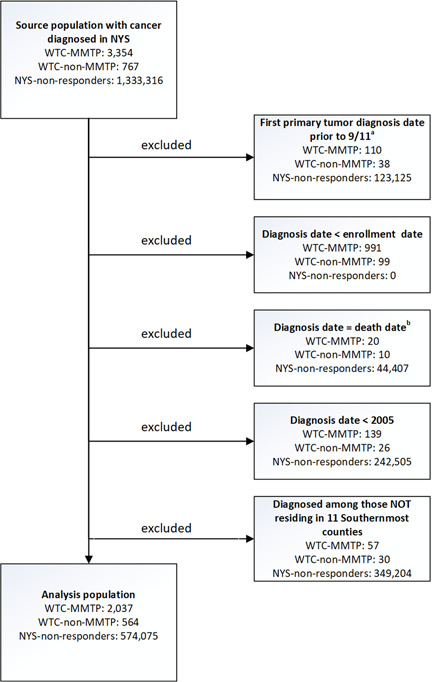
CONSORT diagram. aPersons were excluded from all analyses if their first primary cancer was before September 11, 2001. bPersons were excluded from all analyses if they were diagnosed with cancer on their date of last contact or were diagnosed via autopsy, only. NYS‐non‐responders, all other cancer patients who were residents of the 11‐southernmost counties of New York State and were not included in one of the WTC responder cohorts; WTC‐MMTP, cancer patients enrolled in the World Trade Center Medical Monitoring and Treatment Program; WTC‐non‐MMTP, WTC‐exposed cancer patients who were NOT enrolled in the WTC‐MMTP
2.5. Statistical analysis
We estimated Kaplan–Meier survival functions using event times for those who died from cancer‐specific causes and from all causes. For multivariate analyses, we used piecewise exponential survival models, similar to those used in other WTC studies, 21 , 22 , 23 , 24 to estimate hazard ratios (HR) for cause‐specific and all‐cause mortality. These models generate HRs similar to Cox regression HRs, but also allow parametric estimations of baseline incidence and have rate ratio interpretations. The specific model for our primary analyses was as follows:
where, Y ik is the number of deaths in stratum i (defined by age, calendar year, race/ethnicity, sex, stage, and exposure/enrollment classification) during the time interval indexed by k, with T ik the corresponding person–time at risk for that stratum and x j the exposure/MMTP classification. β j is the log‐relative hazard for contrasts of exposure/MMTP classification; the α k is the log of the baseline hazard, in 1‐year time intervals. The w ik is the dummy variable representing the 1‐year time interval and z il is the covariates' age, sex, race/ethnicity, calendar year, cancer site, and cancer stage. Cancer‐specific mortality (i.e., from any cancer) was derived from ICD‐10 cause of death data; all‐cause mortality was defined as death from any cause. CIs for survival proportions were estimated based on a Poisson distribution of observed deaths.
The analysis was based on two contrasts: WTC‐MMTP responders versus NYS‐non‐responders and WTC‐non‐MMTP responders versus NYS‐non‐responders. Analyses are presented for selected cancer sites among all WTC‐exposed responders and for all cancers.
For primary analyses, follow‐up started on the date of diagnosis and ended at death or December 31, 2016, whichever occurred first. All models controlled for age at diagnosis (in 5‐year groupings), race/ethnicity (Hispanic, non‐Hispanic White, non‐Hispanic Black, non‐Hispanic Asian/Pacific Islander, non‐Hispanic other), sex, year of diagnosis (2005–2015), and cancer site. Cancer incidence data were ascertained through the end of 2015 and mortality data through the end of 2016, therefore, all participants, regardless of when they were diagnosed, had the chance to accrue at least 1 year of follow‐up. To partially control for length bias, models were controlled for cancer stages (localized, regional, and distant).
We conducted three additional analyses. First, we started follow‐up on January 1, 2005, to account for potential lead‐time bias. Second, as smoking status (ever/never) was known for most WTC‐exposed responders but not for NYS‐non‐responders, we repeated the primary analyses with a stratified analysis comparing MMTP and non‐MMTP separately to NYS‐non‐responders forever smokers and never‐smokers, separately. Third, to better understand the impact of access to treatment on all‐cause and cancer‐specific mortality, we examined the 991 participants that were excluded from the primary analyses due to self‐selection into the WTC‐MMTP cohort (i.e., enrolled after cancer diagnosis). Follow‐up for the second and third analysis started at the date of diagnosis.
Analyses were conducted using SAS 9.4 (SAS Institute Inc.) and R v3.6.0. 25 This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines and was approved by Institutional Review Boards at the Albert Einstein College of Medicine, New York City Department of Health and Mental Hygiene, and New York State Department of Health.
3. RESULTS
Descriptive statistics for the study population are presented in Table 1. Overall, among WTC‐exposed responders, 2,601 first primary cancer cases were included in the analysis, of which 2,037 (78.3%) were in WTC‐MMTP responders and 564 (21.7%) were in WTC‐non‐MMTP responders. Among NYS‐non‐responders, 574,075 first primary cancer cases were diagnosed during the study period. We found 303 and 143 deaths among WTC‐MMTP responders and WTC‐non‐MMTP responders, respectively, whereas NYS‐non‐responders had a total of 224,040 deaths. Among these deaths, 248 (82%), 106 (74%), and 158,645 (71%) were due to cancer‐related causes for WTC‐MMTP responders, WTC‐non‐MMTP responders, and NYS‐non‐responders, respectively. Both WTC‐MMTP responders and WTC‐non‐MMTP responders had a higher proportion of cancers diagnosed at an earlier stage and a lower proportion of distant‐stage tumors when compared with NYS‐non‐responders (59% and 57%, respectively, vs. 47% localized; 17% and 19%, respectively, vs. 24% distant).
Table 1.
Selected demographic characteristics for cancers diagnosed between 2005 and 2015
| WTC‐MMTP (n = 2,037) | WTC non‐MMTP (n = 564) | NYS‐non‐responders (n = 574,075) | |
|---|---|---|---|
| Race/ethnicity, n (%) | |||
| Non‐Hispanic White | 1,611 (79.1) | 388 (68.8) | 343,275 (59.8) |
| Non‐Hispanic Black | 213 (10.5) | 91 (16.1) | 104,470 (18.2) |
| Non‐Hispanic Asian/Pacific Islander | 10 (0.5) | 14 (2.5) | 40,025 (7.0) |
| Hispanic | 194 (9.5) | 62 (11.0) | 80,844 (14.1) |
| Non‐Hispanic other | 9 (0.4) | 9 (1.6) | 5,461 (1.0) |
| Sex, n (%) | |||
| Male | 1,870 (91.8) | 431 (76.4) | 285,033 (49.7) |
| Female | 167 (8.2) | 133 (23.6) | 289,042 (50.3) |
| Age at diagnosis, mean (SD) | 55.5 (9.6) | 57.4 (10.0) | 64.1 (14.0) |
| Smoking status, n (%) | |||
| Ever | 885 (43.5) | 302 (53.6) | 0 (0.0) |
| Never | 1,105 (54.3) | 251 (44.5) | 0 (0.0) |
| Unknown | 47 (2.3) | 11 (2.0) | 574,075 (100.0) |
| Year of diagnosis | |||
| 2005–2008 | 457 (22.4) | 204 (36.2) | 206,887 (36.0) |
| 2009–2012 | 825 (40.5) | 188 (33.3) | 209,387 (36.5) |
| 2013–2015 | 755 (37.1) | 172 (30.5) | 157,801 (27.5) |
| Type of primary cancer diagnosis, n (%) | |||
| Prostate | 659 (32.4) | 162 (28.7) | 93,135 (16.2) |
| Melanoma of the skin | 138 (6.8) | 20 (3.5) | 17,436 (3.0) |
| Colon and rectum | 136 (6.7) | 32 (5.7) | 53,767 (9.4) |
| Thyroid | 133 (6.5) | 24 (4.3) | 22,667 (4.0) |
| Lung and bronchus | 110 (5.4) | 42 (7.4) | 58,386 (10.2) |
| Kidney and renal pelvis | 95 (4.7) | 27 (4.8) | 17,756 (3.1) |
| Urinary bladder | 87 (4.3) | 18 (3.2) | 23,459 (4.1) |
| Breast | 58 (2.8) | 54 (9.6) | 85,663 (14.9) |
| Myeloma | 35 (1.7) | 15 (2.7) | 10,183 (1.8) |
| Pancreas | 35 (1.7) | 9 (1.6) | 15,256 (2.7) |
| Brain and other nervous system | 28 (1.4) | 10 (1.8) | 6,327 (1.1) |
| Esophagus | 26 (1.3) | 9 (1.6) | 4,852 (0.9) |
| Liver | 26 (1.3) | 8 (1.4) | 10,588 (1.8) |
| All other sites | 471 (23.1) | 134 (23.8) | 154,600 (26.9) |
| Staging, n (%) | |||
| Localized | 1,200 (58.9) | 324 (57.4) | 270,891 (47.2) |
| Regional | 387 (19.0) | 102 (18.1) | 120,194 (20.9) |
| Distant | 341 (16.7) | 106 (18.8) | 137,277 (23.9) |
| Unknown | 109 (5.4) | 32 (5.7) | 45,713 (8.0) |
| Deaths, n (%) | 303 (14.9) | 143 (25.4) | 224,040 (39.0) |
| Cancer deaths, n (%) | 248 (12.2) | 106 (18.8) | 158,645 (27.6) |
| Survival rate, n (%) | |||
| 1‐year survival a | 1916 (94.1) | 507 (89.9) | 474,895 (82.7) |
| 3‐year survival b | 1346 (88.3) | 372 (81.2) | 326,959 (69.6) |
| 5‐year survival c | 919 (86.1) | 266 (76.0) | 228,933 (62.8) |
| Person–time (year) median (IQR) d | 4.5 (2.3, 7.1) | 4.5 (2.2, 8.0) | 3.7 (1.5, 7.1) |
Abbreviations: IQR, interquartile range; NYS‐non‐responders, all other cancer patients who were residents of the 11‐southernmost counties of New York State and were not included in one of the WTC‐responder cohorts; WTC‐MMTP, cancer patients enrolled in the World Trade Center Medical Monitoring and Treatment Program; WTC‐non‐MMTP, WTC‐exposed cancer patients who were NOT enrolled in the WTC‐MMTP.
Percentages calculated among participants who had an opportunity to accrue at least 1 year of follow‐up.
Percentages calculated among participants who had an opportunity to accrue at least 3 years of follow‐up.
Percentages calculated among participants who had an opportunity to accrue at least 5 years of follow‐up.
Person–time calculated from the time of diagnosis to death or end of follow‐up.
Kaplan–Meier graphs (Figures 2, S1, and S2) show relative survival from 1 to 12 years of follow‐up for WTC‐MMTP, WTC‐non‐MMTP responders, and NYS‐non‐responders for cancer‐specific and all‐cause mortality. In Figure 3, we present cancer‐specific and all‐cause mortality HRs. WTC‐MMTP responders experienced lower cancer‐specific mortality (HR = 0.72; 95% CI = 0.64–0.82) and all‐cause mortality (HR = 0.64; 95% CI = 0.58–0.72) when compared with NYS‐non‐responders. When comparing WTC‐non‐MMTP responders with NYS‐non‐responders, both cancer‐specific mortality (HR = 0.94; 95% CI = 0.78–1.14) and all‐cause mortality (HR = 0.93; 95% CI = 0.79–1.10) was lower but not significantly different. Similar results were found in sensitivity analyses when follow‐up was started on January 1, 2005.
Figure 2.
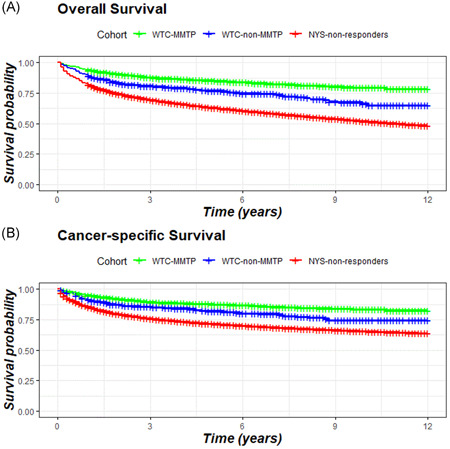
Kaplan–Meier plots: (A) All‐cause and (B) cancer‐specific survival. Follow‐up starts at the time of diagnosis log‐rank p < .001. NYS‐non‐responders, all other cancer patients who were residents of the 11‐southernmost counties of New York State and were not included in one of the WTC‐responder cohorts; WTC‐MMTP, cancer patients enrolled in the World Trade Center Medical Monitoring and Treatment Program; WTC‐non‐MMTP, WTC‐exposed cancer patients who were NOT enrolled in the WTC‐MMTP. [Color figure can be viewed at wileyonlinelibrary.com]
Figure 3.
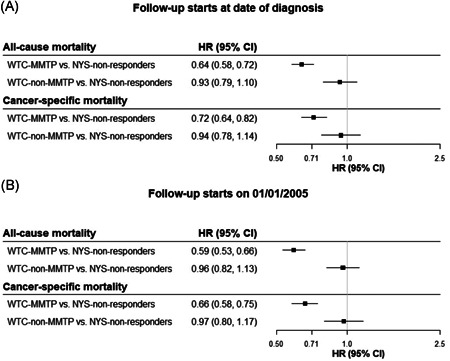
All‐cause and cancer‐specific mortality risk. Models controlled for calendar year, age, race/ethnicity, sex, cancer stage and site. 95% CI, 95% confidence interval; HR, hazard ratio; NYS‐non‐responders, all other cancer patients who were residents of the 11‐southernmost counties of New York State and were not included in one of the WTC‐responder cohorts. WTC‐MMTP, cancer patients enrolled in the World Trade Center Medical Monitoring and Treatment Program; WTC‐non‐MMTP, WTC‐exposed cancer patients who were NOT enrolled in the WTC‐MMTP
Cancers with the largest number of deaths among WTC‐MMTP responders and WTC‐non‐MMTP responders were from the lung (n = 50, n = 27, respectively), prostate (n = 31, n = 14), pancreas (n = 29, n = 9), and colorectal (n = 21, n = 10). Prostate cancer was associated with reduced mortality for WTC‐MMTP responders when compared with NYS‐non‐responders (HR = 0.62; 95% CI = 0.44–0.88). Cancers of the lung (HR = 0.74; 95% CI = 0.56–0.97), colon/rectum (HR = 0.48; 95% CI = 0.31–0.74), and kidney (HR = 0.36; 95% CI = 0.16–0.79) were also associated with reduced mortality for WTC‐MMTP responders when compared with NYS‐non‐responders (Table 2). Comparing WTC‐non‐MMTP responders with NYS‐non‐responders, mortality was significantly elevated for pancreatic (HR = 1.66; 95% CI = 1.15–2.39). Findings were similar when starting follow‐up on January 1, 2005 (Table 2B). Additionally, results were similar for cancer‐specific mortality (Table SI), particularly among cancers with large numbers of deaths such as prostate, lung, and colorectal cancers.
Table 2.
All‐cause mortality risk among cases of selected cancers
| All‐cause mortality by cancer site | WTC‐MMTP versus NYS‐non‐responders | WTC non‐MMTP versus NYS‐non‐responders |
|---|---|---|
| HR (95% CI) | HR (95% CI) | |
| (A) All‐cause mortality risk by cancer site: Follow‐up time starts at diagnosis date | ||
| Prostate | 0.62 (0.44, 0.88) | 0.92 (0.54, 1.55) |
| Lung and bronchus | 0.74 (0.56, 0.97) | 0.88 (0.60, 1.28) |
| Esophagus | 0.65 (0.36, 1.18) | 1.15 (0.55, 2.43) |
| Colon and rectum | 0.48 (0.31, 0.74) | 1.11 (0.60, 2.06) |
| Myeloma | 0.49 (0.22, 1.10) | 0.50 (0.16, 1.54) |
| Pancreas | 1.66 (1.15, 2.39) | 1.18 (0.61, 2.27) |
| Brain and other nervous system | 1.11 (0.70, 1.76) | 0.87 (0.42, 1.83) |
| Liver | 0.74 (0.44, 1.22) | 1.00 (0.50, 2.01) |
| Melanoma of the skin | 0.54 (0.27, 1.08) | 0.82 (0.20, 3.27) |
| Kidney and renal pelvis | 0.36 (0.16, 0.79) | 1.23 (0.51, 2.96) |
| (B) All‐cause mortality risk by cancer site: Follow‐up time starts on January 1, 2005 | ||
| Prostate | 0.59 (0.42, 0.84) | 0.88 (0.52, 1.49) |
| Lung | 0.59 (0.44, 0.78) | 0.76 (0.52, 1.10) |
| Esophagus | 0.60 (0.33, 1.09) | 1.81 (0.86, 3.80) |
| Colon and rectum | 0.50 (0.32, 0.76) | 1.10 (0.59, 2.05) |
| Myeloma | 0.49 (0.22, 1.10) | 0.48 (0.15, 1.48) |
| Pancreas | 1.10 (0.76, 1.58) | 2.31 (1.20, 4.44) |
| Brain and other nervous system | 1.07 (0.67, 1.71) | 0.97 (0.46, 2.04) |
| Liver | 0.73 (0.44, 1.21) | 2.25 (1.12, 4.50) |
| Melanoma of the skin | 0.52 (0.26, 1.05) | 0.90 (0.22, 3.60) |
| Kidney and renal pelvis | 0.37 (0.17, 0.82) | 1.40 (0.58, 3.38) |
Note: All models controlled for calendar year, age, race/ethnicity, sex (except prostate), and stage; models are restricted to participants aged 40 and older. Bold indicates p < 0.05.
Abbreviations: HR, hazard ratio; NYS‐non‐responders, all other cancer patients who were residents of the 11‐southernmost counties of New York State and were not included in one of the WTC‐responder cohorts; WTC‐MMTP, cancer patients enrolled in the World Trade Center Medical Monitoring and Treatment Program; WTC‐non‐MMTP, WTC‐exposed cancer patients who were NOT enrolled in the WTC‐MMTP.
In our secondary analyses, which were stratified by smoking status, we observed a significantly reduced all‐cause mortality for both smoker (HR = 0.73; 95% CI = 0.63–0.85) and nonsmoker (HR = 0.56; 95% CI = 0.47–0.67) WTC‐MMTP responders compared with NYS‐non‐responders of unknown smoking status. All‐cause mortality was similar for smoker (HR = 0.98; 95% CI = 0.80–1.20) and nonsmoker (HR = 0.88; 95% CI = 0.67–1.17) WTC‐non‐MMTP responders when compared with NYS‐non‐responders of unknown smoking status. Cancer‐specific mortality was also reduced for smoker (HR = 0.79; 95% CI = 0.67–0.93) and nonsmoker (HR = 0.66; 95% CI = 0.55–0.81) WTC‐MMTP responders compared with NYS‐non‐responders of unknown smoking status. Cancer‐specific mortality was similar for smoker (HR = 0.95; 95% CI = 0.75–1.21) and nonsmoker (HR = 0.95; 95% CI = 0.68–1.31) in WTC‐non‐MMTP responders when compared with NYS‐non‐responders of unknown smoking status.
In our final secondary analyses, which evaluated the impact of access to treatment among those who self‐selected into a WTC‐MMTP cohort (n = 991) after a cancer diagnosis, there was a similar crude proportion of deaths (n = 120; 12.1%) and cancer‐related deaths (n = 94; 9.5%) compared with those in the main analysis. In multivariable models, protective associations were also observed when comparing this subgroup to NYS‐non‐responders for both all‐cause mortality (HR = 0.29; 95% CI = 0.24, 0.35) and cancer‐cause mortality (HR = 0.30; 95% CI = 0.24, 0.37). Among the 991 participants, the most commonly diagnosed cancers were prostate (n = 250; 25.3%), colorectal (n = 86; 8.7%), non‐Hodgkin lymphoma (n = 64; 6.5%), thyroid (n = 62; 6.3%) and kidney/renal pelvis (n = 60; 6.1%) cancer.
4. DISCUSSION
This is the first analysis of survival among WTC‐exposed responders who developed cancer. After accounting for demographic and temporal factors, we found that both cancer‐specific and all‐cause mortality were lower among WTC‐exposed responders enrolled in MMTP, compared with the general population comparison group (i.e., NYS‐non‐responders with cancer). The same analyses showed WTC‐non‐MMTP responders and NYS‐non‐responders had similar survival outcomes. These results provide evidence that systematic health surveillance and treatment improves survival among cancer patients. It will be important to determine the extent to which medical monitoring and treatment improves survival for cancer when follow‐up is extended and to also investigate how toxic exposures at the WTC disaster site influence this outcome.
WTC‐MMTP responders had consistent monitoring throughout follow‐up and, beginning in October 2012, had no‐cost access to screening and treatment for Program‐certified cancers. 9 It is notable that WTC‐MMTP responders not only had lower cancer‐specific mortality but also had lower all‐cause mortality. Randomized studies of cancer screening in the general population have rarely shown benefits in all‐cause mortality. 5 , 6 , 7 , 8 Restricting our review to randomized studies, we could find only two demonstrating all‐cause mortality benefits: 1 of 11 mammography screening studies 5 and 1 of 6 low‐dose chest CT studies, whereas no studies of fecal occult blood testing, flexible sigmoidoscopy, 26 or prostate‐specific antigen testing 6 showed such effect. 27 , 28 , 29 , 30 , 31 , 32 Meta‐analyses confirmed the lack of benefit across studies. 5 , 6 , 26 , 33 Possible explanations for improved, all‐cause survival benefit found in cancer patients enrolled in MMTPs include differences in cohort characteristics, such as a healthy worker effect among traditional uniformed workers, earlier diagnosis and extensive social support within the cohort, and the provision of not only free screening but also no‐cost treatment for both neoplastic and non‐neoplastic conditions. As the majority of WTC‐MMTP and WTC‐non‐MMTP responders had health insurance, the survival benefit from MMTP enrollment results from additional factors, including (1) the absence of any cost to the WTC‐MMTP responders, whereas there might be copays and deductibles for WTC‐non‐MMTP responders and (2) participation in a formal program with extensive case management likely provides far better adherence to screening and treatment.
Survival may have appeared to improve among WTC‐exposed individuals because of early diagnosis as well as overdiagnosis. Detection of cancer because of closer surveillance is also supported by elevated incidence rates of melanoma of the skin, prostate cancer, and thyroid cancer found among the WTC‐MMTP responders. 10 , 12 , 13 , 14 Although melanoma of the skin and prostate cancer were among the most commonly diagnosed, additional surveillance is unlikely to fully explain the increased incidence among WTC‐MMTP responders, as adjustment for cancer site and stage did not modify the results, and survival improved for several other cancer types. Additionally, WTC‐MMTP and WTC‐non‐MMTP responders had similar proportions of localized and distant tumors, which is further evidence against early detection being responsible for improved survival among WTC‐MMTP responders.
Given that a majority of WTC‐exposed responders were actively working, lower cancer mortality may be attributed to better health, compared with the NYS general population. 34 , 35 , 36 Aging of this population, may have diminished this effect, 34 , 36 however, given the relatively short period of follow‐up, this bias is unlikely to have been pronounced. Furthermore, differences in cancer survival rates between WTC‐MMTP responders and WTC‐non‐MMTP responders suggest that responders who were able to access medical treatment and monitoring received additional benefits that contributed to longer survival, beyond that conveyed by the healthy worker effect. 34 , 36 This was found not only among those who had cancer causes of death but also among those who died from other causes. The MMTPs provide monitoring, diagnostic tests, and treatment, at no charge, for conditions specified by law and certified by NIOSH program administrators as WTC‐related. Non‐cancer‐covered conditions include upper and lower respiratory diseases, gastroesophageal reflux disorders, and mental health conditions, some of which may be cancer precursors. 9 , 37 , 38 Additionally, cardiovascular disease and diabetes, although not covered by the program, may be diagnosed at annual monitoring exams with appropriate referrals provided. These could all have provided potential survival benefits for WTC‐MMTP responders. We aimed at addressing length bias among the medically monitored population, 39 , 40 , 41 by controlling for primary site (in cancer‐specific and all‐cause mortality models) and stage (in all models). Potential lead‐time bias was also addressed by assigning all participants a start time of January 1, 2005.
Although confounding is a possible limitation of all observational research, we attempted to control for available demographic factors that could have influenced relative survival, as well as tobacco smoking. An aspect that we, unfortunately, could not address is the issue of survival bias among participants from the GRC. Unlike the WTCHR, a closed cohort that enrolled all participants between 2003 and 2004, and the FDNY, which predominantly enrolled participants on September 11, 2001, or shortly thereafter, the GRC is an open cohort with continued follow‐up. It is plausible that a small proportion of individuals were diagnosed with cancer and died before enrollment, and thus we cannot fully rule out survival bias for this study. Another limitation is that data related to why those in non‐MMTP are not enrolled in a New York‐based WTC‐MMTP were not available in this study. In addition, non‐MMTP responders may have not enrolled in a New York‐based WTC‐MMTP for various reasons, including enrollment in the non‐FDNY/GRC federal WTC Nationwide Provider Network, 42 barriers related to the enrollment process, despite sustained efforts to inform them about the program, and not meeting eligibility requirements needed to enroll in a WTC‐MMTP. 43 , 44 , 45 It should be noted that these reasons for lack of participation in an MMTP have the potential to bias results either toward or away from the null. For example, enrollment in the federal program would incur a bias toward the null as non‐MMTP participants would be receiving additional healthcare benefits not accounted for in this study, and, conversely, hurdles with respect to the enrollment process, away from the null. In addition to occupation, differences in demographics such as socioeconomic status (SES) between groups may have placed those in the WTC‐MMTP program at an advantage for having a higher likelihood of survival from cancer. We hope to investigate all of these issues in future work.
Another limitation of this study, similar to most cohort studies, is that some individuals may have moved to areas outside of NYS where we could not conduct cancer/mortality linkages. These individuals, who were lost to follow‐up, represent a minority of the cohort. There may have also been imperfect cancer/mortality data linkages and limited power to compare rare cancers across exposure/enrollment classifications. We also could not assess the extent to which comorbidities or underlying medical conditions affected survival for each cohort. Additionally, data were not available to address actual exposures experienced either during or as a consequence of the WTC disaster or related to occupational exposures. This will be important follow‐up work to this study. Finally, we were only partially able to analyze smoking status in stratified analyses since these data were unavailable in the NYS cohort. Indirect methods for assessing the effects of tobacco use in occupational health research, such as those employed by Axelson and Steeland, 46 should be explored in future work but is beyond the scope of the current study. Between WTC‐MMTP responders and WTC‐non‐MMTP responders, differences in smoking rates (44% vs. 54%) were likely not large enough to explain the observed associations (the overall mortality disadvantage from smoking has been estimated to be 17%–38%). 47
On the 20th anniversary of the September 11 attacks, our findings from the first investigation of cancer survival among WTC‐responders demonstrate that enrollment in a health program that includes not only screening but also that no‐cost treatment may provide unique benefits. WTC‐exposed responder cancer patients enrolled in the MMTP had higher survival rates compared with those not enrolled in the MMTP, even after adjusting for some demographic factors and temporal trends, although other occupational and SES factors might have accounted for some of the survival advantages among WTC‐MMTP participants. The importance of these findings, however, extends far beyond the study of WTC health effects as survival benefits from no‐out‐of‐pocket‐cost medical care could have important implications for healthcare policy if applicable to other environmental disasters, both for responders to these events and to the general population.
CONFLICTS OF INTEREST
The authors declare that there are no conflicts of interest.
DISCLOSURE BY AJIM EDITOR OF RECORD
Steven Markowitz declares that he has no conflict of interest in the review and publication decision regarding this article.
AUTHOR CONTRIBUTIONS
The manuscript was conceptualized by David G. Goldfarb, Rachel Zeig‐Owens, Dana Kristjansson, Charles B. Hall, and Paolo Boffetta. The acquisition, analysis, or interpretation of data for the work was done by David G. Goldfarb, Rachel Zeig‐Owens, Dana Kristjansson, Jiehui Li, Mark R. Farfel, James E. Cone, Maria J. Schymura, Charles B. Hall, and Paolo Boffetta. All authors had a role in drafting this study or revising it critically for important intellectual content. All authors have read and agreed for this version to be published.
ETHICS APPROVAL AND INFORMED CONSENT
This study was approved under expedited review and received a waiver of informed consent. This study also received approval from the New York City Department of Health and Mental Hygiene and the New York State Department of Health. The Icahn School of Medicine at Mount Sinai IRB ruled the study as exempt human subjects research.
Supporting information
Supporting information.
Supporting information.
Supporting information.
ACKNOWLEDGMENTS
Rachel Zeig‐Owens has full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Molly Skerker, MPH provided substantial administrative support in the initial phase of this study. She did not receive financial compensation for this study. This study was supported through the National Institute for Occupational Safety and Health (NIOSH) cooperative agreements (U01OH011315, U01 OH011932, U01 OH011681, U01 OH011931, U01 OH011480, and U50/OH009739) and contracts (200‐2011‐39378, 200‐2011‐39383, 200‐2017‐93325, and 200‐2017‐93326). Additionally, this study was supported in part by cooperative agreement 6NU58DP006309 awarded to the New York State Department of Health by the Centers for Disease Control and Prevention (CDC) and by Contract 75N91018D00005 (Task Order 75N91018F00001) and grant P30 CA013330 from the National Cancer Institute (NCI), National Institutes of Health, Department of Health and Human Services. This study was also supported by cooperative agreement U50/ATU272750 from the Agency for Toxic Substances and Disease Registry (ATSDR), CDC, which included support from the National Center for Environmental Health, CDC; and by the New York City Department of Health and Mental Hygiene (NYC DOHMH). The funders have no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Goldfarb DG, Zeig‐Owens R, Kristjansson D, et al. Cancer survival among World Trade Center rescue and recovery workers: a collaborative cohort study. Am J Ind Med. 2021;64:815‐826. 10.1002/ajim.23278
[Correction added on 16 August 2021, after first publication: The caption of figure 2 and 3, and Table 2 were revised at the request of the authors.]
DATA AVAILABILITY STATEMENT
Data that support the findings of the study may be obtained from the corresponding author (Paolo Boffetta) upon reasonable request after approval by the Steering Committee for “Incidence, Latency, and Survival of Cancer Following World Trade Center Exposure” (NIOSH Cooperative Agreement U01 OH011932) in accordance with the study's official Data Sharing Plan.
REFERENCES
- 1. American Cancer Society . Cancer Treatment & Survivorship Facts & Figures 2019‐2021. Atlanta, GA: American Cancer Society; 2019. [Google Scholar]
- 2. Pinsky PF. Principles of cancer screening. Surg Clin North Am. 2015;95(5):953‐966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Walker GV, Grant SR, Guadagnolo BA, et al. Disparities in stage at diagnosis, treatment, and survival in nonelderly adult patients with cancer according to insurance status. J Clin Oncol. 2014;32(28):3118‐3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Miller A. Screening: evidence and practice. Bull World Health Organ. 2008;86(4):320‐320. [Google Scholar]
- 5. Gotzsche PC, Jorgensen KJ. Screening for breast cancer with mammography. Cochrane Database Syst Rev. 2013;2013(6):CD001877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ilic D, Neuberger MM, Djulbegovic M, Dahm P. Screening for prostate cancer. Cochrane Datab Syst Rev. 2013;(1):CD004720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lin JS, Piper MA, Perdue LA, et al. Screening for colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. J Am Med Assoc. 2016;315(23):2576‐2594. [DOI] [PubMed] [Google Scholar]
- 8. Newman DH. Screening for breast and prostate cancers: moving toward transparency. J Natl Cancer Inst. 2010;102(14):1008‐1011. [DOI] [PubMed] [Google Scholar]
- 9. Centers for Disease Control and Prevention . Covered Conditions—World Trade Center Health Program. 2020. https://www.cdc.gov/wtc/conditions.html. Accessed February 1, 2021.
- 10. Boffetta P, Zeig‐Owens R, Wallenstein S, et al. Cancer in World Trade Center responders: findings from multiple cohorts and options for future study. Am J Ind Med. 2016;59(2):96‐105. [DOI] [PubMed] [Google Scholar]
- 11. Santiago‐Colón A, Daniels R, Reissman D, et al. World Trade Center Health Program: first decade of research. Int J Environ Res Public Health. 2020;17(19):7290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li J, Cone JE, Kahn AR, et al. Association between World Trade Center exposure and excess cancer risk. JAMA. 2012;308(23):2479‐2488. [DOI] [PubMed] [Google Scholar]
- 13. Shapiro MZ, Wallenstein SR, Dasaro CR, et al. Cancer in general responders participating in World Trade Center Health Programs, 2003–2013. JNCI Cancer Spect. 2019;4(1):pkz090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Webber MP, Glaser MS, Weakley J, et al. Physician‐diagnosed respiratory conditions and mental health symptoms 7‐9 years following the World Trade Center disaster. Am J Ind Med. 2011;54(9):661‐671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li J, Brackbill RM, Liao TS, et al. Ten‐year cancer incidence in rescue/recovery workers and civilians exposed to the September 11, 2001 terrorist attacks on the World Trade Center. Am J Ind Med. 2016;59(9):709‐721. [DOI] [PubMed] [Google Scholar]
- 16. Solan S, Wallenstein S, Shapiro M, et al. Cancer incidence in World Trade Center rescue and recovery workers, 2001–2008. Environ Health Perspect. 2013;121(6):699‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brackbill RM, Kahn AR, Li J, et al. Combining three cohorts of World Trade Center rescue/recovery workers for assessing cancer incidence and mortality. Int J Environ Res Public Health. 2021;18(4):1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yip J, Webber MP, Zeig‐Owens R, et al. FDNY and 9/11: clinical services and health outcomes in World Trade Center‐exposed firefighters and EMS workers from 2001 to 2016. Am J Ind Med. 2016;59(9):695‐708. [DOI] [PubMed] [Google Scholar]
- 19. Herbert R, Moline J, Skloot G, et al. The World Trade Center disaster and the health of workers: five‐year assessment of a unique medical screening program. Environ Health Perspect. 2006;114(12):1853‐1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Farfel M, DiGrande L, Brackbill R, et al. An overview of 9/11 experiences and respiratory and mental health conditions among World Trade Center Health Registry enrollees. J Urban Health. 2008;85(6):880‐909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Glaser MS, Webber MP, Zeig‐Owens R, et al. Estimating the time interval between exposure to the World Trade Center disaster and incident diagnoses of obstructive airway disease. Am J Epidemiol. 2014;180(3):272‐279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sudenis T, Hall K, Cartotto R. The duration of an exposure response gradient between incident obstructive airways disease and work at the World Trade Center site: 2001‐2011. PLOS Curr. 2015;36:7‐305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu X, Yip J, Zeig‐Owens R, et al. The effect of World Trade Center exposure on the timing of diagnoses of obstructive airway disease, chronic rhinosinusitis, and gastroesophageal reflux disease. Front Public Health. 2017;5:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Weakley J, Hall CB, Liu X, et al. The effect of World Trade Center exposure on the latency of chronic rhinosinusitis diagnoses in New York City firefighters: 2001‐2011. Occup Environ Med. 2016;73(4):280‐283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. R Core, Team . R: A Language and Environment for Statistical Computing. 2019; https://www.R-project.org/. Accessed February 1, 2021.
- 26. Holme O, Bretthauer M, Fretheim A, Odgaard‐Jensen J, Hoff G. Flexible sigmoidoscopy versus faecal occult blood testing for colorectal cancer screening in asymptomatic individuals. Cochrane Database Syst Rev. 2013;9:CD009259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Becker N, Motsch E, Gross ML, et al. Randomized study on early detection of lung cancer with MSCT in Germany: results of the first 3 years of follow‐up after randomization. J Thorac Oncol. 2015;10(6):890‐896. [DOI] [PubMed] [Google Scholar]
- 28. de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced lung‐cancer mortality with volume CT screening in a randomized trial. N Engl J Med. 2020;382(6):503‐513. [DOI] [PubMed] [Google Scholar]
- 29. Infante M, Cavuto S, Lutman FR, et al. Long‐term follow‐up results of the DANTE trial, a randomized study of lung cancer screening with spiral computed tomography. Am J Respir Crit Care Med. 2015;191(10):1166‐1175. [DOI] [PubMed] [Google Scholar]
- 30. National Lung Screening Trial Research T , Aberle DR, Adams AM, et al. Reduced lung‐cancer mortality with low‐dose computed tomographic screening. N Engl J Med. 2011;365(5):395‐409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pastorino U, Rossi M, Rosato V, et al. Annual or biennial CT screening versus observation in heavy smokers: 5‐year results of the MILD trial. Eur J Cancer Prev. 2012;21(3):308‐315. [DOI] [PubMed] [Google Scholar]
- 32. Wille MM, Dirksen A, Ashraf H, et al. Results of the Randomized Danish Lung Cancer Screening Trial with focus on high‐risk profiling. Am J Respir Crit Care Med. 2016;193(5):542‐551. [DOI] [PubMed] [Google Scholar]
- 33. Tang X, Qu G, Wang L, Wu W, Sun Y. Low‐dose CT screening can reduce cancer mortality: a meta‐analysis. Rev Assoc Med Bras (1992). 2019;65(12):1508‐1514. [DOI] [PubMed] [Google Scholar]
- 34. Li CY, Sung FC. A review of the healthy worker effect in occupational epidemiology. Occup Med (Lond). 1999;49(4):225‐229. [DOI] [PubMed] [Google Scholar]
- 35. Stein CR, Wallenstein S, Shapiro M, et al. Mortality among World Trade Center rescue and recovery workers, 2002‐2011. Am J Ind Med. 2016;59(2):87‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wagner NL, Berger J, Flesch‐Janys D, et al. Mortality and life expectancy of professional fire fighters in Hamburg, Germany: a cohort study 1950‐2000. Environ Health. 5, 2006:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chow WH, Finkle WD, McLaughlin JK, Frankl H, Ziel HK, Fraumeni JF, Jr. The relation of gastroesophageal reflux disease and its treatment to adenocarcinomas of the esophagus and gastric cardia. J Am Med Assoc. 1995;274(6):474‐477. [PubMed] [Google Scholar]
- 38. Lagergren J, Bergstrom R, Lindgren A, Nyren O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med. 1999;340(11):825‐831. [DOI] [PubMed] [Google Scholar]
- 39. Duffy SW, Nagtegaal ID, Wallis M, et al. Correcting for lead time and length bias in estimating the effect of screen detection on cancer survival. Am J Epidemiol. 2008;168(1):98‐104. [DOI] [PubMed] [Google Scholar]
- 40. Mahnken JD, Chan W, Freeman DH, Jr. , Freeman JL. Reducing the effects of lead‐time bias, length bias and over‐detection in evaluating screening mammography: a censored bivariate data approach. Stat Methods Med Res. 2008;17(6):643‐663. [DOI] [PubMed] [Google Scholar]
- 41. Morrison AS. The effects of early treatment, lead time and length bias on the mortality experienced by cases detected by screening. Int J Epidemiol. 1982;11(3):261‐267. [DOI] [PubMed] [Google Scholar]
- 42. Centers for Disease Control and Prevention . World Trade Center Health Program Member's Handbook—Nationwide Provider Network. 2019; https://www.cdc.gov/wtc/clinics.html. Accessed April 1, 2021.
- 43. Welch AE, Caramanica K, Debchoudhury I, et al. A qualitative examination of health and health care utilization after the September 11th terror attacks among World Trade Center Health Registry enrollees. BMC Public Health. 2012;12:721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Welch AE, Debchoudhury I, Jordan HT, Petrsoric LJ, Farfel MR, Cone JE. Translating research into action: an evaluation of the World Trade Center Health Registry's Treatment Referral Program. Disaster Health. 2014;2(2):97‐105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Maura F, Diamond B, Maclachlan KH, et al. Initial whole‐genome sequencing of plasma cell neoplasms in first responders and recovery workers exposed to the World Trade Center Attack of September 11, 2001. Clin Cancer Res. 2021;27(7):2111‐2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Axelson O, Steenland K. Indirect methods of assessing the effects of tobacco use in occupational studies. Am J Ind Med. 1988;13(1):105‐118. [DOI] [PubMed] [Google Scholar]
- 47. Warren GW, Kasza KA, Reid ME, Cummings KM, Marshall JR. Smoking at diagnosis and survival in cancer patients. Int J Cancer. 2013;132(2):401‐410. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Supporting information.
Supporting information.
Data Availability Statement
Data that support the findings of the study may be obtained from the corresponding author (Paolo Boffetta) upon reasonable request after approval by the Steering Committee for “Incidence, Latency, and Survival of Cancer Following World Trade Center Exposure” (NIOSH Cooperative Agreement U01 OH011932) in accordance with the study's official Data Sharing Plan.


