Abstract
The mammalian middle ear comprises a chain of ossicles, the malleus, incus and stapes that act as an impedance matching device during the transmission of sound from the tympanic membrane to the inner ear. These ossicles are derived from cranial neural crest cells that undergo endochondral ossification and subsequently differentiate into their final functional forms. Defects that occur during middle ear development can result in conductive hearing loss. In this review, we summarize studies describing the crucial roles played by signaling molecules such as Sonic Hedgehog, Bone Morphogenetic Proteins, Fibroblast Growth Factors, Notch ligands and chemokines during the differentiation of neural crest into the middle ear ossicles. In addition to these cell-extrinsic signals, we also discuss studies on the function of transcription factor genes such as Foxi3, Tbx1, Bapx1, Pou3f4, and Gsc in regulating the development and morphology of the middle ear ossicles.
Keywords: Middle ear, Ossicle, Malleus, incus, stapes, columella, neural crest cells, growth factors, transcription factors
Introduction
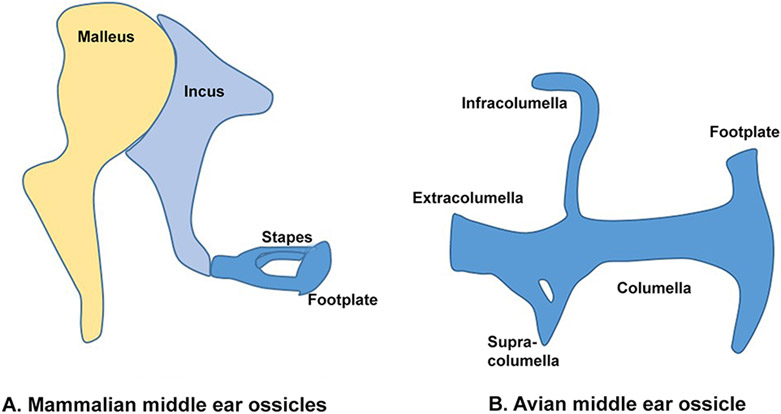
The mammalian hearing apparatus consists of the outer, middle and inner ears, with all three parts being essential for hearing. Sound waves captured by the external ear (also known as the auricle or pinna) cause vibration of the tympanic membrane, which converts sound waves into mechanical vibrations that are conveyed to the chain of middle ear ossicles. These vibrations are applied to the inner ear through the oval window and travel along the cochlear duct, causing frequency-dependent vibrations of the basilar membrane of the cochlea at different positions along its length. These vibrations cause displacement of actin-rich hair bundles on the apical surface of cochlear hair cells, and the subsequent change in membrane potential of hair cells leads to synaptic vesicle release and transmission of nerve impulses along the auditory nerve to the brainstem 1. The mammalian middle ear consists of a chain of three ossicles: the malleus, incus and stapes (Figure1A). One end of the malleus is connected to the tympanic membrane and the other end to the body of incus. The neck of the stapes is connected to the long process of the incus and the stapedial footplate is connected to the oval window of the inner ear 2.
Figure 1: Comparison of the mammalian middle ear ossicles and avian columella.
(A) The mammalian middle ear ossicles consists of the malleus, incus and stapes. The stapes is connected to the oval window of the inner ear through the stapedial footplate. (B) The avian columella is homologous to the mammalian stapes. It consists of the extracolumella, infracolumella, supracolumella, columella and footplate.
Developmental defects in the middle ear ossicles can lead to conductive hearing loss. Some congenital diseases of the middle ear include absence or malformation of any of the middle ear ossicles, stapedial fixation at one or multiple fixed points, and fusion of malleus-incus elements 3-5. Some hereditary syndromes exhibit middle ear malformations, including Branchio-oto-renal (BOR) syndrome, CHARGE syndrome, and DiGeorge syndrome 5-7. Abnormal bone deposition or remodeling around the stapes (otosclerosis) is another cause of conductive hearing loss 8. In children, conductive hearing impairment can also be caused by otitis media with effusion 9,10. Although the genetic contribution to susceptibility to otitis media has been apparent for many years 11, it is only in the last 15 years that human and mouse studies have begun to identify genes associated with this disease 12-16. Pathogenic variants in these genes can lead to altered innate immune responses in the middle ear, altered mucus production and abnormalities in the development of the middle ear cavity and Eustachian tube.
The three middle ear ossicles are largely derived from migrating cranial neural crest cells of the first and second branchial arches. An array of signaling molecules orchestrates the migration and condensation of neural crest into the three ossicle primordia. The production of these developmental signals, and the response of neural crest cells evoked by these signals are regulated by a large number of transcription factors. In this review, we summarize studies on the roles of these secreted signals and transcriptional regulators in regulating the development of the middle ear ossicles. We review Sonic Hedgehog (SHH), Bone Morphogenetic Protein 4 (BMP4), Fibroblast Growth Factors (FGFs), NOTCH and chemokine (CXCL12) signaling molecules, all of which play crucial roles during the early phases of middle ear development. Additionally, we summarize the roles of a number of transcription factors including Foxi3, Tbx1, Bapx1, Pou3f4 and Hoxa2 on middle ear ossicle development.
Evolution of the mammalian middle ear
As ancestral vertebrates began to exploit ecological niches on land, the differing densities and acoustic impedances of air and water presented physical constraints on the efficient transfer of sound wave energy from atmospheric air to the fluid of the inner ear labyrinth. The modern mammalian middle ear functions as an impedance matching device by reducing the loss of energy that occurs at the interface of air and liquid 17,18. This impedance matching is dependent on the geometry and mechanics of the tympanic membrane and the middle ear ossicles. The relative surface areas of the tympanic membrane (large) and the stapedial footplate (small) also act to transfer acoustic energy so as to increase the force per unit area applied to the inner ear 19.
Mammals have a three-ossicle transmission apparatus, whereas most other land vertebrates have a single ossicle. In spite of this simple division, terrestrial middle ears are believed to have evolved independently many times 20,21. Indeed, even the three ossicles of monotreme and therian mammals may have an independent origin 22. It is believed that the hyomandibula of basal vertebrates that allowed the connection of the jaw to the brain case transformed into a sound-conducting bone, the columella, in amniotes (Figure1B) 20,23 and later, the stapes. The synapsid lineage incorporated the columella into the middle ear, but also developed the malleus and incus from the quadrate and articular bones 24, both of which are derived from condensations in the proximal region of Meckel’s cartilage 25. The loss of the original primary jaw articulation allowed the transformation of the proximal region of Meckel’s cartilage into the middle ear ossicles. The exploitation of three fully ossified ear bones that emerged as a consequence of changes to the jaw to allow high frequency hearing has been discussed by Manley (2010). Interestingly, a recent study has shown that marsupials that begin feeding at a very early age still use their middle ear bones to articulate the jaw before the mature jaw articulation forms 26. Although the precise sequence of events leading to the emergence of these two ossicles is still unclear, it is now believed that the miniaturization of the jaw that began in the Triassic-Jurassic period allowed the development of bones small and delicate enough to efficiently conduct high frequency sounds 27.
Development of middle ear and its associated structures
The middle ear ossicles derive largely from neural crest cells. However, a mouse lineage tracing study has shown the stapedial footplate to be derived from mesodermal cells, suggesting that the stapes has a dual origin 28. Dye- and graft-based fate-mapping studies in chicken and mouse embryos show that neural crest migrates from rhombomeres 1-3 into the first pharyngeal arch (PA1) and from rhombomeres 3-5 into the second pharyngeal arch (PA2) 29-33. In mice, genetic fate mapping confirms that Hoxb1- and Hoxa2-expressing crest from rhombomere 4 contribute to PA2 34,35. Some of the neural crest cells migrating into PA1 form the malleus-incus condensation 36, and cells migrating into PA2 form the stapedial condensation 23,37 and the external ear 35. These neural crest cell condensations undergo endochondral ossification to form the middle ear ossicles 38. In the mouse PA1, the malleus-incus forms as a single condensation attached to Meckel’s cartilage at embryonic day (E) 10.5. Later, the malleus and incus separate from each other at E13.5, and the separation of Meckel’s cartilage and malleus occurs postnatally in mice 3,25,37,39, requiring the action of chondroclasts 40. The stapedial condensation forms separately in the PA2 mesenchyme at E10.5 and acquires its characteristic stirrup-like structure at E11.5, due to the stapedial artery passing through it 36. By E15.5, the stapes is connected to the inner ear by the formation of annular ligaments on either side of the stapedial footplate 28,41. The development of these middle ear ossicles is associated with the signals from the endoderm such as Shh and BMP4 36,42, which we describe in more detail later in the review.
In addition to the middle ear ossicles, other elements associated with the definitive mammalian middle ear include the tympanic ring, gonial bone, styloid process, and middle ear cavity. The tympanic ring and gonial bone are neural crest-derived bony structures that anchor the middle ear ossicles to the skull and are generated by intramembranous ossification. They are homologous to the angular and prearticular structures seen in non-mammalian amniote groups 43. The styloid process is another mammalian-specific skeletal element derived from neural crest that forms by endochondral ossification in the PA2 region, and can act as an anchor for cranial muscles and ligaments 44-46.
The middle ear cavity also plays a very important role in sound conduction to the inner ear in addition to housing the middle ear ossicles. Whittmack proposed a developmental model of middle ear cavitation in which the first pharyngeal pouch endoderm that will ultimately form the auditory (Eustachian) tube extends into the middle ear, then expands and envelops the middle ear structures, resulting in endoderm lining the cavity caused by the evacuation of neural crest cells 47. However, fate mapping studies with neural crest- and endoderm-specific Cre-expressing mice revealed that the epithelial cells derived from endoderm surround the eardrum and Eustachian tube, whereas epithelial cells derived from neural crest line more dorsal regions of the middle ear cavity, suggesting a dual origin for the epithelium surrounding the middle ear cavity 21,48.
Direct and indirect roles of secreted signaling molecules on middle ear development
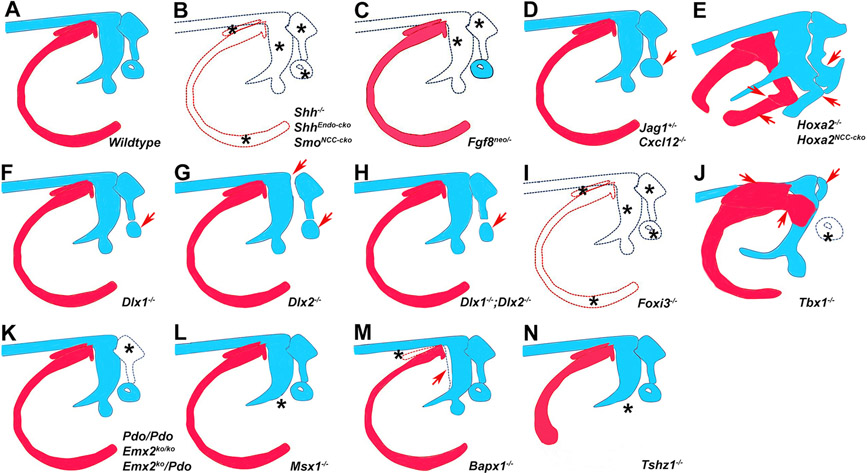
In this section, we review studies on a number of signaling molecules (SHH, BMPs, FGFs, Notch and CXCL12) that play crucial roles in middle ear development, either directly or indirectly. Loss- or gain-of-function of these signals result in middle ear defects including an absence or reduction of middle ear structures, or alterations in the morphology of middle ear structures 23. A summary of these phenotypes is shown in Figure 3 and Table 1.
Figure 3: Summary of middle ear defects observed in different lines of mutant mice.
The middle ear ossicle defects observed in various mouse mutants are summarized in comparison to the wild type ossicles (A). Deletion of genes result in the loss of all three middle ear ossicles including their associated parts such as the tympanic ring and gonial bone (B, I, M), or a subset of the ossicles such as loss of the malleus and incus (C), incus (K) or stapes (E, J). Deletion of some genes can result in malformation of middle ear ossicles, such as a malformed malleus (J, L, M, N), malformed incus (G), malformed stapes (D, F, G, H) or malformed tympanic ring and gonial bone (J, N). Inactivation of Hoxa2 resulted in a mirror image duplication of the malleus, incus, tympanic ring and gonial bone in place of the stapes and other second pharyngeal arch derivatives (E).
These phenotypes are summarized in Table 1. Solid red and blue colors indicate normal bone and cartilages, respectively. Dotted red and blue colors indicate the absence of bone and cartilages, respectively.
Table 1: Middle ear defects observed in various mutant mouse embryos.
A summary of the middle ear defects observed in various mouse embryos shown in Figure 3
| Panel in Fig.3 |
Mouse Mutant | Malleus | Incus | Stapes | Tympanic Ring |
Gonial Bone |
Stapedial Artery |
Stapedial Foramen |
References |
|---|---|---|---|---|---|---|---|---|---|
| A | Wildtype | Normal | Normal | Normal | Normal | Normal | Normal | Normal | |
| B | Shh −/− | Absent | Absent | Absent | Absent | Absent | Absent | Absent | Chiang et al., 1996 |
| Nkx2.5Cre;Shh lox/− | Absent | Absent | Absent | Absent | Absent | Absent | Absent | Billmyre and Klingensmith, 2015 | |
| Wnt1-Cre;Smo lox/lox | Absent | Absent | Absent | Absent | Absent | Absent | Absent | Jeong et al., 2004; Ankamreddy et al., 2019 | |
| C | Fgf8 neo/− | Absent | Absent | Normal | Normal | Normal | Normal | Normal | Abu-Issa et al., 2002 |
| D | Jag1 +/− | Normal | Normal | Malformed | Normal | Normal | Present | Absent | Teng et al., 2017 |
| Cxcl12 −/− | Normal | Normal | Malformed | N/A | N/A | Absent | Absent | Ankamreddy et al., 2020 | |
| E | Hoxa2 −/− | Normal (duplicated) | Normal (duplicated) | Absent | Normal (duplicated) | Normal (duplicated) | Absent | Absent | Kanzler et al., 1998 |
| Wnt1-Cre;Hoxa2 lox/lox | Normal (duplicated) | Normal (duplicated) | Absent | Normal (duplicated) | Normal (duplicated) | Absent | Absent | Santagati et al., 2005 | |
| F | Dlx1 −/− | Normal | Normal | Malformed | Normal | Normal | N/A | N/A | Qiu et al., 1997 |
| G | Dlx2 −/− | Normal | Malformed (Not connected with | Malformed | Normal | Normal | N/A | N/A | Qiu et al., 1995 |
| H | Dlx1−/−; Dlx2−/− | Normal | Normal | Malformed | Normal | Normal | N/A | N/A | Qiu et al., 1997 |
| I | Foxi3 −/− | Absent | Absent | Absent | Absent | Absent | Absent | Absent | Edlund et al., 2014 |
| J | Tbx1 −/− | Normal | Malformed | Absent | Malformed | Malformed | N/A | N/A | Moraes et al., 2005 |
| K | Pdo/Pdo | Normal | Absent | Normal | Normal | Normal | Normal | Normal | Rhodes et al., 2003 |
| Emx2 KO/KO | Normal | Absent | Normal | Normal | Normal | Normal | Normal | Rhodes et al., 2003 | |
| Emx2 KO/Pdo | Normal | Absent | Normal | Normal | Normal | Normal | Normal | Rhodes et al., 2003 | |
| L | Msx1 −/− | Malformed process bravis | Normal | Normal | Normal | Normal | Normal | Normal | Satokata and Maas, 1994 |
SHH:
Hedgehog (HH) signaling plays very important roles in many tissues during embryogenesis and in the adult 49. In brief, HH ligands bind to Patched1 (Ptch1), a transmembrane protein, which inhibits the binding of Ptch1 to Smoothened (Smo), another transmembrane protein. The resulting intracellular signaling cascade downstream from Smo allows proteolytic processing of Gli family transcription factors which translocate into the nucleus and regulate HH target genes 50. There are three HH homologs in amniotes, SHH, Indian HH (IHH), and Desert HH (DHH). Loss of Shh in mice (Shh−/−) causes cyclopia and defects in the outer, middle and inner ears 51. At E10.5, Shh is expressed in the endoderm of the PA1, and its receptor Ptch1 is expressed in the mesenchyme adjacent to the endoderm that will form the malleus-incus anlagen; however, Shh is not expressed in the endoderm beneath the stapes region in PA2, and Ptch1 is also expressed at much lower levels in this region (Figure2B) 36. Moreover, HH co-receptors such as Cdon (Cell-adhesion molecules-related/down-regulated by oncogenes) and Gas1 (Growth arrest-specific 1) are expressed between the middle ear joints and the annular ligaments in a similar pattern to Gdf5, a marker of joints. Gas1 mouse mutants have a range of middle ear defects, including variable dysmorphology of the malleus and incus (with the stapes being less affected), as well as variable defects in the styloid process and tympanic ring 52. Biregional Cdon binding (Boc) is another HH pathway protein expressed surrounding the joints and annular ligaments of the ossicles, similar to Ptch1 expression 41.
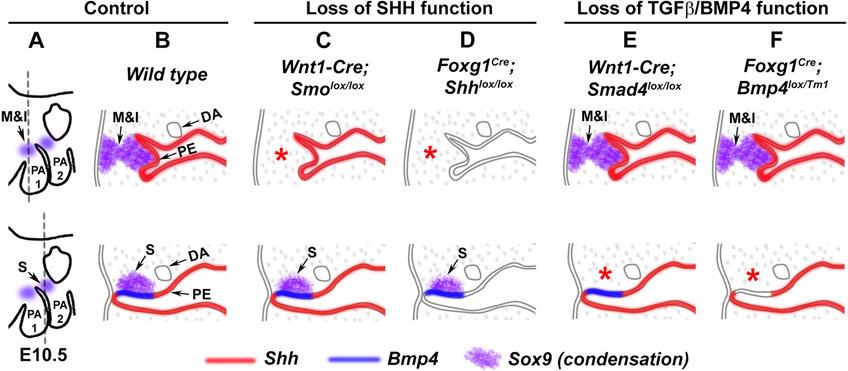
Figure 2: Endoderm acts as a signaling center for developing neural crest derived middle ear ossicles.
Loss of endodermal Shh (Foxg1Cre;Shhlox/lox) function, or inactivation of SHH signaling by deleting Smo in neural crest cells (Wnt1-Cre;Smolox/lox) lead to loss of the malleus and incus condensation, but not the stapes (C, D). Similarly, loss of endodermal Bmp4 (Foxg1Cre;Bmp4lox/Tm1) function, or inactivation of TGF-β/BMP signaling in neural crest cells by deleting Smad4 in neural crest cells resulted in the loss of the stapes condensation, but not the malleus or incus condensations (E,F). M, malleus; I, incus; S, stapes; DA, Dorsal aorta; PE, pharyngeal endoderm. This figure is adopted from Ankamreddy et al., Development (2019) with permission.
Endodermal SHH signaling is essential for neural crest cells to initiate the malleus-incus condensation and is essential for neural crest survival in both the malleus-incus and stapes condensation regions 36. Subsequently, endodermal deletion of Shh or neural crest-specific deletion of Smo results in the loss of middle ear ossicles (Figure 2B-D, 3B) 36,42,53. Conversely, constitutive activation of SHH signaling in neural crest cells resulted in an enlargement of the middle ear condensations and altered the morphology and location of the middle ear ossicles at later stages (E15.5) 36. These studies demonstrate that SHH signaling from pharyngeal endoderm directly regulates early stages of middle ear development.
BMPs:
BMP family members play a number of critical roles in craniofacial development, including neural crest induction and migration, and formation of the facial primordia, teeth, tongue and lips 54. The binding of BMP ligands to BMP-type II receptors (BMPRII, ActIIR or ActRIIB), phosphorylates BMP-type I receptors (ALK1, ALK2, ALK3 and ALK6). These activated BMP-type I receptors phosphorylate receptor Smads (R-Smads), which in the case of Bmp signaling are Smad1, 5 and 8. The activated R-Smads form a complex together with the co-Smad Smad4, translocate into the nucleus, and activate Bmp target genes 55. Noggin is a well-known secreted antagonist of BMP proteins. When BMP signaling was downregulated in cranial neural crest by ectopic expression of Noggin, embryos exhibited a loss of PA2 elements (including the stapes and styloid process) and more caudal arch elements 56. Conversely, Noggin heterozygous (Nog+/−) mutants exhibited ectopic bone formation in the ossicular chain due to incomplete separation of the stapes and styloid process. This ectopic bone led to a mild conductive hearing loss. The joints present between the three middle ear ossicles were also absent due to the fusion of all three middle ear ossicles 44. Inactivation of BMP signaling in neural crest cells by deleting Smad4 with Wnt1-Cre mice, or by deleting Bmp4 in the endoderm using Foxg1-Cre mice, the stapedial condensation failed to form in the PA2 as a result of the absence of neural crest cells in the prospective stapes region (Figure 2E-F) 36. No evidence could be found of stapes formation even at later stages, confirming these defects were not simply due to a developmental delay. These observations suggest that endodermal BMP4 signaling plays a crucial role in early neural crest migration into the prospective stapes region, and normally acts to initiate stapes condensation.
FGF:
FGFs play very important roles during craniofacial development through the regulation of cell proliferation, cell survival and migration 57,58. FGF signaling is mediated by receptor tyrosine kinases (Fgf receptors, Fgfrs; Fgfr1-4) 59. A number of human craniosynostosis syndromes (such as Apert, Pfeiffer, Crouzon, Beare-Stevenson, and Jackson-Weiss syndromes) that are caused by pathogenic variants in FGF receptors can also display inner and middle ear defects 60,61. Among the FGF ligands, Fgf8 is strongly expressed in arch surface ectoderm and the pharyngeal endoderm 62,63. Ectodermal-specific deletion of Fgf8 or hypomorphic Fgf8 mutants exhibit similar phenotypes, including a severely reduced or absent malleus, incus and tympanic ring which are due to the failure in neural crest cell survival. However, the stapes remains unaffected in both of these Fgf8 mutant strains (Figure 3C) 64,65. Among the four FGF receptors, mice carrying a Neo cassette insertion in intron 7 (Fgfr1n7/n7) exhibited a loss of middle ear ossicles 66. In Hush puppy mutant mice, a W691R variant in the Fgfr1 gene resulted in a severe loss of FGFR1 activity. Homozygotes for this variant die at E8.5, however, heterozygotes exhibited hearing loss due to the defects in outer, middle and inner ears. The middle ear phenotypes include an abnormal incus and a wide range of malformations in the stapes 67 including a thinning or absence of the posterior crus in the stapes, or anterior crus malformations 68.
Neural crest-specific deletion of Fgfr2 causes conductive hearing loss due to structural defects in the middle ear ossicles. In these mutants, the measurements of the auditory bulla showed a significant reduction in the overall volume when compared to control littermates. The retrotympanic process and tympanic ring were dysmorphic and hypoplastic. Ectopic bone formations were observed on the manubrium of the malleus, the incudomalleal joint, the ligament insertion site on the anterior process, and the insertion site of the stapedial muscle on the stapes 69.
NOTCH Signaling:
Another important pathway involved in various processes of development is Notch signaling. Unlike secreted growth factors, Notch signaling occurs between two adjacent cells when they contact with each other. The transmembrane ligand present on the signal-sending cell activates transmembrane Notch receptors present on the adjacent signal-receiving cells 70. Human studies showed that mutations in JAGGED1 (JAG1, loss-of-function) were found in 94% of Alagille Syndrome patients. Some of these patients were diagnosed with sensorineural, conductive or mixed types of hearing loss 71. In zebrafish, a jag1b allele isolated in a mutagenesis screen, jag1bb1105 displayed a variety of defects in the hyomandibula, a result that was also seen in morpholino-mediated knockdown of the Notch2 receptor(Zuniga et al., 2010). jag1b is expressed in the most dorsal neural crest contributing to the hyoid and mandibular arches, a region analogous to the region that generates the stapes in mammals. The evolutionary relationship between the fish hyomandibula and the middle ear ossicles raised the possibility that Jag1-Notch2 signaling might be necessary for ossicle formation. Deleting either Jagged1 or Notch2 in neural crest caused significant defects in the stapes, which appeared as a monopodal structure lacking crural regions. The stapedial artery was found passing adjacent to this monopodal structure rather than passing through the stapes 72. As expected, these mice exhibit significant conductive hearing loss. A more detailed examination of Jag1 heterozygous mouse mutants revealed them to have a smaller stapes that lacked a foramen (Figure 3D) 72, suggesting that the primary defect in the stapes is due to its malformation, rather than the lack of the stapedial artery. These results suggest a surprising degree of conservation in pharyngeal arch patterning between mammals and fish.
Chemokine signaling:
The C-X-C motif chemokine ligand 12 (CXCL12), also known as stromal cell-derived factor 1 (SDF1), is a chemokine that mainly signals through two G-protein coupled chemokine receptors, CXCR4 and CXCR7. It plays critical roles in migration of primordial germ cells, gonadotropin-releasing hormone (GnRH) interneurons, and endothelial precursor cells 73. CXCL12 can act also act as a chemoattractant for neural crest cells, as well as regulating the patterning and morphogenesis of neural crest-derived tissues 74-77. In our recent work, we observed that Cxcl12 was expressed in a pattern partially overlapping with the malleus-incus condensation lateral to the pharyngeal endoderm in pharyngeal arch 1, and its expression overlapped with Sox9 and Tfap2a in the stapes region of pharyngeal arch 2. Upon deletion of Cxcl12, the neural crest-derived stapedial condensation initially formed. The primitive stapedial foramen and stapedial artery initially formed at E11.5, but have degenerated by E12.5 due to the loss of CXCL12 signaling, resulting in the loss of the stirrup-shaped stapes (Figure 3D) 78. However, the malleus and incus condensations remained morphologically unaffected in these mutant mice.
Functional roles of transcription factors implicated in middle ear development
In this section, we discuss the roles played by several transcription factors in middle ear ossicle development. Transcription factors including Hoxa2, Dlx genes, Tbx1, Pou3f3, and Foxi3, are known to have direct or indirect functional roles on ossicle development 79-85. Some of these transcription factors also regulate the patterning of neural crest cells in the pharyngeal arch region along either the posterior-anterior or dorsal- ventral axes 81,84,86,87. The middle ear defects observed in the absence of some of the transcription factors were summarized in Figure 3 and Table 1.
Hox genes:
Hox genes play crucial roles in neural and craniofacial development, and also regulate the formation of some neural crest derived-pharyngeal arch elements. Neural crest cells contributing to pharyngeal arch 1 elements do not express Hox genes, but neural crest cells contributing to pharyngeal arch 2-6 elements, including the hyoid and other cartilages forming the neck region, all express Hox genes 88,89. Lack of Hoxa2 results in the homeotic transformation of pharyngeal arch 2 elements into pharyngeal arch 1 elements. As a result, Hoxa2−/− mutant embryos have no stapes, styloid process or hyoid cartilages; instead the malleus, incus, Meckel’s cartilage, tympanic ring and gonial bone elements are duplicated (Figure 3E) 84,87,89,90. Although they do not possess middle ear structures, the arch 1 duplication phenotype is conserved in amphibian and zebrafish models in which Hoxa2 was downregulated, resulting in the homeotic transformation of pharyngeal arch 2 elements into pharyngeal arch 1 91,92. Temporal inactivation of Hoxa2 at pre- or post-neural crest migration results in the duplication of some pharyngeal arch 1 elements in pharyngeal arch 2, suggesting that Hoxa2 plays its role even after the completion of neural crest migration, and also exerts its temporal functions on specific individual neural crest derived elements 89. Additionally, inactivation of Hoxa2 resulted in external ear defects such as microtia 35,89, and pathogenic variants of Hoxa2 can also cause dominant microtia in humans 93.
When Hoxa2 was ectopically expressed in neural crest cells (NCC-Hoxa2) 45, most of the Hoxa2 over-expressing structures were affected. In these NCC-Hoxa2 embryos, the distal parts of the dentary bone and Meckel’s cartilage were normal or less affected, but the proximal parts of the dentary bone were hypoplastic and the proximal part of Meckel’s cartilage was absent. Most of the pharyngeal arch 1 elements such as the malleus, incus, tympanic ring, gonial bone, pterygoid and squamous bones were absent and instead were replaced with a duplicated stapes, styloid process, and the lesser horn of the hyoid cartilage. In neural crest cell-specific ectopic activation of Hoxa2, pharyngeal arch 1 elements underwent homeotic transformation to pharyngeal arch 2 elements 45. Gain-of-function studies of Hoxa2 in the first pharyngeal arch (Hox negative region) in chicken embryos, zebrafish or Xenopus also showed a conserved homeotic transformation of first pharyngeal elements into second pharyngeal elements 92,94,95. In NCC-Hoxa2, the external ear auricle was duplicated, whereas the ear canal and tympanic membrane were absent 45..
Dlx family:
Like Hoxa2, the six amniote Dlx family genes regulate pharyngeal patterning along the dorsal to ventral axis of each arch. Dlx genes arose from an ancestral duplication, and in modern amniotes, the Dlx genes occur in pairs (Dlx1/2, Dlx3/4 and Dlx5/6), transcribed in opposite directions 96. Expression studies of this family showed that Dlx1/2 are expressed throughout the proximo-distal pharyngeal arch mesenchyme, with Dlx5/6 and Dlx3/4 expression being progressively restricted to more distal regions of arch mesenchyme 86. More than 50% of Dlx1−/− mutant mice exhibited an abnormal stapes and styloid process at postnatal day zero (P0) (Figure 3F) 97. P0 Dlx2−/− homozygous mutant pups exhibited abnormal morphogenesis of the proximal parts of the first and second pharyngeal arches, which include an abnormal incus with ectopic palatoquadrate-like cartilage structures, a stapes lacking the central stapedial foramen, and a styloid process not connected to the inner ear (Figure 3G) 98. The malleus in these mutant mice remains unaffected. In most Dlx5−/− homozygous mutants, all three middle ear cartilages were of normal shape, size and position. However, some of these mutants exhibiting exencephaly phenotypes were found to have their stapes missing 79,82. Loss of Dlx6 (Dlx6−/−) gene alone did not affect any of the middle ear ossicles. However, together with the loss of other Dlx genes such as Dlx1, Dlx2 or Dlx5, lack of Dlx6 had abnormal middle ear defects 81,99. Double mutant Dlx5−/−;Dlx6−/− embryos were severely affected and exhibited an exencephalic phenotype. These embryos lack external ear cartilages and their inner ear and middle ear cartilages were fused and severely dysmorphic. The incus was duplicated in these double knockout embryos. Also, the middle ear associated cartilages (Meckel’s cartilage) and bones (tympanic ring and gonial bone) were absent in these double mutant embryos 86,100.
Tbx1:
Tbx1 is a member of the T-box transcription factor family, and is expressed in the ectoderm and endoderm of pharyngeal arches 1-3 at the time of neural crest migration (E9.5). Its expression shifts to the pharyngeal mesoderm (mesodermal core) at E10.5 74,101. Haploinsufficiency of TBX1 in humans can result in DiGeorge Syndrome (also known as 22q11 deletion syndrome 101,102. Some patients with DiGeorge syndrome are reported to present with congenital hearing loss, either conductive (majority of cases), or sensorineural (10%) 80,103. Tbx1−/− neonatal mice exhibit a small and recognizable malleus and tympanic ring, a small cartilaginous nodule-shaped incus and no discernible stapes (Figure 3J). The gonial bone was normal in these mutant pups 103. Although, Tbx1 is not expressed in migrating neural crest, and the failure in the development of the neural crest-derived middle ear ossicles in embryos lacking endodermal Tbx1 suggests that endodermal Tbx1 acts non-autonomously on neural crest cells during the development of the middle ear ossicles 80. Consistent with this result, neural crest cell migration was not affected in Tbx1 mutants and neural crest-specific deletion of Tbx1 does not cause any craniofacial abnormalities 104.
Tbx1 acts indirectly to influence middle ear development through the regulation of a number of the signaling pathways discussed above. In the developing mandibular arch of Tbx1−/− embryos, the downstream targets of FGF8 signaling including Lhx6, Lhx8, Pitx1, Spry1, Spry2 and Erm2 were spatially shifted towards proximal and dorsal regions 104. Bmp4 and its downstream target gene, Alx4 were also shifted towards the proximal side in the developing mandibular arch of Tbx1−/− embryos, and Bmp4 expression was downregulated in the distal oral ectoderm 104. Although Shh expression was not changed, Ptch1 expression was expressed only in the proximal region of the mandibular arch, and diminished in the distal oral ectoderm 104.
Pou3f4:
One of the most frequent causes of X-linked hearing impairments is X-linked mixed hearing loss, characterized by both conductive and progressive sensorineural hearing loss. This non-syndromic hearing loss is due to variants in the coding or regulatory regions of POU3F4, located on chromosome Xq13-q22 105. Pou3f4 mRNA is expressed in the mesenchyme apposed to the stapes footplate, dorsal to the stapes within the developing S-V joint, but is not expressed in the developing malleus and incus. The occurrence of conductive hearing loss in patients with X-linked mixed hearing loss often results from stapedial fixation 106. Replacement of the coding region of mouse Pou3f4 with a Cre recombinase gene results in a functional null allele of Pou3f4 (Pou3f4Y/Cre). These mice exhibit a hypoplastic stapes footplate and oval window dysplasia, contributing to disarticulation of the footplate from the oval window 85. Ephrin-B2 (Efnb2), a type I transmembrane protein, acts as a cellcell interacting ligand for various Ephrin (Eph) receptor tyrosine kinases. Efnb2 plays crucial roles during various developmental processes including epithelial, nervous and brain development. During embryogenesis, Efnb2 regulates neural crest cell migration by acting as a repulsive guidance molecule 107-109. In the inner ear spiral ganglion, Pou3f4 expression in otic mesenchymal cells is essential to activate Epha4, a canonical receptor for Efnb2, and disruption of any of these genes can lead to fasciculation and guidance defects of the spiral ganglion neurons innervating the cochlea 110. In middle ear development, both Efnb2 and Pou3f4 overlap in the stapes footplate, stapedio-vestibular joint, the region dorsal to stapes, and the pharyngeal arch mesenchyme dorsal to the stapedial artery at E12.5-E13.5. However, overlapping expression of Efnb2 and Pou3f4 was not observed in the malleus and incus regions 85. Inactivation of Efnb2 in the mesenchymal and epithelial ear components using Sox9-IRES-Cre driver (Efnb-cko) resulted in the joining of the stapes and styloid process and/or a reduced distance between the stapes and styloid process. This phenotype is similar to the Pou3f4Y/Cre mutant phenotype. The genetic interaction between Pou4f3 and Efnb2 is confirmed by the observation that Pou3f4Y/Cre;Efnb2flox/null compound mutant mice exhibit more severe middle ear defects than Pou3f4Y/Cre mutant mice 85.
Emx2:
Emx2 is a homeobox transcription factor that is necessary for correct middle and inner ear development 111. Emx2 begins to be expressed in the incus primordium from E13.5, distinguishing it from the adjacent malleus condensation 25. Homozygous Emx2 mutants lack both the incus and the malleal structure that articulates with the incus (Figure 3K) 111. This suggests that the malleal articulation requires the incus to form correctly, but is not itself dependent on Emx2 function 111. Although all the middle ear ossicles form in Emx2 heterozygous mutants (Emx2+/−), they also show articulation defects between the malleus and incus. In the same study, a missense mutation in Emx2 identified in the ENU-induced mutant Pardon was shown to have defects in all three ossicles (Figure 3K) 111.
Msx1:
Msx1 is a homeobox-containing transcription factor, which plays a major role in the development of many craniofacial elements, including the palate, mandible and the middle ear ossicles 112. Loss of Msx1 results in deformation of the malleus; the malleus is slightly shorter and lacks a small prominence (Figure 3L) called the process brevis in humans and the orbicular hypophysis in mice 113. However, no other middle ear ossicles were affected in these mice 112,114. X-gal staining of Dlx5lacZ shows expression in the malleus and incus; and similar analysis of Msx1nlacZ mice shows expression in the malleus and cochlea. In Dlx5−/−;Msx1−/− compound mutants, mice show deformation of the malleus, with a lack of the processus brevis (a Msx mutant phenotype), and ectopic gonial bone formation (a Dlx5−/− mutant phenotype). However, no additional defects were observed in these double knockout mouse embryos compared to those observed in either mutant line. This suggests that Msx1 and Dlx5 have independent and non-overlapping functions in ossicular development 112,114.
Foxi3:
Foxi3 is a forkhead transcription factor that plays very important roles in pharyngeal arch development. Foxi3 is expressed in the surface ectoderm and pharyngeal endoderm, but not in migrating neural crest cells that populate the arch 115. It becomes progressively restricted to the cleft/pouch region of each arch, and disappears by E11 115. Mouse and chick Foxi3 transcription factors are homologous to the zebrafish foxi1 transcription factor 116. The unique phenotype observed with the loss of Foxi3 is that it lacks all three components of the ear (outer, middle and inner ear) (Figure 3I). Middle ear associated elements such as tympanic ring and gonial bone elements are absent in Foxi3−/− embryos 83. In these phenotypes, neural crest migration is not affected. However, the pharyngeal arches are greatly reduced and dysmorphic. The cells present within these hypoplastic pharyngeal arches fail to survive, which result in the absence of most of the pharyngeal arch derivatives 83. This phenotype is very similar to a previously characterized deletion of Fgf8 in branchial arch 1 ectoderm 65. Accordingly, Foxi3−/− embryos lack Fgf8 in arch ectoderm, and FGF-responsive genes such as Erm/Etv4 fail to be expressed in neural crest cells invading the first and second arches of Foxi3 mutants, suggesting that the cell death observed in Foxi3 mutants may be due in part to a lack of FGF signaling from the ectoderm to neural crest 83. Cell death of first and second arch neural crest cells and jaw defects is also observed in zebrafish foxi loss of function embryos 117,118. Heat shock activation of either fgf3 or fgf8 is able to rescue the cranial cell death phenotype in foxi1 morphant embryos 83, although these animals were only analyzed at relatively short time points after rescue. It remains to be determined whether Foxi3 regulates other secreted signals in arch ectoderm or endoderm that regulate neural crest cell differentiation and survival in the branchial arches.
c-Fos:
c-Fos is a proto-oncogene which is a component of the AP-1 transcription factor complex. c-Fos acts as a key regulator in osteogenic-macrophage lineage determination 119. During development, the malleus-incus initially form a single condensation together with Meckel’s cartilage. Typically the malleus separates from the proximal part of Meckel’s cartilage at postnatal day 3 in mice 40 and separates from the incus around E13.5 3,36. Chondroclast cells play an important role during the breakdown of the connection between the malleus and Meckel’s cartilage. In c-Fos mutant pups, the malleus fails to separate from the proximal part of Meckel’s cartilage due to a failure of chondroclast differentiation. Treatment of wild type P0 CD1 mouse pups with bisphosphate alderonate (a drug that inhibits clast cell activity) also shows a persistent connection between the malleus and Meckel’s cartilage, similar to the c-Fos phenotype at P3. This phenotype in mice appears to phenocopy the morphology of early Mesozoic mammals 40, suggesting that full separation of the malleus from Meckel’s cartilage was a later mammalian innovation.
Bapx1:
Bapx1 is a bagpipe-related homeobox gene belonging to the NK family of transcription factors. Initially, this family was identified in Drosophila and is considered one of the earliest marker of prechondrogenic cells in vertebrates. Among the three vertebrate Bapx1 genes, two are found in mammals: Nkx3.1 and Bapx1 (Nkx3.2) 120. In humans, the homologue for Bapx1 maps to chromosome 4p16.1, and this region is associated with many skeletal diseases 120. In mice, loss of Bapx1 results in a failure to form many skeletal elements due to early defects in cartilage development. Several genes associated with cartilage differentiation including Sox9, Col2a1 and Indian hedgehog were downregulated in sclerotomal precursors, resulting in cartilage development defects 121. Bapx1 is expressed in the first pharyngeal arch (mandibular region) at E10.5, and its expression is later observed in the region medial to malleus-incus condensations at E11.5, and in the region surrounding the malleus at E13.5. At E12.5, its expression domain is closely associated with Meckel’s cartilage, and in the condensing regions which become the tympanic ring and gonial bones 36,43. In zebrafish, loss of bapx1 resulted in the loss of the jaw joint, whereas its homologous structure in mice (the malleus-incus joint) remains unaffected in the absence of Bapx1 43,122. Middle ear defects were observed in Bapx1 mutant embryos: the width of the malleus, and middle ear-associated elements such as the gonial bone were absent and tympanic ring was hypoplastic (Figure 3M) 43. The gonial bone was also hypoplastic in Bapx1 heterozygous pups 43.
Goosecoid (Gsc):
Goosecoid is a homeobox-containing gene which is conserved among different vertebrates including zebrafish, Xenopus, chicken and mouse. Gsc is initially expressed in the mouse primitive streak during gastrulation at E6, and later during craniofacial development in the mandibular mesenchyme, Eustachian tube, external auditory meatus, and malleus between E10.5 to E14.5 123-125. In Gsc null mutant embryos, the tympanic ring and external auditory meatus are absent and the manubrium and processus brevis of the malleus are reduced compared to controls. The gonial bones in Gsc null mutant mice embryos are either hypoplastic or reduced to small vestiges 125-127. At E10.5, Gsc and Bapx1 expression domains overlap in a small region of first arch mandibular mesenchyme. At later stages (E15.5), Bapx1 was expressed within and surrounding the malleus region and coincides with Gsc expression in the anterior part of the tympanic ring. In the developing external acoustic membrane, Bapx1 and Gsc overlap with each other. The similar expression patterns of Bapx1 and Gsc, and the superficially similar mutant phenotypes suggests that the two transcription factors may be acting in a regulatory network. However, Gsc expression in Bapx1 mutant embryos and Bapx1 expression in Gsc mutant embryos are both unchanged, suggesting these genes act independently regulating tympanic ring development 43.
Tshz1:
TSHZ family members are zinc finger transcription factors which play crucial roles during embryogenesis. Of the three Tshz genes, only Tshz1 is expressed in neural crest derived mesenchymal cells in the first and second pharyngeal arches 128. The mesenchymal expression of Tshz1 in the pharyngeal arches is controlled by the opposing actions of two epithelial-derived signaling molecules FGF8 (which induces Tshz1 expression) and BMP4 (which blocks Tshz1 expression) 129. Tshz1 mutant mice displayed middle ear defects, including an absence of the orbicular apophysis in the malleus (Figure 3N, black asterisk), a shortened and thickened tympanic ring, and an abnormal gonial ring. The incus and stapes developed normally and the stapes inserted normally into the oval window of the inner ear. Analysis of the manubrial area in Tshz1−/− mice showed a small rounded cartilaginous structure between the external acoustic meatus and the tubo-tympanic recess. Although Tshz1 is expressed in pharyngeal arches 1 and 2, it not essential for early patterning of the arches, rather it specifically regulates the development of the malleus and middle ear-associated structures. As discussed above, genes such as Msx1, Gsc or Bapx1 are essential for malleus, tympanic ring and gonial bone development, but expression of these genes in the developing middle ear structures of Tshz1 mutant were unaffected. However, markers for developing bones (Cbfa1/Rux2) were greatly downregulated in the prospective tympanic ring and gonial bone regions of Tshz1 mutants. suggesting that the tympanic ring and gonial bone defects are due to mis-regulation of Cbfa1 130 and placing Tszh1 function either downstream or acting in parallel to Msx1, Gsc and Bapx1 action.
Future directions and unanswered questions in middle ear development research
The work above describes many of the transcriptional regulators and secreted signals that regulate neural crest cell migration into the prospective middle ear region and initiate the condensation of middle ear ossicle primordia. However, many questions remain unanswered. It is currently not clear when neural crest cells migrating into the first and second pharyngeal arches receive patterning information that directs them to form a particular component or structure in the ossicular chain. At one extreme, neural crest cells could start to become distinct from each other as the migrate through the arches, while at the other extreme, they could remain as a relatively homogenous population until they cease migration and begin to condense into cartilage. The advent of single cell RNA sequencing (scRNA-seq) may help to shed light on this question 131. scRNA-seq has already shown that crest cells in different parts of the migratory stream may be transcriptionally different from each other 132, and the ability to analyze single cell transcriptomes to greater read depths may reveal further heterogeneity during the different stages of ossicle formation. Similarly, although the expression patterns of growth factors, including those described in this review, have been described broadly in pharyngeal endoderm and ectoderm, it has been difficult in the past to map the spatial and temporal relationship of different signaling factors at high resolution. Once again, the ability to interrogate the transcriptomes of arch endoderm and ectoderm at the singe cell level may help identify unique populations of cells whose combined signaling output may help shape ossicle formation in their immediate vicinity.
Once neural crest cells cease migration in the first two pharyngeal arches, they undergo endochondral ossification to form cartilages including Meckel’s cartilage, the malleus, incus, stapes, and styloid process. The signaling mechanisms and cell-intrinsic transcriptional codes that distinguish the ossicle cartilage precursors from each other and from other cartilages or bones in the head is not currently clear. Similarly, mouse mutants have given us only a rudimentary understanding of the signals that produce the unique shape and position of each ossicle. To give one example, previous studies showed that the malleus and incus initially develop from a common condensation and eventually separate from each other by forming the malleo-incudal joint. This cells within the prospective malleo-incudal joint down-regulate early condensing markers (Sox9, Col2a1) and up-regulate joint markers (Gdf5) 3,25,36. However, it is still not clear that how the joint between incus and stapes (incudo-stapedial joint) is formed.
A final area of research concerns the large number of genetic disorders that affect the formation of the auditory conduction apparatus. Although the effects of many disorders and syndromes on the middle ear have been characterized, such as DiGeorge or Treacher-Collins syndromes, and a large number of deafness genes affecting inner ear function have been found, it is likely that many new gene variants may contribute to more subtle defects in the development of the middle and external ears. With the advent of large-scale genome and exome sequencing, more potentially pathogenic gene variants associated with conductive hearing loss may be identified. Moreover, the ability to rapidly create mouse models of such variants using CRISPR offers the potential for a new approach to understanding the genetics of middle ear-associated hearing loss.
ACKNOWLEDGEMENTS
The writing of this review was supported by NIH Grant DC013072 to A.K.G.
REFERENCES
- 1.Basch ML, Brown RM 2nd, Jen HI, Groves AK. Where hearing starts: the development of the mammalian cochlea. J Anat. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mallo M. Formation of the middle ear: recent progress on the developmental and molecular mechanisms. Dev Biol. 2001;231(2):410–419. [DOI] [PubMed] [Google Scholar]
- 3.Amin S, Matalova E, Simpson C, Yoshida H, Tucker AS. Incudomalleal joint formation: the roles of apoptosis, migration and downregulation. BMC developmental biology. 2007;7:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park HY, Han DH, Lee JB, Han NS, Choung YH, Park K. Congenital stapes anomalies with normal eardrum. Clinical and experimental otorhinolaryngology. 2009;2(1):33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vincent R, Wegner I, Derks LS, Grolman W. Congenital ossicular chain malformations with mobile stapes in children: Results in 17 cases. Laryngoscope. 2016;126(3):682–688. [DOI] [PubMed] [Google Scholar]
- 6.Fraser FC, Ling D, Clogg D, Nogrady B. Genetic aspects of the BOR syndrome--branchial fistulas, ear pits, hearing loss, and renal anomalies. Am J Med Genet. 1978;2(3):241–252. [DOI] [PubMed] [Google Scholar]
- 7.Verloes A. Updated diagnostic criteria for CHARGE syndrome: a proposal. Am J Med Genet A. 2005;133A(3):306–308. [DOI] [PubMed] [Google Scholar]
- 8.Rudic M, Keogh I, Wagner R, et al. The pathophysiology of otosclerosis: Review of current research. Hear Res. 2015;330(Pt A):51–56. [DOI] [PubMed] [Google Scholar]
- 9.Cai T, McPherson B. Hearing loss in children with otitis media with effusion: a systematic review. Int J Audiol. 2017;56(2):65–76. [DOI] [PubMed] [Google Scholar]
- 10.Kubba H, Pearson JP, Birchall JP. The aetiology of otitis media with effusion: a review. Clin Otolaryngol Allied Sci. 2000;25(3):181–194. [DOI] [PubMed] [Google Scholar]
- 11.Casselbrant ML, Mandel EM. Genetic susceptibility to otitis media. Curr Opin Allergy Clin Immunol. 2005;5(1):1–4. [DOI] [PubMed] [Google Scholar]
- 12.Zheng QY, Hardisty-Hughes R, Brown SD. Mouse models as a tool to unravel the genetic basis for human otitis media. Brain Res. 2006;1091(1):9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hardisty-Hughes RE, Tateossian H, Morse SA, et al. A mutation in the F-box gene, Fbxo11, causes otitis media in the Jeff mouse. Hum Mol Genet. 2006;15(22):3273–3279. [DOI] [PubMed] [Google Scholar]
- 14.Parkinson N, Hardisty-Hughes RE, Tateossian H, et al. Mutation at the Evi1 locus in Junbo mice causes susceptibility to otitis media. PLoS Genet. 2006;2(10):e149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geng R, Wang Q, Chen E, Zheng QY. Current Understanding of Host Genetics of Otitis Media. Front Genet. 2019;10:1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giese APJ, Ali S, Isaiah A, Aziz I, Riazuddin S, Ahmed ZM. Genomics of Otitis Media (OM): Molecular Genetics Approaches to Characterize Disease Pathophysiology. Front Genet. 2020;11:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mason MJ. Structure and function of the mammalian middle ear. II: Inferring function from structure. J Anat. 2016;228(2):300–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nummela S. Scaling of the mammalian middle ear. Hear Res. 1995;85(1-2):18–30. [DOI] [PubMed] [Google Scholar]
- 19.Webster DB. Ear structure and function in modern mammals. Am Zool. 1966;6(3):451–466. [DOI] [PubMed] [Google Scholar]
- 20.Manley GA. An evolutionary perspective on middle ears. Hear Res. 2010;263(1-2):3–8. [DOI] [PubMed] [Google Scholar]
- 21.Tucker AS. Major evolutionary transitions and innovations: the tympanic middle ear. Philos Trans R Soc Lond B Biol Sci. 2017;372(1713). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rich TH, Hopson JA, Musser AM, Flannery TF, Vickers-Rich P. Independent origins of middle ear bones in monotremes and therians. Science. 2005;307(5711):910–914. [DOI] [PubMed] [Google Scholar]
- 23.Chapman SC. Can you hear me now? Understanding vertebrate middle ear development. Front Biosci. 2011;16:1675–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anthwal N, Joshi L, Tucker AS. Evolution of the mammalian middle ear and jaw: adaptations and novel structures. J Anat. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amin S, Tucker AS. Joint formation in the middle ear: lessons from the mouse and guinea pig. Dev Dyn. 2006;235(5):1326–1333. [DOI] [PubMed] [Google Scholar]
- 26.Anthwal N, Fenelon JC, Johnston SD, Renfree MB, Tucker AS. Transient role of the middle ear as a lower jaw support across mammals. Elife. 2020;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lautenschlager S, Gill PG, Luo ZX, Fagan MJ, Rayfield EJ. The role of miniaturization in the evolution of the mammalian jaw and middle ear. Nature. 2018;561(7724):533–537. [DOI] [PubMed] [Google Scholar]
- 28.Thompson H, Ohazama A, Sharpe PT, Tucker AS. The origin of the stapes and relationship to the otic capsule and oval window. Dev Dyn. 2012;241(9):1396–1404. [DOI] [PubMed] [Google Scholar]
- 29.Birgbauer E, Sechrist J, Bronner-Fraser M, Fraser S. Rhombomeric origin and rostrocaudal reassortment of neural crest cells revealed by intravital microscopy. Development. 1995;121(4):935–945. [DOI] [PubMed] [Google Scholar]
- 30.Creuzet S, Couly G, Le Douarin NM. Patterning the neural crest derivatives during development of the vertebrate head: insights from avian studies. J Anat. 2005;207(5):447–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santagati F, Rijli FM. Cranial neural crest and the building of the vertebrate head. Nat Rev Neurosci. 2003;4(10):806–818. [DOI] [PubMed] [Google Scholar]
- 32.Trainor PA, Krumlauf R. Hox genes, neural crest cells and branchial arch patterning. Curr Opin Cell Biol. 2001;13(6):698–705. [DOI] [PubMed] [Google Scholar]
- 33.Trainor PA, Sobieszczuk D, Wilkinson D, Krumlauf R. Signalling between the hindbrain and paraxial tissues dictates neural crest migration pathways. Development. 2002;129(2):433–442. [DOI] [PubMed] [Google Scholar]
- 34.Arenkiel BR, Gaufo GO, Capecchi MR. Hoxb1 neural crest preferentially form glia of the PNS. Dev Dyn. 2003;227(3):379–386. [DOI] [PubMed] [Google Scholar]
- 35.Minoux M, Kratochwil CF, Ducret S, et al. Mouse Hoxa2 mutations provide a model for microtia and auricle duplication. Development. 2013;140(21):4386–4397. [DOI] [PubMed] [Google Scholar]
- 36.Ankamreddy H, Min H, Kim JY, et al. Region-specific endodermal signals direct neural crest cells to form the three middle ear ossicles. Development. 2019;146(2). [DOI] [PubMed] [Google Scholar]
- 37.Anthwal N, Thompson H. The development of the mammalian outer and middle ear. J Anat. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hall BK, Miyake T. All for one and one for all: condensations and the initiation of skeletal development. Bioessays. 2000;22(2):138–147. [DOI] [PubMed] [Google Scholar]
- 39.Miyake T, Cameron AM, Hall BK. Stage-specific onset of condensation and matrix deposition for Meckel's and other first arch cartilages in inbred C57BL/6 mice. J Craniofac Genet Dev Biol. 1996;16(1):32–47. [PubMed] [Google Scholar]
- 40.Anthwal N, Urban DJ, Luo ZX, Sears KE, Tucker AS. Meckel's cartilage breakdown offers clues to mammalian middle ear evolution. Nat Ecol Evol. 2017;1(4):93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shin J-O, Ankamreddy H, Jakka NM, Lee S, Kim U-K, Bok J. Temporal and spatial expression patterns of Hedgehog receptors in the developing inner and middle ear. International Journal of Developmental Biology. 2017;61(8-9):557–563. [DOI] [PubMed] [Google Scholar]
- 42.Billmyre KK, Klingensmith J. Sonic hedgehog from pharyngeal arch 1 epithelium is necessary for early mandibular arch cell survival and later cartilage condensation differentiation. Dev Dyn. 2015;244(4):564–576. [DOI] [PubMed] [Google Scholar]
- 43.Tucker AS, Watson RP, Lettice LA, Yamada G, Hill RE. Bapx1 regulates patterning in the middle ear: altered regulatory role in the transition from the proximal jaw during vertebrate evolution. Development. 2004;131(6):1235–1245. [DOI] [PubMed] [Google Scholar]
- 44.Hwang CH, Wu DK. Noggin heterozygous mice: an animal model for congenital conductive hearing loss in humans. Hum Mol Genet. 2008;17(6):844–853. [DOI] [PubMed] [Google Scholar]
- 45.Kitazawa T, Fujisawa K, Narboux-Neme N, et al. Distinct effects of Hoxa2 overexpression in cranial neural crest populations reveal that the mammalian hyomandibular-ceratohyal boundary maps within the styloid process. Dev Biol. 2015;402(2):162–174. [DOI] [PubMed] [Google Scholar]
- 46.Schmidt OV. Elongated styloid process which interfered with function of a singer's voice; operation and recovery. AMA Arch Otolaryngol. 1951;54(4):417–421. [DOI] [PubMed] [Google Scholar]
- 47.Wittmaack K. Über die normale und die pathologische Pneumatisation des Schläfenbeines. Germany: Gustav Fischer; 1918. [Google Scholar]
- 48.Thompson H, Tucker AS. Dual origin of the epithelium of the mammalian middle ear. Science. 2013;339(6126):1453–1456. [DOI] [PubMed] [Google Scholar]
- 49.Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15(23):3059–3087. [DOI] [PubMed] [Google Scholar]
- 50.McMahon AP, Ingham PW, Tabin CJ. Developmental roles and clinical significance of hedgehog signaling. Curr Top Dev Biol. 2003;53:1–114. [DOI] [PubMed] [Google Scholar]
- 51.Chiang C, Litingtung Y, Lee E, et al. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383(6599):407–413. [DOI] [PubMed] [Google Scholar]
- 52.Seppala M, Depew MJ, Martinelli DC, Fan CM, Sharpe PT, Cobourne MT. Gas1 is a modifier for holoprosencephaly and genetically interacts with sonic hedgehog. J Clin Invest. 2007;117(6):1575–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jeong J, Mao J, Tenzen T, Kottmann AH, McMahon AP. Hedgehog signaling in the neural crest cells regulates the patterning and growth of facial primordia. Genes Dev. 2004;18(8):937–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nie X, Luukko K, Kettunen P. BMP signalling in craniofacial development. Int J Dev Biol. 2006;50(6):511–521. [DOI] [PubMed] [Google Scholar]
- 55.Katagiri T, Watabe T. Bone Morphogenetic Proteins. Cold Spring Harb Perspect Biol. 2016;8(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kanzler B, Foreman RK, Labosky PA, Mallo M. BMP signaling is essential for development of skeletogenic and neurogenic cranial neural crest. Development. 2000;127(5):1095–1104. [DOI] [PubMed] [Google Scholar]
- 57.Moosa S, Wollnik B. Altered FGF signalling in congenital craniofacial and skeletal disorders. Semin Cell Dev Biol. 2016;53:115–125. [DOI] [PubMed] [Google Scholar]
- 58.Neben CL, Merrill AE. Signaling Pathways in Craniofacial Development: Insights from Rare Skeletal Disorders. Curr Top Dev Biol. 2015;115:493–542. [DOI] [PubMed] [Google Scholar]
- 59.Wright TJ, Mansour SL. FGF signaling in ear development and innervation. Curr Top Dev Biol. 2003;57:225–259. [DOI] [PubMed] [Google Scholar]
- 60.Agochukwu NB, Solomon BD, Muenke M. Hearing loss in syndromic craniosynostoses: otologic manifestations and clinical findings. Int J Pediatr Otorhinolaryngol. 2014;78(12):2037–2047. [DOI] [PubMed] [Google Scholar]
- 61.Vallino-Napoli LD. Audiologic and otologic characteristics of Pfeiffer syndrome. Cleft Palate Craniofac J. 1996;33(6):524–529. [DOI] [PubMed] [Google Scholar]
- 62.Crossley PH, Martin GR. The mouse Fgf8 gene encodes a family of polypeptides and is expressed in regions that direct outgrowth and patterning in the developing embryo. Development. 1995;121(2):439–451. [DOI] [PubMed] [Google Scholar]
- 63.Moore-Scott BA, Manley NR. Differential expression of Sonic hedgehog along the anterior-posterior axis regulates patterning of pharyngeal pouch endoderm and pharyngeal endoderm-derived organs. Dev Biol. 2005;278(2):323–335. [DOI] [PubMed] [Google Scholar]
- 64.Abu-Issa R, Smyth G, Smoak I, Yamamura K, Meyers EN. Fgf8 is required for pharyngeal arch and cardiovascular development in the mouse. Development. 2002;129(19):4613–4625. [DOI] [PubMed] [Google Scholar]
- 65.Trumpp A, Depew MJ, Rubenstein JL, Bishop JM, Martin GR. Cre-mediated gene inactivation demonstrates that FGF8 is required for cell survival and patterning of the first branchial arch. Genes Dev. 1999;13(23):3136–3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Trokovic N, Trokovic R, Mai P, Partanen J. Fgfr1 regulates patterning of the pharyngeal region. Genes Dev. 2003;17(1):141–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pau H, Fuchs H, de Angelis MH, Steel KP. Hush puppy: a new mouse mutant with pinna, ossicle, and inner ear defects. Laryngoscope. 2005;115(1):116–124. [DOI] [PubMed] [Google Scholar]
- 68.Calvert JA, Dedos SG, Hawker K, Fleming M, Lewis MA, Steel KP. A missense mutation in Fgfr1 causes ear and skull defects in hush puppy mice. Mamm Genome. 2011;22(5-6):290–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rigueur D, Roberts RR, Bobzin L, Merrill AE. A requirement for Fgfr2 in middle ear development. Genesis. 2019;57(1):e23252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Siebel C, Lendahl U. Notch Signaling in Development, Tissue Homeostasis, and Disease. Physiol Rev. 2017;97(4):1235–1294. [DOI] [PubMed] [Google Scholar]
- 71.Le Caignec C, Lefevre M, Schott JJ, et al. Familial deafness, congenital heart defects, and posterior embryotoxon caused by cysteine substitution in the first epidermal-growth-factor-like domain of jagged 1. Am J Hum Genet. 2002;71(1):180–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Teng CS, Yen HY, Barske L, et al. Requirement for Jagged1-Notch2 signaling in patterning the bones of the mouse and human middle ear. Sci Rep. 2017;7(1):2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang J, Knaut H. Chemokine signaling in development and disease. Development. 2014;141(22):4199–4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Escot S, Blavet C, Faure E, Zaffran S, Duband JL, Fournier-Thibault C. Disruption of CXCR4 signaling in pharyngeal neural crest cells causes DiGeorge syndrome-like malformations. Development. 2016;143(4):582–588. [DOI] [PubMed] [Google Scholar]
- 75.Escot S, Blavet C, Hartle S, Duband JL, Fournier-Thibault C. Misregulation of SDF1-CXCR4 signaling impairs early cardiac neural crest cell migration leading to conotruncal defects. Circ Res. 2013;113(5):505–516. [DOI] [PubMed] [Google Scholar]
- 76.Olesnicky Killian EC, Birkholz DA, Artinger KB. A role for chemokine signaling in neural crest cell migration and craniofacial development. Dev Biol. 2009;333(1):161–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Saito D, Takase Y, Murai H, Takahashi Y. The dorsal aorta initiates a molecular cascade that instructs sympatho-adrenal specification. Science. 2012;336(6088):1578–1581. [DOI] [PubMed] [Google Scholar]
- 78.Ankamreddy H, Koo H, Lee YJ, Bok J. CXCL12 is required for stirrup-shaped stapes formation during mammalian middle ear development. Dev Dyn. 2020;249(9):1117–1126. [DOI] [PubMed] [Google Scholar]
- 79.Acampora D, Merlo GR, Paleari L, et al. Craniofacial, vestibular and bone defects in mice lacking the Distal-less-related gene Dlx5. Development. 1999;126(17):3795–3809. [DOI] [PubMed] [Google Scholar]
- 80.Arnold JS, Braunstein EM, Ohyama T, et al. Tissue-specific roles of Tbx1 in the development of the outer, middle and inner ear, defective in 22q11DS patients. Hum Mol Genet. 2006;15(10):1629–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Beverdam A, Merlo GR, Paleari L, et al. Jaw transformation with gain of symmetry after Dlx5/Dlx6 inactivation: mirror of the past? Genesis. 2002;34(4):221–227. [DOI] [PubMed] [Google Scholar]
- 82.Depew MJ, Liu JK, Long JE, et al. Dlx5 regulates regional development of the branchial arches and sensory capsules. Development. 1999;126(17):3831–3846. [DOI] [PubMed] [Google Scholar]
- 83.Edlund RK, Ohyama T, Kantarci H, Riley BB, Groves AK. Foxi transcription factors promote pharyngeal arch development by regulating formation of FGF signaling centers. Dev Biol. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gendron-Maguire M, Mallo M, Zhang M, Gridley T. Hoxa-2 mutant mice exhibit homeotic transformation of skeletal elements derived from cranial neural crest. Cell. 1993;75(7):1317–1331. [DOI] [PubMed] [Google Scholar]
- 85.Raft S, Coate TM, Kelley MW, Crenshaw EB 3rd, Wu DK. Pou3f4-mediated regulation of ephrin-b2 controls temporal bone development in the mouse. PLoS One. 2014;9(10):e109043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Depew MJ, Lufkin T, Rubenstein JL. Specification of jaw subdivisions by Dlx genes. Science. 2002;298(5592):381–385. [DOI] [PubMed] [Google Scholar]
- 87.Kanzler B, Kuschert SJ, Liu YH, Mallo M. Hoxa-2 restricts the chondrogenic domain and inhibits bone formation during development of the branchial area. Development. 1998;125(14):2587–2597. [DOI] [PubMed] [Google Scholar]
- 88.Hunt P, Wilkinson D, Krumlauf R. Patterning the vertebrate head: murine Hox 2 genes mark distinct subpopulations of premigratory and migrating cranial neural crest. Development. 1991;112(1):43–50. [DOI] [PubMed] [Google Scholar]
- 89.Santagati F, Minoux M, Ren SY, Rijli FM. Temporal requirement of Hoxa2 in cranial neural crest skeletal morphogenesis. Development. 2005;132(22):4927–4936. [DOI] [PubMed] [Google Scholar]
- 90.Rijli FM, Mark M, Lakkaraju S, Dierich A, Dolle P, Chambon P. A homeotic transformation is generated in the rostral branchial region of the head by disruption of Hoxa-2, which acts as a selector gene. Cell. 1993;75(7):1333–1349. [DOI] [PubMed] [Google Scholar]
- 91.Baltzinger M, Ori M, Pasqualetti M, Nardi I, Rijli FM. Hoxa2 knockdown in Xenopus results in hyoid to mandibular homeosis. Dev Dyn. 2005;234(4):858–867. [DOI] [PubMed] [Google Scholar]
- 92.Hunter MP, Prince VE. Zebrafish hox paralogue group 2 genes function redundantly as selector genes to pattern the second pharyngeal arch. Dev Biol. 2002;247(2):367–389. [DOI] [PubMed] [Google Scholar]
- 93.Brown KK, Viana LM, Helwig CC, et al. HOXA2 haploinsufficiency in dominant bilateral microtia and hearing loss. Hum Mutat. 2013;34(10):1347–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Grammatopoulos GA, Bell E, Toole L, Lumsden A, Tucker AS. Homeotic transformation of branchial arch identity after Hoxa2 overexpression. Development. 2000;127(24):5355–5365. [DOI] [PubMed] [Google Scholar]
- 95.Pasqualetti M, Ori M, Nardi I, Rijli FM. Ectopic Hoxa2 induction after neural crest migration results in homeosis of jaw elements in Xenopus. Development. 2000;127(24):5367–5378. [DOI] [PubMed] [Google Scholar]
- 96.Sumiyama K, Tanave A. The regulatory landscape of the Dlx gene system in branchial arches: shared characteristics among Dlx bigene clusters and evolution. Dev Growth Differ. 2020. [DOI] [PubMed] [Google Scholar]
- 97.Qiu M, Bulfone A, Ghattas I, et al. Role of the Dlx homeobox genes in proximodistal patterning of the branchial arches: mutations of Dlx-1, Dlx-2, and Dlx-1 and −2 alter morphogenesis of proximal skeletal and soft tissue structures derived from the first and second arches. Dev Biol. 1997;185(2):165–184. [DOI] [PubMed] [Google Scholar]
- 98.Qiu M, Bulfone A, Martinez S, et al. Null mutation of Dlx-2 results in abnormal morphogenesis of proximal first and second branchial arch derivatives and abnormal differentiation in the forebrain. Genes Dev. 1995;9(20):2523–2538. [DOI] [PubMed] [Google Scholar]
- 99.Jeong J, Li X, McEvilly RJ, Rosenfeld MG, Lufkin T, Rubenstein JL. Dlx genes pattern mammalian jaw primordium by regulating both lower jaw-specific and upper jaw-specific genetic programs. Development. 2008;135(17):2905–2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Robledo RF, Rajan L, Li X, Lufkin T. The Dlx5 and Dlx6 homeobox genes are essential for craniofacial, axial, and appendicular skeletal development. Genes Dev. 2002;16(9):1089–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Garg V, Yamagishi C, Hu T, Kathiriya IS, Yamagishi H, Srivastava D. Tbx1, a DiGeorge syndrome candidate gene, is regulated by sonic hedgehog during pharyngeal arch development. Dev Biol. 2001;235(1):62–73. [DOI] [PubMed] [Google Scholar]
- 102.Lindsay EA, Vitelli F, Su H, et al. Tbx1 haploinsufficieny in the DiGeorge syndrome region causes aortic arch defects in mice. Nature. 2001;410(6824):97–101. [DOI] [PubMed] [Google Scholar]
- 103.Moraes F, Novoa A, Jerome-Majewska LA, Papaioannou VE, Mallo M. Tbx1 is required for proper neural crest migration and to stabilize spatial patterns during middle and inner ear development. Mech Dev. 2005;122(2):199–212. [DOI] [PubMed] [Google Scholar]
- 104.Aggarwal VS, Carpenter C, Freyer L, Liao J, Petti M, Morrow BE. Mesodermal Tbx1 is required for patterning the proximal mandible in mice. Dev Biol. 2010;344(2):669–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.de Kok YJ, Cremers CW, Ropers HH, Cremers FP. The molecular basis of X-linked deafness type 3 (DFN3) in two sporadic cases: identification of a somatic mosaicism for a POU3F4 missense mutation. Hum Mutat. 1997;10(3):207–211. [DOI] [PubMed] [Google Scholar]
- 106.Samadi DS, Saunders JC, Crenshaw EB 3rd. Mutation of the POU-domain gene Brn4/Pou3f4 affects middle-ear sound conduction in the mouse. Hear Res. 2005;199(1-2):11–21. [DOI] [PubMed] [Google Scholar]
- 107.Cramer KS, Miko IJ. Eph-ephrin signaling in nervous system development. F1000Res. 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Davy A, Soriano P. Ephrin-B2 forward signaling regulates somite patterning and neural crest cell development. Dev Biol. 2007;304(1):182–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Miao H, Wang B. Eph/ephrin signaling in epithelial development and homeostasis. Int J Biochem Cell Biol. 2009;41(4):762–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Coate TM, Raft S, Zhao X, Ryan AK, Crenshaw EB 3rd, Kelley MW. Otic mesenchyme cells regulate spiral ganglion axon fasciculation through a Pou3f4/EphA4 signaling pathway. Neuron. 2012;73(1):49–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rhodes CR, Parkinson N, Tsai H, et al. The homeobox gene Emx2 underlies middle ear and inner ear defects in the deaf mouse mutant pardon. J Neurocytol. 2003;32(9):1143–1154. [DOI] [PubMed] [Google Scholar]
- 112.Satokata I, Maas R. Msx1 deficient mice exhibit cleft palate and abnormalities of craniofacial and tooth development. Nat Genet. 1994;6(4):348–356. [DOI] [PubMed] [Google Scholar]
- 113.Mason MJ. Of mice, moles and guinea pigs: functional morphology of the middle ear in living mammals. Hear Res. 2013;301:4–18. [DOI] [PubMed] [Google Scholar]
- 114.Levi G, Mantero S, Barbieri O, et al. Msx1 and Dlx5 act independently in development of craniofacial skeleton, but converge on the regulation of Bmp signaling in palate formation. Mech Dev. 2006;123(1):3–16. [DOI] [PubMed] [Google Scholar]
- 115.Ohyama T, Groves AK. Expression of mouse Foxi class genes in early craniofacial development. Dev Dyn. 2004;231(3):640–646. [DOI] [PubMed] [Google Scholar]
- 116.Edlund RK, Birol O, Groves AK. The role of foxi family transcription factors in the development of the ear and jaw. Curr Top Dev Biol. 2015;111:461–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nissen RM, Yan J, Amsterdam A, Hopkins N, Burgess SM. Zebrafish foxi one modulates cellular responses to Fgf signaling required for the integrity of ear and jaw patterning. Development. 2003;130(11):2543–2554. [DOI] [PubMed] [Google Scholar]
- 118.Solomon KS, Kudoh T, Dawid IB, Fritz A. Zebrafish foxi1 mediates otic placode formation and jaw development. Development. 2003;130(5):929–940. [DOI] [PubMed] [Google Scholar]
- 119.Grigoriadis AE, Wang ZQ, Cecchini MG, et al. c-Fos: a key regulator of osteoclast-macrophage lineage determination and bone remodeling. Science. 1994;266(5184):443–448. [DOI] [PubMed] [Google Scholar]
- 120.Tribioli C, Frasch M, Lufkin T. Bapx1: an evolutionary conserved homologue of the Drosophila bagpipe homeobox gene is expressed in splanchnic mesoderm and the embryonic skeleton. Mech Dev. 1997;65(1-2):145–162. [DOI] [PubMed] [Google Scholar]
- 121.Tribioli C, Lufkin T. The murine Bapx1 homeobox gene plays a critical role in embryonic development of the axial skeleton and spleen. Development. 1999;126(24):5699–5711. [DOI] [PubMed] [Google Scholar]
- 122.Miller CT, Yelon D, Stainier DY, Kimmel CB. Two endothelin 1 effectors, hand2 and bapx1, pattern ventral pharyngeal cartilage and the jaw joint. Development. 2003;130(7):1353–1365. [DOI] [PubMed] [Google Scholar]
- 123.Blum M, Gaunt SJ, Cho KW, et al. Gastrulation in the mouse: the role of the homeobox gene goosecoid. Cell. 1992;69(7):1097–1106. [DOI] [PubMed] [Google Scholar]
- 124.Gaunt SJ, Blum M, De Robertis EM. Expression of the mouse goosecoid gene during mid-embryogenesis may mark mesenchymal cell lineages in the developing head, limbs and body wall. Development. 1993;117(2):769–778. [DOI] [PubMed] [Google Scholar]
- 125.Rivera-Perez JA, Mallo M, Gendron-Maguire M, Gridley T, Behringer RR. Goosecoid is not an essential component of the mouse gastrula organizer but is required for craniofacial and rib development. Development. 1995;121(9):3005–3012. [DOI] [PubMed] [Google Scholar]
- 126.Rivera-Perez JA, Wakamiya M, Behringer RR. Goosecoid acts cell autonomously in mesenchyme-derived tissues during craniofacial development. Development. 1999;126(17):3811–3821. [DOI] [PubMed] [Google Scholar]
- 127.Yamada G, Mansouri A, Torres M, et al. Targeted mutation of the murine goosecoid gene results in craniofacial defects and neonatal death. Development. 1995;121(9):2917–2922. [DOI] [PubMed] [Google Scholar]
- 128.Caubit X, Core N, Boned A, Kerridge S, Djabali M, Fasano L. Vertebrate orthologues of the Drosophila region-specific patterning gene teashirt. Mech Dev. 2000;91(1-2):445–448. [DOI] [PubMed] [Google Scholar]
- 129.Long Q, Park BK, Ekker M. Expression and regulation of mouse Mtsh1 during limb and branchial arch development. Dev Dyn. 2001;222(2):308–312. [DOI] [PubMed] [Google Scholar]
- 130.Core N, Caubit X, Metchat A, Boned A, Djabali M, Fasano L. Tshz1 is required for axial skeleton, soft palate and middle ear development in mice. Dev Biol. 2007;308(2):407–420. [DOI] [PubMed] [Google Scholar]
- 131.Xu J, Liu H, Lan Y, et al. Hedgehog signaling patterns the oral-aboral axis of the mandibular arch. Elife. 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Morrison JA, McLennan R, Wolfe LA, et al. Single-cell transcriptome analysis of avian neural crest migration reveals signatures of invasion and molecular transitions. Elife. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]