Abstract
BACKGROUND
Helicobacter pylori (H. pylori) is a spiral-shaped bacterium responsible for the development of chronic gastritis, gastric ulcer, gastric cancer (GC), and MALT-lymphoma of the stomach. H. pylori can be present in the gastric mucosa (GM) in both spiral and coccoid forms. However, it is not known whether the severity of GM contamination by various vegetative forms of H. pylori is associated with clinical and morphological characteristics and long-term results of GC treatment.
AIM
To establish the features of H. pylori infection in patients with GC and their correlations with clinical and morphological characteristics of diseases and long-term results of treatment.
METHODS
Of 109 patients with GC were included in a prospective cohort study. H. pylori in the GM and tumor was determined by rapid urease test and by immunohistochemically using the antibody to H. pylori. The results obtained were compared with the clinical and morphological characteristics and prognosis of GC. Statistical analysis was performed using the Statistica 10.0 software.
RESULTS
H. pylori was detected in the adjacent to the tumor GM in 84.5% of cases, of which a high degree of contamination was noted in 50.4% of the samples. Coccoid forms of H. pylori were detected in 93.4% of infected patients, and only coccoid-in 68.9%. It was found that a high degree of GM contamination by the coccoid forms of H. pylori was observed significantly more often in diffuse type of GC (P = 0.024), in poorly differentiated GC (P = 0.011), in stage T3-4 (P = 0.04) and in N1 (P = 0.011). In cases of moderate and marked concentrations of H. pylori in GM, a decrease in 10-year relapse free and overall survival from 55.6% to 26.3% was observed (P = 0.02 and P = 0.07, respectively). The relationship between the severity of the GM contamination by the spiral-shaped forms of H. pylori and the clinical and morphological characteristics and prognosis of GC was not revealed.
CONCLUSION
The data obtained indicates that H. pylori may be associated not only with induction but also with the progression of GC.
Keywords: Gastric cancer, Helicobacter pylori, Coccoid and spiral forms of bacteria, Rapid urease test, Relapse free survival, Overall survival
Core Tip: The role of Helicobacter pylori (H. pylori) in the progression of gastric cancer (GC) is not well understood. Our results indicate that in GC, a high degree of gastric mucosa contamination by coccoid forms of H. pylori is associated with advanced stages of the disease and deterioration of long-term results of treatment.
INTRODUCTION
Gastric cancer (GC) continues to be one of the most common malignant diseases in the world[1,2]. Despite a decreasing trend in the incidence of GC in most countries of the world, the treatment results of this pathology cannot be considered satisfactory. In the structure of mortality from malignant neoplasms, this pathology firmly occupies 2nd place in most developed countries of the world, and the 5-year survival rate of radically operated patients does not exceed 15%-30%[3,4].
It is important to note that it is impossible to improve the long-term results of malignant neoplasms treatment without knowledge of the mechanisms associated with their progression[3]. Clinical studies in recent years indicate that inflammatory infiltration of the tumor stroma and surrounding tissues can have an important prognostic value and affect the long-term results of malignant neoplasm treatment[5-9]. A number of studies have shown that inflammatory infiltration of the tumor stroma is associated with the body’s adequate immune response to the tumor, and may be a favorable prognosis factor in various malignant neoplasms[10,11], including GC[12,13]. At the same time, the data obtained by other researchers indicates that pronounced inflammatory infiltration of the tumor stroma, especially T-reg lymphocytes and macrophages, may be a factor contributing to the progression of malignant neoplasms[14,15]. There was a decrease in the overall survival (OS) and relapse-free survival (RFS) of GC patients with a high content of Foxp3 + T-reg in the tumor stroma and regional metastases[16-18] and macrophages[16,19]. It is believed that inflammatory infiltration of the tumor stroma can contribute to tumor progression by activating the mechanisms of angiogenesis, expression of E- and L-selectins, formation of the products of lipid peroxidation and free radicals, destruction of connective tissue matrix and basement membranes of epithelia by proteolytic enzymes, and activation of epithelial-mesenchymal transformation[20-23].
When studying the role of inflammation in the progression of GC, it is impossible to ignore the problem of Helicobacter pylori (H. pylori) infection. H. pylori is a gram-negative, spiral-shaped bacterium, the habitat of which is the gastric mucosa (GM) and duodenum. H. pylori differs from other bacteria in a set of properties that make it possible to colonize the GM and persist for a long time under conditions that are unfavorable for other microorganisms[24,25]. These include: (1) The ability to produce a special enzyme-urease; (2) Synthesis of lytic enzymes that cause the depolymerization and dissolution of gastric mucus, consisting mainly of mucin; (3) The mobility of the bacterium, which is ensured by the presence of 5-6 flagella; (4) The high adhesiveness of bacteria to GM epithelial cells of the GM and elements of connective tissue due to the interaction of bacterial ligands with the corresponding cells receptors; (5) Production of various exotoxins (VacA, CagA, and others); (6) Instability of the H. pylori genome; (7) The presence of vegetative and coccoid forms of bacteria; and (8) possibility of intracellular persistence and translocation outside the GM [26-29].
It should be noted that despite the huge number of studies devoted to H. pylori, it is still not clear whether H. pylori is involved only in the initiation of the tumor process in the stomach, or whether it can affect the mechanisms of tumor progression. The relationship between the severity of H. pylori infection and the clinical and morphological characteristics of GC and long-term results of this pathology treatment remains poorly studied, and in this connection, the question of the expediency of anti-Helicobacter therapy in patients with invasive GC remains open.
Objective
To assess the features of H. pylori infection in patients with Stage I-IIIB GC and their correlation with the clinical and morphological characteristics of the disease, the presence of antibiotic therapy (AT) before surgery, and long-term treatment results.
MATERIALS AND METHODS
The patients
One hundred and nine patients with GC who had undergone radical surgery (R0) between May 2007 and March 2010 at the Orenburg Regional Clinical Oncology Center, were included in this prospective cohort pilot study. Study inclusion criteria were: Histologically proven invasive GC; no evidence of distant metastases; radical surgery (R0); no prior gastric surgery; no previous chemotherapy or radiotherapy. The study did not include patients with decompensation of cardiovascular and renal diseases, exacerbation of chronic inflammatory processes, severe allergic processes, or who received glucocorticoids, antihistamines, and non-steroidal anti-inflammatory drugs. The study was performed in accordance with the Helsinki Declaration and internationally recognized guidelines, and the privacy of patients was protected by decoding the data according to the privacy regulations of the Orenburg Regional Clinical Oncologic Center (Russia, Orenburg). All patients provided written informed consent. The protocol was approved by the Institutional Review Board of the Orenburg State Medical University (Russia, Orenburg).
Clinical and pathological data including age, tumor localization, stage, type of surgery, histology, the presence of AT before surgery, postoperative therapy, and long-term results of treatment were retrieved from the routine reports for analyses. The distribution of patients according to the clinical and pathological characteristics of GC is presented in Table 1.
Table 1.
The distribution of patients according to the clinical and pathological characteristics of gastric cancer
|
Characteristics of gastric cancer
|
n
|
Percent
|
| Age | 61.7 ± 1.03 years (from 24 to 81 years, the median–was 61 years) | |
| Sex | ||
| Men | 72 | 66.1 |
| Women | 37 | 33.9 |
| Tumor localization | ||
| Upper third | 18 | 16.5 |
| Middle third | 32 | 29.4 |
| Lower third | 57 | 52.3 |
| Total gastric cancer | 2 | 1.8 |
| T status | ||
| T1 | 12 | 11 |
| T2 | 30 | 27.5 |
| T3 | 62 | 56.9 |
| T4 | 5 | 4.6 |
| N status | ||
| N0 | 57 | 52.3 |
| N1 | 20 | 18.3 |
| N2 | 32 | 29.3 |
| TNM | ||
| T1-2N0M0 | 40 | 36.7 |
| T3N0M0 | 17 | 15.6 |
| T3-4N1M0 | 20 | 18.3 |
| T3-4N2M0 | 32 | 29.3 |
| Types of GC | ||
| Intestinal type | 50 | 45.9 |
| Diffuse-types | 59 | 54.1 |
| Grade (histolology) | ||
| G1 | 31 | 28.4 |
| G2 | 19 | 17.4 |
| G3 | 33 | 30.3 |
| Signet ring cell carcinoma | 26 | 23.9 |
| Type sugery | ||
| Subtotal distal resection | 85 | 78 |
| Subtotal proximal resection | 18 | 16.5 |
| Gastrectomy | 7 | 6.4 |
GC: Gastric cancer.
When interviewing patients, it was found that 45 patients received AT before admission to the clinic due to a preliminary wrong diagnosis of gastric ulcer or chronic gastritis. The following combinations of antibacterial drugs were most commonly ordered: Amoxicillin + clarithromycin (34 patients), amoxicillin + clarithromycin + metronidazole (6 patients), amoxicillin + metronidazole (3 patients), other drugs (2 patients). Information about the antibacterial drugs, the timing and duration of their intake was entered into the primary patient documentation and then taken into account in the analysis. We considered only those patients who underwent AT in the period from 1 to 1.5 mo before the operation, lasting at least seven days, and using two or more antibacterial drugs.
The long-term results of treatment were assessed for the period from May 12, 2007 to April 12, 2021. The median follow-up period was 86.2 mo. As of April 12, 2021, 26 (24.5%) patients were alive, 54 (50.9%) had died from the progression of GC, 20 (18.9%) had died from causes other than GC, and six (5.6%) left the region at different follow-up periods. Malignant tumors of other localizations were diagnosed in 8 patients at different times after the operation: Non-Hodgkin's lymphomas-in three, prostate cancer-in one, lung cancer-in two, laryngeal cancer-in one, and breast cancer-in one patient. With the exception of one patient with non-Hodgkin's lymphoma, the other patients died from the progression of these diseases. The causes of death were not associated with malignant tumors for the other patients. During the period 2020-2021, seven patients contracted coronavirus disease-19, one of whom died from the disease, but the rest are alive.
Detection H. pylori infection
H. pylori in the GM and tumor was determined by rapid urease test (RUT) and by immunohistochemically (IHC) using the antibody to H. pylori.
RUT
After removal of the stomach (within 30 min) a greater curvature of the organ was opened and biopsy samples were taken from the tumor and the adjacent macroscopically non-tumorous GM at a distance of 3-5 cm from the tumor margin. The samples were placed on test strips (HELPIL–test, “АМА”, Russia) for three minutes. The presence and the severity of the infection were evaluated by the color change of the indicator from yellow to blue.
According to the intensity and the time of the appearance of a blue color, we distinguished three degrees of infection: 3+: Marked (+++)-bright staining in the first minute of the study; 2+: Moderate (++)–for an average intensity of staining for 2 min and 1+ mild (+)-weak staining for three minutes. If the color of the indicator did not change, or became dirty gray, and if after repeated research the same result was received, the test was evaluated as negative. The same samples were later used for histological analysis and IGH.
Immunohistochemistry
The presence and features of H. pylori infection were studied in samples of the GM adjacent to the tumor, in the tumor tissue, in the omentum, and regional lymph nodes of 46 patients, by immunohistochemistry.
The sections for IGH were dewaxed and rehydrated by sequential immersion in xylene and graded ethanol and water. For antigen retrieval, the sections boiling for 10 min in citrate buffer (pH 6) and endogenous peroxidase activity was blocked with 30 mL/L hydrogen peroxide solution. Slides were incubated at room temperature with the anti-H. pylori (RB-9070, Thermo Fisher Scientific, the immunogen is purified H. pylori) rabbit polyclonal antibodies in diluted at 1:1000 for 30 min.
The visualization system included DAB (UltraVision LP Detection System HRP Polymer & DAB Plus Chromogen) and hematoxylin counterstaining. For negative control sections, the primary antibody was replaced with phosphate-buffered saline and processed in the same manner.
The concentration of H. pylori in the GM detected by IGH was graded as 1+ for mild, 2+ for moderate, and 3+ for marked according to the Sydney system[30]. The presence of point inclusions giving a positive reaction with antibodies to H. pylori in the cytoplasm of epithelial cells of deep gastric glands and of the lymphoid cells of the lamina propria of GM as well as in omentum and lymph node, were taken into account.
All sections were carefully and completely scanned by two of the authors (MS and OT) without knowledge of the clinical and pathological data.
The data obtained was compared with clinical features of GC: Stage, localization, histology, the presence of AT before surgery, and 10-year OS and RFS.
Statistics
Statistical analysis was performed using the Statistica 10.0 software. The correlations between different data were evaluated using the nonparametric Spearman's rank correlation or gamma correlation. Chi-square tests were carried out to analyze the difference of distribution among the categorized data. Mann–Whitney U nonparametric test was used to compare the value of the quantitative data. The survival was analyzed using the Kaplan-Meier method. The log-rank test was used to compare survival curves between subgroups of patients. A value of P < 0.05 was considered statistically significant.
RESULTS
The features of H. pylori infection in GC
Of 93 patients (84.5%) demonstrated positive RUT. According to RUT the concentration of H. pylori in GM was mild (1+) in 38 (34.8%) patients, moderate (2+)-in 37 (33.9%) and marked (3+)–in 8 (16.5%). RUT was negative in 16 patients (14.7%).
It was found that urease activity 2+ and 3+ were significantly more frequent in GM than in tumors (in 55.5% and 13.3% of cases, respectively, P = 0.01). In more than half the cases (53.3%), the urease activity in the tumor was lower than in the adjacent GM.
Forty-six samples of GM were stained by IHC. The coccoid forms of H. pylori were found to prevail in GM adjacent to the tumor (Figure 1A). Coccoid forms of H. pylori only were identified in 31 (63.1%) patients, and coccoid and spiral in 12 (30.4%) patients (Figure 1B). No signs of infection were found in 3 (6.5%) patients. The concentration of coccoid and spiral forms of H. pylori in GM according to IGH was 1+ in 24 (52.2%) and 1 (19.6%) patients, 2+-in 11 (23.9%) and 4 (8.7%), and 3+-in 8 (17.4%) and 1 (2.2%) patients. A positive correlation between the concentration of coccoid and spiral forms of H. pylori (gamma = 0.642, P < 0.0001) was noted.
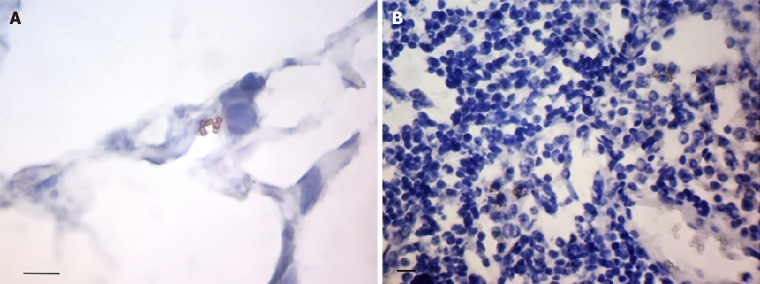
Figure 1.
The features of Helicobacter pylori localization in gastric mucosa in patients with gastric cancer. A: Coccoid forms of Helicobacter pylori (H. pylori) in the gastric pit. The some bacteria within the cytoplasm of epithelium cells (arrows); B: The spiral (black arrows) and coccoid (orange arrows) forms of H. pylori on the surface of superficial-foveolar gastric epithelium; C: The bacteria in the surface mucous gel layer of stomach (arrows); D: The point inclusions giving a positive reaction with antibodies to H. pylori within the cytoplasm of epithelial cells of deep gastric glands (arrows); E: The point inclusions giving a positive reaction with antibodies to H. pylori within the cytoplasm of the immune cells of the lamina propria of gastric mucosa (arrows); F: The point inclusions giving a positive reaction with antibodies to H. pylori within the cytoplasm of intraepithelial lymphocytes (arrows). Immunoperoxidase staining with anti-H. pylori antibody, immersion. Bars: A: 20 μm; B: 10 μm; C: 20 μm; D-F: 10 μm.
The localization of H. pylori in GM was observed not only in the surface mucous gel layer of the stomach (Figure 1C) but also within the cytoplasm of the gastric superficial-foveolar epithelium (Figure 1D). The point inclusions giving a positive reaction with antibodies to H. pylori were also revealed in the cytoplasm of the epithelial cells of deep gastric glands (in 41.3% samples) and of the immune cells (Figure 1E) of the lamina propria of GM (in 43.5% samples), as well as of the intraepithelial lymphocytes (Figure 1F). The close relationship between the presence of point inclusions in immune cells and epithelial cells (gamma = 0.642, P < 0.0001) was noted. The individual cocci or their small clusters, and sometimes the short rod bacterium, were also often detected in the lamina propria of the GM. We also found bacteria in the omentum and lymph nodes. In the omentum the bacteria were presented predominantly by cocci between 0.5 and 1 μm in diameter (Figure 2A). Cocci were arranged most commonly by the small compact groups up to 10-15 cells in the immediate vicinity of the LN capsule. The bacteria were located mainly between the adipocytes. However, it was not clear whether bacteria were outside of the cells or in a narrow rim of cytoplasm of the fat cells.
Figure 2.
The features of Helicobacter pylori localization in omentum and lymph node in patients with gastric cancer. A: The small group of cocci located in the central part of the omentum adipocyte; B: The congestions of bacteria around the nucleus of lymphocytes in the paracortical area of lymph node. Immunoperoxidase staining with anti-Helicobacter pylori antibody, immersion, Bars: 10 μm.
In the tissue of lymph nodes we usually observed the small accumulations of cocci between 10 and 15 cells (Figure 2B). Bacteria were located between lymphocytes of the paracortical area, but not so compact, as in the omentum. Quite often the concentration of bacteria was observed around the nuclei of cells that can testify to their intracellular localization.
Correlations of clinical and pathological characteristics of GC with the severity of H. pylori infection according to RUT data
The gamma correlation coefficient test (gamma) showed that the severity of H. pylori in GM according to RUT positively correlated with the T status (gamma = 0.537, P < 0.00001), N status (gamma = 0.371, P = 0.0007) and stage (gamma = 0.520, P < 0.00001), and negatively correlated with the presence of AT in anamnesis (gamma = -0.418, P = 0.003). The marked (+++) and moderate (++) degrees of H. pylori infection were more often observed in Grade 2 and Grade 3, in T3-4 status, in N1 status, in the T3-4N1-2 stage, and in the absence of AT in anamnesis (Table 2). Correlations of H. pylori concentration in GM according to RUT with 10-year OS and RFS of GC patients were not determined.
Table 2.
Clinical and pathological characteristics of gastric cancer depending on the data rapid urease test
| Characteristics of gastric cancer |
Degrees of infection according to RUT test
|
P value | |||
|
No or mild, n (%) |
Moderate or marked, n (%) |
||||
| Age | 63.8 ± 10.1 | 58.8 ± 11.2 | 0.008 | ||
| T status | |||||
| T1 | 9 | 75 | 3 | 25 | 0.01 |
| T2 | 20 | 66.7 | 10 | 33.3 | |
| T3 | 24 | 38.7 | 38 | 61.3 | |
| T4 | 1 | 20 | 4 | 80 | |
| N status | |||||
| N0 | 37 | 64.9 | 20 | 35.1 | 0.0001 |
| N1 | 2 | 10 | 18 | 90 | |
| N2 | 15 | 46.9 | 17 | 53.1 | |
| TNM | |||||
| T1-2N0M0 | 28 | 70 | 12 | 30 | 0.002 |
| T3N0M0 | 9 | 52.9 | 8 | 47.1 | |
| T3-4N1-2 | 17 | 32.7 | 35 | 67.3 | |
| Types of GC | |||||
| Intestinal type | 27 | 54 | 23 | 46 | 0.39 |
| Diffuse-types | 27 | 45.8 | 32 | 54.2 | |
| Grade (histolology) | |||||
| G1 | 22 | 70 | 9 | 30 | 0.005 |
| G2 | 5 | 26.3 | 14 | 73.7 | |
| G3 | 12 | 36.4 | 21 | 63.6 | |
| Signet ring cell carcinoma | 15 | 57.6 | 11 | 42.3 | |
| Antibiotic therapy before surgery | |||||
| No | 25 | 41.2 | 37 | 68.5 | 0.025 |
| Presence | 28 | 52.8 | 17 | 31.5 | |
RUT: Rapid urease test; GC: Gastric cancer.
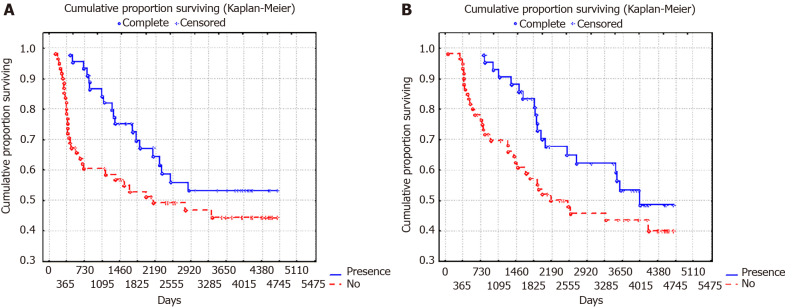
It is important to note that the presence in AT 1-1.5 mo before surgery was associated with a significant improvement in RFS and OS (Figure 3), however, this applied only to patients with local GC (T1-3N0). In advanced GC (T3-4N1 and T3-4N2) there were no significant differences in patient survival (Figure 4).
Figure 3.
10-year overall surviving and relapse-free surviving of patients with gastric cancer depending on the presence of antibiotic therapy 1-1.5 mo before surgery (P = 0.02). A: 10-year overall surviving; B: Relapse-free surviving.
Figure 4.
10-year overall surviving and relapse-free surviving of patients with T3-4N1-2M0 stages of gastric cancer depending on the presence of antibiotic therapy 1-1.5 mo before surgery (P = 0.78). A: 10-year overall surviving; B: Relapse-free surviving.
Correlations of clinical and pathological characteristics of GC with the concentration of spiral and coccoid forms of H. pylori in the GM
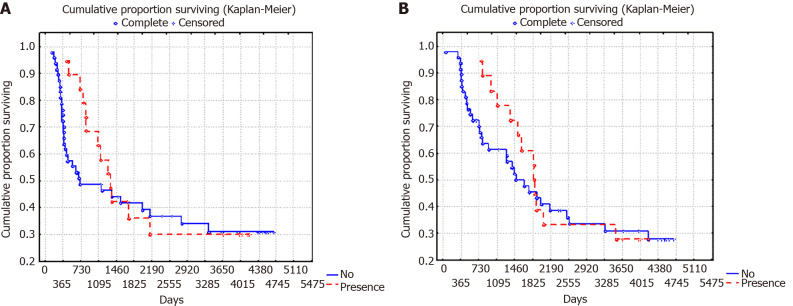
It is important to note that correlations between the clinical and pathological characteristics of GC and the concentration of H. pylori spiral forms in GM were not found. However, the concentration of H. pylori coccoid forms correlated with age (ρ = -0.502, P = 0.0006), histology (gamma = 0.550, P = 0.0004), T status (gamma = 0.709, P = 0.0001), N status (gamma = 0.509, P = 0.002) stage (gamma = 0.636, P = 0.0002), and 10-year RFS (gamma = -0.521, P = 0.008) and OS (gamma = -0.500, P = 0.044). In cases with a moderate and marked concentration (2+ or 3+) of H. pylori coccoid forms in GM compared to cases with a low concentration (1+ or without infection) the patients were younger (57.9 ± 2.5 years vs 66.2 ± 1.4 years, respectively, P = 0.004) and the diffuse type of GC, poorly differentiated tumors (G3), T3-4 stage and N1 stage of GC were more often observed (Table 3). In cases of moderate and marked concentrations of H. pylori in GM, a decrease in 10-year progression-free survival (PFS) and OS survival from 55.6% to 26.3% was observed (P = 0.02 and P = 0.07, respectively). PFS and OS curves, depending on the concentration of coccoid forms of H. pylori in GM, are presented in Figure 5.
Table 3.
Clinical and pathological characteristics of gastric cancer depending on the concentration of Helicobacter pylori coccoid forms in the gastric mucosa
| Characteristics of gastric cancer |
Degrees of infection according to RUT test
|
P value | |||
|
No or mild, n (%)
|
Moderate or marked, n (%)
|
||||
| Age | 66.2 ± 1.4 | 57.9 ± 2.5 | 0.004 | ||
| T status | |||||
| T1 | 6 | 85.7 | 1 | 14.3 | 0.04 |
| T2 | 9 | 81.8 | 2 | 18.2 | |
| T3 | 12 | 44.4 | 15 | 55.6 | |
| T4 | 0 | 0 | 1 | 100 | |
| N status | |||||
| N0 | 18 | 78.3 | 5 | 21.7 | 0.01 |
| N1 | 1 | 16.7 | 5 | 83.8 | |
| N2 | 8 | 47.1 | 9 | 52.9 | |
| TNM | |||||
| T1-2N0M0 | 14 | 82.3 | 3 | 17.6 | 0.02 |
| T3N0M0 | 4 | 66.7 | 2 | 33.3 | |
| T3-4N1-2 | 9 | 39.1 | 14 | 60.9 | |
| Types of GC | |||||
| WIntestinal type | 18 | 75 | 6 | 25 | 0.02 |
| Diffuse-types | 9 | 40.9 | 13 | 59.1 | |
| Grade (histolology) | |||||
| G1 | 14 | 93.3 | 1 | 6.7 | 0.008 |
| G2 | 4 | 44.4 | 5 | 55.6 | |
| G3 | 2 | 28.3 | 5 | 71.4 | |
| Signet ring cell carcinoma | 7 | 46.7 | 8 | 53.3 | |
| Antibiotic therapy before surgery | |||||
| No | 16 | 64.5 | 10 | 38.5 | 0.655 |
| Presence | 11 | 55 | 9 | 45 | |
RUT: Rapid urease test; GC: Gastric cancer.
Figure 5.
10-year overall surviving and relapse-free surviving of patients with gastric cancer depending on the concentration of Helicobacter pylori coccoid forms in the gastric mucosa (P = 0.02). A: 10-year overall surviving; B: Relapse-free surviving.
There were no correlations between the presence of point inclusions in the cytoplasm of epithelial cells of deep gastric glands, in the stroma immune cells, and in the intraepithelial lymphocytes with the clinical and pathological characteristics of GC.
DISCUSSION
A large amount of clinical and experimental data testifies to the important role of H. pylori in the occurrence of GC[31-34], but, there is less research into the features of H. pylori infection in patients with GC and its role in tumor progression, and the results are quite contradictory. These contradictions relate to many aspects, such as: (1) The frequency of infection in patients with GC. This data varies widely and ranges from 36% to 100%[35-40]; (2) The relation of infection with GC prognosis. Some researchers have noted an improvement in the long-term results of GC treatment in infected patients[41,42], while others, on the contrary, found that the presence of H. pylori infection was associated with a decrease in patient survival[43,44]; and (3) The connection between the infection and a histologic type of GC. In some studies, it was noted that patients with an intestinal type of GC are more often infected with H. pylori than patients with the diffuse type of GC[45,46]. Other researchers did not find a difference in H. pylori infection in patients with different histological types of tumors[47].
It is believed that the differences noted are associated with the fact that to reveal the infection the authors used the methods that were significantly different in their sensitivity, and primarily, to coccoid forms of bacteria. Most of the studies were carried out without considering coccoid forms of H. pylori and the concentration of bacteria in GM. The use of the biochemical method for the detection of urease activity and immunohistochemistry for visualization of bacteria in our study allowed us not only to assess the presence of infection in patients with GC, but also to mark some of its features associated with the localization of bacteria in the stomach, with a ratio of cocci and spiral forms, and the degree of bacterial contamination of GM.
Our data for the Orenburg region recorded a high rate of H. pylori infection in patients with GC (84.5%). The coccoid forms of H. pylori, preserving a high degree of urease activity, dominated in GM in patients with GC. They were found in 93.4% of infected patients, with only coccoid forms of H. pylori-in 68.9%.
It is known that the coccoid forms of H. pylori can arise in response to unfavorable environmental factors, such as AT [47,48]. These forms are resistant to AT[49,50] and are able to form biofilms[51] and avoid the immune system[50]. They express a higher rate of cagE mRNA than their spiral counterparts[52], and by increasing the synthesis of tumor necrosis factor-alpha (TNF-α)-inducing protein (Hps), which is introduced into the cytosol and cell nuclei, they can activate nuclear factor-kappaB (NF-κB) and the expression of TNF-α and other cytokines involved in carcinogenesis[53,54]. The effect on the proliferation of gastric epithelial cells in the H. pylori coccoid forms is also stronger than in the helical forms[55], and they can induce the expression of VEGF-A and transforming growth factor-β[56]. The ability to transform into coccoid forms was also found to be characteristic of the most virulent strains of H. pylori[50,54].
It should be noted that the higher infection by coccoid forms of H. pylori in patients with GC, compared to patients with gastritis or gastric ulcer, had been mentioned by other researchers[57]. A number of studies have shown that coccoid forms of H. pylori retained urease activity[58] and the expression of such antigens as CagA, UreA, porin, components of the Cag type IV secretion system (TFSS), antigen-binding adhesin of the blood group BabA and others[59,60].
The use of immunohistochemistry in this study made it possible to detect the bacteria not only in the gastric mucus and on the surface of epithelial cells, but also within the cytoplasm of epithelial and immune cells of GM. Such intracellular expression was characterized by point inclusions giving a specific reaction with antibodies to H. pylori.
The intracellular persistence of H. pylori has been demonstrated by many investigators. They found H. pylori in the cytoplasm of epithelial cells, intercellular spaces, in the lamina propria of GM, and in the lumen of small vessels[61-63]. We assume that the point inclusions in the cytoplasm of epithelial and immune cells giving a positive reaction with antibodies to H. pylori is similar to those particle-rich cytoplasmic structure (PaCS) described earlier in the human superficial-foveolar epithelium and its metaplastic or dysplastic foci[64]. The authors found that the PaCS are a colocalization of VacA, CagA, urease, and outer membrane proteins with NOD1 receptor, ubiquitin-activating enzyme E1, polyubiquitinated proteins, proteasome components, and potentially oncogenic proteins like SHP2 and ERKs[64]. They believe that PaCS is a novel, proteasome-enriched structure arising in ribosome-rich cytoplasm at sites of H. pylori product accumulation.
We believe that the immune cells with point inclusions in the lamina propria of GM are likely to be macrophages. The data obtained by several researchers suggests this conclusion[65,66]. It is noted that even the absorbed bacteria retains their viability in macrophages, which may be associated with the violation of the phagosome maturation[66-68]. The use of confocal microscopy enabled the localization of the bacteria within the cells to be associated with the endosomal and lysosomal markers, and found that H. pylori could use the vesicles of autophagosomes (autophagic vesicles) for its own replication[63,69].
The study found that the concentration of H. pylori coccoid forms in GM was the most significant clinical factor. This factor was associated with the tumor histology, T status, N status, stage, 10-year PFS, and OS. The moderate and marked concentrations of coccoid forms of H. pylori were more often found in the diffuse type of GC (P = 0.024) and T3-4 (P = 0.04) stage. Interestingly, the high concentration of H. pylori is more frequent in Stage N1 than in N2 (at 90.0% and 53.1%, respectively, P = 0.024).
The moderate and marked concentrations of coccoid forms of H. pylori represented a prognostic factor associated with the decrease of 10-year RFS and OS from 55.6% to 26.3% (P = 0.02 and P = 0.07, respectively).
It should be noted that the results of this study do not allow us to unambiguously judge the effect of H. pylori on GC progression. A decrease in OS and DFS in patients with moderate and marked concentrations of H. pylori coccoid forms in the GM may be due to the fact that these patients had more advanced stages and more aggressive forms of GC. Meanwhile, there are more and more studies showing that H. pylori infection can promote GC progression by activating the NF-κB signaling pathway and induction of interleukin-8 secretion[70], the activation of epithelial-mesenchymal transformation[71-74] and angiogenesis[75,76], as well as increasing the invasive properties of tumor cells[77]. It can be assumed that the administration of AT before surgery contributes to the reduction of the inflammatory process activity and normalization of the adhesive properties of tumour cells, which in turn decreases metastasis risk and improves the long-term results of the treatment of GC. The data literature on the improvement of the long-term results of malignant tumours treatment when using antibacterial drugs testify in favour of this hypothesis[78-80].
CONCLUSION
The data obtained indicates that H. pylori may be associated not only with induction but also with the progression of GC. It can be assumed that the prevalence of coccoid forms of bacteria and their intracellular persistence can affect the mechanisms of tumor progression. Further appropriate studies regarding the role of H. pylori in the progression of GC are obviously advisable.
ARTICLE HIGHLIGHTS
Research background
Gastric cancer (GC) continues to be one of the most common malignant diseases in the world. It is known that Helicobacter pylori (H. pylori) infection, initiating the development of a chronic inflammatory process in the gastric mucosa (GM), is the leading risk factor for GC. At the same time, clinical studies indicate that inflammatory infiltration of the tumor stroma and surrounding tissues can have an important prognostic value and affect the long-term results of malignant neoplasm treatment.
Research motivation
It is still not clear whether H. pylori is involved only in the initiation of the tumor process in the stomach, or whether it can affect the mechanisms of tumor progression.
Research objectives
The aim of this study was to establish the features of H. pylori infection in patients with GC and their correlations with clinical and morphological characteristics of diseases and long-term results of treatment.
Research methods
In this prospective observational study, we included all patients with GC who had undergone radical surgery (R0) between May 2007 and March 2010 at the Orenburg Regional Clinical Oncology Center. Features of the H. pylori infection and its severity was determined by rapid urease test and by immunohistochemically using the antibody to H. pylori. The data obtained we compared with clinical features of GC: Stage, localization, histology, the presence of antibiotic therapy (AT) before surgery, and 10-year overall and disease-free survival.
Research results
We found H. pylori infection in the adjacent to the tumor GM in 84.5% of cases. We have established that the coccoid forms of H. pylori predominate in the GM of patients with GC. A high rate of infection by coccoid forms of H. pylori has been associated with more aggressive type of GC, advanced stage, and decline of a 10-year overall and disease-free survival. The presence of AT 1-1.5 mo before the operation was associated with an improvement in the 10-year survival rate of patients with local (T1-3N0M0), but not advanced (T3-4N1-2M0) stages of GC.
Research conclusions
These results indicate that H. pylori may be associated not only with induction but also with the progression of GC.
Research perspectives
The results obtained do not allow one to draw unambiguous conclusions about the role of H. pylori in the progression of GC. Further appropriate prospective studies regarding the role of H. pylori in the progression of GC are obviously advisable.
Footnotes
Institutional review board statement: The study was reviewed and approved by the Institutional Review Board of the Orenburg State Medical University.
Informed consent statement: All patients provided written informed consent.
Conflict-of-interest statement: The authors declare no conflicts of interests related to the publication of this study.
STROBE statement: The authors have read the STROBE Statement—checklist of items, and the manuscript was prepared and revised according to the STROBE Statement—checklist of items.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: Russian Society of Clinical Oncology (RUSSCO).
Peer-review started: April 30, 2021
First decision: June 17, 2021
Article in press: August 31, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Russia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Modun D S-Editor: Fan JR L-Editor: A P-Editor: Guo X
Contributor Information
Marina A Senchukova, Department of Oncology, Orenburg State Medical University, Orenburg 460000, Russia. masenchukova@yandex.com.
Olesya Tomchuk, Department of Histology, Cytology, Embryology, Orenburg State Medical University, Orenburg 460000, Russia.
Elena I Shurygina, Department of Pathology, Orenburg State Medical University, Orenburg 460000, Russia.
Data sharing statement
No additional data are available.
References
- 1.Thrift AP, El-Serag HB. Burden of Gastric Cancer. Clin Gastroenterol Hepatol. 2020;18:534–542. doi: 10.1016/j.cgh.2019.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Baniak N, Senger JL, Ahmed S, Kanthan SC, Kanthan R. Gastric biomarkers: a global review. World J Surg Oncol. 2016;14:212. doi: 10.1186/s12957-016-0969-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 5.Jairath NK, Farha MW, Jairath R, Harms PW, Tsoi LC, Tejasvi T. Prognostic value of intratumoral lymphocyte-to-monocyte ratio and M0 macrophage enrichment in tumor immune microenvironment of melanoma. Melanoma Manag. 2020;7:MMT51. doi: 10.2217/mmt-2020-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farha M, Jairath NK, Lawrence TS, El Naqa I. Characterization of the Tumor Immune Microenvironment Identifies M0 Macrophage-Enriched Cluster as a Poor Prognostic Factor in Hepatocellular Carcinoma. JCO Clin Cancer Inform. 2020;4:1002–1013. doi: 10.1200/CCI.20.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Man YG, Stojadinovic A, Mason J, Avital I, Bilchik A, Bruecher B, Protic M, Nissan A, Izadjoo M, Zhang X, Jewett A. Tumor-infiltrating immune cells promoting tumor invasion and metastasis: existing theories. J Cancer. 2013;4:84–95. doi: 10.7150/jca.5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang M, Li Z, Peng Y, Fang J, Fang T, Wu J, Zhou J. Identification of immune cells and mRNA associated with prognosis of gastric cancer. BMC Cancer. 2020;20:206. doi: 10.1186/s12885-020-6702-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li W, Li M, Wang H, Peng Y, Dong S, Lu Y, Wang F, Xu F, Liu L, Zhao Q. Infiltrating Immune Cells in Gastric Cancer: A Novel Predicting Model for Prognosis. J Cancer. 2021;12:965–975. doi: 10.7150/jca.51079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stanton SE, Disis ML. Clinical significance of tumor-infiltrating lymphocytes in breast cancer. J Immunother Cancer. 2016;4:59. doi: 10.1186/s40425-016-0165-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Öjlert ÅK, Halvorsen AR, Nebdal D, Lund-Iversen M, Solberg S, Brustugun OT, Lingjaerde OC, Helland Å. The immune microenvironment in non-small cell lung cancer is predictive of prognosis after surgery. Mol Oncol. 2019;13:1166–1179. doi: 10.1002/1878-0261.12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang M, Huang YK, Kong JC, Sun Y, Tantalo DG, Yeang HXA, Ying L, Yan F, Xu D, Halse H, Di Costanzo N, Gordon IR, Mitchell C, Mackay LK, Busuttil RA, Neeson PJ, Boussioutas A. High-dimensional analyses reveal a distinct role of T-cell subsets in the immune microenvironment of gastric cancer. Clin Transl Immunology. 2020;9:e1127. doi: 10.1002/cti2.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shin SJ, Kim SY, Choi YY, Son T, Cheong JH, Hyung WJ, Noh SH, Park CG, Kim HI. Mismatch Repair Status of Gastric Cancer and Its Association with the Local and Systemic Immune Response. Oncologist. 2019;24:e835–e844. doi: 10.1634/theoncologist.2018-0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takeuchi Y, Nishikawa H. Roles of regulatory T cells in cancer immunity. Int Immunol. 2016;28:401–409. doi: 10.1093/intimm/dxw025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barua S, Fang P, Sharma A, Fujimoto J, Wistuba I, Rao AUK, Lin SH. Spatial interaction of tumor cells and regulatory T cells correlates with survival in non-small cell lung cancer. Lung Cancer. 2018;117:73–79. doi: 10.1016/j.lungcan.2018.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang W, Liu K, Guo Q, Cheng J, Shen L, Cao Y, Wu J, Shi J, Cao H, Liu B, Tao K, Wang G, Cai K. Tumor-infiltrating immune cells and prognosis in gastric cancer: a systematic review and meta-analysis. Oncotarget. 2017;8:62312–62329. doi: 10.18632/oncotarget.17602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kindlund B, Sjöling Å, Yakkala C, Adamsson J, Janzon A, Hansson LE, Hermansson M, Janson P, Winqvist O, Lundin SB. CD4+ regulatory T cells in gastric cancer mucosa are proliferating and express high levels of IL-10 but little TGF-β. Gastric Cancer. 2017;20:116–125. doi: 10.1007/s10120-015-0591-z. [DOI] [PubMed] [Google Scholar]
- 18.Yuan XL, Chen L, Li MX, Dong P, Xue J, Wang J, Zhang TT, Wang XA, Zhang FM, Ge HL, Shen LS, Xu D. Elevated expression of Foxp3 in tumor-infiltrating Treg cells suppresses T-cell proliferation and contributes to gastric cancer progression in a COX-2-dependent manner. Clin Immunol. 2010;134:277–288. doi: 10.1016/j.clim.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Yang S, Liu T, Cheng Y, Bai Y, Liang G. Immune cell infiltration as a biomarker for the diagnosis and prognosis of digestive system cancer. Cancer Sci. 2019;110:3639–3649. doi: 10.1111/cas.14216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang WJ, Du Y, Zhao X, Ma LY, Cao GW. Inflammation-related factors predicting prognosis of gastric cancer. World J Gastroenterol. 2014;20:4586–4596. doi: 10.3748/wjg.v20.i16.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greten FR, Grivennikov SI. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity. 2019;51:27–41. doi: 10.1016/j.immuni.2019.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suarez-Carmona M, Lesage J, Cataldo D, Gilles C. EMT and inflammation: inseparable actors of cancer progression. Mol Oncol. 2017;11:805–823. doi: 10.1002/1878-0261.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roy S, Glaser S, Chakraborty S. Inflammation and Progression of Cholangiocarcinoma: Role of Angiogenic and Lymphangiogenic Mechanisms. Front Med (Lausanne) 2019;6:293. doi: 10.3389/fmed.2019.00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Brito BB, da Silva FAF, Soares AS, Pereira VA, Santos MLC, Sampaio MM, Neves PHM, de Melo FF. Pathogenesis and clinical management of Helicobacter pylori gastric infection. World J Gastroenterol. 2019;25:5578–5589. doi: 10.3748/wjg.v25.i37.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Šterbenc A, Jarc E, Poljak M, Homan M. Helicobacter pylori virulence genes. World J Gastroenterol. 2019;25:4870–4884. doi: 10.3748/wjg.v25.i33.4870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gu H. Role of Flagella in the Pathogenesis of Helicobacter pylori. Curr Microbiol. 2017;74:863–869. doi: 10.1007/s00284-017-1256-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ansari S, Yamaoka Y. Helicobacter pylori Virulence Factors Exploiting Gastric Colonization and its Pathogenicity. Toxins (Basel) 2019;11 doi: 10.3390/toxins11110677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Necchi V, Candusso ME, Tava F, Luinetti O, Ventura U, Fiocca R, Ricci V, Solcia E. Intracellular, intercellular, and stromal invasion of gastric mucosa, preneoplastic lesions, and cancer by Helicobacter pylori. Gastroenterology. 2007;132:1009–1023. doi: 10.1053/j.gastro.2007.01.049. [DOI] [PubMed] [Google Scholar]
- 29.Semino-Mora C, Doi SQ, Marty A, Simko V, Carlstedt I, Dubois A. Intracellular and interstitial expression of Helicobacter pylori virulence genes in gastric precancerous intestinal metaplasia and adenocarcinoma. J Infect Dis. 2003;187:1165–1177. doi: 10.1086/368133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Servetas SL, Bridge DR, Merrell DS. Molecular mechanisms of gastric cancer initiation and progression by Helicobacter pylori. Curr Opin Infect Dis. 2016;29:304–310. doi: 10.1097/QCO.0000000000000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsuda M, Asaka M, Kato M, Matsushima R, Fujimori K, Akino K, Kikuchi S, Lin Y, Sakamoto N. Effect on Helicobacter pylori eradication therapy against gastric cancer in Japan. Helicobacter. 2017;22 doi: 10.1111/hel.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Franco AT, Johnston E, Krishna U, Yamaoka Y, Israel DA, Nagy TA, Wroblewski LE, Piazuelo MB, Correa P, Peek RM Jr. Regulation of gastric carcinogenesis by Helicobacter pylori virulence factors. Cancer Res. 2008;68:379–387. doi: 10.1158/0008-5472.CAN-07-0824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Noto JM, Gaddy JA, Lee JY, Piazuelo MB, Friedman DB, Colvin DC, Romero-Gallo J, Suarez G, Loh J, Slaughter JC, Tan S, Morgan DR, Wilson KT, Bravo LE, Correa P, Cover TL, Amieva MR, Peek RM Jr. Iron deficiency accelerates Helicobacter pylori-induced carcinogenesis in rodents and humans. J Clin Invest. 2013;123:479–492. doi: 10.1172/JCI64373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arif M, Syed S. Association of Helicobacter pylori with carcinoma of stomach. J Pak Med Assoc. 2007;57:337–341. [PubMed] [Google Scholar]
- 36.Gong EJ, Lee JY, Bae SE, Park YS, Choi KD, Song HJ, Lee GH, Jung HY, Jeong WJ, Cheon GJ, Yook JH, Kim BS. Characteristics of non-cardia gastric cancer with a high serum anti-Helicobacter pylori IgG titer and its association with diffuse-type histology. PLoS One. 2018;13:e0195264. doi: 10.1371/journal.pone.0195264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cho SJ, Choi IJ, Kim CG, Lee JY, Kook MC, Seong MW, Park SR, Lee JS, Kim YW, Ryu KW, Lee JH, Nam BH, Park YI. Helicobacter pylori Seropositivity Is Associated with Gastric Cancer Regardless of Tumor Subtype in Korea. Gut Liver. 2010;4:466–474. doi: 10.5009/gnl.2010.4.4.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sarker KK, Kabir MJ, Bhuyian AKMMU, Alam MS, Chowdhury FR, Ahad MA, Rahman MA, Rahman MM. H. pylori infection and gastric cancer in Bangladesh: a case-control study. Int J Surg Oncol (N Y) 2017;2:e44. doi: 10.1097/IJ9.0000000000000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fang WL, Huang KH, Chang SC, Lin CH, Chen MH, Chao Y, Lo SS, Li AF, Wu CW, Shyr YM. Comparison of the Clinicopathological Characteristics and Genetic Alterations Between Patients with Gastric Cancer with or Without Helicobacter pylori Infection. Oncologist. 2019;24:e845–e853. doi: 10.1634/theoncologist.2018-0742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khan MR, Farooqi NB, Shahzad N. Is Proximal Gastric Cancer A Different Entity From Distal Gastric Cancer? J Ayub Med Coll Abbottabad. 2020;32:194–197. [PubMed] [Google Scholar]
- 41.Fang X, Liu K, Cai J, Luo F, Yuan F, Chen P. Positive Helicobacter pylori status is associated with better overall survival for gastric cancer patients: evidence from case-cohort studies. Oncotarget. 2017;8:79604–79617. doi: 10.18632/oncotarget.18758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li G, Yu S, Xu J, Zhang X, Ye J, Wang Z, He Y. The prognostic role of Helicobacter pylori in gastric cancer patients: A meta-analysis. Clin Res Hepatol Gastroenterol. 2019;43:216–224. doi: 10.1016/j.clinre.2018.08.012. [DOI] [PubMed] [Google Scholar]
- 43.Wang J, Liu X. Correlation Analysis between Helicobacter pylori Infection Status and Tumor Clinical Pathology as Well as Prognosis of Gastric Cancer Patients. Iran J Public Health. 2018;47:1529–1536. [PMC free article] [PubMed] [Google Scholar]
- 44.Li G, Wang Z, Xu J, Cui J, Cai S, Zhan W, He Y. Gastric cancer patients with Helicobacter pylori infection have a poor prognosis. J Surg Oncol. 2013;108:421–426. doi: 10.1002/jso.23417. [DOI] [PubMed] [Google Scholar]
- 45.Zhang XY, Zhang PY, Aboul-Soud MA. From inflammation to gastric cancer: Role of Helicobacter pylori. Oncol Lett. 2017;13:543–548. doi: 10.3892/ol.2016.5506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marsh AM, Buicko JL. Gastric Resection. 2021 Jul 30. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan–. [Google Scholar]
- 47.Reshetnyak VI, Reshetnyak TM. Significance of dormant forms of Helicobacter pylori in ulcerogenesis. World J Gastroenterol. 2017;23:4867–4878. doi: 10.3748/wjg.v23.i27.4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ierardi E, Losurdo G, Mileti A, Paolillo R, Giorgio F, Principi M, Di Leo A. The Puzzle of Coccoid Forms of Helicobacter pylori: Beyond Basic Science. Antibiotics (Basel) 2020;9 doi: 10.3390/antibiotics9060293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kadkhodaei S, Siavoshi F, Akbari Noghabi K. Mucoid and coccoid Helicobacter pylori with fast growth and antibiotic resistance. Helicobacter. 2020;25:e12678. doi: 10.1111/hel.12678. [DOI] [PubMed] [Google Scholar]
- 50.Krzyżek P, Grande R. Transformation of Helicobacter pylori into Coccoid Forms as a Challenge for Research Determining Activity of Antimicrobial Substances. Pathogens. 2020;9 doi: 10.3390/pathogens9030184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Percival SL, Suleman L. Biofilms and Helicobacter pylori: Dissemination and persistence within the environment and host. World J Gastrointest Pathophysiol. 2014;5:122–132. doi: 10.4291/wjgp.v5.i3.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Poursina F, Faghri J, Moghim S, Zarkesh-Esfahani H, Nasr-Esfahani B, Fazeli H, Hasanzadeh A, Safaei HG. Assessment of cagE and babA mRNA expression during morphological conversion of Helicobacter pylori from spiral to coccoid. Curr Microbiol. 2013;66:406–413. doi: 10.1007/s00284-012-0280-7. [DOI] [PubMed] [Google Scholar]
- 53.Loke MF, Ng CG, Vilashni Y, Lim J, Ho B. Understanding the dimorphic lifestyles of human gastric pathogen Helicobacter pylori using the SWATH-based proteomics approach. Sci Rep. 2016;6:26784. doi: 10.1038/srep26784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gladyshev N, Taame M, Kravtsov V. Clinical and laboratory importance of detecting Helicobacter pylori coccoid forms for the selection of treatment. Prz Gastroenterol. 2020;15:294–300. doi: 10.5114/pg.2020.101557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li N, Han L, Chen J, Lin X, Chen H, She F. Proliferative and apoptotic effects of gastric epithelial cells induced by coccoid Helicobacter pylori. J Basic Microbiol. 2013;53:147–155. doi: 10.1002/jobm.201100370. [DOI] [PubMed] [Google Scholar]
- 56.Prevete N, Rossi FW, Rivellese F, Lamacchia D, Pelosi C, Lobasso A, Necchi V, Solcia E, Fiocca R, Ceppa P, Staibano S, Mascolo M, D'Argenio G, Romano M, Ricci V, Marone G, De Paulis A. Helicobacter pylori HP(2-20) induces eosinophil activation and accumulation in superficial gastric mucosa and stimulates VEGF-alpha and TGF-beta release by interacting with formyl-peptide receptors. Int J Immunopathol Pharmacol. 2013;26:647–662. doi: 10.1177/039463201302600308. [DOI] [PubMed] [Google Scholar]
- 57.Chan WY, Hui PK, Leung KM, Chow J, Kwok F, Ng CS. Coccoid forms of Helicobacter pylori in the human stomach. Am J Clin Pathol. 1994;102:503–507. doi: 10.1093/ajcp/102.4.503. [DOI] [PubMed] [Google Scholar]
- 58.Can F, Karahan C, Dolapci I, Demirbilek M, Tekeli A, Arslan H. Urease activity and urea gene sequencing of coccoid forms of H. pylori induced by different factors. Curr Microbiol. 2008;56:150–155. doi: 10.1007/s00284-007-9047-y. [DOI] [PubMed] [Google Scholar]
- 59.Hirukawa S, Sagara H, Kaneto S, Kondo T, Kiga K, Sanada T, Kiyono H, Mimuro H. Characterization of morphological conversion of Helicobacter pylori under anaerobic conditions. Microbiol Immunol. 2018;62:221–228. doi: 10.1111/1348-0421.12582. [DOI] [PubMed] [Google Scholar]
- 60.Morales-Espinosa R, Delgado G, Serrano LR, Castillo E, Santiago CA, Hernández-Castro R, Gonzalez-Pedraza A, Mendez JL, Mundo-Gallardo LF, Manzo-Merino J, Ayala S, Cravioto A. High expression of Helicobacter pylori VapD in both the intracellular environment and biopsies from gastric patients with severity. PLoS One. 2020;15:e0230220. doi: 10.1371/journal.pone.0230220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ozbek A, Ozbek E, Dursun H, Kalkan Y, Demirci T. Can Helicobacter pylori invade human gastric mucosa?: an in vivo study using electron microscopy, immunohistochemical methods, and real-time polymerase chain reaction. J Clin Gastroenterol. 2010;44:416–422. doi: 10.1097/MCG.0b013e3181c21c69. [DOI] [PubMed] [Google Scholar]
- 62.Dudley J, Wieczorek T, Selig M, Cheung H, Shen J, Odze R, Deshpande V, Zukerberg L. Clinicopathological characteristics of invasive gastric Helicobacter pylori. Hum Pathol. 2017;61:19–25. doi: 10.1016/j.humpath.2016.09.029. [DOI] [PubMed] [Google Scholar]
- 63.Huang Y, Wang QL, Cheng DD, Xu WT, Lu NH. Adhesion and Invasion of Gastric Mucosa Epithelial Cells by Helicobacter pylori. Front Cell Infect Microbiol. 2016;6:159. doi: 10.3389/fcimb.2016.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Necchi V, Sommi P, Ricci V, Solcia E. In vivo accumulation of Helicobacter pylori products, NOD1, ubiquitinated proteins and proteasome in a novel cytoplasmic structure. PLoS One. 2010;5:e9716. doi: 10.1371/journal.pone.0009716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ito T, Kobayashi D, Uchida K, Takemura T, Nagaoka S, Kobayashi I, Yokoyama T, Ishige I, Ishige Y, Ishida N, Furukawa A, Muraoka H, Ikeda S, Sekine M, Ando N, Suzuki Y, Yamada T, Suzuki T, Eishi Y. Helicobacter pylori invades the gastric mucosa and translocates to the gastric lymph nodes. Lab Invest. 2008;88:664–681. doi: 10.1038/labinvest.2008.33. [DOI] [PubMed] [Google Scholar]
- 66.Keep S, Borlace G, Butler R, Brooks D. Role of immune serum in the killing of Helicobacter pylori by macrophages. Helicobacter. 2010;15:177–183. doi: 10.1111/j.1523-5378.2010.00750.x. [DOI] [PubMed] [Google Scholar]
- 67.Sit WY, Chen YA, Chen YL, Lai CH, Wang WC. Cellular evasion strategies of Helicobacter pylori in regulating its intracellular fate. Semin Cell Dev Biol. 2020;101:59–67. doi: 10.1016/j.semcdb.2020.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pagliari M, Munari F, Toffoletto M, Lonardi S, Chemello F, Codolo G, Millino C, Della Bella C, Pacchioni B, Vermi W, Fassan M, de Bernard M, Cagnin S. Helicobacter pylori Affects the Antigen Presentation Activity of Macrophages Modulating the Expression of the Immune Receptor CD300E through miR-4270. Front Immunol. 2017;8:1288. doi: 10.3389/fimmu.2017.01288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chu YT, Wang YH, Wu JJ, Lei HY. Invasion and multiplication of Helicobacter pylori in gastric epithelial cells and implications for antibiotic resistance. Infect Immun. 2010;78:4157–4165. doi: 10.1128/IAI.00524-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang X, Zhang J, Lin Y, Xu K, Li N, Chen H, She F. Analysis of the relationship between invasive capability of Helicobacter pylori and gastroduodenal diseases. J Med Microbiol. 2015;64:498–506. doi: 10.1099/jmm.0.000049. [DOI] [PubMed] [Google Scholar]
- 71.Sougleri IS, Papadakos KS, Zadik MP, Mavri-Vavagianni M, Mentis AF, Sgouras DN. Helicobacter pylori CagA protein induces factors involved in the epithelial to mesenchymal transition (EMT) in infected gastric epithelial cells in an EPIYA- phosphorylation-dependent manner. FEBS J. 2016;283:206–220. doi: 10.1111/febs.13592. [DOI] [PubMed] [Google Scholar]
- 72.Choi YJ, Kim N, Chang H, Lee HS, Park SM, Park JH, Shin CM, Kim JM, Kim JS, Lee DH, Jung HC. Helicobacter pylori-induced epithelial-mesenchymal transition, a potential role of gastric cancer initiation and an emergence of stem cells. Carcinogenesis. 2015;36:553–563. doi: 10.1093/carcin/bgv022. [DOI] [PubMed] [Google Scholar]
- 73.Krzysiek-Maczka G, Targosz A, Szczyrk U, Wrobel T, Strzalka M, Brzozowski T, Czyz J, Ptak-Belowska A. Long-Term Helicobacter pylori Infection Switches Gastric Epithelium Reprogramming Towards Cancer Stem Cell-Related Differentiation Program in Hp-Activated Gastric Fibroblast-TGFβ Dependent Manner. Microorganisms. 2020;8 doi: 10.3390/microorganisms8101519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Molina-Castro S, Ramírez-Mayorga V, Alpízar-Alpízar W. Priming the seed: Helicobacter pylori alters epithelial cell invasiveness in early gastric carcinogenesis. World J Gastrointest Oncol. 2018;10:231–243. doi: 10.4251/wjgo.v10.i9.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tafreshi M, Guan J, Gorrell RJ, Chew N, Xin Y, Deswaerte V, Rohde M, Daly RJ, Peek RM Jr, Jenkins BJ, Davies EM, Kwok T. Helicobacter pylori Type IV Secretion System and Its Adhesin Subunit, CagL, Mediate Potent Inflammatory Responses in Primary Human Endothelial Cells. Front Cell Infect Microbiol. 2018;8:22. doi: 10.3389/fcimb.2018.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu N, Zhou N, Chai N, Liu X, Jiang H, Wu Q, Li Q. Helicobacter pylori promotes angiogenesis depending on Wnt/beta-catenin-mediated vascular endothelial growth factor via the cyclooxygenase-2 pathway in gastric cancer. BMC Cancer. 2016;16:321. doi: 10.1186/s12885-016-2351-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li H, Xu CX, Gong RJ, Chi JS, Liu P, Liu XM. How does Helicobacter pylori cause gastric cancer through connexins: An opinion review. World J Gastroenterol. 2019;25:5220–5232. doi: 10.3748/wjg.v25.i35.5220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Takemori N, Ooi HK, Imai G, Hoshino K, Saio M. Possible mechanisms of action of clarithromycin and its clinical application as a repurposing drug for treating multiple myeloma. Ecancermedicalscience. 2020;14:1088. doi: 10.3332/ecancer.2020.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhou B, Xia M, Wang B, Thapa N, Gan L, Sun C, Guo E, Huang J, Lu Y, Cai H. Clarithromycin synergizes with cisplatin to inhibit ovarian cancer growth in vitro and in vivo. J Ovarian Res. 2019;12:107. doi: 10.1186/s13048-019-0570-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Van Nuffel AM, Sukhatme V, Pantziarka P, Meheus L, Sukhatme VP, Bouche G. Repurposing Drugs in Oncology (ReDO)-clarithromycin as an anti-cancer agent. Ecancermedicalscience. 2015;9:513. doi: 10.3332/ecancer.2015.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.