ABSTRACT
Aeromonas salmonicida is an aquatic pathogen that can infect a variety of fish. Phage therapy has been applied to treat bacterial infections. In this study, we obtained three A. salmonicida subsp. masoucida phage isolates from sewage, and one phage (vB_AsM_ZHF) exhibited the best antibacterial effect, based on in vitro kinetics experiments. Sequencing indicated that the vB_AsM_ZHF genome is 161,887 bp (41.24% C+G content) with 237 predicted open reading frames. No antibiotic resistance or virulence genes were detected in the complete genome, which is a requirement for phage therapy safety. Intraperitoneal injection of phage vB_AsM_ZHF into turbot at 8 × 104 PFU/fish rescued turbot from A. salmonicida subsp. masoucida injection and reduced the bacterial burden by 1 order of magnitude. Injection of vB_AsM_ZHF also decreased levels of inflammatory cell infiltration in muscle tissue, cytokines interleukin-1β (IL-1β), tumor necrosis factor alpha (TNF-α), and gamma interferon (IFN-γ) in serum and the expression of the inflammatory factors IL-1β, IL-6, IFN-γ, transforming growth factor β, TNF-α, and hepcidin in the liver, spleen, and head kidney of turbot. Phage vB_AsM_ZHF demonstrated antibacterial ability in vitro and in vivo and significantly reduced mortality in turbot challenged by A. salmonicida subsp. masoucida. This study revealed that phage vB_AsM_ZHF can effectively treat the infection caused by A. salmonicida subsp. masoucida in turbot.
IMPORTANCE A. salmonicida is an aquatic pathogen that can infect different fish and causes economic loss to the global aquaculture industry. Clinical strains of A. salmonicida have developed multidrug resistance, and phage therapy is being evaluated for controlling bacterial infections. Phages are biological antibacterial agents and have the potential to be therapeutic agents against multidrug-resistant bacteria. In this study, three A. salmonicida subsp. masoucida phages were isolated from sewage, and their biological behaviors were characterized. The newly isolated phage vB_AsM_ZHF could inhibit A. salmonicida subsp. masoucida infection in vitro and in vivo, suggesting that it may be an alternative strategy to antibiotics for protecting fish against multidrug-resistant A. salmonicida subsp. masoucida in the aquaculture industry.
KEYWORDS: Aeromonas salmonicida subsp. masoucida, antibacterial, aquaculture, phage therapy, turbot, Scophthalmus maximus
INTRODUCTION
Aeromonas salmonicida is a Gram-negative bacterium and the only nonmotile species in the genus. A. salmonicida is widespread in aquatic environments and infects multiple species of commercial fish, including turbot (Scophthalmus maximus), salmon (Oncorhynchus), Atlantic cod (Gadus morhua), rockfish (Epinephelinae), and sea bream (Sparidae) (1–3). A. salmonicida causes furunculosis and bacterial septicemia with granuloma in muscle, ulceration in skin, and inflammation and necrosis in liver, kidney, and spleen (1). Antibiotics (e.g., β-lactams, florfenicol, quinolones, tetracyclines, and folate-pathway inhibitors) prevent and control A. salmonicida infections in aquaculture. However, the efficacy of antibiotics declines as bacteria acquire antibiotic resistance genes (4). A. salmonicida isolated from turbot has developed resistance to multiple antibiotics (5). Therefore, some alternative strategies are needed to prevent and control multidrug-resistant (MDR) bacteria in aquaculture.
Bacteriophages are viruses that infect bacteria. They were first discovered by Felix d’Herelle and Frederick W. Twort (6). Phage therapy has been used to control human, animal, and plant pathogens, and many successful treatments of MDR bacteria have been reported (7). Commercial phage products such as Salmonlex, Listex, Listshield, and Ecoshield significantly reduce the number of pathogens during food storage, including Salmonella, Escherichia coli, and Listeria monocytogenes during food storage (8). Consequently, phage therapy has the potential to be used in clinics as a biological antibacterial agent.
In the aquaculture industry, phages that infect Vibrio pathogens, including V. parahaemolyticus, V. harveyi, V. anguillarum, and V. splendidus, have been isolated. Some of these phages exhibit therapeutic effects when administered to aquatic organisms such as shrimp and sea cucumber (9, 10). Phages that infect A. salmonicida have also been isolated, and their antibacterial activities have been verified in vitro (11). Phages O, R, and B fail to control A. salmonicida subsp. salmonicida in Atlantic salmon (12). Silve et al. (13) and Imbeault et al. (14) reported that adding A. salmonicida phages to tanks with juvenile Senegalese sole and brook trout decreased the mortality rate in fish challenges with pathogenic bacteria. In addition, the phages reduced the level of host bacteria in the water. Kim et al. (15) demonstrated that intramuscular (i.m.) injection with phage PAS-1 reduced the mortality rate by approximately 30% in rainbow trout exposed to A. salmonicida subsp. salmonicida. However, the treatment of A. salmonicida subsp. masoucida infections in turbot by phage therapy has still not been studied.
This study reports that a virulent A. salmonicida subsp. masoucida phage vB_AsM_ZHF (ZHF) and two other phages were isolated from sewage and identified as members of the Myoviridae family by transmission electron microscopy (TEM). The biological characteristics and complete genome of ZHF were determined. Antibacterial ability was verified in vitro and in vivo. The mortality of turbot challenged with A. salmonicida was reduced by intraperitoneal injection of ZHF. These results suggest that phage therapy with ZHF is a potential strategy to prevent and control bacterial diseases in aquaculture.
RESULTS
Isolation and characterization of A. salmonicida subsp. masoucida AS01.
The pathogen of white smooth colonies was isolated from turbot with skin ulcers in Shandong, China, and named AS01. As shown in Fig. S1A in the supplemental material, strain AS01 was identified as A. salmonicida through phylogenetic tree constructed by 16s rRNA, but it is difficult to distinguish its subspecies (Table 1). The KPI system (see Table S2) and sequence of virulence array protein (vapA) gene (see Fig. S1B) identified strain AS01 as Aeromonas salmonicida subsp. masoucida.
TABLE 1.
Strains used in this study
| Bacterial species | Strain | Accession | Sourcea |
|---|---|---|---|
| Aeromonas salmonicida subsp. masoucida | AS01 | NCBI MZ087214 | Turbot, Shandong, ChinaA,B |
| AS14 | NCBI MZ087944 | Jerk filefish, Shandong, ChinaB | |
| 2018085 | CCTCC M 2018085 | Tongue sole, Shandong, ChinaC | |
| SM1 | NCBI KF263654 | Sewage, Shanghai, ChinaD | |
| Aeromonas salmonicida subsp. achromogene | N-2-2-1 | NCBI KU601307 | Chinese mitten crab, Shanghai, ChinaD |
| I-M-3-1 | NCBI KU570316 | Chinese mitten crab, Shanghai, ChinaD | |
| Aeromonas salmonicida subsp. salmonicida | I-N-3-1 | NCBI KU570320 | Aquaculture water, Shanghai, ChinaD |
| LJ-a | NCBI MZ540812 | Salmon, Shandong, ChinaC | |
| Aeromonas hydrophila | 0856 | NCBI FJ595678 | Turbot, Yantai, ChinaB |
| Edwardsiella tarda | EIB202 | NCBI NC_013508 | Turbot, Yantai, ChinaB |
| Vibrio anguillarum | 425 | NCBI NZ_CP020534 | Turbot, Yellow Sea, ChinaB |
| Pseudomonas plecoglossicida | XSDHY-P | NCBI CP031146 | Large yellow croaker, Zhejiang, ChinaE |
| Vibrio harveyi | HS-T | NCBI MZ093615 | Large yellow croaker, Zhejiang, ChinaB |
| Escherichia coli | 25922 | NCBI X80724 | ATCCF |
Superscripts: A, strain used on the enrichment for phage isolation; B, strains stored in our lab; C, strains provided by Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences; D, strains provided by Shanghai Ocean University; E, strains provided by Zhejiang Ocean University; F, strains purchased from the American Type Culture Collection (ATCC).
Isolation of three A. salmonicida subsp. masoucida phages.
Phages were isolated from the sewage of an aquatic product market in Shanghai and chosen for expansion by their plaque characteristics. Phage morphology and size were determined by TEM to distinguish the isolates (Fig. 1B). Three isolates were characterized and named vB_AsM_ZHA (ZHA), vB_AsM_ZHD (ZHD), and vB_AsM_ZHF (ZHF). All isolates were assigned to the Myoviridae family, order Caudovirales, based on morphology characteristics of the International Committee on Taxonomy of Viruses (ICTV) classification system (16). The sizes of the three phages are shown in Table S1. Phage ZHF formed clear plaques (3.28 ± 0.25 mm in diameter) with distinct halos on the double-layer agar plate, whereas ZHA and ZHD formed turbid plaques of 2.02 ± 0.47 mm and 0.51 ± 0.15 mm in diameter, respectively (Fig. 1A; see also Table S1). Each phage can infect A. salmonicida, including subspecies masoucida and achromogene, but they cannot infect A. salmonicida subsp. salmonicida or other tested species, including A. hydrophila, Edwardsiella tarda, Vibrio anguillarum, Pseudomonas plecoglossicida, Vibrio harveyi, and Escherichia coli (see Fig. S2).
FIG 1.
Plaque and morphology of the A. salmonicida phages vB_AsM_ZHA, vB_AsM_ZHD, and vB_AsM_ZHF. (A) Phage plaque on the double-layer agar plate. Scale bar, 1 cm. (B) Morphology of phages observed by TEM. Scale bar, 100 nm.
Biological characteristics of phages.
One-step growth curves show that growth rates are similar among the three phages. The rise periods of phages ZHA, ZHD, and ZHF are 30, 30, and 20 min, respectively, and the burst sizes are 110, 39, and 284 PFU/cell (Fig. 2A).
FIG 2.
Biological characteristics of A. salmonicida phages vB_AsM_ZHA, vB_AsM_ZHD, and vB_AsM_ZHF. (A) One-step growth curve. (B) pH stability of phages treated with different pH 2 to 11 for 2 h. (C) Thermal stability of phages treated with different temperatures (4, 28, 36, 50, 60, and 70°C) for 2 h. (D) Phage titers at MOIs of 100, 10, 1, 0.1, 0.01, and 0.001. The lowercase letters above the bars indicate significant differences within groups P < 0.05. The data represent the means and SD (n = 3).
Phages were propagated under various environments to evaluate stability, and each phage remains active at pH levels from 6 to 8 at 36°C (Fig. 2B and C). Phage titers drop sharply at pH levels less than 3 and temperatures higher than 50°C. Under conditions of high pH, phage titers decrease by a third.
The optimal multiplicity of infection (MOI) describes the number of host bacteria required for phage growth. The highest yield of phage ZHA was approximately 2 × 109 PFU/ml at an MOI of 1. The optimal MOI of ZHD and ZHF was 0.1, with phage titers reaching approximately 3 × 109 PFU/ml (Fig. 2D).
Growth curves of A. salmonicida subsp. masoucida infected with phages at various MOIs were measured by monitoring absorbance for 60 h to evaluate the in vitro antibacterial efficacy of each phage. Negative controls received phosphate-buffered saline (PBS) instead of phage, and positive controls received polymyxin B (Col). Each phage isolate exhibited acceptable antibacterial ability in the first 25 h at 0.01 to 100 MOI (Fig. 3). A. salmonicida infected with phage ZHD at 100 MOI began to grow again at 25 h, whereas cultures infected with ZHA and ZHF recovered at 40 h and 45 h, respectively. Bacteria infected with phage ZHF have the lowest recovery at 60 h. This isolate also has the largest burst size, the longest time until bacterial regrowth, and the lowest optimal MOI. These characteristics suggest that ZHF has the best antibacterial activity on the in vitro growth of A. salmonicida subsp. masoucida. Therefore, phage ZHF was used in the following experiments.
FIG 3.
Growth curves displaying the antibacterial effect of phages (vB_AsM_ZHA, vB_AsM_ZHD, and vB_AsM_ZHF) on A. salmonicida (AS) growth at MOIs of 100, 10, 1, 0.1, and 0.01. The negative control was host bacteria only; the positive control included host bacteria and polymyxin B (Col). Values indicate means (n = 3).
Genomic analysis of phage vB_AsM_ZHF.
The genome of vB_AsM_ZHF was sequenced and submitted to GenBank under accession number MW584871. A circle map of the genome is displayed in Fig. 4A. The phage ZHF genome is a linear double-strand DNA consisting of 161,887 bp and a G+C content of 41.24%. There are 237 predicted open reading frames (ORFs) accounting for 149,784 bp (92.52%) of the genome, with an average length of 632 bp. The ORFs consist of 40 structural and packing protein genes, 27 DNA replication/modification genes, 16 transcriptional regulation genes, 14 metabolism genes, 3 host-lysis genes, and 107 hypothetical protein genes. No virulence or antibiotic resistance genes are detected in analyses using the Virulence Factor Database (VFDB) and the Comprehensive Antibiotic Research Database (CARD). ZHF appears to be a virulent phage because no integrase gene is found in the genome, making it a phage suitable for phage therapy.
FIG 4.
Genome characteristics and comparative analysis of A. salmonicida phage vB_AsM_ZHF. (A) Circle map of vB_AsM_ZHF genome. GC skew and GC content are showed in the inner circle. ORFs are visualized in the outer circle by arrows in the transcription direction. (B) Heatmap of the ANI values for 10 complete genomes of A. salmonicida phage phiAS4 (HM452125), A. salmonicida phage AS-gz (NC_042019), Stenotrophomonas phage IME13 (JX306041.1), A. salmonicida phage Aes508 (JN377894), A. salmonicida phage 25 (DQ529280), Escherichia phage CJ20 (MT533174.1), Klebsiella phage PKO111 (NC_031095), Serratia phage CBH8 (MF036691), and Acinetobacter phage vB_ApiM_fHyAci03 (NC_049438). The ANI values, ranging from 60 (blue) to 100 (red), were calculated by OrthoANI.
The average nucleotide identity (ANI) values of 10 complete genomes were calculated and are presented as a heatmap to illustrate the similarities between ZHF and other phages (Fig. 4B). No known phage genome exhibited 100% identical sequence homology with the ZHF genome, confirming its novelty. Phage ZHF has a high ANI value compared to other Aeromonas phages. Whole genomic sequence alignment shows that phage ZHF has more cross wires with Aeromonas phages compared to other phage strains (see Fig. S3). Although multiple genes of A. salmonicida phage AS-gz are ordered differently along the genome, they retain high ANI values with ZHF. Many genes of A. salmonicida phage phiAS4 and AES508 and Stenotrophomonas phage IME13 have gene orders identical to ZHF. Other analyzed phages have structural proteins with homology to those in ZHF, but fewer similarities with other proteins.
Therapeutic efficacy of phage vB_AsM_ZHF against A. salmonicida subsp. masoucida infection in turbot.
A challenge model of A. salmonicida subsp. masoucida was established in turbot (see Fig. S4). Groups of 20 turbot each were challenged with 1 × 104, 1 × 105, or 1 × 106 CFU/fish by i.m. injection. The survival rates are 60, 10, and 0%, respectively, at 20 days postinoculation (dpi). We chose 8 × 104 CFU/fish as the challenge dose for A. salmonicida subsp. masoucida in the experiments that follow.
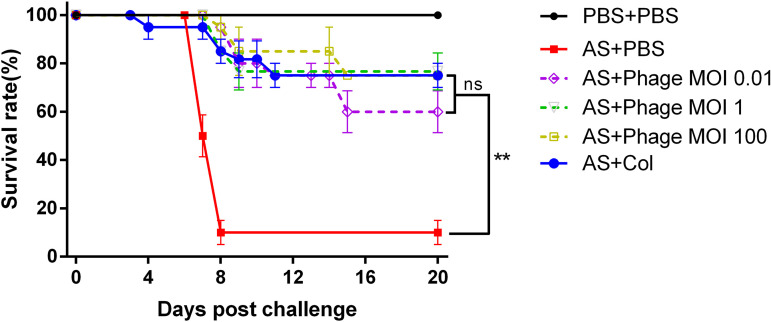
Turbot infected with A. salmonicida subsp. masoucida were administered ZHF at different MOIs (0.01, 1, and 100) by i.p. injection, and the mortality of each group was recorded to measure the therapeutic efficacy of ZHF. Negative controls received i.p. injections of PBS instead of ZHF, and positive controls received polymyxin B. As shown in Fig. 5, the survival rates of phage-treated groups (8 × 102, 8 × 104, and 8 × 106 PFU/fish) are 60, 70, and 75%, respectively. These rates are similar to the positive control (75%). These results suggest that ZHF has the potential to protect turbot against A. salmonicida subsp. masoucida infection.
FIG 5.
Survival of turbot challenged by A. salmonicida (i.m.) with 8 × 104 CFU/fish and treated with phage vB_AsM_ZHF (i.p.) at doses of 8 × 102 PFU/fish (MOI of 0.01), 8 × 104 PFU/fish (MOI of 1), and 8 × 106 PFU/fish (MOI of 100). Negative control, A. salmonicida (i.m.) and PBS (i.p.); positive control, A. salmonicida (i.m.) and Col (i.p.); blank control, PBS (i.m. and i.p.). The values indicate the means and SD (n = 3). The statistical significance of final survival rates (20 dpi) was set as P ≤ 0.01 (**).
The bacterial burden and phage titer in vivo were assessed to characterize the dynamics of ZHF against A. salmonicida subsp. masoucida in turbot (Fig. 6). There were similar change trends of bacteria burden and phage titer in the liver and spleen. Bacterial colonization is not detected in the liver and spleen during the first 2 days but gradually increased over the next few days. The bacterial burden of the treatment group rose to approximately 103 CFU/g compared to the negative control of 104 CFU/g. In head kidney tissues, the bacterial burden of the treatment group remains lower than that of the negative control over 8 days with steady growth. The phage titer exhibits no difference between the treatment and control groups in the first 2 days, although the phage titer of the treatment group decreases more slowly than that of the control group. In the liver and spleen, the phage titer decreases rapidly by 8 dpi in the treated group, whereas phage ZHF is entirely cleared in controls that were not challenged with A. salmonicida. ZHF is cleared more slowly from the head kidney than from other organs. In summary, ZHF effectively reduces the bacterial burden in infected A. salmonicida subsp. masoucida and delays the clearance of phages by the immune system in turbot.
FIG 6.
Bacterial burdens (left axis) and phage titers (right axis) in the liver, spleen, and kidney at 1, 2, 4, and 8 dpi. Treatment group, AS 8 × 104 CFU/fish (i.m.) and ZHF 8 × 104 PFU/fish (i.p.); negative control of bacterial burden, AS 8 × 104 CFU/fish (i.m.); negative control of phage titer, ZHF 8 × 104 PFU/fish (i.p.). The values indicate means and SD (n = 3).
Anti-inflammatory effect of A. salmonicida subsp. masoucida phage therapy in vivo.
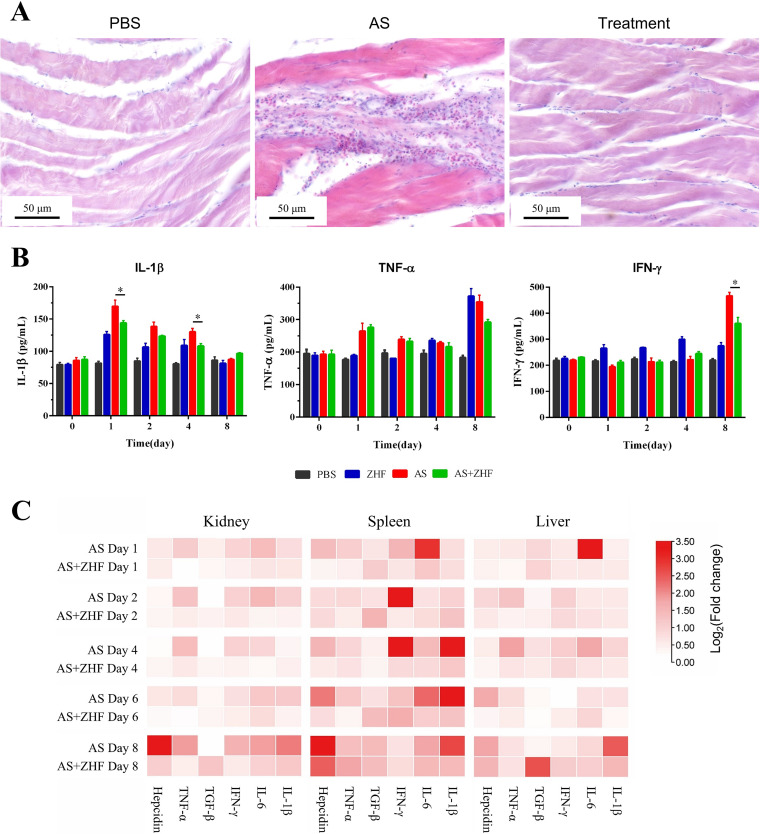
Histopathology of muscle tissue (Fig. 7A) shows that A. salmonicida subsp. masoucida causes infiltration of inflammatory cells by 8 dpi. However, phage therapy significantly reduces the number of inflammatory cells to levels seen in the PBS group. ZHF decreases the level of interleukin-1β (IL-1β) in serum relative to the bacterial infection group 1 to 4 dpi, and the serum levels of TNF-α and gamma interferon (IFN-γ) are lower than the control group at 8 dpi (Fig. 7B). The relative expression levels of inflammatory factors (IL-1β, IL-6, IFN-γ, transforming growth factor β [TGF-β], tumor necrosis factor alpha [TNF-α], and hepcidin) on 1, 2, 4, 8 dpi in the liver, spleen, and head kidney were measured by real-time quantitative PCR (RT-qPCR) and visualized by heatmaps (Fig. 7C). TGF-β levels in the treatment group are higher than the infection group, especially at 8 dpi. In contrast, the levels of IL-1β at 8 dpi, IL-6 at 1 dpi, IFN-γ in spleen at 2 dpi and 4 dpi, and hepcidin at 8 dpi in the treatment group are lower than those in the infection group. These results suggest that phage therapy effectively reduces the level of inflammation in vivo.
FIG 7.
Anti-inflammatory effect of phage therapy in vivo. (A) Histopathology of muscle tissues from the turbot of the PBS group, the bacterial group (AS), and the treatment group (AS+ZHF) at 8 dpi. Scale bar, 100 μm. (B) IL-1β, TNF-α, and IFN-γ in sera from turbot from the PBS group, the bacterial group (AS), the phage group (ZHF), and the treatment group (AS+ZHF) at 0, 1, 2, 4, snf 8 dpi. (C) Heat map of the relative expression levels of inflammatory factors (IL-1β, IL-6, IFN-γ, TGF-β, TNF-α, and hepcidin) at 1, 2, 4, and 8 dpi in the liver, spleen, and kidney from turbot of the bacterial group (AS) and treatment group (AS+ZHF). The values in panel B indicate means and SD (n = 3; *, P ≤ 0.05; **, P ≤ 0.01).
DISCUSSION
Multidrug-resistant bacterial infections of fish and shellfish result in economic losses to the global aquaculture industry. A. salmonicida is a fish pathogen that causes furunculosis and bacterial septicemia in wild and cultured fish, such as salmonids and turbot (17). Therefore, alternatives to antibiotics are needed to control and prevent multidrug-resistant bacterial infections. One option is phage therapy because of its efficacy against multidrug-resistant bacteria, high specificity to host bacteria, rapid growth in host bacteria, and few side effects. At present, phage therapy has been used to inhibit multidrug-resistant bacteria that infect humans, such as Klebsiella pneumoniae and Acinetobacter baumannii (18). Phages are also used to control A. salmonicida and reduce mortality in trout (14, 15). This study reports the isolation and characterization of a novel A. salmonicida subsp. masoucida phage vB_AsM_ZHF. Its therapeutic efficacy was evaluated in turbot.
In total, three novel virulent phages (ZHA, ZHD, and ZHF) that infect A. salmonicida subsp. masoucida were isolated in sewage from an aquatic product market, an appropriate source of phages that attack aquatic bacteria (19). TEM revealed that the phages have symmetric icosahedral heads and straight contractile tails (Fig. 1B; see also Table S1). These characteristics identify ZHA, ZHD, and ZHF as members of the Myoviridae family, Caudovirales order, according to their morphology and size (20). The phages with tail attach better to the bacterial receptors, so they have more potential for therapeutic use (21). ZHA, ZHD, and ZHF form clear or turbid plaques on A. salmonicida subsp. masoucida and achromogenes hosts but do not infect A. salmonicida subsp. salmonicida or other tested bacteria (see Fig. S2).
The biological characteristics of phages should be understood before using them for treatment. The burst size is a primary indicator of phage lysis efficiency. ZHF has a burst size of 284 PFU/cell (Fig. 2A), which is significantly higher than the burst sizes of ZHA and ZHD. ZHF has a burst size higher than other reported Aeromonas phages, such as AS-szw, AS-yj, AS-zj, AS-sw, and AS-g (ranging from 86 to 145 PFU/cell) (22), suggesting that ZHF has potential as a biological antibacterial agent. ZHF remains steady in fluctuating environments with pH levels ranging from 6 to 8 (Fig. 2B) and temperatures lower than 36°C (Fig. 2C), similar to other phage isolates (10, 11, 15). ZHA, ZHD, and ZHF have different amplification patterns at MOI ranging from 0.01 to 100 and achieve their highest titers at an MOI of 0.1 or 1 (Fig. 2D). These results suggest that phage vB_AsM_ZHF is likely to produce the desired antibacterial effect.
ZHF exhibited the best antibacterial activity in vitro (Fig. 3). The bacterial recovery growth time for ZHF is the longest and the optical density of recovered cultures is the lowest among the three phages. Chen et al. (11) isolated five A. salmonicida phages and found that phage-resistant strains began to recover after 4 h at 0.1 to 10 MOI. In contrast, ZHF, which inhibits bacterial recovery for at least 45 h, has superior antibacterial activity than other A. salmonicida phages. However, almost all phages will select phage-resistant strains from the dominant strain under bacteriostatic pressure (23), but the A. salmonicida subsp. masoucida strain resistant to ZHF exhibited poor growth, which is a notable advantage in phage therapy. Hays et al. (24) found that lysis inhibition and resistance evolution of Vibrio cholerae occurs at high phage MOIs. Phage-resistant bacteria proliferated rapidly under phage ZHD pressure at an MOI of 100, confirming that exceedingly high doses might decrease the antibacterial efficacy. Therefore, ZHF was the optimum phage among the three phage based on its antibacterial activity.
The genome of vB_AsM_ZHF was sequenced and analyzed to gain an understanding of this newly discovered phage. Currently, the complete genomes of 48 A. salmonicida phages have been reported in the NCBI database (http://www.ncbi.nlm.nih.gov/pubmed, accessed 17 February 2021). ZHF was confirmed to be a novel phage belonging to the Myoviridae phage family, which is consistent with the results of TEM observation. The genome is 161,887 bp, similar to other A. salmonicida phages in the Myoviridae family, such as A. salmonicida phage phiAS4 (HM452125). Three ORFs were predicted to encode proteins involved in the lysis of bacteria: holin, lysozyme, and lysis inhibition regulator. These proteins form a holin-lysozyme-spanin system that lyses A. salmonicida cells and releases progeny phages (25). Comparative genome analyses revealed that there is a close relationship between vB_AsM_ZHF and other A. salmonicida phages, such as phage phiAS4 (ANI value 96.32%) (Fig. 4B; see also Fig. S3). No putative virulence factors or antibiotic resistance genes were found in the vB_AsM_ZHF genome, based on searches in VFDB and ARDB, implying that ZHF is suitable for phage therapy. Moreover, no integrase gene was found in the ZHF genome, indicating that ZHF is more conducive to phage therapy than lysogenic phages. These results suggest that A. salmonicida phage vB_AsM_ZHF is a virulent phage belonging to Myoviridae family that has the potential to be a safe biological antibacterial agent with a satisfactory ability to lyse A. salmonicida.
Turbot were challenged by A. salmonicida subsp. masoucida injection i.m. and then divided into five groups to determine the therapeutic effect of ZHF. Phages were administered i.p. at three different dosages (treatment groups), whereas positive- and negative-control groups received polymyxin B (Col) and PBS, respectively. As shown in Fig. 5, the phage therapy groups receiving 8 × 106 PFU/fish (MOI = 100) and 8 × 104 PFU/fish (MOI = 1) exhibited survival rates equal to that of the polymyxin B (positive control). The lowest dosage of ZHF, 8 × 102 PFU/fish (MOI = 0.01), decreased mortality to 40%, which is an acceptable level of therapeutic effect. Moreover, phage treatment decreased the abundance of host bacteria in vivo. Rorbo et al. (26) explored the phage KVP40, which reduced the CFU of four different V. anguillarum strains in Atlantic cod and turbot. In this study, the bacterial count of the treatment group (AS+ZHF) was approximately 1 order of magnitude lower than the negative-control group (AS) in liver, spleen, and head kidney tissues at 8 dpi. Typically, phages are eliminated by the immune and reticuloendothelial systems (RES) within several days (27, 28). As shown in Fig. 6, ZHF was completely cleared in vivo by 8 dpi. In summary, i.p. injection of A. salmonicida subsp. masoucida phage ZHF protects turbot by controlling the density of pathogenic bacteria; therefore, the use of protectants, such as sodium alginate and liposomes, should be considered to prolong phage survival in vivo (29).
Bacterial infections cause inflammatory responses. In previous studies, phages reduced inflammation in vivo. Hua et al. (30) reported that intranasal administration of the phage SH-Ab15519 rescued mice injected with carbapenem-resistant Acinetobacter baumannii (CRAB) based on lung inflammation histopathology and TNF-α and IL-6 levels. Jia et al. (31) reported that phage IME-JL8 reduced the serum levels of IL-1β, TNF-α, and IFN-γ elicited by Citrobacter freundii infection. Our results indicate that vB_AsM_ZHF therapy in turbot challenged with A. salmonicida reduces the infiltration of inflammatory cells in muscle tissue, lowers serum levels of IL-1β, TNF-α, and IFN-γ, and decreases gene expression of IL-1β, IL-6, IFN-γ, TGF-β, TNF-α, and hepcidin in the liver, spleen, and head kidney. However, the mechanisms by which phages ZHF induces anti-inflammatory effects remain unknown.
Phage therapy is considered a safe treatment for infectious diseases, and no side effects have been reported after phage administration in the early clinical treatments and gene expression analyses (32, 33). Phages typically cause a weak inflammatory response within the body (34). In this study, i.p. injection of a high dose (1010 PFU/ml) of phages into turbot elicited no mortality or apparent adverse effects, and little inflammatory cell infiltration was observed in muscle (see Fig. S5). However, phage injection caused a low-level immune response, due to phage elimination by immune system, which would not be considered an adverse effect in clinical use (28). In summary, the phage vB_AsM_ZHF is a safe biological antibacterial agent that reduces the inflammatory responses elicited by A. salmonicida infection in turbot.
This study reports the isolation of ZHF, an A. salmonicida subsp. masoucida phage, and confirms the therapeutic effect of its i.p. injection in turbot. However, the issues of phage resistance and the length of phage survival in vivo remain to be solved. Future studies are likely to evaluate the design of phage cocktails (11) and the development of phage protectants (29). For convenience in actual aquaculture, the oral administration models of phages need to be established (35).
Conclusions.
We isolated a novel A. salmonicida subsp. masoucida phage vB_AsM_ZHF from sewage and identified its biological characteristics. The complete genome was sequenced and analyzed, and no antibiotic resistance or virulence genes were found. A phage therapy model of i.p. administration was established and verified in turbot in vivo. ZHF significantly reduced mortality, bacterial burden, and inflammatory responses without apparent side effects. In conclusion, our work represents a strategy for developing phage therapies to control multi-antibiotic-resistant Aeromonas salmonicida subsp. masoucida in aquaculture.
MATERIALS AND METHODS
Bacterial strains.
Liver, spleen, and kidney tissues were collected from turbot with skin ulcers in Yantai (Shandong, China). The single colony isolation was screened out through plate streaking method from ground tissues and cultured at 22°C for 48 h. The dominant bacterial colonies were named as strain AS01 and stored in 40% (vol/vol) glycerol at −80°C and cultured overnight at 22°C with shaking in tryptic soy broth (TSB; Shengsi Biotech, Shanghai, China).
The pathogenic strain Aeromonas salmonicida AS01 was verified by 16S rRNA and vapA gene sequencing (36). The phylogenetic trees were produced by the neighbor-joining method in MEGA 7 (37) with 1,000 bootstrap replicates. The subspecies was identified using an API 20NE kit (bioMérieux, France) and determined using the online Apiweb. The API system and the sequence of vapA identified strain AS01 as Aeromonas salmonicida subsp. masoucida. The other strains used in this study are shown in Table S1.
Isolation and purification of bacteriophages.
The bacteriophages ZHA, ZHD, and ZHF were isolated by the double-layer agar plate method (38) in sewage collected from an aquatic product market in Shanghai, China. Briefly, sewage was centrifuged for 10 min at 8,000 × g and the supernatant was filtered using 0.22 μm nitrocellulose membranes (Millipore, Billerica, MA). The filtrate (25 ml) was cocultured overnight with 25 ml of 2× TSB (4 mM CaCl2) and 100 μl of A. salmonicida AS01 (OD600 = 0.4 to 0.6) at 28°C with shaking. The mixed culture was centrifuged at 8,000 × g for 5 min at 4°C, and the supernatant was filtered using 0.22-μm membranes to remove bacteria. The diluted phage culture (100 μl) was mixed with 100 μl of A. salmonicida (OD600 = 0.4 to 0.6), followed by incubation at room temperature for 10 min. TSB with 0.7% agar (55 to 60°C) was added to the mixture, and the top agar was poured onto a TSA plate. The double-layer agar plate was cultured at 28°C for 12 h. The isolation on double-layer agar plates was repeated at least three times to purify the phages. Phages were stored at −80°C in 40% (vol/vol) glycerol. The host range of each phage was also determined by the double-layer agar plate method.
Phage cultures were concentrated and purified by NaCl-PEG precipitation and cesium chloride (CsCl) gradient ultracentrifugation with modification (39). Briefly, the phage lysates were incubated with 1 M NaCl at 4°C overnight and then centrifuged at 12,000 × g for 30 min at 4°C. The supernatant was precipitated overnight by PEG8000 at 4°C and then centrifuged at 12,000 × g for 30 min at 4°C. Subsequently, the pellet was resuspended in Super Micro buffer (SM buffer; 200 mM NaCl, 16 mM MgSO4, 0.1 M Tris-HCl, 0.02% gelatin [pH 7.5]). The concentrated phage was deposited onto a CsCl step-gradient composed of 1.3-, 1.5-, and 1.7-g/ml layers and centrifuged at 200,000 × g for 1 h at 4°C. The band that sedimented between 1.3 and 1.5 g/ml was collected and stored at 4°C.
Phage characteristics.
Morphological characteristics were determined by TEM. Purified phages (>1010 PFU/ml) were deposited onto carbon-coated copper grids and allowed to adsorb for 5 min. Phosphotungstic acid (2% [pH 7.0]) was added for 30 s to stain phage particles negatively. Copper grids were observed by TEM (JEM-1400plus; JEOL, Japan) at an acceleration voltage of 80 kV. Six images of each phage strain were obtained.
One-step growth curves were generated as previously described with some modifications (40). Briefly, each phage was mixed with 1 ml of A. salmonicida AS01 at an MOI of 0.1 for 10 min. Samples were then centrifuged at 12,000 × g for 2 min at 4°C, and the pellet was resuspended in 1 ml of TSB; this process was repeated three times. The final suspension was added to 10 ml of TSB and cultured at 28°C with shaking. Phage titers were measured at 10-min intervals in triplicate using the double-layer agar method. The burst size was calculated by dividing the final phage titer by the initial phage titer.
Phages were incubated at various temperatures (4, 28, 40, 50, 60, and 70°C) and different pH levels (pH 2 to 12) for 1 h. Samples were diluted with SM buffer, and titers were determined by the double-layer agar method using three biological replicates and three technical replicates.
Different titers of phages were cocultured with A. salmonicida AS01 at MOIs ranging from 0.001 to 100 for 8 h at 28°C with shaking to determine the optimal MOI. Phage titers were measured by the double-layer agar method using three biological replicates and three technical replicates, and the optimal MOI refers to the MOI of the phage sample with the highest titer.
Antibacterial effect in vitro.
A. salmonicida AS01 were cultured (OD600 = 0.5), and the bacterial pellets were resuspended in PBS. Phages were cultured and washed with PBS using the NaCl-PEG method, and then titers were determined by the double-layer agar method. Host bacterial suspensions (2 μl) and 2× TSB (100 μl) were added to 100-well plates with phages (50 μl) at MOIs ranging from 0.01 to 100. Deionized water was added to a final volume of 200 μl. Host bacteria treated with polymyxin B (20 μg/ml) were used as positive controls, and host bacteria treated with PBS served as negative controls. Plates were cultured for 60 h at 28°C with shaking, and the OD600 was monitored every 30 min using a Bioscreen plate reader (Bioscreen C; OY Growth Curves, Finland). Each treatment was performed in triplicate.
Genome sequencing and bioinformatic analysis.
Genomic DNA was extracted from high titer phage particles (>1010 PFU/ml) using a TIANamp virus DNA/RNA kit (Tiangen, Beijing, China) and stored at −20°C. Sequencing libraries were constructed using the TruSeq DNA sample preparation kit (Illumina, USA) on an Illumina MiSeq platform. A5-miseq (41) and SPAdes (42) were used to construct contigs, and then MUMmer (43) and Pilon (44) were used to assemble data. The complete genome of ZHF was sequenced and assembled by Personal Biotechnology Company (Shanghai, China).
ORFs were analyzed using GeneMarkS (45), and functional protein-coding genes were predicted by searching against the National Center for Biotechnology Information Nonredundant (NR) Database using Diamond software (46). Virulence genes and antibiotic resistance genes were searched in the VFDB (47) and CARD (48) databases. A circle map of the ZHF genome was generated with CGView (49). ANI values were determined between complete phage genomes with the OrthoANIu tool (50), and the heatmap was generated with TBTools (51). The genome comparisons between phages were performed with Easyfig 2.2.5 (52).
Therapeutic efficacy of vB_AsM_ZHF against A. salmonicida subsp. masoucida infection in turbot.
Turbot (35.0 ± 5.0 g) were obtained from an aquaculture farm (Tianyuan, Shandong, China) and temporarily placed in the aquaculture system without feed for a week. The environment was maintained at 16 to 18°C, with a salinity of 30 to 35 g/liter, dissolved oxygen levels of 6 to 8 mg/liter, and pH values of 7.5 to 8.0. A total of 90% of the water content was exchanged with fresh seawater every 2 days.
Each turbot was challenged with A. salmonicida AS01 (8 × 104 CFU/fish) by i.m. injection and then divided into five groups of 20 fish each. Phage therapy groups received i.p. injections of ZHF at 8 × 102, 8 × 104, or 8 × 106 PFU/fish. Positive-control groups received polymyxin B (Col; 20 μg/ml). Negative control group received bacteria (i.m.) and PBS (i.p.); the blank control group received PBS (i.m. and i.p.). The volume of each injection was 100 μl. Each group of fish was placed in a glass tank (60 liters of seawater), and the seawater was changed every 2 days. The environment in each tank was the same as described above. Cumulative mortalities of each group were recorded every day for 3 weeks. Experiments were performed in triplicate. Liver, spleen, and head kidney tissues and sera from three fish were collected at 0, 1, 2, 4, and 8 dpi as triplicate samples for the following experiments.
Bacterial burden and phage titer in vivo.
Liver, spleen, and head kidney samples from three turbot were each weighed and ground in PBS. The bacterial burden was determined by inoculating serial dilutions on tryptic soy agar plates with 20 μg/ml ampicillin at 28°C for 36 h. Ground tissues were centrifuged at 12,000 × g for 5 min. The titer of each supernatant was measured by the double-layer agar method using dilutions of three biological replicates and three technical replicates. The in vivo bacterial burdens and phage titers were expressed as CFU and PFU, respectively, per tissue weight (g).
Histological evaluation of muscle tissues.
Muscle tissues collected from the PBS (blank control), A. salmonicida (negative control), and phage therapy (8 × 104 PFU/fish) groups were fixed in 4% paraformaldehyde for 24 h. Samples were sequentially dehydrated through a gradient of ethanol solutions and embedded in paraffin wax. Slices (4 μm) were cut and stained with hematoxylin and eosin after rehydration through a gradient of ethanol solutions. The prepared tissue slices were observed under a microscope.
Inflammatory cytokine assays in tissue and serum.
Inflammatory cytokine gene expression was measured in liver, spleen, and head kidney tissues by RT-qPCR. Total RNA was extracted from turbot samples by TRIzol (Thermo, USA) according to the manufacturer’s instructions. Total RNA (1 mg) was reverse transcribed into cDNA, and genomic DNA was removed using a FastKing RT kit with gDNase (Tiangen, China). Products were stored at −80°C. RT-qPCR was carried out on three biological replicates and three technical replicates using MonAmp ChemoHS qPCR Mix (Monad, Wuhan, China) according to the manufacturer’s instructions and detected with an ABI 7500 RealTime detection system (Applied Biosystems, USA). The primers used to amplify each gene are listed in Table 2. The relative gene expression of each sample was calculated by using the 2−ΔΔCT method with β-actin as a reference gene.
TABLE 2.
Primers used in this study
| Name | Sequence (5′–3′) |
|---|---|
| β-Actin-F | TGAACCCCAAAGCCAACAGG |
| β-Actin-R | AGAGGCATACAGGGACAGCAC |
| IL-1β-F | GAGAGCATCGTGGAAGAACA |
| IL-1β-R | GTTTCGGACCAGAACGAAGT |
| IL-6-F | TATCAGCGCCTCTTCCCAAA |
| IL-6-R | AAACTGACGGTCTAAGGCCA |
| TNF-α-F | TGGAGTGGAAGAACGGTCAA |
| TNF-α-R | GAGACGCGTTGAAGCCTAAG |
| IFN-γ-F | CGTCTCAGCAAGGATGAACA |
| IFN-γ-R | CCGACCATGAACAGCTTCTT |
| Hepcidin-F | CGAGTCACATCAGGCAGAAG |
| Hepcidin-R | TCCTCAGAACTTGCAGCAGA |
| TGF-β-F | ACCAAAACCATCCCAATGCC |
| TGF-β-R | ATGAAACGGGAAGCGAGGTA |
| 27-F | AGAGTTTGATCMTGGCTCAG |
| 1492-R | TACGGYTACCTTGTTACGACTT |
| vapA-F | CTGGACTTCTCCACTGCTCA |
| vapA-R | ACGTTGGTAATCGCGAAATC |
Blood samples were collected from three turbot in each group and centrifuged at 3,000 × g for 15 min to obtain serum. Inflammatory cytokines (IL-1β, TNF-α, and IFN-γ) were measured with enzyme-linked immunosorbent assay kits (Lai Er Bio-Tech, Hefei, China). The data represent the mean values and standard deviations of three biological replicates and three technical replicates.
Statistical analysis.
Statistical analyses were performed with GraphPad Prism 7.0. Results are expressed as means ± the standard deviations (SD). One-way analysis of variance (ANOVA) was used to determine the statistical significance, and the significance level is indicated in the figures by asterisks (*, P ≤ 0.05; **, P ≤ 0.01).
Ethics statement.
The conduct and procedures involved in the present work were approved by the Animal Ethics Committee of East China University of Science and Technology (ECUST IACUC 30201051).
Data availability.
The sequence data for the Aeromonas phage vB_AsM_ZHF were deposited at GenBank under accession no. MW584871.
ACKNOWLEDGMENTS
This study was supported by grants from the Innovation Group Project of Southern Marine Science and Engineering Guangdong Laboratory (Zhuhai) (311020005), the National Natural Science Foundation of China (32025038), and the Fundamental Research Funds for the Central Universities (22221062017019).
We have no conflicts of interest to declare.
Footnotes
Supplemental material is available online only.
Contributor Information
Shuai Shao, Email: shaoscott@163.com.
Qin Liu, Email: qinliu@ecust.edu.cn.
Charles M. Dozois, INRS—Institut Armand-Frappier
REFERENCES
- 1.Kim DH, Choi SY, Kim CS, Oh MJ, Jeong HD. 2013. Low-value fish used as feed in aquaculture were a source of furunculosis caused by atypical Aeromonas salmonicida. Aquaculture 408-409:113–117. 10.1016/j.aquaculture.2013.05.014. [DOI] [Google Scholar]
- 2.Zorrilla I, Chabrillón M, Arijo S, Díaz-Rosales P, Martínez-Manzanares E, Balebona MC, Moriñigo MA. 2003. Bacteria recovered from diseased cultured gilthead sea bream (Sparus aurata L.) in southwestern Spain. Aquaculture 218:11–20. 10.1016/S0044-8486(02)00309-5. [DOI] [Google Scholar]
- 3.Ringo E, Jutfelt F, Kanapathippillai P, Bakken Y, Sundell K, Glette J, Mayhew TM, Myklebust R, Olsen RE. 2004. Damaging effect of the fish pathogen Aeromonas salmonicida subsp. salmonicida on intestinal enterocytes of Atlantic salmon (Salmo salar L.). Cell Tissue Res 318:305–312. 10.1007/s00441-004-0934-2. [DOI] [PubMed] [Google Scholar]
- 4.Watts JEM, Schreier HJ, Lanska L, Hale MS. 2017. The rising tide of antimicrobial resistance in aquaculture: sources, sinks and solutions. Mar Drugs 15. 10.3390/md15060158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McIntosh D, Cunningham M, Ji B, Fekete FA, Parry EM, Clark SE, Zalinger ZB, Gilg IC, Danner GR, Johnson KA, Beattie M, Ritchie R. 2008. Transferable, multiple antibiotic, and mercury resistance in Atlantic Canadian isolates of Aeromonas salmonicida subsp. salmonicida is associated with carriage of an IncA/C plasmid similar to the Salmonella enterica plasmid pSN254. J Antimicrob Chemother 61:1221–1228. 10.1093/jac/dkn123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Altamirano FLG, Barr JJ. 2019. Phage therapy in the postantibiotic era. Clin Microbiol Rev 32:e00066-18. 10.1128/CMR.00066-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Law N, Aslam S. 2020. Phage therapy: primer and role in the treatment of MDROs. Curr Infect Dis Rep 22. 10.1007/s11908-020-00742-x. [DOI] [Google Scholar]
- 8.Soffer N, Woolston J, Li M, Das C, Sulakvelidze A. 2017. Bacteriophage preparation lytic for Shigella significantly reduces Shigella sonnei contamination in various foods. PLoS One 12:e0175256. 10.1371/journal.pone.0175256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Z, Li X, Zhang J, Wang X, Wang L, Cao Z, Xu Y. 2016. Use of phages to control Vibrio splendidus infection in the juvenile sea cucumber Apostichopus japonicus. Fish Shellfish Immunol 54:302–311. 10.1016/j.fsi.2016.04.026. [DOI] [PubMed] [Google Scholar]
- 10.Chen L, Fan J, Yan T, Liu Q, Yuan S, Zhang H, Yang J, Deng D, Huang S, Ma Y. 2019. Isolation and characterization of specific phages to prepare a cocktail preventing Vibrio sp. Va-F3 infections in shrimp (Litopenaeus vannamei). Front Microbiol 10:2337. 10.3389/fmicb.2019.02337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen L, Yuan S, Liu Q, Mai G, Yang J, Deng D, Zhang B, Liu C, Ma Y. 2018. In vitro design and evaluation of phage cocktails against Aeromonas salmonicida. Front Microbiol 9:1476. 10.3389/fmicb.2018.01476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verner-Jeffreys DW, Algoet M, Pond MJ, Virdee HK, Bagwell NJ, Roberts EG. 2007. Furunculosis in Atlantic salmon (Salmo salar L.) is not readily controllable by bacteriophage therapy. Aquaculture 270:475–484. 10.1016/j.aquaculture.2007.05.023. [DOI] [Google Scholar]
- 13.Silva YJ, Moreirinha C, Pereira C, Costa L, Rocha RJM, Cunha A, Gomes NCM, Calado R, Almeida A. 2016. Biological control of Aeromonas salmonicida infection in juvenile Senegalese sole (Solea senegalensis) with phage AS-A. Aquaculture 450:225–233. 10.1016/j.aquaculture.2015.07.025. [DOI] [Google Scholar]
- 14.Imbeault S, Parent S, Lagace M, Uhland CF, Blais JF. 2006. Using bacteriophages to prevent furunculosis caused by Aeromonas salmonicida in farmed brook trout. J Aquat Anim Health 18:203–214. 10.1577/H06-019.1. [DOI] [Google Scholar]
- 15.Kim JH, Choresca CH, Shin SP, Han JE, Jun JW, Park SC. 2015. Biological control of Aeromonas salmonicida subsp. salmonicida infection in rainbow trout (Oncorhynchus mykiss) using Aeromonas phage PAS-1. Transbound Emerg Dis 62:81–86. 10.1111/tbed.12088. [DOI] [PubMed] [Google Scholar]
- 16.King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ. 2012. Virus taxonomy: classification and nomenclature of viruses: ninth report of the International Committee on Taxonomy of Viruses. Elsevier Academic Press, Inc, New York, NY. [Google Scholar]
- 17.Dalsgaard I, Gudmundsdóttir BK, Helgason S, Høie S, Thoresen OF, Wichardt UP, Wiklund T. 1998. Identification of atypical Aeromonas salmonicida: inter-laboratory evaluation and harmonization of methods. J Appl Microbiol 84:999–1006. 10.1046/j.1365-2672.1998.00435.x. [DOI] [PubMed] [Google Scholar]
- 18.Kortright KE, Chan BK, Koff JL, Turner PE. 2019. Phage therapy: a renewed approach to combat antibiotic-resistant bacteria. Cell Host Microbe 25:219–232. 10.1016/j.chom.2019.01.014. [DOI] [PubMed] [Google Scholar]
- 19.Yang M, Liang Y, Huang S, Zhang J, Wang J, Chen H, Ye Y, Gao X, Wu Q, Tan Z. 2020. Isolation and characterization of the novel phages vB_VpS_BA3 and vB_VpS_CA8 for lysing Vibrio parahaemolyticus. Front Microbiol 11:259. 10.3389/fmicb.2020.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ackermann HW. 2009. Basic phage electron microscopy. Methods Mol Biol 501:113–126. 10.1007/978-1-60327-164-6_12. [DOI] [PubMed] [Google Scholar]
- 21.Nobrega FL, Vlot M, de Jonge PA, Dreesens LL, Beaumont HJE, Lavigne R, Dutilh BE, Brouns SJJ. 2018. Targeting mechanisms of tailed bacteriophages. Nat Rev Microbiol 16:760–773. 10.1038/s41579-018-0070-8. [DOI] [PubMed] [Google Scholar]
- 22.Duarte J, Pereira C, Moreirinha C, Salvio R, Lopes A, Wang D, Almeida A. 2018. New insights on phage efficacy to control Aeromonas salmonicida in aquaculture systems: an in vitro preliminary study. Aquaculture 495:970–982. 10.1016/j.aquaculture.2018.07.002. [DOI] [Google Scholar]
- 23.Labrie SJ, Samson JE, Moineau S. 2010. Bacteriophage resistance mechanisms. Nat Rev Microbiol 8:317–327. 10.1038/nrmicro2315. [DOI] [PubMed] [Google Scholar]
- 24.Hays SG, Seed KD. 2020. Dominant Vibrio cholerae phage exhibits lysis inhibition sensitive to disruption by a defensive phage satellite. Elife 9:e53200. 10.7554/eLife.53200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Young R. 2014. Phage lysis: three steps, three choices, one outcome. J Microbiol 52:243–258. 10.1007/s12275-014-4087-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rorbo N, Ronneseth A, Kalatzis PG, Rasmussen BB, Engell-Sorensen K, Kleppen HP, Wergeland HI, Gram L, Middelboe M. 2018. Exploring the effect of phage therapy in preventing Vibrio anguillarum infections in cod and turbot larvae. Antibiotics (Basel) 7. 10.3390/antibiotics7020042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsuzaki S, Yasuda M, Nishikawa H, Kuroda M, Ujihara T, Shuin T, Shen Y, Jin Z, Fujimoto S, Nasimuzzaman MD, Wakiguchi H, Sugihara S, Sugiura T, Koda S, Muraoka A, Imai S. 2003. Experimental protection of mice against lethal Staphylococcus aureus infection by novel bacteriophage phi MR11. J Infect Dis 187:613–624. 10.1086/374001. [DOI] [PubMed] [Google Scholar]
- 28.Dabrowska K. 2019. Phage therapy: what factors shape phage pharmacokinetics and bioavailability? Systematic and critical review. Med Res Rev 39:2000–2025. 10.1002/med.21572.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loh B, Gondil VS, Manohar P, Khan FM, Yang H, Leptihn S. 2020. Encapsulation and delivery of therapeutic phages. Appl Environ Microbiol 87:e01979-20. 10.1128/AEM.01979-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hua Y, Luo T, Yang Y, Dong D, Wang R, Wang Y, Xu M, Guo X, Hu F, He P. 2018. Phage therapy as a promising new treatment for lung infection caused by carbapenem-resistant Acinetobacter baumannii in mice. Front Microbiol 8:2659. 10.3389/fmicb.2017.02659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jia K, Yang N, Zhang X, Cai R, Zhang Y, Tian J, Raza SHA, Kang Y, Qian A, Li Y, Sun W, Shen J, Yao J, Shan X, Zhang L, Wang G. 2020. Genomic, morphological and functional characterization of virulent bacteriophage IME-JL8 targeting Citrobacter freundii. Front Microbiol 11:585261. 10.3389/fmicb.2020.585261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gindin M, Febvre HP, Rao S, Wallace TC, Weir TL. 2019. Bacteriophage for gastrointestinal health (PHAGE) study: evaluating the safety and tolerability of supplemental bacteriophage consumption. J Am Coll Nutr 38:68–75. 10.1080/07315724.2018.1483783. [DOI] [PubMed] [Google Scholar]
- 33.McCallin S, Alam Sarker S, Barretto C, Sultana S, Berger B, Huq S, Krause L, Bibiloni R, Schmitt B, Reuteler G, Brüssow H. 2013. Safety analysis of a Russian phage cocktail: from MetaGenomic analysis to oral application in healthy human subjects. Virology 443:187–196. 10.1016/j.virol.2013.05.022. [DOI] [PubMed] [Google Scholar]
- 34.Dufour N, Delattre R, Chevallereau A, Ricard JD, Debarbieux L. 2019. Phage therapy of pneumonia is not associated with an overstimulation of the inflammatory response compared to antibiotic treatment in mice. Antimicrob Agents Chemother 63:e00379-19. 10.1128/AAC.00379-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vinner GK, Rezaie-Yazdi Z, Leppanen M, Stapley AGF, Leaper MC, Malik DJ. 2019. Microencapsulation of Salmonella-specific bacteriophage Felix O1 using spray-drying in a pH-responsive formulation and direct compression tableting of powders into a solid oral dosage form. Pharmaceuticals (Basel) 12. 10.3390/ph12010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gulla S, Lund V, Kristoffersen AB, Sørum H, Colquhoun DJ. 2016. vapA (A-layer) typing differentiates Aeromonas salmonicida subspecies and identifies a number of previously undescribed subtypes. J Fish Dis 39:329–342. 10.1111/jfd.12367. [DOI] [PubMed] [Google Scholar]
- 37.Kumar S, Stecher G, Tamura K. 2016. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874. 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Echeverria-Vega A, Morales-Vicencio P, Saez-Saavedra C, Gordillo-Fuenzalida F, Araya R. 2019. A rapid and simple protocol for the isolation of bacteriophages from coastal organisms. MethodsX 6:2614–2619. 10.1016/j.mex.2019.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sambrook J, Russell DW, Sambrook J, Russell DW. 2001. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 40.Ellis EL, Delbrück M. 1939. The growth of bacteriophage. J Gen Physiol 22:365–384. 10.1085/jgp.22.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coil D, Jospin G, Darling AE. 2015. A5-miseq: an updated pipeline to assemble microbial genomes from Illumina MiSeq data. Bioinformatics 31:587–589. 10.1093/bioinformatics/btu661. [DOI] [PubMed] [Google Scholar]
- 42.Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Son P, Prjibelski AD, Pyshkin AV, Sirotkin AV, Vyahhi N, Tesler G, Alekseyev MA, Pevzner PA. 2012. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol 19:455–477. 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kurtz S, Phillippy A, Delcher AL, Smoot M, Shumway M, Antonescu C, Salzberg SL. 2004. Versatile and open software for comparing large genomes. Genome Biol 5:R12. 10.1186/gb-2004-5-2-r12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walker BJ, Abeel T, Shea T, Priest M, Abouelliel A, Sakthikumar S, Cuomo CA, Zeng Q, Wortman J, Young SK, Earl AM. 2014. Pilon: an integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS One 9:e112963. 10.1371/journal.pone.0112963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Besemer J, Borodovsky M. 2005. GeneMark: web software for gene finding in prokaryotes, eukaryotes and viruses. Nucleic Acids Res 33:W451–W454. 10.1093/nar/gki487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buchfink B, Xie C, Huson DH. 2015. Fast and sensitive protein alignment using DIAMOND. Nat Methods 12:59–60. 10.1038/nmeth.3176. [DOI] [PubMed] [Google Scholar]
- 47.Chen LH, Yang J, Yu J, Ya ZJ, Sun LL, Shen Y, Jin Q. 2005. VFDB: a reference database for bacterial virulence factors. Nucleic Acids Res 33:D325–D328. 10.1093/nar/gki008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McArthur AG, Waglechner N, Nizam F, Yan A, Azad MA, Baylay AJ, Bhullar K, Canova MJ, De Pascale G, Ejim L, Kalan L, King AM, Koteva K, Morar M, Mulvey MR, O’Brien JS, Pawlowski AC, Piddock LJV, Spanogiannopoulos P, Sutherland AD, Tang I, Taylor PL, Thaker M, Wang W, Yan M, Yu T, Wright GD. 2013. The comprehensive antibiotic resistance database. Antimicrob Agents Chemother 57:3348–3357. 10.1128/AAC.00419-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stothard P, Wishart DS. 2005. Circular genome visualization and exploration using CGView. Bioinformatics 21:537–539. 10.1093/bioinformatics/bti054. [DOI] [PubMed] [Google Scholar]
- 50.Yoon SH, Ha SM, Lim J, Kwon S, Chun J. 2017. A large-scale evaluation of algorithms to calculate average nucleotide identity. Antonie Van Leeuwenhoek 110:1281–1286. 10.1007/s10482-017-0844-4. [DOI] [PubMed] [Google Scholar]
- 51.Chen C, Chen H, Zhang Y, Thomas HR, Frank MH, He Y, Xia R. 2020. TBtools: an integrative toolkit developed for interactive analyses of big biological data. Mol Plant 13:1194–2102. 10.1016/j.molp.2020.06.009. [DOI] [PubMed] [Google Scholar]
- 52.Sullivan MJ, Petty NK, Beatson SA. 2011. Easyfig: a genome comparison visualizer. Bioinformatics 27:1009–1010. 10.1093/bioinformatics/btr039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1 and S2, Fig. S1 to S5. Download AEM.01468-21-s0001.pdf, PDF file, 7.0 MB (7MB, pdf)
Data Availability Statement
The sequence data for the Aeromonas phage vB_AsM_ZHF were deposited at GenBank under accession no. MW584871.