Abstract
Women exhibit less burden of anatomic obstructive coronary atherosclerotic disease as compared with men of the same age, but contradictorily show similar or higher cardiovascular mortality rates. The higher prevalence of nonexertional cardiac symptoms and nonobstructive coronary atherosclerotic disease in women may lead to lack of recognition and appropriate management, resulting in undertesting and undertreatment. Leaders in women’s health from the American College of Cardiology’s Cardiovascular Disease in Women Committee present novel imaging cases that may provoke thought regarding the broad clinical spectrum of myocardial infarction and ischemia with nonobstructive coronary arteries in women. These unique imaging approaches are based on the concept of targeting sex-specific differences in acute and stable ischemic heart disease.
Keywords: atherosclerosis, coronary microvascular dysfunction, ischemia and no obstructive coronary arteries, INOCA, MINOCA, myocardial infarction with nonobstructive coronary arteries, sex, women
AS WE INTRODUCE THESE CASES, READERS SHOULD TAKE NOTE THAT THESE ARE PRESENTED TO PROMPT discussion regarding unique aspects of cardiovascular risk attributed to women. Through these cases, we present the concept that imaging may be applied to target relevant biology in women and that there are sex-specific phenotypes for which tailored detection strategies can reveal the actual high-risk status of some women who are otherwise considered to be at lower risk. These cases illustrate the increasingly recognized high prevalence of nonobstructive coronary artery disease (CAD) in symptomatic women, including among those with acute myocardial infarction (myocardial infarction with nonobstructive coronary arteries [MINOCA]) and stable ischemia (ischemia and no obstructive coronary arteries [INOCA]), and how imaging may uncover their increased risk. Some of these cases were completed as part of specialized female-specific evaluation pathways developed within women’s health centers, and others were completed as part of novel research to define the role of anatomic or functional parameters to identify women at risk. Evaluation pathways tailored to important patient subgroups are part of the goals of precision medicine, and are here presented with a focus on unique imaging approaches for women. These cases were assembled by the American College of Cardiology’s Cardiovascular Disease in Women Committee to introduce readers to the thought-provoking clinical spectrum of MINOCA and INOCA, particularly prevalent in women.
CASES
CASE 1: MINOCA WITH CORONARY ARTERY DISSECTION.
A 69-year-old White woman presented to a cardiologist with stable exertional chest pain. She had a history of hypertension, for which she was taking antihypertensive medication. Physical examination revealed a lean woman with a body mass index of 22 kg/m2 and no abnormalities. Her resting electrocardiogram (ECG) showed normal sinus rhythm. Further cardiac evaluation included an inconclusive ECG treadmill stress test, as well as noncontrast and contrast coronary computed tomography (CT) performed with a single-energy 320-slice CT scanner (Aquillon ONE, Toshiba Medical System). Her coronary artery calcium (CAC) score was 71 (70th percentile age-sex-race adjusted). Her coronary CT angiography (CCTA) demonstrated a CAD-RADS (Coronary Artery Disease Reporting and Data System) 2. In more detail, minimal to mild coronary plaques were observed in the proximal right coronary artery (RCA) and in the left main to mid left anterior descending artery (LAD) with a quantified maximal diameter stenosis of 12% and 36%, respectively (Figure 1). Since her 10-year atherosclerotic cardiovascular disease risk was >7.5% (12.1%) and coronary artery calcium (CAC) score was >0, lipid-lowering therapy with statins was the preferred choice for treatment as recommended by current guidelines (1). However, following completion of all tests and a thorough clinician-patient risk discussion involving shared decision making, the patient declined medical therapy. Regardless, lifestyle changes were strongly advised by her cardiologist.
FIGURE 1. Coronary Computed Tomography Angiography of Case 1.
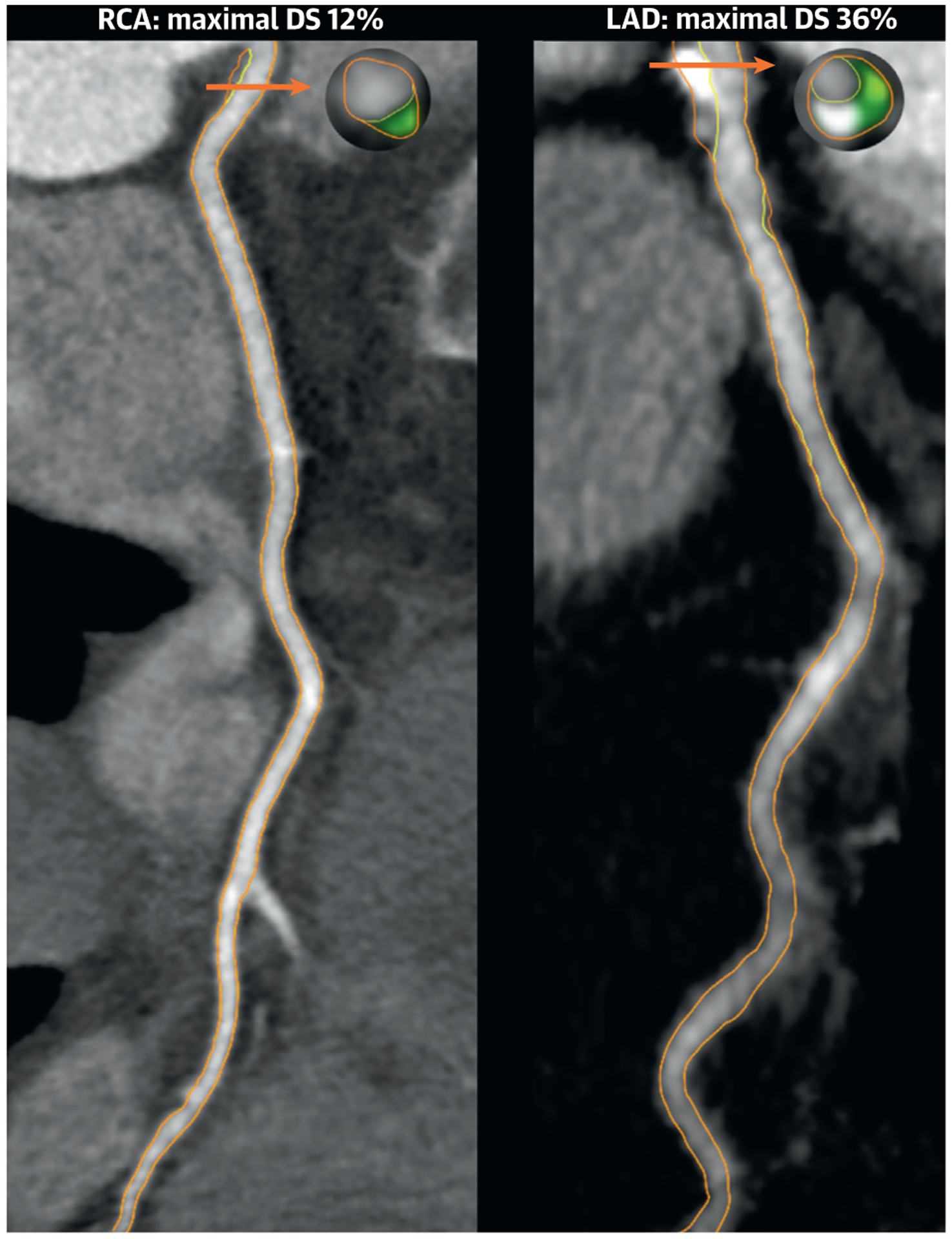
Orange arrows indicate the cross section of maximal diameter stenosis (DS). On the cross sectional level, light green indicates fibrofatty plaque 31–130 Hounsfield units (HU); dark green indicates fibrous plaque 131 to 350 HU; and gray indicates calcified plaque >350 HU. LAD = left anterior descending artery; RCA = right coronary artery.
Three years later, she was admitted to the emergency department with acute chest pain. She complained of retrosternal discomfort with radiation to the right arm, which started during exercise while riding her bicycle 2 h before. On physical examination, vital signs were normal. Her ECG showed sinus rhythm with premature ventricular contractions in bigeminy, small Q waves in the inferior leads and ST-segment elevation in the inferolateral leads (Figure 2A). Cardiac biomarkers were abnormal, and confirmed the initial diagnosis of an ST-segment elevation myocardial infarction (STEMI). Invasive coronary angiography (ICA) was performed, which demonstrated minimal CAD in the LAD and left circumflex artery (LCx) (Figure 2B) and a spontaneous coronary artery dissection (SCAD) of the right posterior descending artery with Thrombolysis In Myocardial Infarction flow grade 0 (Figure 2C). The SCAD was classified as a type 2B using the Saw angiographic classification, because of the long diffuse and smooth stenosis that extended to the distal tip of the artery (2). Her echocardiogram showed apical hypokinesis with a preserved left ventricular ejection fraction (LVEF). As she was hemodynamically stable without left main or proximal multivessel involvement, she was treated conservatively with aspirin and clopidogrel to encourage stabilization of the dissection. She was monitored and remained hospitalized for 5 days. Reperfusion with primary percutaneous coronary intervention in SCAD remains challenging due to the potential of false lumen entry, iatrogenic dissection extension or intramural hematoma dissemination (2). As noted by a recent observational report, revascularization rates in STEMI due to SCAD are therefore significantly lower as compared with STEMI due to atherosclerosis (70% vs. 97%), likely demonstrating more conservative use of primary percutaneous coronary intervention in hemodynamically stable SCAD with distal dissection (3). To date, the use of (dual) antiplatelet therapy in SCAD remains controversial with divergent practice, given the lack of randomized trial data. One of the main concerns is that use of antiplatelet therapy might further increase bleeding risk, given the presence of an intramural hematoma. Nevertheless, data from observational studies potentially allow for this strategy in the acute phase of SCAD (2).
FIGURE 2. Myocardial Infarction With Nonobstructive Coronary Arteries of Case 1.
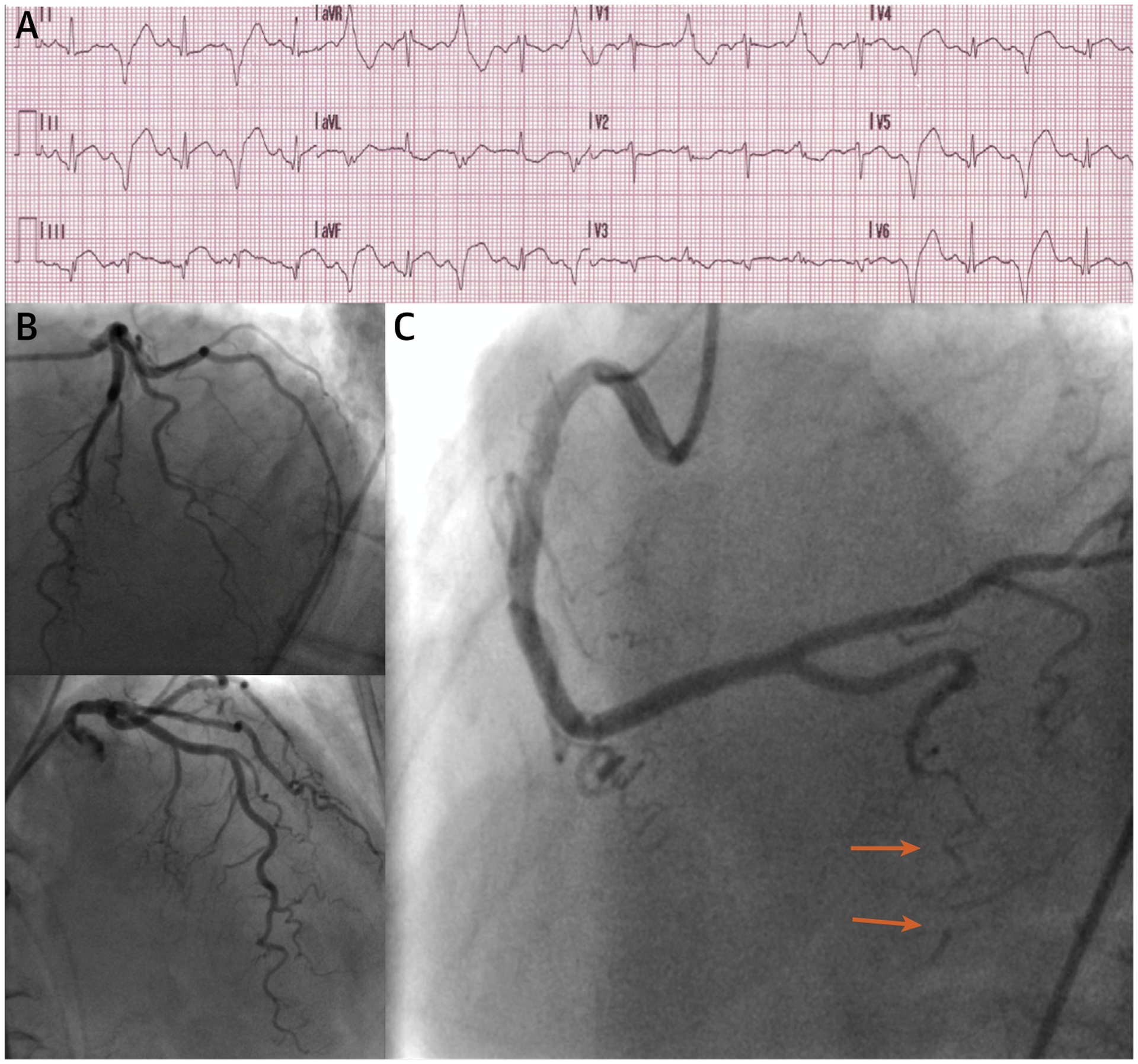
(A) Electrocardiogram showing an ST-segment elevation myocardial infarction. (B) Invasive coronary angiography (ICA) showing minimal coronary artery disease in the left anterior descending artery and left circumflex artery. (C) ICA showing a spontaneous coronary artery dissection of the right posterior descending artery, classified as a type 2B due to a long diffuse and smooth stenosis that extends to the distal tip of the artery (orange arrows).
In conclusion, her final diagnosis was a MINOCA due to SCAD, for which she was treated conservatively and later discharged in asymptomatic condition. In her case, hypertension and aerobic exercise could be hypothesized as probable predisposing and precipitating factors. Information about parity or the use of postmenopausal hormone replacement therapy was unknown. No screening for extracoronary arteriopathies was performed, but according to current guidelines this should always be advised in SCAD survivors as systemic arteriopathies are present in 70% to 80% of cases (4). No other SCAD-specific risk factors such as systemic inflammatory diseases, connective tissue diseases, or substance abuse were identified. Thus far, no subsequent major adverse cardiac events have occurred. Further research is warranted to unravel the true pathophysiology and long-term clinical impact of SCAD.
CASE 2: MINOCA WITH NONOBSTRUCTIVE CORONARY ATHEROSCLEROTIC DISEASE.
A 66-year-old White woman presented to a cardiologist with stable exertional chest pain. She had a history of untreated hypertension and dyslipidemia, and also reported a family history of stroke in a first-degree relative. Physical examination revealed a lean woman with a body mass index of 22 kg/m2 and no abnormalities. Her resting ECG showed normal sinus rhythm. Further cardiac evaluation included noncontrast and contrast CT performed with a dualenergy 128-slice CT scanner (SOMATOM Definition Flash, Siemens). Her CAC score was 115 (81st percentile age-sex-race adjusted). Her CCTA demonstrated a CAD-RADS 2/V. In more detail, multiple minimal to mild coronary plaques were observed in all major epicardial arteries with a quantified maximal diameter stenosis ranging from 17% to 36% (Figure 3). Considering her 10-year atherosclerotic cardiovascular disease risk was >7.5% (7.8%) and CAC score >0, lipid-lowering therapy with a moderate-intensity statin was initiated. Addition of risk-reducing antihypertensive medication would also have been appropriate. Lifestyle changes were strongly advised by her cardiologist.
FIGURE 3. Coronary Computed Tomography Angiography of Case 2.
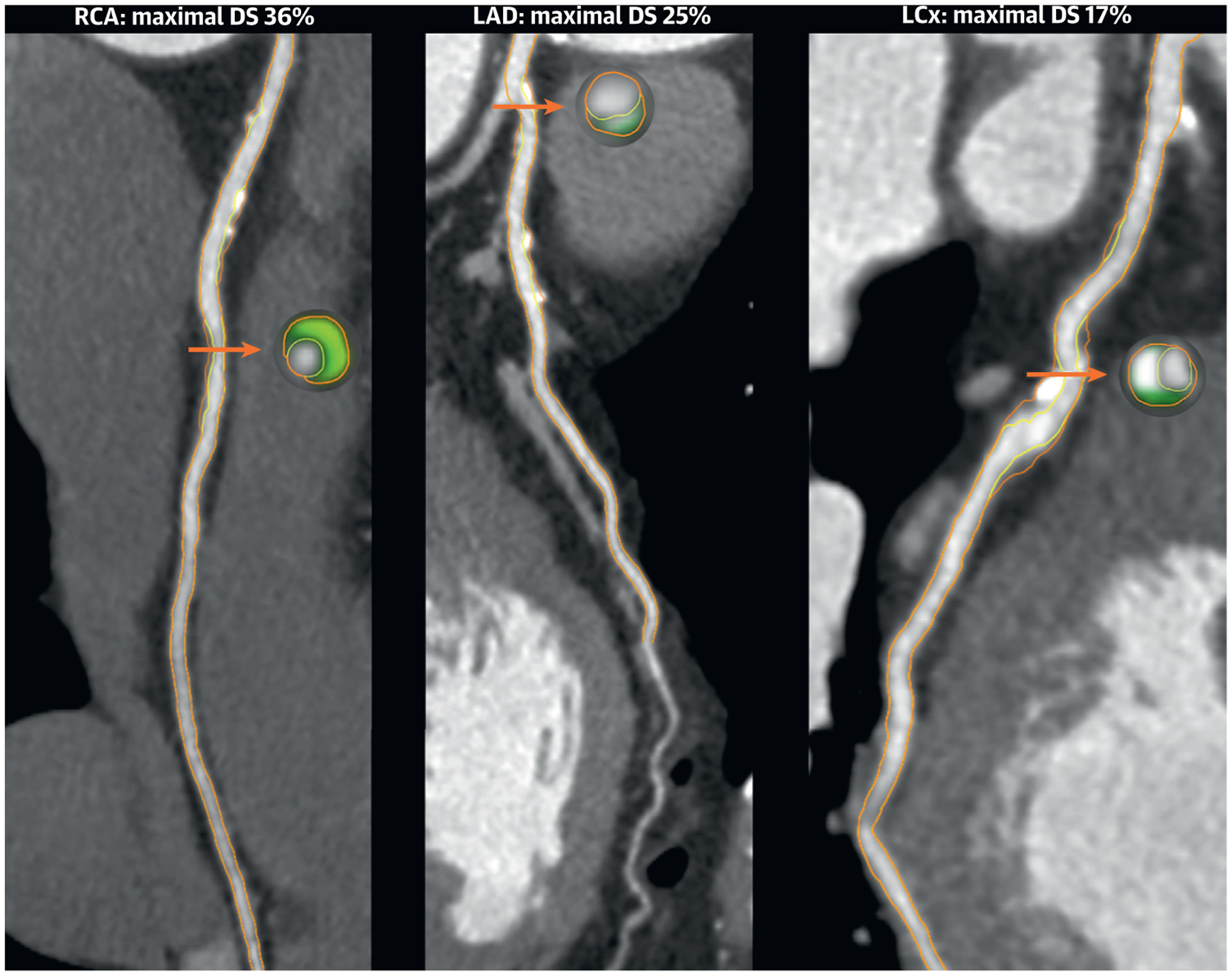
Orange arrows indicate the cross section of maximal DS. On the cross sectional level, light green indicates fibrofatty plaque 31–130 HU; dark green indicates fibrous plaque 131–350 HU; and gray indicates calcified plaque >350 HU. LCx = left circumflex artery; other abbreviations as in Figure 1.
Four years later, she presented again to a cardiologist, this time with acute chest pain radiating to the right clavicle, which started 1 h after an elective colonoscopy. She had no recent history of dyspnea, night sweats, or infection. On physical examination, she was afebrile and vital signs were normal. Her ECG showed sinus rhythm with an incomplete right bundle branch block with nonspecific T-wave and ST-segment abnormalities. Focused echocardiogram performed in the emergency department showed a preserved LVEF by visual estimation, and no signs of pericardial effusion or aortic dissection. Blood samples showed no signs of inflammation, with normal white blood cell count and C-reactive protein level. Cardiac biomarkers were abnormal, and suggested an initial diagnosis of non-STEMI. ICA was performed, which demonstrated mild CAD in the mid RCA (Figure 4A) and minimal CAD in the LAD and LCx. There was no evidence of SCAD on her invasive angiogram. Evident tortuosity, corkscrew signs, and intravessel and multivessel symmetry signs were observed throughout her coronary system (Figure 4B). In her case, coronary tortuosity was scored as severe using a simplified definition of ≥1 curvature of ≥90° in ≥2 major epicardial arteries. Invasive ventriculography showed a preserved LVEF without regional wall motion abnormalities. As no evident culprit lesion was observed, she was treated conservatively with aspirin, nebivolol, lisinopril, atorvastatin, and high-dose pantoprazole.
FIGURE 4. Myocardial Infarction With Nonobstructive Coronary Arteries of Case 2.
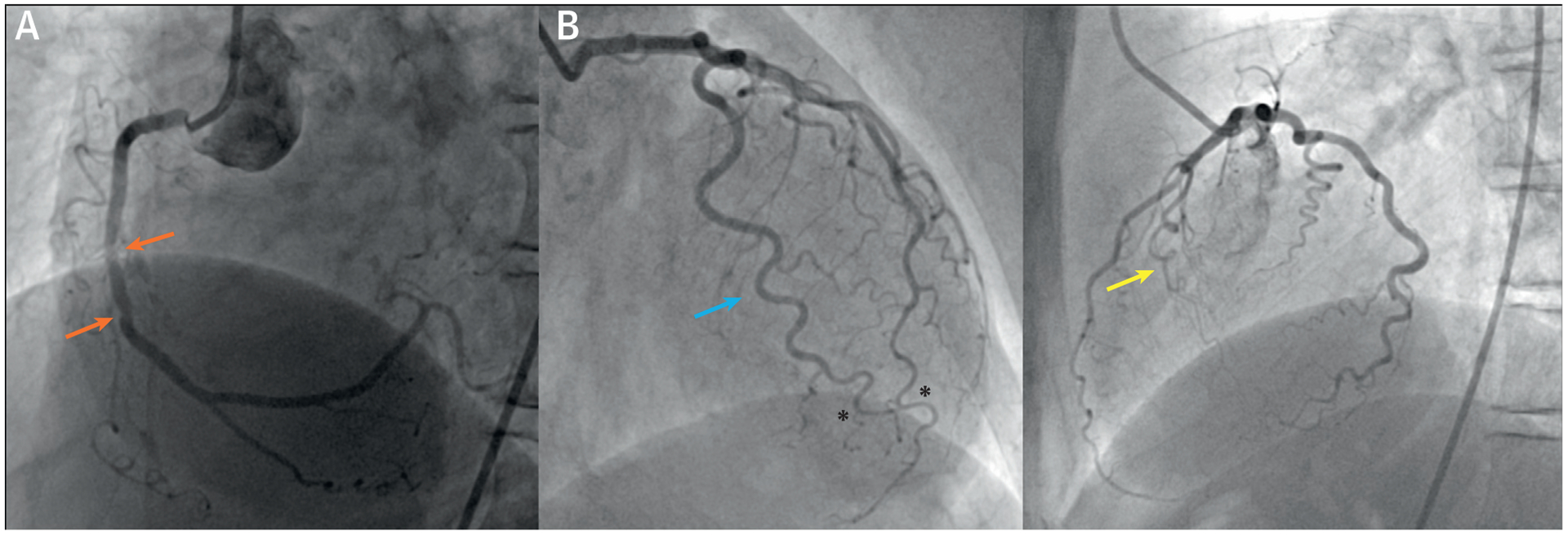
(A) Invasive coronary angiography (ICA) showing mild coronary artery disease (orange arrows) in the mid right coronary artery. (B) ICA showing severe tortuosity with corkscrew signs (yellow arrow), intravessel symmetry signs (blue arrow), and multivessel symmetry signs (black asterisks).
In conclusion, her final diagnosis was a MINOCA due to nonobstructive coronary atherosclerotic disease, for which she was treated and later discharged in stable condition. In this case, no further etiologic testing, such as intravascular ultrasound or optical coherence tomography, was performed. Current recommendations from expert consensus statements on MINOCA recommend to consider additional tests like intravascular imaging, pressure-Doppler wire assessment, provocative spasm testing, or cardiac magnetic resonance for exact further specification of etiology (5). There is evidence that coronary tortuosity is more commonly observed in women as compared with men. It has been postulated that bending of the vessel can lead to loss of coronary perfusion pressure, as well as to changes in wall shear stress. Changes in wall shear stress are, in the presence of atherosclerotic disease, associated with progression and vulnerability of coronary plaque. However, true associations between tortuosity and future MINOCA remain to be determined.
CASE 3: INOCA WITH NONOBSTRUCTIVE CORONARY ATHEROSCLEROTIC DISEASE.
A 78-year-old Asian woman was scheduled by a cardiologist for elective ICA to evaluate stable nonexertional chest pain and dyspnea. She had a history of hypertension and type 2 diabetes mellitus, for which she was taking multiple antihypertensive medications, a statin, and oral hypoglycemic agents. Physical examination revealed an overweight woman with a body mass index of 29.7 kg/m2, an elevated systolic blood pressure of 154/65 mm Hg, and no further abnormalities. Her resting ECG showed normal sinus rhythm. Prior cardiac evaluation included an intermediate-risk ECG treadmill stress test, as well as a contrast CT performed with a dual-energy 128-slice CT scanner (SOMATOM Definition Flash, Siemens). Her CCTA demonstrated CAD-RADS 2/V. In more detail, multiple mild coronary plaques were observed in all major epicardial arteries with a quantified maximal diameter stenosis ranging from 34% to 46%. Semi-automated quantification of the coronary plaque in the mid RCA showed a completely noncalcified plaque with a total plaque and necrotic core volume of 48 mm3 and 7 mm3, respectively (Figure 5A). The percent atheroma volumes of total plaque and necrotic core were relatively large, at 30% and 4%, respectively. Within both the proximal and mid RCA, high-risk plaques were observed exhibiting both positive remodeling and low-attenuation plaque with <30 Hounsfield units. In particular, fractional flow reserve derived from CT (FFR-CT) of the RCA showed a pressure drop with a value of 0.78 (Figure 5C). In light of her persistent symptoms and intermediate-risk ECG treadmill stress test, she was clinically referred for ICA with 3-vessel invasive FFR (iFFR) measurements and enrolled in the CREDENCE (Computed TomogRaphic Evaluation of Atherosclerotic DEtermiNants of Myocardial IsChEmia) trial. ICA demonstrated mild CAD in the proximal and mid RCA, in the mid LAD and in the proximal LCx with a maximal diameter stenosis using quantitative coronary angiography of 32%, 41% and 35%, respectively (Figure 5B). These invasive measures of stenosis were almost identical to the aforementioned noninvasive measures. Additionally, iFFR was measured distally to the most stenotic lesion in all major epicardial arteries: iFFR was 0.77 in the RCA, 0.86 in the LAD, and 0.93 in the LCx. Because of persistent symptoms and evidence of provocative epicardial ischemia, percutaneous coronary intervention of the RCA was performed with a drug-eluting stent. Post-intervention iFFR was 0.89, and medications were further optimized. Although inconsistent with current guidelines, this interventional approach is likely born out of a lack of clinical trial evidence or more guidance on treatment strategies from expert consensus statements for nonobstructive coronary atherosclerotic disease. In this case, optimized preventive therapy should always be the primary option.
FIGURE 5. Integrated Imaging Findings of Case 3.
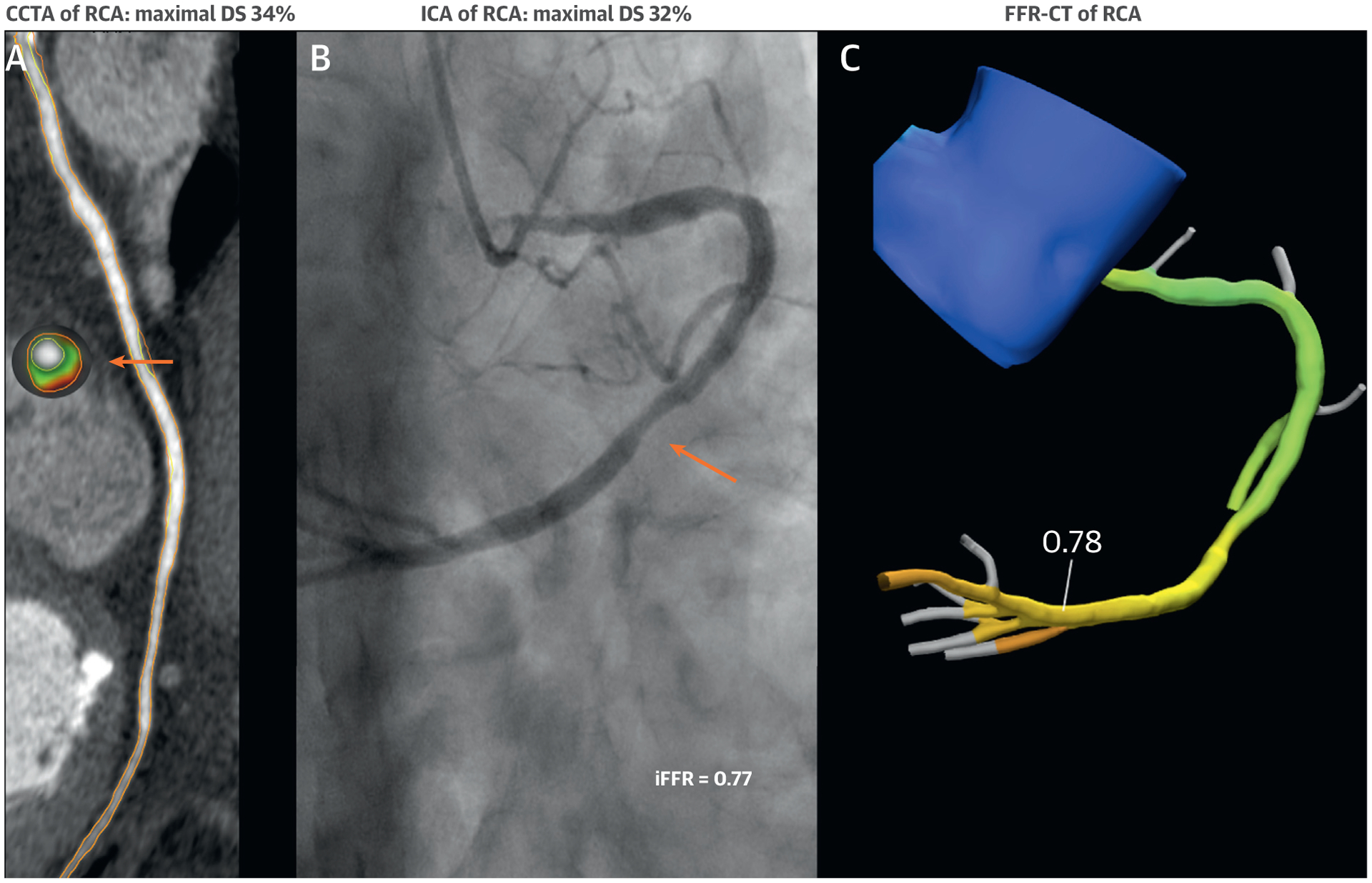
(A) Coronary computed tomography angiography (CCTA) of the RCA. Orange arrows indicate the cross section of maximal DS. On the cross-sectional level, red indicates necrotic core <30 HU; light green indicates fibrofatty plaque 31–130 HU; and dark green indicates fibrous plaque 131–350 HU. (B) Invasive coronary angiography (ICA) of the RCA with invasive fractional flow reserve (iFFR) measurements. (C) FFR derived from computed tomography (FFR-CT) of the RCA. Abbreviations as in Figure 1.
In conclusion, her final diagnosis was INOCA due to nonobstructive coronary atherosclerotic disease exhibiting multiple characteristics of vulnerable plaque. This case illustrates the possibility that additional high-risk markers on CCTA beyond stenosis alone are potentially able to provoke ischemia. Nonetheless, it is important to note that (semi-)automated quantification of coronary plaque is at present still investigational, and not yet ready for routine reporting in clinical practice.
CASE 4: INOCA WITH PRIOR UNRECOGNIZED MYOCARDIAL INFARCTION.
A 54-year-old White postmenopausal woman presented to a cardiologist for a second opinion regarding persistent exertional and rest chest pain. She had a history of gestational diabetes, hypertension, dyslipidemia, and postmenopausal vasomotor and vaginal symptoms, for which she was taking hydrochlorothiazide, oral progesterone, and estradiol vaginal tablets. Physical examination revealed an obese woman with a body mass index of 31 kg/m2, an elevated systolic blood pressure of 150/65 mm Hg, and no further abnormalities. Her resting ECG showed normal sinus rhythm. Prior cardiac evaluation included a normal echocardiogram. She was referred for invasive coronary reactivity testing, which revealed a myocardial bridge in the mid LAD without significant systolic compression (Figure 6A), mild atherosclerosis on intravascular ultrasound, and an abnormal coronary flow reserve (CFR) to intracoronary adenosine at 1.8. An invasive CFR <2.32 has been shown to predict major adverse cardiovascular outcomes in women with suspected ischemia and nonobstructive coronary arteries. During the study, she also developed a spontaneous coronary vasospasm in the distal LAD (Figure 6B), which reproduced her chest pain and resolved with intracoronary nitroglycerin (Figure 6C). Intracoronary acetylcholine to evaluate coronary endothelial dysfunction was not necessary per usual protocol in the setting of severe spontaneous coronary spasm. She was diagnosed with ischemic heart disease with nonobstructive coronary atherosclerotic disease, coronary microvascular dysfunction (CMD) and epicardial coronary vasospasm. Her mid-LAD myocardial bridge was not felt to be contributory to her angina. She was started on aspirin, amlodipine, carvedilol, atorvastatin, and sublingual nitroglycerin. Her diuretic and hormone therapy were discontinued, and she was referred to cardiac rehabilitation. Stress cardiac magnetic resonance, performed as part of the WISE-CVD (Women’s Ischemia Syndrome Evaluation-Coronary Vascular Dysfunction) study, demonstrated stress-induced circumferential subendocardial hypoperfusion on adenosine-stress first-pass perfusion (Figure 7A). She also had evidence of a small distal anterior subendocardial scar seen on cardiac magnetic resonance late gadolinium enhancement (Figure 7B).
FIGURE 6. Invasive Coronary Reactivity Testing of Case 4.
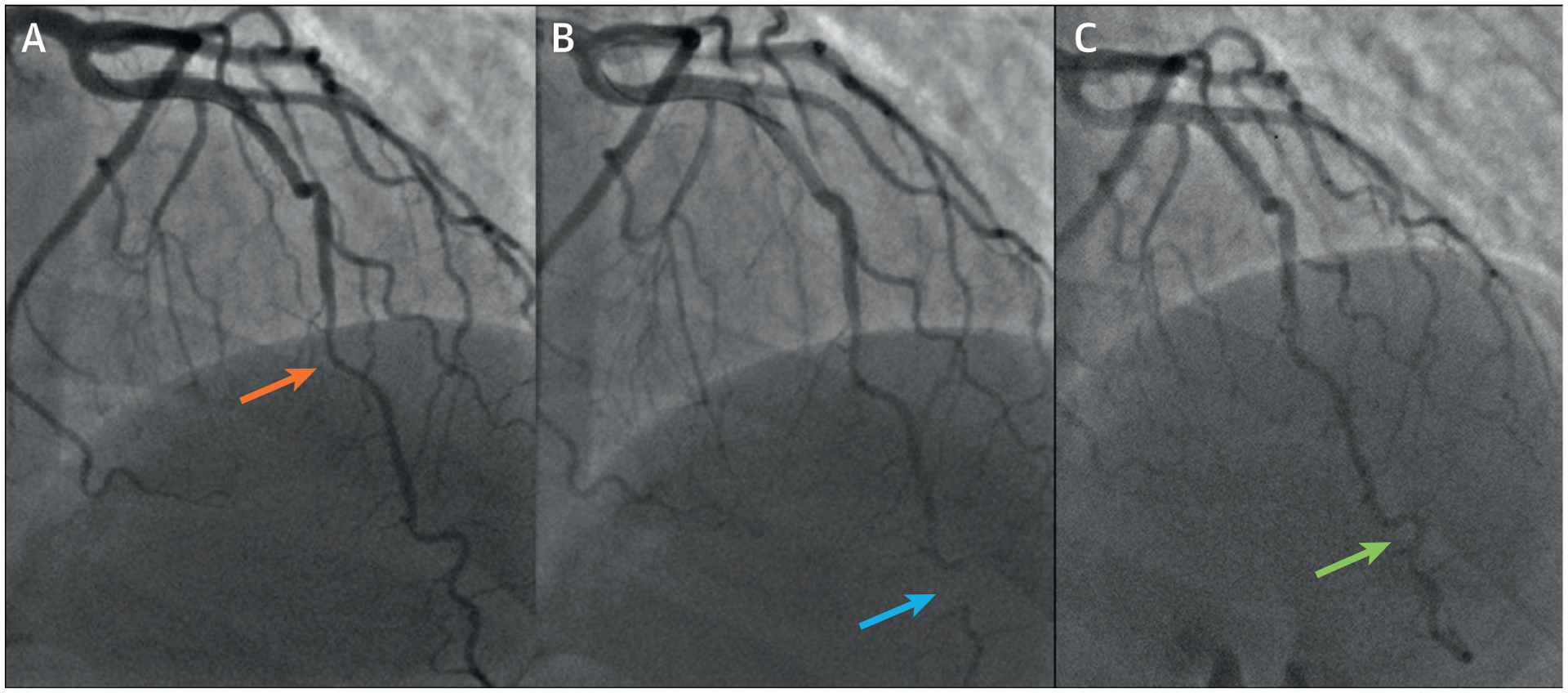
(A) Baseline invasive coronary angiography demonstrating a myocardial bridge (orange arrow) in the mid–left anterior descending artery without significant systolic compression and mild atherosclerosis. Coronary flow reserve to intracoronary adenosine was abnormal at 1.8. (B) Post-adenosine, a spontaneous coronary vasospasm (blue arrow) was visualized in the distal left anterior descending artery with reproduction of chest pain. (C) Intracoronary nitroglycerin resulted in resolution of the coronary vasospasm (green arrow) and chest pain.
FIGURE 7. Stress Cardiac Magnetic Resonance of Case 4.
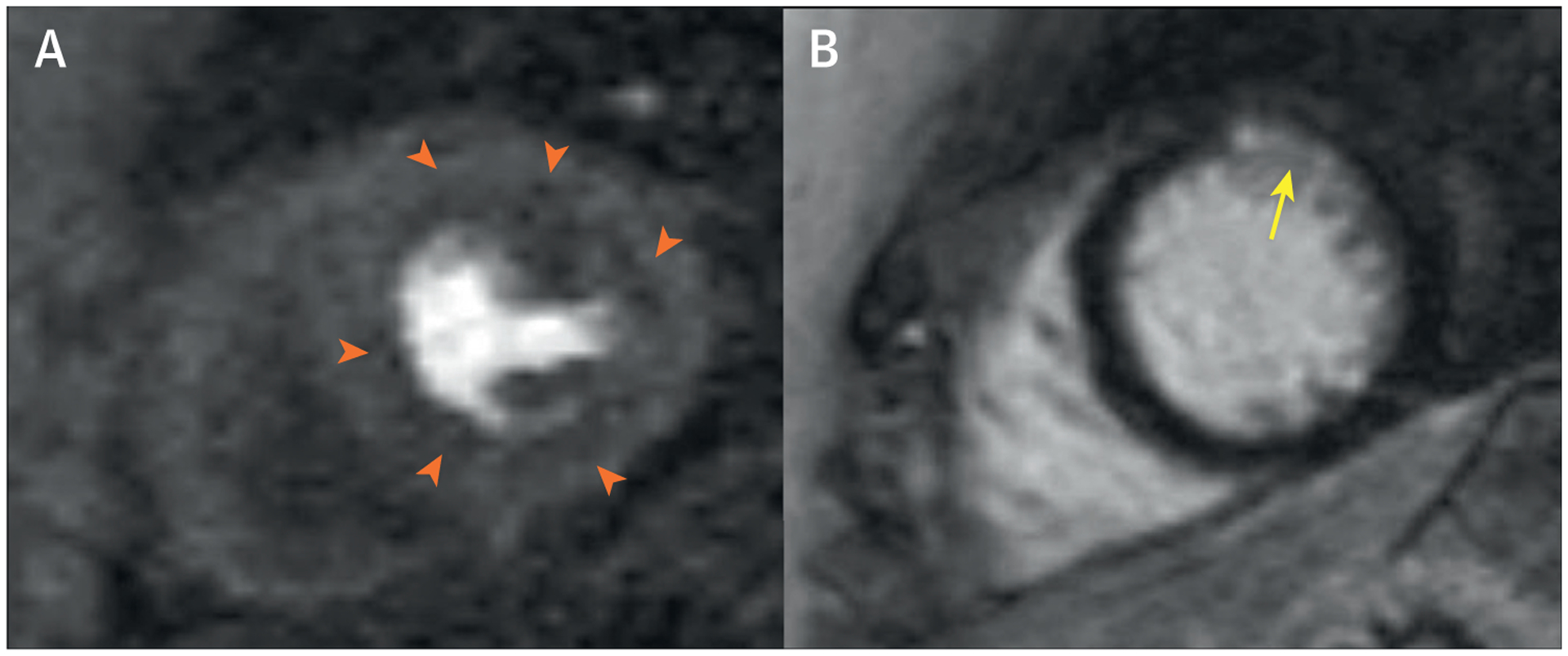
(A) Stress cardiac magnetic resonance demonstrating adenosine-induced circumferential subendocardial hypoperfusion (orange arrowheads) on first pass perfusion. (B) Small distal anterior subendocardial myocardial scar (yellow arrow) detected on cardiac magnetic resonance late gadolinium enhancement.
In conclusion, her final diagnosis was INOCA due to CMD and coronary vasospasm with prior unrecognized myocardial infarction. Her symptoms improved with targeted medical therapy, and she has not experienced any subsequent myocardial infarction or hospitalizations for angina to date. The WISE-CVD study found that myocardial scar was prevalent (8%) in this population, with an annual scar incidence of 1%. One-third of the participants with scar did not have a prior diagnosis of myocardial infarction, suggesting that women with suspected ischemia and nonobstructive coronary atherosclerotic disease not uncommonly have clinically underdiagnosed myocardial infarction. This case is an example in which provocative coronary testing and noninvasive imaging provided additional diagnostic and prognostic information beyond a traditional ICA.
CASE 5: INOCA WITH CORONARY MICROVASCULAR DYSFUNCTION.
A 60-year-old White woman was scheduled by a cardiologist for noninvasive cardiac stress testing to evaluate stable dyspnea, fatigue, and chest discomfort. She had a history of hypertension, obesity, and rheumatoid arthritis. Physical examination revealed an obese woman with a body mass index of 30 kg/m2 and bilateral swelling and tenderness of the small joints of the hands, wrist, and ankles. Her resting ECG showed normal sinus rhythm. She was unable to exercise due to joint pain, and therefore she underwent a pharmacological stress myocardial perfusion positron emission tomography CT. Her ECG response to vasodilator stress was ischemic with 1-mm horizontal ST-segment depression in leads V3 to V5, which was associated with transient chest discomfort similar to the symptoms prompting testing. Gated images demonstrated a normal biventricular size and an LVEF of 67% with normal regional wall motion. Myocardial perfusion images demonstrated a medium-sized, severe perfusion defect involving the LV apical segments and true apex, with near-complete reversibility, reflecting 14.7% ischemic myocardium (Figure 8A). Absolute stress myocardial blood flows and, concordantly, CFR values were reduced in all coronary territories with an abnormal global left ventricular CFR of 1.71 (Figure 8B). She was referred for ICA, which demonstrated absence of obstructive CAD (Figure 8C). The procedure report concluded that she had a false positive stress test. In this context, it should be noted that despite the fact that she did not manifest obstructive epicardial CAD as the etiology of her symptoms, it would be incorrect to assume that her stress test was a false positive result. This patient with multiple cardiac comorbidities, including autoimmune inflammatory disease, had severe INOCA and CMD. An important takeaway from the recent ISCHEMIA (International Study of Comparative Health Effectiveness with Medical and Invasive Approaches) trial was that >20% of enrolled patients (who were required to demonstrate moderate-severe ischemia on objective, core laboratory–verified diagnostic testing) were actually not randomized because they lacked obstructive CAD on anatomic evaluation; most of these patients were women. Of patients enrolled in the ISCHEMIA trial, women had more frequently angina than men despite manifesting less extensive anatomic CAD. Data from the CorMicA (CORonary MICrovascular Angina) trial revealed that approximately 45% of patients referred for the invasive evaluation of angina or ischemia did not have obstructive CAD, and nearly 90% of these patients may demonstrate objective evidence of coronary vasomotor dysfunction using published criteria, including 81% with CMD. As such, many patients with chronic angina, dyspnea, and myocardial ischemia do not have obstructive CAD. Yet, as in her counterpart with obstructive CAD, she requires aggressive medical therapy and risk factor reduction, including optimal management of blood pressure, lipids, and weight. Efforts are ongoing to more precisely phenotype patients like this one, who demonstrate nonobstructive CAD and CMD, to determine which additional interventions may be effective. Newer therapies targeting residual cholesterol and inflammatory risk, neurohormonal activation and glucose handling in the kidneys, and even surgical weight loss may demonstrate improved outcomes in these patients.
FIGURE 8. Integrated Imaging Findings of Case 5.
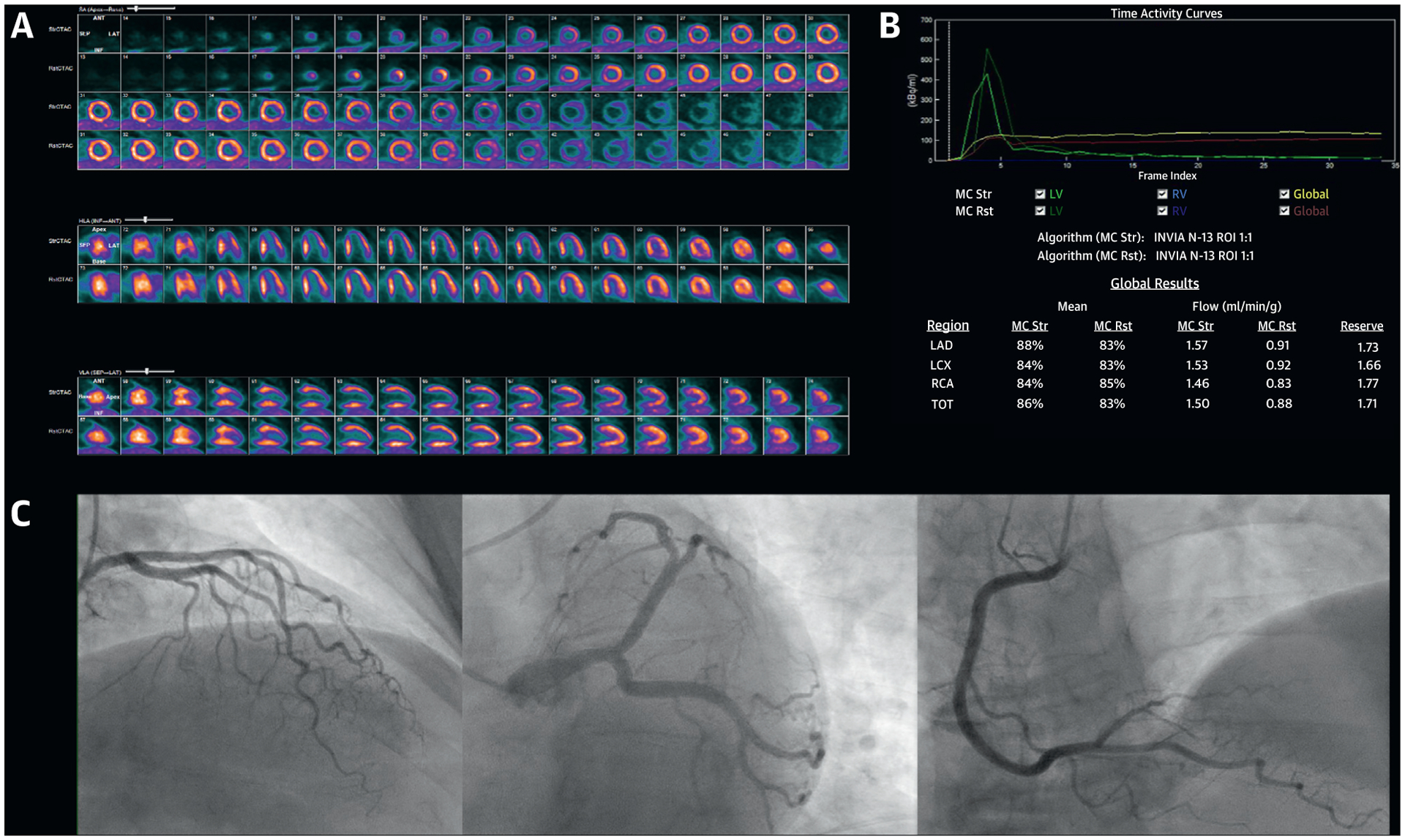
(A) Stress and rest 13N-NH3 myocardial perfusion positron emission tomography demonstrating severe ischemia involving the typical distribution of the mid to distal left anterior descending artery. (B) Positron emission tomography quantitative myocardial blood flow values demonstrating globally reduced coronary flow reserve. (C) Invasive coronary angiography demonstrating absence of obstructive epicardial coronary artery disease.
In conclusion, her final diagnosis was INOCA with CMD. This case illustrates how defining CAD not only as an anatomic problem, but also as a functional one (for which imaging tools may be leveraged in future clinical trials to evaluate the full extent of the coronary circulation), may lead to a more complete understanding of the pathophysiology of ischemic heart disease, especially in women.
CONCLUSIONS
These cases were assembled by the American College of Cardiology’s Cardiovascular Disease in Women Committee to introduce readers to the unique and thought-provoking clinical spectrum of MINOCA and INOCA more common in women. Through these cases, we present the concept that imaging may be applied to target relevant biology in women and that there are sex-specific phenotypes that require tailored detection strategies to reveal the actual high-risk status among women who may erroneously be perceived at lower risk. Invasive and noninvasive imaging can help in our understanding of the underlying pathophysiological mechanisms within and beyond the context of coronary atherosclerotic disease. These mechanisms need to be further elucidated to facilitate more definitive and specific treatment strategies tailored to this important subgroup of women with nonobstructive CAD.
Footnotes
AUTHOR RELATIONSHIP WITH INDUSTRY
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Cardiovascular Imaging author instructions page.
REFERENCES
- 1.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019;73:e285–350. [DOI] [PubMed] [Google Scholar]
- 2.Saw J, Humphries K, Aymong E, et al. Spontaneous coronary artery dissection: clinical outcomes and risk of recurrence. J Am Coll Cardiol 2017;70:1148–58. [DOI] [PubMed] [Google Scholar]
- 3.Lobo AS, Cantu SM, Sharkey SW, et al. Revascularization in patients with spontaneous coronary artery dissection and ST-segment elevation myocardial infarction. J Am Coll Cardiol 2019;74: 1290–300. [DOI] [PubMed] [Google Scholar]
- 4.Hayes SN, Kim ESH, Saw J, et al. Spontaneous Coronary Artery Dissection: Current State of the Science: a Scientific Statement From the American Heart Association. Circulation 2018;137: e523–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agewall S, Beltrame JF, Reynolds HR, et al. ESC working group position paper on myocardial infarction with non-obstructive coronary arteries. Eur Heart J 2017;38:143–53. [DOI] [PubMed] [Google Scholar]


