Abstract
Objective
Hydroxychloroquine (HCQ) is commonly prescribed for the treatment of systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), and other rheumatic diseases. To limit retinal toxicity, the 2016 American Academy of Ophthalmology (AAO) guidelines recommended limiting the HCQ dose to 5 mg/kg/day or less. Our objective was to develop a quality improvement program to improve adherence to these guidelines.
Methods
We performed a retrospective analysis of 801 adult patients receiving HCQ for SLE and RA in a single academic rheumatology practice. In 2018, we calculated weight‐based doses of HCQ at two time points at least 6 months apart. We surveyed provider opinions regarding the 2016 AAO guidelines and implemented a quality improvement intervention during which dosing data were shared with all prescribers (individually and in aggregate) and nurse‐aided decision support was provided for HCQ refill requests. One year after the initial analysis and intervention, we again assessed weight‐based doses of HCQ for the 674 patients still taking HCQ.
Results
At both measured time points during 2018, 22.8% of patients received doses greater than 5 mg/kg/day. For 60% of those patients, the dose of HCQ was reduced to 5 mg/kg/day or less by the study end. Between the second time point in 2018 and the postintervention time point in 2019, there was a statistically significant increase in the proportion of patients receiving of dose of 5 mg/kg/day or less (from 74% to 87%; P < 0.0001).
Conclusion
We observed a significant increase in adherence with current AAO guidelines for weight‐based HCQ dosing after providing feedback to providers regarding their prescribing data and reviewing weight‐based dosing prior to refilling prescriptions.
INTRODUCTION
Hydroxychloroquine (HCQ) is a commonly prescribed medication for the treatment of systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), and other rheumatic diseases. Its safety profile is favorable compared with those of most other medications available for these conditions. Retinal toxicity is a rare but well‐recognized complication of HCQ use, and the risk of HCQ‐induced retinopathy correlates with daily dose and duration of use (1). In addition to ophthalmologic screening examinations, the 2016 guidelines of the American Academy of Ophthalmology (AAO) recommend limiting the dose of HCQ to 5 mg/kg/day or less of actual body weight to reduce the risk of retinopathy (1).
Prior studies examined HCQ prescribing practices after the introduction of these guidelines (2, 3, 4, 5). In these studies, between 30% and 56% of patients taking HCQ received a dose greater than the 2016 guidelines, with higher dosing among patients with low body weight (2, 3, 5). The goal of this study was to determine the current proportion of patients in a large tertiary care, academic rheumatology practice whose HCQ doses complied with the AAO guidelines, to assess provider perspectives on the guidelines, and to implement measures to improve adherence over time.
PATIENTS AND METHODS
Preintervention analysis (2018)
We performed a single‐center retrospective analysis of 1066 adult patients receiving HCQ for SLE or RA in the rheumatology practice of Beth Israel Deaconess Medical Center between January 1, 2018, and December 31, 2018. To account for any “loading dose” adjustments during initiation, we included only patients with two rheumatology provider interactions separated by at least 6 months (Figure 1). For patients who discontinued the drug, the reason for discontinuation was noted, but these patients, as well as those lost to follow‐up or who died during the period of study, were excluded from analysis (Figure 1). For the remaining 801 patients, we collected each patient’s weight and HCQ dose (using both prescription list from the online medical record and pharmacy fill history). We used these data to determine the daily weight‐based dose at each time point and calculated aggregate dosing averages for the practice and dosing data for individual providers. Using data from the second (preintervention) time point, we stratified patients into three dosing categories (≤5 mg/kg/day, >5 to <6.5 mg/kg/day, and ≥6.5 mg/kg/day). We calculated the median age, age range, sex distribution, average body weight, and rheumatology provider’s number of years in independent practice. We performed one‐way ANOVA and t‐tests to assess for statistical significance across groups.
Figure 1.
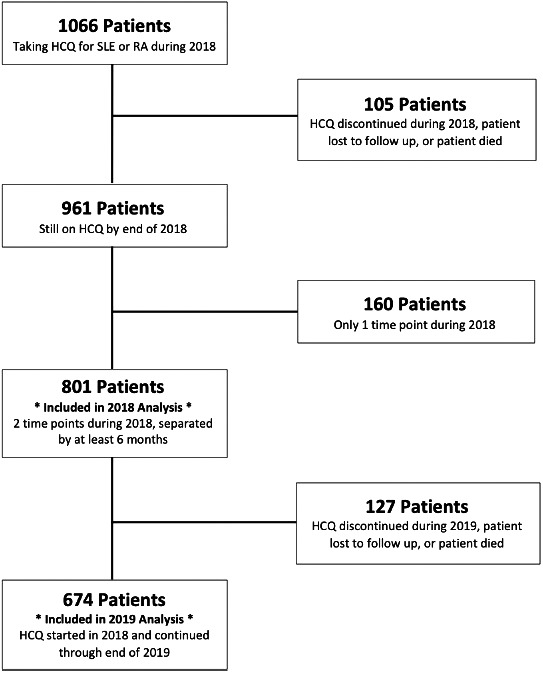
Patients included in the 2018 and 2019 analyses. HCQ, hydroxychloroquine; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus.
Quality improvement intervention
Through mid‐2019, we implemented a two‐pronged quality improvement intervention. First, we shared the practice’s aggregate, anonymized dosing averages with all 19 providers in the practice, including 13 attending physicians and six fellows‐in‐training. We then provided each prescriber with their own dosing data, including a list of medical record numbers for patients receiving a dose greater than 5 mg/kg/day.
The second part of our intervention involved nursing‐aided decision support for HCQ refill requests. For each request, nurses forwarded the patient’s weight‐based dose (using the most recent weight documented in the medical record) to the prescribing physician for approval, alerting the physician if the dose was greater than 5 mg/kg/day.
Provider survey
Shortly after sharing the 2018 prescribing data with individual providers, we distributed an anonymous electronic survey to all providers in the practice. We asked the following four questions regarding their attitudes toward the AAO dosing guidelines:
“Do you support the current recommended weight‐based dosing limit for HCQ of 5mg/kg/day proposed by the American Academy of Ophthalmology?”
“Under what circumstances would you consider exceeding the recommended 5mg/kg/day dosing guideline for HCQ?”
“After receiving the data of your own prescribing practices for HCQ, was any of the information unexpected?”
“Having received this data of your own prescribing practices for HCQ, will this change how you prescribe the medication?”
Postintervention analysis (2019)
In early 2020, we reviewed the same 801 patients included in the 2018 analysis who continued to take HCQ through the 2019 calendar year. We excluded patients with a single time point in 2018 as well as those who were newly started on the medication in 2019. We also excluded patients who died during 2019, who were lost to follow‐up, or for whom the drug was discontinued (Figure 1). For the remaining 674 patients, we recorded the most recent weight and HCQ dosage from the second half of 2019, after our quality improvement intervention. We used these data to calculate aggregate dosing averages for the practice and dosing changes for individual patients.
Using SAS‐JMP software, we performed McNemar’s test to assess for statistical significance between our second preintervention time point and our postintervention time point. We chose the second date from 2018 as our preintervention time point in order to account for any loading dose adjustments that may have been used for patients newly started on HCQ in early 2018. We also performed a multivariate analysis, adjusting for patient age, patient sex, patient weight, and prescribing provider.
Institutional review board statement
This study was reviewed and approved by the Institutional Review Board of Beth Israel Deaconess Medical Center.
RESULTS
Preintervention analysis
Of the 801 patients included in the preintervention analysis, 68.4% received 5 mg/kg/day or less at both dates and an additional 10.3% were prescribed 5 mg/kg/day or less for at least one of the two dates. Of this 10.3%, nearly two‐thirds had doses that trended down from Date 1 to Date 2, whereas the rest had doses that trended up across time points. Of all the patients in the analysis, 21.3% received a dose greater than 5 mg/kg/day for both dates in 2018. Table 1 includes patient characteristics stratified by dosing range at the second preintervention time point. Among these patients, there was a statistically significant decrease in average patient weight with increasing HCQ dosing interval (P < 0.0001), with mean weights of 80.7 kg for patients who received 5 mg/kg/day or less (95% confidence interval [CI], 79.1‐82.3), 67.0 kg for patients who received greater than 5 mg/kg/day and less than 6.5 mg/kg/day (95% CI, 63.9‐70.1), and 54.4 kg for patients who received 6.5 mg/kg/day or more (95% CI, 48.2‐60.6). Additionally, providers for patients with HCQ doses of 6.5 mg/kg/day or more had a higher average number of years in independent practice (12.1 years; 95% CI, 11.3‐13.0) compared with providers for patients with HCQ doses of 5 mg/kg/day or less (17.12 years; 95% CI, 13.9‐20.3) (P = 0.01). Because our study population was mostly female (87%), we were unable to assess differences by sex across dosing groups.
Table 1.
Patient characteristics at the second preintervention time point
| Dosing Range at Date 2 | Median Age, n (range) | Female Patients, n (%) | Male Patients, n (%) | Average Body Weight, kg | Provider With 0‐10 Years in Practice, n (%) |
Provider with 10+ Years in Practice n (%) |
|---|---|---|---|---|---|---|
| <5 mg/kg/day | 55 (19‐98) | 508 (73) | 90 (86) | 80.7* | 264 (78) | 335 (73) |
| >5 to <6.5 mg/kg/day | 47.5 (19‐84) | 147 (21) | 15 (14) | 67.0* | 62 (18) | 100 (22) |
| >6.5 mg/kg/day | 50.5 (24‐94) | 40 (6) | 0 (0) | 54.4* | 13 (4) | 27 (6) |
P < 0.0001.
Provider survey
Of the 19 providers whose patients were included in the preintervention analysis, two left the practice before the survey was sent. Of the remaining 17 providers, 13 responded (response rate: 76%). Eleven of 13 respondents (85%) supported the current recommended weight‐based dosing limit for HCQ of 5 mg/kg/day or less proposed by the AAO. Of the two who did not fully endorse the guidelines, one proposed that dosing based on ideal body weight would be safer. The other suggested that as long as a patient receives annual screening eye examinations to detect early retinal changes, HCQ at doses more than 5 mg/kg/day may be safer and less immunosuppressive for selected patients than other agents available for SLE and RA.
In fact, respondents expressed several circumstances in which they would prescribe doses of HCQ that exceed the guidelines. Most providers (77%) felt that dosing more than 5 mg/kg/day for a limited amount of time is safe. One provider suggested that a patient with SLE whose disease is flaring might temporarily require a higher‐than‐usual dose of HCQ. Another provider suggested that because HCQ can take several weeks to provide clinical benefit and because the ideal dose for an individual patient is uncertain, a patient might temporarily warrant a higher dose when first prescribed. Nearly half of providers (46%) also noted that they would endorse exceeding dosing guidelines in order to avoid potentially more toxic medications. Moreover, two (15%) noted that they may exceed HCQ dosing guidelines in order to simplify a patient’s medication regimen (as HCQ is available only in 200‐mg tablets in the United States).
Of the 13 respondents, 9 (69%) felt that the data describing their own HCQ prescribing practices were as expected. Four (31%) noted unexpected findings. For two of these providers, the data uncovered scenarios that had led to a higher‐than‐anticipated HCQ dose for their patients. For example, one respondent noted that some patients postponed follow‐up and remained on higher doses for longer than planned. Another noted that a patient’s substantial weight loss warranted a dose adjustment that had not yet been made. Nearly half of the responding providers (including all providers who described their individual data as containing unexpected findings) planned to change their prescribing behavior on the basis of the data provided.
Postintervention analysis
Of the 801 patients included in the 2018 analysis, 674 continued to receive care within the practice during 2019 and continued HCQ through the end of the 2019 calendar year. Among these 674 patients, 154 had received more than 5 mg/kg/day for both Dates 1 and 2 during 2018; 93 (60%) of them had dose reductions to 5 mg/kg/day or less by Date 3. Of those who still received a dose greater than the guidelines at Date 3, 50 (82%) received a dose between 5 mg/kg/day and 6.5 mg/kg/day, and 11 (18%) received doses greater than 6.5 mg/kg/day.
Our quality improvement intervention was associated with a statistically significant reduction in HCQ dose between Dates 2 and 3 (Figure 2). By Date 2, all patients had been taking HCQ for at least 6 months. Between Dates 2 and 3, the proportion of patients receiving a dose of 5 mg/kg/day or less increased from 74% to 87% (P < 0.0001). Notably, among providers who continued to prescribe doses above the guidelines by the end of the study, two documented that if the patients’ disease control remained adequate, they planned to lower the dose in the future.
Figure 2.
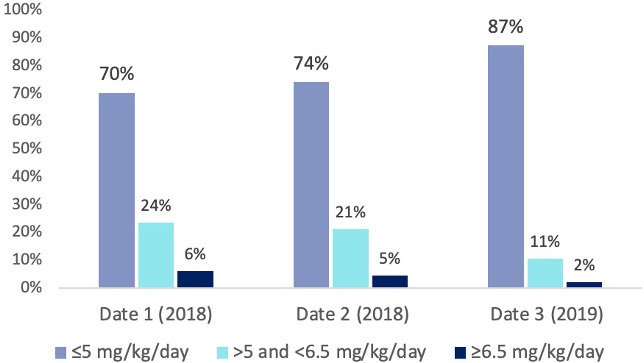
Change in hydroxychloroquine weight‐based dosing over time. HCQ, hydroxychloroquine.
In a multivariate analysis adjusting for the patient age, weight, and sex and the prescribing provider’s number of years in independent practice, the specific provider was a significant effect modifier (P < 0.01). This aligned with the pattern we observed in that certain providers tended to prescribe lower doses of HCQ or reduce their patients’ HCQ doses more often than others. We did not find an association between provider sex or years in practice and the likelihood of dose adjustment, nor did we detect an association between whether a provider responded to the survey and the likelihood of dose adjustment.
DISCUSSION
This study assessed adherence to the 2016 AAO dosing guidelines for HCQ in an academic tertiary care practice, surveyed attitudes of prescribers regarding the guidelines, and evaluated the impact of quality improvement interventions to increase adherence. The preintervention rate of HCQ doses of more than 5 mg/kg/day (24%) in our practice was lower than rates described in the literature shortly after publication of the 2016 AAO guidelines (2, 3, 4). Perhaps this is related to increased time elapsed since publication of the guidelines, or it may reflect geographical variation. Consistent with prior studies (2, 3, 5), we found that patients receiving a dose of more than 5 mg/kg/day were more likely to be of lower body weight compared with patients receiving doses according to the guidelines. More experienced physicians were somewhat more likely to prescribe higher doses of HCQ; this could be due to longstanding practice habits that were resistant to change after the 2016 guidelines were released or greater confidence in the safety of HCQ despite changes in guidelines. Notably, the effectiveness of our intervention was not significantly different between more or less experienced physicians. After providing feedback regarding prescribing data to physicians in our practice and alerting them regarding refill requests for doses that exceed current guidelines, we observed a significant increase in the proportion of patients whose HCQ doses were 5 mg/kg/day or less.
The 2016 AAO guidelines modified prior recommendations by decreasing the recommended HCQ dosing limit from 6.5 mg/kg/day of ideal body weight or less to 5 mg/kg/day of actual body weight or less (6, 7). Significant nonadherence with these guidelines has been observed and may increase risk of retinopathy, particularly with long‐term use of HCQ (7). One criticism of these guidelines has been that they do not take into account the clinical nuances of treating patients with SLE (8), RA, and other rheumatic diseases. That said, a recent multispecialty joint statement (including six rheumatologists) expressed support of the 2016 AAO guidelines while still emphasizing the medication’s importance (9). Adherence with the AAO guidelines could certainly have unintended consequences, including increased disease activity and loss of other potential benefits from HCQ, including reduced mortality (10), reduced risk of thrombosis, and reduced metabolic and cardiovascular disease (11). There is no well‐established minimum effective dose for HCQ (4), and it is unclear what dose is necessary to confer these other potential benefits. Further research into this area would be helpful to guide dose reduction guidelines in the future.
Our survey and quality improvement intervention identified some important insights into HCQ prescribing practices at our institution. Providers within our practice acknowledged that there are times that they would not follow these guidelines (such as increased disease activity), but some providers were clear that they would follow the guidelines more closely if made more aware of their practice patterns. Our practice had near complete consensus to try to follow the guidelines more often, and our intervention appeared to be effective in that regard.
The interventions described in this study involved minimal effort by individual prescribers. Sharing a list of patients receiving doses greater than the guidelines provided a targeted, actionable list for the practice and each prescriber. Real‐time feedback to providers at the time of HCQ prescription refill requests also required little prescriber time or effort. Reminder systems, repeated feedback, and the use of a combination of interventions simultaneously have been demonstrated within the quality improvement literature as effective methods for changing clinical practice (12). Other approaches, including electronic health record (EHR) systems that provide reminders or “force functions” can encourage compliance with dosing guidelines as well (13). However, not all practices have access to such EHRs or programmers to implement such a process.
There are several potential limitations of our study. Medication nonadherence is a nontrivial issue for patients taking HCQ (14). Though we did assess each patient’s listed dose in the medical record and attempted to confirm their refill history, details regarding adherence and provider instructions were not available for all patients. Therefore, the HCQ dose actually taken by patients may have been overestimated. Some details regarding individual patients’ history of HCQ use were not available, including how long each patient had been on the drug, glucocorticoid use, past efforts to taper HCQ dose, or assessments of disease activity while taking various doses. Additionally, because our quality improvement intervention included two components, we are unable to determine which had the larger impact on prescribing practices. Given that there was a modest reduction in patients receiving doses greater than the guidelines from the first preintervention time point to the second time point, it is possible that some providers may have made dose adjustments independent of our intervention. As this was a single‐center study in a tertiary care setting, the results may not be generalizable to other clinical settings.
Because many patients remain on HCQ for years, or even decades, it is our hope that improved weight‐based dosing will reduce the long‐term risk of HCQ‐induced retinopathy without leading to loss of disease control or other unintended consequences. It is likely that the quality improvement interventions described here can be readily adopted by other practices in a variety of settings with an expectation of similar or even better results.
AUTHOR CONTRIBUTIONS
Drs. Skorupa and Shmerling drafted the article, revised it critically for important intellectual content, approved the final version to be published, and take responsibility for the integrity of the data and the accuracy of the data analysis.
ACKNOWLEDGMENT
The authors of this study would like to acknowledge the participation of the rheumatologists at Beth Israel Deaconess Medical Center and their investment in ongoing healthcare quality improvement.
Funding for this project was provided by the Rheumatology Research Foundation.
No potential conflicts of interest relevant to this article were reported.
REFERENCES
- 1. Marmor MF, Kellner U, Lai TY, Melles RB, Mieler WF, American Academy of Ophthalmology . Recommendations on screening for chloroquine and hydroxychloroquine retinopathy (2016 revision). Ophthalmology 2016;123:1386–94. [DOI] [PubMed] [Google Scholar]
- 2. Braslow RA, Shiloach M, Macsai MS. Adherence to hydroxychloroquine dosing guidelines by rheumatologists: an electronic medical record‐based study in an integrated health care system. Ophthalmology 2017;124:604–8. [DOI] [PubMed] [Google Scholar]
- 3. Gianfrancesco MA, Schmajuk G, Haserodt S, Trupin L, Izadi Z, Jafri K, et al. Hydroxychloroquine dosing in immune‐mediated diseases: implications for patient safety. Rheumatol Int 2017;37:1611–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Melles RB, Jorge AM, Marmor MF, Zhang Y, Choi HK. Sharp decline in hydroxychloroquine dosing‐analysis of 17,797 initiators from 2007 to 2016. Clin Rheumatol 2018;37:1853–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jorge AM, Melles RB, Zhang Y, Lu N, Rai SK, Young LH, et al. Hydroxychloroquine prescription trends and predictors for excess dosing per recent ophthalmology guidelines. Arthritis Res Ther 2018;20:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marmor MF, Kellner U, Lai TY, Lyons JS, Mieler WF, American Academy of Ophthalmology . Revised recommendations on screening for chloroquine and hydroxychloroquine retinopathy. Ophthalmology 2011;118:415–22. [DOI] [PubMed] [Google Scholar]
- 7. Melles RB, Marmour MF. The risk of toxic retinopathy in patients on long‐term hydroxychloroquine therapy. JAMA Opthalmol 2014;132:1453–60. [DOI] [PubMed] [Google Scholar]
- 8. Pullen LC. Rheumatologists debate hydroxychloroquine dosing guidelines for lupus. The Rheumatologist. 2019. URL: https://www.the‐rheumatologist.org/article/rheumatologists‐debate‐hydroxychloroquine‐dosing‐guidelines‐for‐lupus/.
- 9. Rosenbaum JT, Costenbader KH, Desmarais J, Ginzler EM, Fett N, Goodman SM, et al. American College of Rheumatology, American Academy of Dermatology, Rheumatologic Dermatology Society, and American Academy of Ophthalmology 2020 joint statement on hydroxychloroquine use with respect to retinal toxicity. Arthritis Rheumatol 2021;73:908–11. [DOI] [PubMed] [Google Scholar]
- 10. Alarcón GS, McGwin G, Bertoli AM, Fessler BJ, Calvo‐Alén J, Bastian HM, et al. Effect of hydroxychloroquine on the survival of patients with systemic lupus erythematosus: data from LUMINA, a multiethnic US cohort (LUMINA L). Ann Rheum Dis 2007;66:1168–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rempenault C, Combe B, Barnetche T, Cécile Gaujoux‐Viala C, Lukas C, Morel J, et al. Metabolic and cardiovascular benefits of hydroxychloroquine in patients with rheumatoid arthritis: a systematic review and meta‐analysis. Ann Rheum Dis 2018;77:98–103. [DOI] [PubMed] [Google Scholar]
- 12. Strom KL. Quality improvement interventions: what works? [Review]. J Healthc Qual 2001;23:4–14. [DOI] [PubMed] [Google Scholar]
- 13. Koppikar S, Gotthei S, Farrer C, Gakhal N. Improving hydroxychloroquine dosing and toxicity screening at a tertiary care ambulatory centre: a quality improvement initiative. J Rheumatol 2021;48:138–44. [DOI] [PubMed] [Google Scholar]
- 14. Hachulla E, le Gouellec N, Launay D, Balquet MH, Maillard H, Azar R, et al. Adherence to hydroxychloroquine in patients with systemic lupus: contrasting results and weak correlation between assessment tools. Joint Bone Spine 2020;87:603–10. [DOI] [PubMed] [Google Scholar]


