Abstract
Objective
Biological agents have shown markedly different response rates by baseline C‐reactive protein (CRP). Here, we determine the response of patients with nonradiographic axial spondyloarthritis (nr‐axSpA) to etanercept stratified by their baseline CRP level.
Methods
The EMBARK trial was a phase 3, randomized, double‐blind, placebo‐controlled study of etanercept in nr‐axSpA. The primary endpoint was Assessment of Spondyloarthritis International Society (ASAS) 40 at Week 12, the conclusion of the double‐blind phase. It recruited patients who met the ASAS criteria for axial spondyloarthritis, and sacroiliac joint magnetic resonance scans were completed on all patients. In this post hoc analysis, we analyzed outcomes by baseline C‐reactive protein (CRP) level of less than 5 mg/L, 5 mg/L to 10 mg/L, and greater than 10 mg/L. The clinical trial outcome data were accessed via the Vivli platform.
Results
In the less than 5 mg/L CRP group treated with etanercept, the ASAS20 response, ASAS40 response, Ankylosing Spondylitis Disease Activity Score‐CRP (ASDAS‐CRP), and ASDAS‐ESR (erythrocyte sedimentation rate) outcomes were 49% (P = 0.84), 26% (P = 0.14), 42% (P = 0.002), and 44% (P = 0.006), respectively. In the 5 to 10 mg/L CRP group treated with etanercept, the ASAS20 response, ASAS40 response, ASDAS‐CRP, and ASDAS‐ESR outcomes were 56% (P = 0.99), 31% (P = 0.40), 56% (P = 0.16), and 50% (P = 0.11), respectively. In the greater than10 mg/L CRP group treated with etanercept, the ASAS20 response, ASAS40 response, ASDAS‐CRP, and ASDAS‐ESR outcomes were 74% (P = 0.02), 68% (P = 0.003), 82% (P = 0.005), and 50% (P = 0.001), respectively.
Conclusion
Although there are reduced ASAS20 and ASAS40 response rates in the groups with baseline CRP less than 10 mg/L, there remain clinically relevant responses when the composite outcome measures ASDAS‐CRP or ASDAS‐ESR were used, and this should be considered when deciding on thresholds for reimbursement.
INTRODUCTION
Nonradiographic axial spondyloarthritis (nr‐axSpA) has a growing number of effective therapies (1). These treatments aim to address the significant symptom load and functional impairment this disease causes (2). Previous studies of biologics in axial spondyloarthritis (axSpA) have identified that baseline magnetic resonance imaging (MRI) and C‐reactive protein (CRP) status predict treatment response (3, 4). One study has shown that when objective inflammation is lacking, no clinical efficacy of etanercept treatment was demonstrable (5). This has led many regulators and funders to require the demonstration of objective inflammation as part of the label or for reimbursement, respectively. This is potentially based on health economic assessments, which conclude that, in those who lack objective inflammation, the cost of the therapy does not justify the clinical response achieved or quality‐adjusted life years gained. There are different opinions in the field about the ability to accurately diagnose axSpA in the absence of signs of objective inflammation (6, 7, 8). Therefore, it is very important to understand how baseline CRP level impacts treatment response in nr‐axSpA.
The EMBARK trial studied the treatment efficacy of etanercept in patients with nr‐axSpA, enrolling patients with active nr‐axSpA who had chronic back pain for less than 3 months (9). Patients received etanercept for 12 weeks before continuing in the open‐label phase of the trial. In this post hoc analysis of the EMBARK trial, we have determined treatment response after patients were stratified into three groups according to their baseline CRP level. In some countries (eg, Australia), access to anti‐tumor necrosis factor (anti‐TNF) for nr‐axSpA is restricted to patients with CRP greater than 10 mg/dl. This analysis was specifically completed to determine whether patients with low (<5 mg/L) or low‐positive (5‐10 mg/L) CRP levels achieved clinically important treatment responses relevant in some jurisdictions (eg, Australia) where patients with nr‐axSpA are not eligible for reimbursed therapy if CRP levels are low (<5 mg/L) or low normal (5‐10 mg/L) at baseline.
PATIENTS AND METHODS
The EMBARK trial was a multicenter, randomized, double‐blind, placebo‐controlled trial of etanercept in nr‐axSpA. We obtained the clinical trial data from the EMBARK trial through the Vivli clinical trial data platform (www.vivli.org). The full EMBARK trial protocol can be found in the original publication (9). Briefly, patients aged 18 years or more but less than 50 years with chronic back pain for greater than 3 months and less than 5 years and active nr‐axSpA (as determined by the 2009 Assessment of Spondyloarthritis International Society [ASAS] axSpA criteria and a Bath axSpA Disease Activity Index [BASDAI] score ≥4) were recruited. They were required to have had an inadequate response to two or more nonsteroidal anti‐inflammatory drugs taken separately for a total duration of more than 4 weeks. A total of 215 patients entered the study; 106 were randomized into the etanercept treatment group, and 109 were randomized to the placebo group. The trial had the following two phases: a 12‐week double‐blind phase and a 12‐week open‐label extension. This paper reports on the data from the 12‐week double‐blind phase.
The data analysis was carried out in R studio and Prism. The primary objective was to determine the response rates by baseline CRP level. The patients were stratified into the following three levels: CRP of less than 5 mg/L, CRP of 5 mg/L to 10 mg/L, and CRP of more than 10 mg/L. For the purposes of this manuscript, these are referred to as low, low‐positive, and high, respectively. Patients who did not have an ASAS20 or ASAS40 response documented were removed from the analysis. The odds ratio (OR) and number needed to treat (NNT) to achieve an ASAS20 and ASAS40 response were calculated. A Fisher exact test for each CRP group was calculated to compare the placebo and etanercept groups.
As a secondary objective, we wanted to determine whether the baseline CRP level could predict the effect of etanercept on other measures of disease activity. The other outcome measures assessed included axSpA Disease Activity Score‐CRP (ASDAS‐CRP), ASDAS‐ESR, BASDAI, and Spondyloarthritis Research Consortium of Canada (SPARCC) sacroiliac (SIJ) MRI score. If patients were missing any of the scores, they were excluded from the final analysis. Details of their composition are detailed elsewhere (10). Each of the scores was plotted against the baseline CRP level, and a linear regression was performed using Spearman’s correlation.
The change in ASDAS scores can be assessed on the basis of previously published thresholds (10). A decrease in ASDAS scores of less than 1.1 was considered as no clinical improvement. Minimal clinical improvements were defined as a change in scores of 1.1 to 2.0. Major clinical improvements were changes in scores of 2.0 or more. The ASDAS‐CRP or ASDAS‐ESR scores at baseline were subtracted from the scores at Week 12. The scores were stratified by the change in ASDAS score and baseline CRP group. A χ2 score was calculated to determine whether there was a significant number of patients with a major clinical benefit. A χ2 analysis was performed to determine whether there were any significant differences in proportions. To calculate an OR and NNT, we categorized the patients into two groups, including those with no clinical benefit (change in score <1.1) and those with clinical benefit (change in score ≥1.1).
For BASDAI and SPARCC SIJ scores, patients can be categorized into those with improvement and those without improvement. Decreases in BASDAI scores greater than 50% are indicative of clinical improvement. SPARCC SIJ score decreases greater than 2.5 were categorized as clinical improvement. The patients were again stratified by baseline CRP levels and contingency tables. A Fisher exact test was performed to determine whether there were significant differences between the placebo and etanercept groups. The OR and NNT were also calculated for these indices.
RESULTS
Data were provided from 214 patients in total (placebo = 108; etanercept = 106). The patients were stratified by their baseline CRP levels. After stratification, there were 144 patients (placebo = 73; etanercept = 71), 31 patients (placebo = 15; etanercept = 16), and 39 patients (placebo = 20; etanercept = 19), in the low, low‐positive, and high CRP baseline groups, respectively. Eight patients were missing ASAS20 and ASAS40 outcome data and were excluded from the final analysis. After the missing data were excluded, there were 138 patients (placebo = 72; etanercept = 66), 30 patients (placebo = 14; etanercept = 16), and 38 patients (placebo = 19; etanercept = 19), in the low, low‐positive, and high CRP baseline groups, respectively. Patients treated with etanercept had higher ASAS20 and ASAS40 responses (Figure 1 and Supplementary Table 1). Of placebo‐treated patients with baseline CRP levels less than 5 mg/L, 33% had an ASAS20 response and 15% had an ASAS40 response. In comparison, 48% and 26% of etanercept‐treated patients had an ASAS20 and ASAS40 response, respectively. Of placebo‐treated patients with a baseline CRP level between 5 mg/L and 10 mg/L, 57% and 14% of patients had an ASAS20 and ASAS40 response, respectively. At the same baseline CRP level, 56% and 31% of etanercept‐treated patients had an ASAS20 and ASAS40 response, respectively. However, the differences were not significantly different at the low and low‐positive CRP baseline levels (P ≥ 0.05). The difference between placebo‐ and etanercept‐treated patients was significantly different in patients in the high CRP group (P < 0.05). In these patients, an ASAS20 response was achieved in 32% of placebo‐treated patients and 74% of etanercept‐treated patients. The ASAS40 response rate was similar, with 16% of placebo‐treated patients and 68% of etanercept‐treated patients achieving an ASAS40 response.
Figure 1.
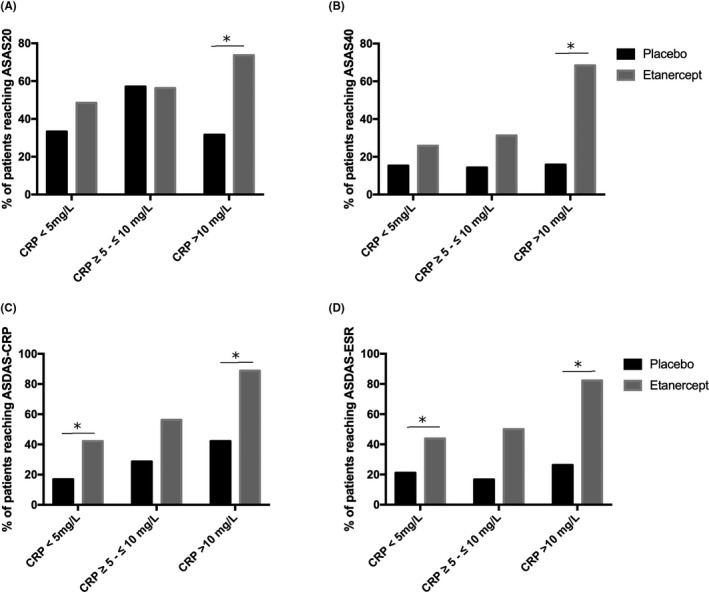
Outcomes by C‐reactive protein (CRP) group. The percentage of patients reaching Assessment of Spondyloarthritis International (ASAS) 20 (A), ASAS40 (B), Ankylosing Spondylitis Disease Activity Score‐CRP (ASDAS‐CRP) (C), or Ankylosing Spondylitis Disease Activity Score‐Erythrocyte Sedimentation Rate (ASDAS‐ESR) (D) criteria for clinical improvement were plotted after being stratified into their appropriate baseline CRP levels. *Significant differences between the placebo and etanercept group (P < 0.05).
We also investigated response according to the composite ASDAS‐CRP and ASDAS‐ESR score. Patients with ASDASs of 1.1 or less were considered to have clinical improvement. The percentage of patients responding to etanercept was similar using the ASDAS‐CRP or ASDAS‐ESR score (Figure 1 and Supplementary Table 1). In patients with a baseline CRP level of less than 5 mg/L, there were 17% were responders with the ASDAS‐CRP and 21% were responders with the ASDAS‐ESR. Patients treated with etanercept had a significantly higher percentage of patients with clinical response (42% and 44% using the ASDAS‐CRP and ASDAS‐ESR, respectively). Similarly, patients with a baseline CRP level of more than10 mg/L also had a significantly higher percentage of patients who responded to etanercept as compared with placebo, as per the ASDAS‐CRP and ASDAS‐ESR response. In the placebo group, 42% and 26% of patients responded to the ASDAS‐CRP and ASDAS‐ESR, respectively. Conversely, in patients treated with etanercept, 89% and 82% had a clinical response as per the ASDAS‐CRP and ASDAS‐ESR, respectively. Although patients with low and high CRP levels responded to etanercept, patients with a baseline CRP level between 5 mg/L and 10 mg/L did not have a significant clinical response over those treated with placebo. In placebo‐treated patients, 29% and 17% were responders according to the ASDAS‐CRP and ASDAS‐ESR, respectively. Of etanercept‐treated patients, 56% and 50% were responders according to the ASDAS‐CRP and ASDAS‐ESR, respectively.
The OR and NNT were calculated at each of the CRP baseline levels (Table 1). Patients treated with etanercept had a greater likelihood of reaching an ASAS20 or ASAS40 response. However, only patients with a high baseline CRP level had significantly higher odds of response (P < 0.05).
Table 1.
The odds ratio and NNT for patients treated with etanercept
| Odds Ratio | 95% Lower Confidence Interval | 95% Upper Confidence Interval | NNT | N (n Placebo, n Etanercept) | |
|---|---|---|---|---|---|
| ASAS20 | |||||
| CRP <5 mg/L | 1.9 | 1.0 | 3.8 | 6.6 | 138 (72, 66) |
| CRP 5‐10 mg/L | 1.0 | 0.2 | 3.8 | 112 | 30 (14, 16) |
| CRP >10 mg/L | 6.1 | 1.5 | 27.8 | 2.4 | 38 (19, 19) |
| ASAS40 | |||||
| CRP <5 mg/L | 1.9 | 0.9 | 4.3 | 9.5 | 138 (72, 66) |
| CRP 5‐10 mg/L | 2.7 | 0.4 | 15.4 | 5.9 | 30 (14, 16) |
| CRP >10 mg/L | 11.6 | 2.4 | 45.06 | 1.9 | 38 (19, 19) |
| ASDAS‐CRP (for clinical improvement) | |||||
| CRP <5mg/L | 3.6 | 1.7 | 8.2 | 4.0 | 135 (71, 64) |
| CRP 5‐10 mg/L | 3.2 | 0.76 | 12.0 | 3.6 | 30 (14, 16) |
| CRP >10 mg/L | 11.0 | 1.9 | 55.0 | 2.1 | 37 (19, 18) |
| ASDAS‐ESR (for clinical improvement) | |||||
| CRP <5 mg/L | 2.9 | 1.4 | 6.0 | 4.4 | 137 (71, 66) |
| CRP 5‐10 mg/L | 5.0 | 0.9 | 27.0 | 3.0 | 28 (12, 16) |
| CRP >10 mg/L | 13.1 | 2.5 | 52.3 | 1.8 | 36 (19, 17) |
| BASDAI | |||||
| CRP <5 mg/L | 2.0 | 0.9 | 4.0 | 7.0 | 138 (72, 66) |
| CRP 5‐10 mg/L | 2.5 | 0.6 | 9.4 | 4.7 | 30 (14, 16) |
| CRP >10 mg/L | 6.4 | 1.5 | 22.2 | 2.4 | 38 (19, 19) |
| SPARCC SIJ score | |||||
| CRP <5 mg/L | 0.6 | 0.2 | 1.4 | 12.0 | 135 (72, 63) |
| CRP 5‐10 mg/L | 0.6 | 0.1 | 3.3 | 8.4 | 29 (14, 15) |
| CRP >10 mg/L | 0.3 | 0.1 | 1.6 | 4.8 | 38 (19, 19) |
Abbreviation: ASAS, Assessment of Spondyloarthritis International Society; ASDAS‐CRP, Ankylosing Spondylitis Disease Activity Score‐CRP; ASDAS‐ESR, Ankylosing Spondylitis Disease Activity Score‐Erythrocyte Sedimentation Rate; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; CRP, C‐reactive protein; NNT, number needed to treat; SIJ, sacroiliac; SPARCC, Spondyloarthritis Research Consortium of Canada.
We then studied patient response to etanercept based on additional outcome measures. The measures studied were the ASDAS‐CRP, the ASDAS‐ESR, the BASDAI, and the SPARCC SIJ scores. In our initial analysis of these four scores, the baseline CRP levels were plotted against each score; then, a linear regression was performed with Spearman’s correlation (Figure 2). In the placebo patients, there was no significant correlation between the baseline CRP score and response to treatment. Conversely, etanercept‐treated patients had increasing treatment responses as the baseline CRP level increased.
Figure 2.
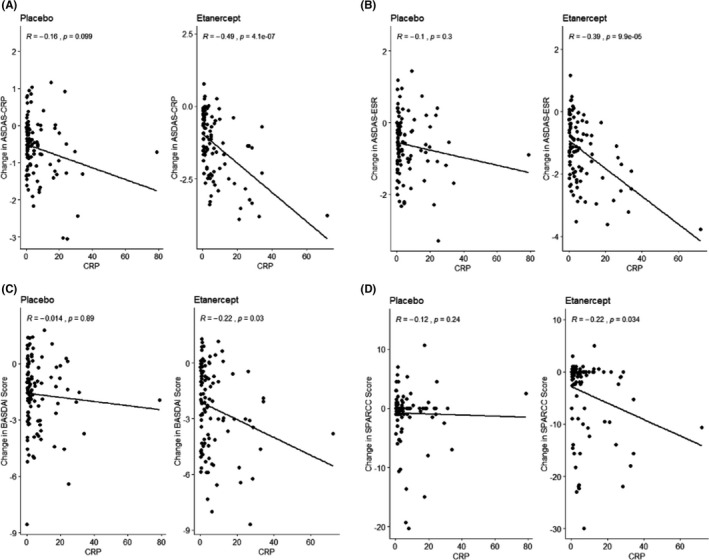
The continuous relationship between C‐reactive protein (CRP) and outcome. The graph of baseline CRP serum levels (mg/L) against the Ankylosing Spondylitis Disease Activity Score‐CRP (ASDAS‐CRP) (A), Ankylosing Spondylitis Disease Activity Score‐Erythrocyte Sedimentation Rate (ASDAS‐ESR) (B), Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) (C), and Spondyloarthritis Research Consortium of Canada (SPARCC) sacroiliac (SIJ) (D) scores.
We studied each of the scores in further detail. The ASDAS responses can be categorized by the change in the score from the baseline. The scores are divided into clinical worsening, no improvement, minor clinical improvement, and major clinical improvement (see methods). A χ2 analysis demonstrated that there were significantly different proportions of patients in each of the different groups between the etanercept‐ and placebo‐treated groups (P < 0.05). As with the ASAS responses, we stratified the patients on the basis of their baseline CRP level to determine whether the baseline CRP level affected etanercept treatment response. In the ASDAS‐CRP and ASDAS‐ESR, the response proportions were significantly different at the low and high CRP levels (P < 0.05) but not in the low‐positive levels (Supplemental Figure 1) (P > 0.05). When the patients were categorized more broadly into those with no clinical improvement and those with any clinical improvement, the results were unchanged (Figure 3). The low and high CRP levels remained statistically significant, but the low‐positive baseline CRP level remained not significant (Supplementary Figure 1).
Figure 3.
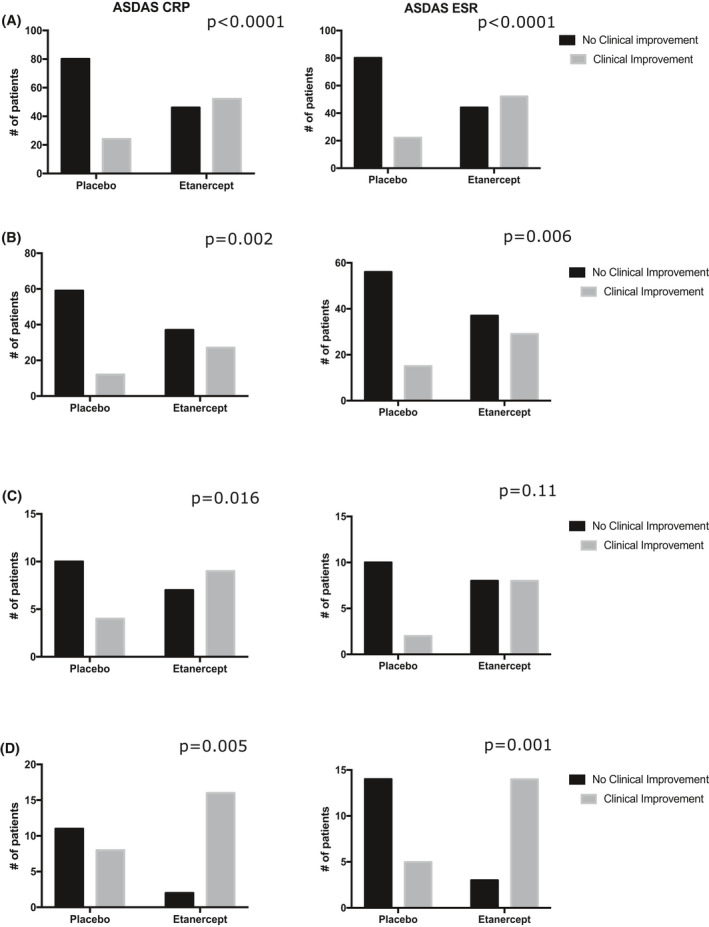
Clinical improvement in Ankylosing Spondylitis Disease Activity Score C‐reactive protein (ASDAS‐CRP) and Ankylosing Spondylitis Disease Activity Score‐Erythrocyte Sedimentation Rate (ASDAS‐ESR). The patients were stratified based on baseline C‐reactive protein (CRP) level. The number of patients that had clinical improvement and no clinical improvement are shown. (A) All patients (Not stratified based on CRP). (B) Patients with a CRP level of less than 5 mg/L. (C) Patients with a CRP level of 5 to 10 mg/L. (D) Patients with a CRP level greater than 10 mg/L. The P values for each Fischer exact test are displayed on their respective graphs. P < 0.05 was considered significant.
The ORs for ASDAS‐CRP and ASDAS‐ESR were in the 3 to 5 range for the low and middle baseline CRP levels. The NNTs for these levels ranged from 3 to 5 as well. Significantly, in patients with the highest baseline CRP levels, the OR was 11.0 and 13.1 for the ASDAS‐CRP and ASDAS‐ESR, respectively. Similarly, the NNT was 2.1 and 1.8 for the ASDAS‐CRP and ASDAS‐ESR, respectively (Table 1).
The BASDAI score can also be categorized into clinical improvement or no clinical improvement. Similar to the ASDAS score, the proportion of all patients showing benefit was significantly different between the etanercept‐ and placebo‐treated groups (P < 0.05). Low and low‐positive CRP levels showed no significant differences in proportions between etanercept‐ and placebo‐treated patients. Conversely, patients who were treated with etanercept and had a baseline CRP level of more than 10 mg/L had a significantly lower proportion of people with a positive response (Supplementary Figure 2).
Similarly, we performed the same analysis with the SPARCC SIJ scores. However, there was no significant difference in proportions between etanercept‐ and placebo‐treated patients, regardless of the baseline CRP level (Table 1 and Supplementary Figure 2).
DISCUSSION
In this study, we performed a post hoc analysis on data from the EMBARK trial. The original EMBARK trial stratified patients into high and low baseline CRP levels. The aim of baseline CRP stratification was to determine response rates based on finer graduations of baseline CRP.
We found that, in patients with nr‐axSpA, the baseline CRP level was correlated with a positive response to etanercept treatment as per the ASAS20, ASAS40, ASDAS‐CRP, ASDAS‐ESR, BASDAI, and change in SPARCC SIJ score outcomes. All the scores (except the change in SPARCC SIJ score) that we studied showed that a CRP level of more than 10 mg/L was associated with a significant response to etanercept; the ASDAS‐CRP and ASDAS‐ESR scores (composite outcome measures) also demonstrated significant improvement in the low CRP group. The finding of an NNT of 112 in the 5 to 10 mg/L CRP group for the ASAS20 outcome is not in keeping with the pattern of results from the other outcome measures and is potentially the result of low numbers in this group.
Multiple investigators have shown that a higher baseline CRP level predicts a higher response to treatment in patients with radiographic axSpA and nr‐axSpA (3, 4, 11, 12, 13, 14). In a large study by Rudwaleit et al, 1250 patients with early axSpA were randomized to placebo or adalimumab treatment (3). Of patients with a baseline CRP level greater than 12 mg/L, more than 60% of patients had an ASAS40 response. Similarly, in patients in the EMBARK trial with a CRP level greater than10 mg/L, 68% of etanercept‐treated patients had a positive ASAS40 response. A previous post hoc analysis of the EMBARK trial found that patients with objective inflammation, as shown by positive SIJ MRI results or raised CRP level, correlated with a greater percentage of patients with clinical improvement (11).
Our present study investigated baseline CRP level and its relationship to disease response. However, predicting disease response to biologics is likely more complicated than a baseline CRP level. Indeed, logistic regression of patients treated with TNF inhibitors showed that younger age, duration of disease, human leucocyte antigen (HLA) status, and TNF inhibitor naivety also correlated with a positive response to treatment (3, 12, 15). Furthermore, the baseline CRP level only captures inflammation in a patient at a specific time. In patients with active axSpA and a “normal” baseline CRP level, approximately 50% of these patients may have an increased CRP level when followed longitudinally (16). Similarly, another study has demonstrated that patients with active disease, but no MRI or baseline CRP evidence of inflammation, developed inflammatory changes when followed longitudinally (17). The baseline CRP level (and other serum inflammatory markers) may be too nonspecific to serve as the only predictor of disease response (18). The low specificity of CRP to axSpA disease process may explain the lack of significance for the change in SPARCC SIJ score in patients with a CRP level greater than 10 mg/L, although the numbers were also small in this group.
Despite these drawbacks, our study further confirms previous studies demonstrating that higher baseline CRP levels are correlated with response to etanercept in patients with nr‐axSpA. In addition, this analysis importantly highlights that the ASDAS‐CRP and ASDAS‐ESR, which are considered better disease activity measures than BASDAI or ASAS responses, are able to demonstrate the value of biologic treatment across CRP groups (19, 20, 21). This should precipitate a reconsideration of the widespread use of the ASAS40 as a primary outcome measure in clinical trials. However, CRP may be too nonspecific to the axSpA disease process. Therefore, CRP may serve as part of an algorithm to more accurately predict response to biologic treatment. Further research is required to determine the factors that predict response to biologics. We know that patients treated earlier in the course of their disease have a higher likelihood of response to biologics (12, 15, 22). Furthermore, the patients that respond to biologics are likely to have a continued response to biologics years after the commencement of treatment (18). Overall, it is important to note that patients with low and low‐positive baseline CRP levels do have clinically meaningful responses to etanercept in nr‐axSpA.
AUTHOR CONTRIBUTIONS
Drs. Robinson and Tam were involved in drafting the article. All authors were involved in revising the article critically for important intellectual content. Dr. Nash revised the article critically for important intellectual content. All authors approved the final version of the article to be published. Dr. Robinson had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design
Tam, Robinson.
Acquisition of data
Tam, Robinson.
Analysis and interpretation of data
Tam, Nash, Robinson.
Supporting information
Fig S1
Fig S2
Table S1
This publication is based on research using data from Pfizer that has been made available through Vivli. Vivli has not contributed to or approved and is not in any way responsible for the contents of this publication.
Dr. Nash reports speaking for, participating in data safety monitoring boards for, and receiving research grants from Pfizer. Dr. Robinson reports receiving personal fees from Abbvie, Atom Biosciences, Eli Lilly, Gilead, Janssen, Novartis, UCB, Roche, and Pfizer; meeting attendance support from Bristol Myers Squibb, Eli Lilly, Pfizer, and UCB Pharma; and receiving grant funding from Janssen, Novartis, Pfizer, and UCB Pharma.
Funding: None.
REFERENCES
- 1. Robinson PC, Sengupta R, Siebert S. Non‐radiographic axial spondyloarthritis (nr‐axSpA): advances in classification, imaging and therapy. Rheumatol Ther 2019;6:165–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boonen A, Sieper J, van der Heijde D, Dougados M, Bukowski JF, Valluri S, et al. The burden of non‐radiographic axial spondyloarthritis. Semin Arthritis Rheum 2015;44:556–62. [DOI] [PubMed] [Google Scholar]
- 3. Rudwaleit M, Claudepierre P, Wodsworth P, Cortina EL, Sieper J, Kron M, et al. Effectiveness, safety, and predictors of good clinical response in 1250 patients treated with adalimumab for active ankylosing spondylitis. J Rheumatol 2009;36:801–8. [DOI] [PubMed] [Google Scholar]
- 4. Braun J, Deodhar A, Landewé R, Baraliakos X, Miceli‐Richard C, et al. Impact of baseline C‐reactive protein levels on the response to secukinumab in ankylosing spondylitis: 3‐year pooled data from two phase III studies. RMD Open 2018;4:e000749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rusman T, van der Weijden MA, Nurmohamed MT, Landewé RB, de Winter JJ, Boden BJ, et al. Is treatment in patients suspected of non‐radiographic Axial Spondyloarthritis effective? Six months results of a placebo‐controlled trial. Arthritis Rheumatol 2020;73:806–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Robinson PC, van der Linden A, Khan MA, Taylor WJ. Axial spondyloarthritis: concept, construct, classification, and implications for therapy. Nat Rev Rheumatol 2020;17:109–18. [DOI] [PubMed] [Google Scholar]
- 7. Van der Linden S, Akkoc N, Brown MA, Robinson PC, Kahn MA. The ASAS criteria for axial spondyloarthritis: strengths, weaknesses, and proposals for a way forward. Curr Rheumatol Rep 2015;17:62. [DOI] [PubMed] [Google Scholar]
- 8. Rudwaleit M, van der Heijde D, Landewé R, Listing J, Akkoc N, Brandt J, et al. The development of Assessment of Spondyloarthritis International Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 2009;68:777–83. [DOI] [PubMed] [Google Scholar]
- 9. Dougados M, van der Heijde D, Sieper J, Braun J, Maksymowych WP, Citera G, et al. Symptomatic efficacy of etanercept and its effects on objective signs of inflammation in early nonradiographic axial spondyloarthritis: a multicenter, randomized, double‐blind, placebo‐controlled trial. Arthritis Rheumatol 2014;66:2091–102. [DOI] [PubMed] [Google Scholar]
- 10. Landewe R, van Tubergen A. Clinical tools to assess and monitor spondyloarthritis. Curr Rheumatol Rep 2015;17:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brown MA, Bird PA, Robinson PC, Mease PJ, van den Bosch F, Surian C, et al. Evaluation of the effect of baseline MRI sacroiliitis and C reactive protein status on etanercept treatment response in non‐radiographic axial spondyloarthritis: a post hoc analysis of the EMBARK study. Ann Rheum Dis 2018;77:1091–3. [DOI] [PubMed] [Google Scholar]
- 12. Vastesaeger N, van der Heijde D, Inman RD, Wang Y, Deodhar A, Hsu B, et al. Predicting the outcome of ankylosing spondylitis therapy. Ann Rheum Dis 2011;70:973–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Davis JC, van der Heijde DM, Dougados M, Braun J, Cush JJ, Clegg DO, et al. Baseline factors that influence ASAS 20 response in patients with ankylosing spondylitis treated with etanercept. J Rheumatol 2005;32:1751–4. [PubMed] [Google Scholar]
- 14. Baraliakos X, Szumski A, Koenig AS, Jones H. The role of C‐reactive protein as a predictor of treatment response in patients with ankylosing spondylitis. Semin Arthritis Rheum 2019;48:997–1004. [DOI] [PubMed] [Google Scholar]
- 15. Baraliakos X, Koenig AS, Jones H, Szumski A, Collier D, Bananis E. Predictors of clinical remission under anti‐tumor necrosis factor treatment in patients with ankylosing spondylitis: pooled analysis from large randomized clinical trials. J Rheumatol 2015;42:1418–26. [DOI] [PubMed] [Google Scholar]
- 16. Landewe R, Nurminen T, Davies O, Baeten D. A single determination of C‐reactive protein does not suffice to declare a patient with a diagnosis of axial spondyloarthritis 'CRP‐negative'. Arthritis Res Ther 2018;20:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Baraliakos X, Sieper J, Chen S, Pangan AL, Anderson JK. Non‐radiographic axial spondyloarthritis patients without initial evidence of inflammation may develop objective inflammation over time. Rheumatology 2017;56:1162–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Turina MC, Yeremenko N, van Gaalen F, van Oosterhout M, Berk IJ, Ramonda R, et al. Serum inflammatory biomarkers fail to identify early axial spondyloarthritis: results from the SpondyloArthritis Caught Early (SPACE) cohort. RMD Open 2017;3:e000319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Machado P, Landewé R. Spondyloarthritis: is it time to replace BASDAI with ASDAS? [Commentary]. Nat Rev Rheumatol 2013;9:388–90. [DOI] [PubMed] [Google Scholar]
- 20. Machado P, Landewé RB, Braun J, Baraliakos X, Hermann KG, et al. MRI inflammation and its relation with measures of clinical disease activity and different treatment responses in patients with ankylosing spondylitis treated with a tumour necrosis factor inhibitor. Ann Rheum Dis 2012;71:2002–5. [DOI] [PubMed] [Google Scholar]
- 21. Pedersen SJ, Sørensen IJ, Garnero P, Johansen JS, Madsen OR, Tvede N, et al. ASDAS, BASDAI and different treatment responses and their relation to biomarkers of inflammation, cartilage and bone turnover in patients with axial spondyloarthritis treated with TNFα inhibitors. Ann Rheum Dis 2011;70:1375–81. [DOI] [PubMed] [Google Scholar]
- 22. Robinson PC, Brown MA. The window of opportunity: a relevant concept for axial spondyloarthritis. Arthritis Res Ther 2014;16:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Table S1


