Synopsis
Melanins, the main pigments of the skin and hair in mammals, are synthesized within membrane-bound organelles of melanocytes called melanosomes. Melanosome structure and function are determined by a cohort of resident transmembrane proteins, many of which are expressed only in pigment cells and localize specifically to melanosomes. Defects in the genes that encode melanosome-specific proteins or components of the machinery required for their transport in and out of melanosomes underlie various forms of ocular or oculocutaneous albinism, characterized by hypopigmentation of the hair, skin, and eyes and by visual impairment. We review major components of melanosomes, including the enzymes that catalyze steps in melanin synthesis from tyrosine precursors, solute transporters that allow these enzymes to function, and structural proteins that underlie melanosome shape and melanin deposition. We then review the molecular mechanisms by which these components are biosynthetically delivered to newly forming melanosomes—many of which are shared by other cell types that generate cell type-specific lysosome-related organelles. We also highlight unanswered questions that need to be addressed by future investigation.
Introduction—melanosome morphology and function
Skin and hair pigmentation in mammals are a consequence of cellular activities and cooperation between two cell types: melanocytes that make melanins and keratinocytes that distribute melanins throughout the skin. The transfer of melanins from melanocytes to keratinocytes and the intracellular events within keratinocytes that allow for the proper distribution of the transferred pigments are discussed in the companion article by Benito-Martinez et al. (2021). This review will focus on the cell biology within melanocytes responsible for melanin synthesis.
Within epidermal and follicular melanocytes, and also in developing pigment cells of the eye (including retinal pigment epithelial cells, iris and ciliary body pigment epithelial cells, and choroidal and iris melanocytes), melanins are synthesized within membrane-bound intracellular compartments called melanosomes. Melanosomes belong to the lysosome-related organelle (LRO) family, members of which share some features of conventional lysosomes (e.g., low intracellular pH at some maturation stage, and some content of lysosomal proteins), while displaying a unique composition and function to fulfill physiological needs of the organism (Bowman et al. 2019; Delevoye et al. 2019). The specific physiological functions of each LRO are conferred by the cell type-specific components that reside within them. Moreover, these components must be delivered, either following their synthesis within the LRO-producing cell or following uptake from extracellular sources, specifically to the LRO. Hence, this review will focus first on the melanosome contents that are required for melanin synthesis and subsequent transfer, and then on the mechanisms by which these contents are delivered to melanosomes. The latter mechanisms likely apply to multiple cell types that generate LROs. Of note, genetic defects in either the melanosome components themselves or in their delivery pathways result in one of many forms of oculocutaneous albinism (OCA) or ocular albinism (OA; Garrido et al. 2021). Analyses of the products of the affected genes and melanocytes from patients or mouse models of OCA and OA have been instrumental in defining the mechanisms of melanosome biogenesis.
In order to appreciate melanosome function, one must first understand melanosome ultrastructure, as detected by electron microscopy. Melanosomes within pigment cells that synthesize primarily the black and brown eumelanins develop through four morphologically distinct stages (Seiji et al. 1961).1 Stage I melanosomes correspond to conventional vacuolar early endosomal compartments: round organelles integrated into the endocytic pathway with an electron-lucent lumen displaying a planar clathrin coat and few intraluminal vesicles (ILVs; Raposo et al. 2001). Throughout this review, we will refer to these organelles either as Stage I melanosomes or early endosomes. In melanocytes and developing eye pigment cells, these organelles are unique in that the ILVs within them serve as sites to seed the formation of unusual fibrillar structures (Seiji et al. 1961; Berson et al. 2001; Raposo et al. 2001; Hurbain et al. 2008) that will be discussed further below. These fibrils elongate and assemble laterally into fibrillar sheets as Stage I melanosomes mature into Stage II; in cross-sections, Stage II melanosomes appear to contain parallel or concentric proteinaceous fibrils (Seiji et al. 1961; Moyer 1966). Concomitantly, the sheets distend the shape of Stage II melanosomes into ellipsoidal organelles. The sheets ultimately serve to template the deposition of melanins as they are synthesized in Stages III and IV melanosomes. Hence, Stages I and II are unpigmented organelles, and may be referred to as “premelanosomes.”
Melanin synthesis begins in Stage III, in which the fibrous sheets become laden with melanin. Stage III melanosomes are thus defined as those in which melanin-laden fibrous sheets are still visible, whereas Stage IV melanosomes contain sufficient melanin content to mask the underlying fibrillar structure (Seiji et al. 1961). Therefore, Stages III and IV are the melanin-containing organelles that are easily detected in melanocyte cell cultures as dark granules by bright-field light microscopy (Benito-Martinez et al. 2020). Melanin in stage IV melanosomes is fated for transfer to keratinocytes. Importantly, while Stage I melanosomes are also intermediates in the endocytic pathway and accessible to endocytosed tracers, melanosomes of Stages II–IV are not accessible; rather, the endocytosed tracers from Stage I melanosomes are eventually delivered to bona fide lysosomes (Raposo et al. 2001). Thus, melanosome and endolysosomal maturation diverge at the level of Stage I melanosomes.
What is the nature of the proteinaceous fibrillar sheets upon which melanins deposit within melanosomes? How are melanins synthesized within melanosomes? How are the components of these distinct stages delivered to maturing melanosomes? How are these processes disrupted in various forms of albinism? These questions will be addressed further below. Many of the proteins discussed below as either melanosome components or effectors of melanosome maturation or melanosome cargo delivery are listed in Table 1, and a glossary of terms is included in Box 1.
Table 1.
Protein contents of melanosomes and proteins that influence melanosome biogenesis
| Protein name | Associated disease/human phenotype | Protein function |
|---|---|---|
| I. Melanosome contents | ||
| a. Enzymes and structural proteins | ||
| TYR | OCA1 | Integral membrane enzyme; catalyzes oxidation of tyrosine to dopaquinone |
| DCT | OCA8 | Integral membrane enzyme; catalyzes tautomerization of dopachrome to DHICA |
| TYRP1 | OCA3 | Integral membrane enzyme; catalyzes polymerization of DHICA to melanin? Chaperones TYR? Buffers against oxidants? |
| PMEL | Pigmentary glaucoma | Structural protein: forms the fibrillar amyloid matrix of eumelanin-containing melanosomes |
| b. Channels and transporters | ||
| V-ATPase | ATP-dependent proton pump; required for acidification of melanosomes, endosomes, lysosomes, TGN | |
| OCA2 | OCA2 | Cl− channel; required to neutralize maturing melanosomes |
| SLC45A2 | OCA4 | Putative H+/sugar symporter; required to neutralize maturing melanosomes |
| TPC2 | Blonde hair | Cation channel; supports melanosome acidification |
| ATP7A | Menkes Disease | ATP-dependent copper transporter; required for activation of TYR |
| c. Other | ||
| GPR143 | OA1 | GPCR on melanosomes; required for segregation of endolysosomes from melanosomes |
| MART1/melan-a | — | May chaperone PMEL and/or GPR143 |
| CD63 | — | Tetraspanin; regulates intraluminal sorting of PMEL on endosomes, present throughout melanosome maturation and endolysosomes |
| II. Transporters on other organelles that impact pigmentation | ||
| NCKX5 (SLC24A5) | OCA6 | K+-dependent Na+/Ca2+ antiporter on Golgi or mitochondria; required for proper pigmentation |
| CLC7 | Autosomal recessive osteopetrosis;autosomal dominant hypopigmentation, organomegaly, and delayed myelinationand development | Cl− channel on late endosomes/lysosomes and perhaps melanosomes; supports lysosomal pH regulation |
| MFSD12 | Polymorphism associated with skinpigment differences in African and SouthAmerican populations | Cysteine transporter on late endosomes/lysosomes and perhaps melanosomes; required to provide substrate for pheomelanin synthesis |
| TRPM1 | Congenital stationary night blindness | Ca2+ channel; required for proper pigmentation—localization not clear |
| ABCB6 | Dyschromatosis universalis, others | ATP-dependent transporter on late endosomes/lysosomes and early stage melanosomes; required for PMEL fibril formation |
| III. Melanosome protein trafficking effectors | ||
| a. Regulators of early stage melanosome maturation | ||
| PIKFyve | Fleck corneal dystrophy, Charcot-Marie-Tooth disease 4J | Phosphatidylinositol-3-phosphate 5-kinase; regulates endosome maturation, required for early stage melanosomes to interact with late endosomes |
| APOE | Hyperlipoproteinemia, lipoproteinglomerulopathy, familial Alzheimerdisease, cardiovascular disease, and others | A component of very low density, intermediate density, and high-density lipoprotein particles and chylomicrons; found on intralumenal vesicles of Stage I melanosomes with PMEL, and required for PMEL localization and maturation |
| b. HPS complexes | ||
| BLOC-1 | HPS types 7, 8, 9, and 11 | Eight-subunit complex; initiates cargo transport from endosomes to maturing melanosomes |
| BLOC-2 | HPS types 3, 5, and 6 | Three-subunit complex; participates in BLOC-1-dependent transport from endosomes to maturing melanosomes |
| BLOC-3 | HPS types 1 and 4 | Two-subunit complex; activates GTP exchange onto RAB32 and RAB38; functions in recycling from melanosomes, perhaps also forward traffic to melanosomes |
| AP-3 | HPS types 2 and 10 | Four-subunit coat protein complex; TYR sorting into clathrin-coated vesicles and sorting of VAMP7 and other cargo into BLOC-1-dependent endosomal tubules destined for melanosomes |
| c. Related proteins/protein complexes | ||
| AP-2 | Sigma subunit gene is defective in familialhypocalciuric hypercalcemia type III | Four-subunit clathrin-associated coat protein complex at the plasma membrane responsible for sorting many cargoes into endocytic vesicles; required for PMEL localization to early stage melanosomes |
| AP-1 | Sigma subunit genes are defective inMEDNIK syndrome and Pettigrewsyndrome | AP-3-like four-subunit coat protein complex; cargo sorting by tubules or vesicles from endosomes or the Golgi; also binds to KIF13A, contributes with KIF13A to generate and position BLOC-1-dependent melanosome-bound tubules |
| d. Rab GTPases and effectors | ||
| RAB6A/A′ | — | Ras-related small GTPase; regulates trafficking of MART-1 and DCT to melanosomes |
| RAB11A | — | Ras-related small GTPase; regulates recycling endosome formation, impacts formation of BLOC-1-dependent tubules in non-pigment cells, role in melanocytes is not known |
| RAB22A | — | Ras-related small GTPase; may regulate the BLOC-1-dependent cargo transport pathway |
| RAB32 | — | Ras-related small GTPase; regulates membrane trafficking to (DCT) and from (VAMP7) melanosomes—activated by BLOC-3, may also associate with BLOC-2 |
| RAB38 | — | Ras-related small GTPase; regulates membrane trafficking to and from melanosomes—activated by BLOC-3, may also associate with BLOC-2 |
| ELKS | — | RAB6 effector; facilitates RAB6 vesicle targeting and cargo transfer from the Golgi to melanosomes |
| APPL1 | Maturity-onset diabetes of the young | RAB5 effector; interacts with Phosphatidylinositol-3-kinase and impacts signaling from a subset of early endosomes; knockdown in melanocytes impairs pigmentation |
| VARP (ANKRD27) | — | Scaffolding protein; interacts with VAMP7 and RAB32/RAB38 on melanosomes, likely sorts VAMP7 into retrograde transport carriers |
| e. Additional regulators | ||
| VAMP7 | — | A SNARE protein; functions in the fusion of BLOC-1-dependent transport carriers with melanosomes |
| Syntaxin 12/13 (STX12/13) | — | A SNARE protein present throughout the early endosomal system; required for cargo transport to melanosomes, at least in part by associating with VAMP7 |
| Syntaxin 3 (STX3) | — | A SNARE protein; implicated in cargo transport to melanosomes |
| KIF13A | — | Heavy chain of kinesin-3 microtubule-based motor; required to pull BLOC-1-dependent membrane endosomal tubules along microtubules |
| Annexin A2 (ANXA2) | — | A peripheral membrane actin polymerization initiator; functions on endosomes in the initiation of BLOC-1-dependent tubular cargo transport |
| ARP2/3 | ARPC1B subunit is defective inimmunodeficiency 71 with inflammatorydisease and congenital thrombocytopenia | Mutlisubunit complex that nucleates branched actin filaments; functions together with Annexin A2 during initiation of BLOC-1-dependent tubular cargo transport and with Myosin VI to release retrograde tubules emerging from melanosomes |
| ESCRT-0, 1, 2, and 3 | Syndromic congenital dyserythropoieticanemia, frontotemporal dementia, amyotrophic lateral sclerosis, hereditaryspastic paraplegia, cancer | ESCRT; a series of multisubunit protein complexes that regulate multivesicular body formation among many other membrane shaping and repair roles. Depletion of ESCRT-1 impairs BLOC-1-dependent cargo transport to melanosomes |
| Myosin VI | Deafness | Actin-based motor; regulates release of retrograde tubules emerging from melanosomes |
| OPTN | Adult-onset primary open angle glaucoma | A partner of Myosin VI; contributes to the release of tubules emerging from melanosomes |
| WASH | Autosomal recessive mental retardation, autosomal dominant spasticparaplegia, Ritscher–Schinzelsyndrome-1 | A multisubunit peripheral membrane actin polymerization initiator; functions together with Myosin VI to release retrograde tubules emerging from melanosomes |
| HOPS and CORVET | Hypomyelinating leukodystrophy, mucopolysaccharidosis-plus syndrome | Two multisubunit protein complexes that share a core of four subunits; function as both tethers and SNARE pairing regulators in the endosomal system. The common core subunit VPS33A is implicated in melanosome biogenesis |
| LYST (CHS1) | Chediak–Higashi syndrome | The product of the gene defective in Chediak–Higashi syndrome; required for proper melanosome biogenesis. Function unknown |
Box 1 Glossary of terms
Amyloid: an ordered protein aggregate in a beta-sheet-rich protein fold that usually forms fibrillary structures. Typically associated with neurodegenerative and other diseases, PMEL forms amyloid fibers upon which melanins deposit in melanosomes.
Antiporter: a type of transmembrane protein that transports ions or other small molecules across the membrane in one direction and a second ion or small molecule in the other direction. NCKX5 functions as a Na+/Ca2+ antiporter, likely at the Golgi.
BLOCs (biogenesis of lysosome-related organelles complexes): a series of three protein complexes (BLOC-1, -2, and -3), each of which is composed of subunits that are disrupted in variants of the Hermansky–Puldak syndromes, that associate peripherally with membranes and effect the movement of transmembrane proteins between endosomes and melanosomes during melanosome biogenesis.
Cargo: a term typically applied to transmembrane and soluble proteins that are transferred from one compartment to another.
Cargo transport carriers: vesicles or tubules that transport transmembrane and soluble proteins from one organelle to another.
Channel: a type of transmembrane protein that, when open, allows for free movement of ions or other small molecules across the membrane according to their concentration gradient. OCA2 and TPC2 are channels on melanosomes for chloride and for cations, respectively.
Coat proteins: protein complexes that are recruited to membranes from the cytosol and that facilitate sorting of membrane-bound proteins into vesicles or tubules for delivery elsewhere. Some coats also support membrane bending to facilitate vesicle formation.
DHI: dihydroxyindole, one of the major building blocks of eumelanins.
DHICA: dihydroxyindole carboxylic acid, one of the major building blocks of eumelanins and a strong anti-oxidant.
Early endosomes/sorting endosomes: cellular compartments that are the first site of accumulation of internalized material and that serve as major sorting stations for cargo transport to other organelles. They consist of vacuolar domains that concentrate lumenal material destined for late endosomes/lysosomes and tubulovesicular domains that sort membrane proteins to other destinations.
ESCRT (endosomal sorting complexes required for transport): a series of four protein complexes (ESCRT-0, -1, -2, and -3) that associate peripherally with maturing early endosomes to facilitate the sorting of transmembrane proteins into patches and the inward invagination of these patches to form intralumenal vesicles. ESCRT-0 and -1 most often recognize ubiquitylated proteins as cargoes.
Eumelanin: black and brown melanin polymers of indole structures formed by successive oxidation reactions starting with tyrosine.
G protein-coupled receptors (GPCRs): a large family of seven transmembrane domain-containing proteins that recognize various ligands and that couple with heterotrimeric G proteins and arrestin family members to transmit signals to cells. GPR143 is an intracellular GPCR in pigment cells, and several plasma membrane GPCRs, including MC1R, regulate melanogenesis.
GWAS: genome-wide association study, an experimental approach to correlate genetic polymorphisms with phenotypic traits or diseases.
ILVs: small vesicles (usually 40–60 nm in diameter) present within endosomes and melanosomes that form from invagination of the limiting membrane.
L-3,4-dihydroxyphenylalanine (L-dopa): an intermediate/side-product of melanin synthesis in melanosomes. Also a major neurotransmitter in the central nervous system (generated by a different pathway catalyzed by the cytoplasmic enzyme, tyrosine hydroxylase).
Lysosome-related organelles (LROs): a family of cell type-specific subcellular membrane-bound organelles that derive at least in part from the endolysosomal pathway. Melanosomes are members of the LRO family.
Melanogenesis: the process by which melanin pigments are synthesized.
Melanosomes: LROs within skin and eye melanocytes and pigment epithelia of the retina, iris and ciliary body within which melanin pigments are synthesized and stored.
Oculocutaneous albinism (OCA): genetic conditions resulting from impaired melanin synthesis in the skin and eyes.
Ocular albinism (OA): genetic conditions resulting from impaired melanin synthesis predominantly in the eye.
Pheomelanin: red and yellow melanin polymers formed from intermediates in which dopaquinone is modified by cysteine.
Phosphatidylinositol phosphates: Phosphatidylinositol (PtdIns) is a minor component of total cellular phospholipids, and consists of the six-carbon inositol ring covalently linked to the diacylglycerol backbone. Three of the five available carbons (C3, C4, or C5) can be modified by phosphorylation. Most relevant to melanosome biogenesis, PtdIns-3-phosphate is most prevalent on early endosomal membranes, including Stage I melanosomes, and PtdIns-(3,5)-bisphosphate regulates maturation of Stage I melanosomes and PMEL processing.
Premelanosomes: a term sometimes used to describe the non-pigmented early stages of melanosome maturation.
Rab GTPases: small GTPases that serve as molecular switches during membrane trafficking and vesicle and organelle motility. When bound to GTP they bind effectors that perform work (such as cytoskeletal motors or membrane fusion machinery). Different Rab proteins regulate distinct steps in melanosome biogenesis.
Recycling endosomes: a subset of early endosomes that facilitate delayed delivery of internalized cargo to the plasma membrane or to the trans-Golgi network. The tubular transport carriers generated in a BLOC-1-dependent manner have features of recycling endosomes.
Sec1-Munc18 (SM) proteins: SNARE-binding proteins that function both to chaperone/stabilize unpaired syntaxin-family SNARE proteins and to stabilize four-helix bundles during SNARE-dependent fusion.
SNARE proteins: integral membrane proteins with long, alpha helical domains that function in fusion of transport carriers with target membranes. Fusion requires formation of a four-helix bundle by three or four specific cohorts of SNARE proteins.
Sorting signals: linear sequences of amino acids or conformational determinants that are recognized by coat proteins or sorting adaptors for sorting of cargo on a source compartment into transport carriers destined for another compartment. In the context of melanosome biogenesis, linear sequences on the cytoplasmic domains of integral membrane proteins serve as sorting signals that are recognized by heterotetrameric adaptors AP-1 or AP-3.
Symporter: a type of transmembrane protein that transports ions or other small molecules across the membrane together with a second ion or small molecule in the same direction. SLC45A2 functions in heterologous cell types as a symporter for protons and sucrose from the lumen/extracellular space to the cytoplasm.
Tethering proteins: typically, elongated proteins or protein complexes that serve to bridge transport carriers with target organelles during cargo transport. Typically function in association with specific Rab and SNARE proteins.
Ubiquitylation: the process by which the small protein, ubiquitin, is conjugated to proteins as a signal, often for degradation in the cytoplasm. In endosomal compartments, ubiquitin conjugation typically serves as a signal for cargo sorting onto intralumenal membranes.
Vacuolar-type H(+)-ATPase (V-ATPase): a multisubunit transmembrane protein complex on melanosomes and all endosomes, lysosomes, and the trans Golgi network that pumps protons into the organelle in an ATP-dependent manner, thereby acidifying the organelle.
Melanosome contents and pigmentation
Melanin is the primary determinant of mammalian pigmentation and can be found in two forms: eumelanins (black and brown pigments) and pheomelanins (red and yellow pigments; D'Alba and Shawkey 2019). As discussed above, mutations in genes required for melanin synthesis result in OCA. Eight subtypes of non-syndromic OCA (OCA1–8) have been described, and except for two subtypes—OCA5, whose gene is uncloned, and OCA7, whose gene encodes the leucine-rich melanocyte differentiation-associated protein of unknown function—the genes mutated in OCA encode either enzymes or ion transport proteins that impact melanogenesis.
The tyrosinase family
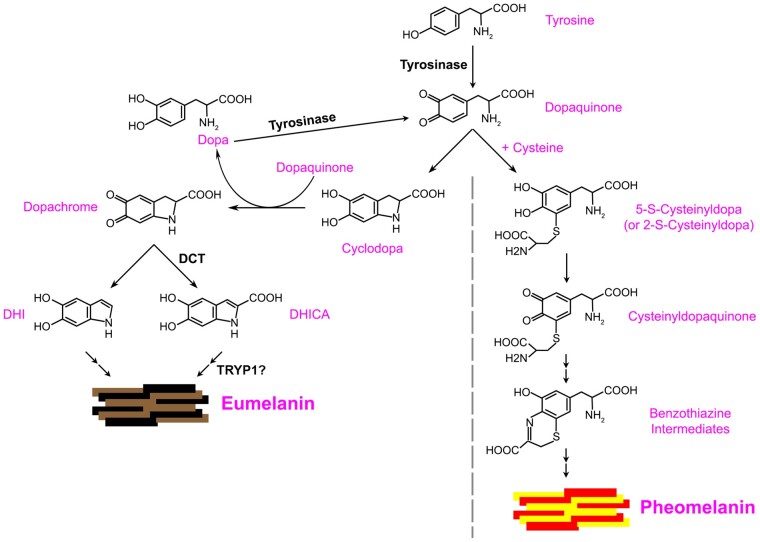
Both eumelanin and pheomelanin are synthesized via a series of reactions that are initiated by the oxidation of tyrosine (or of L-3,4-dihydroxyphenylalanine [L-dopa]) to dopaquinone, catalyzed by the enzyme tyrosinase (TYR; Fig. 1; Raper and Wormall 1923; Raper 1926, 1927; Raper and Speakman 1926; Evans and Raper 1937; Mason 1948; Cooksey et al. 1997; Ramsden and Riley 2014). In the presence of cysteine, dopaquinone is spontaneously converted to either 2-S- or 5-S-cysteinyldopa, which are both intermediates in the autocatalytic synthesis of pheomelanins (Fig. 1, right side). In the absence of cysteine, dopaquinone is spontaneously converted to cyclodopa and then to dopachrome, intermediates in the formation of eumelanin (Fig. 1, left side; see below; Ito and Wakamatsu 2008). While TYR is the only enzyme required for melanin formation, the structurally related TYR-related protein 1 (TYRP1) and dopachrome tautomerase (DCT, a.k.a. TYRP2) modulate the eumelanin synthesis pathway. Impaired function of any one of these proteins results in OCA (Tomita et al. 1992; Oetting and King 1994a, 1994b; Boissy et al. 1996; Montoliu et al. 2014; Pennamen et al. 2020b).
Fig. 1.
Model of eumelanin synthesis. TYR catalyzes the oxidation of tyrosine to generate dopaquinone. In the presence of cysteine, dopaquinone is spontaneously converted to pheomelanin via 5-S-cysteinyldopa or 2-S-cysteinyldopa, cysteinyldopaquinone, and benzothiazine intermediates. In the absence of cysteine, dopaquinone can spontaneously cyclize to cyclodopa, and subsequent spontaneous redox exchange of cyclodopa with dopaquinone gives rise to dopachrome and L-dopa (Cooksey et al. 1997; Ramsden and Riley 2014); L-dopa can then be oxidized by TYR to form more dopaquinone. Dopachrome can spontaneously reorganize to form DHI or can undergo tautomerization by DCT to form DHICA. Both DHI and DHICA undergo oxidation and polymerization to form eumelanins. TYRP1 might support DHICA polymerization either directly or indirectly, or alternatively serve either as a chaperone for TYR or as an antioxidant sink. Adapted from Ito and Wakamatsu (2008).
TYR, TYRP1, and DCT (Fig. 2) belong to the TYR family of enzymes, all of which contain a cysteine-rich domain, two metal-binding sites, a single transmembrane domain, and a short cytosolic C-terminal tail (Solano 2018). The metals bound by these proteins yield insight into their enzymatic functions. TYR is a Cu2+-binding protein, which allows it to oxidize its substrates tyrosine and L-dopa to generate dopaquinone (Fig. 1; Olivares and Solano 2009; Ramsden and Riley 2014; Solano 2018). Mutations in TYR cause OCA1, typically the most severe form of OCA, due to the requirement for TYR in both eumelanin and pheomelanin production (Gronskov et al. 2007; Montoliu et al. 2014). DCT is a Zn2+-binding protein that catalyzes the tautomerization of dopachrome to 5,6-dihydroxyindole (DHI)-2-carboxylic acid (DHICA) downstream of dopaquinone in the eumelanin synthesis pathway (Fig. 1; Tsukamoto et al. 1992; Solano et al. 1996; Olivares and Solano 2009). Mutations in DCT cause OCA8 (Pennamen et al. 2020b), a milder form of OCA, as eumelanin can also be formed independently of DCT activity via the spontaneous formation of DHI from dopachrome (Fig. 1). While both DHI and DHICA can be oxidized and assembled into eumelanin polymers, DHICA melanins are more anti-oxidative and have distinct pigmentation properties (Guyonneau et al. 2004; Jiang et al. 2010); thus, DCT activity determines the composition of eumelanin.
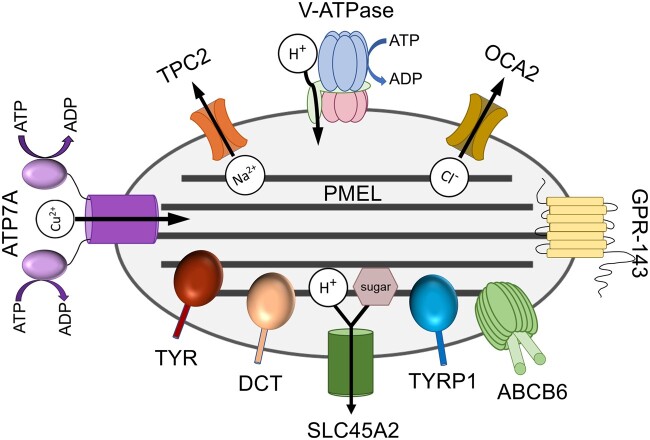
Fig. 2.
Major melanogenic components of melanosomes. Shown is a schematic of the protein components discussed in the text and their localization within melanosomes of multiple stages (melanosome stage is not indicated). For transporters, the substrate transported is indicated in white circles (and/or the mauve sugar for SLC45A2), and direction of transport is indicated by arrows.
While the functions of TYR and DCT have been well-characterized, the function of TYRP1 is controversial. Mutations in TYRP1 result in OCA3, a mild form of “rufous” OCA characterized by reddish skin and hair; mutations in the mouse ortholog similarly result in brown rather than black coat color (Tomita et al. 1992; Oetting and King 1994a, 1994b; Boissy et al. 1996). However, it is unclear whether TYRP1 functions as an enzyme, a modifier of TYR activity, or an anti-oxidant (Orlow et al. 1994; Kobayashi et al. 1998). This conundrum is exacerbated by differences in function ascribed to the highly homologous human and mouse TYRP1 orthologs (Jimenez-Cervantes et al. 1994; Kobayashi et al. 1994; Boissy et al. 1998). Purified preparations of mouse TYRP1 were shown to catalyze the oxidation of DHICA to form melanins (Jimenez-Cervantes et al. 1994; Kobayashi et al. 1994). In contrast, human TYRP1 does not appear to share this function (Boissy et al. 1998); consistently, the crystal structure of the luminal domain of human TYRP1 shows that its metal-binding site contains Zn2+—an ion that lacks redox activity—supporting a model in which human TYRP1 cannot function as an oxidative enzyme (Williams 1987; Furumura et al. 1998; McCall et al. 2000; Lai et al. 2017). While it is possible that mouse TYRP1 binds Cu2+ in the same site to support the oxidation of DHICA, the few amino acid differences between human and mouse TYRP1 are not consistent with this model (Solano 2018).
In addition to its proposed enzymatic function, there are two alternative models for TYRP1 function. First, TYRP1 may be a chaperone for TYR that either regulates its activity or protects it from degradation. This model is based on the instability of TYR in cells lacking functional TYRP1 (Kobayashi et al. 1998), the prolonged activity of the purified TYR luminal domain in vitro upon coincubation with the purified TYRP1 luminal domain (Dolinska et al. 2019), and the detection of high molecular weight complexes containing both TYR and TYRP1 from melanoma cell lysates (Orlow et al. 1994). However, inconsistencies between these reports and potential differences between mouse and human TYRP1 warrant re-evaluation of the conclusions. Moreover, a concern with both this model and the enzymatic model of TYRP1 activity is that TYRP1 localizes nearly exclusively to the limiting membrane of melanosomes (Raposo et al. 2001), whereas active TYR localizes to both the limiting membrane and internal membranes (Turner et al. 1975; Theos et al. 2005). As TYRP1 could only chaperone TYR on the same membrane, the chaperone model would suggest that the two cohorts of TYR may have different functions, properties, or stabilities. A second non-mutually exclusive alternative hypothesis for TYRP1 function comes from two groups showing that loss of TYRP1 in human and mouse melanocytes caused cell death in cells actively synthesizing melanin, indicating that TYRP1 functions to buffer cells from cytotoxicity of melanin synthesis (Johnson and Jackson 1992; Luo et al. 1994; Rad et al. 2004). This might reflect the pro-oxidant properties of DHI-based eumelanin compared to the antioxidant properties of DHICA-based or mixed DHI- and DHICA-based eumelanins (Guyonneau et al. 2004; Jiang et al. 2010), and suggests that TYRP1 may either shift the eumelanin synthesis pathway toward DHICA-based eumelanin or serve as a sink for pro-oxidant activity. While more work needs to be done to define TYRP1 function, the causative role of its mutations in OCA3 indicates that TYRP1 is important for the eumelanin synthesis pathway.
PMEL and the control of melanosome shape
Within melanosomes, eumelanins are deposited on a matrix of intraluminal fibrous sheets that appear as arrays of parallel fibrils in Stage II melanosomes by thin section transmission electron microscopy (Seiji et al. 1961; Birbeck 1963; Moyer 1966; Hurbain et al. 2008). The sheets are assembled from amyloid-like fibrils composed of proteolytic fragments of the pigment cell-specific protein, pre-melanosome protein (PMEL a.k.a Pmel17, gp100, or silver; Watt et al. 2013) (Fig. 2). Like all known melanosomal proteins, PMEL is synthesized as an integral membrane glycoprotein in the endoplasmic reticulum (ER), but during trafficking through the Golgi complex to early endosome intermediates, it is processed by two proteases: a proprotein convertase (potentially furin; Leonhardt et al. 2011) and beta-amyloid convertase enzyme 2, releasing a luminal fragment into endosomes (Berson et al. 2001; Raposo et al. 2001; Berson et al. 2003; Rochin et al. 2013). This fragment has biophysical properties of amyloids (Fowler et al. 2006) and assembles into fibrils in association with the intraluminal membranes of Stage I melanosomes/multivesicular early endosomes (Berson et al. 2001; Reggiori and Pelham 2001; Hurbain et al. 2008). ILV formation and consequent PMEL amyloid formation require the tetraspanin CD63 and apolipoprotein E, a component of low-density lipoprotein particles (van Niel et al. 2011; 2015), but not the classical endosomal sorting complexes required for transport (ESCRT) machinery for multivesicular body formation (Theos et al. 2006b). The amyloid core consists of a peptide at the border of the structurally uncharacterized N-terminal domain and the polycystic kidney disease repeat domain (Hee et al. 2017). Amyloid formation by this region requires (1) interactions with several PMEL luminal domains (Hoashi et al. 2006; Theos et al. 2006b; Leonhardt et al. 2013) and (2) disulfide bond exchange between the amyloidogenic fragment and a cysteine-rich juxtamembrane region that is not retained within melanosomes (Ho et al. 2016). A heavily glycosylated mucin-like domain of direct repeats that was initially thought to be required for fibril formation (Hoashi et al. 2006; Theos et al. 2006b) is now understood to be necessary for lateral association of the fibrils into sheets, and likely for stability (Leonhardt et al. 2013; Graham et al. 2019). Why the amyloid fold was exploited as a scaffold for melanin deposition is unclear.
The PMEL fibrillar sheets are responsible for the ovoid shape of melanosomes (Theos et al. 2006a; Hellström et al. 2011), which is important for the circadian migration of melanosomes into the apical processes of retinal pigment epithelial cells during phagocytosis of damaged photoreceptor outer segments (Burgoyne et al. 2015). However, PMEL is not required for pigmentation per se, and PMEL deficiency does not result in albinism. Primary or immortalized melanocytes from Pmel−/− mice that lack PMEL expression or from silver mice with a Pmel mutation that prevents accumulation in melanosomes are highly pigmented, and the mice themselves are only modestly hypopigmented (Dunn and Thigpen 1930; Theos et al. 2006a; Hellström et al. 2011). The skin/hair hypopigmentation likely reflects impaired melanocyte health in the absence of PMEL, a phenotype that is exacerbated by certain dominant mutations in PMEL such as in the Dominant white chicken or the Silver horse (Hamilton 1940; Kerje et al. 2004; Andersson et al. 2011; Watt et al. 2011). How PMEL promotes melanocyte health is not clear, but may reflect adsorption of oxidative melanin intermediates that would otherwise damage the melanosome membrane and potentially attack cytoplasmic constituents; the dominant PMEL mutants might damage the membrane directly as has been proposed for pathogenic amyloids (Sciacca et al. 2018). Indeed, damage to iris or ciliary body pigment cells likely underlies the pigmentary glaucoma observed in human patients with PMEL mutations (Lahola-Chomiak et al. 2019). A better understanding of how PMEL protects melanocytes and eye pigment cells, how melanosome shape influences its function in all pigment cell types, and why PMEL is so highly conserved in mammals (Theos et al. 2005) despite a modest impact of its loss of expression on whole animal pigmentation and vision are questions that warrant answers.
Melanosome pH control
Mutations in genes encoding ion transport proteins account for three subtypes of OCA, highlighting the importance of ion regulation in melanogenesis. OCA types 2, 4, and 6 (OCA2, OCA4, and OCA6) are, respectively, caused by mutations in OCA2, SLC45A2, and SLC24A5 (Getting and King 1994; Manga and Orlow 1999; Brilliant 2001; Newton et al. 2001; Gronskov et al. 2007; Wei et al. 2013; Montoliu et al. 2014; Morice-Picard et al. 2014). At least two of these genes, OCA2 and SLC45A2, directly impact the pH of maturing melanosomes (Fig. 2). Stages I and II melanosomes are acidic organelles due to the proton importing activity of the ubiquitously expressed vacuolar-type H(+)-ATPase (V-ATPase; Bhatnagar and Ramalah 1998; Tabata et al. 2008); Fig. 2 this is necessary for the processing of PMEL to its amyloid form and potentially for the lateral association of the amyloid fibrils into sheets (Berson et al. 2001, 2003; Graham et al. 2019). However, TYR is only weakly active below pH 6 (Townsend et al. 1984; Ancans et al. 2001), and hence melanosomes must neutralize as they mature to promote optimal TYR activity (Saeki and Oikawa 1985; Ancans et al. 2001; Fuller et al. 2001; Raposo et al. 2001).
The protein OCA type 2 (OCA2, a.k.a. p-protein) is a Cl−-selective ion channel that localizes to a subset of maturing melanosomes (Sitaram et al. 2009; Bellono et al. 2014; Le et al. 2020). OCA2 allows Cl− ions to flow from the melanosomal lumen into the cytoplasm (Bellono et al. 2014). This promotes melanosome pH neutralization, presumably because Cl− ion efflux reduces melanosome membrane potential, thereby decreasing V-ATPase activity (Bellono et al. 2014). Interestingly, OCA2 has a short half-life (<4 h; Sitaram et al. 2009), is physically associated with melanin (Donatien and Orlow 1995), and has been shown by immuno-electron microscopy analysis to localize predominantly to ILVs of pigmented melanosomes (Sitaram et al. 2012) [a cohort of TYR also localizes to ILVs of mature melanosomes (Theos et al. 2005), which have been observed by electron microscopy since the 1970s (Turner et al. 1975; Jimbow et al. 1979)]. These observations suggest that OCA2 resides in its active form on the limiting membrane of melanosomes only transiently and then associates with invaginating membranes of newly forming ILVs, much like growth factor receptors on endosomes of other cell types (Hanson and Cashikar 2012). Since OCA2 is an ungated Cl− channel, its removal from the limiting membrane and localization to ILVs may be one mechanism to regulate its activity.
How OCA2 is sorted to ILVs and whether this sorting regulates OCA2 function is as yet unknown. The major pathway for cargo sorting into ILVs requires cargo ubiquitylation, followed by its recognition by ESCRT-0, -I, -II, and -III (Gruenberg 2020), but it is unknown if OCA2 sorting to ILVs is ubiquitin/ESCRT-dependent or -independent. Additionally, OCA2 is detected on only a subset of maturing melanosomes that partially overlaps with the subset harboring TYRP1 and TYR (Le et al. 2020). While OCA2 exploits the same Biogenesis of LRO Complex-1 (BLOC-1)-dependent trafficking pathway used by TYRP1 to access melanosomes (Sitaram et al. 2012; discussed later), it is unknown whether OCA2 trafficking to melanosomes is restricted to a certain melanosome stage or if OCA2 is constantly delivered to the melanosome followed by clearance from the membrane. Finally, cells lacking OCA2 also appear to have defects in TYR trafficking and assembly and altered sensitivity to ER stress (Manga et al. 2001; Chen et al. 2002; Cheng et al. 2013). How these activities might be regulated by ion channel activity on melanosomes is not understood.
SLC45A2 localizes to mature melanosomes and, like OCA2, is necessary to maintain a more neutral melanosomal lumen (Le et al. 2020). Homologs of SLC45A2 in plants and Drosophila are plasma membrane proteins that function as sucrose/proton symporters, transporting both sucrose and a proton into the cytosol in a single step (Sun et al. 2010). When expressed in yeast, mouse SLC45A2 supports the translocation of sucrose and other mono and disaccharides across the plasma membrane from the extracellular space to the cytosol in a proton-dependent manner (Bartolke et al. 2014). If SLC45A2 were to function similarly in melanosomes, it would expel a sugar molecule and a proton from the lumen into the cytosol, thereby supporting pH neutralization. Whether SLC45A2 transports the same substrates from melanosomes, however, is unclear and difficult to test. Its family members SLC45A1, SLC45A3, and SLC45A4, act as sugar transporters in yeast and localize to the plasma membrane in mammalian cells, where they have been shown to affect sugar levels (Shimokawa et al. 2002; Shin et al. 2012; Vitavska et al. 2016; Vitavska and Wieczorek 2017). If SLC45A2 indeed functions as a melanosomal sugar/proton symporter, the source of the sugar substrate is intriguing. Little is known about the sugar content of melanosomes except as components of glycoproteins. All known resident melanosomal proteins are integral membrane glycoproteins that are heavily glycosylated (Kwon et al. 1987; Halaban and Moellmann 1990; Tsukamoto et al. 1992; Jimenez-Cervantes et al. 1993; Schiaffino et al. 1996; Yamaguchi et al. 1996; Sitaram et al. 2009). Lysosomal hydrolases that catabolize mature glycoproteins into peptides and monosaccharides can also be found in melanosomes (Diment et al. 1995), and thus may provide a potential source of sugar molecules that could impact melanosome pH neutralization. However, it remains unclear whether sugar export from the melanosome is required for melanosome biogenesis or whether it serves solely as a means for proton export. Data indicate that the latter is true since OCA2 overexpression can compensate for the loss of SLC45A2 (Le et al. 2020).
Like OCA2 and SLC45A2, two-pore channel 2 (TPC2); Fig. 2 localizes to melanosomes and directly affects melanosome pH. Unlike OCA2 and SLC45A2, which are expressed only in melanocytes and eye pigment cells, TPC2 is ubiquitously expressed and localizes not only on melanosomes but also to endosomes and lysosomes (Ambrosio et al. 2016; Bellono et al. 2016). Mutations in TPC2 are not associated with OCA, but non-pathological variants are linked to pigmentation variation in humans (Sulem et al. 2008; Chao et al. 2017). TPC2 is a gated cation channel that is activated by phosphatidylinositol 3,5-bisphosphate [PtdIns(3,5)P2] (Wang et al. 2012). Although TPC2 has been proposed to be nonselective in its cation specificity and to open in response to nicotinic acid adenine dinucleotide phosphate (NAADP; Calcraft et al. 2009), careful electrophysiology experiments indicate that TPC2 is 100-fold more selective for sodium (Na2+) than for calcium (Ca2+) and is not directly responsive to NAADP (Wang et al. 2012; Bellono et al. 2016). Nevertheless, TPC2 knockout in a melanoma cell line led to reduced Ca2+ release from melanosomes (Ambrosio et al. 2016). A recent report that screened for TPC2 agonists suggested that TPC2 can be selective for either Na2+ or Ca2+ depending on whether the agonist is PtdIns(3,5)P2 or NAADP, respectively (Gerndt et al. 2020); however, whether this is physiologically relevant is unclear. TPC2 knockdown or knockout in mouse melanocytes results in increased melanosome pH, melanin content, and melanosome size, while TPC2 overexpression results in decreased melanosome pH and melanin content, indicating that TPC2 negatively regulates melanosome biogenesis (Ambrosio et al. 2016; Bellono et al. 2016). As such, TPC2 may function to increase membrane potential by allowing Na2+ efflux from the melanosome, thereby enhancing V-ATPase activity and promoting melanosomal acidification (Bellono et al. 2016). Indeed, overexpression of TPC2 in melanocytes antagonized the increased pigmentation caused by OCA2 overexpression, indicating that the functions of these two ion transporters oppose each other (Bellono et al. 2016). However, it is unknown whether TPC2 and OCA2 normally localize to the same or different melanosome stages. TPC2 might localize to immature melanosomes to promote acidification, or to late-stage melanosomes to halt melanogenesis. Further work is required to understand how and when TPC2, OCA2, and SLC45A2 coordinate to regulate melanosome pH.
Additional ion transporters that impact melanogenesis
OCA6 is caused by mutations in SLC24A5, a gene encoding the intracellular potassium (K+)-dependent Na2+–Ca2+ exchanger 5 (NCKX5) protein (Wei et al. 2013), and pigment variation within many world populations correlates with sequence variation at the SLC24A5 locus (Lamason et al. 2005; Soejima and Koda 2007; Crawford et al. 2017; Martin et al. 2017). NCKX5 is a Na2+/Ca2+ antiporter that exploits the K+ gradient from the cytosol to the extracellular space/organelle lumen for the movement of Na2+ into the cytosol and of Ca2+ toward the extracellular space/organelle lumen (Altimimi and Schnetkamp 2007; Szerencsei et al. 2016). SLC24A5 knockout or knockdown results in decreased melanin content, decreased expression of melanosomal proteins, and reduced melanosomal Ca2+ content (Ginger et al. 2008; Vogel et al. 2008; Wilson et al. 2013; Zhang et al. 2019). Interestingly, NCKX5 does not appear to localize to melanosomes but rather to either the trans-Golgi network (TGN) or mitochondria (Ginger et al. 2008; Wilson et al. 2013; Rogasevskaia et al. 2019; Zhang et al. 2019). This suggests that ion regulation in other organelles impacts pigmentation in melanosomes. However, the mechanism by which NCKX5 activity in non-melanosomal organelles influences melanogenesis is unclear. It has been proposed that Ca2+ released from the TGN may act as a second messenger to regulate melanogenesis (although this might be via the regulation of melanosome gene expression; Dolman and Tepikin 2006; Wilson et al. 2013), potentially by replenishing TGN Ca2+ stores to facilitate multiple rounds of signaling (Ginger et al. 2008). Zhang et al. propose that NCKX5, which they suggest localizes to mitochondria, is required for Ca2+ storage in melanosomes (Zhang et al. 2019). While it is unclear how a Ca2+ importer on mitochondria would support Ca2+ storage in another organelle, it may involve direct transfer of Ca2+ from mitochondria to melanosomes at mitochondria–melanosome junctions, which have been shown to be critical for proper melanogenesis (Daniele et al. 2014). Nevertheless, while Ca2+ is abundant in melanosomes, its function within the organelle is not known (Bush and Simon 2007).
Five other solute transport proteins—ATPase copper transporting alpha (ATP7A), transient receptor potential cation channel, subfamily M, member 1 (TRPM1, a.k.a. melastatin), chloride channel 7 (ClC-7), major facilitator superfamily domain containing 12 (MFSD12), and ATP binding cassette protein, subfamily B, family member 6 (ABCB6)—influence, but are not required for, melanogenesis. Mutations in the genes encoding these proteins cause pigmentation variation in humans and animal models but do not cause OCA (Levinson et al. 1994; Mercer et al. 1994; Chalhoub et al. 2003; Lange et al. 2006; Oancea et al. 2009; Que et al. 2015; Crawford et al. 2017; Adhikari et al. 2019; Lona-Durazo et al. 2019).
ATP7A (Fig. 2) is a P-type ATP-dependent copper transporter present on most cells that is required for copper import into the secretory pathway and for copper export under conditions of copper overload (Lutsenko et al. 2007). In most cells, it functions at the TGN and/or in endosomes to provide copper as a cofactor for secreted or endomembrane proteins (La Fontaine and Mercer 2007). Loss of ATP7A function results in Menkes Disease, a severe developmental disorder characterized by impaired growth, rapid neurodegeneration, and hypopigmentation (Chelly et al. 1993; Mercer et al. 1993; Vulpe et al. 1993). The pigmentation defects are a consequence of the requirement for copper as a cofactor for TYR activity (Petris et al. 2000). Indeed, although histochemical assays suggest that copper is loaded onto TYR in the Golgi (Novikoff et al. 1968), a cohort of ATP7A in melanocytes that localizes to melanosomes is necessary for copper loading onto TYR in that organelle (Setty et al. 2008). How copper is stripped from TYR en route from the Golgi to melanosomes (Setty et al. 2008) and why copper needs to be reloaded onto TYR in melanosomes is not understood.
TRPM1 is a Ca2+ channel that localizes to unidentified nonmelanosome organelles and to the plasma membrane in melanocytes (Xu et al. 2001; Oancea et al. 2009). TRPM1 mRNA levels directly correlate with melanin content in human melanocytes (Oancea et al. 2009), indicating that TRPM1 somehow modulates melanogenesis. More work is required to identify the role of Ca2+ in melanin synthesis and whether TRPM1 plays a similar role as NCKX5 in regulating this process.
ClC-7 is a Cl−/H+ antiporter that localizes to lysosomes in nonpigmented cells (Lange et al. 2006; Graves et al. 2008), although its localization in pigmented cells is not yet known. On lysosomes, ClC-7 imports Cl− ions into the lumen in exchange for a proton in a 2:1 Cl−:H+ ratio (Graves et al. 2008). Knockdown of Clcn7, the gene encoding ClC-7, results in reduced lysosomal acidification (Graves et al. 2008), presumably due to the requirement for a counterion to promote V-ATPase activity (Wagner et al. 2004; Carraro-Lacroix et al. 2009). Mice with loss-of-function mutations in Clcn7 or Ostm1, the gene encoding its partner protein OSTM1, have a gray coat color rather than the expected agouti (Chalhoub et al. 2003; Lange et al. 2006), suggesting that ClC-7 may be required for pheomelanin synthesis. However, whether ClC-7 affects melanin synthesis directly by modulating ion transport at sites of melanin synthesis or indirectly by modulating lysosomal pH is as yet unknown. Additionally, whether pheomelanin and eumelanin synthesis occur in the same melanosomes, separate melanosomes, or in a different organelle requires further investigation.
MFSD12 is part of a large family of ion transport proteins with diverse substrates, and the MFSD12 gene has been identified in several genome-wide association studies as associated with pigmentation variation (Crawford et al. 2017; Adhikari et al. 2019; Lona-Durazo et al. 2019). Like mice with mutations in Clcn7, Mfsd12−/− mice have a gray rather than agouti coat color, indicating a requirement for MFSD12 in pheomelanin synthesis but not eumelanin synthesis (Crawford et al. 2017). Consistently, MFSD12 is required for cysteine import into lysosomes and/or melanosomes, providing a necessary precursor for pheomelanin production (Adelmann et al. 2020). However, dark skin in humans correlates with reduced MFSD12 expression (Crawford et al. 2017), and knock down of Mfsd12 in mouse melanocytes or melanoma cells results in increased pigmentation, suggesting an influence of MFSD12 on eumelanin production as well (Crawford et al. 2017; Adelmann et al. 2020). In melanocytes, MFSD12 localizes predominantly to late endosomes/lysosomes rather than melanosomes (Crawford et al. 2017), suggesting that MFSD12 might exert its impact on eumelanin synthesis indirectly through its activity in lysosomes rather than directly in melanosomes as has also been proposed (Adelmann et al. 2020).
ABCB6 (Fig. 2) is an ATP-dependent transporter whose substrate has not yet been conclusively identified and for which mutations are associated with a variety of human genetic disorders, including Dyschromatosis universalis hereditaria—characterized by hyper and hypopigmented macules over the skin (Zhang et al. 2013). Although it has been reported to function on mitochondria, the plasma membrane, or the endolysosomal system in various cell types (Krishnamurthy et al. 2006; Paterson et al. 2007; Kiss et al. 2015), ABCB6 localizes to late endosomes/lysosomes and to early stage melanosomes in a pigmented human melanoma cell line (Bergam et al. 2018). Depletion of ABCB6 in these cells or expression of human disease-associated variants resulted in disruption of PMEL fibril formation and accumulation of PMEL in multivesicular endosomal structures, although it had little impact on pigmentation per se (Bergam et al. 2018). How this transporter might impact PMEL fibril formation remains to be determined.
Additional as yet unidentified ion transporters on melanosomes or other melanocyte organelles likely influence melanogenesis by directly regulating melanosome pH and enzyme activity, ion content in organelles that crosstalk with melanosomes, or as yet undiscovered mechanisms. Further studies are required to determine how ion transport proteins cooperatively function to regulate these processes in pigmented cells and how their substrate ions impact melanogenesis.
Melanosome biogenesis and membrane dynamics
As described in the preceding section, most of the components contributing to melanin synthesis are transmembrane proteins associated with the limiting membrane of the melanosome. As melanin factories, melanosomes are dedicated subcellular spaces for melanin chemistry, but also serve as a safe location to preserve cells from the dispersion of harmful oxidative radicals generated during melanogenesis. Therefore, the biogenesis of the melanosome and the development of the membrane trafficking pathways that deliver melanogenic enzymes, transporters, and structural proteins are key processes for skin pigmentation. The contents of melanosomes are, like those of a subset of other LROs, primarily derived from the endosomal system (Raposo et al. 2001), but some—such as DCT and MART-1—are delivered directly from the secretory pathway (Patwardhan et al. 2017; Fig. 3). Recent reviews have described the complexity underlying these trafficking routes and the cellular components involved (Bowman et al. 2019; Delevoye et al. 2019). This section will describe our current understanding of the mechanisms governing the delivery of contents to maturing melanosomes and raise some questions that still need to be answered.
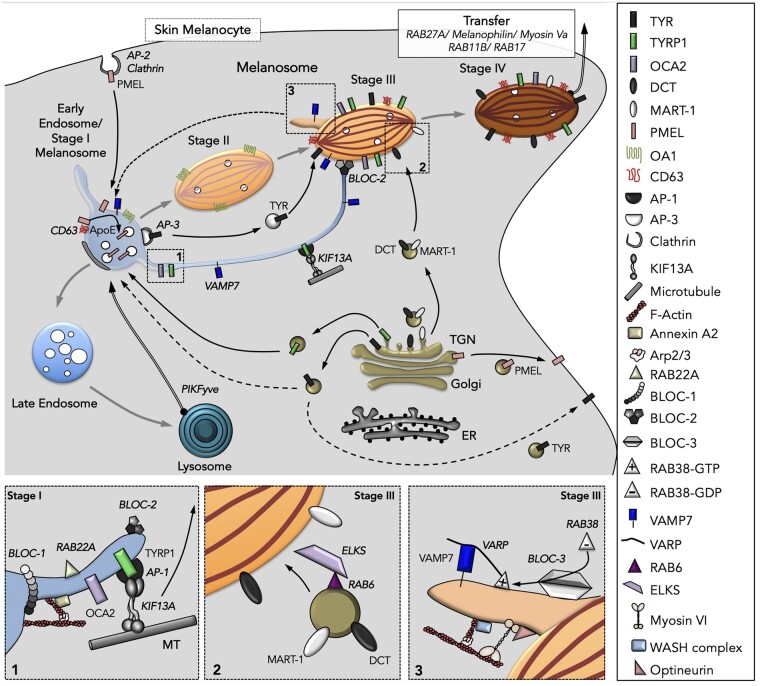
Fig. 3.
Working model of intracellular trafficking during melanosome biogenesis. Key molecules indicated in the figure are shown to the right. All melanosomal proteins are synthesized in the ER and transit the Golgi complex and the TGN en route to melanosomes. Transport of cargoes such as TYR, TYRP1, and DCT between compartments from the TGN and beyond are indicated by solid (if known) or dashed (if not yet determined) black arrows. The early endosome/Stage I melanosome progressively matures (gray arrows) to late endosomes and lysosomes or to Stage IV pigmented melanosomes through the sequential delivery of components that originate from the endocytic and exocytic pathways. PMEL is targeted from the TGN to the plasma membrane, from where it is endocytosed PMEL and delivered to Stage I melanosomes. It is then sorted to ILVs, from which it forms elongated amyloid fibrils that distend the organelle to form Stage II melanosomes. This process requires CD63, OA1, Apoliprotein E (ApoE), and other effectors described in the text. Melanin synthesis begins in Stage III due to the delivery of TYR, TYRP1, OCA2, ATP7A, and other cargoes from endosomes/Stage I melanosomes—to which TYRP1 is delivered without passing through the cell surface—and of DCT and MART-1 from the Golgi/TGN. Two transport pathways originate from Stage I melanosomes, a vesicular route requiring AP-3 and a tubular route (box 1) requiring BLOC-1, AP-1, RAB22A, KIF13A, microtubules, and branched actin-associated machineries for tubule formation. The tubules are targeted along microtubules toward maturing Stage III melanosomes in a process requiring BLOC-2, and fuse with these organelles in a VAMP7-dependent manner. A third route (box 2) is mediated by secretory-like vesicles bearing MART-1 and DCT that bud from the Golgi apparatus/TGN in a process requiring RAB6 and are targeted toward maturing Stage III melanosomes in an ELKS-dependent manner. From Stage III melanosomes, some components (e.g., VAMP7 bound to the scaffolding protein VARP) are removed (box 3) via membrane tubules that require BLOC-3, RAB38/RAB32, Myosin VI, OPTN, the WASH complex, and branched actin filaments to promote their formation and release; these tubules might be transported to Stage I melanosomes (dashed arrow). Stage IV melanosomes require the tripartite complex RAB27A, Melanophilin, Myosin Va, and other RAB GTPases to tether to the peripheral actin cytoskeleton prior to transfer to keratinocytes, a poorly understood process that is detailed in the companion paper in this issue (Benito-Martinez et al. 2021). Adapted from Bowman et al. (2019) and Delevoye et al. (2019).
The enigmatic Stage I melanosome
Melanocytes harbor both melanosomes and classical organelles of the late endolysosomal pathway common to all cells, both of which presumably originate from the same Stage I melanosome/early endosome (Raposo et al. 2001). The co-existence of melanosomes and late endosomes/lysosomes ensures that melanocytes maintain both pigmentation and degradative/metabolic capacities. However, we do not yet understand how Stage I melanosomes transform into both Stage II melanosomes and late endosomes.
One hypothetical mechanism for this step is that Stage I melanosomes divide into Stage II and late endosomal compartments. Such a split might be driven by signaling from GPR143 (a.k.a. OA type 1, OA1); Fig. 2 a pigment cell-specific intracellular G Protein-Coupled Receptor (Schiaffino et al. 1999; Innamorati et al. 2006) that is defective in OA1 patients (Schiaffino et al. 1996). Quantitative immuno-electron microscopy analyses show that GPR143 associates with multiple endolysosomal compartments but is most enriched in early stage melanosomes (Fig. 3) where it regulates early melanogenesis and the segregation of the endosomal and melanosomal lineages (Giordano et al. 2009), as well as melanosome number, size, composition, and subcellular positioning (Palmisano et al. 2008; Giordano et al. 2009; Falletta et al. 2014; Burgoyne et al. 2015). GRP143 expression in nonpigment cells can block fusion of a subset of late endosomes with lysosomes (Burgoyne et al. 2013), but how GPR143-mediated signaling mediates this effect is not clear. GPR143-mediated stimulation of melanosome segregation requires the integrity of melanosome–mitochondrial membrane contacts and mitochondrial ATP production (Daniele et al. 2014) and may be modulated by ubiquitylation and ESCRT-dependent clearance from the endosomal/melanosomal limiting membrane (Giordano et al. 2011). L-dopa was proposed as the ligand to activate GPR143 (Lopez et al. 2008), but this has yet to be verified. Alternatively, it is possible that GPR143 in some way senses a threshold level of PMEL accumulation—for example, via membrane deformation—to trigger a split from the conventional endolysosomal pathway.
A second hypothesis to explain the segregation of melanosomes and late endosomes from Stage I melanosomes is that distinct subpopulations of early endosomes co-exist, one giving rise to the LRO and the other to late endosomes/lysosomes. In nonpigment cells, a subset of early endosomal compartments, called signaling endosomes, receive internalized cargoes for further recycling (Miaczynska et al. 2004; Kalaidzidis et al. 2015). Signaling endosomes bear specific molecular signatures that include the adaptor protein, phosphotyrosine interacting with PH domain and leucine zipper (APPL) proteins (Miaczynska et al. 2004). Interestingly, depletion of APPL1 from pigmented melanoma cells decreased pigmentation (Kedlaya et al. 2011); however, an associated reduction in TYR expression suggests that this effect reflects signaling, perhaps mediated by MC1R, rather than a specific trafficking pathway toward melanosomes (Yardman-Frank and Fisher 2020). Nevertheless, whether additional early endosomal subpopulations contribute directly to melanosome biogenesis remains unknown.
A third and nonmutually exclusive hypothesis would be that late endosomes themselves trigger the Stage I to Stage II transformation. Stage I identity and membrane dynamics rely on the activity of the lipid kinase PIKfyve, which controls the fusion and content mixing of late endosomes/lysosomes with Stage I/II melanosomes (Bissig et al. 2019; Fig. 3). PIKfyve is a phosphatidylinositol (PtdIns)-3-phosphate 5-kinase responsible for generating PtdIns(3,5)bisphosphate on late endosomes. In other cell types, PIKfyve regulates late endosome maturation by controlling import and export from the maturing organelle (Rutherford et al. 2006; de Lartigue et al. 2009). PIKfyve exists as a complex with the PtdIns(3,5)bisphosphatase, FIG4, and mouse models deficient in this complex showed coat-color dilution (Chow et al. 2007) associated with abnormal melanosome identity, morphology, and PMEL fibrillation in pigment cell culture (Bissig et al. 2019). Therefore, endolysosomes might contribute to the maintenance and function of melanosomes and other LROs. Similarly, depletion of autophagy regulators such as WIPI1, Beclin, and ULK1 modulates melanogenesis in pigmented melanoma cell lines, at least in part by modulating melanogenic gene expression (Ganesan et al. 2008; Ho et al. 2011; Kalie et al. 2013); however, the same mechanisms might not operate in nontransformed melanocytes (Zhang et al. 2015; Yun et al. 2016; Yang et al. 2021).
Whether elements of all three hypotheses co-exist or whether they reflect alternative mechanisms used by melanocytes to tune early melanogenesis remains to be defined.
Late melanogenesis: three membrane trafficking pathways to deliver multiple components
During melanosome maturation, Stage I melanosomes not only give rise to Stage II, but also facilitate maturation to Stage III by serving as biosynthetic transport intermediates for melanogenic cargoes. At least two types of transport carriers—spherical vesicles and extended tubules—emerge from the limiting membrane of Stage I melanosomes and are destined for maturing Stage III melanosomes (Fig. 3). The major cargo identified to date in the vesicles is TYR (Huizing et al. 2001; Theos et al. 2005), whereas tubules deliver cargoes that promote TYR activity, including TYRP1, ATP7A, OCA2, and perhaps SLC45A2 (Setty et al. 2007, 2008; Delevoye et al. 2009; Sitaram et al. 2012; Delevoye et al. 2016; Dennis et al. 2016; Le et al. 2020). The extended, long-lived tubules have features of recycling endosomes and directly connect the Stage I melanosomes with maturing Stage III melanosomes without severing (Delevoye et al. 2009; Dennis et al. 2015; Fig. 3, box 1). Whereas tubules from multiple endosomal vacuoles can fuse with and provide cargoes to a single Stage III melanosome (Dennis et al. 2015), whether tubules can physically bridge each Stage I with multiple Stage III melanosomes is not yet known.
The biogenesis, protein sorting ability, and motility of Stage I melanosome-derived vesicular and tubular carriers are controlled by various trafficking components, including protein complexes that are targeted by mutations in patients with Hermansky–Pudlak syndrome (HPS; see below; Bowman et al. 2019; Delevoye et al. 2019). But how these different membrane remodeling events are coordinated at the limiting membrane of Stage I melanosomes is not known. Stage III melanosomes may comprise multiple distinct maturation stages that could each receive distinct cohorts of melanosomal components in a spatiotemporally regulated manner. The potential existence of such subsets is based on the differential content of SLC45A2 and OCA2 in maturing melanosomes (Le et al. 2020). However, it is not yet clear if this differential content represents distinct stages of cargo delivery or distinct fates of these cargoes upon arrival at the Stage III melanosome limiting membrane.
Stage I melanosomes are not the only source of biosynthetic transport intermediates bearing cargoes to maturing Stage III melanosomes. Indeed, a third pathway originates from the Golgi apparatus/TGN and contributes to melanin synthesis by direct delivery of vesicles bearing the cargoes DCT and MART-1 to Stage III melanosomes (Patwardhan et al. 2017; Fig. 3, box 2). This observation places melanosomes at the crossroads of both endocytic and exocytic routes. The Golgi-to-melanosome route relies on well-established secretory effectors (i.e., RAB6 and ELKS) and thus appears to be an adaptation of the conventional Golgi-to-plasma membrane secretory pathway. However, many unsolved questions remain about this pathway. What drives the specific recognition and packaging of melanosomal proteins among a myriad of Golgi-derived components? How are theoretically similar secretory vesicles directed to different target membranes (i.e., melanosomes or the plasma membrane)? How are these vesicles instructed to bud from the TGN and traffic to their final destination? Finally, why are some melanosomal components delivered directly from the Golgi while others use the endosomal sorting station? Addressing these questions will be important not only for understanding melanosome biogenesis, but for post-Golgi organelle maturation in general.
The existence of these three distinct delivery pathways likely permits sequential delivery of melanin-synthesizing enzymes and transporters to optimize function and permit the gradual maturation and pigmentation of Stage III melanosomes. Furthermore, the existence of distinct pathways suggests that melanosomal components must remain physically separated prior to their arrival in melanosomes, likely to prevent generation of oxidative melanin intermediates that could wreak havoc on conserved secretory and endosomal functions. Indeed, the two confirmed enzymes in the melanin pathway (TYR and DCT) and the third potential one (TYRP1) each takes a distinct pathway for delivery to maturing melanosomes, indicating that only when these three pathways converge can pigmentation ensue. As distinct as they are, these trafficking pathways must coordinate with each other, suggesting that Stage I melanosomes, the TGN, and transport intermediates are not only conveyors, but possibly also “messengers.” In any case, a better understanding of the roles of the melanosomal components together with their trafficking functions is needed to unlock the proverbial Stage III black box.
Finding a needle in a haystack: the role of the APs in cargo sorting
A challenge faced by pigment cells is to identify and select the melanosomal resident proteins among the plethora of proteins destined for secretion or for other compartments. Such specific delivery requires membrane-associated sorting machineries that can detect, bind, and sort cargo proteins to melanosomes. Sorting is largely achieved by the heterotetrameric adaptor protein (AP) complexes that engage integral membrane proteins within the endomembrane system. Five APs have been identified to date (Sanger et al. 2019), among which AP-1, AP-2, and AP-3 play known functions associated with melanocyte pigmentation (Bowman et al. 2019). Recognition of transmembrane proteins is triggered by the binding of specific cytoplasmic-exposed amino acid signals by APs on specific membranes (Sanger et al. 2019). AP-2 functions at the plasma membrane to sort cargoes for endocytosis, whereas AP-1 and AP-3 function on endosomes and at the TGN. In general, AP-1, AP-2, and a cohort of AP-3 also recruit clathrin, thereby promoting both the selection and packaging of specific cargo into vesicular transport carriers at the appropriate membrane.
The clearest role for APs in cargo sorting to melanosomes is for AP-3 (Fig. 3), the first known membrane trafficking complex for which subunit mutations were identified in HPS patients (Dell'Angelica et al. 1999). Among the symptoms of HPS is OCA, and so the identification of AP-3 subunit mutations in HPS types 2 and 10 suggested an important role in pigmentation. Indeed, AP-3 engages a cytoplasmic sorting signal in TYR and, accordingly, AP-3-deficient melanocytes from HPS2 patients or mouse models are hypopigmented and mislocalize TYR to endosomes (Huizing et al. 2001; Theos et al. 2005). A fraction of TYR is still targeted to melanosomes in the absence of AP-3, resulting in partial pigmentation; this reflects the fact that AP-1 binds to the same sorting signal, and is capable of targeting a cohort of TYR to melanosomes via a distinct pathway in the absence of AP-3 (Theos et al. 2005). Thus, each AP defines a distinct but parallel trafficking mechanism to melanosomes that can, to some extent, compensate for each other. A separate cohort of AP-3 is also required for proper trafficking of OCA2 to melanosomes via the second pathway discussed further below, as well as for sorting of fusion proteins into that pathway.
The prototypical AP-1-dependent melanosomal cargo is TYRP1 (Fig. 3, box 1), which binds exclusively to AP-1 (Theos et al. 2005) and thus is largely (but incompletely; Bowman et al. 2021) targeted to melanosomes even in the absence of AP-3 (Huizing et al. 2001). As such, destabilization of AP-1 leads to TYRP1 sequestration in endosomes, concomitant TYRP1 depletion from Stage III melanosomes, and pigment dilution in melanocyte cultures (Delevoye et al. 2009). AP-1 can also recognize sorting signals in OCA2 and ATP7A for melanosome delivery (Setty et al. 2008; Sitaram et al. 2009; Holloway et al. 2013). AP-1 also recruits the kinesin-3 motor KIF13A (Nakagawa et al. 2000) to endosomes to generate the tubular transport carriers (see below and Fig. 3, box 1). Thus, the impact of AP-1 depletion on cargo transport is complicated, as AP-1 is required both for cargo sorting and for the generation of the transport carriers into which the cargoes are sorted (Delevoye et al. 2009). Intriguingly, AP-3 also functions in sorting some cargoes, such as OCA2 (Sitaram et al. 2009) and SNARE fusion proteins (Bowman et al. 2021), into the canonical AP-1-dependent pathway. While OCA2 recognition by AP-1 can confer steady-state localization within endosomes, its recognition by AP-3 enables the sorting of OCA2 to melanosomes (Sitaram et al. 2012). Nevertheless, in contrast to TYR, OCA2 is transported through tubules and requires BLOC-1 for melanosome delivery (see below; Sitaram et al. 2012). These observations highlight how AP-3 and AP-1 play unique functions in both distinct and common trafficking routes.
Trafficking of PMEL has also been proposed to require APs. At least a cohort of PMEL within endosomes/Stage I melanosomes is targeted following internalization from the plasma membrane (Theos et al. 2006a; Fig. 3) via recognition of a cytoplasmic internalization signal by AP-2 (Theos et al. 2006a; Robila et al. 2008). Although AP-1 was also proposed to regulate PMEL trafficking (Valencia et al. 2006), depletion of AP-1 does not interfere with PMEL fibril formation or Stage II melanosome morphogenesis (Delevoye et al. 2009) and hence is unlikely to impact PMEL.
While APs are clearly involved in selection and/or trafficking of melanosomal cargoes, they also likely affect melanosome biogenesis indirectly. For instance, AP-3 plays an important role in organizing the endosomal membrane system, as depletion of AP-3 results in substantial expansion of early endosomal networks (Peden et al. 2004; Theos et al. 2005). AP-3 could potentially organize endosomal membrane subdomains fated for melanosomal cargo sorting by coordinating with RAB GTPases, like RAB4A (Nag et al. 2018). Alternatively, other membrane shaping components, such as the ESCRT machinery (Giordano et al. 2009; Truschel et al. 2009), tetraspanins (van Niel et al. 2011), or membrane lipids (Sprong et al. 2001; Groux-Degroote et al. 2008; van Niel et al. 2015; Bissig et al. 2019) might establish and maintain endosomal domains needed to correctly select, sort, and traffic various melanosomal cargoes from endosomes to melanosomes. Compared to endosomes, much less is known about how Golgi/TGN membranes might be organized to support melanosomal cargo sorting and trafficking. For instance, whether RAB6 could potentially coordinate with APs or other sorting machineries in melanocytes is not known. However, the RAB6 effector ELKS that promotes the docking and fusion of Golgi-derived vesicles with the plasma membrane (Grigoriev et al. 2007, 2011) controls the direct delivery of RAB6-vesicles to pigmented melanosomes (Patwardhan et al. 2017; Fig. 3, box 2), suggesting that ELKS in melanocytes has a similar targeting function at melanosomes and the plasma membrane.
Formation of recycling tubules and targeting to melanosomes: roles of BLOC-1 and BLOC-2
Once integral membrane melanosomal components are selected at the source organelle, they must be loaded into the proper transport carrier for delivery to melanosomes. As in other well-characterized trafficking steps (Bonifacino and Glick 2004), cargo sorting must be coupled to carrier biogenesis and transport. Importantly, key components in membrane reshaping and transport in and out of the melanosome were identified as genes mutated in HPS (Bowman et al. 2019).
BLOC-1 and tubule formation
Membrane remodeling during cargo transport is best understood for the tubular recycling endosome-to-melanosome pathway, in which tubular structures emerge from the limiting membrane of vacuolar endosomes/Stage I melanosomes and extend toward maturing Stage III melanosomes (Delevoye et al. 2009; Bowman et al. 2019; Delevoye et al. 2019; Fig. 3). The biogenesis of a narrow tubule from a relatively flat membrane (e.g., Stage I) requires the initiation of membrane curvature and its stabilization as a spherical bud that is then elongated. Among the factors needed to shape the recycling tubule, BLOC-1 is a master regulator. BLOC-1 consists of eight small subunits, four of which are mutated in HPS subtypes (HPS7, HPS8, HPS9, and HPS11), a fifth is mutated in an unique mouse model, and the remaining three are shared with a separate complex, BLOC-1 related complex (BORC), that regulates lysosomal positioning in all cell types (Bowman et al. 2019; Pennamen et al. 2020a). The loss of expression of any single BLOC-1 subunit leads to destabilization and loss of function of the entire complex, resulting in decreased pigmentation in human patients, animal models, and melanocyte culture (Bowman et al. 2019). The hypopigmentation is caused by a malformation of the recycling tubules from endosomes (Delevoye et al. 2016) that leads to the missorting and endosomal accumulation of melanosomal components including TYRP1, OCA2, and ATP7A (Di Pietro et al. 2006; Setty et al. 2007, 2008; Sitaram et al. 2012). At the limiting membrane of endosomes, BLOC-1 coordinates the formation of recycling tubules in coordination with actin polymerization and KIF13A (Delevoye et al. 2016), which pulls the membrane tubule along microtubules (Fig. 3, box 1). This process is not restricted to pigment cells in mammals (Delevoye et al. 2014). As discussed earlier, AP-1 not only functions in cargo sorting into the newly formed recycling tubules but also facilitates the recruitment of KIF13A, thereby mobilizing tubules toward the cell periphery in the vicinity of Stage III melanosomes (Delevoye et al. 2009). Hence, uncoupling the AP-1-KIF13A interaction leads to the malformation and malfunction of pigmented melanosomes in melanocyte culture and in reconstructed pigmented epidermis (Campagne et al. 2018). Thus, BLOC-1 supports the biogenesis of recycling tubules by engaging cytoskeleton-associated machineries, while AP-1 bridges the membrane-associated cargoes to microtubules for transport to melanosomes (Fig. 3, box 1).
Many questions regarding the “BLOC-1 pathway” are still unsolved. A key open question is the molecular function of BLOC-1 and its intrinsic membrane remodeling capacity. A recombinant form of BLOC-1 adopts a curvilinear banana-like structure (Lee et al. 2012), suggesting that BLOC-1 might sense, generate, and/or stabilize membrane curvature needed for tubule formation, much like SNX-BAR proteins (van Weering and Cullen 2014). This sensing could be coupled to the lipid composition of the membranes since BLOC-1 can be copurified with PI4KIIα (Salazar et al. 2009; Gokhale et al. 2012)—a lipid kinase that produces PtdIns-4-phosphate on endosomal membranes. RAB GTPases could also potentially play a role in organizing membrane domains suitable for BLOC-1-dependent tubule formation. For example, RAB22A is necessary for proper melanosome biogenesis and interacts with BLOC-1 and other regulators of tubule formation (Shakya et al. 2018), and RAB11A interacts with KIF13A to form and transport recycling tubules in nonpigment cells (Delevoye et al. 2014). However, whether RAB11A cooperates with BLOC-1 in melanocytes remains questionable given that decreased expression of RAB11A raised melanocyte pigmentation (Beaumont et al. 2011; Tarafder et al. 2014). Indeed, BLOC-1 may function in a cell type-specific manner, as it coordinates with distinct effectors—Annexin A2 or the WASH complex—to mediate actin polymerization in melanocytes or neurons and HEK 293 cells, respectively (Ryder et al. 2013; Delevoye et al. 2016). Future studies are needed to better delineate the functions and impact of “BLOC-1 and friends” on pigmentation and how their alterations could lead to unrecognized forms of OCA.
BLOC-2 and tubule targeting
Compared to our understanding of tubular transport carrier formation, little is known regarding how BLOC-1-dependent tubules are specifically targeted to maturing melanosomes. Targeting of the recycling tubules to melanosomes requires BLOC-2 (Fig. 3), a complex composed of three subunits encoded by genes that are defective in HPS variants (HPS3, 5, and 6; Di Pietro et al. 2004). Accordingly, BLOC-2-deficient HPS patients, mouse HPS models, and derived melanocytes are hypopigmented (Bowman et al. 2019), and TYRP1 in melanocytes is mislocalized, largely to target organelles to which recycling endosomes would normally deliver cargo—the TGN, endosomes, and the plasma membrane (Helip-Wooley et al. 2007; Huizing et al. 2009; Dennis et al. 2015). BLOC-2 physically interacts with a cohort of BLOC-1 and can be detected on endosomal tubules near pigmented melanosomes (Di Pietro et al. 2006), suggesting that BLOC-2 may function downstream of BLOC-1 along the recycling endosome-to-melanosome route. Consistently, BLOC-2-deficient melanocytes generate normal numbers of tubules, but they are shorter lived and fail to contact melanosomes (Dennis et al. 2015). Exactly how BLOC-2 supports tubule contact with melanosomes is not clear. It might function either as a membrane tether to melanosomes or as a linker to stabilize the membrane tubules through interactions with microtubule components. BLOC-2 appears to associate with RAB22A (Shakya et al. 2018) and/or the cell type-restricted RAB32 and RAB38 (Bultema et al. 2012), but the consequences of these interactions are not clear. While the HPS6 subunit was proposed to associate with the dynein/dynactin complex, a microtubule motor that ferries cargo in the opposite direction as most kinesins (Li et al. 2014), it remains to be determined if this reflects the aggregation of an overexpressed isolated HPS6 subunit that mobilizes to the microtubule organizing center (Garcia-Mata et al. 1999) rather than a physiological function of BLOC-2. Future studies are needed to decipher the molecular basis for BLOC-2 function.
Endosomal membrane fusion with maturing melanosomes
To deliver cargos to melanosomes, the transport carriers must fuse with the limiting membrane of melanosomes. Membrane fusion within the endomembrane system is mediated by interactions between SNARE proteins, a conserved family of coiled-coil proteins (Jahn and Scheller 2006). Typically, a v-SNARE on the vesicle or tubule membrane engages a compound t-SNARE complex on the target membrane to form a stable four-helix bundle; zipping of this bundle brings apposing membranes into close apposition, resulting in disruption of the lipid bilayer and ultimate fusion. Each fusion step in the endomembrane system is mediated by a specific pairing of v- and t-SNAREs. In addition, tethering factors typically mediate long-range interactions between apposing membranes prior to SNARE engagement, and other components such as Sec1-Munc18 (SM) family members often promote SNARE fusion (Cai et al. 2007; Rizo and Südhof 2012).
Our understanding of the SNARE proteins and their regulators that specifically mediate membrane fusion processes with melanosomes is very limited. In the BLOC-1-dependent pathway, the v-SNARE utilized appears to be VAMP7 (Fig. 3), an ubiquitous SNARE protein that mediates fusion within the late endosomal/lysosomal and autophagy pathways in other cell types. This is supported by the following observations: depletion of VAMP7 disrupts TYRP1 targeting to melanosomes and reduces pigmentation (Tamura et al. 2011; Jani et al. 2015; Dennis et al. 2016); a GFP-VAMP7 fusion protein localizes at steady state to melanosomes (Dennis et al. 2016); and, like TYRP1, VAMP7 is trapped in early endosomes of BLOC-1-deficient cells (Dennis et al. 2016). The specific t-SNAREs involved, however, are unclear. Syntaxin-13 (a.k.a. syntaxin-12) is needed for pigmentation and TYRP1 trafficking (Jani et al. 2015) and binds in vitro to BLOC-1 (Huang et al. 1999; Moriyama and Bonifacino 2002; Ghiani et al. 2010), but its restriction to tubules and lack of association with melanosomes upon fusion (Dennis et al. 2015; Delevoye et al. 2016) suggest that it is not a requisite fusion partner for VAMP7. Intriguingly, we recently found that syntaxin-13 forms a complex with VAMP7 (likely with two additional SNARE helices) in endosomes that is sorted into BLOC-1-dependent transport carriers. Sorting is mediated by redundant recognition of either syntaxin-13 by BLOC-1 or of VAMP7 by AP-3 within a BLOC-1-AP-3 super-complex; disruption of either recognition mode does not affect cargo transport, but disruption of both impairs delivery of VAMP7 and BLOC-1-dependent cargo to melanosomes (Bowman et al. 2021). This explains the requirement for syntaxin-13 in BLOC-1-dependent cargo transport. Syntaxin-3 was reported to be required for TYRP1 delivery to melanosomes (Yatsu et al. 2013), but this SNARE localizes to the plasma membrane at steady state and is thus unlikely to function as the melanosomal t-SNARE (D. Harper and M. S. Marks, unpublished observations). A number of SNARE proteins are upregulated during differentiation of a mouse melanoma to a pigmented phenotype, and thus might be involved in melanogenesis (Wade et al. 2001), but to date, only VAMP7 has been directly implicated. In addition, essentially nothing is known about the tethering protein complexes and SM family members involved in melanosome biogenesis. A point mutation in the VPS33A subunit of HOPS and CORVET tethering/SM complexes underlies an HPS-like phenotype in buff mice (Suzuki et al. 2003), but how the mutation impacts VPS33A function and whether it is involved directly in melanosome biogenesis is not clear. Identifying the SNARE proteins and additional fusion regulators for all of the melanosome trafficking pathways will be necessary to complete the picture of cargo transfer to melanosomes.
Delivering is not enough: recycling from Stage III melanosomes
The trafficking pathways described above consist of membrane carriers targeting the maturing melanosomes. However, like any other organelle, melanosomes must control their size to maintain their homeostasis and function. This can only happen if some materials are removed from melanosomes to counter the anterograde flow of components.
Insight into retrograde transport from melanosomes came from the analysis of the trafficking of VAMP7 in the BLOC-1-dependent pathway. In melanocytes, VAMP7 dynamically associates with pigmented melanosomes, from where it is retrieved in tubular transport carriers that are distinct from those carrying forward-moving cargos (Dennis et al. 2016; Fig. 3, box 3). These melanosome-derived tubules are devoid of classical melanosomal and endosomal components, suggesting that their role is primarily to retrieve unwanted melanosomal contents, including VAMP7, for eventual return to early endosomes to promote multiple rounds of forward trafficking (Dennis et al. 2016). The retrieved VAMP7 associates with VARP (Dennis et al. 2016), a scaffolding partner that stabilizes VAMP7 in a nonfusogenic conformation (Burgo et al. 2012; Schäfer et al. 2012), and with RAB38, another VARP-binding partner (Wang et al. 2008) that associates with melanosomes (Wasmeier et al. 2006; Bultema et al. 2012; Gerondopoulos et al. 2012; Fig. 3, box 3). VARP association with melanosomes requires GTP-bound RAB38 and/or its close homolog RAB32 (Dennis et al. 2016), both of which are activated by the guanine nucleotide exchange factor BLOC-3 (Gerondopoulos et al. 2012). BLOC-3 is composed of HPS1 and HPS4 subunits (Chiang et al. 2003; Martina et al. 2003; Nazarian et al. 2003), mutations in which result in the most common forms of HPS (Seward and Gahl 2013). BLOC-3-deficient melanocytes are impaired in the formation and release of retrograde VAMP7-containing melanosomal carriers (Dennis et al. 2016), suggesting that BLOC-3 and RAB32/RAB38 function primarily in the retrograde pathway.
Release of VAMP7-containing retrograde tubular carriers from melanosomes requires the cooperation of the Myosin VI actin-based motor and its partner Optineurin (OPTN) with branched actin filament-associated machineries (Ripoll et al. 2018; Fig. 3, box 3). Together, these components promote the constriction of the tubule “neck” prior to its membrane fission. Impairing the release of the melanosomal tubules by depletion or inhibition of any of these components generates abnormally large melanosomes with increased melanosomal protein content, a more acidic pH, and reduced tendency for melanin secretion and transfer to keratinocytes, resulting in increased melanin content of the melanocytes (Loubéry et al. 2012; Ripoll et al. 2018). It remains unclear if the myosin VI-dependent release pathway is regulated by BLOC-3 and RAB38 or RAB32, but if so, it would suggest that skin and hair hypopigmentation in HPS1 and HPS4 patients might be due to suboptimal melanin transfer from melanocytes to keratinocytes. Another myosin, Myosin Vc, also interacts with RAB32/38 and its depletion in pigment cells phenocopied the loss of Myosin VI, suggesting that different myosins can cooperate during melanosome maturation (Bultema et al. 2014).
Analyses of retrograde trafficking from melanosomes have provided an unprecedented link between the homeostasis, maturation, and secretory capacity of melanosomes. Additional studies will be needed to better delineate how incoming traffic to melanosomes is coordinated with cargo loading and release of the retrograde tubules. Moreover, a better understanding of additional components within the retrograde tubules that guide them to their ultimate destination is needed. Indeed, it remains to be validated whether these tubules are targeted to early endosomes/Stage I melanosomes as proposed (Dennis et al. 2016). Interestingly, the volume of melanosome-derived tubules can reach one-third of the total melanosome volume (Ripoll et al. 2018), indicating that a large fraction of luminal solutes is also removed with VAMP7, perhaps contributing to pH regulation. Finally, although the melanosomal tubules did not contain melanin-laden PMEL fibers, they aligned preferentially along their longest axis (Ripoll et al. 2018). This suggests that membrane deformation at the limiting membrane of melanosomes does not occur randomly, and that the fibrils could impose steric hindrance to serve as an organelle-intrinsic mechanical signal for melanosomes to initiate retrograde trafficking prior to peripheral capture and subsequent transfer to keratinocytes.
Conclusion
Cell biological and biochemical analyses of mammalian skin melanocytes and the products of genes that are defective in various forms of nonsyndromic and syndromic forms of albinism have provided invaluable information about how a pigment cell exploits both specific and ubiquitous mechanisms to build the functional melanosome. This has allowed us to better understand not only the molecular basis of pigmentation but also basic principles of functional amyloid formation, organellar ion flux, organelle maturation, and membrane transport that apply to many other physiological systems. Importantly, the membrane dynamics and trafficking within skin melanocytes that are responsible for melanosome maturation serve as a model for the mechanisms shared by other LRO-generating cells during the assembly of their specific organelles—particularly the platelet dense granules, lung alveolar type II cell lamellar bodies, and immune organelles that are disrupted in the various forms of HPS (Bowman et al. 2019; Delevoye et al. 2019). Further analyses of melanosome components, membrane trafficking pathways, and identification of new functional partners of already-identified components will likely not only deepen our understanding of the biogenesis of melanosomes and other LROs but also provide new candidates for genes targeted in forms of albinism and other pigmentary disorders that currently lack a molecular diagnosis.
More generally, a major challenge in understanding how the cellular machineries such as APs, BLOCs, and SNAREs specifically regulate melanosome biogenesis in pigment cells is their ubiquitous expression and role in trafficking to ubiquitous organelles in other cell types. For example, BLOC-1 is required for recycling endosome morphogenesis in HeLa cells (Delevoye et al. 2014) and for delivery of certain cargoes to the primary cilium (Monis et al. 2017). Given that other LROs are malformed in HPS, but conventional endolysosomal organelles are largely intact, it is likely that cell type-restricted factors or modifications, such as RAB GTPases, effector isoforms, or post-translational modifications such as phosphorylation or ubiquitylation, play a role in defining the specificity of cargo selection, transport carrier targeting, and fusion in melanocytes and other cell types. Solving these enigmas will extend beyond the context of pigmentation and will help to solve a bigger mystery about how a common intracellular toolbox can be exploited by specialized cell types to create a new organelle.
Funding
This work was supported by National Institutes of Health grants R01 EY015625 (to M.S.M. and G.R.) from the National Eye Institute, R01 AR076241 (to M.S.M, Sarah Tishkoff, and Elena Oancea) and R01 AR071382 (to M.S.M. and Elena Oancea) from the National Institute for Arthritis, Musculoskeletal and Skin Diseases; the Fondation pour la Recherche Médicale (FRM team label DEQU201903007827 to G.R. and SPF201909009097 to J.S.C.); the Fondation ARC pour la Recherche sur le Cancer (ARCPJA22020060002267 to C.D.); the Agence Nationale de la Recherche (ANR: “MYOACTIONS” ANR-17-CE11-0029-03, to C.D.); GENESPOIR (to C.D.); LabEx Cell(n)Scale (ANR-11-LABX-0038); L'Oréal Research and Development (to G.R.), Institut Curie (to G.R.), l’Institut National de la Santé et de la Recherche Médicale (INSERM, to C.D.), and the Centre National de la Recherche Scientifique (CNRS, to G.R.).
Acknowledgments
We thank past and present members of the Raposo and Marks laboratories for many contributions that are presented in this work and Leslie B. King for careful editing of the manuscript.
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Melanosomes in melanocytes that make predominantly red and yellow pheomelanins have a distinct structure and have not been well characterized. This review will thus focus exclusively on melanosomes that make primarily eumelanins.
From the Symposium “SICB-Wide Symposium: The integrative biology of pigment organelles” presented at the virtual annual meeting of the Society for Integrative and Comparative Biology, January 3–7, 2020.
References
- Adelmann CH, Traunbauer AK, Chen B, Condon KJ, Chan SH, Kunchok T, Lewis CA, Sabatini DM.. 2020. MFSD12 mediates the import of cysteine into melanosomes and lysosomes. Nature 588:699–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adhikari K, Mendoza-Revilla J, Sohail A, Fuentes-Guajardo M, Lampert J, Chacon-Duque JC, Hurtado M, Villegas V, Granja V, Acuna-Alonzo V, et al. 2019. A GWAS in Latin Americans highlights the convergent evolution of lighter skin pigmentation in Eurasia. Nat Commun 10:358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altimimi HF, Schnetkamp PP.. 2007. Na+/Ca2+-K+ exchangers (NCKX): functional properties and physiological roles. Channels (Austin) 1:62–9. [DOI] [PubMed] [Google Scholar]
- Ambrosio AL, Boyle JA, Aradi AE, Christian KA, Di Pietro SM.. 2016. TPC2 controls pigmentation by regulating melanosome pH and size. Proc Natl Acad Sci U S A 113:5622–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ancans J, Tobin DJ, Hoogduijn MJ, Smit NP, Wakamatsu K, Thody AJ.. 2001. Melanosomal pH controls rate of melanogenesis, eumelanin/phaeomelanin ratio and melanosome maturation in melanocytes and melanoma cells. Exp Cell Res 268:26–35. [DOI] [PubMed] [Google Scholar]
- Andersson LS, Lyberg K, Cothran G, Ramsey DT, Juras R, Mikko S, Ekesten B, Ewart S, Lindgren G.. 2011. Targeted analysis of four breeds narrows equine Multiple Congenital Ocular Anomalies locus to 208 kilobases. Mamm Genome 22:353–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolke R, Heinisch JJ, Wieczorek H, Vitavska O.. 2014. Proton-associated sucrose transport of mammalian solute carrier family 45: an analysis in Saccharomyces cerevisiae. Biochem J 464:193–201. [DOI] [PubMed] [Google Scholar]
- Beaumont KA, Hamilton NA, Moores MT, Brown DL, Ohbayashi N, Cairncross O, Cook AL, Smith AG, Misaki R, Fukuda M, et al. 2011. The recycling endosome protein Rab17 regulates melanocytic filopodia formation and melanosome trafficking. Traffic 12:627–43. [DOI] [PubMed] [Google Scholar]
- Bellono NW, Escobar IE, Lefkovith AJ, Marks MS, Oancea E.. 2014. An intracellular anion channel critical for pigmentation. eLife 3:e04543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellono NW, Escobar IE, Oancea E.. 2016. A melanosomal two-pore sodium channel regulates pigmentation. Sci Rep 6:26570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito-Martinez S, Zhu Y, Jani RA, Harper DC, Marks MS, Delevoye C.. 2020. Research techniques made simple: cell biology methods for the analysis of pigmentation. J Invest Dermatol 140:257–68 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito-Martinez S, Salavessa L, Raposo G, Marks MS, Delevoye C. 2021. Melanin transfer and fate within human keratinocytes in human skin pigmentation. Integr Comp Biol (doi: 10.1093/icb/icab094). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergam P, Reisecker JM, Rakvacs Z, Kucsma N, Raposo G, Szakacs G, van Niel G.. 2018. ABCB6 resides in melanosomes and regulates early steps of melanogenesis required for PMEL amyloid matrix formation. J Mol Biol 430:3802–18. [DOI] [PubMed] [Google Scholar]
- Berson JF, Harper D, Tenza D, Raposo G, Marks MS.. 2001. Pmel17 initiates premelanosome morphogenesis within multivesicular bodies. Mol Biol Cell 12:3451–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berson JF, Theos AC, Harper DC, Tenza D, Raposo G, Marks MS.. 2003. Proprotein convertase cleavage liberates a fibrillogenic fragment of a resident glycoprotein to initiate melanosome biogenesis. J Cell Biol 161:521–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar V, Ramalah A.. 1998. Characterization of Mg2+-ATPase activity in isolated B16 murine melanoma melanosomes. Mol Cell Biochem 189:99–106. [DOI] [PubMed] [Google Scholar]
- Birbeck MSC. 1963. Electron microscopy of melanocytes: the fine structure of hair-bulb premelanosomes. Ann NY Acad Sci 100:540–7. [DOI] [PubMed] [Google Scholar]
- Bissig C, Croisé P, Heiligenstein X, Hurbain I, Lenk GM, Kaufman E, Sannerud R, Annaert W, Meisler MH, Weisman LS, et al. 2019. The PIKfyve complex regulates the early melanosome homeostasis required for physiological amyloid formation. J Cell Sci 132:229500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boissy RE, Sakai C, Zhao H, Kobayashi T, Hearing VJ.. 1998. Human tyrosinase related protein-1 (TRP-1) does not function as a DHICA oxidase activity in contrast to murine TRP-1. Exp Dermatol 7:198–204. [DOI] [PubMed] [Google Scholar]
- Boissy RE, Zhao H, Oetting WS, Austin LM, Wildenberg SC, Boissy YL, Zhao Y, Sturm RA, Hearing VJ, King RA, et al. 1996. Mutation in and lack of expression of tyrosinase-related protein-1 (TRP-1) in melanocytes from an individual with brown oculocutaneous albinism: a new subtype of albinism classified as “OCA3”. Am J Hum Genet 58:1145–56. [PMC free article] [PubMed] [Google Scholar]
- Bonifacino JS, Glick BS.. 2004. The mechanisms of vesicle budding and fusion. Cell 116:153–66. [DOI] [PubMed] [Google Scholar]
- Bowman SL, Bi-Karchin J, Le L, Marks MS.. 2019. The road to LROs: insights into lysosome-related organelles from Hermansky-Pudlak syndrome and other rare diseases. Traffic 20:404–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman SL, Le L, Zhu Y, Harper DC, Sitaram A, Theos AC, Sviderskaya EV, Bennett DC, Raposo G, Owen DJ, et al. 2021. A BLOC-1-AP-3 super-complex sorts a cis-SNARE complex into endosome-derived tubular transport carriers. J Cell Biol 220:e202005173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brilliant MH. 2001. The mouse p (pink-eyed dilution) and human P genes, oculocutaneous albinism type 2 (OCA2), and melanosomal pH. Pigment Cell Res 14:86–93. [DOI] [PubMed] [Google Scholar]
- Bultema JJ, Ambrosio AL, Burek CL, Di Pietro SM.. 2012. BLOC-2, AP-3, and AP-1 function in concert with Rab38 and Rab32 to mediate protein trafficking to lysosome-related organelles. J Biol Chem 287:19550–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bultema JJ, Boyle JA, Malenke PB, Martin FE, Dell’Angelica EC, Cheney RE, Di Pietro SM.. 2014. Myosin vc interacts with rab32 and rab38 proteins and works in the biogenesis and secretion of melanosomes. J Biol Chem 289:33513–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgo A, Proux-Gillardeaux V, Sotirakis E, Bun P, Casano A, Verraes A, Liem RK, Formstecher E, Coppey-Moisan M, Galli T.. 2012. A molecular network for the transport of the TI-VAMP/VAMP7 vesicles from cell center to periphery. Dev Cell 23:166–80. [DOI] [PubMed] [Google Scholar]
- Burgoyne T, Jolly R, Martin-Martin B, Seabra MC, Piccirillo R, Schiaffino MV, Futter CE.. 2013. Expression of OA1 limits the fusion of a subset of MVBs with lysosomes - a mechanism potentially involved in the initial biogenesis of melanosomes. J Cell Sci 126:5143–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne T, O’Connor MN, Seabra MC, Cutler DF, Futter CE.. 2015. Regulation of melanosome number, shape and movement in the zebrafish retinal pigment epithelium by OA1 and PMEL. J Cell Sci 128:1400–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush WD, Simon JD.. 2007. Quantification of Ca(2+) binding to melanin supports the hypothesis that melanosomes serve a functional role in regulating calcium homeostasis. Pigment Cell Res 20:134–9. [DOI] [PubMed] [Google Scholar]
- Cai H, Reinisch K, Ferro-Novick S.. 2007. Coats, tethers, rabs, and SNAREs work together to mediate the intracellular destination of a transport vesicle. Dev Cell 12:671–82. [DOI] [PubMed] [Google Scholar]
- Calcraft PJ, Ruas M, Pan Z, Cheng X, Arredouani A, Hao X, Tang J, Rietdorf K, Teboul L, Chuang KT, et al. 2009. NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature 459:596–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campagne C, Ripoll L, Gilles-Marsens F, Raposo G, Delevoye C.. 2018. AP-1/KIF13A blocking peptides impair melanosome maturation and melanin synthesis. Int J Mol Sci 19:568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carraro-Lacroix LR, Lessa LM, Fernandez R, Malnic G.. 2009. Physiological implications of the regulation of vacuolar H+-ATPase by chloride ions. Braz J Med Biol Res 42:155–63. [DOI] [PubMed] [Google Scholar]
- Chalhoub N, Benachenhou N, Rajapurohitam V, Pata M, Ferron M, Frattini A, Villa A, Vacher J.. 2003. Grey-lethal mutation induces severe malignant autosomal recessive osteopetrosis in mouse and human. Nat. Med 9:399–406. [DOI] [PubMed] [Google Scholar]
- Chao YK, Schludi V, Chen CC, Butz E, Nguyen ONP, Muller M, Kruger J, Kammerbauer C, Ben-Johny M, Vollmar AM, et al. 2017. TPC2 polymorphisms associated with a hair pigmentation phenotype in humans result in gain of channel function by independent mechanisms. Proc Natl Acad Sci U S A 114:E8595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelly J, Tumer Z, Tonnesen T, Petterson A, Ishikawa-Brush Y, Tommerup N, Horn N, Monaco AP.. 1993. Isolation of a candidate gene for Menkes disease that encodes a potential heavy metal binding protein. Nat Genet 3:14–9. [DOI] [PubMed] [Google Scholar]
- Chen K, Manga P, Orlow SJ.. 2002. Pink-eyed dilution protein controls the processing of tyrosinase. Mol Biol Cell 13:1953–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng T, Orlow SJ, Manga P.. 2013. Loss of Oca2 disrupts the unfolded protein response and increases resistance to endoplasmic reticulum stress in melanocytes. Pigment Cell Melanoma Res 26:826–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang PW, Oiso N, Gautam R, Suzuki T, Swank RT, Spritz RA.. 2003. The Hermansky-Pudlak syndrome 1 (HPS1) and HPS4 proteins are components of two complexes, BLOC-3 and BLOC-4, involved in the biogenesis of lysosome-related organelles. J Biol Chem 278:20332–7. [DOI] [PubMed] [Google Scholar]
- Chow CY, Zhang Y, Dowling JJ, Jin N, Adamska M, Shiga K, Szigeti K, Shy ME, Li J, Zhang X, et al. 2007. Mutation of FIG4 causes neurodegeneration in the pale tremor mouse and patients with CMT4J. Nature 448:68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooksey CJ, Garratt PJ, Land EJ, Pavel S, Ramsden CA, Riley PA, Smit NP.. 1997. Evidence of the indirect formation of the catecholic intermediate substrate responsible for the autoactivation kinetics of tyrosinase. J Biol Chem 272:26226–35. [DOI] [PubMed] [Google Scholar]
- Crawford NG, Kelly DE, Hansen MEB, Beltrame MH, Fan S, Bowman SL, Jewett E, Ranciaro A, Thompson S, Lo Y, et al.; NISC Comparative Sequencing Program. 2017. Loci associated with skin pigmentation identified in African populations. Science 358:eaan8433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Alba L, Shawkey MD.. 2019. Melanosomes: biogenesis, properties, and evolution of an ancient organelle. Physiol Rev 99:1–19. [DOI] [PubMed] [Google Scholar]
- Daniele T, Hurbain I, Vago R, Casari G, Raposo G, Tacchetti C, Schiaffino MV.. 2014. Mitochondria and melanosomes establish physical contacts modulated by Mfn2 and involved in organelle biogenesis. Curr Biol 24:393–403. [DOI] [PubMed] [Google Scholar]
- de Lartigue J, Polson H, Feldman M, Shokat K, Tooze SA, Urbé S, Clague MJ.. 2009. PIKfyve regulation of endosome-linked pathways. Traffic 10:883–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delevoye C, Heiligenstein X, Ripoll L, Gilles-Marsens F, Dennis MK, Linares RA, Derman L, Gokhale A, Morel E, Faundez V, et al. 2016. BLOC-1 brings together the actin and microtubule cytoskeletons to generate recycling endosomes. Curr Biol 26:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delevoye C, Hurbain I, Tenza D, Sibarita JB, Uzan-Gafsou S, Ohno H, Geerts WJC, Verkleij AJ, Salamero J, Marks MS, et al. 2009. AP-1 and KIF13A coordinate endosomal sorting and positioning during melanosome biogenesis. J Cell Biol 187:247–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delevoye C, Marks MS, Raposo G.. 2019. Lysosome-related organelles as functional adaptations of the endolysosomal system. Curr Opin Cell Biol 59:147–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delevoye C, Miserey-Lenkei S, Montagnac G, Gilles-Marsens F, Paul-Gilloteaux P, Giordano F, Waharte F, Marks MS, Goud B, Raposo G.. 2014. Recycling endosome tubule morphogenesis from sorting endosomes requires the kinesin motor KIF13A. Cell Rep 6:445–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell’Angelica EC, Shotelersuk V, Aguilar RC, Gahl WA, Bonifacino JS.. 1999. Altered trafficking of lysosomal proteins in Hermansky-Pudlak syndrome due to mutations in the b3A subunit of the AP-3 adaptor. Mol Cell 3:11–21. [DOI] [PubMed] [Google Scholar]
- Dennis MK, Delevoye C, Acosta-Ruiz A, Hurbain I, Romao M, Hesketh GG, Goff PS, Sviderskaya EV, Bennett DC, Luzio JP, et al. 2016. BLOC-1 and BLOC-3 regulate VAMP7 cycling to and from melanosomes via distinct tubular transport carriers. J Cell Biol 214:293–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis MK, Mantegazza AR, Snir OL, Tenza D, Acosta-Ruiz A, Délevoye C, Zorger R, Sitaram A, de Jesus-Rojas W, Ravichandran K, et al. 2015. BLOC-2 targets recycling endosomal tubules to melanosomes for cargo delivery. J Cell Biol 209:563–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pietro SM, Falcon-Perez JM, Dell'Angelica EC.. 2004. Characterization of BLOC-2, a complex containing the Hermansky-Pudlak syndrome proteins HPS3, HPS5 and HPS6. Traffic 5:276–83. [DOI] [PubMed] [Google Scholar]
- Di Pietro SM, Falcon-Perez JM, Tenza D, Setty SRG, Marks MS, Raposo G, Dell'Angelica EC.. 2006. BLOC-1 interacts with BLOC-2 and the AP-3 complex to facilitate protein trafficking on endosomes. Mol Biol Cell 17:4027–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diment S, Eidelman M, Rodriguez GM, Orlow SJ.. 1995. Lysosomal hydrolases are present in melanosomes and are elevated in melanizing cells. J Biol Chem 270:4213–5. [DOI] [PubMed] [Google Scholar]
- Dolinska MB, Wingfield PT, Young KL 2nd, Sergeev YV.. 2019. The TYRP1-mediated protection of human tyrosinase activity does not involve stable interactions of tyrosinase domains. Pigment Cell Melanoma Res 32:753–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolman NJ, Tepikin AV.. 2006. Calcium gradients and the Golgi. Cell Calcium 40:505–12. [DOI] [PubMed] [Google Scholar]
- Donatien PD, Orlow SJ.. 1995. Interaction of melanosomal proteins with melanin. Eur J Biochem 232:159–64. [DOI] [PubMed] [Google Scholar]
- Dunn LC, Thigpen LW.. 1930. The silver mouse: a recessive color variation. J Heredity 21:495–8. [Google Scholar]
- Evans WC, Raper HS.. 1937. The accumulation of l-3:4-dihydroxyphenylalanine in the tyrosinase-tyrosine reaction. Biochem J 31:2162–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falletta P, Bagnato P, Bono M, Monticone M, Schiaffino MV, Bennett DC, Goding CR, Tacchetti C, Valetti C.. 2014. Melanosome-autonomous regulation of size and number: the OA1 receptor sustains PMEL expression. Pigment Cell Melanoma Res 27:565–79. [DOI] [PubMed] [Google Scholar]
- Fowler DM, Koulov AV, Alory-Jost C, Marks MS, Balch WE, Kelly JW.. 2006. Functional amyloid formation within mammalian tissue. PLoS Biol 4:e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller BB, Spaulding DT, Smith DR.. 2001. Regulation of the catalytic activity of preexisting tyrosinase in black and Caucasian human melanocyte cell cultures. Exp Cell Res 262:197–208. [DOI] [PubMed] [Google Scholar]
- Furumura M, Solano F, Matsunaga N, Sakai C, Spritz RA, Hearing VJ.. 1998. Metal ligand-binding specificities of the tyrosinase-related proteins. Biochem Biophys Res Commun 242:579–85. [DOI] [PubMed] [Google Scholar]
- Ganesan AK, Ho H, Bodemann B, Petersen S, Aruri J, Koshy S, Richardson Z, Le LQ, Krasieva T, Roth MG, et al. 2008. Genome-wide siRNA-based functional genomics of pigmentation identifies novel genes and pathways that impact melanogenesis in human cells. PLoS Genet 4:e1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Mata R, Bebok Z, Sorscher EJ, Sztul ES.. 1999. Characterization and dynamics of aggresome formation by a cytosolic GFP-chimera. J Cell Biol 146:1239–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido G, Fernandez A, Montoliu L.. 2021. HPS11 and OCA8: two new types of albinism associated with mutations in BLOC1S5 and DCT genes. Pigment Cell Melanoma Res 34:10–2. [DOI] [PubMed] [Google Scholar]
- Gerndt S, Chen CC, Chao YK, Yuan Y, Burgstaller S, Scotto Rosato A, Krogsaeter E, Urban N, Jacob K, Nguyen ONP, et al. 2020. Agonist-mediated switching of ion selectivity in TPC2 differentially promotes lysosomal function. Elife 9:e54712.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerondopoulos A, Langemeyer L, Liang JR, Linford A, Barr FA.. 2012. BLOC-3 mutated in Hermansky-Pudlak syndrome is a Rab32/38 guanine nucleotide exchange factor. Curr Biol 22:2135–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getting WS, King RA.. 1994. Molecular basis of Oculocutaneous albinism. J Invest Dermatol 103:S131–6. [DOI] [PubMed] [Google Scholar]
- Ghiani CA, Starcevic M, Rodriguez-Fernandez IA, Nazarian R, Cheli VT, Chan LN, Malvar JS, de Vellis J, Sabatti C, Dell’angelica EC.. 2010. The dysbindin-containing complex (BLOC-1) in brain: developmental regulation, interaction with SNARE proteins and role in neurite outgrowth. Mol Psychiatry 15:204–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginger RS, Askew SE, Ogborne RM, Wilson S, Ferdinando D, Dadd T, Smith AM, Kazi S, Szerencsei RT, Winkfein RJ, et al. 2008. SLC24A5 encodes a trans-Golgi network protein with potassium-dependent sodium-calcium exchange activity that regulates human epidermal melanogenesis. J Biol Chem 283:5486–95. [DOI] [PubMed] [Google Scholar]
- Giordano F, Bonetti C, Surace EM, Marigo V, Raposo G.. 2009. The Ocular Albinism type 1 (OA1) G-protein coupled receptor functions with MART-1 at early stages of melanogenesis to control melanosome identity and composition. Hum Mol Genet 18:4530–45. [DOI] [PubMed] [Google Scholar]
- Giordano F, Simoes S, Raposo G.. 2011. The ocular albinism type 1 (OA1) GPCR is ubiquitinated and its traffic requires endosomal sorting complex responsible for transport (ESCRT) function. Proc Natl Acad Sci U S A 108:11906–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokhale A, Larimore J, Werner E, So L, Moreno-De-Luca A, Lese-Martin C, Lupashin VV, Smith Y, Faundez V.. 2012. Quantitative proteomic and genetic analyses of the schizophrenia susceptibility factor dysbindin identify novel roles of the biogenesis of lysosome-related organelles complex 1. J Neurosci 32:3697–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham M, Tzika AC, Mitchell SM, Liu X, Leonhardt RM.. 2019. Repeat domain-associated O-glycans govern PMEL fibrillar sheet architecture. Sci Rep 9:6101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves AR, Curran PK, Smith CL, Mindell JA.. 2008. The Cl-/H+ antiporter ClC-7 is the primary chloride permeation pathway in lysosomes. Nature 453:788–92. [DOI] [PubMed] [Google Scholar]
- Grigoriev I, Splinter D, Keijzer N, Wulf PS, Demmers J, Ohtsuka T, Modesti M, Maly IV, Grosveld F, Hoogenraad CC, et al. 2007. Rab6 regulates transport and targeting of exocytotic carriers. Dev Cell 13:305–14. [DOI] [PubMed] [Google Scholar]
- Grigoriev I, Yu KL, Martinez-Sanchez E, Serra-Marques A, Smal I, Meijering E, Demmers J, Peranen J, Pasterkamp RJ, van der Sluijs P, et al. 2011. Rab6, Rab8, and MICAL3 cooperate in controlling docking and fusion of exocytotic carriers. Curr Biol 21:967–74. [DOI] [PubMed] [Google Scholar]
- Gronskov K, Ek J, Brondum-Nielsen K.. 2007. Oculocutaneous albinism. Orphanet J Rare Dis 2:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groux-Degroote S, van Dijk SM, Wolthoorn J, Neumann S, Theos AC, De Mazière AM, Klumperman J, van Meer G, Sprong H.. 2008. Glycolipid-dependent sorting of melanosomal from lysosomal membrane proteins by lumenal determinants. Traffic 9:951–63. [DOI] [PubMed] [Google Scholar]
- Gruenberg J. 2020. Life in the lumen: the multivesicular endosome. Traffic 21:76–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyonneau L, Murisier F, Rossier A, Moulin A, Beermann F.. 2004. Melanocytes and pigmentation are affected in dopachrome tautomerase knockout mice. Mol Cell Biol 24:3396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halaban R, Moellmann G.. 1990. Murine and human b locus pigmentation genes encode a glycoprotein (gp75) with catalase activity. Proc Natl Acad Sci U S A 87:4809–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton H. 1940. A study of the physiological properties of melanophores with special reference to their role in feather coloration. Anat Rec 78:525–48. [Google Scholar]
- Hanson P, Cashikar A.. 2012. Multivesicular body morphogenesis. Annu Rev Cell Dev Biol 28:337–62. [DOI] [PubMed] [Google Scholar]
- Hee JS, Mitchell SM, Liu X, Leonhardt RM.. 2017. Melanosomal formation of PMEL core amyloid is driven by aromatic residues. Sci Rep 7:44064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helip-Wooley A, Westbroek W, Dorward HM, Koshoffer A, Huizing M, Boissy RE, Gahl WA.. 2007. Improper trafficking of melanocyte-specific proteins in Hermansky-Pudlak syndrome type-5. J Invest Dermatol 127:1471–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellström AR, Watt B, Fard SS, Tenza D, Mannström P, Narfström K, Ekesten B, Ito S, Wakamatsu K, Larsson J, et al. 2011. Inactivation of the Pmel gene alters melanosome shape but has only a subtle effect on visible pigmentation. PLoS Genet 7:e1002285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho H, Kapadia R, Al-Tahan S, Ahmad S, Ganesan AK.. 2011. WIPI1 coordinates melanogenic gene transcription and melanosome formation via TORC1 inhibition. J Biol Chem 286:12509–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho T, Watt B, Spruce LA, Seeholzer SH, Marks MS.. 2016. The Kringle-like domain facilitates post-endoplasmic reticulum changes to PMEL oligomerization and disulfide bond configuration and promotes amyloid formation. J Biol Chem 291:3595–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoashi T, Muller J, Vieira WD, Rouzaud F, Kikuchi K, Tamaki K, Hearing VJ.. 2006. The repeat domain of the melanosomal matrix protein Pmel17/gp100 is required for the formation of organellar fibers. J Biol Chem 281:21198–2208. [DOI] [PubMed] [Google Scholar]
- Holloway ZG, Velayos-Baeza A, Howell GJ, Levecque C, Ponnambalam S, Sztul E, Monaco AP.. 2013. Trafficking of the Menkes copper transporter ATP7A is regulated by clathrin-, AP-2-, AP-1-, and Rab22-dependent steps. Mol Biol Cell 24:1735–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Kuo YM, Gitschier J.. 1999. The pallid gene encodes a novel, syntaxin 13-interacting protein involved in platelet storage pool deficiency. Nat Genet 23:329–32. [DOI] [PubMed] [Google Scholar]
- Huizing M, Pederson B, Hess RA, Griffin A, Helip-Wooley A, Westbroek W, Dorward H, O’Brien KJ, Golas G, Tsilou E, et al. 2009. Clinical and cellular characterisation of Hermansky-Pudlak syndrome type 6. J Med Genet 46:803–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizing M, Sarangarajan R, Strovel E, Zhao Y, Gahl WA, Boissy RE.. 2001. AP-3 mediates tyrosinase but not TRP-1 trafficking in human melanocytes. Mol Biol Cell 12:2075–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurbain I, Geerts WJC, Boudier T, Marco S, Verkleij A, Marks MS, Raposo G.. 2008. Electron tomography of early melanosomes: implications for melanogenesis and the generation of fibrillar amyloid sheets. Proc Natl Acad Sci U S A 105:19726–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innamorati G, Piccirillo R, Bagnato P, Palmisano I, Schiaffino MV.. 2006. The melanosomal/lysosomal protein OA1 has properties of a G protein-coupled receptor. Pigment Cell Res 19:125–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S, Wakamatsu K.. 2008. Chemistry of mixed melanogenesis–pivotal roles of dopaquinone. Photochem Photobiol 84:582–92. [DOI] [PubMed] [Google Scholar]
- Jahn R, Scheller RH.. 2006. SNAREs — engines for membrane fusion. Nat Rev Mol Cell Biol 7:631–43. [DOI] [PubMed] [Google Scholar]
- Jani RA, Purushothaman LK, Rani S, Bergam P, Setty SRG.. 2015. STX13 regulates cargo delivery from recycling endosomes during melanosome biogenesis. J Cell Sci 128:3263–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S, Liu XM, Dai X, Zhou Q, Lei TC, Beermann F, Wakamatsu K, Xu SZ.. 2010. Regulation of DHICA-mediated antioxidation by dopachrome tautomerase: implication for skin photoprotection against UVA radiation. Free Radic Biol Med 48:1144–51. [DOI] [PubMed] [Google Scholar]
- Jimbow K, Oikawa O, Sugiyama S, Takeuchi T.. 1979. Comparison of eumelanogenesis in retinal and follicular melanocytes; role of vesiculo-globular bodies in melanosome differentiation. J Invest Dermatol 73:278–84. [DOI] [PubMed] [Google Scholar]
- Jimenez-Cervantes C, Garcia-Borron JC, Valverde P, Solano F, Lozano JA.. 1993. Tyrosinase isoenzymes in mammalian melanocytes. 1. Biochemical characterization of two melanosomal tyrosinases from B16 mouse melanoma. Eur J Biochem 217:549–56. [DOI] [PubMed] [Google Scholar]
- Jimenez-Cervantes C, Solano F, Kobayashi T, Urabe K, Hearing VJ, Lozano JA, Garcia-Borron JC.. 1994. A new enzymatic function in the melanogenic pathway. The 5,6-dihydroxyindole-2-carboxylic acid oxidase activity of tyrosinase-related protein-1 (TRP1). J Biol Chem 269:17993–8000. [PubMed] [Google Scholar]
- Johnson R, Jackson IJ.. 1992. Light is a dominant mouse mutation resulting in premature cell death. Nat Genet 1:226–9. [DOI] [PubMed] [Google Scholar]
- Kalaidzidis I, Miaczynska M, Brewińska-Olchowik M, Hupalowska A, Ferguson C, Parton RG, Kalaidzidis Y, Zerial M.. 2015. APPL endosomes are not obligatory endocytic intermediates but act as stable cargo-sorting compartments. J Cell Biol 211:123–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalie E, Razi M, Tooze SA.. 2013. ULK1 regulates melanin levels in MNT-1 cells independently of mTORC1. PLoS One 8:e75313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedlaya R, Kandala G, Liu TF, Maddodi N, Devi S, Setaluri V.. 2011. Interactions between GIPC-APPL and GIPC-TRP1 regulate melanosomal protein trafficking and melanogenesis in human melanocytes. Arch Biochem Biophys 508:227–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerje S, Sharma P, Gunnarsson U, Kim H, Bagchi S, Fredriksson R, Schütz K, Jensen P, von Heijne G, Okimoto R, et al. 2004. The Dominant white, Dun and Smoky color variants in chicken are associated with insertion deletion polymorphisms in the PMEL17 gene. Genetics 168:1507–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss K, Kucsma N, Brozik A, Tusnady GE, Bergam P, van Niel G, Szakacs G.. 2015. Role of the N-terminal transmembrane domain in the endo-lysosomal targeting and function of the human ABCB6 protein. Biochem J 467:127–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Imokawa G, Bennett DC, Hearing VJ.. 1998. Tyrosinase stabilization by Tyrp1 (the brown locus protein). J Biol Chem 273:31801–5. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Urabe K, Winder A, Jiménez-Cervantes C, Imokawa G, Brewington T, Solano F, García-Borrón JC, Hearing VJ.. 1994. Tyrosinase related protein 1 (TRP1) functions as a DHICA oxidase in melanin biosynthesis. EMBO J 13:5818–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy PC, Du G, Fukuda Y, Sun D, Sampath J, Mercer KE, Wang J, Sosa-Pineda B, Murti KG, Schuetz JD.. 2006. Identification of a mammalian mitochondrial porphyrin transporter. Nature 443:586–9. [DOI] [PubMed] [Google Scholar]
- Kwon BS, Halaban R, Kim GS, Usack L, Pomerantz S, Haq AK.. 1987. A melanocyte-specific complementary DNA clone whose expression is inducible by melanotropin and isobutylmethyl xanthine. Mol Biol Med 4:339–55. [PubMed] [Google Scholar]
- La Fontaine S, Mercer JF.. 2007. Trafficking of the copper-ATPases, ATP7A and ATP7B: role in copper homeostasis. Arch Biochem Biophys 463:149–67. [DOI] [PubMed] [Google Scholar]
- Lahola-Chomiak AA, Footz T, Nguyen-Phuoc KNeil GJ, Fan B, Allen KF, Greenfield DS, Parrish RK, Linkroum K, Pasquale LR , et al. 2019. Non-synonymous variants in premelanosome protein (PMEL) cause ocular pigment dispersion and pigmentary glaucoma. Hum Mol Genet 28:1298–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai X, Wichers HJ, Soler-Lopez M, Dijkstra BW.. 2017. Structure of human tyrosinase related protein 1 reveals a binuclear zinc active site important for melanogenesis. Angew Chem Int Ed Engl 56:9812–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamason RL, Mohideen MAPK, Mest JR, Wong AC, Norton HL, Aros MC, Jurynec MJ, Mao X, Humphreville VR, Humbert JE, et al. 2005. SLC24A5, a putative cation exchanger, affects pigmentation in zebrafish and humans. Science 310:1782–6. [DOI] [PubMed] [Google Scholar]
- Lange PF, Wartosch L, Jentsch TJ, Fuhrmann JC.. 2006. ClC-7 requires Ostm1 as a -subunit to support bone resorption and lysosomal function. Nature 440:220–3. [DOI] [PubMed] [Google Scholar]
- Le L, Escobar IE, Ho T, Lefkovith AJ, Latteri E, Haltaufderhyde KD, Dennis MK, Plowright L, Sviderskaya EV, DC B, et al. 2020. SLC45A2 protein stability and regulation of melanosome pH determine melanocyte pigmentation. Mol Biol Cell 31:2687–702.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HH, Nemecek D, Schindler C, Smith WJ, Ghirlando R, Steven AC, Bonifacino JS, Hurley JH.. 2012. Assembly and architecture of biogenesis of lysosome-related organelles complex-1 (BLOC-1). J Biol Chem 287:5882–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonhardt RM, Vigneron N, Hee JS, Graham M, Cresswell P.. 2013. Critical residues in the PMEL/Pmel17 N-terminus direct the hierarchical assembly of melanosomal fibrils. Mol Biol Cell 24:964–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonhardt RM, Vigneron N, Rahner C, Cresswell P.. 2011. Proprotein convertases process Pmel17 during secretion. J Biol Chem 286:9321–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson B, Vulpe C, Elder B, Martin C, Verley F, Packman S, Gitschier J.. 1994. The mottled gene is the mouse homologue of the Menkes disease gene. Nat Genet 6:369–73. [DOI] [PubMed] [Google Scholar]
- Li K, Yang L, Zhang C, Niu Y, Li W, Liu JJ.. 2014. HPS6 interacts with dynactin p150Glued to mediate retrograde trafficking and maturation of lysosomes. J Cell Sci 127:4574–88. [DOI] [PubMed] [Google Scholar]
- Lona-Durazo F, Hernandez-Pacheco N, Fan S, Zhang T, Choi J, Kovacs MA, Loftus SK, Le P, Edwards M, Fortes-Lima CA, et al. 2019. Meta-analysis of GWA studies provides new insights on the genetic architecture of skin pigmentation in recently admixed populations. BMC Genet 20:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez VM, Decatur CL, Stamer WD, Lynch RM, McKay BS.. 2008. L-DOPA is an endogenous ligand for OA1. PLoS Biol 6:e236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loubéry S, Delevoye C, Louvard D, Raposo G, Coudrier E.. 2012. Myosin VI regulates actin dynamics and melanosome biogenesis. Traffic 13:665–80. [DOI] [PubMed] [Google Scholar]
- Luo D, Chen H, Jimbow K.. 1994. Cotransfection of genes encoding human tyrosinase and tyrosinase-related protein-1 prevents melanocyte death and enhances melanin pigmentation and gene expression of Lamp-1. Exp Cell Res 213:231–41. [DOI] [PubMed] [Google Scholar]
- Lutsenko S, Barnes NL, Bartee MY, Dmitriev OY.. 2007. Function and regulation of human copper-transporting ATPases. Physiol Rev 87:1011–46. [DOI] [PubMed] [Google Scholar]
- Manga P, Boissy RE, Pifko-Hirst S, Zhou BK, Orlow SJ.. 2001. Mislocalization of melanosomal proteins in melanocytes from mice with oculocutaneous albinism type 2. Exp Eye Res 72:695–710. [DOI] [PubMed] [Google Scholar]
- Manga P, Orlow SJ.. 1999. The pink-eyed dilution gene and the molecular pathogenesis of tyrosinase-positive albinism (OCA2). J Dermatol 26:738–47. [DOI] [PubMed] [Google Scholar]
- Martin AR, Lin M, Granka JM, Myrick JW, Liu X, Sockell A, Atkinson EG, Werely CJ, Moller M, Sandhu MS, et al. 2017. An unexpectedly complex architecture for skin pigmentation in Africans. Cell 171:1340–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martina JA, Moriyama K, Bonifacino JS.. 2003. BLOC-3, a protein complex containing the Hermansky-Pudlak syndrome gene products HPS1 and HPS4. J Biol Chem 278:29376–84. [DOI] [PubMed] [Google Scholar]
- Mason HS. 1948. The chemistry of melanin; mechanism of the oxidation of dihydroxyphenylalanine by tyrosinase. J Biol Chem 172:83–99. [PubMed] [Google Scholar]
- McCall KA, Huang C, Fierke CA.. 2000. Function and mechanism of zinc metalloenzymes. J Nutr 130:1437S–1446S. [DOI] [PubMed] [Google Scholar]
- Mercer JF, Grimes A, Ambrosini L, Lockhart P, Paynter JA, Dierick H, Glover TW.. 1994. Mutations in the murine homologue of the Menkes gene in dappled and blotchy mice. Nat Genet 6:374–8. [DOI] [PubMed] [Google Scholar]
- Mercer JF, Livingston J, Hall B, Paynter JA, Begy C, Chandrasekharappa S, Lockhart P, Grimes A, Bhave M, Siemieniak D.. 1993. Isolation of a partial candidate gene for Menkes disease by positional cloning. Nat Genet 3:20–5. [DOI] [PubMed] [Google Scholar]
- Miaczynska M, Christoforidis S, Giner A, Shevchenko A, Uttenweiler-Joseph S, Habermann B, Wilm M, Parton RG, Zerial M.. 2004. APPL proteins link Rab5 to nuclear signal transduction via an endosomal compartment. Cell 116:445–56. [DOI] [PubMed] [Google Scholar]
- Monis WJ, Faundez V, Pazour GJ.. 2017. BLOC-1 is required for selective membrane protein trafficking from endosomes to primary cilia. J Cell Biol 216:2131–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoliu L, Gronskov K, Wei AH, Martinez-Garcia M, Fernandez A, Arveiler B, Morice-Picard F, Riazuddin S, Suzuki T, Ahmed ZM, et al. 2014. Increasing the complexity: new genes and new types of albinism. Pigment Cell Melanoma Res 27:11–8. [DOI] [PubMed] [Google Scholar]
- Morice-Picard F, Lasseaux E, Francois S, Simon D, Rooryck C, Bieth E, Colin E, Bonneau D, Journel H, Walraedt S, et al. 2014. SLC24A5 mutations are associated with non-syndromic oculocutaneous albinism. J Invest Dermatol 134:568–71. [DOI] [PubMed] [Google Scholar]
- Moriyama K, Bonifacino JS.. 2002. Pallidin is a component of a multi-protein complex involved in the biogenesis of lysosome-related organelles. Traffic 3:666–77. [DOI] [PubMed] [Google Scholar]
- Moyer FH. 1966. Genetic variations in the fine structure and ontogeny of mouse melanin granules. Am Zool 6:43–66. [DOI] [PubMed] [Google Scholar]
- Nag S, Rani S, Mahanty S, Bissig C, Arora P, Azevedo C, Saiardi A, van der Sluijs P, Delevoye C, van Niel G, et al. 2018. Rab4A organizes endosomal domains for sorting cargo to lysosome-related organelles. J. Cell Sci 131:jcs216226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Setou M, Seog D, Ogasawara K, Dohmae N, Takio K, Hirokawa N.. 2000. A novel motor, KIF13A, transports mannose-6-phosphate receptor to plasma membrane through direct interaction with AP-1 complex. Cell 103:569–40. [DOI] [PubMed] [Google Scholar]
- Nazarian R, Falcón-Pérez JM, Dell’Angelica EC.. 2003. Biogenesis of lysosome-related organelles complex 3 (BLOC-3): a complex containing the Hermansky-Pudlak syndrome (HPS) proteins HPS1 and HPS4. Proc Natl Acad Sci U S A 100:8770–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton JM, Cohen-Barak O, Hagiwara N, Gardner JM, Davisson MT, King RA, Brilliant MH.. 2001. Mutations in the human orthologue of the mouse underwhite gene (uw) underlie a new form of oculocutaneous albinism, OCA4. Am J Hum Genet 69:981–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novikoff AB, Albala A, Biempica L.. 1968. Ultrastructural and cytochemical observations on B-16 and Harding-Passey mouse melanomas. The origin of premelanosomes and compound melanosomes. J Histochem Cytochem 16:299–319. [DOI] [PubMed] [Google Scholar]
- Oancea E, Vriens J, Brauchi S, Jun J, Splawski I, Clapham DE.. 2009. TRPM1 forms ion channels associated with melanin content in melanocytes. Sci Signal 2:ra21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oetting WS, King RA.. 1994a. Analysis of tyrosinase mutations associated with tyrosinase-related oculocutaneous albinism (OCA1). Pigment Cell Res 7:285–90. [DOI] [PubMed] [Google Scholar]
- Oetting WS, King RA.. 1994b. Molecular basis of oculocutaneous albinism. J Invest Dermatol 103:131S–6S. [DOI] [PubMed] [Google Scholar]
- Olivares C, Solano F.. 2009. New insights into the active site structure and catalytic mechanism of tyrosinase and its related proteins. Pigment Cell Melanoma Res 22:750–60. [DOI] [PubMed] [Google Scholar]
- Orlow SJ, Zhou B-K, Chakraborty AK, Drucker M, Pifko-Hirst S, Pawelek JM.. 1994. High-molecular-weight forms of tyrosinase and the tyrosinase-related proteins: evidence for a melanogenic complex. J Invest Dermatol 103:196–201. [DOI] [PubMed] [Google Scholar]
- Palmisano I, Bagnato P, Palmigiano A, Innamorati G, Rotondo G, Altimare D, Venturi C, Sviderskaya EV, Piccirillo R, Coppola M, et al. 2008. The ocular albinism type 1 protein, an intracellular G protein-coupled receptor, regulates melanosome transport in pigment cells. Hum Mol Genet 17:3487–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson JK, Shukla S, Black CM, Tachiwada T, Garfield S, Wincovitch S, Ernst DN, Agadir A, Li X, Ambudkar SV, et al. 2007. Human ABCB6 localizes to both the outer mitochondrial membrane and the plasma membrane. Biochemistry 46:9443–52. [DOI] [PubMed] [Google Scholar]
- Patwardhan A, Bardin S, Miserey-Lenkei S, Larue L, Goud B, Raposo G, Delevoye C.. 2017. Routing of the RAB6 secretory pathway towards the lysosome related organelle of melanocytes. Nat Commun 8:15835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peden AA, Oorschot V, Hesser BA, Austin CD, Scheller RH, Klumperman J.. 2004. Localization of the AP-3 adaptor complex defines a novel endosomal exit site for lysosomal membrane proteins. J Cell Biol 164:1065–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennamen P, Le L, Tingaud-Sequeira A, Fiore M, Bauters A, Van Duong Beatrice N, Coste V, Bordet JC, Plaisant C, Diallo M, et al. 2020a. BLOC1S5 pathogenic variants cause a new type of Hermansky-Pudlak syndrome. Genet Med 22:1613–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennamen P, Tingaud-Sequeira A, Gazova I, Keighren M, McKie L, Marlin S, Gherbi HS, Kaplan J, Delevoye C, Lacombe D, et al. 2020b. Dopachrome tautomerase variants in patients with oculocutaneous albinism. Genet Med 23:479– 87. [DOI] [PubMed] [Google Scholar]
- Petris MJ, Strausak D, Mercer JF.. 2000. The Menkes copper transporter is required for the activation of tyrosinase. Hum Mol Genet 9:2845–51. [DOI] [PubMed] [Google Scholar]
- Que SK, Weston G, Suchecki J, Ricketts J.. 2015. Pigmentary disorders of the eyes and skin. Clin Dermatol 33:147–58. [DOI] [PubMed] [Google Scholar]
- Rad HH, Yamashita T, Jin HY, Hirosaki K, Wakamatsu K, Ito S, Jimbow K.. 2004. Tyrosinase-related proteins suppress tyrosinase-mediated cell death of melanocytes and melanoma cells. Exp Cell Res 298:317–28. [DOI] [PubMed] [Google Scholar]
- Ramsden CA, Riley PA.. 2014. Tyrosinase: the four oxidation states of the active site and their relevance to enzymatic activation, oxidation and inactivation. Bioorg Med Chem 22:2388–95. [DOI] [PubMed] [Google Scholar]
- Raper HS. 1926. The Tyrosinase-Tyrosine reaction: production of l-3.4-Dihydroxyphenylalanine from Tyrosine. Biochem J 20:735–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raper HS. 1927. The Tyrosinase-tyrosine reaction: production from Tyrosine of 5: 6-Dihydroxyindole and 5: 6-dihydroxyindole-2-carboxylic acid-the precursors of melanin. Biochem J 21:89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raper HS, Speakman HB.. 1926. The Tyrosinase-Tyrosine reaction: note on the identity of Tyrosinase from different sources. Biochem J 20:69–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raper HS, Wormall A.. 1923. The Tyrosinase-Tyrosine reaction. Biochem J 17:454–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo G, Tenza D, Murphy DM, Berson JF, Marks MS.. 2001. Distinct protein sorting and localization to premelanosomes, melanosomes, and lysosomes in pigmented melanocytic cells. J. Cell Biol 152:809–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reggiori F, Pelham HR.. 2001. Sorting of proteins into multivesicular bodies: ubiquitin-dependent and -independent targeting. EMBO J 20:5176–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripoll L, Heiligenstein X, Hurbain I, Domingues L, Figon F, Petersen KJ, Dennis MK, Houdusse A, Marks MS, Raposo G, et al. 2018. Myosin VI and branched actin filaments mediate membrane constriction and fission of melanosomal tubule carriers. J Cell Biol 217:2709–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizo J, Südhof TC.. 2012. The membrane fusion enigma: SNAREs, Sec1/Munc18 proteins, and their accomplices–guilty as charged? Annu Rev Cell Dev Biol 28:279–308. [DOI] [PubMed] [Google Scholar]
- Robila V, Ostankovitch M, Altrich-Vanlith ML, Theos AC, Drover S, Marks MS, Restifo N, Engelhard VH.. 2008. MHC class II presentation of gp100 epitopes in melanoma cells requires the function of conventional endosomes and is influenced by melanosomes. J. Immunol 181:7843–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochin L, Hurbain I, Serneels L, Fort C, Watt B, Leblanc P, Marks MS, De Strooper B, Raposo G, van Niel G.. 2013. BACE2 processes PMEL to form the melanosome amyloid matrix in pigment cells. Proc Natl Acad Sci USA 110:10658–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogasevskaia TP, Szerencsei RT, Jalloul AH, Visser F, Winkfein RJ, Schnetkamp PPM.. 2019. Cellular localization of the K(+) -dependent Na(+) -Ca(2+) exchanger NCKX5 and the role of the cytoplasmic loop in its distribution in pigmented cells. Pigment Cell Melanoma Res 32:55–67. [DOI] [PubMed] [Google Scholar]
- Rutherford AC, Traer C, Wassmer T, Pattni K, Bujny MV, Carlton JG, Stenmark H, Cullen PJ.. 2006. The mammalian phosphatidylinositol 3-phosphate 5-kinase (PIKfyve) regulates endosome-to-TGN retrograde transport. J Cell Sci 119:3944–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder PV, Vistein R, Gokhale A, Seaman MN, Puthenveedu M, Faundez V.. 2013. The WASH complex, and endosomal Arp2/3 activator, interacts with the Hermansky-Pudlak syndrome complex BLOC-1 and its cargo phosphatidylinositol-4 kinase type II alpha. Mol Biol Cell 24:2269–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeki H, Oikawa A.. 1985. Stimulation by ionophores of tyrosinase activity of mouse melanoma cells in culture. J Invest Dermatol 85:423–5. [DOI] [PubMed] [Google Scholar]
- Salazar G, Zlatic S, Craige B, Peden AA, Pohl J, Faundez V.. 2009. Hermansky-pudlak syndrome protein complexes associate with phosphatidylinositol-4-kinase type II alpha in neuronal and non-neuronal cells. J Biol Chem 284:1790–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger A, Hirst J, Davies AK, Robinson MS.. 2019. Adaptor protein complexes and disease at a glance. J Cell Sci 132:jcs222992. [DOI] [PubMed] [Google Scholar]
- Schäfer IB, Hesketh GG, Bright NA, Gray SR, Pryor PR, Evans PR, Luzio JP, Owen DJ.. 2012. The binding of Varp to VAMP7 traps VAMP7 in a closed, fusogenically inactive conformation. Nat Struct Mol Biol 19:1300–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiaffino MV, Baschirotto C, Pellegrini G, Montalti S, Tacchetti C, De Luca M, Ballabio A.. 1996. The ocular albinism type 1 gene product is a membrane glycoprotein localized to melanosomes. Proc Natl Acad Sci U S A 93:9055–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiaffino MV, d’Addio M, Alloni A, Baschirotto C, Valetti C, Cortese K, Puri C, Bassi MT, Colla C, De Luca M, et al. 1999. Ocular albinism: evidence for a defect in an intracellular signal transduction system. Nat Genet 23:108–12. [DOI] [PubMed] [Google Scholar]
- Sciacca MFM, Tempra C, Scollo F, Milardi D, La Rosa C.. 2018. Amyloid growth and membrane damage: current themes and emerging perspectives from theory and experiments on Abeta and hIAPP. Biochim Biophys Acta Biomembr 1860:1625–38. [DOI] [PubMed] [Google Scholar]
- Seiji M, Fitzpatrick TB, Birbeck MSC.. 1961. The melanosome: a distinctive subcellular particle of mammalian melanocytes and the site of melanogenesis. J Invest Dermatol 36:243–52. [DOI] [PubMed] [Google Scholar]
- Setty SRG, Tenza D, Sviderskaya EV, Bennett DC, Raposo G, Marks MS.. 2008. Cell-specific ATP7A transport sustains copper-dependent tyrosinase activity in melanosomes. Nature 454:1142–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setty SRG, Tenza D, Truschel ST, Chou E, Sviderskaya EV, Theos AC, Lamoreux ML, Di Pietro SM, Starcevic M, Bennett DC, et al. 2007. BLOC-1 is required for cargo-specific sorting from vacuolar early endosomes toward lysosome-related organelles. Mol Biol Cell 18:768–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seward SL Jr, Gahl WA.. 2013. Hermansky-Pudlak syndrome: health care throughout life. Pediatrics 132:153–60. [DOI] [PubMed] [Google Scholar]
- Shakya S, Sharma P, Bhatt AM, Jani RA, Delevoye C, Setty SR.. 2018. Rab22A recruits BLOC-1 and BLOC-2 to promote the biogenesis of recycling endosomes. EMBO Rep 19:e45918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimokawa N, Okada J, Haglund K, Dikic I, Koibuchi N, Miura M.. 2002. Past-A, a novel proton-associated sugar transporter, regulates glucose homeostasis in the brain. J Neurosci 22:9160–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin D, Howng SY, Ptacek LJ, Fu YH.. 2012. miR-32 and its target SLC45A3 regulate the lipid metabolism of oligodendrocytes and myelin. Neuroscience 213:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitaram A, Dennis MK, Chaudhuri R, De Jesus-Rojas W, Tenza D, Setty SRG, Wood CS, Sviderskaya EV, Bennett DC, Raposo G, et al. 2012. Differential recognition of a dileucine-based sorting signal by AP-1 and AP-3 reveals a requirement for both BLOC-1 and AP-3 in delivery of OCA2 to melanosomes. Mol Biol Cell 23:3178–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitaram A, Piccirillo R, Palmisano I, Harper DC, Dell’Angelica EC, Schiaffino MV, Marks MS.. 2009. Localization to mature melanosomes by virtue of cytoplasmic dileucine motifs is required for human OCA2 function. Mol Biol Cell 20:1464–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soejima M, Koda Y.. 2007. Population differences of two coding SNPs in pigmentation-related genes SLC24A5 and SLC45A2. Int J Legal Med 121:36–9. [DOI] [PubMed] [Google Scholar]
- Solano F. 2018. On the metal cofactor in the Tyrosinase family. Int J Mol Sci 19:633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solano F, Jimenez-Cervantes C, Martinez-Liarte JH, Garcia-Borron JC, Jara JR, Lozano JA.. 1996. Molecular mechanism for catalysis by a new zinc-enzyme, dopachrome tautomerase. Biochem J 313: 447–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprong H, Degroote S, Claessens T, van Drunen J, Oorschot V, Westerink BHC, Hirabayashi Y, Klumperman J, van der Sluijs P, van Meer G.. 2001. Glycosphingolipids are required for sorting melanosomal proteins in the Golgi complex. J Cell Biol 155:369–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulem P, Gudbjartsson DF, Stacey SN, Helgason A, Rafnar T, Jakobsdottir M, Steinberg S, Gudjonsson SA, Palsson A, Thorleifsson G, et al. 2008. Two newly identified genetic determinants of pigmentation in Europeans. Nat Genet 40:835–7. [DOI] [PubMed] [Google Scholar]
- Sun Y, Reinders A, LaFleur KR, Mori T, Ward JM.. 2010. Transport activity of rice sucrose transporters OsSUT1 and OsSUT5. Plant Cell Physiol 51:114–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Oiso N, Gautam R, Novak EK, Panthier JJ, Suprabha PG, Vida T, Swank RT, Spritz RA.. 2003. The mouse organellar biogenesis mutant buff results from a mutation in Vps33a, a homologue of yeast vps33 and Drosophila carnation. Proc Natl Acad Sci U S A 100:1146–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szerencsei RT, Ginger RS, Green MR, Schnetkamp PP.. 2016. Identification and characterization of K(+)-dependent Na(+)-Ca(2+) exchange transport in pigmented MEB4 cells mediated by NCKX4. Biochemistry 55:2704–12. [DOI] [PubMed] [Google Scholar]
- Tabata H, Kawamura N, Sun-Wada GH, Wada Y.. 2008. Vacuolar-type H(+)-ATPase with the a3 isoform is the proton pump on premature melanosomes. Cell Tissue Res 332:447–60. [DOI] [PubMed] [Google Scholar]
- Tamura K, Ohbayashi N, Ishibashi K, Fukuda M.. 2011. Structure-function analysis of VPS9-ankyrin-repeat protein (Varp) in the trafficking of tyrosinase-related protein 1 in melanocytes. J Biol Chem 286:7507–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarafder AK, Bolasco G, Correia MS, Pereira FJ, Iannone L, Hume AN, Kirkpatrick N, Picardo M, Torrisi MR, Rodrigues IP, et al. 2014. Rab11b mediates melanin transfer between donor melanocytes and acceptor keratinocytes via coupled exo/endocytosis. J Invest Dermatol 134:1056–66. [DOI] [PubMed] [Google Scholar]
- Theos AC, Berson JF, Theos SC, Herman KE, Harper DC, Tenza D, Sviderskaya EV, Lamoreux ML, Bennett DC, Raposo G, et al. 2006a. Dual loss of ER export and endocytic signals with altered melanosome morphology in the silver mutation of Pmel17. Mol Biol Cell 17:3598–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theos AC, Tenza D, Martina JA, Hurbain I, Peden AA, Sviderskaya EV, Stewart A, Robinson MS, Bennett DC, Cutler DF, et al. 2005. Functions of AP-3 and AP-1 in tyrosinase sorting from endosomes to melanosomes. Mol Biol Cell 16:5356–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theos AC, Truschel ST, Tenza D, Hurbain I, Harper DC, Berson JF, Thomas PC, Raposo G, Marks MS.. 2006b. A lumenal domain-dependent pathway for sorting to intralumenal vesicles of multivesicular endosomes involved in organelle morphogenesis. Dev Cell 10:343–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita Y, Takeda A, Matsunaga J, Okinaga S, Shibahara S, Tagami H.. 1992. Molecular bases of tyrosinase-negative oculocutaneous albinism: a single base insertion or a missense point mutation in the tyrosinase gene. Pigment Cell Res 2:96–100. [DOI] [PubMed] [Google Scholar]
- Townsend D, Guillery P, King RA.. 1984. Optimized assay for mammalian tyrosinase (polyhydroxyl phenyloxidase). Anal Biochem 139:345–52. [DOI] [PubMed] [Google Scholar]
- Truschel ST, SImoes S, Setty SRG, Harper DC, Tenza D, Thomas PC, Herman KE, Sackett SD, Cowan DC, Theos AC, et al. 2009. ESCRT-1 function is required for Tyrp1 transport from early endosomes to the melanosome limiting membrane. Traffic 10:1318–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto K, Jackson IJ, Urabe K, Montague PM, Hearing VJ.. 1992. A second tyrosinase-related protein, TRP-2, is a melanogenic enzyme termed DOPAchrome tautomerase. EMBO J 11:519–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner WA Jr., Taylor JD, Tchen TT.. 1975. Melanosome formation in the goldfish: the role of multivesicular bodies. J Ultrastruct Res 51:16–31. [DOI] [PubMed] [Google Scholar]
- Valencia JC, Watabe H, Chi A, Rouzaud F, Chen KG, Vieira WD, Takahashi K, Yamaguchi Y, Berens W, Nagashima K, et al. 2006. Sorting of Pmel17 to melanosomes through the plasma membrane by AP1 and AP2: evidence for the polarized nature of melanocytes. J Cell Sci 119:1080–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Niel G, Bergam P, Di Cicco A, Hurbain I, Lo Cicero A, Dingli F, Palmulli R, Fort C, Potier MC, Schurgers LJ, et al. 2015. Apolipoprotein E regulates amyloid formation within endosomes of pigment cells. Cell Rep 13:43–51. [DOI] [PubMed] [Google Scholar]
- van Niel G, Charrin S, Simoes S, Romao M, Rochin L, Saftig P, Marks MS, Rubinstein E, Raposo G.. 2011. The Tetraspanin CD63 integrates ESCRT-independent and dependent sorting at a common endosome. Dev Cell 21:708–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Weering JR, Cullen PJ.. 2014. Membrane-associated cargo recycling by tubule-based endosomal sorting. Semin Cell Dev Biol 31:40–7. [DOI] [PubMed] [Google Scholar]
- Vitavska O, Edemir B, Wieczorek H.. 2016. Putative role of the H(+)/sucrose symporter SLC45A3 as an osmolyte transporter in the kidney. Pflugers Arch 468:1353–62. [DOI] [PubMed] [Google Scholar]
- Vitavska O, Wieczorek H.. 2017. Putative role of an SLC45 H(+)/sugar cotransporter in mammalian spermatozoa. Pflugers Arch 469:1433–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel P, Read RW, Vance RB, Platt KA, Troughton K, Rice DS.. 2008. Ocular albinism and hypopigmentation defects in Slc24a5-/- mice. Vet Pathol 45:264–79. [DOI] [PubMed] [Google Scholar]
- Vulpe C, Levinson B, Whitney S, Packman S, Gitschier J.. 1993. Isolation of a candidate gene for Menkes disease and evidence that it encodes a copper-transporting ATPase. Nat Genet 3:7–13. [DOI] [PubMed] [Google Scholar]
- Wade N, Bryant NJ, Connolly LM, Simpson RJ, Luzio JP, Piper RC, James DE.. 2001. Syntaxin 7 complexes with mouse Vps10p tail interactor 1b, Syntaxin 6, vesicle-associated membrane protein (VAMP)8, and VAMP7 in B16 melanoma cells. J Biol Chem 276:19820–7. [DOI] [PubMed] [Google Scholar]
- Wagner CA, Finberg KE, Breton S, Marshansky V, Brown D, Geibel JP.. 2004. Renal vacuolar H+-ATPase. Physiol Rev 84:1263–314. [DOI] [PubMed] [Google Scholar]
- Wang F, Zhang H, Zhang X, Wang Y, Ren F, Zhang X, Zhai Y, Chang Z.. 2008. Varp interacts with Rab38 and functions as its potential effector. Biochem Biophys Res Commun 372:162–7. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhang X, Dong XP, Samie M, Li X, Cheng X, Goschka A, Shen D, Zhou Y, Harlow J, et al. 2012. TPC proteins are phosphoinositide- activated sodium-selective ion channels in endosomes and lysosomes. Cell 151:372–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasmeier C, Romao M, Plowright L, Bennett DC, Raposo G, Seabra MC.. 2006. Rab38 an Rab32 control early post-Golgi trafficking of melanogenic enzymes. J Cell Biol 175:271–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt B, Tenza D, Lemmon MA, Kerje S, Raposo G, Andersson L, Marks MS.. 2011. Mutations in or near the transmembrane domain alter PMEL amyloid formation from functional to pathogenic. PLoS Genet 7:e1002286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt B, van Niel G, Raposo G, Marks MS.. 2013. PMEL: a pigment cell-specific model for functional amyloid formation. Pigment Cell Melanoma Res 26:300–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei AH, Zang DJ, Zhang Z, Liu XZ, He X, Yang L, Wang Y, Zhou ZY, Zhang MR, Dai LL, et al. 2013. Exome sequencing identifies SLC24A5 as a candidate gene for nonsyndromic oculocutaneous albinism. J Invest Dermatol 133:1834–40. [DOI] [PubMed] [Google Scholar]
- Williams RJP. 1987. The biochemistry of zinc. Polyhedron 6:61–9. [Google Scholar]
- Wilson S, Ginger RS, Dadd T, Gunn D, Lim FL, Sawicka M, Sandel M, Schnetkamp PP, Green MR.. 2013. NCKX5, a natural regulator of human skin colour variation, regulates the expression of key pigment genes MC1R and alpha-MSH and alters cholesterol homeostasis in normal human melanocytes. Adv Exp Med Biol 961:95–107. [DOI] [PubMed] [Google Scholar]
- Xu XZ, Moebius F, Gill DL, Montell C.. 2001. Regulation of melastatin, a TRP-related protein, through interaction with a cytoplasmic isoform. Proc Natl Acad Sci U S A 98:10692–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y, Heiny ME, Suzuki M, Gitlin JD.. 1996. Biochemical characterization and intracellular localization of the Menkes disease protein. Proc Natl Acad Sci U S A 93:14030–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HL, Lin CP, Vudhya Gowrisankar Y, Huang PJ, Chang WL, Shrestha S, Hseu YC.. 2021. The anti-melanogenic effects of ellagic acid through induction of autophagy in melanocytes and suppression of UVA-activated alpha-MSH pathways via Nrf2 activation in keratinocytes. Biochem Pharmacol 185:114454. [DOI] [PubMed] [Google Scholar]
- Yardman-Frank JM, Fisher DE.. 2020. Skin pigmentation and its control: from ultraviolet radiation to stem cells. Exp. Dermatol. 30:560– 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatsu A, Ohbayashi N, Tamura K, Fukuda M.. 2013. Syntaxin-3 is required for melanosomal localization of Tyrp1 in melanocytes. J Invest Dermatol 133:2237–46. [DOI] [PubMed] [Google Scholar]
- Yun WJ, Kim EY, Park JE, Jo SY, Bang SH, Chang EJ, Chang SE.. 2016. Microtubule-associated protein light chain 3 is involved in melanogenesis via regulation of MITF expression in melanocytes. Sci Rep 6:19914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Li D, Zhang J, Chen X, Huang M, Archacki S, Tian Y, Ren W, Mei A, Zhang Q, et al. 2013. Mutations in ABCB6 cause dyschromatosis universalis hereditaria. J Invest Dermatol 133:2221–8. [DOI] [PubMed] [Google Scholar]
- Zhang CF, Gruber F, Ni C, Mildner M, Koenig U, Karner S, Barresi C, Rossiter H, Narzt MS, Nagelreiter IM, et al. 2015. Suppression of autophagy dysregulates the antioxidant response and causes premature senescence of melanocytes. J Invest Dermatol 135:1348–57. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Gong J, Sviderskaya EV, Wei A, Li W.. 2019. Mitochondrial NCKX5 regulates melanosomal biogenesis and pigment production. J Cell Sci 132:jcs.232009. [DOI] [PMC free article] [PubMed] [Google Scholar]