Abstract
Background:
Exposure to high air temperature in late pregnancy is increasingly recognized as a risk factor for preterm birth (PTB). However, the combined effects of heatwaves with air pollution and green space are still unexplored. In the context of climate change, investigating the interaction between environmental factors and identifying communities at higher risk is important to better understand the etiological mechanisms and design targeted interventions towards certain women during pregnancy.
Objectives:
To examine the combined effects of heatwaves, air pollution and green space exposure on the risk of PTB.
Methods:
California birth certificate records for singleton births (2005–2013) were obtained. Residential zip code-specific daily temperature during the last week of gestation was used to create 12 definitions of heatwave with varying temperature thresholds and durations. We fit multi-level Cox proportional hazard models with time to PTB as the outcome and gestational week as the temporal unit. Relative risk due to interaction (RERI) was applied to estimate the additive interactive effect of air pollution and green space on the effect of heatwaves on PTB.
Results:
In total, 1,967,300 births were included in this study. For PM2.5, PM10 and O3, we found positive additive interactions (RERIs >0) between heatwaves and higher air pollution levels. Combined effects of heatwaves and green space indicated negative interactions (RERIs <0) for less intense heatwaves (i.e., shorter duration or relatively low temperature), whereas there were potential positive interactions (RERIs >0) for more intense heatwaves.
Conclusion:
This study found synergistic harmful effects for heatwaves with air pollution, and potential positive interactions with lack of green space on PTB. Implementing interventions, such as heat warning systems and behavioral changes, targeted toward pregnant women at risk for high air pollution and low green space exposures may optimize the benefits of reducing acute exposure to extreme heat before delivery.
Keywords: Preterm birth, heatwave, temperature, air pollution, green space, built environment, interaction
INTRODUCTION
Preterm birth (PTB), defined as birth before 37 weeks of gestation (March of Dimes et al., 2012), occurs in 11% of births globally, and the rate is increasing in most countries (Harrison & Goldenberg, 2016; Petrou, 2003). PTB is a leading cause of infant mortality and morbidity (Liu et al., 2016), and child mortality (Harrison & Goldenberg, 2016; Liu et al., 2016); it is also associated with an increased risk of multiple long-term adverse effects, including physical, cognitive, behavioral, and developmental problems (Chehade et al., 2018; McCormick et al., 2011; Saigal & Doyle, 2008).
In epidemiological studies, exposure to high ambient air temperature is increasingly recognized as a risk factor for PTB (Arroyo et al., 2016; Auger et al., 2014; Bekkar et al., 2020; Kent et al., 2014; Schifano et al., 2013; Sun et al., 2019; Vicedo-Cabrera et al., 2015; Wang et al., 2013; Wang et al., 2020; Zhang et al., 2017). We recently reported that acute exposure to extreme heat during the last week of gestation may trigger an earlier delivery with important variability according to distinct heat wave definitions (Ilango et al., 2020). The potential biological mechanism by which heat stress leads to PTB is that high temperature could cause dehydration and stimulate secretion of antidiuretic hormone and oxytocin. These may reduce uterine blood flow, which could affect fetal metabolic responses and trigger contractions, and result in PTB (Dreiling & Carman, 1991; Stan et al., 2013). It is expected that the frequency, intensity, and duration of heatwaves will increase due to climate change (Hoegh-Guldberg et al., 2018; Schär, 2015), which will add to the existing PTB burden. Understanding the effect of heatwaves on PTB and identifying optimal thresholds to activate interventions targeting pregnant women is particularly important in the context of climate change.
In addition to the direct effect of heat on PTB, climate change could indirectly threaten population health through several pathways (Watts et al., 2015). For example, heat stress can increase concentrations of tropospheric ozone (O3) and exacerbate the toxicity of ground-level airborne pollutants such as particulate matter (PM) and O3 (Ebi & McGregor, 2008; Gordon et al., 2014; Hou & Wu, 2016). Air pollution may increase oxidative stress and inflammatory responses, cause endocrine disruption, and change hemodynamic status in the body (Feng et al., 2016; Kannan et al., 2006; Moore et al., 2018; Vadillo-Ortega et al., 2014), which may be associated with PTB. Several studies have reported that ambient air pollution exposure during pregnancy increases the risk of PTB (Kloog et al., 2012; Lamichhane et al., 2015; Laurent et al., 2016; X. Li et al., 2017; Malley et al., 2017; Olsson et al., 2013; Pereira et al., 2014; Sheridan et al., 2019; Stieb et al., 2012; Wang et al., 2018; Wilhelm et al., 2011; Wu et al., 2009) particularly toward the end of gestation. Zanobetti and Peters called for further studies on the combined adverse health effects of air pollution and weather since their joint effects may be larger than estimated for single exposure (Zanobetti & Peters, 2015). Investigating their joint effects may contribute to better understanding of etiological mechanisms and the ability to identify high risk communities for which targeted interventions could be designed (Kloog, 2019). However, previous studies on the combined effect of air pollution and temperature have predominantly focused on mortality, hospitalization rates, cardiovascular disease, and respiratory outcomes (Benmarhnia et al., 2014; De Sario et al., 2013; J. Li et al., 2017; Shaposhnikov et al., 2014). Only one recent study in Guangzhou, China reported moderately intensive heatwaves (i.e., shorter or with relatively low temperature thresholds) may act synergistically with ambient particulate matter < 2.5 μm (PM2.5) exposure to increase the risk of PTB (Wang et al., 2020). Further studies in other geographical regions and considering other air pollutants would provide insight toward the combined effect between heatwaves and air pollutants on PTB.
Furthermore, effective adaptation measures to climate changes, such as the development of green infrastructure, may help reduce the burden of heatwaves (Mimura et al., 2014). Green space influences ambient temperature through transpiration, shading and modified convection. Green spaces could provide substantial cooling benefits for their surroundings, especially when urban heat islands are most intense (Gunawardena et al., 2017). The protective associations of green space on PTB have been shown in previous studies (Casey et al., 2016; Grazuleviciene et al., 2015; Hystad et al., 2014; Laurent et al., 2013; Sun et al., 2020). A recent study in Rome observed no effect modification for the Normalized Difference Vegetation Index (NDVI) on the relationship between maximum apparent temperature and PTB (Asta et al., 2019). To our knowledge, no prior study has examined interactions between heatwaves and green space exposure during pregnancy and their joint impact on PTB. Understanding their potential interactions could help confirm the benefits of green space on mitigating the effects of climate change and the need to enhance green space infrastruture, especially in urban areas (Kloog, 2019).
To expand our understanding of heatwave in relation to PTB, we aimed to examine the potential interaction of heatwaves, air pollution and green space exposure on the risk of PTB, using data from California births between 2005 and 2013.
METHODS
Study Population
We created a cohort of mothers who gave birth in California between 2005 and 2013 using Birth Data Files 2005–2013, maintained and provided by the California Department of Public Health. We focused on births during the warm season (May through September) as defined in previous studies of extreme heat and health in California (Avalos et al., 2017; Basu et al., 2017; Basu et al., 2010; Gershunov & Guirguis, 2012; Ilango et al., 2020). This analysis was restricted to live, singleton births and excluded mothers with missing residential zip code and births with missing gestational age. Mothers who gave birth after September 30 were considered at-risk for preterm birth in September but were not included as events. Further details of the cohort have been previously described (Ilango et al., 2020). The final sample included 1,967,300 mother/infant pairs (Appendix A). This study was approved by the University of California, Irvine (IRB #2019–5242).
Outcome
The outcome of interest for this analysis was time-to-preterm birth, with gestational weeks as the time scale. Preterm birth was defined as gestational age less than 37 weeks. Preterm birth was further categorized into extremely PTB (<28 weeks gestation), very PTB (28–<32 weeks gestation), and late PTB (32-<37 weeks gestation) in sensitivity analyses. Clinical estimates of gestational age were obtained from the Birth Data Files and were reviewed for implausible combinations of birth weight and gestational age (Alexander et al., 1996). Births that occurred outside of the risk period were censored.
Exposure
Heatwave
Our classification of heatwave has been previously described (Ilango et al., 2020). Briefly, we created 12 definitions of heatwave with varied temperature thresholds (75th, 90th, 95th, and 98th percentiles) and durations (at least 2, 3, or 4 consecutive days) for each Zip Code Tabulation Area (ZCTA), representing zip codes defined by the U.S. Census Bureau. Temperature data were derived from National Ocean and Atmosphere Administration Cooperative Observer stations and then assigned to population-weighted zip code centroids for zip code-specific daily estimates of maximum temperature (Livneh et al., 2015). For each definition of a heatwave, exposure was dichotomized and mothers were considered exposed if they experienced an extreme heat episode the week before birth (i.e., the six days preceding delivery and date of birth).
Air Pollutants
PM2.5 and O3 were the main air pollutants of interest in this analysis. PM2.5 and O3 were estimated using 24-hour daily means and 8-hour daily maximums, respectively, sampled and analyzed by the US Environmental Protection Agency Air Quality System. Measured concentrations within a 20 km radius of each population-weighted centroid were used for interpolation. Values were interpolated using an inverse distance weighting approach, where monitoring stations closer to the point of interest contributed more to the estimated concentration than stations farther away. Exposure was set to missing if there was no measured concentration within 20 km of the zip code centroid. Daily-, zip code-specific pollutant estimates were then linked to each participant’s residential zip code with a moving average exposure from the week before birth. In addition, we conducted analyses regarding PM10 and nitrogen dioxide (NO2) and we used the same source of data and a similar interpolation approach to assign exposure to these pollutants during pregnancy to our study participants.
Green Space
Green space was evaluated using NDVI, a measure that estimates surrounding green space from a satellite-derived index of vegetation. Details on estimation of NDVI has been previously published (Sun et al., 2020). Briefly, NDVI ranges from −1 to 1 and describes the difference between visible and near-infrared reflectance of vegetation cover, where higher values indicate more greenness. The NDVI estimates were based on every eight-day MODIS products with a spatial resolution of 250 m x 250 m. Average NDVI was calculated and assigned to each zip code based on the NDVI values in all 250 m grids within the zip code. Annual-, zip code-specific NDVI estimates were linked to each participant zip code and date of birth and were evaluated per 0.1 unit increase in regression analyses. Tree canopy, measured as the population-weighted percentage of the zip code with tree canopy, was used as an alternative estimate of green space in sensitivity analyses (Delaney et al., 2018). The tree canopy data was obtained from the California Healthy Places Index (HPI) that was modeled from the National Land Cover Database (Homer et al., 2015).
Covariates
Covariates included in the regression model were selected a priori as potential confounders and well documented risk factors of preterm birth. We ascertained information on covariates from birth data. All models included the following maternal characteristics: age, race/ethnicity (American Indian/Alaskan Native, Asian, Hispanic, Native Hawaiian/Pacific Islander, Non-Hispanic Black, Non- Hispanic White, and other), education (less than high school, high school diploma, less than four years of college, four years of college, and more than four years college), health insurance (Medicaid, private health insurance, and other), parity (1, 2, 3, ≥4), plus sex of infant (female or male), and season of birth (spring, summer, fall).
Statistical Analysis
Descriptive statistics of maternal, infant, and environmental characteristics were computed for the total study population and stratified by preterm (<37 weeks) and term (⩾37 weeks) births.
A time-to-event framework was implemented for the main analysis. We fit multi-level Cox proportional hazard models with time to PTB as the outcome and gestational week as the temporal unit. Mothers with term births were censored and their zip codes were included as a random effect to account for any spatial clustering of PTB. We ran separate models for each heat wave definition. Exposure was assigned using week before birth, as seen in previous work (Auger et al., 2014; Ilango et al., 2020). All models included an interaction term between heatwave and air pollutant or green space and were adjusted for maternal age, race/ethnicity, education, health insurance, parity, infant sex and season of birth. We generated hazard ratios and 95% confidence intervals for each heat wave definition.
In this analysis, we used the relative risk due to interaction (RERI) to estimate the additive interactive effect of heatwave exposure on PTB with respective two-way interactions with one week of air pollution exposure (PM10, PM2.5 per 10 ug/m3 and O3, NO2 per 10 ppb) and annual green space (per 0.1 decrease in NDVI) (VanderWeele & Knol, 2014). This qualitative measure represents interaction departure from additivity, which is a priority when evaluating etiologic research questions. For example, preventing the effect of heat waves among mothers exposed to high air pollution or low NDVI may have a greater benefit by reducing PTB risk. RERI = 𝐻𝑅11 − 𝐻𝑅10 − 𝐻𝑅01 + 𝐻𝑅00, where 𝐻𝑅𝑥𝑦 = (𝐷 = 1|𝑋 = 𝑥, 𝑌 = 𝑦) the risk of the outcome D when X is value x and Y is value y.
Sensitivity Analyses
First, we applied the Cox proportional hazard models considering the interaction of green space and heatwaves on PTB restricting to only urban zip codes as a sensitivity analysis. Urban zip codes were defined as those with a rural-urban commuting area of less than three, which indicates that at least 30% of the population commutes to an urban area for work (Cromartie, 2005). Next, we considered varying exposure windows of 4 days and 2 weeks prior to delivery to consider more acute and less acute periods of exposure to heat wave and air pollution. We also considered tree canopy, as an alternative estimate of green space (Ulmer et al., 2016). The RERIs were calculated per one unit increase in tree canopy percentage, which was a population-weighted percentage of the census tract area with tree canopy defined by the Healthy Places Index (Delaney et al., 2018), averaged by zip code. Lastly, chronic exposure to each air pollutants were considered as alternative measures of air pollution exposure, which was the average exposure concentration over the entire gestational period. All analyses were performed with SAS version 9.4 (SAS Institute, Inc., Cary, North Carolina).
RESULTS
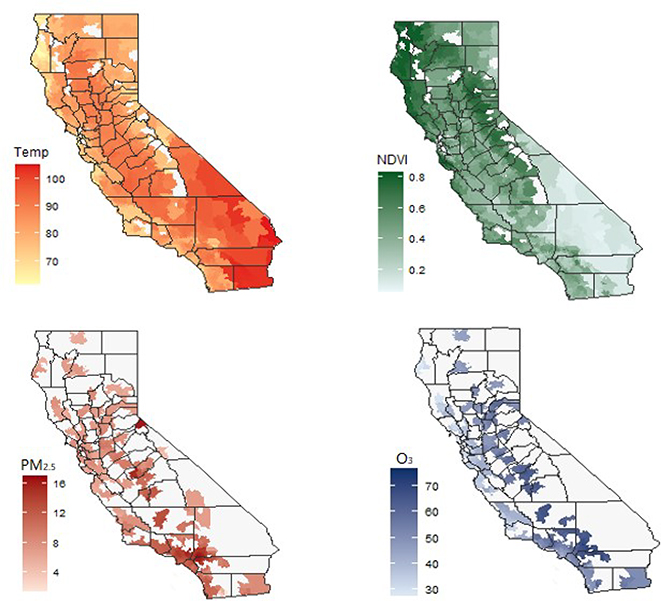
Among 1,967,300 births included in our study population, 127,039 (6.72 %) PTBs, 4,975 (0.25%) extremely PTBs, 11,894 (0.60%) very PTBs, and 114,949 (5.84%) late PTBs were identified. The distribution of selected population characteristics and environmental conditions is presented in Table 1. The ZCTA-specific mean temperatures, PM2.5 and O3 concentrations, and NDVI values are depicted in Figure 1. Overall, ambient temperature and air pollution levels in southern California were higher than those in northern California.
Table 1.
Description of the study population and environmental conditions, by gestational age category in California, May-September 2005 – 2013.
| Characteristics | Preterm birth (< 37 weeks) n = 127,039 | Term birth (≥ 37 weeks) n = 1,763,247 | Total births* n = 1,967,300 |
|---|---|---|---|
|
| |||
| Maternal age, years, mean (SD) | 28.36 (6.7) | 28.29 (6.2) | 28.30 (6.3) |
| Maternal race/ethnicity, n (%) | |||
| American Indian/Alaskan Native | 667 (0.5) | 7582 (0.4) | 8557 (0.4) |
| Asian | 14630 (11.5) | 220431 (12.5) | 244711 (12.4) |
| Hispanic | 69499 (54.7) | 896736 (50.9) | 1005799 (51.1) |
| Native Hawaiian/Pacific Islander | 804 (0.6) | 8918 (0.5) | 10138 (0.5) |
| Non-Hispanic Black | 10723 (8.4) | 94844 (5.4) | 109931 (5.6) |
| Non-Hispanic White | 28635 (22.5) | 505522 (28.7) | 555511 (28.2) |
| Maternal education, n (%) | |||
| ≤ 8th grade | 37341 (30.5) | 425993 (25.0) | 482210 (25.4) |
| 9th grade – high school | 33139 (27.1) | 435511 (25.6) | 487902 (25.7) |
| At least come college | 51979 (42.5) | 841046 (49.4) | 929120 (48.9) |
| Health insurance, n (%) | |||
| Medicaid | 66787 (52.8) | 815952 (46.4) | 918890 (46.8) |
| Private health insurance | 51910 (41.0) | 829836 (47.1) | 917391 (46.7) |
| Other | 7847 (6.2) | 114598 (6.5) | 127523 (6.5) |
| Parity, n (%) | |||
| 1 | 48634 (38.6) | 699535 (39.7) | 779126 (39.7) |
| 2 | 35146 (27.9) | 561898 (31.9) | 620926 (31.6) |
| 3 | 22001 (17.5) | 299279 (17.0) | 334441 (17.0) |
| 4 or more | 20093 (16.0) | 201457 (11.4) | 230483 (11.7) |
| Sex of infant, n (%) | |||
| Female | 56891 (44.8) | 863502 (49.0) | 958057 (48.7) |
| Male | 70139 (55.2) | 899741 (51.0) | 1009228 (51.3) |
| Season of birth, n (%) | |||
| Spring (May - June) | 50647 (39.9) | 668504 (37.9) | 719151 (36.6) |
| Summer (July - September) | 76392 (60.1) | 1094743 (62.1) | 1171135 (59.5) |
| Fall (October) | NA | NA | 77014 (3.9) |
| Environmental condition during the week preceding birth, mean (SD) | |||
| Temperature, °F | 83.52 (9.9) | 83.00 (9.8) | 83.12 (9.8) |
| PM2.5, μg/m3 | 11.48 (4.7) | 11.19 (4.6) | 11.26 (4.7) |
| PM10, μg/m3 | 30.34 (12.6) | 29.48 (12.3) | 29.69 (12.4) |
| O3, ppb | 50.36 (12.7) | 49.37 (12.5) | 49.39 (12.5) |
| NO2, ppb | 12.68 (6.2) | 12.27 (6.1) | 12.58 (6.4) |
| NDVI | 0.37 (0.1) | 0.37 (0.1) | 0.37 (0.1) |
SD, standard deviation; F, Fahrenheit.; ppb, parts per billion.
Birth outcomes are censored if the mother was at-risk during the study period (May to September) but gave birth outside this window. This study included 1,890,286 births and 77,014 fetus-at-risk.
Figure 1. Mean temperature, NDVI, air pollution, California, May-September, 2005–2013.
Number of zip codes: Temperature: 1759; NDVI: 1759; PM2.5: 1202; O3: 1356.
Heatwave definitions used in this study are shown in Table 2. During the warm season (May-September), the maximum proportion of days using different heatwave definitions was 17.97% [least conservative definition: mean maximum temperature = 88.11 °F (the 75th percentile) lasting for at least 2 days] and the minimum proportion was 0.20% [most conservative definition: mean maximum temperature = 98.11 °F (the 98th percentile) lasting for at least 4 days].
Table 2.
Description of heatwave definitions used in these analyses.
| Heatwave Definition | Threshold percentile and mean maximum temperature (°Fahrenheit) | Duration (days) | Number of days (%) |
|---|---|---|---|
|
| |||
| HWD1 | 75 (88.11) | 2 | 489,140 (17.97) |
| HWD2 | 75 (88.1l) | 3 | 351,812 (12.93) |
| HWD3 | 75 (88.1l) | 4 | 257,301 (9.45) |
| HWD4 | 90 (92.46) | 2 | 166,943 (6.13) |
| HWD5 | 90 (92.46) | 3 | 103,197 (3.79) |
| HWD6 | 90 (92.46) | 4 | 6,4824 (2.38) |
| HWD7 | 95 (95.14) | 2 | 75,787 (2.78) |
| HWD8 | 95 (95.14) | 3 | 42,406 (1.56) |
| HWD9 | 95 (95.14) | 4 | 23,797 (0.87) |
| HWD10 | 98 (98.11) | 2 | 26,336 (0.97) |
| HWD11 | 98 (98.11) | 3 | 11,886 (0.44) |
| HWD12 | 98 (98.11) | 4 | 5,553 (0.20) |
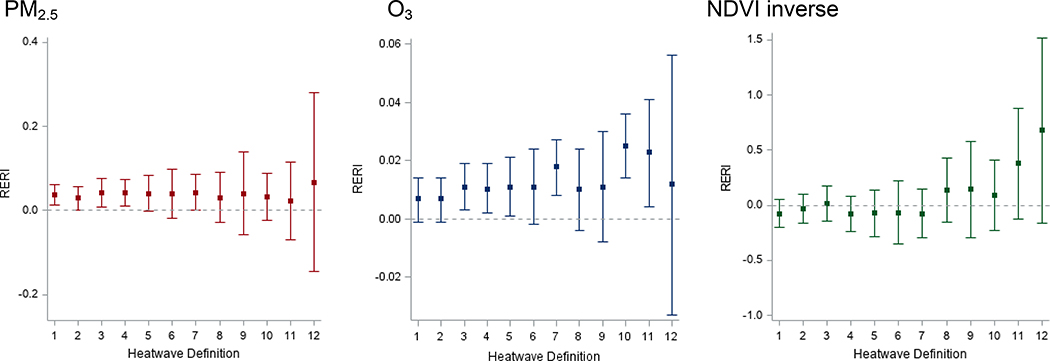
Results of the combined effects of heat waves, air pollution, and green space are presented in Figure 2 and Appendix B. Independent effects of heat waves have been previously published and independent effects of air pollution and green space are described in Appendix C (Ilango et al., 2020). For PM2.5, PM10 and O3, we found consistent positive additive interactions between heatwaves and higher air pollution levels. RERIs >0 indicated the presence of positive additive interaction, suggesting joint effects of heatwaves and air pollution on PTB are greater than expected based on the estimated effects of each exposure alone (i.e., synergistic). However, the RERIs for NO2 were consistently negative (see Appendix B). Overall, estimates for additive interactions of heatwaves and NDVI-based green space indicated negative additive interactions (RERIs <0) for less intense heatwaves (HWD1–7), whereas there was a potential synergistic effect of more intense heatwaves (HWD8–12) and green space exposure (RERIs >0).
Figure 2. RERI of heatwave and PM2.5, O3, NDVI inverse on preterm birth.
RERI: Relative excess risks due to interaction.
All models adjusted for maternal age, race/ethnicity, education, insurance, parity, infant sex, season; maternal residential ZIP code was fitted as a random effect.
RERI calculated for 10 μg/m3 increase in PM2.5; 10 parts per billion (ppb) increase in O3; 0.1 unit decrease in NDVI.
In sensitivity analyses, interaction analyses were performed using varying exposure windows, four days and two weeks prior to the delivery date, to investigate the acute effects of heatwave exposures on PTB. Results of RERI indicated similar trend for the shorter exposure window of four days, whereas the combined effects of heatwaves with PM and NDVI were weaker and imprecise for longer exposure period of two weeks (Appendix D). We also examined the combined effects of heatwaves and chronic exposure to air pollution over the full gestational period. RERIs suggested similar pattern for the long-term effects compared to the acute effects during the final gestational week (Appendix E). Furthermore, we calculated the RERIs of heatwave and lack of green space on PTB restricted to urban zip codes and found the joint effects were similar between urban population and the whole population (Appendix F). When considering tree canopy as a measure of green space in the sensitivity analysis, potential additive interactions were observed between relatively intense heatwaves and decreasing tree canopy levels (Appendix G). RERIs stratified by PTB type were shown in Appendix H. Results for late PTB were similar to PTB, but no precise trends were observed for extremely PTB and very PTB.
DISCUSSION
To the best of our knowledge, this is the first study to examine the potential combined effects of heatwaves, air pollutants, and green space exposure on the risk of PTB. In this large obstetric population from the entire state of California from 2005 to 2013, we found synergistic effects for heatwaves with high air pollution (PM2.5, PM10 and O3) and potential interaction with low green space exposures, indicating that those with higher levels of air pollution and lower green space may benefit more from preventing acute exposure to extreme heat before birth.
Relationships between environmental factors are complex. Studies which do not consider combined exposures may have underestimated their effects (Klompmaker et al., 2019b; Klompmaker et al., 2019a). An increasing number of epidemiological studies have suggested that an elevated risk of PTB were associated with high temperature exposure toward the end of gestation (Arroyo et al., 2016; Asta et al., 2019; Bekkar et al., 2020; He et al., 2016; Schifano et al., 2013; Sun et al., 2019; Vicedo-Cabrera et al., 2015; Wang et al., 2013; Wang et al., 2020; Zhang et al., 2017). In a recent review and meta-analysis (Bekkar et al., 2020), five US studies (Avalos et al., 2017; Basu et al., 2017; Basu et al., 2010; Ha et al., 2017; Kloog et al., 2015) examined the association between heat exposure and PTB. Four of the 5 studies (80%) found a positive association between heat exposure during pregnancy and PTB, and PTB risk increased 11.6% per 5.6 °C increase. In our previous work, we also found consistent evidence of an effect of acute exposure to heat stress before delivery and PTB (Ilango et al., 2020). However, the potential interactions of heatwave exposure and other environmental factors have not been studied extensively.
Air quality is highly sensitive to extreme meteorological events. For instance, heatwaves can increase ozone concentrations by affecting the production and transport of air pollutants (Carmichael et al., 1998; Hou & Wu, 2016; Jacob et al., 1993). To date, only one study in Guangzhou, China evaluated the interactive effects of heatwaves and PM2.5 on PTB. The authors reported that the joint effects of PM2.5 and heatwaves appeared to be synergistic (RERIs >0) for less extreme heatwaves but were less than additive (RERIs <0) for more intense heatwaves in their study population (Wang et al., 2020). In our study, we observed consistent positive additive interactions (RERIs >0) between heatwaves and PM and O3 on PTB for all heatwave definitions, suggesting that acute exposure to heatwaves may be more harmful for women who are exposed to higher levels of air pollution during the last week of gestation. We also found similar combined effects of acute exposure to heatwaves and long-term exposure to air pollution over the full gestational period. This synergistic effect of heatwave and air pollution on PTB may imply underlying biological mechanisms. Heat stress can worsen toxic outcome in humans through a variety of mechanisms. For example, air pollution may add oxidative stress and inflammatory responses in the body that may lead to preterm labor (Moore et al., 2018; Vadillo-Ortega et al., 2014), and raising ambient temperature may accelerate the intake of air pollutants, such as PM and O3, through heat-induced sweating, elevation in skin blood flow, and pulmonary ventilation. Moreover, heat stress combined with work or exercise is likely to worsen toxicity (Gordon et al., 2014). We also found that RERIs for NO2 and heat were negative for most heatwave definitions, suggesting the effect of heat on PTB is lower when NO2 exposure increases in late pregnancy. Such result can be explained by the fact that nitrogen oxides act as precursors for ozone. Indeed, as O3 levels are expected to increase during heat events and that we found synergistic effects between O3 and heat events, it can explain such pattern. Yet, it would be interesting in future work to explore modern statistical approaches to study environmental exposure mixtures (Hamra & Buckley, 2018; Keil et al., 2020).
The combined effects of heatwaves and green space on PTB have not been studied previously. A previous review reported that micro-urban heat islands that mostly use NDVI to capture lack of greenness have been shown to exacerbate the effects of extreme heat on mortality and hospital admissions (Schinasi et al., 2018). Green space from remotely sensed imagery, such as NDVI, cannot reflect types of vegetation (e.g., tree canopy, low-lying vegetation, grass), which might have varying impacts on health through different pathways (Astell-Burt & Feng, 2019; Zhang & Tan, 2019). For instance, NDVI may be relatively insensitive to changes in canopy structure due to variations between vegetation types (e.g., shrubs and grassland) and canopy types (e.g., evergreen vs. deciduous) (Gamon et al., 1995). We used NDVI and tree canopy to capture different aspects of green space exposure. A study in Rome reported no effect modification for NDVI within 100 m from residential address on the relationship between maximum apparent temperature and PTB (Asta et al., 2019). In our study, we found some interesting interaction patterns between heatwaves and green space on PTB. Although RERIs were mostly imprecise, the results implied potential positive interactions between heatwaves and low NDVI-based green space exposure for more conservative heatwave definitions. Further, RERIs of the tree canopy cover showed positive additive interactions, even for less conservative heatwave definitions. Green space can offer substantial cooling benefits to the surrounding area and tree-dominated green space (i.e., canopy-layer) provides greater heat stress relief when most required (Gunawardena et al., 2017).
The main strengths of our study include the large size and diversity of the obstetric population; a broad set of definitions that capture heatwaves of varying intensity and duration; and the time-to-event analytical framework that could account for the impacts of time-varying temperature, air pollution and green space exposures, and different exposure windows.
However, this study has limitations, which suggest avenues for further research. First, estimated exposures were assessed solely based on the maternal residential zip code reported on the infant birth certificate, which could not capture local-scale exposure. Exposure misclassification could also be caused by lack of information on the residential mobility during pregnancy, individual activity patterns, and heterogeneity in indoor and outdoor exposures. Future studies may include the exact maternal addresses, residential change, and use personal monitors to examine real-time personal-level heatwave, air pollution and green space exposures. Next, although we conducted interaction analyses using two green space indicators, they do not allow us to distinguish between different vegetation species and types of green space (e.g., street trees, gardens, and parks). Further research is needed using more informative and multiple green space indicators, such as proximity to green spaces and greenness based on street view image, to represent different aspects of green space exposure and explore the potential roles of different green space exposures on the associations between heat and health. Moreover, future research may take into account health status, behaviors and other individual factors, such as chronic diseases, maternal complications, physical activity, and smoking status, which may modify the effect of heat, air pollution, and green space on the risk of PTB. Further, the meteorological characteristics and environmental factors could vary across regions and might cause different health impacts. Thus, there is also a need to conduct studies in other geographical settings with different climate, air pollution and green space levels.
This study found synergistic effects for heatwaves with air pollution, and potential positive interactions with lack of green space on PTB, suggesting that reducing acute exposure to extreme heat before delivery may be more beneficial for pregnant women with high air pollution and low green space exposures. Targeted interventions, such as extreme heat warning systems, reduced outdoor activities, increased cooling zones, and protective behaviors, conducted among women who are exposed to more air pollution and less green space may optimize the potential benefits of reducing heat exposure during pregnancy.
Supplementary Material
Acknowledgements
This study was supported by the National Institute of Environmental Health Sciences (NIEHS; ES030353) and the National Cancer Institute (NCI; 1R01CA228147). Any opinions, findings, and conclusions or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the views of the NIH.
References
- Alexander GR, Himes Jh Fau - Kaufman RB, Kaufman Rb Fau - Mor J, Mor J Fau - Kogan M, & Kogan M (1996). A United States national reference for fetal growth. (0029–7844 (Print)). [DOI] [PubMed]
- Arroyo V, Diaz J, Ortiz C, Carmona R, Saez M, & Linares C (2016). Short term effect of air pollution, noise and heat waves on preterm births in Madrid (Spain). Environ Res, 145, 162–168. doi: 10.1016/j.envres.2015.11.034 [DOI] [PubMed] [Google Scholar]
- Asta F, Michelozzi P, Cesaroni G, De Sario M, Badaloni C, Davoli M, & Schifano P (2019). The Modifying Role of Socioeconomic Position and Greenness on the Short-Term Effect of Heat and Air Pollution on Preterm Births in Rome, 2001–2013. Int J Environ Res Public Health, 16(14). doi: 10.3390/ijerph16142497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astell-Burt T, & Feng X (2019). Association of Urban Green Space With Mental Health and General Health Among Adults in Australia. JAMA Netw Open, 2(7), e198209. doi: 10.1001/jamanetworkopen.2019.8209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auger N, Naimi I, A. I., Smargiassi A, Lo E, & T., K. (2014). Extreme Heat and Risk of Early Delivery Among Preterm and Term Pregnancies. Epidemiology, 25(3), 344–350. [DOI] [PubMed] [Google Scholar]
- Avalos LA, Chen H, Li DK, & Basu R (2017). The impact of high apparent temperature on spontaneous preterm delivery: a case-crossover study. Environ Health, 16(1), 5. doi: 10.1186/s12940-017-0209-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu R, Chen H, Li DK, & Avalos LA (2017). The impact of maternal factors on the association between temperature and preterm delivery. Environ Res, 154, 109–114. doi: 10.1016/j.envres.2016.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu R, Malig B, & Ostro B (2010). High ambient temperature and the risk of preterm delivery. Am J Epidemiol, 172(10), 1108–1117. doi: 10.1093/aje/kwq170 [DOI] [PubMed] [Google Scholar]
- Bekkar B, Pacheco S, Basu R, & DeNicola N (2020). Association of Air Pollution and Heat Exposure With Preterm Birth, Low Birth Weight, and Stillbirth in the US: A Systematic Review. JAMA Netw Open, 3(6), e208243. doi: 10.1001/jamanetworkopen.2020.8243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benmarhnia T, Oulhote Y, Petit C, Lapostolle A, Chauvin P, Zmirou-Navier D, & Deguen S (2014). Chronic air pollution and social deprivation as modifiers of the association between high temperature and daily mortality. Environmental Health, 13(1), 53. doi: 10.1186/1476-069X-13-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael GR, Uno I, Phadnis MJ, Zhang Y, & Sunwoo Y (1998). Tropospheric ozone production and transport in the springtime in east Asia. Journal of Geophysical Research: Atmospheres, 103(D9), 10649–10671. doi: 10.1029/97jd03740 [DOI] [Google Scholar]
- Casey JA, James P, Rudolph KE, Wu CD, & Schwartz BS (2016). Greenness and Birth Outcomes in a Range of Pennsylvania Communities. Int J Environ Res Public Health, 13(3). doi: 10.3390/ijerph13030311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chehade H, Simeoni U, Guignard JP, & Boubred F (2018). Preterm Birth: Long Term Cardiovascular and Renal Consequences. Curr Pediatr Rev, 14(4), 219–226. doi: 10.2174/1573396314666180813121652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromartie J (2005). Rural-urban commuting area codes.
- De Sario M, Katsouyanni K, & Michelozzi P (2013). Climate change, extreme weather events, air pollution and respiratory health in Europe. Eur Respir J, 42(3), 826–843. doi: 10.1183/09031936.00074712 [DOI] [PubMed] [Google Scholar]
- Delaney T, Dominie W, Dowling H, Maizlish N, Chapman D, Hill L, … Woolf S (2018). Healthy Places Index. Public Health Alliance of Southern California: Long Beach, CA, USA. [Google Scholar]
- Dreiling CE, & Carman FS (1991). Maternal Endocrine and Fetal Metabolic Responses to Heat Stress. J Dairy Sci, 74312–74327. [DOI] [PubMed] [Google Scholar]
- Ebi KL, & McGregor G (2008). Climate change, tropospheric ozone and particulate matter, and health impacts. Environ Health Perspect, 116(11), 1449–1455. doi: 10.1289/ehp.11463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S, Gao D, Liao F, Zhou F, & Wang X (2016). The health effects of ambient PM2.5 and potential mechanisms. Ecotoxicol Environ Saf, 128, 67–74. doi: 10.1016/j.ecoenv.2016.01.030 [DOI] [PubMed] [Google Scholar]
- Gamon JA, Field CB, Goulden ML, Griffin KL, Hartley AE, Joel G, … Valentini R (1995). Relationships Between NDVI, Canopy Structure, and Photosynthesis in Three Californian Vegetation Types. Ecological Applications, 5(1), 28–41. doi: 10.2307/1942049 [DOI] [Google Scholar]
- Gershunov A, & Guirguis K (2012). California heat waves in the present and future. Geophysical Research Letters, 39(18). doi: 10.1029/2012gl052979 [DOI] [Google Scholar]
- Gordon CJ, Johnstone AF, & Aydin C (2014). Thermal stress and toxicity. Compr Physiol, 4(3), 995–1016. doi: 10.1002/cphy.c130046 [DOI] [PubMed] [Google Scholar]
- Grazuleviciene R, Danileviciute A, Dedele A, Vencloviene J, Andrusaityte S, & et al. (2015). Surrounding greenness, proximity to city parks and pregnancy outcomes in Kaunas cohort study. Int J Hyg Environ Health, 218, 358–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunawardena KR, Wells MJ, & Kershaw T (2017). Utilising green and bluespace to mitigate urban heat island intensity. Sci Total Environ, 584–585, 1040–1055. doi: 10.1016/j.scitotenv.2017.01.158 [DOI] [PubMed] [Google Scholar]
- Ha S, Liu D, Zhu Y, Kim SS, Sherman S, & Mendola P (2017). Ambient Temperature and Early Delivery of Singleton Pregnancies. Environ Health Perspect, 125(3), 453–459. doi: 10.1289/EHP97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamra GB, & Buckley JP (2018). Environmental exposure mixtures: questions and methods to address them. Curr Epidemiol Rep, 5(2), 160–165. doi: 10.1007/s40471-018-0145-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison MS, & Goldenberg RL (2016). Global burden of prematurity. Semin Fetal Neonatal Med, 21, 74–79. [DOI] [PubMed] [Google Scholar]
- He JR, Liu Y, Xia XY, Ma WJ, Lin HL, Kan HD, … Muglia LJ (2016). Ambient Temperature and the Risk of Preterm Birth in Guangzhou, China (2001–2011). Environ Health Perspect, 124(7), 1100–1106. doi: 10.1289/ehp.1509778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoegh-Guldberg O, Jacob D, Taylor M, Bindi M, Brown S, Camilloni I, … Engelbrecht F (2018). Impacts of 1.5 C global warming on natural and human systems Global warming of 1.5° C.: An IPCC Special Report (pp. 175–311): IPCC Secretariat. [Google Scholar]
- Homer C, Dewitz J, Yang L, Jin S, Danielson P, Xian G, … Megown K (2015). Completion of the 2011 National Land Cover Database for the Conterminous United States - Representing a Decade of Land Cover Change Information. Photogrammetric Engineering and Remote Sensing, 81, 346–354. doi: 10.14358/PERS.81.5.345 [DOI] [Google Scholar]
- Hou P, & Wu S (2016). Long-term Changes in Extreme Air Pollution Meteorology and the Implications for Air Quality. Sci Rep, 6, 23792. doi: 10.1038/srep23792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hystad P, Davies HW, Frank L, Van Loon J, Gehring U, Tamburic L, & Brauer M (2014). Residential greenness and birth outcomes: evaluating the influence of spatially correlated built-environment factors. Environ Health Perspect, 122(10), 1095–1102. doi: 10.1289/ehp.1308049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilango SD, Weaver M, Sheridan P, Schwarz L, Clemesha RES, Bruckner T, … Benmarhnia T (2020). Extreme heat episodes and risk of preterm birth in California, 2005–2013. Environ Int, 137, 105541. doi: 10.1016/j.envint.2020.105541 [DOI] [PubMed] [Google Scholar]
- Jacob DJ, Logan JA, Yevich RM, Gardner GM, Spivakovsky CM, Wofsy SC, … Zimmerman PR (1993). Simulation of summertime ozone over North America. Journal of Geophysical Research, 98(D8), 14797. doi: 10.1029/93jd01223 [DOI] [Google Scholar]
- Kannan S, Misra DP, Dvonch JT, & Krishnakumar A (2006). Exposures to airborne particulate matter and adverse perinatal outcomes: a biologically plausible mechanistic framework for exploring potential effect modification by nutrition. Environ Health Perspect, 114(11), 1636–1642. doi: 10.1289/ehp.9081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil AP, Buckley JP, O’Brien KM, Ferguson KK, Zhao S, & White AJ (2020). A Quantile-Based g-Computation Approach to Addressing the Effects of Exposure Mixtures. Environ Health Perspect, 128(4), 47004. doi: 10.1289/EHP5838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent ST, McClure LA, Zaitchik BF, Smith TT, & Gohlke JM (2014). Heat waves and health outcomes in Alabama (USA): the importance of heat wave definition. Environ Health Perspect, 122(2), 151–158. doi: 10.1289/ehp.1307262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klompmaker JO, Hoek G, Bloemsma LD, Wijga AH, van den Brink C, Brunekreef B, … Janssen NAH (2019b). Associations of combined exposures to surrounding green, air pollution and traffic noise on mental health. Environ Int, 129, 525–537. doi: 10.1016/j.envint.2019.05.040 [DOI] [PubMed] [Google Scholar]
- Klompmaker JO, Janssen NAH, Bloemsma LD, Gehring U, Wijga AH, van den Brink C, … Hoek G (2019a). Residential surrounding green, air pollution, traffic noise and self-perceived general health. Environ Res, 179(Pt A), 108751. doi: 10.1016/j.envres.2019.108751 [DOI] [PubMed] [Google Scholar]
- Kloog I (2019). Air pollution, ambient temperature, green space and preterm birth. Curr Opin Pediatr, 31(2), 237–243. doi: 10.1097/MOP.0000000000000736 [DOI] [PubMed] [Google Scholar]
- Kloog I, Melly S, Ridgway W, Coull B, & Schwartz J (2012). Using new satellite based exposure methods to study the association between pregnancy PM2.5 exposure, premature birth and birth weight in Massachusetts. Environ Health, 11, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloog I, Melly SJ, Coull BA, Nordio F, & Schwartz JD (2015). Using Satellite-Based Spatiotemporal Resolved Air Temperature Exposure to Study the Association between Ambient Air Temperature and Birth Outcomes in Massachusetts. Environ Health Perspect, 123(10), 1053–1058. doi: 10.1289/ehp.1308075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamichhane DK, Leem JH, Lee JY, & Kim HC (2015). A meta-analysis of exposure to particulate matter and adverse birth outcomes. Environ Health Toxicol, 30, e2015011. doi: 10.5620/eht.e2015011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent O, Hu J, Li L, Kleeman MJ, Bartell SM, Cockburn M, … Wu J (2016). A Statewide Nested Case-Control Study of Preterm Birth and Air Pollution by Source and Composition: California, 2001–2008. Environ Health Perspect, 124(9), 1479–1486. doi: 10.1289/ehp.1510133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent O, Wu J, Li L, & Milesi C (2013). Green spaces and pregnancy outcomes in Southern California. Health Place, 24, 190–195. doi: 10.1016/j.healthplace.2013.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Woodward A, Hou XY, Zhu T, Zhang J, Brown H, … Liu Q (2017). Modification of the effects of air pollutants on mortality by temperature: A systematic review and meta-analysis. Sci Total Environ, 575, 1556–1570. doi: 10.1016/j.scitotenv.2016.10.070 [DOI] [PubMed] [Google Scholar]
- Li X, Huang S, Jiao A, Yang X, Yun J, Wang Y, … Xiang H (2017). Association between ambient fine particulate matter and preterm birth or term low birth weight: An updated systematic review and meta-analysis. Environ Pollut, 227, 596–605. doi: 10.1016/j.envpol.2017.03.055 [DOI] [PubMed] [Google Scholar]
- Liu L, Oza S, Hogan D, Chu Y, Perin J, Zhu J, … Black RE (2016). Global, regional, and national causes of under-5 mortality in 2000–15: an updated systematic analysis with implications for the Sustainable Development Goals. The Lancet, 388(10063), 3027–3035. doi: 10.1016/s0140-6736(16)31593-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livneh B, Bohn TJ, Pierce DW, Munoz-Arriola F, Nijssen B, Vose R, … Brekke L (2015). A spatially comprehensive, hydrometeorological data set for Mexico, the U.S., and Southern Canada 1950–2013. Sci Data, 2, 150042. doi: 10.1038/sdata.2015.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malley CS, Kuylenstierna JC, Vallack HW, Henze DK, Blencowe H, & Ashmore MR (2017). Preterm birth associated with maternal fine particulate matter exposure: A global, regional and national assessment. Environ Int, 101, 173–182. doi: 10.1016/j.envint.2017.01.023 [DOI] [PubMed] [Google Scholar]
- March of Dimes, PMNCH, Save the Children, & WHO. (2012). Born Too Soon: The Global Action Report on Preterm Birth.
- McCormick MC, Litt JS, Smith VC, & Zupancic JA (2011). Prematurity: an overview and public health implications. Annu Rev Public Health, 32, 367–379. doi: 10.1146/annurev-publhealth-090810-182459 [DOI] [PubMed] [Google Scholar]
- Mimura N, Pulwarty R, Duc D, & et al. (2014). Adaptation planning and implementation. Climate change 2014: impacts, adaptation, and vulnerability Working Group II Contribution to the IPCC 5th Assessment Report, Cambridge University Press, Cambridge, UK and New York, NY. [Google Scholar]
- Moore TA, Ahmad IM, & Zimmerman MC (2018). Oxidative Stress and Preterm Birth: An Integrative Review. Biol Res Nurs, 20(5), 497–512. doi: 10.1177/1099800418791028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson D, Mogren I, & Forsberg B (2013). Air pollution exposure in early pregnancy and adverse pregnancy outcomes: a register-based cohort study. BMJ Open, 3(2). doi: 10.1136/bmjopen-2012-001955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira G, Belanger K, Ebisu K, & Bell ML (2014). Fine particulate matter and risk of preterm birth in Connecticut in 2000–2006: a longitudinal study. Am J Epidemiol, 179(1), 67–74. doi: 10.1093/aje/kwt216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrou S (2003). Economic consequences of preterm birth and low birthweight. BJOG: An International Journal of Obstetrics and Gynaecology, 110, 17–23. doi: 10.1016/s1470-0328(03)00013-2 [DOI] [PubMed] [Google Scholar]
- Saigal S, & Doyle LW (2008). An overview of mortality and sequelae of preterm birth from infancy to adulthood. The Lancet, 371(9608), 261–269. doi: 10.1016/s0140-6736(08)60136-1 [DOI] [PubMed] [Google Scholar]
- Schär C (2015). The worst heat waves to come. Nature Climate Change, 6(2), 128–129. doi: 10.1038/nclimate2864 [DOI] [Google Scholar]
- Schifano P, Lallo A, Asta F, De Sario M, Davoli M, & Michelozzi P (2013). Effect of ambient temperature and air pollutants on the risk of preterm birth, Rome 2001–2010. Environ Int, 61, 77–87. doi: 10.1016/j.envint.2013.09.005 [DOI] [PubMed] [Google Scholar]
- Schinasi LH, Benmarhnia T, & De Roos AJ (2018). Modification of the association between high ambient temperature and health by urban microclimate indicators: A systematic review and meta-analysis. Environ Res, 161, 168–180. doi: 10.1016/j.envres.2017.11.004 [DOI] [PubMed] [Google Scholar]
- Shaposhnikov D, Revich B, Bellander T, Bedada GB, Bottai M, Kharkova T, … Pershagen (2014). Mortality Related to Air Pollution with the Moscow Heat Wave and Wildfire of 2010. Epidemiology, 25(3), 359–364. doi: 10.1097/ede.0000000000000090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan P, Ilango S, Bruckner TA, Wang Q, Basu R, & Benmarhnia T (2019). Ambient Fine Particulate Matter and Preterm Birth in California: Identification of Critical Exposure Windows. Am J Epidemiol, 188(9), 1608–1615. doi: 10.1093/aje/kwz120 [DOI] [PubMed] [Google Scholar]
- Stan CM, Boulvain M, Pfister R, & Hirsbrunner-Almagbaly P (2013). Hydration for treatment of preterm labour. Cochrane Database Syst Rev(11), CD003096. doi: 10.1002/14651858.CD003096.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieb DM, Chen L, Eshoul M, & Judek S (2012). Ambient air pollution, birth weight and preterm birth: a systematic review and meta-analysis. Environ Res, 117, 100–111. doi: 10.1016/j.envres.2012.05.007 [DOI] [PubMed] [Google Scholar]
- Sun S, Weinberger KR, Spangler KR, Eliot MN, Braun JM, & Wellenius GA (2019). Ambient temperature and preterm birth: A retrospective study of 32 million US singleton births. Environ Int, 126, 7–13. doi: 10.1016/j.envint.2019.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Sheridan P, Laurent O, Li J, Sacks DA, Fischer H, … Wu J (2020). Associations between green space and preterm birth: Windows of susceptibility and interaction with air pollution. Environment International, 142, 105804. doi: 10.1016/j.envint.2020.105804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmer J, Wolf K, Backman D, Tretheway R, Blain C, O’Neil-Dunne J, & Frank L (2016). Multiple health benefits of urban tree canopy: The mounting evidence for a green prescription. Health & Place, 42, 54–62. doi: 10.1016/j.healthplace.2016.08.011 [DOI] [PubMed] [Google Scholar]
- Vadillo-Ortega F, Osornio-Vargas A, Buxton MA, Sanchez BN, Rojas-Bracho L, Viveros-Alcaraz M, … O’Neill MS (2014). Air pollution, inflammation and preterm birth: a potential mechanistic link. Med Hypotheses, 82(2), 219–224. doi: 10.1016/j.mehy.2013.11.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderWeele TJ, & Knol MJ (2014). A Tutorial on Interaction. Epidemiologic Methods, 3(1). doi: 10.1515/em-2013-0005 [DOI] [Google Scholar]
- Vicedo-Cabrera AM, Olsson D, & Forsberg B (2015). Exposure to seasonal temperatures during the last month of gestation and the risk of preterm birth in Stockholm. Int J Environ Res Public Health, 12(4), 3962–3978. doi: 10.3390/ijerph120403962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Williams G, Guo Y, Pan X, & Tong S (2013). Maternal exposure to heatwave and preterm birth in Brisbane, Australia. BJOG, 120(13), 1631–1641. doi: 10.1111/1471-0528.12397 [DOI] [PubMed] [Google Scholar]
- Wang Q, Benmarhnia T, Zhang H, Knibbs LD, Sheridan P, Li C, … Huang C (2018). Identifying windows of susceptibility for maternal exposure to ambient air pollution and preterm birth. Environ Int, 121(Pt 1), 317–324. doi: 10.1016/j.envint.2018.09.021 [DOI] [PubMed] [Google Scholar]
- Wang Q, Li B, Benmarhnia T, Hajat S, Ren M, Liu T, … Huang C (2020). Independent and Combined Effects of Heatwaves and PM2.5 on Preterm Birth in Guangzhou, China: A Survival Analysis. Environ Health Perspect, 128(1), 17006. doi: 10.1289/EHP5117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts N, Adger WN, Agnolucci P, Blackstock J, Byass P, Cai W, … Costello A (2015). Health and climate change: policy responses to protect public health. The Lancet, 386(10006), 1861–1914. doi: 10.1016/s0140-6736(15)60854-6 [DOI] [PubMed] [Google Scholar]
- Wilhelm M, Ghosh J, Su J, Cockburn M, Jerrett M, & Ritz B (2011). Traffic-related air toxics and preterm birth: a population-based case-control study in Los Angeles County, California. Environ Health, 10, 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Ren C, Delfino RJ, Chung J, Wilhelm M, & Ritz B (2009). Association between local traffic-generated air pollution and preeclampsia and preterm delivery in the south coast air basin of California. Environ Health Perspect, 117(11), 1773–1779. doi: 10.1289/ehp.0800334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanobetti A, & Peters A (2015). Disentangling interactions between atmospheric pollution and weather. Epidemiol Community Health, 69(7), 613–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, & Tan Y (2019). Associations between Urban Green Spaces and Health are Dependent on the Analytical Scale and How Urban Green Spaces are Measured. Int J Environ Res Public Health, 16(4). doi: 10.3390/ijerph16040578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Yu C, & Wang L (2017). Temperature exposure during pregnancy and birth outcomes: An updated systematic review of epidemiological evidence. Environ Pollut, 225, 700–712. doi: 10.1016/j.envpol.2017.02.066 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.