SHORT ABSTRACT:
Stroke is a global issue with minimal treatment options and no current clinical therapy for regenerating lost brain tissue. Here we describe methods for creating precise photothrombotic stroke in the motor cortex of rodents, as well as subsequent injection of hydrogel biomaterials to study their effects on tissue regeneration after stroke.
Keywords: Stroke, photothrombotic stroke, biomaterials, hydrogel, injectable hydrogels, hydrogel scaffolds, neurobiology, biomedical engineering
LONG ABSTRACT:
Stroke is the leading cause of disability and the fifth-leading cause of death in the United States. Approximately 87% of all strokes are ischemic strokes and are defined as the sudden blockage of a vessel supplying blood to the brain. Within minutes of the blockage, cells begin to die and result in irreparable tissue damage. Current therapeutic treatments focus on clot removal or lysis to allow reperfusion and prevent more severe brain damage. Although transient brain plasticity may salvage some of the damaged tissue over time, a significant fraction of patients are left with neurological deficits that will never resolve. There is a lack of therapeutic options to treat neurological deficits caused by stroke, emphasizing the need to develop new strategies to treat this growing patient population. Injectable biomaterials are currently being designed to enhance brain plasticity and improve endogenous repair through the delivery of active agents or stem cells. One method to test these approaches is to utilize a rodent stroke model, inject the biomaterial into the stroke core, and assess repair. Knowing the precise location of the stroke core is imperative for accurate treatment after stroke, therefore a stroke model that results in a predictable stroke location is preferable to avoid the need for imaging prior to injection. The following methods will cover how to induce a photothrombotic stroke, how to inject a hydrogel in a controlled and precise manner, and how to extract and cryosection the brain while keeping the biomaterial intact. In addition, we will highlight how these same hydrogel materials can be used for the co-delivery of stem cells. This protocol can be generalized to the use of other injectable biomaterials into the stroke core.
INTRODUCTION:
Stroke is the leading cause of disability and the fifth-leading cause of death in the United States1. Approximately 87% of all strokes are ischemic, while a majority of the remaining 13% are hemorrhagic2. An ischemic stroke is defined as the blockage of blood flow in an artery to the surrounding tissue. This occlusion results in oxygen deprivation and subsequent necrosis that often leads to permanent disability in surviving patients. While there has been a decrease in the mortality rate of stroke3, its prevalence is expected to increase by 3.4 million people by 20304. This increase in disabled survivors and consequent economic burden has led to a push for stroke research that focuses on mechanisms of neural repair. Following stroke there is an inflammatory period that leads to the formation of a scar that prevents the necrotic region from expanding. The region surrounding the necrotic core is termed “peri-infarct” and there is strong evidence that the plasticity in this region, which includes increased angiogenesis, neurogenesis, and axonal sprouting, is directly linked to the observed recovery in animal models and humans 5. Since there are no in vitro models that can properly replicate the complex interactions following stroke, animal models are essential for stroke research.
There are several in vivo models that can be used to produce ischemic stroke. One of the most common models used in mice is middle cerebral artery occlusion, or MCAo, through distal or proximal (via intra-arterial filament) occlusion. The proximal model, also known as filament MCAo (fMCAo), typically result in large ischemic strokes that encompass anywhere from 5% to 50% of the cerebral hemisphere, dependent upon a number of factors6. In these models, a suture or filament is advanced from the internal carotid artery into the base of the middle cerebral artery (MCA) and kept in place for a specific period of time. This method for occlusion, which can be made temporary or permanent, produces an infarct that is centered in the striatum and may or may not involve overlying cortex6. The resulting stroke size is highly variable, and imaging techniques, such as laser doppler, are required to confirm the effectiveness of the procedure in each mouse. Intra-arterial or intra-luminal filament occlusion that lasts greater than 30 minutes produces strokes at the larger end of the size range. Some investigators have focused on shorter filament occlusion times, which require substantial experimental focus and lab validation7. Filament MCAo models in mice follow similar stages of cell death, ischemic progression, and formation of a peri-infarct region seen in human stroke cases; however, the larger strokes more closely resemble the disease state of malignant cerebral infarctions, which are less common, less treatable human strokes6. Meanwhile, distal MCA occlusion requires a more involved surgery and craniectomy. In this model, the distal part of the MCA that runs along the surface of the brain is directly occluded with a suture tie or cauterization. In some variations of the technique, the carotid arteries are unilaterally or transiently-bilaterally occluded. A benefit of the distal MCAo is that it produces a cortical-based stroke that is less variable in size than the filament model. However, the distal model produces poorer behavioral output due to transection of the ECA, which is also a concern with fMCAo6.
An alternative stroke model that is known for being less invasive is the photothrombotic (PT) model. The PT model results in a well-defined location of ischemia and is associated with a high survival rate8. The technique relies on a photosensitive dye injected intraperitoneally that allows for intravascular photo-oxidation simply by irradiating the desired tissue with a light or laser. Upon excitation, oxygen radicals are formed that cause endothelial damage, which activates platelet aggregation and clot formation in the irradiated area8. The tight control over stroke size and location, as well as high reproducibility of the PT model, makes it ideal for studying biomaterials. While precision is made possible through use of a laser and stereotaxic coordinates, there are some disadvantages that may make this model less ideal for particular studies. Unlike the fMCAo model, the PT stroke model is not able to be reperfused. Therefore, materials for investigating neuroprotective agents specific to damages following reperfusion or the mechanisms following reperfusion would not be useful here8. Additionally, due to the microvascular insult of the PT model, relatively little ischemic penumbra is seen. Instead, local vasogenic edema occurs, which is uncharacteristic for human stroke, making this model undesirable for preclinical drug studies focused on the peri-infarct area6, 8.
The overall goal of biomaterial strategies in stroke is to either deliver bioactive agents or to act as a surrogate extracellular matrix for brain tissue growth. One strategy that we will explore using our methods is to deliver hydrogel directly into the stroke core, as opposed to the peri-infarct tissue where many current cell therapies are delivered9. The rationale for this approach is that delivery into the necrotic tissue found in the core will avoid disrupting the surrounding healthy or recovering tissue. We assume diffusion of any active agents included within the biomaterial will be able to reach the peri-infarct from the core, especially since we find that delivery of hydrogel biomaterials reduces the thickness of the glial scar10. This is important since the peri-infarct region has been shown to exhibit neuroplasticity after stroke, making it an attractive target. Furthermore, the delivery of a surrogate matrix to the stroke core can be loaded with angiogenic11 or neurogenic12 factors to guide the formation of new tissue, as well as cells for delivery13. Cell delivery is greatly enhanced by using a matrix because it protects cells from the harsh injection forces and local environment present during delivery, as well as encourages differentiation and engraftment14.
These injectable therapeutic biomaterials have clinical relevance in stroke applications, as there are currently no medical therapies that stimulate neuronal recovery after stroke. The underlying neural circuits involved in recovery lie in brain tissue that is adjacent to the stroke core15, while the stroke core itself is devoid of viable neural tissue. We anticipate that delivering a biomaterial into the necrotic stroke core has potential to stimulate the adjacent tissue toward regenerative processes through a number of mechanisms previously mentioned, including depot release of growth factors12, stimulation of tissue in-growth and promotion of recovering brain tissue development10, 11, alteration of immune responses16, and delivery of stem cell-derived therapeutics13, 17. However, to effectively study the possibility of these applications, a consistent and reproducible method for inducing stroke and injecting biomaterials is needed. The PT stroke model uses techniques that offer precise control over the orientation and location of the stroke. A laser attached to the stereotaxic device guides orientations, and pumps attached to the stereotaxic devices control the injection rate of the material without the need for additional forms of imaging. Therefore, we have chosen to describe the methods for performing a PT stroke in the motor cortexes of mice and for injecting biomaterials into the stroke core. Here, we use microporous annealed particle (MAP) hydrogels as the biomaterial for injection with no added cells or growth factors. Additionally, we explain how to successfully retrieve the brain with intact biomaterial, and we discuss immunochemistry assays used to analyze the stroke outcome with and without injection of biomaterials.
PROTOCOL:
Ethics statement
The experiments reported in this video were conducted in accordance with IUCAC at Duke University and University of California Los Angeles. 8 to 12-week-old male C57Bl/6J mice were used in this study. The animals were housed under controlled temperature (22 ± 2 °C), with a 12hr light-dark cycle period and access to pelleted food and water ad libitum. Analgesia and sedation protocols are described as approved by the IUCAC but might differ from protocols used in other laboratories.
1. Preparation of the Material and Instruments
1.1 Prepare photothrombotic dye prior to surgery. Weigh out 15 mg of Rose Bengal into a 2 mL Eppendorf tube. CAUTION: Rose Bengal can cause serious eye damage/irritation.
1.1.1 Dissolve the Rose Bengal in 1.5 mL of sterile filtered 1xPBS to make a working concentration of 10mg/mL.
1.1.2 Vortex the tube until no clumps are visible, indicating the dye has fully dissolved, then cover in foil until further use. Note: Amount of dye required will depend on the number of mice, where dye is injected IP at a concentration of 10 μL/g, e.g., 200 μL for a 20 g mouse.
1.2 Turn on heating pad in order to maintain the mouse body temperature during anesthesia (37°C).
1.3 Prepare autoclaved scissors, forceps, cotton, vet eye ointment, surgical glue or suture material, a blue or black surgical marker, and sterile alcohol and beta iodine wipes. Prepare bone drill with sterilized drill bit heads.
1.4 Turn on bead sterilization to 160°C to allow for sterilization of instruments between mice. Set aside a 50mL conical tube of sterile Saline or 1xPBS solution for later use in maintaining a hydrated operation area.
1.5 Attach the laser to the stereotaxic device. CAUTION: Use laser safety goggles whenever the laser is on following this point in the procedure. Wearing laser safety goggles, measure the power of the laser (mW).
1.5.1 Adjust the current on the laser console, following the laser manufacturing instructions, until the laser power output reads 10mW, and set the current as a pre-set on the home screen console. Then adjust the current again to establish another pre-set where the laser output power reads 40mW. Now turn the laser off until further use. Note: The 10 mW will be used to orient the laser prior to use.
1.6 Prepare a clean cage for mice after surgery. Then, place the mouse into the induction chamber with an isoflurane concentration of 4% to anesthetize it until spontaneous movement stops and its breathing comes to a slow, steady rate.
1.7 Once asleep, weigh the mouse to determine how much rose bengal dye to inject. Then, transfer and secure the mouse to the stereotaxic device with a maintenance isoflurane concentration of 1.5%. Note: Increasing isoflurane concentrations can dilate vessels and prevent sufficient occlusion or consistently-sized strokes between mice.
1.8 Apply vet eye ointment to both eyes. Note: watch breathing and temperature throughout surgical procedure and pinch toes occasionally to ensure the mouse cannot feel anything.
2. Photothrombotic Stroke Model
2.1 Shave the fur off of the mouse’s head and aseptically prepare the area by using an alcohol wipe followed by a beta iodine wipe. Repeat three times. Note: If necessary, use an extra alcohol wipe at the end to clean off all beta iodine as it causes skin irritation which leads the mice to itch their skull after surgery.
2.2 Using small operation scissors, make a lateral incision between the ears to the eyes, about 1–2cm. Note: It is easiest to do this by pulling the skin upward with forceps to create a tent, cutting once downwards, then inserting the scissors into the opening and creating a continuous midline incision.
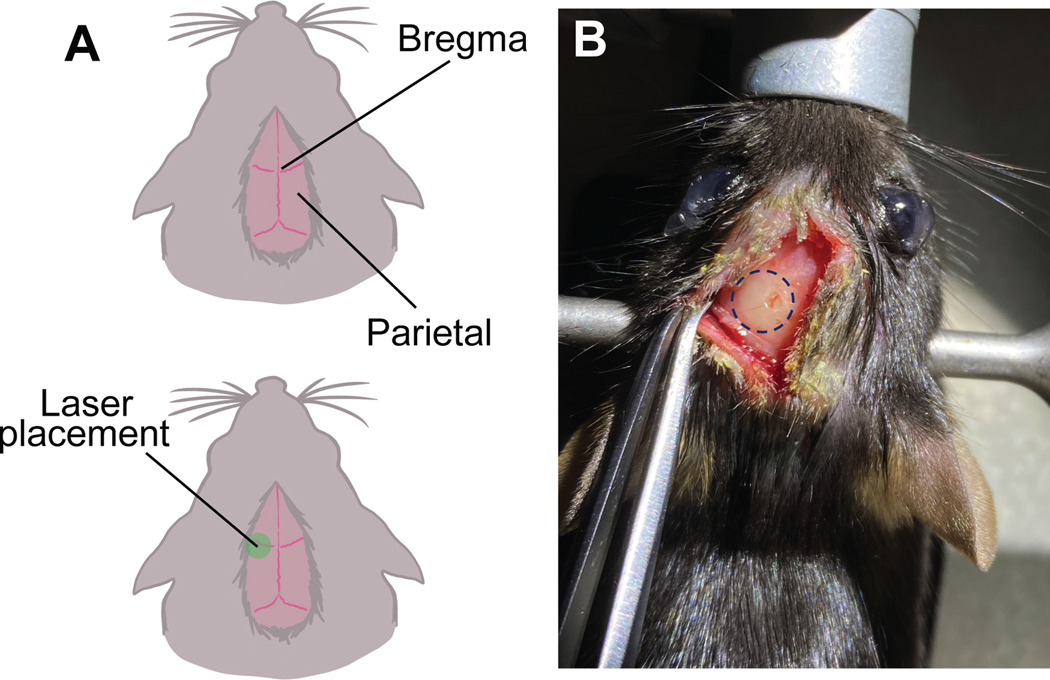
2.3 Separate the skin and press lightly on the parietal bones with the forceps to locate the bregma. Mark the bregma with a dot using the surgical marker. Note: Bone fragment movement highlights the frontal border of the parietal bones. The bregma is the center point at which the frontal and parietal bone fragments meet (Figure 1a).
Figure 1. Schematic representation of bregma and localization of stroke.
a. The parietal lobes meet at the top to form the bregma. The laser placement is centered 2.0mm left of the bregma. b. Visual conformation of stroke and burr hole can be seen 5 days after stroke.
2.4. Wearing laser safety goggles, move the laser over the skull of the mouse, fixing the stereotaxic device at a 90° angle. Select the 10 mW pre-set and turn on the laser.
2.4.1 Adjust the laser’s x- and y-axis until it is directly over the bregma. Reset the x and y on the digital display console, and then move the laser 1.8 mm left of the bregma. Now bring the laser as close to the skull as possible without letting it touch the skull.
2.4.2 Move the laser to 2.2 mm left of the bregma to ensure that it can move without any obstructions. Turn off the laser.
2.5 Inject the appropriate amount of rose bengal dye intraperitoneally (IP) using a disposable 29G needle. Immediately start a timer for 7 min to allow dye to circulate systemically. Select the pre-set for 40mW without turning the laser on. Note: Once comfortable with the procedure, the dye can be injected before aseptic preparation of the mouse and finished before the 7 min are over to save time.
2.6 Turn on the laser (40mW) when the 7 min timer goes off, while wearing laser safety goggles, and set a 10 min timer.
2.6.1 After 10 min, move the laser to 2.2mm left of the bregma without turning it off, and set another 10 min timer.
2.6.2 Once the second 10 min timer has gone off, change the laser back to 10mW and move the laser to 2.0 mm left of the bregma. Mark this spot with the surgical marker. Then turn off the laser.
2.6.3 Lift the laser and unlock it from the 90° angle so it can be moved away from the mouse. At this point, apply the sterile Saline or PBS with cotton if the surgical area is dry.
2.7 Drill in small bursts at the 2.0 mm mark perpendicular to the surface of the skull. Note: Be careful to drill slowly to avoid drilling too deeply and hitting the brain. There will be a slight drop/change in resistance once the drill has gone through the skull, and drilling may cause bleeding.
2.7.1 Use cotton to absorb any excess blood. Note: It is typical to see some blood from nicked arteries in the skull after drilling – excessive blood means the drill bit hit the brain. If this happens, record the mouse identifier and move on.
2.8 Place a small wipe next to the mouse. Using the forceps, pull the skin close together to prepare for gluing. Note: Suturing can be done here instead. Suturing typically requires only 3 sutures.
2.8.1 Using the forceps, grab the two sides of the skin and pull them together and upward. Dab off any large drops of surgical glue at the end of the tip on the wipe before applying to the mouse.
2.8.2 Apply the surgical glue sparingly and hold skin together with forceps for 10 seconds. Continue this method until there are no visible openings. Note: The glue comes out quickly – do not squeeze the bottle. Avoid gluing the skin to the skull or getting glue in the eye.
2.9 After gluing, or suturing, place the mouse in the new cage to recover from anesthesia. Note: It takes 5–10 min for the animal to recover, and it helps to rest half of the cage on a heating pad.
3. Sham Operation
Perform all procedures identically to the operation described above except inject 1xPBS or Saline instead of rose bengal dye.
4. Injection of hydrogel or other biomaterials
Injection of biomaterials are performed at a user-defined time. We have performed injections between 5- and 7-days post-stroke. 2 – 6 μL of hydrogel are injected (user-defined).
4.1 Turn on heating pad in order to maintain the mouse body temperature during anesthesia (37°C).
4.1.2 Prepare autoclaved scissors, forceps, cotton, vet eye ointment, surgical glue or suture material, and sterile alcohol and beta iodine wipes. Prepare the bone drill with sterile drill bit heads and the 25 μL glass Hamilton syringes with metal 30G needles.
4.1.3 Turn on bead sterilization to 160°C to allow for sterilization of instruments between mice.
4.1.4 Attach the injection pumps to the stereotaxic device. Set flow rate to 1 μL/min and set volume to 4 μL.
4.1.5 Prepare a clean cage for the mice after surgery.
4.2 Place the mouse into the induction chamber with an isoflurane concentration of 4% to anesthetize it until spontaneous movement stops and its breathing comes to a slow, steady rate.
4.2.1 Once asleep, transfer and secure the mouse to the stereotaxic device with a maintenance isoflurane concentration of 1.5%. Apply vet eye ointment to both eyes.
4.2.3 Shave any fur that may have regrown of the mouse’s head, and aseptically prepare the area by using an alcohol wipe followed by a beta iodine wipe. Repeat three times. Note: If necessary, use an extra alcohol wipe at the end to clean off all the beta iodine as it causes skin irritation and leads the mice to itch their skull after surgery.
4.3 Using the small scissors and/or forceps, reopen the skin above the brain. Use cotton with sterile saline or PBS to clean any debris from the skull. Note: A white-yellow circle (the stroke, Figure 1b) and the burr hole from the drill should be visible. If the dead tissue is not visible, likely no stroke occurred. If the burr hole is not centered above the stroke, re-drill to center it.
4.4 Orient the injection pump over the mouse and lock at 90°. Clean the syringe with sterile saline or PBS solution before backloading material.
4.4.1 Once clean, disassemble the glass syringe so that there is no needle. Backload the syringe using a 25 μL positive displacement pipette with the 10 μL of hydrogel (or other biomaterial here with or without cells).
4.4.2 Using the syringe plunger, push the gel all the way to the front of the syringe. Then, reassemble the syringe and continue pushing until the gel visibly exudes from the needle.
4.5 Place the syringe onto the pump. Note: The back of the pump may need to be adjusted so the syringe fits. Do this by adjusting the flow rate to 30 uL/min, select WITHDRAWAL or INJECT depending on the direction it needs to move, and then press START. Hit STOP when the back of the pump is at the correct location.
4.5.1 Screw the back of the pump so the syringe is secure. If adjustments were previously made, make sure the flow rate is set back to 1 μL/min and is set to inject.
4.6. Move the pump in the x and y direction to orient it over the hole. Move the pump down in the z direction until the needle of the syringe touches the top of the hole.
4.7 Reset the z on the digital display console. Then, move the syringe down in the z direction 0.750 mm. Note: If the syringe bends or there is resistance, the skull either healed or was not completely drilled through and needs to be re-drilled.
4.8 Press START on the pump console to inject 4 – 6 μL of the hydrogel at a rate of 1 μL/min.
4.9 Set a 5 min timer when the pump stops injecting to allow the gel to begin crosslinking.
4.10 After 5 min, slowly pull up the syringe by turning the z nob. Watch to see if any material is accidently pulled or leaking from the hole. Unlock the pump and move it away from the mouse when the needle is far enough away from the skull.
4.11. Using forceps, surgical glue, or sutures, close the skin above the head. Place the mouse in the clean cage and observe its recovery. It should take about 5 – 10 min for the mouse to recover from anesthesia.
5. Perfusion
5.1 Anesthetize the mouse using isoflurane.
5.2 Fix the animal in the supine position and open the abdominal cavity with a median cut. Optional: Clamp the descending abdominal aorta to use less fixative and PBS.
5.3 Cut open the diaphragm and move up the sides of the rib cage to open the chest cavity. Insert a perfusion cannula into the left ventricle and cut an opening in the right atrium. Start to slowly perfuse with 10–20 mL of PBS or until the liquid runs semi-clear.
5.4 Once clear, switch to 4% PFA for another 10 – 20 mL. Note: Unpinning the arms allows them to contract as the PFA infuses. Once they stop moving, wait another 30 seconds.
5.5 Decapitate the animal and pull back the skin to expose the skull. Using blunt, thin forceps, carefully remove the pieces of the skull to expose the brain. Note: Special care needs to be taken when removing the skull above the stroke site. Small surgical scissors can be used here to help cut away the skull. The biggest challenge is gel getting stuck to the skull and coming out of the brain as the skull is removed.
5.6 Once the brain is detached, place in 10 – 15 mL of 4% PFA overnight (no longer than 24 hrs).
5.7 Move the brains from PFA to 30% sucrose the following day. Note: The brain is ready for cryosection once it sinks to the bottom (about 2 – 3 days). It can also be left for storage at this point.
6. Cryosectioning and staining
6.1 Take the brain out of sucrose and place on a glass slide. Cut a small portion of the back of the brain off with a razor blade to create a flat portion to be mounted onto a chuck.
6.1.1 Place a dollop of Optimal Cutting Temperature Compound (OCT) onto the chuck, and then place the brain, flat side down, onto the chuck in the OCT. Place this specimen on dry ice until frozen or in the cryostat on the freezing block. Note: OCT can be added to fully encompass the brain to help prevent the material from separating from the brain during sectioning.
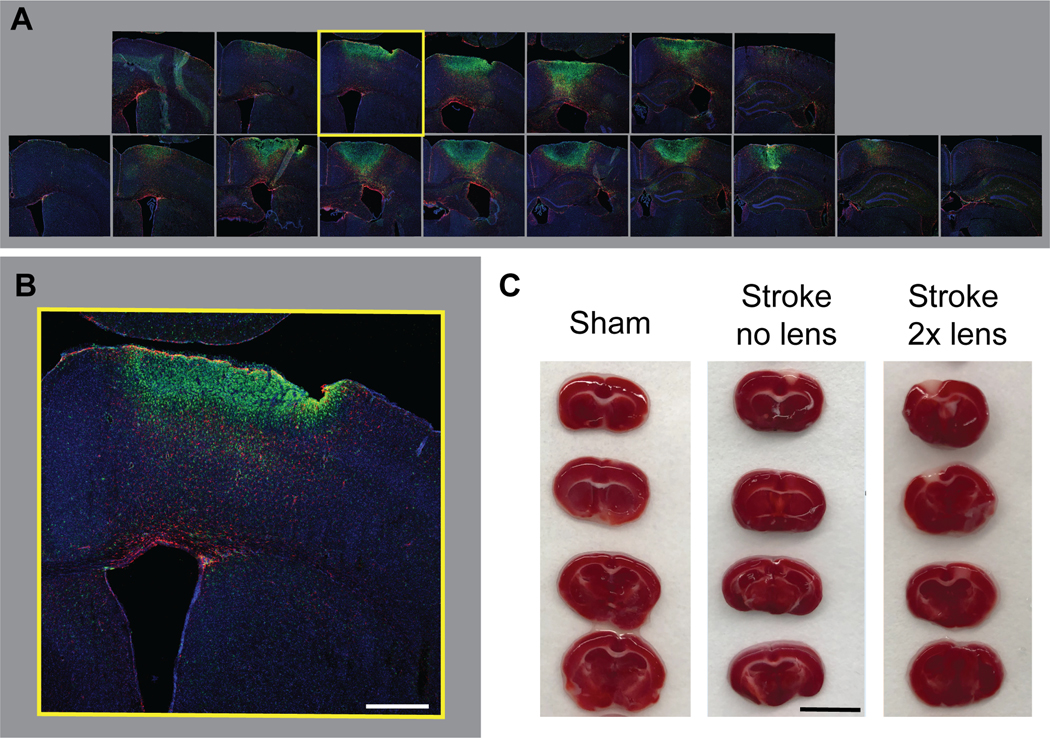
6.2 Cut the brains serially on a cryostat at −20°C at 30 μm thick sections across 10 gelatin coated slides. Note: Take care when sectioning so as not to lose the hydrogel. If this part is difficult, place some OCT on the top of the brain over the gel (Figure 2). Store at −80°C until further use.
Figure 2. Injection and visualization of biomaterial.
a. Visual of gel injection during surgery. b. The gel can be visualized by eye when removing the skull. c. Once frozen and mounted, the gel will be visible in the brain while sectioning.
6.3 Take slides out of the −80°C and place on a staining tray to allow them to dry. Note: Slides that have not be frozen but just sectioned can also be stained immediately.
6.4 Using a hydrophobic pen, draw a circle around the brain slices on the slide. Once dry, rehydrate the brain slices with 1x filtered PBS and place on a belly dancer at room temperature for 15 min. Note: This takes about 1 mL of buffer per slide with 9 – 12 brain sections.
6.5 Wash the slides with 1x filtered PBS for 5 min at room temperature, repeat three times.
6.6 Block slides for 30 min to an hour with 10% donkey serum in 0.01% PBS-Triton X at room temperature on the belly dancer. Note: This takes about 200 μL per slide.
6.7 Incubate the primary antibody in 10% donkey serum with 0.01% PBS-Triton X overnight at 4°C on a shaker or belly dancer. Test primaries first to determine correct concentrations. Note: Here we used GFAP (1:400), Iba1 (1:250), Glut1 (1:500) (Figure 3).
Figure 3. Immunohistochemistry stains.
a. Schematic of cryosectioning brains 30um thick. Several sections in the middle are chosen for image analysis. b. Stroke only, immunohistochemistry (IHC) stains 15 days after stroke. Astrocytes (GFAP+) cells are shown in red, microglia (Iba1+) are green, and vessels (GLUT1+) are white. Stroke + Hydrogel condition, IHC 10d post injection, 15 days after stroke. Astrocytes (GFAP+) cells are shown in red, microglia (Iba1+) are green, hydrogel material is white.
6.8 Wash slides with 1x filtered PBS the following day for 5 min at room temperature, repeat three times.
6.9 Incubate with secondary antibody in 10% donkey serum in 0.01% PBS-Triton X and DAPI for 2 hrs on the belly dancer at room temperature. Note: 1:1000 was used for all secondaries and DAPI.
6.10 Wash the slides for 5 min with 1x filtered PBS.
6.11 Allow sections to dry at room temperature in the dark. Note: This can take 20 min to 4 hrs; placing the slides in a desiccator can speed up the process.
6.12 Dehydrate slides in ascending concentrations of alcohol (1 min per solution) of 50%, 70%, 95%, 100%, and 100%.
6.13 De-fat in first bath of xylene for 1 min, then second bath of xylene for 5 min.
6.14 Quickly take the slides out one-by-one and add mounting medium while brain sections are wet with xylene. Quickly add a glass coverslip on top. Note: Use a cover slip that is correct length and thickness needed for the slide and microscope used for imaging, respectively.
6.15 Get rid of any bubbles under the coverslip by pressing gently with a pipette tip in an outward motion from the center of the slide. Allow DPX to dry for 4 hrs to overnight in the dark at room temperature.
6.16 Use a razor blade to scrape off any dried mounting medium that spilled onto the slides. Wipe down slides with lens cleaner. Slides can now be imaged with fluorescence microscopy. Note: It is best to store slide in the dark or at 4°C until imaging is finished.
DISCUSSION:
Here we demonstrate an easily reproducible, minimally invasive, permanent stroke model and describe how to inject a biomaterial into the infarct five days after stroke. The use of the photothrombotic dye rose bengal and a 520 nm collimated laser connected to the stereotaxic device gives us the ability to position the stroke at the motor cortex of the mouse with enhanced precision. Five days after stroke, the location of the infarct is visible by eye at the center of irradiation, 2.0 mm medio-lateral to the bregma. Hydrogel is then accurately injected into the stroke core using syringe pumps. Mortality during the surgery and after stroke in this model is virtually absent, with only a handful of mice dying after surgery due to anesthesiological complications. Despite the advantages of using PT stroke for biomaterial applications, there are several biological limitations and technical difficulties that should be addressed.
In terms of biological limitations, PT is a not a stroke model that offers the option for cerebral reperfusion, a process that can occur spontaneously in human strokes or in response to endovascular clot retrieval/lysis. Additionally, unlike the MCAo model that mimics the etiology of most human strokes by occluding a major cerebral vessel, PT impairs blood flow through many smaller vessels. A technical difficulty of injecting hydrogel biomaterials is their tendency to adhere to the syringe during surgery or to the skull during extractions. Designing a hydrogel that remains as a liquid during injection and then gels in situ can address the first issue; however, preventing the gel from sticking to the dura mater is often unavoidable since the injection site and stroke core reside at the surface of the brain. This can make it difficult to extract the brain tissue while keeping the biomaterial intact. As such, special care must be taken when removing pieces of skull from the brain to ensure that the dura mater does not lift with the skull and unintentionally uproot the gel. Cryosectioning brains also requires technical skill because both the handling process and blade transection can easily detach the gel from its surrounding tissue, thereby limiting data collection regarding cell infiltration. Detachment is exacerbated when the material is not fully integrated with the tissue. Preparing the sample with OCT creates a reinforcement shell that helps to minimize material separation during cutting.
Before injecting biomaterials, we recommend optimizing the desired stroke size depending upon rodent strain and laboratory setup. Smaller or larger infarcts can be made by adjusting three main parameters: laser output intensity, beam magnification, and irradiation time. Other factors that have also been shown to affect stroke size include concentration of isoflurane18 and amount of time the dye is allowed to circulate prior to irradiation. Regarding laser output intensity (power/area or mW/cm2), we found that a laser power of 10 mW was sufficient to produce a small infarct with horizontal diameter slightly smaller to that of the laser beam. Higher laser power yielded marginally wider but substantially deeper infarcts (data not shown) due to the laser beam having a gaussian irradiation profile. By increasing the intensity, the center point of maximum irradiance can penetrate deeper past the skull, as well as cause the slight increase in the spot size penetration of the laser. Other stroke models have thinned the skull by sanding it prior to applying light or laser in order to increase light penetration and subsequent reproducibility of clotting while maintaining a lower laser intensity19; however, this can cause hemorrhaging in the skull and takes a fair amount of time during surgery and skill to master without accidently damaging the brain in the process. Similar to intensity, increasing the laser beam diameter also increased infarct size; however, laser intensity and beam diameter do not scale linearly with respect to one another but follow the equation, , where r is the radius of the beam. In terms of irradiation time, we found that a minimum of 10 min was needed to yield reproducible strokes. To increase the horizontal diameter of the infarct while keeping the vertical depth constant, we applied the laser to adjacent locations for 10 min each, sequentially. To address the influence of isoflurane concentration and circulation time of rose bengal, we found that 1.5% isoflurane concentration and 7 min of circulation after IP injection resulted in consistent formation of strokes. These five parameters were optimized for our particular use. We required a large enough stroke to cause behavioral changes yet not interfere with the subventricular zone, where we aimed to study the effect of our material on neural progenitor cells. In addition to stroke size, we also needed to determine the optimal gel volume to inject. At minimum, we needed enough gel to entirely fill the stroke cavity because we found that gaps between gel and surrounding tissue led to similar results as our control groups, while contact between gel and tissue resulted in fewer reactive astrocytes and increased cellular infiltration. We arrived at a lower limit of 4–6 μL of gel, which produced no adverse effects after injected (data not shown).
Regarding assessing stroke outcome, there are several options beyond IHC staining, such as magnetic resonance imaging, behavioral tests, histological analysis, and TTC (2,3,5-triphenyltetrazolium chloride) staining. It is highly recommended to use TTC for preliminary optimization of stroke due to its ease of use and immediate results. Previous behavioral testing in our lab has looked at the Cylinder test/spontaneous forelimb task, the grid-walking test, and the pasta test11, 20. It should be noted that rotarod testing did not show significant decreases in behavioral outcomes after the PT stroke method was implemented (data not shown). Thus, behavioral tests should assess very complex tasks that require significant cortical input.
Here we demonstrated the technique of injecting hydrogels after stroke without the delivery of growth factors or cells. However, hydrogels have also been utilized for cell transplantation, as well as drug and growth factor delivery, and may prove to be a valuable resource for both studying biology after stroke and investigating new methods of therapy. In the context of stroke, our lab has injected non-porous hyaluronic acid hydrogels encapsulating induced pluripotent stem cell-derived neural progenitor cells13, 17 at concentrations ranging from 25,000 cells/μL to 50,000 cells/μL of gel. The use of a hydrogel resulted in an increase in cell viability and differentiation. Other labs have also reported cell differentiation and increased tissue regeneration in response to hydrogels used to transplant stem cells after stroke 21–23. Additionally, we have injected hydrogels with growth factors after stroke that lead to tissue regeneration and behavioral improvement after stroke12, 13. In terms of material design, injectability is a valuable feature that serves to minimize invasiveness; therefore, hydrogels should be formulated as either sol-gels (liquid-to-solid), shear thinning (G’ > G” under static conditions; G” > G’ during flow), or granular gels comprised of building blocks whose diameter is less than the inner diameter of a needle11, 24, 25. Optimization should then be performed to test diffusion of drugs or growth factors, cell viability under a range of cell concentrations, gel conditions, and injection parameters, such as speed, needle gauge, injection depth, and day of injection relative to when stroke was induced. For example, day of material injection has ranged from one day post-stroke to as long as three weeks post-stroke26, 27. Here, we report five days but have previously injected seven days after stroke when transplanting cells. These days were chosen based on the timeline of the inflammatory response as well as the peak activity of angiogenesis and neurogenesis seen one week after stroke5, 28–30. Volume characterization should also be performed at every injection time point, as the stroke volume will decrease over time due to atrophy. Given that these parameters are optimized prior to injection, the methods here are sufficient to guide users to inject biomaterials with the addition of cells and/or growth factors and drugs.
| Name | Company | Catalog Number | Comments |
|---|---|---|---|
| 10% Normal Goat Serum | VWR | 100504–028 | For blocking buffer |
| 2-ply alcohol pre pad, Sterile, Medium | Medline | MDS090735 | |
| 25uL Hamilton Syringe 702RN, no needle | Fishcer Scientific | 14824663 | Syringes used to inject biomaterials |
| 25uL Positive displacement pipette | Gilson | M-25 | |
| 2x Beam Expander, 400–650nm | Thorlabs | GBE02-A | Laser beam expander |
| Adjustable Stage Platform | Kopf Instruments | 901 | |
| Anti-Glucose Transporter GLUT1 antibody, rabbit | Abcam | ab113435 | |
| Anti-Iba1 Antibody, goat | abcam | ab5076 | |
| BD Vacutainer Safety-Lok Blood Collection Sets. 25G, 12” | Medsupply | 367294 | For perfusions |
| BKF12- Matte Black Aluminum Foil | Thorlabs | BKF12 | To cover anything that is reflective when using laser. |
| Bone Iris Mini Scissors - 3-1/2” | Sklar surgical instruments | 64-2035 | |
| C57BL/6 Mice | Jackson Laboratory | 000664 | 8-12 weeks of age |
| Cage Assembly Rob | Thorlabs | ER3-P4 | 3” Long, diameter 6mm, 4 pack - for attaching laser to sterotax |
| Carbon Steel Burrs −0.5mm Diameter | Fine Science Tools | 19007-05 | For creating burr hole |
| Chromium(III) potassium sulfate dodecahydrate | VWR | EM1.01036.0250 | |
| Compact Controller for pigtailed lasers | Thorlabs | CLD1010LP | |
| Cotton Swabs | VWR | 89031-288 | |
| CP 25 pipette tips | Gilson | F148012 | |
| Donkey anti-goat IgG H&L (488) | abcam | ab150129 | |
| Donkey Anti-rabbit IgG H&L (647) | abcam | ab150075 | |
| Donkey Anti-rat IgG H&L (555) | abcam | ab150154 | |
| EMS DPX Mountant | Elecron Microscopy Sciences | 13512 | Mounting solution for slides |
| EMS Gelatin Powder Type A 300 Bloom | Electron Microscopy Sciences | 16564 | For gelatin coating slides |
| EMS Paraformaldehyde, Granular | VWR | 100504-162 | For making 45 PFA |
| ESD Worstation kit | Elmstat | WSKK5324SB | Need for setting up the laser |
| Fiber Bench Wall Plate, unthreaded | Thorlabs | HCA3 | Need for connecting laser to Kopf shaft |
| FiberPort | Thorlabs | PAF-X-5-A | FC/APC& APC, f=4.6mm, 350–700nm, diameter 0.75mm |
| Fine Scissors - straight/sharp-blunt/10cm | Fine Science Tools | 14028-10 | |
| GFAP Antibody, rat | Thermo Fisher Scientific | 13-0300 | |
| Heating Plate | Kopf Instruments | HP-4M | |
| ImmEdge Hydrophobic Barrier Pen | Vector Laboratories | H-4000 | For staining slides |
| IMPAC 6-Integrated Multi Patient Anesthesia Center | VetEquip | 901808 | |
| Iodine Prep Pads | Medx Supple | MED MDS093917H | |
| Jewlers Forceps #5 | GFS chemicals | 46085 | |
| Laser Safety Glasses | Thorlabs | LG10B | Amber Lenses, 35% Visible light (googles versions available too) |
| M27-1084 Powerful LED Dual Goose-neck | United Scope | LED-11C | |
| Medical USP Grade Oxygen | Airgas | OX USP250 | |
| Miltex Adson Dressing Forceps, Disecting-grade | Intefra Miltex | V96-118 | |
| Mini Cord/Cordless Small Animal Trimmer | Harvard aparatus | 72-6110 | |
| Mini-pump variable flow | Thomas Scientific | 70730-064 | Pump for perfusions |
| Mouse Brain Matrices, Coronal Slices, 1mm | Kent Scientific | RBMA-200c | For TTC slices |
| Mouse Gas Anethesia Head Holder | Kopf Instruments | 923-B | |
| Nanojet Control Box | Chemyx | 10050 | |
| Nanojet pump header | Chemxy | 10051 | Attach to stereotaxic device for injecting biomaterial |
| Needle RN 30G PT STY 3, 0.5 inch | Fishcer Scientific | NC9459562 | |
| Non-rupture ear bars 60º | Kopf Instruments | 922 | |
| PBS buffer pH 7.4 | VWR | 97062 338 | |
| Pigtaled laser 520 nm, 100mW, 5G Pin | Thorlabs | LP520-MF100 | |
| Positive charge glass slides | Hareta | AHS90-WH | |
| Power engergy meter | Thorlabs | PM100D | Used to measure your mW laser output |
| Puralube Vet Ointment | Dr. Foster Smith | 9N-76855 | |
| Rectal Probe Mouse | kopf Instruments | Ret-3-ISO | |
| Rose Bengal Dye 95% | Sigma-Aldrich | 330000-5G | |
| Shaft Modified 8–32 threaded hole 1/2” depth | Kopf Instruments | 1770-02 | For connecting laser to sterotaxic device |
| Slim photodiode power sensor | Thorlabs | S130VC | Used with power energy meter |
| SM1-Threaed 30 mm Cage Plate 0.35" thick 2 Retaining | Thorlabs | CP02 | For connecting laser expander |
| Sol-M U-100 Insuline syringe with 1/2 unit markings 0.5 mL | VWR | 10002-726 | To inject rose bengal |
| StainTray Slide Staining System | Simport Scientific | M920-2 | For staining slides |
| Sterotaxic device | Kopf Instruments | 940 | Small Animal Stereotaxic Instrument |
| Student Adson Forceps −1×2 teeth | Fine Science Tools | 91127-12 | |
| Student Fine Forceps - straight/broad Shanks | Fine Science Tools | 91113-10 | |
| Temperature Controller | Kopf Instruments | TCAT-2LV | |
| Tissue-Tek OCT compound | VWR | 25608-930 | |
| Triton X-100 | VWR | 97063-864 | |
| Upper Bracket Clamp | Kopf Instruments | 1770-c | For connecting laser to sterotaxic device |
| Vetbond Tissue Adhessive 3mL | Santa Cruz Biotechnology | sc-361931 | |
| Vogue Professional My Manicurist | Bargin Source | 6400 | For Burrs |
| VWR Bead Sterilizers | VWR | 75999-328 | |
| Tissue Tek OCT compound | Sakura | 4583 | For tissue embeding |
ACKNOWLEDGMENTS:
We like to acknowledge the National Institutes of Health and the National Institute of Neurological Disorders and Stroke for funding (R01NS079691).
Footnotes
DISCLOSURES:
Enter text here.
REFERENCES:
- 1.National Center for Health Statistics (US). Health, United States, 2015: With Special Feature on Racial and Ethnic Health Disparities. Hyattsville, MD: U.S. Department of Health and Human Services. No. 2016–123, (2016) [PubMed] [Google Scholar]
- 2.Benjamin EJ, et al. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation. 135 (10), e229–e445, 10.1161/CIR.0000000000000485 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Am Heart Association Heart. Disease and Stroke Statistics—2007. Circulation. 115 (5), e69–e171, 10.1161/CIRCULATIONAHA.106.179918 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Ovbiagele B, et al. Forecasting the future of stroke in the united states: A policy statement from the American heart association and American stroke association. Stroke. 44 (8), 2361–2375, 10.1161/STR.0b013e31829734f2 (2013). [DOI] [PubMed] [Google Scholar]
- 5.Carmichael ST Themes and strategies for studying the biology of stroke recovery in the poststroke epoch. Stroke. 39 (4), 1380–1388, 10.1161/STROKEAHA.107.499962 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carmichael S Thomas. Rodent models of focal stroke: size, mechanism, and purpose. NeuroRx: the journal of the American Society for Experimental NeuroTherapeutics, 2(3), 396–409, 10.1602/neurorx.2.3.396 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qin L, Jing D, Parauda S, Carmel J, Ratan RR, Lee FS, Cho S. An adaptive role for BDNF Val66Met polymorphism in motor recovery in chronic stroke. Journal of Neuroscience, 34(7), 2493–502, 10.1523/JNEUROSCI.4140-13.2014 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fluri F, Schuhmann MK, and Kleinshnitz C, Animal models of ischemic stroke and their application in clinical research. Drug Design, Development and Therapy. 9, 3445–3454, 10.2147/DDDT.S56071 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tuladhar A, Payne SL, and Scoichet MS, Harnessing the potential of biomaterials for brain repair after stroke. Frontiers. 5, id. 14, 10.3389/fmats.2018.00014 (2018). [DOI] [Google Scholar]
- 10.Nih LR, Sideris E Carmicahel ST, and Segura T Injection of microporous annealing particle (MAP) hydrogels in the stoke cavity reduces gliosis and inflammation and promotes NPC migration to the lesion. Advance Materials. 29 (32), 10.1002/adma.201606471 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nih LR, Gojgini S, Carmichael ST, and Segura T Duel-function injectable angiogenic biomaterial for the repair of brain tissue following stroke. Nature Materials. 17, 642–651, 10.1038/s41563-018-0083-8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cook DJ, et al. Hydrogel-delivered brain-derived neurotrophic factor promotes tissue repair and recovery after stroke. Journal of Cerebral Blood Flow & Metabolism. 37, 1030–1045, 10.1177/0271678X16649964, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lam J, Lowry WE, Carmichael ST, Segura T Delivery of iPS-NPCs to the stroke cavity within a hyaluronic acid matrix promotes the differentiation of transplanted cells. Advance Functional Materials. 24, 7053–7062, 10.1002/adfm.201401483 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nih, Lina R et al. Engineered HA hydrogel for stem cell transplantation in the brain: Biocompatibility data using a design of experiment approach. Data in Brief. 10, 202–209, 10.1016/j.dib.2016.11.069 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carmichael ST, The 3 Rs of Stroke Biology: Radial, Relayed, and Regenerative. Neurotherapeutics. 13, 348–59, 10.1007/s13311-015-0408-0 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caicco MJ, Cooke MJ, Wang Y, Tuladhar A, Morshead CM, Shoichet MS A hydrogel composite system for sustained epi-cortical delivery of Cyclosporin A to the brain for treatment of stroke. Journal of Controled Release. 166(3), 197–202, 10.1016/j.jconrel.2013.01.002 (2013). [DOI] [PubMed] [Google Scholar]
- 17.Moshayedi P, et al. Systematic optimization of an engineered hydrogel allows for selective control of human neural stem cell survival and differentiation after transplantation in the stroke brain. Biomaterials. 105, 145–55, 10.1016/j.biomaterials.2016.07.028 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu H, et al. Hemodynamic effects of intraoperative anesthetics administration in photothrombotic stroke model: a study using laser speckle imaging. BMC Neuroscience. 18 (10), 10.1186/s12868-016-0327-y (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wiersma AM and Winship IR Induction of Photothrombotic Stroke in the Sensorimotor Cortex of Rats and Preparation of Tissue for Analysis of Stroke Volume and Topographical Cortical Localization of Ischemic Infarct. Bio-protocol. 8(10), 10.21769/BioProtoc.2861 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang H, et al. Region-specific and activity-dependent regulation of SVZ neurogenesis and recovery after stroke. PNAS, 116 (27), 13621–13630, 10.1073/pnas.1811825116 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Payne SL, Anandakumaran PN, Varga BV, Morshead CM, Nagy A, and Shoichet MS In vitro maturation of human iPSC-derived neuroepithelial cells influences transplant survival in the stroke-injured rat brain. Tissue Engineering Part A. 24, 351–360. 10.1089/ten.tea.2016.0515 (2018). [DOI] [PubMed] [Google Scholar]
- 22.Lee IH, et al. Delayed epidural transplantation of human induced pluripotent stem cell-derived neural progenitors enhances functional recovery after stroke. Science Reports. 7, 1943, 10.1038/s41598-017-02137-w (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Somaa FA, et al. Peptide-based scaffolds support human cortical progenitor graft integration to reduce atrophy and promote functional repair in a model of stroke. Cell Reports. 20, 1964–1977, 10.1016/j.celrep.2017.07.069 (2017). [DOI] [PubMed] [Google Scholar]
- 24.Griffin DR, Weaver WM, Scumpia P, Di Carlo D, and Segura T Accelerated wound healing by injectable microporous gel scaffolds assembled from annealed building blocks. Nature Materials. 14 (7), 737–744, 10.1038/nmat4294 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sideris E Yu Aaron, Chen J Carmichael ST, and Segura T Hyaluronic acid particle hydrogels decrease cerebral atrophy and promote pro-reparative astrocyte/axonal infiltration in the core after ischemic stroke. bioRxiv. 10.1101/768291 (2019). [DOI] [Google Scholar]
- 26.Yu, et al. Combinated Transplantation of Neural Stem Cells and Collagen Type I Promote Functional Recovery After Cerebral Ischemia in Rats. The Anatomical Record. 293 (5), 10.1002/ar.20941 [DOI] [PubMed] [Google Scholar]
- 27.Jin K, et al. Transplantation of Human Neural Precursor Cells in Matrigel Scaffolding Improves Outcome from Focal Cerebral Ischemia after Delayed Postischemic Treatment in Rats. Journal of Cerebral Blood Flow & Metabolism. 30 (3), 534–544, 10.1038/jcbfm.2009.219 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gelderblom M, et al. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke. 40 (5), 1849–57, 10.1161/STROKEAHA.108.534503 (2009). [DOI] [PubMed] [Google Scholar]
- 29.Wei L, Erinjeri JP, Rovainen CM, and Woolsey TA Collateral growth and angiogenesis around cortical stroke. Stroke. 32 (9), 2179–84, 10.1161/hs0901.094282 (2001). [DOI] [PubMed] [Google Scholar]
- 30.Zhang R, et al. Activated Neural Stem Cells Contribute to Stroke-Induced Neurogenesis and Neuroblast Migration toward the Infarct Boundary in Adult Rats. Journal of Cerebral Blood Flow & Metabolism. 24 (4), 441–448, 10.1097/00004647-200404000-00009 (2004). (2010). [DOI] [PubMed] [Google Scholar]