Abstract
Social isolation gained discussion momentum due to the COVID-19 pandemic. Whereas many studies address the effects of long-term social isolation in post-weaning and adolescence and for periods ranging from 4 to 12 weeks, little is known about the repercussions of adult long-term social isolation in middle age. Thus, our aim was to investigate how long-term social isolation can influence metabolic, behavioural, and central nervous system-related areas in middle-aged mice. Adult male C57Bl/6 mice (4 months-old) were randomly divided into Social (2 cages, n = 5/cage) and Isolated (10 cages, n = 1/cage) housing groups, totalizing 30 weeks of social isolation, which ended concomitantly with the onset of middle age of mice. At the end of the trial, metabolic parameters, short-term memory, anxiety-like behaviour, and physical activity were assessed. Immunohistochemistry in the hippocampus (ΔFosB, BDNF, and 8OHDG) and hypothalamus (ΔFosB) was also performed. The Isolated group showed impaired memory along with a decrease in hippocampal ΔFosB at dentate gyrus and in BDNF at CA3. Food intake was also affected, but the direction depended on how it was measured in the Social group (individually or in the group) with no alteration in ΔFosB at the hypothalamus. Physical activity parameters increased with chronic isolation, but in the light cycle (inactive phase), with some evidence of anxiety-like behaviour. Future studies should better explore the timepoint at which the alterations found begin. In conclusion, long-term social isolation in adult mice contributes to alterations in feeding, physical activity pattern, and anxiety-like behaviour. Moreover, short-term memory deficit was associated with lower levels of hippocampal ΔFosB and BDNF in middle age.
Keywords: Social Isolation, Hippocampus, Memory, Behaviour, Physical activity
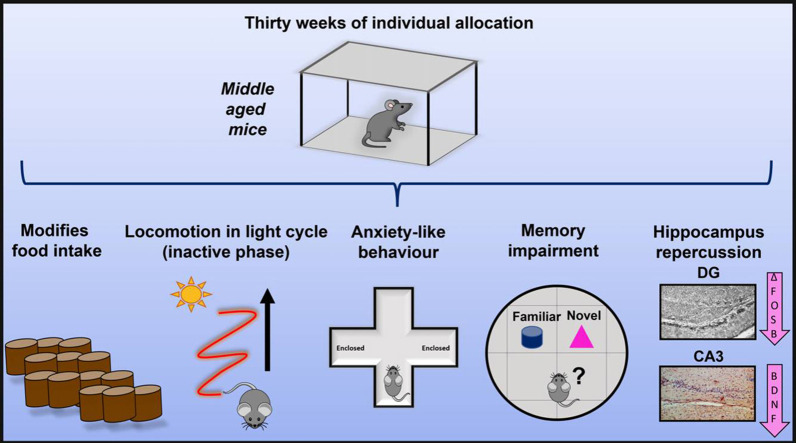
Graphical Abstract
Long-term social isolation results in memory impairment associated with decreased hippocampal ΔFoSB at dentate gyrus and in BDNF at CA3. Also, it results in increased physical activity in inactive phase (light cycle), anxiety-like behaviour and food intake modifications.
1. Introduction
Social isolation is related to an increased risk of morbidity and mortality [1], [2], [3]. This risk associated with social isolation and loneliness is comparable with well-established risk factors for health, like physical inactivity, obesity, and substance abuse [2]. Social isolation is also associated with cardiovascular disease in humans and animals by exacerbating atherogenesis [4]. Currently, due to the COVID-19 pandemic, social isolation has become a highlighted topic, providing an opportunity to recognize the importance of social connection for health [5].
With aging, social relationships may change for a variety of reasons including death or disability among social network members and personal factors including a decline in physical or cognitive abilities [6], contributing to less interaction. Furthermore, social isolation is associated with negative changes in several health-related behaviours [7], [8]. In older individuals, social isolation was related to a greater likelihood of being physically inactive and smoking [7], [8]. Besides, prolonged isolation or loneliness may in themselves act as stressors [7]. Indeed, it is robustly linked with cognitive and neural impairments [9].
Moreover, many experimental protocols rely on keeping mice separated from each other, as is the case for studies with calorie restriction, that are usually related to longevity [10], [11], [12], [13]. Yet, several animal researches investigate social isolation beginning at childhood or adolescence [14], [15], [16], a period of maturation and development, or address the effects of long-term social isolation in mice lasting 4–12 weeks [14], [16], [17], [18], [19], [20], and few with an interval over 16 weeks [15], [21], [22]. Yet, even 72 h of social isolation is sufficient to negatively impact the central nervous system, reducing hippocampal long-term potentiation in 4 and 6 month-old C57BL/6 and A/J male mice [23]. When isolation was kept for seven weeks, significant changes in behaviour were observed, such as increased locomotor activity, decreased habituation response, and impaired memory. However, these effects can be strain-dependent (C57BL/6 J and DBA/2 mice) on many occasions [24].
Less is known on how long-term social isolation can affect a mature and developed mouse, especially when they reach middle age, a period in which some age-related metabolic and behavioural alterations start to manifest [25], [26], [27]. We have shown that middle-aged mice displayed decreased locomotor activity and impaired glucose tolerance [28], while others have demonstrated a decline in cognitive function as a result of hyperglycemia [29]. Indeed, the decline in cognitive parameters during aging can be explained by an increase in oxidative stress [30] and a decrease in neurotrophins, like brain-derived neurotrophic factor (BDNF) [31]. Social isolation could exacerbate all these alterations.
As highlighted by Leser &Wagner (2015), unravelling the biological processes underlying the damaging effects of partial or perceived social isolation in adulthood on mental health should be highly beneficial, since social isolation was found by many studies to have a negative influence on human mental health and cognition in all ages [32], [33]. However, only a few studies have dealt with this issue so far in a mechanistic approach, mainly due to the lack of a proper animal model [32]. Tools like ΔFosB and BDNF are a great ally in studies that explores parameters of neuronal activation [34] and neuroplasticity [35], respectively. Furthermore, previous studies reported the role of both in memory [36], [37]. Thus, our aim was to investigate the effects of long-term (30 weeks) social isolation on behavioural, metabolic, and central aspects in middle-aged mice. Our hypothesis is that the lack of social interaction beginning in adult life can result in negative repercussions on central and behavioural parameters at middle age.
2. Materials and methods
2.1. Animal procedures
The experiments were approved by the Institutional Ethics Committee on Animal Use (CEUA n. 5541040218). Male C57Bl/6J mice were obtained from the Centre for Development of Animal Models for Medicine and Biology (CEDEME, Federal University of São Paulo). They were kept at the animal house of the Department of Bioscience in a temperature-controlled room (22 °C) with a 12:12-h light-dark cycle (7:00–19:00 h), with free access to water and food (AIN-93 M diet).
2.2. Social isolation
Adult four-month-old mice were randomly divided into either Social (2 cages, n = 5/cage) or Isolated (10 cages, n = 1/cage) housing groups. For both groups, cages were microisolators, coupled with a ventilated rack (Alesco), with individual input/exit air for each cage, not allowing the animals to smell each other and blocking most external noise. Mice were followed up from early 4 (4 M) to late 10 months of age (10 M), totalizing 30 weeks of social isolation. All cages of both groups were enriched with cotton and paper towels.
2.3. General parameters
To assess how long-term social isolation could affect some metabolic parameters, body weight gain, food intake, and fast blood glucose were investigated. Body weight gain was calculated by subtracting 10 M from 4 M body weight. Food intake was determined individually by subtracting the weight of the remaining food after 24 h from the weight of food given, with care taken to account for spillage. In the Social group, food intake was also measured while mice were in their collective cages. The total cage consumption was registered and divided by the number of mice, with the result presented as two observations (n = 2 cages). The individual measures were performed simultaneously to the 24 h physical activity analysis, described below.
For fast blood glucose, food was first removed overnight. In the morning, food was offered for 1 h ad libitum for both groups, and then removed again. After 6 h of fasting, blood was collected from the tip of the tail to assess blood glucose, using Accu-Check Advantage II. This protocol ensures that animals were submitted to the same fasting period [28].
2.4. Open field
Anxiety-like behaviour was evaluated in the open field test. The open field is a circular wooden apparatus (diameter = 50 cm, height = 40 cm) without a roof and with no top. A camera was positioned on the open field at a height of 230 cm. Mice were acclimated in the room for 1 h [38]. Then, each animal was placed in the centre of the circular field (5 min), under low light conditions (30 lux) [39], [40]. For behavioural analysis, the arena was divided into distal, middle, and central circular zones [40]. Distance travelled (m), mean speed (m/s), maximum speed (m/s), line crossing number, time(s) and entries in external, intermediate, and centre zones were evaluated. In addition, rearing and grooming behaviours were also analysed. The data were assessed using OpenFLD (developed by Stéfano Pupe Johann, Brazil) and ANY-maze® (Stoelting Co, IL, United States of America) software.
2.5. Novel object recognition test (NOR)
The effects of social deprivation on short-term memory were evaluated by the NOR test. The NOR test is a popular method for testing the neurobiology of non-spatial memory in rodents and is widely used for assessing hippocampal function in rodent models [41].
NOR was performed in the open field arena. The absence of a top in the open field allowed the animals to use distal cues to perform the task. Different objects were used, each with two copies. They were made with the same material, differing in colour, size, and shape. The objects were heavy enough to not be moved by the animals. The objects, as well as the open field, were cleaned with 5% alcohol before every mouse was tested, to avoid the presence of olfactory hints. No object had an etiological significance.
A pilot test was initially carried out to assess whether the animals showed any preference for the selected objects. Prior to the experiment day, for habituation, mice were placed individually in the empty apparatus for 5 min. The experiment day consisted of a training phase, in which each mouse was presented to two identical objects. After a retention interval of 1 h, they were submitted to the test phase, in which they were presented to a familiar object in the same location as the training phase and to a novel object. The objects used in the training and testing phases were different between mice. The sessions lasted 5 min each, under low light conditions (30 lux). The criteria for object contact were time spent sniffing and touching the object. The time that the mice spent to climb and stay on top of the object was discarded, as they were considered to be behaviour linked to escape and non-exploitation [42]. The analyses were performed using OpenFLD software (developed by Stéfano Pupe Johann, Brazil).
For analysis, the percentage of time spent in each object was used. For Familiar Object: (Time in Familiar Object x 100) / (Time in Familiar Object + Time in Novel Object); for Novel Object: (Time in Novel Object x 100) / (Time in Novel Object + Time in Familiar Object). We also calculated the object recognition index (IR) as IR = Novel object time / (Novel object time + familiar object time) x 100 and the object discrimination index (ID), using the formula ID = (novel object time – familiar object time) / (novel object time + familiar object time). A positive value indicates more time exploring the new object. A discrimination index of zero indicates equal time spent with the two objects and negative that the animal spent more time on the familiar object [43].
2.6. Elevated plus maze (EPM)
Anxiety-like behaviour was also evaluated by the EPM test. The elevated plus maze is a wooden cross-shaped apparatus that contains two closed arms (27.5 cm × 6.5 cm × 18 cm) as opposed to two open arms (27.5 cm × 6.5 cm), 46 cm away from the floor. The background of the apparatus was painted white to increase the contrast and favour mice recognition by the tracking programme. Each mouse was placed in the centre of the plus-maze facing an open arm and recorded for 5 min, under low light conditions (30 lux). After each record, the apparatus was cleaned with 5% alcohol. The assessments included the percentage of time spent, number of entrances, percentage of the number of entries in arms and travelled distance (m) in the apparatus. For risk assessment, latency to enter in the open arm, head-dips, and stretch-attend posture (defined by contraction and stretching of body to its original position without locomotion) were analysed. Time and frequency of head-dips were evaluated. Protected head dips were identified as behaviour when animals performed head dips into the open arm, but with the body inside the enclosed arm. Unprotected head dips corresponded to head dips into the open arm with body inside the open arm. Finally, head dips at 1/3 end of open arm consisted of head dips into the open arm with body in the final end border of the open arm.
The analysis was performed using ANY-maze (Stoelting Co, IL, United States of America) and PlusMZ (developed by Stéfano Pupe Johann, Brazil) software.
2.7. Physical activity parameters
To better explore mice physical activity parameters in a full cycle as well as separated by active/dark and inactive/light cycle, an actimeter system with infrared (IR) beam sensors was used. The measurement was performed in a cage different from their home cage. Before starting the IR actimeter, the animals were acclimatized for 2 h in the equipment and in the room [44]. IR actimeter was composed of a 2 dimensional (X and Y axes) square frame (25 × 25 cm), each frame containing 16 × 16 infrared beams separated 1.3 cm from each other (Panlab-Harvard Apparatus, Barcelona, Spain). Spontaneous physical activity (SPA) was recorded individually at the end of 10 months, for consecutive 24 h and it was determined using the ActiTrack software v2.7 (Panlab-Harvard Apparatus, Barcelona, Spain). The software allowed the determination of SPA (sum of stereotypes, defined as the number of samples where the position of the subject is different from its position during the previous sample and equal to its position during the second sample back in time, and locomotion, the number of samples where the position of the subject is different from its position during the previous sample and different from the position of the second sample back in time), average speed (AS) and percentage of time at resting (RT).
2.8. Immunohistochemistry
The central repercussions of long-term social isolation were investigated evaluating the expression of the neuronal activity marker, ΔFosB. It was also of our interest to investigate whether the lack of social interaction could affect oxidative stress, via expression of 8-hydroxy-2'-deoxyguanosine (8-OHdG), and the expression of the brain-derived neurotrophic factor (BDNF), given its importance for neuroplasticity. At 10 M, mice were anesthetized with a mixture of ketamine (75 mg / kg), xylazine (10 mg / kg), fentanyl (0.5 mg / kg) and acepromazine (1 mg / kg) administered intraperitoneally. After ensuring unresponsiveness by the toe pinch-response method, the mice were perfused. Their brain was removed, postfixed, and cryoprotected. Coronal sections 30 µm thick were obtained in cryostat (CM1850, Leica) and kept in 6-well plates containing antifreeze solution (18.5% sucrose and 37.5% ethylene glycol in 0.05 M PBS, pH 7.4) at −20 ºC. Immunoperoxidase reactions were performed for ΔFosB (Anti FosB, Sigma-Aldrich, 1:1000, code AV32519/ secondary: Goat Anti-Rabbit IgG (H+L), Vector Laboratories, 1: 1000, code BA-1000), BDNF (Anti BDNF, Abcam, 1:75, code ab108319/secondary: Anti-rabbit IgG peroxidase produced in goat, Sigma-Aldrich, 1:600, code A0545) and 8OHdG (Anti DNA/RNA damage, Abcam, 1:325, code ab62623 [15A3]/ secondary: Goat anti-mouse IgG (H+L), Vector Laboratories, 1:750, code BA9200) analysis and revealed using a solution containing 3,3'diaminobenzidine tetrahydrochloride (DAB 0.05%, Sigma), nickel (only for ΔFosB) and 0.01% H2O2 (Sigma) for approximately 10 min. The reaction was blocked by transferring the sections to a location containing distilled water. The CA1, CA2, CA3, dentate gyrus (DG), lateral hypothalamus (LHA) and dorsomedial hypothalamus (DMH) were photographed (bregma interval considered: from −1.58 mm/ interaural 2.22 mm to bregma 1.94 mm/ interaural 1.86 mm) with a conventional light microscope (Axio Observer D1, Zeiss), with the assistance of a stereotactic atlas of mouse brain [45]. The images were subjected to a blind semi-quantitative analysis of the immunostained cells, performed using Image ProPlus 6.0 software.
2.9. Statistical analysis
Results are shown as a mean + confidence interval of 95% (CI 95%). Statistical analyses were performed using the software SPSS v.22. Graphical illustration and outlier detection was performed using GraphPad Prism, v.8. Outliers detected by Grubbs' test, also called the ESD method (extreme studentized deviate) were removed. Shapiro-Wilk test was adopted to assess the data normality.
Unpaired t-student test, Mann-Whitney (for non-parametric data), two-way ANOVA, and Generalized Estimation Equations (GEE) were used. For t-student, T value and for Mann-Whitney, U value, were presented. For ANOVA, F value, degrees of freedom, and the p-value were presented, and when the effect of interaction was observed, the Tukey post hoc test was employed. GEE was followed by Bonferroni post hoc test when an interaction between the factors was observed. To determine the best distribution model, the independence model criterion (QIC) was used as a reference, and based on that, Gamma Log distribution was chosen. For GEE, the values of Wald's chi-square, degrees of freedom, and the p-value of the model were attributed. In addition, for some parameters, the effect size (Cohen's d for equal sample or Hedges' g for no equal sample) was shown. Significance was set at p < 0.05.
3. Results
3.1. Long-term social isolation results in differences in food intake, without change in fast blood glucose
Food intake was measured in two different conditions for the Social group. When mice of the Social group were placed in individual cages for separated intake records, food ingestion was lower in Isolated compared to Social ( Table 1). However, when food intake was measured while they remained in their collective cage, the result is reverse (Social = 2.52 ± [2.46 – 2.58]; Isolated = 2.89 ± [2.76 – 3.03]; U = 5; p = 0.0003; n = 2 for Social group (number of cages) and n = 9 for Isolated group). Body weight gain (Table 1) was not statistically different. However, the Hedges' g value was 0.78, indicating an effect size from medium to large. In relation to fast blood glucose (Table 1), we did not observe differences between groups.
Table 1.
General parameters of socially isolated and group housed animals.
| Metabolic parameter | Housing condition | Mean | CI 95% Lower | CI 95% Upper | t/U value | p value |
|---|---|---|---|---|---|---|
| Body weight gain (g) | Social | 3.53 | 2.19 | 4.86 | 27 | 0.14 |
| Isolated | 4.98 | 3.58 | 6.37 | |||
| Food intake (g) | Social | 3.29 | 3.02 | 3.55 | 2.886 | 0.01* |
| Isolated | 2.89 | 2.76 | 3.03 | |||
| Fast blood glucose (mg/dL) | Social | 182.50 | 161.20 | 203.80 | 0.375 | 0.71 |
| Isolated | 178.30 | 166.30 | 190.40 |
Metabolic parameters in different housing conditions. Food intake was measured in two different conditions for the Social group. When mice of the Social group were placed in individual cages for separated intake records, food ingestion was lower in Isolated compared to Social (shown in the Table). However, when food intake was measured while they remained in their collective cages (total cage consumption divided by the number of mice), this result is reversed (Social = 2.52 ± [2.46 – 2.58]; Isolated = 2.89 ± [2.76 – 3.03]; U = 5; p = 0.0003; n = 2 for Social group (number of cages) and n = 9 for Isolated group, data not shown in the Table). Body weight gain was not statistically different as shown. However, the Hedges' g value was 0.78, indicating an effect size from medium to large. Unpaired t-student test or Mann-Whitney (for non-parametric data), n = 9–10/group, * p < 0.05.
3.2. Long-term social isolation negatively affected short-term memory
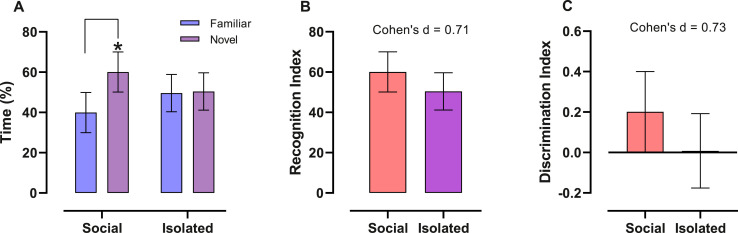
Two-way ANOVA showed an effect of object (F (1,36) = 6.02; p = 0.01) and interaction between object and housing (F (1,36) = 5.173; p = 0.02), with no effect of housing (F = (1,36) = 1.74-31; p > 0.99). The effect of object was noted in Social group, since permanence time was higher in novel than in familiar object. Tukey post-hoc detected those mice in the Isolated group failed to distinguish the novel from the familiar object (p = 0.99), unlike Social group (p = 0.01), that spent more time in the novel than in the familiar object ( Fig. 1 A). Despite the lower mean values for both IR and ID (Fig. 1B and C, respectively) in the Isolated compared to the Social group, we did not find any statistical difference (IR, t = 1.60, p = 0.12; ID, t = 1.60, p = 0.12). However, we observed a medium-large effect size in IR (0.71) and ID (0.73). There was no statistical difference for the time (seconds) spent exploring the two objects in the training phase of the behavioural test (data not shown graphically) (Social = Familiar Object A: 7.26 ± [4.48 – 10.04], Familiar Object B: 6.44 ± [3.86 – 9.02]; t = 1.534; p = 0.15; Isolated = Familiar Object A: 9.92 ± [6.40 – 13.45] / Familiar Object B: 8.47 ± [6.0 – 10.92]; t = 1.203; p = 0.25).
Fig. 1.
Short-term memory performance of socially isolated vs. group housed mice in the novelty recognition test. Mean ± CI95. (A) Permanence time on familiar and novel object. Isolated mice fail to distinguish novel object; Two-way ANOVA with Tukey post hoc test, n = 10/group, *= p < 0.05. (B, C) Recognition (IR) and Discrimination (ID) indexes, respectively. Even with no statistical differences, effect size for both were medium to large; Unpaired t-student test, Cohen’s effect size, n = 10/group.
3.3. Long-term social isolation results in minor anxiety-like behaviour
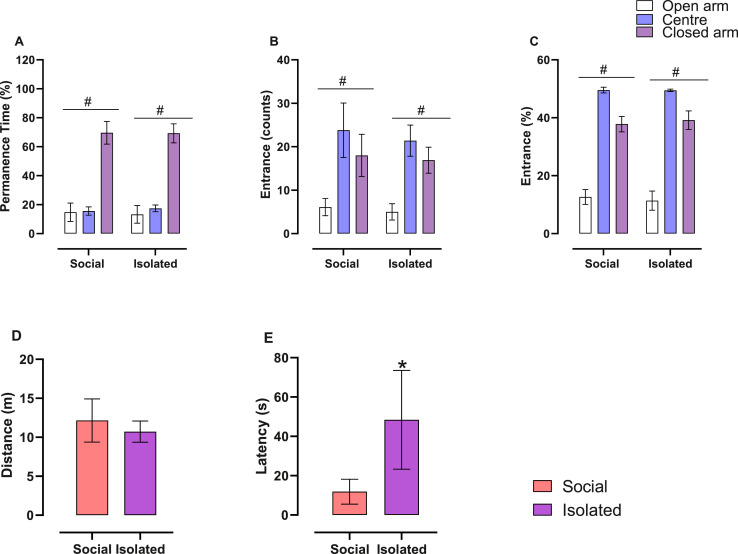
In the elevated plus maze, for permanence time in either closed arm, open arms, and centre ( Fig. 2A), GEE only detected an effect of type of arm (Wald = 444.011; df = 2; p = 0.0001), without effect of housing type (Wald = 0.000; df = 1; p = 0.99) and interaction (Wald = 1.88; df = 2; p = 0.40). Mice of both groups stayed longer in the closed arms (Fig. 2A).
Fig. 2.
Impact of different housing conditions in anxiety-like behaviour in the elevated plus-maze. Mean ± CI95. (A, B, C) % Permanence time, number of entrances and % entrances, respectively, in each arm in the elevated plus maze. The only effect noted was the effect of the arm, with no effect of housing and interaction; GEE with Bonferroni post hoc test, n = 10/group, # = effect of arm (p < 0.05). (D-E) Distance and latency to enter the open arm, respectively. Isolated mice showed higher latency to enter the open arm, suggesting that long-term isolation results in anxiety-like behaviour. Unpaired t-student test or Mann-Whitney (for non-parametric data), n = 9–10/group, * p < 0.05.
In relation to the number of entrances (Fig. 2B), we also observed in GEE only an effect of type of arm (Wald = 6314; df = 2; p = 0.0001), without effects of housing type (Wald = 0.792; df = 1; p = 0.37) or interaction (Wald = 1.215; df = 2; p = 0.54). Mice of both groups had a higher number of entries in the centre and all arms were different from each other (Fig. 2B). The % entrances (Fig. 2C) also presented only effect of arm (Wald = 3284.121; df = 2; p < 0.0001), without effect of housing (Wald = 0.483; df = 1; p = 0.48) and interaction (Wald = 0.642; df = 2; p = 0.72). Distance travelled (Fig. 2D) (t = 1.05; p = 0.30) was not different between groups. On the other hand, Isolated mice presented a higher latency (t = 3.040; p = 0.007; Fig. 2E) to enter the open arm. For head dips and stretch-attend posture ( Table 2), we noted that Isolated mice spent less time performing unprotected dip at the final 1/3 of the open arm.
Table 2.
Differences of housing conditions in head dips and stretch-attend posture in the elevated plus maze.
| Elevated plus-maze parameter | Housing conditions | Mean | CI 95% Lower | CI 95% Upper | t/U value | p value |
|---|---|---|---|---|---|---|
| Protected dips time (s) | Social | 6.70 | 4.88 | 8.52 | 0.902 | 0.37 |
| Isolated | 5.56 | 3.36 | 7.76 | |||
| Protected dips frequency (count) | Social | 6.60 | 4.90 | 8.29 | 0.093 | 0.92 |
| Isolated | 6.70 | 4.97 | 8.42 | |||
| Unprotected dips time (s) | Social | 3.13 | 1.92 | 4.34 | 0.565 | 0.57 |
| Isolated | 2.65 | 1.16 | 4.14 | |||
| Unprotected dips frequency (count) | Social | 3.11 | 2.06 | 4.16 | 41 | 0.76 |
| Isolated | 3.60 | 1.38 | 5.81 | |||
| Unprotected dips at 1/3 end of open arm time (s) | Social | 5.13 | 3.10 | 7.15 | 2.371 | 0.02* |
| Isolated | 2.59 | 1.26 | 3.92 | |||
| Unprotected dips at 1/3 end of open arm frequency (count) | Social | 4.00 | 2.34 | 5.65 | 1.190 | 0.24 |
| Isolated | 2.90 | 1.61 | 4.18 | |||
| Stretch-attend time (s) | Social | 11.21 | 9.31 | 13.11 | 0.270 | 0.78 |
| Isolated | 11.65 | 8.54 | 14.75 | |||
| Stretch-attend frequency (count) | Social | 19.20 | 15.90 | 22.50 | 29 | 0.11 |
| Isolated | 24.10 | 19.67 | 28.53 |
Social isolation decreased unprotected head dips time at the end 1/3 of open arm in the elevated plus maze, a behaviour related to anxiety-like behaviour, with no additional differences. Unpaired t-student test or Mann-Whitney (for non-parametric data), n = 9–10/group, * p < 0.05.
In the open field, rearing time, rearing frequency, grooming time, grooming frequency, distance, mean speed, maximum speed, line crossing, entries in external zone, entries in intermediate zone, entries in centre, and time in centre ( Table 3) showed no differences between groups. However, Isolated mice spent less time in the external zone and more time in the intermediate zone (Table 3).
Table 3.
Comparisons of open field parameters of socially isolated and group housed mice.
| Open field parameter | Housing condition | Mean | CI 95% Lower | CI 95% Upper | t/U value | p value |
|---|---|---|---|---|---|---|
| Rearing (sec) | Social | 23.36 | 17.08 | 29.64 | 0.400 | 0.69 |
| Isolated | 22.06 | 18.67 | 25.45 | |||
| Rearing (count) | Social | 26.50 | 18.87 | 34.13 | 0.240 | 0.81 |
| Isolated | 27.60 | 20.61 | 34.59 | |||
| Grooming (sec) | Social | 12.58 | 8.28 | 16.88 | 0.749 | 0.46 |
| Isolated | 10.80 | 7.55 | 14.05 | |||
| Grooming (count) | Social | 15.50 | 11.28 | 19.72 | 0.665 | 0.51 |
| Isolated | 13.60 | 8.71 | 18.49 | |||
| Distance (m) | Social | 19.45 | 16.76 | 22.14 | 43 | 0.63 |
| Isolated | 17.91 | 14.09 | 21.73 | |||
| Mean speed (m/min) | Social | 0.06 | 0.05 | 0.07 | 44.50 | 0.69 |
| Isolated | 0.05 | 0.04 | 0.07 | |||
| Max speed (m/min) | Social | 0.20 | 0.18 | 0.22 | 49.50 | 0.98 |
| Isolated | 0.21 | 0.16 | 0.26 | |||
| Line crossing (count) | Social | 273.00 | 239.8 | 306.20 | 48.50 | 0.92 |
| Isolated | 259.50 | 208.4 | 310.60 | |||
| External zone time (sec) | Social | 224.60 | 213.00 | 236.20 | 2.150 | 0.04* |
| Isolated | 196.90 | 170.00 | 223.70 | |||
| External zone entries (count) | Social | 36.10 | 31.94 | 40.26 | 35 | 0.26 |
| Isolated | 39.50 | 31.51 | 47.49 | |||
| Intermediate zone time (sec) | Social | 64.00 | 55.25 | 72.75 | 2.230 | 0.03* |
| Isolated | 87.77 | 65.30 | 110.20 | |||
| Intermediate zone entries (count) | Social | 46.90 | 40.55 | 53.25 | 35.50 | 0.28 |
| Isolated | 52.20 | 40.42 | 63.98 | |||
| Centre time (sec) | Social | 11.37 | 7.89 | 14.85 | 1.168 | 0.25 |
| Isolated | 15.35 | 8.47 | 22.23 | |||
| Centre entries (count) | Social | 11.50 | 8.71 | 14.29 | 1.258 | 0.22 |
| Isolated | 14.60 | 9.76 | 19.43 |
Social isolation slightly modifies open field parameters, decreasing time in external and increasing time in intermediate zones. Unpaired t-student test or Mann-Whitney (for non-parametric data), n = 9–10/group, * p < 0.05.
3.4. Long-term social isolation increases physical activity in the light cycle (inactive phase)
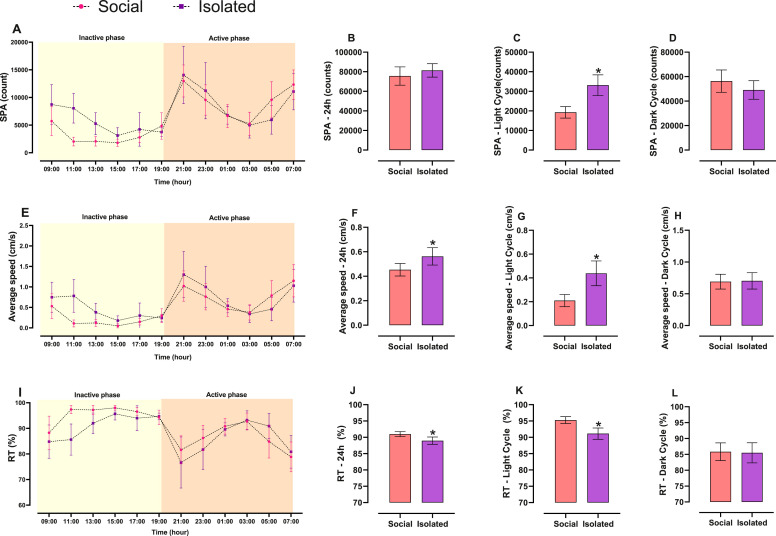
Twenty-four hours spontaneous physical activity was not different between groups (t = 1.109; p = 0.28; Fig. 3B). However, average speed (U = 10.50; p = 0.006; Fig. 3F) of locomotion was higher in the Isolated group. Accordingly, resting time was lower in Isolated compared to Social group (U = 5; p = 0.0007; Fig. 3J).
Fig. 3.
Differences between socially isolated vs. group housed mice in physical activity parameters in 24 h and divided by light (inactive phase) and dark (active phase) cycles. Mean ± CI95. Line graphs show physical activity parameters behaviour in 24 h, displaying data every 2 h. The different background colours separate light from dark cycle. Column graphs represent the total sum of count or sum separated by cycle. The statistical differences found in full cycle were the result of changes in physical activity pattern in the light cycle. Unpaired t-student test or Mann-Whitney (for non-parametric data), n = 9–10/group, * p < 0.05.
When light (inactive phase) and dark (active phase) cycles were analysed separately, the effects of housing condition on cage activity become clearer. In the light cycle, the Isolated group presented higher spontaneous physical activity (t = 5.198; p < 0.0001; Fig. 3C) and average speed of locomotion (t = 4.451; p = 0.0003; Fig. 3G) and lower resting time (t = 4.666; p = 0.0002; Fig. 3K), whereas no difference was observed in the dark cycle for any parameter (Spontaneous physical activity: t = 1.373; p = 0.18; Fig. 3D / Average speed: t = 0.171; p = 0.86; Fig. 3H/Resting time: t = 0.186; p = 0.85; Fig. 3L).
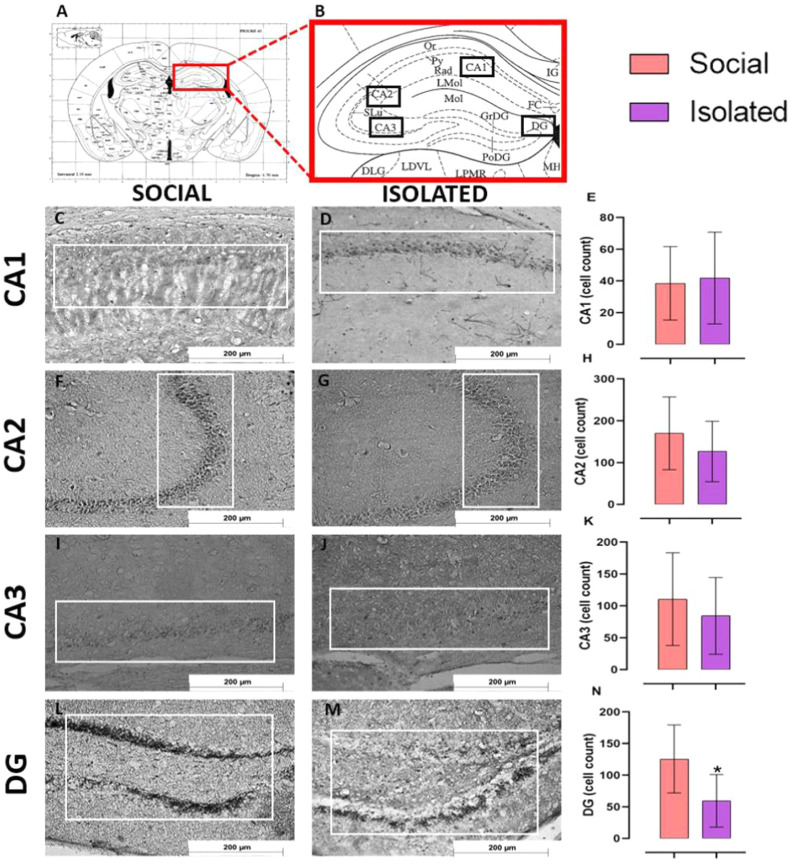
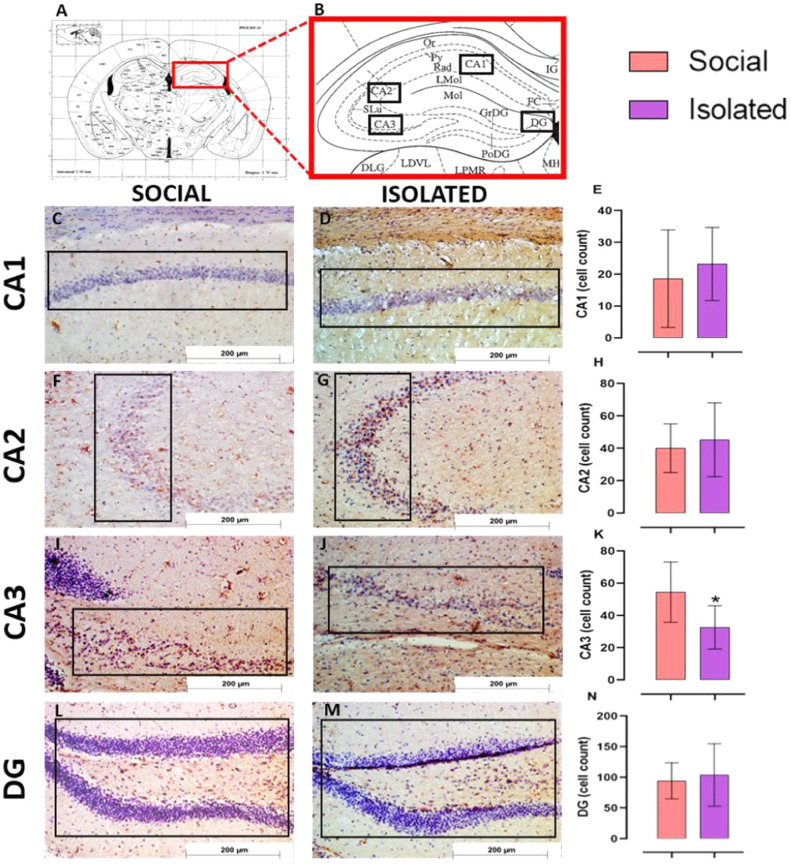
3.5. Long-term social isolation decreased neuronal activity and BDNF expression in some hippocampal areas, with no evidence of oxidative stress
The expression of ΔFosB ( Fig. 4), a marker of neuronal activity, was decreased at DG of the Isolated group compared with the Social group (t = 2.468; p = 0.03). We did not observe any difference between groups for CA1 (t = 0.219; p = 0.83), CA2 (t = 0.987; p = 0.34) and CA3 (t = 0.713; p = 0.49). For BDNF ( Fig. 5), isolation decreased its expression at CA3 (t = 2.632; p = 0.03) but not in CA1 (U = 8; p = 0.41), CA2 (t = 0.529; p = 0.61) and DG (t = 0.451; p = 0.66).
Fig. 4.
Immunoreactivity of ΔFosB in the hippocampus of mice housed in social vs isolated conditions. (A-B) Bregma reference used for immunohistochemistry [45]. Representative images of ΔFosB immunostaining in hippocampus of Social/Isolated mice and quantitative analyses for CA1 (C-E), CA2 (F-H), CA3 (I-K), and DG (L-N). In E, H, K, N, results are mean ± CI95. Social isolation decreased ΔFosB, a neuronal activity marker, at DG. Unpaired t-student test or Mann-Whitney (for non-parametric data), n = 6/group, * p < 0.05. Thionine staining.
Fig. 5.
Immunoreactivity of BDNF in the hippocampus of mice housed in social vs isolated conditions. (A-B) Bregma reference used for immunohistochemistry [45]. Representative images of BDNF immunostaining in hippocampus of Social/Isolated mice and quantitative analyses for CA1 (C-E), CA2 (F-H), CA3 (I-K) and, DG (L-N). In E, H, K, N, results are mean ± CI95. Social isolation decreased BDNF, a neurotrophic factor important for neuroplasticity, at CA3. Unpaired t-student test or Mann-Whitney (for non-parametric data), n = 5/group, * p < 0.05. Hematoxylin staining.
Housing type had no effect on oxidative stress marker 8-OHdG expression ( Table 4) in any of the hippocampus regions studied.
Table 4.
Immunoreactivity of 8-OHdG in hippocampus of socially isolated vs. group housed mice.
| Hippocampus area | Housing condition | Mean | CI 95% Lower | CI 95% Upper | t/U value | p value |
|---|---|---|---|---|---|---|
| CA1 | Social | 21.20 | 10.95 | 31.45 | 0.890 | 0.39 |
| Isolated | 29.17 | 9.85 | 48.48 | |||
| CA2 | Social | 45.83 | 31.20 | 60.47 | 0.824 | 0.42 |
| Isolated | 39.00 | 23.52 | 54.48 | |||
| CA3 | Social | 44.33 | 26.37 | 62.30 | 0.202 | 0.84 |
| Isolated | 41.83 | 15.71 | 67.96 | |||
| DG | Social | 94.20 | 77.28 | 111.10 | 1.166 | 0.27 |
| Isolated | 106.50 | 85.57 | 127.40 |
Immunohistochemistry for 8-OHdG at hippocampus. Social isolation does not modify 8-OHdG, a marker of oxidative stress, in hippocampus areas. Unpaired t-student test, n = 5–6/group.
3.6. Long-term social isolation did not change ΔFosB in LHA and DMH
ΔFosB expression was not different between groups in both LHA and DMH ( Table 5).
Table 5.
Immunoreactivity of ΔFosB in hypothalamus of socially isolated vs. group housed mice.
| Hypothalamus area | Housing condition | Mean | CI 95% Lower | CI 95% Upper | t/U value | p value |
|---|---|---|---|---|---|---|
| Dorsomedial hypothalamus (cell count) | Social | 323.50 | -135.40 | 782.40 | 0.168 | 0.87 |
| Isolated | 296.20 | 47.75 | 544.60 | |||
| Lateral hypothalamus (cell count) | Social | 369.30 | 255.60 | 483.10 | 0.292 | 0.77 |
| Isolated | 343.80 | 121.20 | 566.40 |
Immunohistochemistry for ΔFosB at hypothalamus. Changes in food intake, were not a result of hypothalamus changes. Unpaired t-student test, n = 5–6/group.
4. Discussion
Understanding the effects of social isolation has gained momentum due to the COVID-19 pandemic and the consequent social distancing measures. Here, we investigated the effects of long-term social isolation on metabolic, behavioural, and central nervous system-related areas in middle-aged mice. This is a period of life characterized by the beginning of some age-related metabolic and behavioural alterations [25], [26], [27], [28], thus being particularly important but yet not so explored. Our main finding was that long-term social isolation can impair short-term memory, which was associated with a decrease in ΔFosB and BDNF expression at hippocampal DG and CA3, respectively. Other aspects also deserve attention, as physical activity, anxiety-related behaviour, and food intake were also affected.
Isolated mice failed to distinguish the novel from the familiar object in the NOR test, indicating short-term memory deficit. It is important to highlight that performance impairment in the NOR test can be related to hippocampal and/or cortical dysfunctions [42]. In the NOR test, there is no reward and animals explore the novel object due to their natural propensity to novelty, being a simple tool to assess memory [42]. As addressed by Cohen & Stackman (2015), the hippocampus is necessary for the retention of object recognition memory when a delay greater than 10 min is imposed between the NOR sample and test sessions [41], as in our approach. The theory proposed by Cohen & Stackman (2015), suggests a partnership between the perirhinal cortex and the hippocampus in object memory processing [41]. However, neither the hippocampus nor the perirhinal cortex is solely responsible for object memory as assessed by the NOR test [41]. Despite the use of indexes like IR and ID, the difference in the exploration time of the novel and familiar objects is an important measure of memory [42].
Due to the NOR result, we investigated the hippocampus, since it is the main central area related to memory [46], [47]. We observed a significant decrease in ΔFosB and BDNF expression at DG and CA3, respectively. DG is intimately connected to CA3 where, in animals, an auto-associative network enables the recall of complete memories to underpin object/event recognition [48], [49]. Recurrent collaterals in the CA3 subfield of the hippocampus form an auto-associative system, allowing the recall of previously stored objects and events [48], [49]. As prolonged isolation or loneliness may in themselves act as stressors [7], chronic stress can result in hippocampal dysfunction and deficits in learning and memory [50], which can explain the results found in the study. We believe that the differences were only noted at DG and CA3 because these are the regions of the hippocampus that enable accurate object recognition and pattern separation, respectively [48]. One simplified view of this neural network is that pattern separation at DG helps separate memories of similar events to protect against erroneous object recognition at CA3 [48]. Changes in oxidative stress-mediated mitochondrial function, inflammatory factors, neurotrophic factors, and fos proteins are also observed as a pathophysiological consequence of social isolation, which induces neurological disorders [51]. Our results support these findings, except for the oxidative stress marker 8-OHdG, and demonstrates that the brain was unable to adapt to the chronic stress caused by social isolation.
ΔFosB is a transcription factor whose expression throughout the brain is modulated by chronic exposure to stress and a variety of other stimuli [36]. It is widely used as a marker of neuronal activity since it remains stable for long periods of time when compared to other neuronal activation markers [34]. Moreover, ΔFosB has been shown to regulate synaptic plasticity and behaviour [36]. Silencing of the transcriptional activity of hippocampal ΔFosB impaired learning and memory in male mice across a battery of hippocampal-dependent memory tasks (NOR test) [36]. Besides, ΔFosB was induced in hippocampus CA1 and DG subfields by spatial learning and novel environmental exposure. These results demonstrated for the first time that the transcription factor ΔFosB is important for hippocampal-dependent learning and memory [36]. In our study, a decrease of ΔFosB at DG in isolated mice associated with impairment in the NOR test may be associated with the role of hippocampal ΔFosB in memory. Interestingly, according to Gajewski et al., lower levels of ΔFosB and/or other FosB isoforms hippocampus in humans may in part underlie the cognitive deficits associated with depression and addiction, or contribute to the comorbidity of these psychiatric disorders [52]. With respect to BDNF, it is a well-stablished regulator of neurogenesis and is critical in memory formation, plasticity and cell proliferation [53], and is found in high concentrations in the hippocampus and cortex. A decrease in BDNF signalling could influence age-related memory impairment [53]. Geist et al. (2017) found that globally reduced BDNF levels in rats impaired novelty recognition and fear memory retention [37]. Thus, long-term social isolation results in reduction of an important marker of neuronal activation and neurotrophic factor related to hippocampus neurogenesis, negatively affecting memory.
Housing type also influenced physical activity. We noted that the higher 24 h average speed and the lower resting time in the Isolated group, were due to changes in the light cycle. In addition, even though the 24 h spontaneous physical activity was not different, when cycles were analysed separately, the activity was also greater in the light cycle of Isolated compared to the Social group. These results indicate a possible circadian alteration in response to the stress of isolation. Guo et al. observed the effect of 1–4 months of social isolation in young mice and found that the isolated male mice had higher locomotor activity than the isolated female and group-housed ones, suggesting that male mice might be more sensitive than females to social isolation regarding locomotor activity [21]. However, in this study, locomotion activity was measured only in the light cycle [21]. Interestingly, in a review, Arakawa (2018) discussed that some studies found that isolated rats become less active and have higher anxiety than socially reared rats and that the effects of isolation have no relationship to stress responses or emotional reactions [54]. In contrast, Juczewski et al. (2020), observed strong individual differences between animals, suggesting 3 different types of mouse behaviour in response to stress: animal movement increases, decreases, or no change [55].
We believe that the lack of a consensus and the divergent data regarding locomotion are due to different isolation protocols, age of mice, sex differences, test duration, and methodology adopted. In our study, we assessed for 24 h using an infrared-based system, which also allowed an analysis separated by cycles. Curiously, it should be noted that behavioural performance in some memory-related tests can be influenced by an alteration of the locomotor activity or anxiety levels of the animals [54]. Our results go in line with this hypothesis since, besides alteration in activity, we also observed some differences in the elevated plus-maze. Isolated mice presented higher latency to enter the open arm and spent less time at the final 1/3 of the open arm, indicating an anxious state. However, more studies must be carried out since the classical anxiety-related parameters have not changed. Indeed, other studies reported that mice submitted to chronic social isolation presented higher anxiety and depression [56], [57].
With respect to body weight gain, despite no statistical differences, the effect size was considerable (medium to large). Another study has found that chronic social isolation led to exacerbated body weight gain, in line with our result [58]. In addition, an interesting result emerged from our food intake analysis. Whereas it was higher in the Isolated group when the measurement in the Social group was performed collectively, better representing the chronic experimental condition and being in line with the body weight gain, the result was opposite when mice of the Social group were separated for 24 h for individual intake registration. Yamada et al. [59] add to the complexity of the effects of housing condition on food intake. They observed that mice submitted to 2 weeks of social isolation respond differently according to their age It increased in young-isolated but not in aged-isolated mice compared with the group-housed control [59]. Thus, short- and long-term social isolation seems to affect food intake differently, and age may also play a role. Moreover, our results suggest caution in the choice of the protocol and the interpretation of food intake data, as both individual and collective measures have limitations.
The food intake data led us to investigate the hypothalamus, as it is one of the main areas responsible for the control of homeostatic food intake. Curiously, when we analysed LHA and DMH, two important hypothalamic nuclei for food intake control [60], we did not observe any effects of type of housing. However, due to the strong stressor component of social isolation, areas responsible for controlling non-homeostatic food intakes (food intake not related to body energy status) such as the prefrontal cortex, orbitofrontal cortex, and the mesolimbic pathway [61] could have been affected.
It is important to mention that hippocampus has also been implicated in food behaviour. In a review, Kanoski and Grill detailed how multiple hippocampal subregions constitute an important neural substrate linking the external context, the internal context, and mnemonic and cognitive information to control both appetitive and food behaviour [62]. Hippocampal neurons also receive energy balance-relevant information from circulating endocrine signals such as leptin, GLP-1, and ghrelin, participating in the regulation of food intake and food-reward driven appetitive behaviours [62]. Even though social isolation can be included in the environmental factors related to the development of obesity and type 2 diabetes [63], no differences in fast blood glucose were observed between groups.
Comparing our results with studies that explored acute social isolation, some points need to be addressed. Different times of social isolation can result in different responses, even when the same parameter is analysed, memory being a good example. Long, but not short-term social recognition memory, is abolished by one week of social isolation in male adult mice [20], [32], [64]. In addition, Gusmão et al. (2012) observed that one week of social isolation in adult male C57BL/6 J mice is not detrimental to inhibitory avoidance memory, object recognition, anxiety, or odour habituation, and discrimination [20]. In contrast, animals isolated during 4 weeks presented an anxiety-like phenotype [20]. As highlighted by Arakawa, results from studies with behaviour analyses, such as anxiety and locomotor activity, are commonly confused and controversial, not by the isolation period itself, but due to sex differences, the animal model selected and period of life in which isolation occurs [54].
One limitation of our study is that experiments were performed only at the end of the protocol. As such, it is difficult to establish when memory impairments and changes in the other parameters have arisen. Other limitations include not investigating other areas linked to memory, such as the prefrontal cortex, retrosplenial cortex, perirhinal cortex, parietal cortex and regions surrounding hippocampus [41], [65] and non-homeostatic food intake, such as the prefrontal cortex, orbitofrontal cortex, nucleus accubems, and ventral tegmental area [61]. Importantly, for an accurate measurement of food intake, it was necessary to separate mice in Social group for 24 h and, as we have shown, even this short period may be enough to alter feeding.
Putting together, long-term social isolation starting in adulthood resulted in memory impairment of middle-aged mice, associated with negative repercussions in the hippocampus. It also changed the circadian pattern of physical activity and modified food intake. All these alterations were accompanied by anxiety-related behaviours, but not the classical ones. Finally, experimental designs requiring social isolation need to be careful to interpret data, especially when analysing central and behavioural parameters.
5. Conclusion
In conclusion, long-term social isolation contributes to changes in food intake, the pattern of physical activity parameters, anxiety-related behaviours, and memory of middle-aged mice. Furthermore, short-term memory deficit was associated to lower expression of ΔFosB and BDNF in DG and CA3 at hippocampus, respectively. Future studies should better explore the timepoint at which the alterations found begin.
Funding
This study was financed in part by São Paulo Research Foundation (FAPESP), grants #2011/05932–3; 2017/04528–0; 2017/26075–8 and by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001.
CRediT authorship contribution statement
Camila Aparecida Machado de Oliveira, Izabelle Dias Benfato: Conceived the study, Designed the experiments, Wrote the manuscript, Analysed the data and obtained the funding. Izabelle Dias Benfato, Ana Carolina Silvares Quintanilha, Jessica Salles Henrique, Melyssa Alves Souza, Barbara dos Anjos Rosário, Jose Ivo Araújo Beserra Filho, Robson Luiz Oliveira Santos: Performed the experiments. Luciana Le Sueur Maluf, Alessandra Mussi Ribeiro: Contributed to the acquisition of data, Performed a critical revision of the manuscript. All authors have read the manuscript and approved its contents.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgements
Not applicable.
Availability of data and material
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
- 1.Serra M., Marongiu F., Laconi E. Long-term moderate caloric restriction and social isolation synergize to induce anorexia-like behavior in rats. Nutrition. 2021;86 doi: 10.1016/j.nut.2021.111177. [DOI] [PubMed] [Google Scholar]
- 2.Holt-Lunstad J., Smith T.B., Baker M., Harris T., Stephenson D. Loneliness and social isolation as risk factors for mortality: a meta-analytic review. Perspect. Psychol. Sci. 2015;10(2):227–237. doi: 10.1177/1745691614568352. [DOI] [PubMed] [Google Scholar]
- 3.Cacioppo J.T., Cacioppo S., Capitanio J.P., Cole S.W. The neuroendocrinology of social isolation. Annu. Rev. Psychol. 2015;66:733–767. doi: 10.1146/annurev-psych-010814-015240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li H., Xia N. The role of oxidative stress in cardiovascular disease caused by social isolation and loneliness. Redox Biol. 2020;37 doi: 10.1016/j.redox.2020.101585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith B.J., Lim M.H. How the COVID-19 pandemic is focusing attention on loneliness and social isolation. Public Health Res. Pr. 2020;30(2) doi: 10.17061/phrp3022008. [DOI] [PubMed] [Google Scholar]
- 6.Cudjoe T.K., Roth D.L., Szanton S.L., Wolff J.L., Boyd C.M., Thorpe R.J., Jr. The epidemiology of social isolation: national health and aging trends study. J. Gerontol.: Ser. B. 2020;75(1):107–113. doi: 10.1093/geronb/gby037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shankar A., McMunn A., Banks J., Steptoe A. Loneliness, social isolation, and behavioral and biological health indicators in older adults. Health Psychol. 2011;30(4):377–385. doi: 10.1037/a0022826. [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi L.C., Steptoe A. Social isolation, loneliness, and health behaviors at older ages: longitudinal cohort study. Ann. Behav. Med. 2018;52(7):582–593. doi: 10.1093/abm/kax033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.White C.N., VanderDrift L.E., Heffernan K.S. Social isolation, cognitive decline, and cardiovascular disease risk. Curr. Opin. Psychol. 2015;5:18–23. [Google Scholar]
- 10.Del Arco A., Segovia G., de Blas M., Garrido P., Acuña-Castroviejo D., Pamplona R., Mora F. Prefrontal cortex, caloric restriction and stress during aging: studies on dopamine and acetylcholine release, BDNF and working memory. Behav. Brain Res. 2011;216(1):136–145. doi: 10.1016/j.bbr.2010.07.024. [DOI] [PubMed] [Google Scholar]
- 11.Vermeij W., Dollé M., Reiling E., Jaarsma D., Payan-Gomez C., Bombardieri C.R., Wu H., Roks A., Botter S., Van Der Eerden B. Restricted diet delays accelerated ageing and genomic stress in DNA-repair-deficient mice. Nature. 2016;537(7620):427–431. doi: 10.1038/nature19329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salvatore M.F., Terrebonne J., Fields V., Nodurft D., Runfalo C., Latimer B., Ingram D.K. Initiation of calorie restriction in middle-aged male rats attenuates aging-related motoric decline and bradykinesia without increased striatal dopamine. Neurobiol. Aging. 2016;37:192–207. doi: 10.1016/j.neurobiolaging.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parikh I., Guo J., Chuang K.H., Zhong Y., Rempe R.G., Hoffman J.D., Armstrong R., Bauer B., Hartz A.M., Lin A.L. Caloric restriction preserves memory and reduces anxiety of aging mice with early enhancement of neurovascular functions. Aging (Albany NY) 2016;8(11):2814–2826. doi: 10.18632/aging.101094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsumoto K., Nomura H., Murakami Y., Taki K., Takahata H., Watanabe H. Long-term social isolation enhances picrotoxin seizure susceptibility in mice: up-regulatory role of endogenous brain allopregnanolone in GABAergic systems. Pharmacol. Biochem. Behav. 2003;75(4):831–835. doi: 10.1016/s0091-3057(03)00169-2. [DOI] [PubMed] [Google Scholar]
- 15.Wang B., Wu Q., Lei L., Sun H., Michael N., Zhang X., Wang Y., Zhang Y., Ge B., Wu X. Long-term social isolation inhibits autophagy activation, induces postsynaptic dysfunctions and impairs spatial memory. Exp. Neurol. 2019;311:213–224. doi: 10.1016/j.expneurol.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 16.Farbstein D., Hollander N., Peled O., Apter A., Fennig S., Haberman Y., Gitman H., Yaniv I., Shkalim V., Pick C.G. Social isolation in mice: behavior, immunity, and tumor growth. Stress. 2020;24:1–10. doi: 10.1080/10253890.2020.1777976. [DOI] [PubMed] [Google Scholar]
- 17.Karpova I., Bychkov E., Marysheva V., Mikheev V., Shabanov P. Effects of oxytocin on the levels and metabolism of monoamines in the brain of white outbred mice during long-term social isolation. Bull. Exp. Biol. Med. 2017;163(6):714–717. doi: 10.1007/s10517-017-3887-7. [DOI] [PubMed] [Google Scholar]
- 18.O’Keefe L.M., Doran S.J., Mwilambwe-Tshilobo L., Conti L.H., Venna V.R., McCullough L.D. Social isolation after stroke leads to depressive-like behavior and decreased BDNF levels in mice. Behav. Brain Res. 2014;260:162–170. doi: 10.1016/j.bbr.2013.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun M., Choi E.Y., Magee D.J., Stets C.W., During M.J., Lin E.-J.D. Metabolic effects of social isolation in adult C57BL/6 mice. Int. Sch. Res. Not. 2014;2014(2014 ():1–9. doi: 10.1155/2014/690950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gusmão I.D., Monteiro B.M., Cornélio G.O., Fonseca C.S., Moraes M.F., Pereira G.S. Odor-enriched environment rescues long-term social memory, but does not improve olfaction in social isolated adult mice. Behav. Brain Res. 2012;228(2):440–446. doi: 10.1016/j.bbr.2011.12.040. [DOI] [PubMed] [Google Scholar]
- 21.Guo M., Wu C.F., Liu W., Yang J.Y., Chen D. Sex difference in psychological behavior changes induced by long-term social isolation in mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2004;28(1):115–121. doi: 10.1016/j.pnpbp.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 22.Huang H.-J., Liang K.-C., Ke H.-C., Chang Y.-Y., Hsieh-Li H.M. Long-term social isolation exacerbates the impairment of spatial working memory in APP/PS1 transgenic mice. Brain Res. 2011;1371:150–160. doi: 10.1016/j.brainres.2010.11.043. [DOI] [PubMed] [Google Scholar]
- 23.Kamal A., Ramakers G., Altinbilek B., Kas M. Social isolation stress reduces hippocampal long-term potentiation: effect of animal strain and involvement of glucocorticoid receptors. Neuroscience. 2014;256:262–270. doi: 10.1016/j.neuroscience.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 24.Voikar V., Polus A., Vasar E., Rauvala H. Long‐term individual housing in C57BL/6J and DBA/2 mice: assessment of behavioral consequences. Genes, Brain Behav. 2005;4(4):240–252. doi: 10.1111/j.1601-183X.2004.00106.x. [DOI] [PubMed] [Google Scholar]
- 25.K. Flurkey, J.M. Currer, The Jackson Laboratory handbook on genetically standardized mice, Jackson Laboratory, 2009.
- 26.Kevin Flurkey J.M.C., Harrison D.E. The Mouse in Biomedical Research. Second edition., Chapter 20 – Mouse Models in Aging Research; 2007. [Google Scholar]
- 27.Stowie A.C., Glass J.D. Longitudinal study of changes in daily activity rhythms over the lifespan in individual male and female C57BL/6J mice. J. Biol. Rhythms. 2015;30(6):563–568. doi: 10.1177/0748730415598023. [DOI] [PubMed] [Google Scholar]
- 28.Benfato I.D., Moretto T.L., de Carvalho F.P., Barthichoto M., Ferreira S.M., Costa Júnior J.M., Lazzarin M.C., de Oliveira F., Martinez C., Prado de França Carvalho C., de Oliveira C.A.M. Spontaneous physical activity and mediators of energy homeostasis in the hypothalamus of mice from 4 to 10 months of age. Exp. Physiol. 2017;102(11):1524–1534. doi: 10.1113/EP086265. [DOI] [PubMed] [Google Scholar]
- 29.Pugazhenthi S., Qin L., Reddy P.H. Common neurodegenerative pathways in obesity, diabetes, and Alzheimer’s disease. Biochim. Et. Biophys. Acta (BBA)-Mol. Basis Dis. 2017;1863(5):1037–1045. doi: 10.1016/j.bbadis.2016.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mecocci P., Boccardi V., Cecchetti R., Bastiani P., Scamosci M., Ruggiero C., Baroni M. A long journey into aging, brain aging, and Alzheimer’s disease following the oxidative stress tracks. J. Alzheimer’s Dis. 2018;62(3):1319–1335. doi: 10.3233/JAD-170732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miranda M., Morici J.F., Zanoni M.B., Bekinschtein P. Brain-derived neurotrophic factor: a key molecule for memory in the healthy and the pathological brain. Front. Cell. Neurosci. 2019;13:363. doi: 10.3389/fncel.2019.00363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leser N., Wagner S. The effects of acute social isolation on long-term social recognition memory. Neurobiol. Learn. Mem. 2015;124:97–103. doi: 10.1016/j.nlm.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 33.Cacioppo J.T., Hawkley L.C. Perceived social isolation and cognition. Trends Cogn. Sci. 2009;13(10):447–454. doi: 10.1016/j.tics.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dos Anjos Rosário B., De Fátima Santana De Nazaré M., Lemes J.A., De Andrade J.S., Da Silva R.B., Pereira C.D.S., Ribeiro D.A., De Barros Viana M. Repeated crack cocaine administration alters panic-related responses and delta FosB immunoreactivity in panic-modulating brain regions. Exp. Brain Res. 2021;239(4):1179–1191. doi: 10.1007/s00221-020-06031-2. [DOI] [PubMed] [Google Scholar]
- 35.Leal G., Bramham C.R., Duarte C.B. BDNF and hippocampal synaptic plasticity. Vitam. Horm. 2017;104:153–195. doi: 10.1016/bs.vh.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 36.Eagle A.L., Gajewski P.A., Yang M., Kechner M.E., Al Masraf B.S., Kennedy P.J., Wang H., Mazei-Robison M.S., Robison A.J. Experience-dependent induction of hippocampal ΔFosB controls learning. J. Neurosci. 2015;35(40):13773–13783. doi: 10.1523/JNEUROSCI.2083-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Geist P.A., Dulka B.N., Barnes A., Totty M., Datta S. BNDF heterozygosity is associated with memory deficits and alterations in cortical and hippocampal EEG power. Behav. Brain Res. 2017;332:154–163. doi: 10.1016/j.bbr.2017.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Gould T.D., Dao D.T., Kovacsics C.E. The open field test. Mood Anxiety Relat. Phenotypes Mice. 2009:1–20. [Google Scholar]
- 39.Santos J.R., Cunha J.A., Dierschnabel A.L., Campêlo C.L., Leão A.H., Silva A.F., Engelberth R.C., Izídio G.S., Cavalcante J.S., Abílio V.C. Cognitive, motor and tyrosine hydroxylase temporal impairment in a model of parkinsonism induced by reserpine. Behav. Brain Res. 2013;253:68–77. doi: 10.1016/j.bbr.2013.06.031. [DOI] [PubMed] [Google Scholar]
- 40.Beserra-Filho J.I., de Macêdo A.M., Leão A.H., Bispo J.M.M., Santos J.R., de Oliveira-Melo A.J., Menezes P.D.P., Duarte M.C., de Souza Araújo A.A., Silva R.H. Eplingiella fruticosa leaf essential oil complexed with β-cyclodextrin produces a superior neuroprotective and behavioral profile in a mice model of Parkinson’s disease. Food Chem. Toxicol. 2019;124:17–29. doi: 10.1016/j.fct.2018.11.056. [DOI] [PubMed] [Google Scholar]
- 41.Cohen S.J., Stackman R.W., Jr. Assessing rodent hippocampal involvement in the novel object recognition task. A review. Behav. Brain Res. 2015;285:105–117. doi: 10.1016/j.bbr.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Antunes M., Biala G. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn. Process. 2012;13(2):93–110. doi: 10.1007/s10339-011-0430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Denninger J.K., Smith B.M., Kirby E.D. Novel object recognition and object location behavioral testing in mice on a budget. J. Vis. Exp. 2018;141 doi: 10.3791/58593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Quintanilha A.C.S., Benfato I.D., Santos R.L.O., Antunes H.K.M., de Oliveira C.A.M. Effects of acute exercise on spontaneous physical activity in mice at different ages. BMC Sports Sci. Med Rehabil. 2021;13(1):78. doi: 10.1186/s13102-021-00311-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.G. Paxinos, K.B. Franklin, The mouse brain in stereotaxic coordinates, Gulf Professional Publishing, 2004.
- 46.O’keefe J., Nadel L. Oxford University Press; 1978. The hippocampus as a cognitive map. [Google Scholar]
- 47.Eichenbaum H. The role of the hippocampus in navigation is memory. J. Neurophysiol. 2017;117(4):1785–1796. doi: 10.1152/jn.00005.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dillon S.E., Tsivos D., Knight M., McCann B., Pennington C., Shiel A.I., Conway M.E., Newson M.A., Kauppinen R.A., Coulthard E.J. The impact of ageing reveals distinct roles for human dentate gyrus and CA3 in pattern separation and object recognition memory. Sci. Rep. 2017;7(1):1–13. doi: 10.1038/s41598-017-13853-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marr D., Willshaw D., McNaughton B. Springer; 1991. Simple Memory: A Theory for Archicortex, From the Retina to the Neocortex; pp. 59–128. [Google Scholar]
- 50.Lee V., MacKenzie G., Hooper A., Maguire J. Reduced tonic inhibition in the dentate gyrus contributes to chronic stress‐induced impairments in learning and memory. Hippocampus. 2016;26(10):1276–1290. doi: 10.1002/hipo.22604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mumtaz F., Khan M.I., Zubair M., Dehpour A.R. Neurobiology and consequences of social isolation stress in animal model—a comprehensive review. Biomed. Pharmacother. 2018;105:1205–1222. doi: 10.1016/j.biopha.2018.05.086. [DOI] [PubMed] [Google Scholar]
- 52.Gajewski P.A., Turecki G., Robison A.J. Differential expression of FosB proteins and potential target genes in select brain regions of addiction and depression patients. PLOS One. 2016;11(8) doi: 10.1371/journal.pone.0160355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Erickson K.I., Miller D.L., Roecklein K.A. The aging hippocampus. Neuroscientist. 2012;18(1):82–97. doi: 10.1177/1073858410397054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arakawa H. Ethological approach to social isolation effects in behavioral studies of laboratory rodents. Behav. Brain Res. 2018;341:98–108. doi: 10.1016/j.bbr.2017.12.022. [DOI] [PubMed] [Google Scholar]
- 55.Juczewski K., Koussa J.A., Kesner A.J., Lee J.O., Lovinger D.M. Stress and behavioral correlates in the head-fixed method: stress measurements, habituation dynamics, locomotion, and motor-skill learning in mice. Sci. Rep. 2020;10(1):1–19. doi: 10.1038/s41598-020-69132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wallace D.L., Han M.-H., Graham D.L., Green T.A., Vialou V., Iniguez S.D., Cao J.-L., Kirk A., Chakravarty S., Kumar A. CREB regulation of nucleus accumbens excitability mediates social isolation–induced behavioral deficits. Nat. Neurosci. 2009;12(2):200–209. doi: 10.1038/nn.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arzate-Mejía R.G., Lottenbach Z., Schindler V., Jawaid A., Mansuy I.M. Long-term impact of social isolation and molecular underpinnings. Front. Genet. 2020;11:1285. doi: 10.3389/fgene.2020.589621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pereda-Pérez I., Popović N., Otalora B.B., Popović M., Madrid J.A., Rol M.A., Venero C. Long-term social isolation in the adulthood results in CA1 shrinkage and cognitive impairment. Neurobiol. Learn Mem. 2013;106:31–39. doi: 10.1016/j.nlm.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 59.Yamada C., Saegusa Y., Nahata M., Sadakane C., Hattori T., Takeda H. Influence of aging and gender differences on feeding behavior and ghrelin-related factors during social isolation in mice. PLOS One. 2015;10(10) doi: 10.1371/journal.pone.0140094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Suyama S., Yada T. New insight into GABAergic neurons in the hypothalamic feeding regulation. J. Physiol. Sci. 2018;68(6):717–722. doi: 10.1007/s12576-018-0622-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu C.M., Kanoski S.E. Homeostatic and non-homeostatic controls of feeding behavior: distinct vs. common neural systems. Physiol. Behav. 2018;193:223–231. doi: 10.1016/j.physbeh.2018.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kanoski S.E., Grill H.J. Hippocampus contributions to food intake control: mnemonic, neuroanatomical, and endocrine mechanisms. Biol. Psychiatry. 2017;81(9):748–756. doi: 10.1016/j.biopsych.2015.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nonogaki K., Nozue K., Oka Y. Social isolation affects the development of obesity and type 2 diabetes in mice. Endocrinology. 2007;148(10):4658–4666. doi: 10.1210/en.2007-0296. [DOI] [PubMed] [Google Scholar]
- 64.Kogan J.H., Franklandand P.W., Silva A.J. Long‐term memory underlying hippocampus‐dependent social recognition in mice. Hippocampus. 2000;10(1):47–56. doi: 10.1002/(SICI)1098-1063(2000)10:1<47::AID-HIPO5>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 65.Chao O.Y., de Souza Silva M.A., Yang Y.-M., Huston J.P. The medial prefrontal cortex-hippocampus circuit that integrates information of object, place and time to construct episodic memory in rodents: behavioral, anatomical and neurochemical properties. Neurosci. Biobehav. Rev. 2020;113:373–407. doi: 10.1016/j.neubiorev.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.