Abstract
Inflammation and disease are closely related. Inflammation can induce various diseases, and diseases can promote inflammatory response, and two possibly induces each other in a bidirectional loop. Inflammation is usually treated using synthetic anti-inflammatory drugs which are associated with several adverse effects hence are not safe for long-term use. Therefore, there is need for anti-inflammatory drugs which are not only effective but also safe. Several researchers have devoted to the research and development of effective anti-inflammatory drugs with little or no side effects. In this review, we studied some small molecules with reported anti-inflammatory activities and hence potential sources of anti-inflammatory agents. The information was retrieved from relevant studies published between January 2019 and May, 2021 for review. This review study was aimed to provide relevant information towards the design and development of effective and safe anti-inflammation agents.
Keywords: Anti-inflammatory, synthesis, biological activity, structure–activity relationship
1. Introduction
Inflammation is a complex response to noxious signals that for the host survival, it has been implicated in the pathogenesis of many human diseases1. Pro-inflammatory mediators, such as cytokines that are secreted in excess from inflammatory cells leads to development of arteriosclerosis when they chronically affect blood vessels. They also cause tissue degeneration and/or dysfunction to various organs. Chronic inflammation is also involved in sarcopenia that brings hypofunction in the elderly, dementia, osteoporosis, or cancer, several chronic diseases, and negatively affects life expectancy2. It has been reported that patients with neuropsychiatric disorders have increased levels of inflammatory mediators. Depression facilitates inflammatory reactions whereas inflammation promotes depression and other neuropsychiatric disorders. Patients with neuropsychiatric disorders exhibit all cardinal features of inflammation, including increased circulation levels of inflammatory inducers, activated sensors, and inflammatory mediators targeting various tissues. Therefore, neuropsychiatric disorders and inflammation are closely intertwined, and they possibly induce each other in a bidirectional loop3,4. Inflammation also plays an important role in the development of several age-related diseases, such as frailty. It has been reported that low-grade chronic inflammation can also increase the risk of atherosclerosis and insulin resistance which are the leading mechanisms in the development of cardiovascular diseases5. Inflammation is usually associated with the development and progression of cancer6. The relationships between inflammation and cancer are varied and complex. However, an important connection between inflammation and cancer development is DNA damage. Reactive oxygen and nitrogen species are generated during inflammation to combat pathogens and to stimulate tissue repair as well as regeneration. However, these chemicals can also damage DNA, causing mutations that initiate and promote cancer7. Currently, there are several experimental and clinical evidence that atherosclerosis is a chronic inflammatory disease8. Further, inflammation is closely related to several other diseases and hence deserves our attention.
The anti-inflammatory drugs currently used are associated with several side effects at varying degrees including gastrointestinal toxicities, cardiovascular risks, renal injuries, and hepatotoxicity as well as hypertension, sudden cardiac death, and other minor disorders9–13. Most of the anti-inflammatory drugs in clinical practice are becoming superseded due to their potential adverse effects. These are found to be highly unsafe for long-term use14. Therefore, it is of urgently significance to develop anti-inflammatory drugs with low toxicity and good efficacy. At present, the design of anti-inflammatory compounds has great limitations due to undesirable side effects but this has not curtailed enthusiasm of researchers in exploring for potent anti-inflammatory drugs15.
This review focuses on the latest developments in the design and synthesis of anti-inflammatory small molecules since 2019, as well as the corresponding structure–activity relationship (SAR) research. The compounds discussed in this report have shown significant anti-inflammatory activity in pharmacological experiments or potential candidates for anti-inflammatory drugs. It is hoped that this article will help in the identification of potent scaffolds, which can provide new ideas for the development of new effective anti-inflammatory drugs.
2. Recent development in anti-inflammatory agents
2.1. Pyrazole derivatives as anti-inflammatory agents
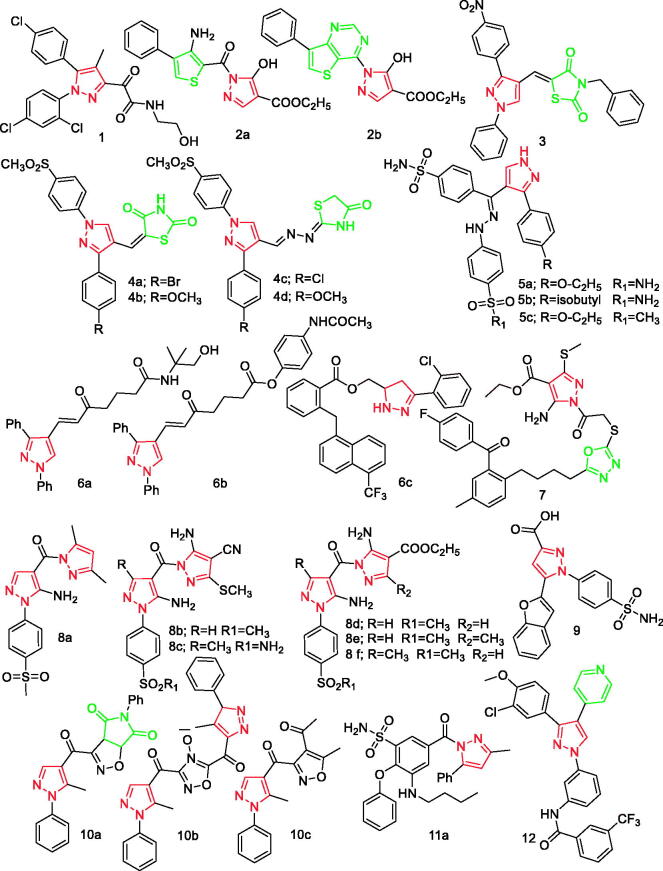
According to Kim et al. compound 1 (Figure 1) was developed and screened for its anti-inflammatory mechanisms following lipopolysaccharide (LPS)-induced neuroinflammation in vitro and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced neurotoxicity in vivo. It was found that prophylactic treatment with compoud 1 decreased pro-inflammatory molecules through NF-κB and p38 mitogen-activated protein kinase (MAPK) signalling. These results suggest that compound 1 is a promising neuroprotective agent for prevention and treatment of microglia-mediated neuroinflammatory conditions16.
Figure 1.
Pyrazole derivatives 1–12 reported as anti-inflammatory agents.
In another study, El-Shoukrofy et al. designed and developed a new thiophene and annulated thiophene-pyrazole hybrids. The hybrids were screened for their in vitro COX-1/COX-2 enzymatic inhibition and in vivo anti-inflammatory activities. The thiophene analogue 2a (Figure 1) and the thienopyrimidine derivative 2b (Figure 1) are promising anti-inflammatory candidates that exert moderate selective COX-2 inhibition with acceptable physicochemical properties. The SAR study has found that replacement of the 5-hydroxypyrazole-4-carboxylate moiety by dimethylpyrazole ring system or diarylpyrazole functionality decreased the in vivo anti-inflammatory activity especially in the acute mode. It was found that replacement of the hydroxyl group in compound 2b with amino group decreases the in vivo anti-inflammatory activity17.
A total of 14 novel diaryl pyrazolyl thiazolidinediones were synthesised by Bansal et al. Compound 3 (Figure 1) was found to be potent anti-inflammatory agent in terms of reducing inflammatory markers (TNF-α, IL-β, and MDA)18.
According to Abdellatif et al., two new series (thiazolidinedione series and thiazolidinone series) were synthesised containing pyrazole ring with vicinal diaryl rings as selective COX-2 moiety. Of these compounds, four compounds 4a, 4b, 4c, and 4d (Figure 1) had higher COX-2 selectivity index (SI) values (8.69–9.26) than the COX-2 selective drug celecoxib (COX-2 SI = 8.60) and showed the highest anti-inflammatory activities as well as the lowest ulcerogenicity than other derivatives19.
Further, Abdellatif et al. also designed and synthesised three novel series of diarylpyrazole and triarylpyrazole derivatives. It was found that all the synthetic compounds were tested for both in vitro and in vivo inhibitory activity. All compounds were more selective for COX-2 isozyme than COX-1 isozyme and had excellent anti-inflammatory activity. All compounds showed significant COX-2 isozyme inhibitory activities (IC50 = 0.023–0.125 µM) and SI = 85.3–185.2 µM which was comparable to reference drug celecoxib (IC50 = 0.063 µM, SI = 142.2 µM). Compounds 5a, 5b, and 5c (Figure 1) are more potent than celecoxib. The bisaminosulphonyl compounds showed higher anti-inflammatory activity compared with aminosulfonyl-methylsulfonyl compounds and were comparable to celecoxib20.
Taher et al. reported novel pyrazole and pyrazoline derivatives. It was observed that compounds 6a, 6b, and 6c (Figure 1) possessed significant anti-inflammatory activity (28.57–30.95%) in which 6a gave comparable potency to that shown by reference drug, Indomethacin (30.95%). These results suggested that the length of carbon chain plays an important role in both anti-inflammatory activities. The lengthening of carbon chain in compounds 6a and 6b gave higher anti-inflammatory activities. Further, cyclisation of chalcones into pyrazolines gave compounds 6c more potent anti-inflammatory effects21.
According to Zabiulla et al. a series of benzophenones conjugated with oxadiazole sulphur bridge pyrazole pharmacophores were synthesised by incorporating fluoro, cholro, bromo, methoxy, and methyl groups at different positions of the benzoyl ring of benzophenone. After these were evaluated for anti-inflammatory activities, it was found that compound 7 (Figure 1) with electron withdrawing fluoro group at the para position of the benzoyl ring of benzophenone was characterised by the highest IC50 values (11.18, 0.10 µM) for both COX-1 and COX-2 inhibition22.
In a separate study by Abdellatif et al. 13 pyrazole derivatives were synthesised and evaluated for their anti-inflammatory activity (in vitro and in vivo). All the compounds were found to be more potent against COX-2 than COX-1 isozyme and showed significant anti-inflammatory activity in vivo. Compounds 8a–8f (Figure 1) showed good COX-2 SI (246.8–353.8) in comparison with the COX-2 selective drug: celecoxib (326.7). Furthermore, these compounds showed appreciable anti-inflammatory activity with oedema inhibition (51–86 and 83–96%) relative to celecoxib (60.6 and 82.8%) after 3 and 5 h, respectively 23.
Gedawy et al. designed new series of pyrazole sulphonamide derivatives. In vitro assessment of all the designed derivatives, COX-1, COX-2, and 5-LOX inhibitory activities were performed in the study. It was reported that compound 9 (Figure 1) showed the most potent analgesic and anti-inflammatory activities that surpassed the effectiveness of conventional drugs, celecoxib, and indomethacin. The compound 9 (Figure 1) showed potent COX-1, COX-2, and 5-LOX inhibitory activity with IC50 of 5.40, 0.01, and 1.78 µM, respectively. This indicated a SI of 344.56 that was more effective than the reference standards and its parent. In addition, it was confirmed that changing the carboxyl group in the structure of compound 9 with an ester derivative does not change activity of the compound 924.
Abdelall et al. reported novel isoxazoles and the furoxan derivative as new safe anti-inflammatory agents. According to Abdelall et al., all these compounds were evaluated for COX-1/COX-2 and most of them were reported to show promising selectivity. The furoxan derivative 10b (Figure 1) gave 59% inhibitory activity using carrageenan-induced paw rat oedema model. Compounds 10a and 10c (Figure 1) were more found to be more selective than celecoxib and nearly with equal potency to celecoxib as anti-inflammatory agents in vivo. However, compounds 10a and 10b were found to be equally safe compared with celecoxib25.
Ibrahim et al. designed and developed new benzenesulfonamide derivatives as selective COX-2 inhibitors based on bumetanide scaffold. Compounds 11a (Figure 1), 11b, and 11c (Figure 13) were good inhibitors of COX-2 with IC50 values of 0.32, 0.28, and 0.17 µM, respectively. Further, compounds 11a, 11b, and 11c were 12.6, 14.4, and 23.7-fold more potent than celecoxib, respectively. These compounds are selective on COX-2 with SI = 61.13 (11a), 71.93 (11b), and 115.82 (11c). Based on in vitro and in vivo experiment results, compounds 11b and 11c exhibited the highest anti-inflammatory activities and lowest incidence of pepticulcer26.
Figure 13.
Triazol derivatives 77–80, 11b, 11c reported as anti-inflammatory agents.
It has been reported that the development of new triarylpyrazole derivatives and their biological activities at molecular, cellular, and in vivo levels. According to El-Gamal et al. compound 12 (Figure 1) was the most potent inhibitor of p38α/MAPK14 kinase (IC50 = 22 nM) among this series. It was reported that compound 12 inhibited p38α/MAPK14 kinase inside HEK293 cells in nanoBRET cellular kinase assay with EC50 value of 0.55 mM, which was comparable to the potency of dasatinib. Further, it was shown that compound 12 inhibits production of TNF-α in LPS-induced THP-1 cells with IC50 value of 58 nM. The in vivo anti-inflammatory effect of compound 12 was comparable to the effects of diclofenac with no ulcerogenic side effect on stomach27.
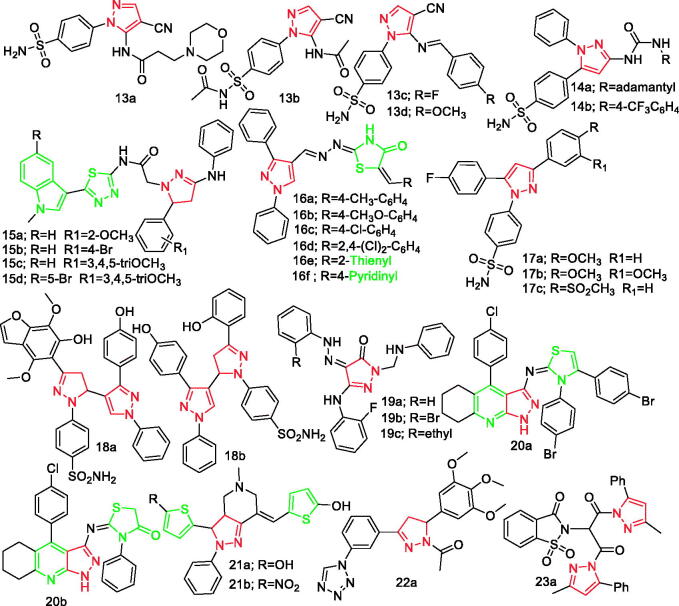
New pyrazole derivatives were developed and evaluated for their COX-1 and COX-2 inhibitory activity in vitro by Hassan et al. It was reported that compounds 13a, 13b, 13c, and 13d (Figure 2) exhibited IC50 towards COX-2 enzyme of 39.43, 61.24, 38.73, and 39.14 nM, respectively. Furthermore, compounds 13a, 13b, 13c, and 13d exhibited SIs of 22.21, 14.35, 17.47, and 13.10, respectively. In the in vivo anti-inflammatory assay, it was reported that these compounds showed higher or comparable activity to positive control, celecoxib28.
Figure 2.
Pyrazole derivatives 13a–23a reported as anti-inflammatory agents.
In separate studies, Abdelazeem et al. developed two 1, 5-diarylpyrazole series of urea- linked and amide-linked compounds. The two compounds were later evaluated as dual COX-2/sEH inhibitors using recombinant enzyme assays in vitro. Compounds 14a and 14b (Figure 2) showed the highest inhibitory activities against both COX-2 and sEH (IC50 of COX-2 = 1.85 and 1.24 µM; sEH = 0.55 and 0.40 nM, respectively). Further, the two compounds, 14a and 14b have a more favourable cardio-profile than celecoxib with much less cardiovascular risks associated with the common selective COX-2 inhibitors29.
Mehta et al. developed a series of novel derivatives of N-(5–(1-methyl-indol-3-yl)-1, 3, 4-thiadiazol-2-yl)-2–(5-substitutedphenyl)-3-(phenylamino)-4 and 5-dihydropyrazol-1-yl) acetamide. They used carrageenan-induced inflammation in rat paw oedema model to screen for the anti-inflammatory activities of the derivatives. Compounds 15a, 15b, 15c, and 15d (Figure 2) were found to possess good anti-inflammatory activity having percentage of inhibition to the extent of 46.8%, 48.1%, 49.4%, and 48.5%, respectively, whereas the effects of the standard drug, diclofenac were 50.0%30.
In another study, Abd et al. designed and developed a new set of pyrazole-thiazolidinone conjugates. Experimental data showed that the compounds 16a−16f (Figure 2) were the most promising anti-inflammatory candidates producing rapid onset of action. Further, the SAR study showed that the benzylidene scaffold without any substitution revealed no activity. In addition, the activity was improved via the insertion of electron-donating groups at para-position (4-methyl or methoxy groups) whereas alteration to meta-position caused a significant drop in the activity. Substitution of the benzylidene moiety with electron drawing groups (CN, F, or Br) weakened the anti-inflammatory activity whereas the reverse results were obtained by the Cl-substituted compounds afforded promising potency. However, it was reported that the heterocyclic rings can produce wide variations in the activity31.
Another study by Abdellatif et al. designed and developed twelve halogenated triarylpyrazoles. All the target compounds in this study showed appreciable in vitro COX-2 inhibitory activity (IC50 = 0.043–0.17 µM) over COX-1 (IC50 = 7.8–15.4 µM) relative to celecoxib (COX-1/IC50 = 9.87 µM and COX-2/IC50 = 0.055 µM). Compounds 17a, 17b, and 17c (Figure 2) displayed remarkable potency (IC50 = 0.049, 0.057, and 0.054 µM, respectively) which was marginally closer to the potency effect of positive control, celecoxib. The compounds (compounds 17a, 17b, and 17c; Figure 2) displayed superior anti-inflammatory activity at all-time intervals (% oedema inhibition = 42.1–87.9) and showed lower ulcer index values (UI = 1.25–2.5) than indomethacin (UI = 14) and close to celecoxib (UI = 1.75). Through their research study, it was revealed that differences in size and electronegativity of the halo substituent have a remarkable effect on biological activity32.
New hydroxybenzofuranyl-pyrazolyl chalcones, hydroxyphenyl-pyrazolyl chalcones and the corresponding pyrazolylpyrazolines were developed by Ragab et al. It was reported that there was no statistical deference in in vivo anti-inflammatory activities of compounds 18a and 18b (Figure 2) as compared with the effects of the standard drug, celecoxib. It was shown that derivatives 18a and 18b with obvious selectivity against COX-2 and still sustaining similar degree of COX-1 inhibition may have lower cardiovascular side effects than those with exclusive inhibition of COX-233.
According to Mohamed et al., the newly developed pyrazoles and pyrazolo [3, 4-b] pyridines were COX-2 inhibitors. It was found that compounds 19a, 19b, and 19c (Figure 2) were the most active and selective as COX-2 inhibitors with IC50 values 0.071, 0.048, and 0.055 µM. Further, they were the most effective in protection from oedema and had the least ulcerogenic effect among all derivatives34.
The synthesis of 13 chlorinated tetrahydro-1H-pyrazolo [3, 4-b] quinoline by Mroueh et al. was reported to serve as tacrine analogues with both lower hepatotoxicity and high COX-2 inhibitory activity. Compounds 20a and 20b (Figure 2) were able of inhibiting LPS-induced COX-2 protein expression at a concentration as low as of 10 µg/mL with COX-2 inhibitory IC50 of 0.76 and 0.84 µM, respectively, from levels analogous to non-stimulated control cells35.
Elsewhere Dennis et al. developed pyrazolopyridines as anti-inflammatory agents. It was found that, in all in vitro and in silico studies, compounds 21a and 21b (Figure 2) showed the highest effects which were similar to the effect of the positive drug indomethacin, but higher than that the effects of indomethacin36.
Elsewhere, Labib et al. reported the design and development of 4 novel sets of tetrazole derivatives as selective COX-2 inhibitors. Compounds 22a (Figure 2) and 22b (Figure 19) exhibited potent COX-2 inhibitory activity (IC50 = 0.039–0.065 µM) in vitro. For their in vivo anti-inflammatory activity applying carrageenan-induced paw oedema assay in rats at different time intervals, compounds 22 b (% oedema inhibition = 29.209–41.379) and 22a (% oedema inhibition =30.120–42.643) were more effective than reference celecoxib (% oedema inhibition = 28.694–40.114). Further, it was found that, trimethoxy derivatives were more potent inhibitors of COX-2, PGE2, TNF-α, and IL-637.
Figure 19.
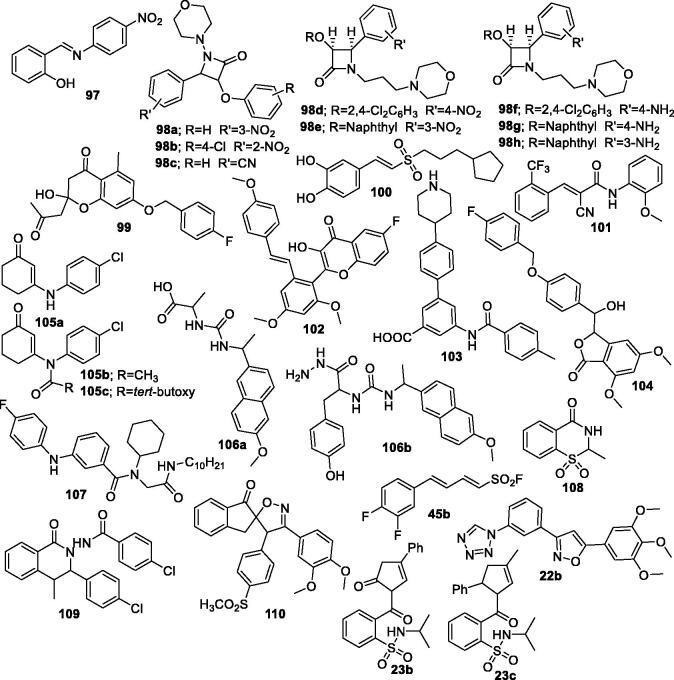
Other derivatives 97–110, 22b, 23b, 23c, and 45b reported as anti-inflammatory agents.
Taher et al. synthesised two new series of 1, 2-benzisothiazol-3(2H)-one-1, 1-dioxide derivatives and evaluated for their in vitro COX-1/COX-2 inhibitory activity. In vivo anti-inflammatory evaluation revealed that derivatives 23b (Figure 19), 23c (Figure 19), and its cyclised form 23a (Figure 2) exhibited the highest anti-inflammatory activity with comparable potency to standard drug, celecoxib38.
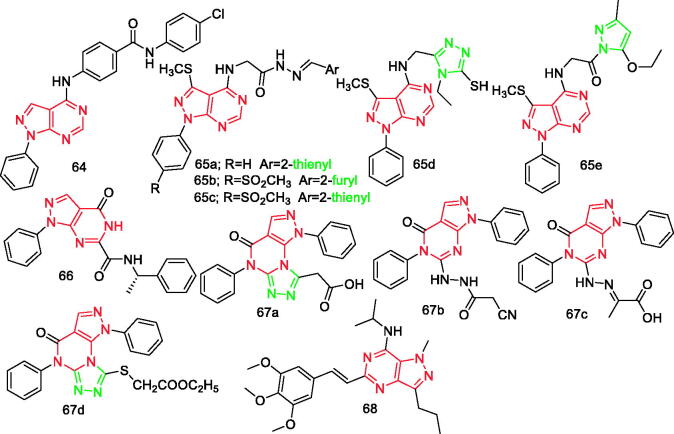
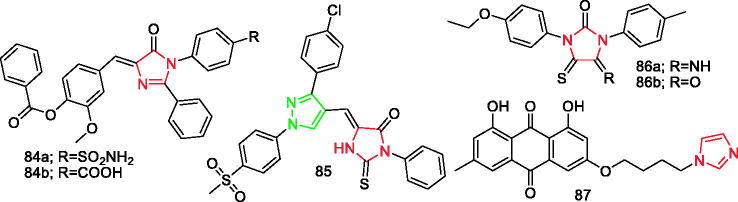
In addition, there is a pyrazole ring in the structure of compound 28 (Figure 3), compound 65e (Figure 9), and compound 85 (Figure 15).
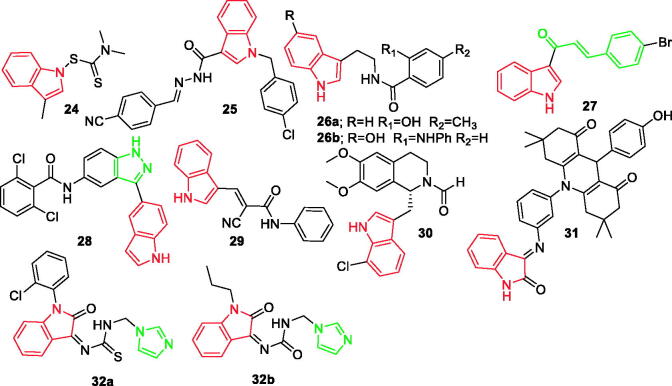
Figure 3.
Indole derivatives 24–32b reported as anti-inflammatory agents.
Figure 9.
Pyrazolo[3,4-d]pyrimidine derivatives 64–68 reported as anti-inflammatory agents.
Figure 15.
Imidazole derivatives 8ā4a–87 reported as anti-inflammatory agents.
2.2. Indole derivatives as anti-inflammatory agents
A total of 29 novel indole-dithiocarbamate compounds were prepared and evaluated by Song et al. for their anti-inflammatory activity. Most of these compounds exhibited high potency on inhibiting the releasing of tumour necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6). It was also noted that compound 24 (Figure 3) exhibited a dose-dependent inhibition effect on both TNF-α and IL-6 production. Moreover, it was reported that compound 24 effectively suppressed the expression of TNF-α, IL-6, IL-1β, VCAM-1, and ICAM-1 at the mRNA level in lung tissues39.
In different study, Ju et al. designed a series of substituted indole analogues and tested for their inhibitory activity against COX-1 and COX-2. Compound 25 (Figure 3) showed the most potent inhibition of the both enzymes with higher selectivity to COX-2, close to that of diclofenac. In addition, a possible anti-inflammatory mechanism of compound 25 was proposed based on the in vitro results. It was found that compound 25 can first inhibit COX-2, leading to a decrease in reactive oxygen species (ROS) production. Decreased ROS production culminates in to the suppression of NF-κB activation and endonuclear translocation, thereby attenuating the expression of pro-inflammatory mediators, such as iNOS, COX-2, TNF-α, and IL-640.
According to Fan et al. the newly synthesised 23 derivatives of N-salicyloyl tryptamine as multifunctional agents and revealed their new application for anti-neuroinflammation. Two of the compounds, 26a and 26b (Figure 3) displayed highly preferable COX-2 inhibition. On the other hand, compounds 26a and 26b reduced GFAP and Iba-1 levels in the hippocampus and displayed neuroprotection in Nissl staining in vivo. Besides, both compounds 26a and 26b had remarkable safety with LD50 > 1000 mg/kg41.
The anti-inflammatory effect of indole-based chalcone derivative 27 (Figure 3) was evaluated on LPS activated murine macrophages RAW264.7 cells and carrageenan-induced acute in rats models. The IC-9 at a low dose (7.5 mg/kg bwt) showed more potent activity on carrageenan-induced inflammation in animal model. The study demonstrated that the compounds 27 responsive mechanism appeared to be dependent on NF-jB-sensitive transcriptional regulatory mechanism42.
A new series of 3-(indol-5-yl)-indazoles was also designed, developed, and evaluated for their anti-inflammatory activities in macrophages. Among the compounds generated, compound 28 (Figure 3) inhibited LPS-induced expression of TNF-α and IL-6 in macrophages with IC50 values of 0.89 and 0.53 µM, respectively. The SAR analysis showed modification of electron-donating groups like 3-methyl, 3, 5-dimethoxy group or heterocycles, which led to a dramatic loss in inhibitory activity against both IL-6 and TNF-α. Halogen group substituted benzamide and this was found to be crucial for the anti-inflammatory effect. However, the hydroxyethyl group may have not contributed to the anti-inflammatory benefits43.
In the paw oedema assay, the compound 29 of newly designed and developed (E)-2-cyano-3-(1H-indol-3-yl)-N-phenylacrylamide by Silva et al. with 50 mg/kg (Figure 3) showed satisfactory anti-inflammatory activity. Moreover, in the peritonitis assay that assesses leukocyte migration, the compound 29 also exhibited promising results. Therefore, these preliminary studies demonstrated that compound 29 is a strong candidate for development of an anti-inflammatory drug with an improved gastrointestinal safety profile as compared to the conventional anti-inflammatory drugs44. Zhang et al. synthesised a series of novel tetrahydroisoquinoline derivatives based on the crystal structure of phosphodiesterase 4 (PDE4). In vivo studies demonstrated that a topical administration of 30 (Figure 3) achieved more significant efficacy than calcipotriol to improve the features of psoriasis-like skin inflammation45.
Compound 31 (Figure 3) was developed by Periyasami et al. and was found to have excellent anti-inflammatory pharmacological efficiency in in vivo biological experiments. Anti-inflammatory activity of this compound was equivalent to that of the standard drug, indomethacin. The molecular mechanisms of target molecule might be related to the decline of NF-kB, TNF-α, IL-6, and IL-1β that may possibly result in inhibition of COX-2 and iNOS expression and its product (PGE2 and nitric oxide [NO])46.
A wide range of substituted imidazoles was developed by Kumar et al. synthesised through a one-pot approach. The anti-inflammatory property of imidazoles was analysed by human red blood cell (HRBC) membrane stabilisation method. Imidazole moieties 32a and 32b (Figure 3) were adjudged to be the promising dual purpose drugs to treat both inflammation and breast cancer. These compounds also showed appreciable H2O2 scavenging activity47.
In addition, there is an indole ring in the structure of compounds 15a−15d (Figure 2).
2.3. Chalcone derivatives as anti-inflammatory agents
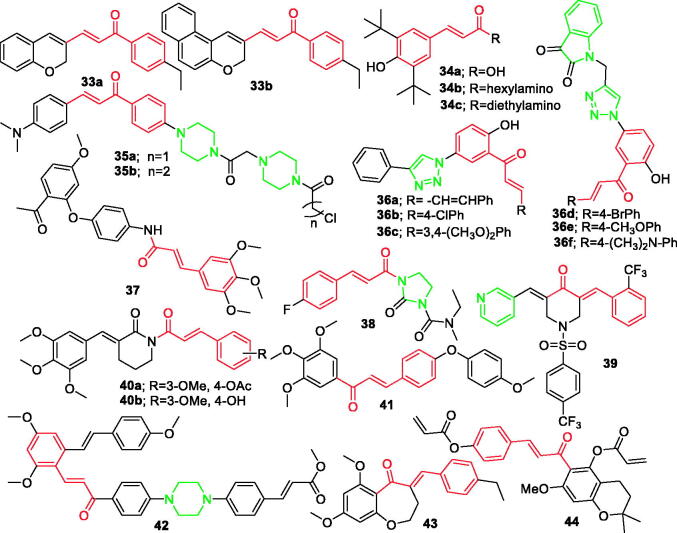
According to Fu et al. 38 chalcone derivatives bearing a chromen or benzo[f]chromen moiety were designed and synthesised. Six compounds bearing a chromen moiety were weak inhibitors of the COX-1 isozyme but showed moderate COX-2 isozyme inhibitory effects (IC50s from 0.37 to 0.83 µM). The compounds bearing a benzo[f]chromen moiety were more selective towards COX-2 than those bearing a chromen moiety with IC50s from 0.25 to 0.43 µM. Compounds 33a and 33b (Figure 4) were found to be the most active and selective COX-2 inhibitors with IC50 values of 0.39 and 0.43 µM. The order of activity for the compounds containing substitutions on the phenyl ring was p-F >o-F>m-F, p-Cl>o-Cl> m-Cl, and p-Br>o-Br>m-Br. Further, the order of activity for compounds containing the benzo [f] chromen moiety with substitutions on the phenyl ring was o-F > m-F > p-F and the order of activity for Br-substituted positions was o-Br>m-Br>p-Br. For compounds containing the electron-donating groups on the phenyl ring, the anti-inflammatory activities were in the order of 4-CH2CH3 >3, 4-(OCH3)2>4-OCH3 >4-N (CH3)2 >4-CH3>4-NH2>3, 4-(CH3)2. Compounds containing the benzo[f]chromen moiety had activities in the order of 4-CH2CH3 >4-OCH3>4-CH3>4-N (CH3)2>4-NH2>3, 4-(OCH3)2>3, 4-(CH3)248.
Figure 4.
Chalcone derivatives 33–44 reported as anti-inflammatory agents.
Ribeiro et al. synthesised a new series of cinnamic acid derivatives and evaluated in human blood for their inhibitory activity of COX-1 and COX-2 enzymes. The relationships between structure and activity showed that phenolic hydroxyl groups are essential for both COX-1 and COX-2 inhibition. In addition, the presence of bulky hydrophobic di-tert-butyl groups in the phenyl ring strongly contributes to selective COX-2 inhibition. This study revealed three effective selective inhibitors of COX-2, namely compound 34a (Figure 4) with IC50=3.0 ± 0.3 µM, compound 34b (Figure 4) with IC50=2.4 ± 0.6 µM and 34c (Figure 4) with IC50=1.09 ± 0.09 µM49.
In another study, Tang et al. developed a series of novel chalcone derivatives bearing bispiperazine linker and screened in vitro anti-inflammatory. The IC50 values of compounds 35a (Figure 4) and 35b (Figure 4) were 0.42 and 0.82 µM, respectively. The preliminary SAR analysis indicated that the type of bispiperazinochalcone derivatives influences the anti-inflammatory activities as well as the linkers of bispiperazine. Further, it was found that the amide linker of the compounds had an obvious influence on anti-inflammatory activities50.
In another study Boshra et al. designed and developed two novel series of 2′-hydroxychalconetriazole hybrid molecules. All the compounds were evaluated in vitro and in vivo for anti-inflammatory activity. Most of compounds were selective inhibitors of COX-2. Compounds 36a–36f (Figure 4) demonstrated highly potent dual inhibition of COX-2 (IC50=0.037–0.041 µM) and 15-LOX (IC50=1.41–1.80 µM) 51.
A series of paeonol derivatives were also developed by Hu et al. Compound 37 (Figure 4) was found to have low toxicity, which showed 96.32% inhibitory activity at 20 µM and IC50=6.96 µM against LPS-induced over expression of NO in RAW 264.7 macrophages. Preliminary mechanism indicated that compound 37 could significantly suppress LPS-induced expressions of iNOS, COX-2, and inhibit NO production through NF-κB/MAPΚ signalling pathway in a concentration-dependent manner. It was also found that the anti-inflammatory activity could be improved when the strong electron-withdrawing group was substituted by the aromatic ring, such as -CF3, -OCH3, and -F52.
Dozens of piperlongumine analogues were developed and tested on PC12 cells to examine neuroprotective effects against H2O2 and 6-OHDA induced damage Ji et al. designed and synthesised. Among them, compound 38 (Figure 4) was found to alleviate the accumulation of ROS, inhibit the production of NO, and downregulate the level of IL-6. Preliminary mechanism confirmed that compound 38 exerted antioxidant and anti-inflammatory activities by activating Nrf2 signalling pathway53.
Elsewhere, Yao et al. designed and developed a series of dissymmetric pyridyl-substituted 3, 5-bis (arylidene)-4-piperidones. It was reported that compound 39 (Figure 4) effectively promoted cell apoptosis through up-regulating cleaved caspase-3 and Bax expression and down-regulating Bcl-2 expression. Furthermore, compound 39 significantly inhibited NF-κB pathway activation by blocking the phosphorylation of IκBα and exhibited the strongest inhibitory effect against both TNF-α and IL-6 expression with inhibition rate of upto 15%. HE staining and immunohistochemistry results also proved that the antitumor and anti-inflammatory effect of compound 39 (10 mg/kg) is comparable to the positive drug54.
In a separate study, Qian et al. prepared a novel series of di-carbonyl analogues of curcumin and evaluated for their anti-inflammatory properties. It was found that compounds 40a and 40b (Figure 4) showed the most potent anti-inflammatory activities. In addition, the two compounds had higher structural stability, orally bioavailability in vitro and markedly alleviated LPS-induced acute lung injury (ALI)55. Two chalcone derivatives as anti-inflammatory agents were developed by Emam et al. They reported that compound 41 (Figure 4) exhibited the highest anti-inflammatory activity by inhibition of NO concentration in LPS-induced RAW264.7 macrophages (IC50=11.2 µM). Moreover, compound 41 decreased the expression of NF-κB and phosphorylated IκB in LPS-stimulated macrophages56. In a different study, Ma et al. synthesised a series of novel derivatives between resveratrol and chalcone possessing piperazine moiety. Derivative 42 (Figure 4) was found to be the most potent anti-inflammatory agent (IC50=4.13 ± 0.07 µM) 57.
A series of new benzoxepane derivatives were designed and synthesised by Gao et al. According to the study, compound 43 (Figure 4) (inhibition of TNF-α release IC50 values of 5.2 µM) was the most effective compound in vitro with low toxicity. Further, compound 43 was shown to display anti-inflammatory effect in vitro and in vivo via inhibiting PKM2-mediated glycolysis and NLRP3 activation. The SAR analysis showed the position and number of methoxyl groups could affect the activity, OCH3>para-OCH3 >3, 4, 5-trimethoxyl. It was found that when replacing benzene ring to pyridine ring, the activity remained the same whereas the toxicity levels were aggravated. In addition, it was reported that insertion of a triple bond between double bond and benzene ring resulted in loss of the activity58.
Ajiaikebaier et al. synthesised 25 derivatives of pyranochalcone and evaluated in vitro activities against TNF-α induced NF-κB inhibition in HEK293T cells. It was found that several displaying the same acrylate moiety on the B ring exhibited potent inhibition, with IC50 values ranging from 0.29 to 10.46 µM. It was reported that the most potent was compound 44 (Figure 4) with IC50 values of 0.29 µM. The study demonstrated that the inhibitory effect of compound 44 on LPS-stimulated inflammatory mediator production in the mouse macrophage cell line RAW 264.7 correlates with the suppression of the NF-κB and the MAPK signalling pathways59.
2.4. Pyridine derivatives as anti-inflammatory agents
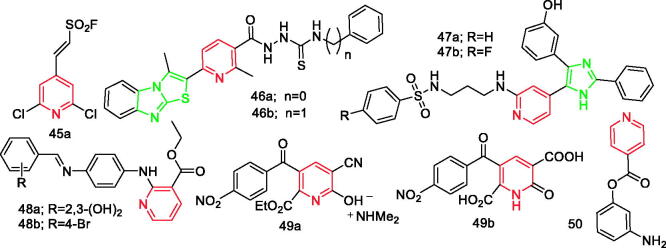
Jiang et al. synthesised a series of (hetero) arylethenesulfonyl fluorides and screened for their in vitro anti-inflammatory activities. Compounds 45a (Figure 5) and 45b (Figure 19) showed excellent anti-inflammatory activity with IC50 values of 18.77 ± 1.10 and 16.06 ± 1.09 µg/mL, respectively. Compounds containing electron-withdrawing (–Cl, –NO2, –F, and –Br) groups on the phenyl ring were found to be the most potent anti-inflammatory agents. Notably, the presence of -SO2F group played a crucial role in increasing anti-inflammatory activities60.
Figure 5.
Pyridine derivatives 46a–50 reported as anti-inflammatory agents.
El-Kerdawy et al. synthesised a new series of benzimidazothiazole derivatives and conducted in vitro and in vivo anti-inflammatory screening of the compounds. Results revealed that the thiourea derivatives (46a and 46b (Figure 5)) had the most active compounds, with IC50 values of 4.52 and 16.02 nM, respectively. These values were equal to 1/10 and 1/2 values of celcoxib, respectively. In addition, the pharmacological data indicated the importance of the thiourea moiety for anti-inflammatory activity in contrast to the isocyanate ones61.
Ali et al. synthesised a series of B-RAFV600E imidazol-5-yl pyridine inhibitors to inhibit P38α kinase. According to the obtained results, compounds 47a and 47b (Figure 5) were the most potent inhibitors (IC50=47 and 45 nM, respectively). In addition, compound 47b effectively inhibited production of TNF-α, 1 L-6, and 1 L-1β proinflammatory cytokines in LPS-induced RAW 264.7 macrophages with IC50 values of 78.03, 17.6, and 82.15 nM, respectively. Compound 47b exhibited significant inhibitory activity for the production of PGE2 and NO with IC50 values of 0.29 and 0.61 µM, respectively62.
El-Dash et al. synthesised two novel series derived from nicotinic acid and evaluated their inhibitory activity against cyclooxygenases (COX-1 and COX-2). All compounds showed highly potent COX-2 inhibitory activity and higher selectivity towards COX-2 inhibition compared to indomethacin. Moreover, compounds 48a and 48b (Figure 5) demonstrated SIs of 1.8–1.9 fold higher than celecoxib. Gastric toxicity and histopathological studies showed that compounds 48a and 48b had no gastric toxicity, which was consistent with their highly selective inhibitory effect of COX-2 enzyme in vitro63.
Gonçalves et al. synthesised 35 novel 2-pyridone derivatives and 14 novel pyridinium salts. Among them, the pyridinium salt 49a (Figure 5) was the most active, and it selectively inhibited COX-2 enzyme activity at a concentration of 1.95 µM. Compound 49b (Figure 5) was the most effective in reducing ear oedema, indicating that carboxylic acid plays a significant role in the anti-inflammation activity. The findings of the study suggest that 2-pyridone is an attractive scaffold for design of novel anti-inflammatory agents and its substitution pattern directly influences the anti-inflammatory activity profile. Furthermore, compound 49a can be an interesting lead compound for developing novel selective inhibitors of COX-264.
Yaqoob et al.65 synthesised novel scaffolds containing isonicotinoyl motif derivatives and screened for their in vitro anti-inflammatory activity. Results showed that compound 50 (Figure 5) exhibited an exceptional IC50 value (1.42 ± 0.1 µg/mL) with 95.9% inhibition at 25 µg/mL, which is eight folds better than the standard drug ibuprofen (11.2 ± 1.9 µg/mL)65.
It should be noted that there is a pyridine ring in the structure of compound 12 (Figure 1) and compound 16f (Figure 2).
2.5. Pyrimidine derivatives as anti-inflammatory agents
Sun et al. synthesised a series of fluoro-substituted hexahydropyrido[4,3-d]pyrimidines (PPMs) . Compound 51 (Figure 6), substituted by electron-withdrawing substitutes (3-F and 4-CF3) exhibited less toxicity and higher anti-inflammatory activity. Preliminary mechanistic studies revealed that compound 51 induced dose-dependent cell apoptosis at cell and protein levels. On the other hand, it inhibited NF-κB activation by suppressing LPS-induced phosphorylation levels of p65, IκBα, and Akt, and by indirectly suppressing MAPK signalling and inhibiting the nuclear translocation of NF-κB induced by TNF-α or LPS66.
Figure 6.
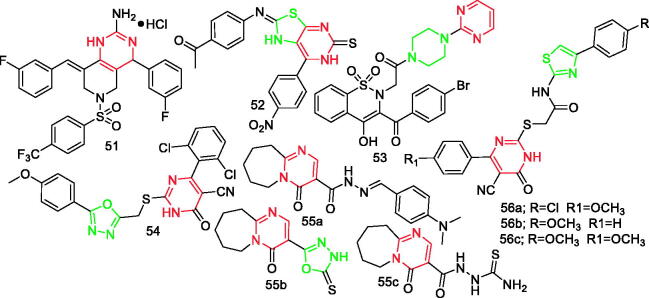
Pyrimidine derivatives 51–56c reported as anti-inflammatory agents.
Bakr et al.67 synthesised novel candidates of thiazolo[4,5-d]pyrimidines and screened for their anti-inflammatory activity. Compound 52 (Figure 6), with IC50=0.87 µM, was the most active derivative with 57%, 88%, and 88% inhibition of inflammation after 1, 3, and 5 h, respectively. In comparison, celecoxib showed 43%, 43%, and 54% inhibition after 1, 3, and 5 h, respectively. In addition, the inhibitory effect of compound 52 on COX-2 was higher than that of celecoxib, a selective COX-2 inhibitor drug67.
Szczęśniak-Sięga et al.68 designed and synthesised novel arylpiperazine-1,2-benzothiazine derivatives as potential anti-inflammatory agents. Subsequent in vitro and molecular docking experiments showed that compound 53 (Figure 6) had the highest COX-2 selectivity compared to meloxicam (0.79 vs. 0.71)68.
Alfayomy et al.69 prepared two new series of 1,3,4-oxadiazole and coumarin derivatives based on pyrimidine-5-carbonitrile scaffold, and then assessed their COX-1/COX-2 inhibitory activity. Compound 54 (Figure 6) was found to be the most potent and selective inhibitor of COX-2 (IC50=0.081 µM, SI = 170.37). The in vivo anti-inflammatory activity study of COX-2 inhibitors indicated that the anti-inflammatory activity of compound 54 was higher than that of the reference drug celecoxib. In addition, it showed better safety compared to celecoxib in the ulcerogenic liability test69.
Hassanein et al.70 synthesised new hexahydropyrimido[1,2-a]azepine derivatives as anti-inflammatory agents with better safety profiles. Compounds 55a, 55b, and 55c (Figure 6) were found to be the most selective COX-2 inhibitors in vitro. These derivatives showed in vivo anti-inflammatory activity that was comparable to celecoxib in the carrageenan-induced rat paw oedema method70.
Abdel-Aziz et al.71 synthesised a novel series of pyrimidine-5-carbonitrile derivatives and evaluated their anti-inflammatory activity against COX-1/COX-2 activity. Results indicated that compounds 56a, 56b, and 56c were potent and selective COX-2 inhibitors (IC50=1.03–1.71 µM) compared to celecoxib (IC50=0.88 µM). Compounds 56a, 56b, and 56c showed in vivo anti-inflammatory activity up to 90%, 94%, and 86% of meloxicam, respectively, after a 4 h interval. Moreover, the compounds showed higher gastric safety profiles than meloxicam71.
Notably, there is a pyrimidine ring in the structure of compound 2b (Figure 1).
2.6. Pyridazine derivatives as anti-inflammatory agents
Loksha et al. synthesised some novel derivatives of 2-alkyl 6-substituted pyridazin-3(2H) –ones and screened their cyclooxygenase (COX)-1/COX-2 inhibition activity and their abilities as analgesic and anti-inflammatory agents. Compounds 57a, 57b, and 57c (Figure 7) showed anti‐inflammatory activities with up to 65%, 62%, and 60% inhibition of oedema, respectively, which were higher than that of diclofenac (58%) at a dose of 10 mg/kg72.
Figure 7.
Pyridazine derivatives 57a–59c reported as anti-inflammatory agents.
Ahmed et al. designed and synthesised a series of pyridazinone derivatives, which were then screened for preferential inhibition of COX-2 over COX-1 isoforms. Results showed that compounds 58a, 58b, 58c, and 58d (Figure 7) exhibited the most prominent COX-2 inhibitory activity, with IC50 values ranging between 15.56 and 19.77 nM. In addition, their sensitivity indices (SIs) were 24, 38, 35, and 24, respectively, which were 1.4–2.2 folds higher than celecoxib (SI 17). The results obtained after performing the carrageenan-induced rat paw oedema method and ulcerogenic liability test showed that compounds 58b, 58c, and 58d demonstrated superior anti-inflammatory activity compared to both indomethacin and celecoxib, and none of these compounds showed gastric ulcerogenic effect73.
Ahmed et al. designed and synthesised new pyridazinone and pyridazinthione derivatives. All the synthesised derivatives were then evaluated for cyclooxygenase inhibitory activity and COX-2 selectivity using celecoxib and indomethacin as reference drugs. According to the results, compounds 59a, 59b, and 59c (Figure 7) exhibited significantly increased potency towards COX-2 enzyme compared to celecoxib. Their IC50 values were 67.23, 43.84, and 53.01 nM, respectively, which were 1.1–1.7 folds more potent than celecoxib (IC50=73.53 nM) and extremely much more potent than indomethacin (IC50=739.2 nM). Compound 59b was the most selective COX-2 inhibitor with a SI of 11, which was as high as that of celecoxib. Notably, the in vivo anti-inflammatory activity of compound 59b was comparable to indomethacin and equipotent to celecoxib. Moreover, compound 59b showed better gastric profile compared to both celecoxib and indomethacin74.
2.7. Quinoline derivatives as anti-inflammatory agents
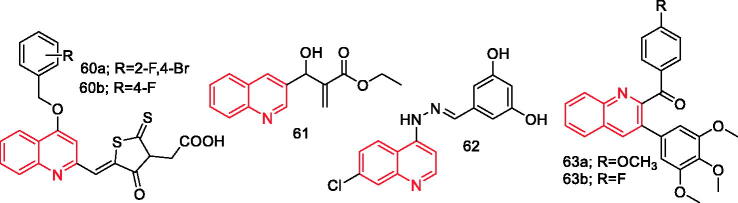
Zhang et al. synthesised 25 quinoline derivatives. Some compounds showed potential anti-inflammatory activity against the 12-O-tetradecanoylphorbol-13-acetate-induced mouse ear inflammation. The most potent compounds 60a and 60b (Figure 8), significantly inhibited the mRNA expression levels of cytokines and inflammatory mediators, including IL-17A, IL-22, IL-23, p65, TNF-α, and IFN-γ75.
Figure 8.
Quinoline derivatives 60a–63b reported as anti-inflammatory agents.
Zuo et al. synthesised a series of novel arylpropionic esters and evaluated their biological activity in LPS-stimulated RAW264.7 cells. Compound 61 (Figure 8) exhibited the most potent activity through dose-dependent inhibition of NO (IC50=3.52 µM), TNF-α, and IL-6 (84.1% and 33.6%, respectively) production, as well as suppressing the expression of iNOS, COX-2, and TLR4 proteins76.
Debnath et al. synthesised a series of aryl 7-chloroquinolinyl hydrazone derivatives and evaluated their anti-inflammatory activities based on their ability to inhibit secretion of pro-inflammatory cytokines from the macrophages after stimulation with LPS. It was found that compound 62 (Figure 8) was the most promising lead with IC50=12.39 ± 0.97 µM77.
Yang et al. synthesised 2-substituted 3-arylquinoline derivatives and evaluated their anti-inflammatory effects in LPS-activated murine J774A.1 macrophage cells. Among the compounds, 63a and 63b (Figure 8) were the most active and they could inhibit production of NO at non-cytotoxic concentrations. Moreover, the two compounds had significant anti-inflammatory activities on LPS-activated macrophages through inhibiting production of NO, TNF-α, and IL-6, attenuating the activity of NF-êB, repressing iNOS expression, and suppressing phosphorylation of MAPKs78.
2.8. Pyrazolo[3,4-d]pyrimidine derivatives as anti-inflammatory agents
Somakala et al. synthesised nine new N-substituted-4-((1-phenyl-1H-pyrazolo[3,4-d]pyrimidin-4-yl)amino)benzamides compounds. Compound 64 (Figure 9) bearing the 4-chlorophenyl group showed in vitro anti-inflammatory activity (82.35 ± 4.04) comparable to the standard drug diclofenac sodium (84.13 ± 1.63) and better p38α MAP kinase inhibitory activity (IC50=0.032 ± 1.63 µM) than the prototypic inhibitor SB203580 (IC50 = 0.041 ± 1.75 µM). However, the activity was decreased when the 4-chlorophenyl group was replaced by 4-methoxyphenyl, 4-methylphenyl, and 4-fluorophenyl groups, respectively. It seems that the electron- donating properties of the methoxy group and the methyl group at the fourth position of the benzene ring are not as good as that of chlorophenyl group, which reduces the activity. When the 4-position of the benzene ring is substituted with a fluorine group, it may be due to its high electronegativity and small size, so the activity is not as good as compound 6479.
Abdelall et al. prepared four pyrazolopyrimidine series with the fourth position substituted with Schiff base, triazole, oxadiazole, and pyrazole moieties, respectively. With regard to the anti-inflammatory activity, compounds 65a, 65c, 65d, and 65e (Figure 9) showed higher activity compared to celecoxib. Compounds 65e, 65b, and 65c (Figure 9) were closely selective to celecoxib. In addition, histopathological analysis indicated that compounds 65a and 65b were safer than indomethacin and similar to celecoxib. The long-lasting anti-inflammatory activity was observed in derivatives with the Schiff base, especially those bearing SO2CH3 pharmacophores. Moreover, the triazole derivative showed a good inhibitory activity compared to celecoxib80.
Atatreh et al. synthesised novel pyrazolo[3,4-d]pyrimidine analogues. They then conducted enzymatic COX-1 and COX-2 inhibition assays to determine the in vitro ability of the compounds to inhibit COX-1 and COX-2. In addition, the in vivo anti-inflammatory activity of the compounds was evaluated using two different screening protocols, the carrageenan-induced paw oedema method and the turpentine oil-induced granuloma pouch bioassay. Compound 66 (Figure 9) showed promising experimental activity (COX-1 IC50 of 28 µM and COX-2 IC50 of 23 µM), but only slight selectivity towards COX-2. However, the selectivity can be further enhanced by optimising the pyrazolopyrimidine ring binding so that it reaches the selectivity region in the COX-2 active site81.
Tageldin et al. reported synthesis of some new pyrazolo[3,4-d] pyrimidinone and pyrazolo[4,3-e][1,2,4]triazolo[4,3-a]pyrimidinone derivatives, and evaluation of their anti-inflammatory activities. Compounds 67a, 67b, 67c, and 67d (Figure 9) showed COX-2 inhibitory potency (IC50 =0.58, 0.38, 0.74, and 0.29 µM, respectively) and selectivity indices (SI = 7.46, 12.23, 7.93, and 9.82, respectively) higher than celecoxib (IC50=0.78 µM and SI = 7.23). Results obtained after conducting in vitro formalin-induced paw oedema and cotton-pellet induced granuloma assays revealed that compounds 67a–67d displayed anti-inflammatory activity (AI = 64.3–78.6%) higher than celecoxib (AI = 46.4%) in the acute model, whereas compounds 67a and 67b possessed potent anti-inflammatory activity (AI = 43.4% and 46.9%) superior to both references (AI = 8.6% and 36.1% for celecoxib and diclofenac sodium, respectively) in the chronic model. All the tested compounds exhibited safe gastrointestinal profile, with the exception of compound 67a82.
Shi et al. designed and synthesised four series of total 90 new pyrazolo[4,3- d]pyrimidine compounds and evaluated their anti-inflammatory activities by inhibiting LPS-induced NO production. Results indicated that 3,4,5-trimethoxystyryl-1 H-pyrazolo[4,3-d]pyrimidine was the most active scaffold. Among them, compound 68 (Figure 9) was the most potent anti-inflammatory agent (IC50=3.17 µM), with low toxicity and strong inhibition of NO release (IR = 90.4% at 10 µM). It also showed potent inhibition of iNOS with IC50 value of 1.12 µM. Furthermore, preliminary mechanism studies indicated that it could interfere with the stability and formation of active dimeric iNOS83.
2.9. Pyrrolidinedione derivatives as anti-inflammatory agents
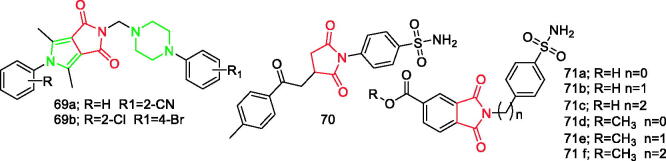
Redzicka et al.84 designed and synthesised a series of N-substituted 3,4-pyrroledicarboximides. All compounds exhibited stronger inhibition of COX-2 than the reference drug (meloxicam) and compounds 69a and 69b (Figure 10) were proven to be the most effective against COX-2 enzyme. Replacement of aryl substituent in a series of piperazine derivatives with heteroaryl or cycloalkyl substitutes led to a significant reduction in COX-1 inhibition. Moreover, introduction of hydrophilic hydroxyethyl substitutes led to a decrease in the inhibitory activity in relation to both enzymes, while introduction of an OH group in a series of arylpiperidine derivatives significantly reduced COX-1 inhibition84.
Figure 10.
Pyrrolidinedione derivatives 69a–71f reported as anti-inflammatory agents.
Jan et al.85 synthesised N-substituted pyrrolidine-2,5-dione derivatives and determined their in-vitro, in-vivo, and acute toxicity as multitarget anti-inflammatory agents. It was found that compound 70 (Figure 10), with IC50 value 0.98 mM and SI of 31.5, was the most potent inhibitor of COX-285.
Abdel-Aziz et al.86 prepared trimellitimides and evaluated their in vivo anti-inflammatory and ulcerogenic effects, and in vitro cytotoxicity. Results showed that compounds 71a–71f (Figure 10) were the most potent COX-2 inhibitors (IC50=0.14, 0.13, 0.10, 0.25, 0.25, and 0.20 µM, respectively) and were comparable to celecoxib (IC50=0.12 µM)86.
It is worth noting that there is a pyrrolidinedione ring in the structure of compound 10a (Figure 1).
2.10. Thiazolidinone derivatives as anti-inflammatory agents
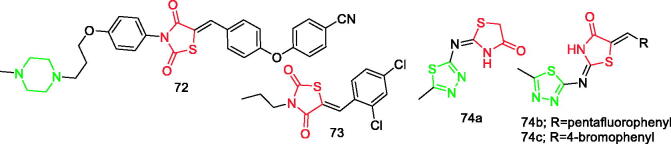
Elkamhawy et al. synthesised a series of thiazolidine-2,4-dione derivatives as irreversible allosteric covalent inhibitors of IKK-b with potential in vivo anti-inflammatory activity. Almost all derivatives elicited submicromolar IC50 values, with the most potent IKK-b inhibitor (IC50=0.20 µM) being compound 72 (Figure 11). Most derivatives with a linker attached to the meta position were less effective, but the activity would increase when the 4-substituent on the phenoxy ring was the electron-withdrawing group nitro or cyano87.
Figure 11.
Thiazolidinone derivatives 72–74 reported as anti-inflammatory agents.
Ranjan et al. synthesised a series of 13 novel 2,4-thiazolidinedione derivatives. Compound 73 (Figure 11) showed great potential because it not only had maximum antidiabetic activity, but also possessed excellent anti-inflammatory and antioxidant potential. The anti-inflammatory activity of compound 73 was closer to the standard compound. Results obtained after performing the in vivo anti-inflammatory activity test indicated that the group at the fourth position of arylidene substituent attached to thiazolidnedione is vital for good anti-inflammatory activity88.
Omar et al. designed and synthesised new 15-LOX/COX dual inhibitors based on 1,3-thiazolidin-4-one (15-lipoxygenase pharmacophore) and 1,3,4-thiadiazole (COX pharmacophore) scaffolds. Compounds 74a, 74b, and 74c (Figure 11) were capable of inhibiting 15-LOX at 2.74, 13.11, and 10.21 µM, respectively, and COX-2 at 0.32, 0.1, and 0.1 µM, respectively. The compounds showed in vivo anti-inflammatory activity comparable to the reference drug (celecoxib)89.
Notably, there is a thiazolidinone ring in the structure of compound 3 (Figure 1), compounds 4a–4d (Figure 1), compounds 16a–16f (Figure 2), and compound 20b (Figure 2).
2.11. Thiazole derivatives as anti-inflammatory agents
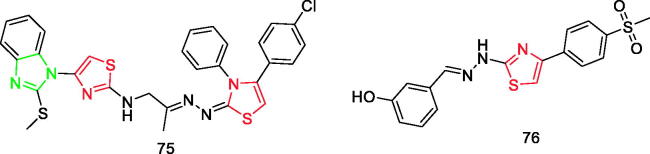
Maghraby et al. prepared new molecular hybrids of 2-methylthiobenzimidazole linked to various anti-inflammatory pharmacophores using a 2-aminothiazole linker. All hybrids revealed potent 15-LOX inhibitory activity (IC50=1.67–6.56 µM). From the results, it was evident that compound 75 (Figure 12) was the most potent dual COX-2 (IC50=0.045 µM, SI = 294) inhibitor compared to celecoxib (COX-2; IC50=0.045 µM, SI = 327), with double inhibitory activity versus 15-LOX enzyme (IC50=1.67 µM) relative to quercetin (IC50=3.34 µM)90.
Figure 12.
Thiazole derivatives 75–76 reported as anti-inflammatory agents.
Sağlık et al. synthesised a diverse series of thiazolylhydrazine-methyl sulphonyl derivatives and used an in vitro fluorometric method to evaluate their ability to inhibit COX enzymes. Results showed that compound 76 (Figure 12) had the highest COX-2 inhibitory activity (IC50=0.140 ± 0.006 mM). Besides, it had a more potent inhibition profile, at least 12-fold, than nimesulide (IC50 =1.684 ± 0.079 mM). However, its inhibitory activity was similar to that of celecoxib (IC50=0.132 ± 0.005 mM)91.
In addition, there is a thiazole ring in the structure of compound 20a (Figure 2), compounds 46a–46b (Figure 5), compound 52 (Figure 6), and compounds 56a–56c (Figure 6).
2.12. Triazol derivatives as anti-inflammatory agents
Li et al. developed a new method for synthesis of 1,2,4-triazole-3-carboxylates. Compound 77 (Figure 13) showed extraordinary COX-2 inhibition (IC50=17.9 nM) and the best selectivity (COX-1/COX-2 = 1080). Furthermore, 5 mg/kg of compound 77 exhibited better in vivo anti-inflammation and gastric protection results compared to 10 mg/kg Indomethacin. SAR suggested that –CH2Cl moiety is the essential moiety, substitution of benzene ring with p-F possesses the best IC50 inhibition, and -CO2Me is an assisting group92.
Avci et al. designed and synthesised a new series of 1,2,4-triazole-5-thione Mannich derivatives containing a naproxen moiety. Results obtained after performing acetic acid induced-writhing and carrageenan-induced paw oedema tests revealed that all compounds induced peripherally-mediated antinociceptive activities, as well as notable anti-inflammatory effects. The results of hotplate and tail-clip tests indicated that compounds 78a–78f (Figure 13) centrally mediated anti-nociceptive activities in addition to their peripherally-mediated effects93.
Shen et al. synthesised a series of 5-alkyl-4-oxo-4,5-dihydro-[1, 2, 4]triazolo[4, 3-a] quinoxaline-1-carboxamide derivatives and evaluated their anti-inflammatory effects using RAW264.7 cells. Results revealed that compound 79 (Figure 13) had the highest anti-inflammatory activity. Further studies showed that compound 79 reduced the levels of NO, TNF-α, and IL-6, and that its anti-inflammatory activity involves inhibition of COX-2 and iNOS, and downregulation of the MAPK signal pathway. Moreover, it displayed more prominent anti-inflammatory activity than the positive control (ibuprofen) in the in vivo acute inflammatory model94.
Munir et al. synthesised 1,3,4-oxadiazole, 1,2,4-triazole, Schiff base, and 3,5-disubstituted pyrazole derivatives starting from salicylic acid and acyl acid hydrazides and tested their inhibitory activity against COX-1 and COX-2. Based on the results, compound 80 (Figure 13) showed excellent COX-2 inhibition, with IC50 value of 1.76 µM95.
In addition, there is a triazol ring in the structure of compounds 11b–11c (Figure 13), compounds 36a–36f (Figure 4), compound 65d (Figure 9), compound 67a (Figure 9), and compound 67d (Figure 9).
2.13. Oxazolidinone derivatives as anti-inflammatory agents
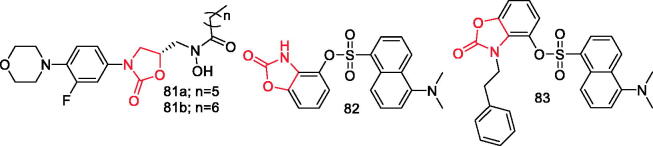
Phillips et al. synthesised oxazolidinone hydroxamic acid derivatives and evaluated their inhibitory activity against leukotriene (LT) biosynthesis in three in vitro cell-based test systems and on direct inhibition of recombinant human 5-lipoxygenase (5-LO). Compounds 81a and 81b (Figure 14) had outstanding potencies (IC50<1 mM) comparable to that of zileuton, the prototype 5-LO inhibitor. Pronounced in vivo activity of compounds 81a and 81b was demonstrated in zymosan-induced peritonitis mice model96.
Figure 14.
Oxazolidinone derivatives 81a–83 reported as anti-inflammatory agents.
Tang et al. synthesised 22 new 4‐sulfonyloxy/alkoxy benzoxazolone derivatives. The most active compound 82 (Figure 14), exhibited the strongest inhibitory activity against NO, IL-1β, and IL-6 production, with IC50 values of 17.67, 20.07, and 8.61 µΜ, respectively. After 2 h, the induced effects were comparable or stronger than those of the positive control (celecoxib). Compound 82 also displayed higher activity in vivo than celecoxib in a mouse model of xylene-induced ear oedema. Further molecular analysis revealed that compound 82 could inhibit activation of the MAPK-NF-κB/iNOS pathway, thereby reducing excessive release of NO, IL-1β, and IL-697.
Tang et al. synthesised eighteen new disubstituted benzoxazolone derivatives and evaluated their activity against NO production in an LPS-induced RAW264.7 cells. According to obtained results, compound 83 (Figure 14) showed NO inhibitory activity (IC50 value = 14.72 µM) and iNOS inhibitory activity (IC50 value = 3.342 µM). Furthermore, it could significantly protect against the LPS-induced ALI. All results demonstrated that compound 83 could be a promising lead structure for iNOS inhibitors, with anti-inflammatory activity, to treat LPS-induced ALI98.
2.14. Imidazole derivatives as anti-inflammatory agents
Metwally et al. synthesised new imidazol-5-one derivatives and screened for their in vivo anti-inflammatory activity using a standard acute carrageenan-induced rat paw oedema method. Compound 84b (Figure 15) produced the maximum effect (49.0%) compared to celecoxib (43.1%). After studying the interaction of the derivatives with COX-2 enzyme compared to celecoxib, it was found that the inhibitory effect of compound 84a (Figure 15) exhibited a high selectivity towards COX-2 inhibition (IC50 =0.087 µM)99.
Abdellatif et al. synthesised a new series of hybrid structures containing thiohydantoin and evaluated their COX inhibition ability. Results revealed that all compounds were more potent against COX-2 than COX-1, and compound 85 (Figure 15) had higher COX-2 inhibitory activity (IC50 =0.76 µM) than celecoxib (IC50 =0.84 µM). In addition, all derivatives were significantly less ulcerogenic (ulcer indexes = 2.64–3.87) than ibuprofen (ulcer index = 20.25). Among all the compounds, compound 85 showed the highest COX-2 SI = 10.72, while the SI value for celecoxib was 8.60. In this series of compounds, the methoxy substituted analogues showed higher cytotoxic activities than the respective unsubstituted derivatives100.
El-Sharief et al. synthesised new series of imidazolidineiminothione and imidazolidin-2,5-dione derivatives and evaluated for their anti-inflammatory activity. All the tested compounds exhibited high activity compared to the reference drug. Results showed that compound 86b (Figure 15) was the most potent active agent (IC50=0.0003 µM) compared to celecoxib (IC50=2.8 µM). In addition, the IC50 value of compound 86a (Figure 15) was 0.0021 µM. The study also determined the IC50 of all compounds against COX-2, with results showing that all of the synthesised products exhibited very low values reflecting their high potency101.
Zhu et al.102 synthesised 12 azole derivatives of emodin and assessed their anti-inflammatory activity. Among them, compound 87 (Figure 15) was the most potent anti-inflammatory agent, which showed the best inhibition of NO production. The IC50 of compound 87 in NO production was 1.35 µM, which was lower than that of indomethacin. Therefore, it can be deduced that compound 87 inhibited LPS-induced expression of inflammatory mediators in RAW 264.7 cells through blocking NF-κB pathways102.
In addition, there is an imidazole ring in the structure of compounds 32a–32b (Figure 3), compound 38 (Figure 4), compounds 46a–46b (Figure 5), compounds 47a–47b (Figure 5), and compound 75 (Figure 12).
2.15. Pyrrol derivatives as anti-inflammatory agents
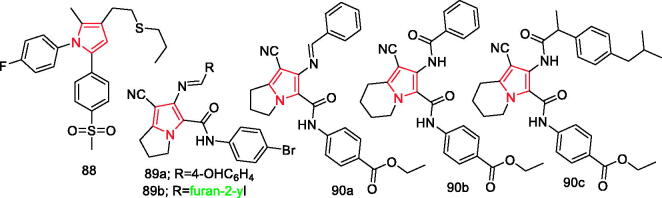
Reale et al. synthesised and characterised a novel series of 1,5-diarylpyrrol-3-sulphur derivatives as COX-2 selective inhibitors endowed with anti-inflammatory/antinociceptive profile. Compound 88 (Figure 16), with IC50 =0.011 µM, showed a significant anti-inflammatory and antinociceptive activity in vivo, thereby providing insights into the development of a novel anti-inflammatory and antinociceptive agent103.
Figure 16.
Pyrrol derivatives 88–90c reported as anti-inflammatory agents.
Shawky et al. synthesised two new series of pyrrolizine-5-carboxamides and evaluated their anticancer and anti-inflammatory activities. Results showed that compound 89a (Figure 16) was the most potent COX-2 inhibitor (IC50=0.64 ± 0.02 nM), while compound 89b (Figure 16) was the most active COX-1 inhibitor (IC50=13.16 ± 0.64 nM)104.
Attalah et al. designed and synthesised two new series of ethyl benzoate bearing pyrrolizine and indolizine moieties. According to the obtained results, compounds 90a, 90b, and 90c (Figure 16) displayed in vivo anti-inflammatory and analgesic activity nearly equal to or slightly higher than that of ibuprofen. Further mechanistic study of these compounds revealed inhibitory activity against COX-1/2 with preferential inhibition of COX-2105.
In addition, there is a pyrrol ring in the structure of compounds 15a–15d (Figure 2) and compounds 69a–69b (Figure 10).
2.16. Furan derivatives as anti-inflammatory agents
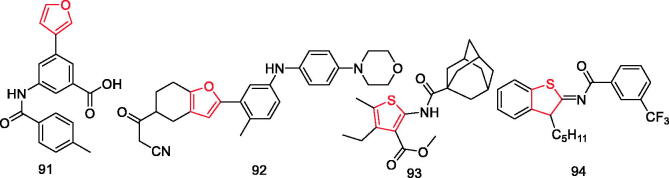
Lu et al. designed a series of 5-aryl-3-amide benzoic acid derivatives as novel P2Y14 antagonists with improved pharmacokinetic properties. Among them, compound 91 (Figure 17) showed the most potent P2Y14 antagonising activity, with an IC50 value of 2.18 nM. Moreover, it exerted promising in vivo efficacy in alleviating paw swelling and inflammatory infiltration in monosodium urate-induced acute gouty arthritis mice model. Elucidation of the preliminary mechanism of compound 91 indicated that it blocked pyroptosis of macrophages through inhibiting activation of NLRP3 inflammasome106.
Figure 17.
Furan derivatives 91–92 and Thiophene derivative 93–94 reported as anti-inflammatory agents.
Wang et al. designed and synthesised 4–(4,5,6,7-tetrahydrofuro[3,2-c]pyridin-2-yl) pyrimidin-2-amino derivatives as JAK inhibitors. The in vivo investigation revealed that compound 92 (Figure 17) possessed favourable pharmacokinetic properties and displayed slightly better anti-inflammatory efficacy than tofacitinib at the same dosage107.
In addition, there is a furan ring in the structure of compound 65b (Figure 9) and compound 88b (Figure 16).
2.17. Thiophene derivatives as anti-inflammatory agents
Mugnaini et al. synthesised a set of selective cannabinoid type 2 receptor (CB2R) ligands based on the thiophene scaffold. Results revealed that compound 93 (Figure 17) was able to completely abolish release of the human monocyte chemotactic protein-2 in HaCaT cells at 10 mM concentration, which suggested that it has potential to treat skin inflammatory disease. The SAR showed that 3-carboxylate and 2-carboxamido moieties are not decisive for receptor binding. On the other hand, insertion of substituents at the fifth position seems to be more effective since it led to a progressive increase of CB2R affinity, often accompanied by higher selectivity, and can be noticed in compounds bearing an increasingly larger substituent. However, polar groups, such as the sulphone group, or isosteric replacement of thiophene with more polar heterocycles led to far less active compounds108.
Ghonim et al. designed and synthesised novel thiazole and benzothiazole derivatives and screened for their binding affinity and functional activity on two G-protein coupled receptors (CB1 and CB2). Among them, compound 94 (Figure 17) exhibited remarkable protection against dextran sulphate sodium-induced acute colitis in mice model109.
In addition, there is a thiophene ring in the structure of compounds 2a–2b (Figure 1), compound 16e (Figure 2), compounds 21a–21b (Figure 2), compound 65a (Figure 9), and compound 65c (Figure 9).
2.18. Piperazine derivatives as anti-inflammatory agents
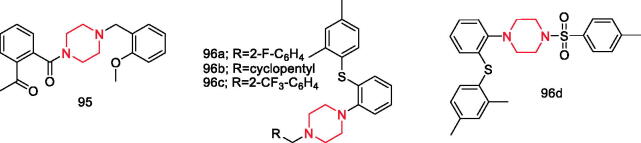
Liu et al. synthesised 20 novel talmapimod analogues and evaluated the in vivo anti-inflammatory activities. Results indicated that compound 95 (Figure 18) was the most potent. The in vitro enzymatic experiment identified compound 95 as a potent inhibitor against both p38a MAPK (IC50 =1.95 µM) and COX-2 (IC50 =0.036 µM). Furthermore, it had the ability to downregulate NF-κB and MAPK signalling pathways110.
Figure 18.
Piperazine derivatives 95–96d reported as anti-inflammatory agents.
Talmon et al. synthesised a series of novel vortioxetine derivatives. Among them, compound 96a (Figure 18) had significant antioxidant and anti-COX-1 activity, while compound 96b (Figure 18) exhibited good antioxidant and mild anti-COX-1/2 inhibitor activities, while compound 96c (Figure 18) was a good anti-COX-1/2 inhibitor and compound 96d (Figure 18) was more specific versus COX-2111.
In addition, there is a piperazine ring in the structure of compounds 35a–35b (Figure 4), compound 42 (Figure 4), compound 53 (Figure 6), compounds 69a–69b (Figure 10), compound 72 (Figure 11), compounds 78a–78f (Figure 13), and compound 79 (Figure 13).
2.19. Other derivatives as anti-inflammatory agents
Roriz et al. synthesised a nitro-Schiff base derivative and examined its anti-inflammatory effects using in vivo models of inflammation such as neutrophil migration, paw oedema, and exudation assays. The production of NO was also estimated in vivo and in vitro. Results showed that the compound 97 (Figure 19) efficiently inhibited inflammation and might be a good candidate for the treatment of inflammatory-associated conditions112.
Heiran et al. synthesised and characterised 29 monocyclic β-lactam derivatives bearing a morpholine ring substituent on the nitrogen. Compounds 98a–98h (Figure 19) showed higher activity with anti-inflammatory ratio values of 38, 62, 51, 72, 51, 35, 55, and 99, respectively, compared to the reference compound (dexamethasone) which had an anti-inflammatory ratio value of 32. This suggests that these compounds can be considered as potent iNOS inhibitors. The compounds also exhibited IC50 values of 0.48 ± 0.04, 0.51 ± 0.01, 0.22 ± 0.02, 0.12 ± 0.00, 0.25 ± 0.05, 0.82 ± 0.07, 0.44 ± 0.04, and 0.60 ± 0.04 mM, respectively, against HepG2 cells, and biocompatibility and non-toxic behaviour comparable to doxorubicin (IC50<0.01 mM)113.
Liu et al. reported a concise total synthesis of an exceedingly potent anti-inflammatory agent (violacin A) and screened its anti-inflammatory activity against NO production using LPS-induced Raw264.7 cells. The derivative, compound 99 (Figure 19), showed stronger inhibition of NO, IL-1β, IL-6, and TNF-α production via inhibiting the NF-κB signalling pathway in Raw 264.7 cells114.
Chen et al. designed and synthesised (E)-3,4-dihydroxystyryl alkyl sulphones, as new analogues of neurodegenerative agents. The results showed that compound 100 (Figure 19) displayed better anti-inflammatory activity (IC50=1.6 µM). Further activity studies indicated that the length of the linker and the conformation of cycloalkyl play critical roles in anti-inflammatory activities115.
Gu et al. synthesised a series of phenylacrylamide derivatives as novel anti-oxidant and anti-inflammatory agents. Compound 101 (Figure 19) could inhibit NO production and the activity of NF-kB in LPS-stimulated HBZY-1 mesangial cells, indicating its potential anti-inflammatory activity. Notably, compound 101 is associated with activation of the Nrf2 signalling pathway116.
Chen et al. designed and synthesised thirty-seven new resveratrol-based flavonol derivatives. The preliminary experiment revealed that compound 102 (Figure 19) suppressed LPS-induced expressions of iNOS and COX-2, and production of IL-6, TNF-a, and NO through the NF-kB/MAPK signalling pathway in a concentration-dependent manner. According to SARs, the introduction of a hydroxyl group intoflavonoid is beneficial to the anti-inflammatory activity117.
Zhang et al. synthesised a series of 3-amide benzoic acid derivatives as novel and potent P2Y14 receptor antagonists. The most promising antagonist, compound 103 (Figure 19), showed potent anti-inflammation in monosodium urate-treated THP-1 cells, which was comparable to PPTN (IC50=1.77 nM). Compound 103 can be further optimised towards the development of novel anti-inflammatory agents118.
Chen et al. designed and synthesised a total of 31 novel phthalide derivatives and screened their anti-inflammatory activities both in vitro and in vivo. Compound 104 (Figure 19) was found to be the most active one with low toxicity and it showed 95.23% inhibitory rate at 10 µM with a IC50 value of 0.76 mM against LPS-induced NO overexpression. Preliminary mechanism studies indicated that compound 104 activated the Nrf2/HO-1 signalling pathway via accumulation of ROS and blocking the NF-κB/MAPK signalling pathway. Moreover, the in vivo anti-inflammatory activity showed that compound 104 had obvious therapeutic effects against the adjuvant-induced rat arthritis model119.
Kumar et al. synthesised new cyclic enaminone derivatives and evaluated their inhibitory activities against cyclooxygenase (COX-1 and COX-2). Results demonstrated that three compounds (105a, 105b, and 105c (Figure 19)) predominantly inhibited COX-2, with SIs of 74.09, 108.68, and 19.45, respectively. The anti-inflammatory potency of compound 105a was comparable to that of celecoxib at a dose of 12.5 mg/kg, and compounds 105b and 105c were more/equally effective compared to celecoxib at 12.5 and 25 mg/kg doses120.
Elhenawy et al. designed and synthesised a safe nonsteroidal anti-inflammatory drug agent based on the Naproxen scaffold. According to the obtained results, compounds 106a and 106b (Figure 19) showed a higher anti-inflammatory potency than Naproxen121.
Chávez-Riveros et al. synthesised 16 diphenylamine derivatives and evaluated the in vivo anti-inflammatory activity through an ear oedema model using 12-O-tetradecanoylpholbol-13-acetate. Results revealed that compound 107 (Figure 19) analogue was more active than the commercial drug indomethacin in the in vivo study (92% and 89% inhibition of oedema, respectively). In addition to its remarkable anti-inflammatory capability, compound 107 was innocuous against RAW 264.7 cell line, with a comparable lethality in Artemia salina relative to diclofenac122.
Li et al. synthesised a series of 1,3-benzothiazinone derivatives. All the compounds had no obvious cytotoxicity in the in vitro assay. Compound 108 (Figure 19) displayed lower gastrointestinal toxicity than meloxicam. Besides, compound 108 decreased the swelling in carrageenan-induced paw oedema inflammation models and significantly reduced the PGE2 level. Importantly, the study found that 1,3-benzothiazinone derivatives are unique scaffolds with anti-inflammatory activity and low toxicity123.
Sakr et al. reported a new series of 1,4-dihydroquinazolin-3(2H)-yl benzamide derivatives as anti-inflammatory and analgesic agents, and COX-1/2 inhibitors. Compound 109 (Figure 19) showed the best in vitro COX-2 inhibitory activity (IC50 =0.05 µM). In addition, it showed the best results in most aspects where its oedema inhibition percentage (48%) was better than that of diclofenac sodium (37.8%), and the analgesic effect (3.4 writhes) was better than that of both indomethacin (12.6 writhes) and diclofenac sodium (17.4 writhes). The SAR study showed substitution of both phenyl rings was favourable because compounds with an unsubstituted phenyl ring showed lower activities in all the tests compared to their substituted analogues. Compounds carrying two nitro groups showed good in vitro COX inhibitory activity but with lower in vivo anti-inflammatory activity, which can be attributed to the high polarity of the two nitro groups that decreases the lipophilicity of the compound and hence decreases its bioavailability. To have good anti-inflammatory activity, at least one of the substituents should be halogen. This may be due to the lipophilicity of halogens which improves the bioavailability124.
Abolhasani et al. synthesised new indanone compounds containing spiroisoxazoline derivatives and evaluated their inhibitory activities for COX enzymes. Compound 110 (Figure 19) showed superior selectivity with higher potency of inhibiting COX-2 enzyme. Furthermore, compound 110 exhibited the highest COX-2 inhibitory activity, and displayed the most potent cytotoxicity on MCF-7 cells, with an IC50 value of 0.03 ± 0.01 µM which was comparable to that of doxorubicin (IC50 of 0.062 ± 0.012 µM). Moreover, it can lead to apoptosis of MCF-7 cells, which may be mediated by upregulating the expression of Bax/Bcl-2 and activating the caspase-3 pathway125.
3. Discussion and conclusion
This article screened and reviewed 110 articles of small molecules with good anti-inflammatory activity published from the literature since 2019. The figures in the article list 218 active compound structures. The statistical results from the elemental composition of these compounds are as follows: (1) Only the structures of compounds 20a (Figure 2), 24 (Figure 3), 65d (Figure 9), 75 (Figure 12), and 96a–96c (Figure 18) do not contain an O element, notably these structures contain S element; (2) only the structures of compounds 33a–33b (Figure 4), 34a (Figure 4), 41 (Figure 4), 43 (Figure 4), and 100 (Figure 19) do not contain N element, almost all of them are chalcone derivatives; (3) the S element appears in 105 active structures described in 52 articles; (4) the F element appears in 37 active structures described in 31 articles; (5) the Cl element appears published in 31 active structures in 28 articles; (6) the Br element appears discussed in 13 active structures in 13 articles. The statistical results from the functional group of these compounds are as follows: 60 pyrazole derivatives in 36 articles, 15 indole derivatives in 10 articles, 22 chalcone derivatives in 12 articles, 12 pyridine derivatives in eight articles, 11 pyrimidine derivatives in seven articles, 10 pyridazine derivatives in three articles, six quinoline derivatives in four articles, 12 pyrazolo[3,4-d]pyrimidine derivatives in five articles, 10 pyrrolidinedione derivatives in four articles, 16 thiazolidinone derivatives in seven articles, nine thiazole derivatives in six articles, 20 triazol derivatives in nine articles, four oxazolidinone derivatives in three articles, 14 imidazole derivatives in nine articles, 12 pyrrol derivatives in five articles, four furan derivatives in four articles, nine thiophene derivatives in seven articles, 19 piperazine derivatives in nine articles, and 12 morpholine derivatives 13a (Figure 2), 81a–81b (Figure 14), 92 (Figure 17), and 98a–98h (Figure 2) in four articles. From these results, it is evident that the structure containing the pyrazole ring as the selective COX-2 agent is now a research hot spot, and many pyrazole derivatives with good anti-inflammatory activity have been synthesised. Interestingly, there are two active compounds (14a (Figure 2) and 93 (Figure 17)) having adamantyl in the structure in two of the reviewed articles. All compounds, except 45a (Figure 5), 55b–55c (Figure 6), 58b (Figure 7), and 74a (Figure 11), contain a benzene ring structure. It is well known that the type and position of the substituents on the benzene ring have a significant impact on the biological activity of the structure. Therefore, we counted the substitutions of all benzene rings. Results indicated that there were many derivatives with a monosubstituted benzene ring: 54 unsubstituted derivatives of benzene ring in 30 articles, five derivatives with p-NO2-phenyl in four articles, two derivatives with o-F- phenyl in two articles, two derivatives with m-F-phenyl in two articles, 20 derivatives with p-F-phenyl in 17 articles, one derivative with o-Br-phenyl in one article, 11 derivatives with p-Br-phenyl in 11 articles, three derivatives with o-Cl-phenyl in three articles, 14 derivatives with p-Cl-phenyl in 12 articles, three derivatives with o-CF3-phenyl in three articles, one derivative with m-CF3-phenyl in one article, three derivatives with p-CF3-phenyl in three articles, one derivative with o-CH3-phenyl in one article, three derivatives with m-CH3 in two articles, eight derivatives with p-CH3 in eight articles, three derivatives with o-OCH3-phenyl in three articles, and 13 derivatives with p-OCH3-phenyl in 11 articles. Importantly, it was found that there are no m-Br, m-Cl, and m-OCH3 substituted compounds among all the compounds with benzene ring. In addition, we found some derivatives with a multi-substituted benzene ring: five derivatives with 2,4-(Cl)2-phenyl in four articles, two derivatives with 2,6-(Cl)2-phenyl in two articles, three derivatives with 3,4-(OCH3)2-phenyl in three articles, and 11 derivatives with 3,4,5-(OCH3)3-phenyl in eight articles. Moreover, the applications of some basic functional groups of all compounds were counted and the obtained results were as follows: six derivatives with -SO2NR1R2 (R1≠H, R2≠H) in six articles, 16 derivatives with -SO2CH3 in eight articles, 32 derivatives with –SO2NH2 in 13 articles, 113 derivatives with amide in 68 articles (among them are 66 cyclic lactams derivatives in 30 articles), 12 derivatives with –NH2 (–SO2NH2 not counted) in six articles, 14 derivatives with –COOH in 10 articles, 34 derivatives with –OH in 26 articles, and 24 derivatives with –CN in 14 articles. It was also found that the –C=N– bond is often used in the design and synthesis of anti-inflammatory compounds for molecular hybridisation or splicing of different structures (25 derivatives in 17 articles). Based on the above statistics, it is speculated that the above basic functional groups can be applied to the design of future anti-inflammatory drugs. Therefore, it is easier and direct to design compounds with good anti-inflammatory activity through hybridisation of many heterocycles with good anti-inflammatory activity (such as pyrazole) and some basic functional group molecules that perform well in anti-inflammatory compounds. However, researchers should not be limited to this because we have shown that there are many compounds with a simple structure but have good anti-inflammatory effects.
In summary, this review has discussed the active compound structures, biological activities, and SARs of newly reported derivatives with good anti-inflammatory activity. We hope that this article will provide more insights into the design of anti-inflammatory compounds, which will aid in the development of numerous anti-inflammatory compounds with good efficacy and low toxicity by researchers.
Funding Statement
This work was supported by the National Natural Science Funding of China [No. 81860798] and the Science and Technology Planning Project of Inner Mongolia Autonomous Region [No. 2020SGG3689].
Disclosure statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article.
References
- 1. Parikh NS, Merkler AE, Iadecola C.. Inflammation, autoimmunity, infection, and stroke: epidemiology and lessons from therapeutic intervention. Stroke 2020;51:711–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suzuki K. Chronic inflammation as an immunological abnormality and effectiveness of exercise. Biomolecules 2019;9:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bauer ME, Teixeira AL.. Inflammation in psychiatric disorders: what comes first? Ann NY Acad Sci 2019;1437:57–67. [DOI] [PubMed] [Google Scholar]
- 4.Beurel E, Toups M, Nemeroff CB.. The bidirectional relationship of depression and inflammation: double trouble. Neuron 2020;107:234–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soysal P, Arik F, Smith L, et al. Inflammation, frailty and cardiovascular disease. Adv Exp Med Biol 2020;1216:55–64. [DOI] [PubMed] [Google Scholar]
- 6.Singh N, Baby D, Rajguru JP, et al. Inflammation and cancer. Ann Afr Med 2019;18:121–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kay J, Thadhani E, Samson L, et al. Inflammation-induced DNA damage, mutations and cancer. DNA Repair 2019;83:102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolf D, Ley K.. Immunity and inflammation in atherosclerosis. Circ Res 2019;124:315–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu Y, Li W, Han Y, et al. Regulatory effects of non-steroidal anti-inflammatory drugs on cardiac ion channels Nav1.5 and Kv11.1. Chem Biol Interact 2021;338:109425. [DOI] [PubMed] [Google Scholar]
- 10.Bindu S, Mazumder S, Bandyopadhyay U.. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: a current perspective. Biochem Pharmacol 2020;180:114147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naumov AV, Tkacheva ON, Khovasova NO.. Safety of nonsteroidal anti-inflammatory drugs in patients with cardiovascular risk. Ter Arkh 2019;91:108–13. [DOI] [PubMed] [Google Scholar]
- 12.Poly TN, Islam M, Yang HC, et al. Non-steroidal anti-inflammatory drugs and risk of Parkinson’s disease in the elderly population: a meta-analysis. Eur J Clin Pharmacol 2019;75:99–108. [DOI] [PubMed] [Google Scholar]
- 13.Braun J, Baraliakos X, Westhoff T.. Nonsteroidal anti-inflammatory drugs and cardiovascular risk - a matter of indication. Semin Arthritis Rheum 2020;50:285–8. [DOI] [PubMed] [Google Scholar]
- 14.Sharma S, Kumar D, Singh G, et al. Recent advancements in the development of heterocyclic anti-inflammatory agents. Eur J Med Chem 2020;200:112438. [DOI] [PubMed] [Google Scholar]
- 15.Mahesh G, Anil KK, Reddanna P.. Overview on the discovery and development of anti-inflammatory drugs: should the focus be on synthesis or degradation of PGE2? J Inflamm Res 2021;14:253–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim B, Park J, Cho D, et al. 2-(5-(4-Chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazol-3-yl)-N-(2-hydroxyethyl)-2-oxoacetamide (CDMPO) has anti-inflammatory properties in microglial cells and prevents neuronal and behavioral deficits in MPTP mouse model of Parkinson's disease. Neuropharmacology 2020;166:107928. [DOI] [PubMed] [Google Scholar]
- 17.El-Shoukrofy MS, Abd El Razik HA, AboulWafa OM, et al. Pyrazoles containing thiophene, thienopyrimidine and thienotriazolopyrimidine as COX-2 selective inhibitors: design, synthesis, in vivo anti-inflammatory activity, docking and in silico chemo-informatic studies. Bioorg Chem 2019;85:541–57. [DOI] [PubMed] [Google Scholar]
- 18.Bansal G, Singh S, Monga V, et al. Synthesis and biological evaluation of thiazolidine-2,4-dione-pyrazole conjugates as antidiabetic, anti-inflammatory and antioxidant agents. Bioorg Chem 2019;92:103271. [DOI] [PubMed] [Google Scholar]
- 19.Abdellatif KRA, Fadaly WAA, Kamel GM, et al. Design, synthesis, modeling studies and biological evaluation of thiazolidine derivatives containing pyrazole core as potential anti-diabetic PPAR-γ agonists and anti-inflammatory COX-2 selective inhibitors. Bioorg Chem 2019;82:86–99. [DOI] [PubMed] [Google Scholar]
- 20.Abdellatif KRA, Abdelall EKA, Labib MB, et al. Design, synthesis of celecoxib-tolmetin drug hybrids as selective and potent COX-2 inhibitors. Bioorg Chem 2019;90:103029. [DOI] [PubMed] [Google Scholar]
- 21.Taher AT, Mostafa Sarg MT, El-Sayed Ali NR, et al. Design, synthesis, modeling studies and biological screening of novel pyrazole derivatives as potential analgesic and anti-inflammatory agents. Bioorg Chem 2019;89:103023. [DOI] [PubMed] [Google Scholar]
- 22.Zabiulla Gulnaz AR, Mohammed Y, et al. Design, synthesis and molecular docking of benzophenone conjugated with oxadiazole sulphur bridge pyrazole pharmacophores as anti inflammatory and analgesic agents. Bioorg Chem 2019;92:103220. [DOI] [PubMed] [Google Scholar]
- 23.Abdellatif KRA, Abdelall EKA, Lamie PF, et al. New pyrazole derivatives possessing amino/methanesulphonyl pharmacophore with good gastric safety profile: design, synthesis, cyclooxygenase inhibition, anti-inflammatory activity and histopathological studies. Bioorg Chem 2020;95:103540. [DOI] [PubMed] [Google Scholar]
- 24.Gedawy EM, Kassab AE, El Kerdawy AM.. Design, synthesis and biological evaluation of novel pyrazole sulfonamide derivatives as dual COX-2/5-LOX inhibitors. Eur J Med Chem 2020;189:112066. [DOI] [PubMed] [Google Scholar]
- 25.Abdelall EKA. Synthesis and biological evaluations of novel isoxazoles and furoxan derivative as anti-inflammatory agents. Bioorg Chem 2020;94:103441. [DOI] [PubMed] [Google Scholar]
- 26.Ibrahim TS, Salem IM, Mostafa SM, et al. Design, synthesis, and pharmacological evaluation of novel and selective COX-2 inhibitors based on bumetanide scaffold. Bioorg Chem 2020;100:103878. [DOI] [PubMed] [Google Scholar]
- 27.El-Gamal MI, Anbar HS, Tarazi H, et al. Discovery of a potent p38α/MAPK14 kinase inhibitor: synthesis, in vitro/in vivo biological evaluation, and docking studies. Eur J Med Chem 2019;183:111684. [DOI] [PubMed] [Google Scholar]
- 28.Hassan GS, Abdel Rahman DE, Abdelmajeed EA, et al. New pyrazole derivatives: synthesis, anti-inflammatory activity, cycloxygenase inhibition assay and evaluation of mPGES. Eur J Med Chem 2019;171:332–42. [DOI] [PubMed] [Google Scholar]
- 29.Abdelazeem AH, Safi El-Din AG, Abdel-Fattah MM, et al. Discovery of novel urea-diarylpyrazole hybrids as dual COX-2/sEH inhibitors with improved anti-inflammatory activity and highly reduced cardiovascular risks. Eur J Med Chem 2020;205:112662. [DOI] [PubMed] [Google Scholar]
- 30.Mehta DK, Taya P, Das R, et al. Design, synthesis and molecular docking studies of novel thiadiazole analogues with potential antimicrobial and antiinflammatory activities. Antiinflamm Antiallergy Agents Med Chem 2019;18:91–109. [DOI] [PubMed] [Google Scholar]
- 31.Abd El-Karim SS, Mohamed HS, Abdelhameed MF, et al. Design, synthesis and molecular docking of new pyrazole-thiazolidinones as potent anti-inflammatory and analgesic agents with TNF-α inhibitory activity. Bioorg Chem 2021;111:104827. [DOI] [PubMed] [Google Scholar]
- 32.Abdellatif KRA, Abdelall EKA, Labib MB, et al. Synthesis of novel halogenated triarylpyrazoles as selective COX-2 inhibitors: anti-inflammatory activity, histopatholgical profile and in-silico studies. Bioorg Chem 2020;105:104418. [DOI] [PubMed] [Google Scholar]
- 33.Ragab F, Mohammed EI, Abdel JG, et al. Synthesis of hydroxybenzofuranyl-pyrazolyl and hydroxyphenyl-pyrazolyl chalcones and their corresponding pyrazoline derivatives as COX inhibitors, anti-inflammatory and gastroprotective agents. Chem Pharm Bull (Tokyo) 2020;68:742–52. [DOI] [PubMed] [Google Scholar]
- 34.Mohamed LW, Shaaban MA, Zaher AF, et al. Synthesis of new pyrazoles and pyrozolo [3,4-b] pyridines as anti-inflammatory agents by inhibition of COX-2 enzyme. Bioorg Chem 2019;83:47–54. [DOI] [PubMed] [Google Scholar]
- 35.Mroueh M, Faour WH, Shebaby WN, et al. Synthesis, biological evaluation and modeling of hybrids from tetrahydro-1H-pyrazolo[3,4-b]quinolines as dual cholinestrase and COX-2 inhibitors. Bioorg Chem 2020;100:103895. [DOI] [PubMed] [Google Scholar]
- 36.Dennis Bilavendran J, Manikandan A, Thangarasu P, et al. Synthesis and discovery of pyrazolo-pyridine analogs as inflammation medications through pro- and anti-inflammatory cytokine and COX-2 inhibition assessments. Bioorg Chem 2020;94:103484. [DOI] [PubMed] [Google Scholar]
- 37.Labib MB, Fayez AM, EL-Nahass E, et al. Novel tetrazole-based selective COX-2 inhibitors: design, synthesis, anti-inflammatory activity, evaluation of PGE2, TNF-α, IL-6 and histopathological study. Bioorg Chem 2020;104:104308. [DOI] [PubMed] [Google Scholar]
- 38.Taher ES, Ibrahim TS, Fares M, et al. Novel benzenesulfonamide and 1,2-benzisothiazol-3(2H)-one-1,1-dioxide derivatives as potential selective COX-2 inhibitors. Eur J Med Chem 2019;171:372–82. [DOI] [PubMed] [Google Scholar]
- 39.Song Z, Zhou Y, Zhang W, et al. Base promoted synthesis of novel indole-dithiocarbamate compounds as potential anti-inflammatory therapeutic agents for treatment of acute lung injury. Eur J Med Chem 2019;171:54–65. [DOI] [PubMed] [Google Scholar]
- 40.Ju Z, Su M, Hong J, et al. Design of balanced COX inhibitors based on anti-inflammatory and/or COX-2 inhibitory ascidian metabolites. Eur J Med Chem 2019;180:86–98. [DOI] [PubMed] [Google Scholar]
- 41.Fan X, Li J, Deng X, et al. Design, synthesis and bioactivity study of N-salicyloyl tryptamine derivatives as multifunctional agents for the treatment of neuroinflammation. Eur J Med Chem 2020;193:112217. [DOI] [PubMed] [Google Scholar]
- 42.Sasidharan R, Sreedharannair LM, Mohanan R, et al. Anti-inflammatory effect of synthesized indole-based chalcone (2E)-3-(4-bromophenyl)-1-(1H-indol-3-yl) prop-2-en-1-one: an in vitro and in vivo studies. Immunopharmacol Immunotoxicol 2019;41:568–76. [DOI] [PubMed] [Google Scholar]
- 43.Liu Z, Chen L, Yu P, et al. Discovery of 3-(Indol-5-yl)-indazole derivatives as novel myeloid differentiation protein 2/Toll-like receptor 4 antagonists for treatment of acute lung injury. J Med Chem 2019;62:5453–69. [DOI] [PubMed] [Google Scholar]
- 44.Silva P, de Almeida M, Silva J, et al. (E)-2-Cyano-3-(1H-Indol-3-yl)-N-phenylacrylamide, a hybrid compound derived from indomethacin and paracetamol: design, synthesis and evaluation of the anti-inflammatory potential. Int J Mol Sci 2020;21:2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang R, Li H, Zhang X, et al. Design, synthesis, and biological evaluation of tetrahydroisoquinolines derivatives as novel, selective PDE4 inhibitors for antipsoriasis treatment. Eur J Med Chem 2021;211:113004. [DOI] [PubMed] [Google Scholar]
- 46.Periyasami G, Antonisamy P, Perumal K, et al. A competent synthesis and efficient anti-inflammatory responses of isatinimino acridinedione moiety via suppression of in vivo NF-κB, COX-2 and iNOS signaling. Bioorg Chem 2019;90:103047. [DOI] [PubMed] [Google Scholar]
- 47.Rajesh Kumar M, Violet Dhayabaran V, Sudhapriya N, et al. p-TSA.H2O mediated one-pot, multi-component synthesis of isatin derived imidazoles as dual-purpose drugs against inflammation and cancer. Bioorg Chem 2020;102:104046. [DOI] [PubMed] [Google Scholar]
- 48.Fu ZY, Jin QH, Qu YL, et al. Chalcone derivatives bearing chromen or benzo[f]chromen moieties: design, synthesis, and evaluations of anti-inflammatory, analgesic, selective COX-2 inhibitory activities. Bioorg Med Chem Lett 2019;29:1909–12. [DOI] [PubMed] [Google Scholar]
- 49.Ribeiro D, Proença C, Varela C, et al. New phenolic cinnamic acid derivatives as selective COX-2 inhibitors. Design, synthesis, biological activity and structure-activity relationships. Bioorg Chem 2019;91:103179. [DOI] [PubMed] [Google Scholar]
- 50.Tang Y, Zheng X, Qi Y, et al. Synthesis and anti-inflammatory evaluation of new chalcone derivatives bearing bispiperazine linker as IL-1β inhibitors. Bioorg Chem 2020;98:103748. [DOI] [PubMed] [Google Scholar]
- 51.Boshra AN, Abdu-Allah HHM, Mohammed AF, et al. Click chemistry synthesis, biological evaluation and docking study of some novel 2'-hydroxychalcone-triazole hybrids as potent anti-inflammatory agents. Bioorg Chem 2020;95:103505. [DOI] [PubMed] [Google Scholar]
- 52.Hu YS, Han X, Yu PJ, et al. Novel paeonol derivatives: design, synthesis and anti-inflammatory activity in vitro and in vivo. Bioorg Chem 2020;98:103735. [DOI] [PubMed] [Google Scholar]
- 53.Ji L, Qu L, Wang C, et al. Identification and optimization of piperlongumine analogues as potential antioxidant and anti-inflammatory agents via activation of Nrf2. Eur J Med Chem 2021;210:112965. [DOI] [PubMed] [Google Scholar]
- 54.Yao B, Sun Y, Chen S, et al. Dissymmetric pyridyl-substituted 3,5-bis(arylidene)-4-piperidones as anti-hepatoma agents by inhibiting NF-κB pathway activation. Eur J Med Chem 2019;167:187–99. [DOI] [PubMed] [Google Scholar]
- 55.Qian J, Chen X, Shu S, et al. Design and synthesis novel di-carbonyl analogs of curcumin (DACs) act as potent anti-inflammatory agents against LPS-induced acute lung injury (ALI). Eur J Med Chem 2019;167:414–25. [DOI] [PubMed] [Google Scholar]
- 56.Emam SH, Sonousi A, Osman EO, et al. Design and synthesis of methoxyphenyl- and coumarin-based chalcone derivatives as anti-inflammatory agents by inhibition of NO production and down-regulation of NF-κB in LPS-induced RAW264.7 macrophage cells. Bioorg Chem 2021;107:104630. [DOI] [PubMed] [Google Scholar]
- 57.Ma Y, Zheng X, Zhu P, et al. Novel resveratrol-chalcone derivatives: synthesis and biological evaluation. Mini Rev Med Chem 2019;19:424–36. [DOI] [PubMed] [Google Scholar]
- 58.Gao CL, Hou GG, Liu J, et al. Synthesis and target identification of benzoxepane derivatives as potential anti-neuroinflammatory agents for ischemic stroke. Angew Chem Int Ed Engl 2020;59:2429–39. [DOI] [PubMed] [Google Scholar]
- 59.Ajiaikebaier D, Li Z, Lin T, et al. Synthesis of pyranochalcone derivatives and their inhibitory effect on NF-κB activation. Bioorg Med Chem Lett 2021;42:128042. [DOI] [PubMed] [Google Scholar]
- 60.Jiang Y, Rakesh KP, Alharbi NS, et al. Radical scavenging and anti-inflammatory activities of (hetero)arylethenesulfonyl fluorides: synthesis and structure-activity relationship (SAR) and QSAR studies. Bioorg Chem 2019;89:103015. [DOI] [PubMed] [Google Scholar]
- 61.El-Kerdawy MM, Ghaly MA, Darwish SA, et al. New benzimidazothiazole derivatives as anti-inflammatory, antitumor active agents: synthesis, in-vitro and in-vivo screening and molecular modeling studies. Bioorg Chem 2019;83:250–61. [DOI] [PubMed] [Google Scholar]
- 62.Ali EMH, Abdel-Maksoud MS, Hassan RM, et al. Design, synthesis and anti-inflammatory activity of imidazol-5-yl pyridine derivatives as p38α/MAPK14 inhibitor. Bioorg Med Chem 2021;31:115969. [DOI] [PubMed] [Google Scholar]
- 63.El-Dash Y, Khalil NA, Ahmed EM, et al. Synthesis and biological evaluation of new nicotinate derivatives as potential anti-inflammatory agents targeting COX-2 enzyme. Bioorg Chem 2021;107:104610. [DOI] [PubMed] [Google Scholar]
- 64.Gonçalves DS, de S, Melo SM, et al. Synthesis of novel 3,5,6-trisubstituted 2-pyridone derivatives and evaluation for their anti-inflammatory activity. Bioorg Med Chem 2020;28:115549. [DOI] [PubMed] [Google Scholar]
- 65.Yaqoob S, Nasim N, Khanam R, et al. Synthesis of highly potent anti-inflammatory compounds (ROS inhibitors) from isonicotinic acid. Molecules 2021;26:1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sun Y, Gao Z, Yan W, et al. Discovery of novel NF-кB inhibitor based on scaffold hopping: 1,4,5,6,7,8-hexahydropyrido[4,3-d]pyrimidine. Eur J Med Chem 2020;198:112366. [DOI] [PubMed] [Google Scholar]
- 67.Bakr RB, Ghoneim AA, Azouz AA.. Selective cyclooxygenase inhibition and ulcerogenic liability of some newly prepared anti-inflammatory agents having thiazolo[4,5-d]pyrimidine scaffold. Bioorg Chem 2019;88:102964. [DOI] [PubMed] [Google Scholar]
- 68.Szczęśniak-Sięga BM, Wiatrak B, Czyżnikowska Ż, et al. Synthesis and biological evaluation as well as in silico studies of arylpiperazine-1,2-benzothiazine derivatives as novel anti-inflammatory agents. Bioorg Chem 2021;106:104476. [DOI] [PubMed] [Google Scholar]
- 69.Alfayomy AM, Abdel-Aziz SA, Marzouk AA, et al. Design and synthesis of pyrimidine-5-carbonitrile hybrids as COX-2 inhibitors: anti-inflammatory activity, ulcerogenic liability, histopathological and docking studies. Bioorg Chem 2021;108:104555. [DOI] [PubMed] [Google Scholar]
- 70.Hassanein HH, Abdel Rahman DE, Fouad MA, et al. Synthesis of new hexahydropyrimido[1,2-a]azepine derivatives bearing functionalized aryl and heterocyclic moieties as anti-inflammatory agents. Future Med Chem 2021;13:625–41. [DOI] [PubMed] [Google Scholar]
- 71.Abdel-Aziz SA, Taher ES, Lan P, et al. Design, synthesis, and biological evaluation of new pyrimidine-5-carbonitrile derivatives bearing 1,3-thiazole moiety as novel anti-inflammatory EGFR inhibitors with cardiac safety profile. Bioorg Chem 2021;111:104890. [DOI] [PubMed] [Google Scholar]
- 72.Loksha YM, Abd Alhaseeb MM.. Synthesis and biological screening of some novel 6-substituted 2-alkylpyridazin-3(2H)-ones as anti-inflammatory and analgesic agents. Arch Pharm (Weinheim) 2020;353:e1900295. [DOI] [PubMed] [Google Scholar]
- 73.Ahmed EM, Kassab AE, El-Malah AA, et al. Synthesis and biological evaluation of pyridazinone derivatives as selective COX-2 inhibitors and potential anti-inflammatory agents. Eur J Med Chem 2019;171:25–37. [DOI] [PubMed] [Google Scholar]
- 74.Ahmed EM, Hassan MSA, El-Malah AA, et al. New pyridazine derivatives as selective COX-2 inhibitors and potential anti-inflammatory agents; design, synthesis and biological evaluation. Bioorg Chem 2020;95:103497. [DOI] [PubMed] [Google Scholar]
- 75.Zhang W, Li P, Zhao M, et al. Synthesis and identification of quinoline derivatives as topoisomerase I inhibitors with potent antipsoriasis activity in an animal model. Bioorg Chem 2019;88:102899. [DOI] [PubMed] [Google Scholar]
- 76.Zuo J, Wang S, Jiang X, et al. Design, synthesis and biological evaluation of novel arylpropionic esters for the treatment of acute kidney injury. Bioorg Chem 2020;105:104455. [DOI] [PubMed] [Google Scholar]
- 77.Debnath U, Mukherjee S, Joardar N, et al. Aryl quinolinyl hydrazone derivatives as anti-inflammatory agents that inhibit TLR4 activation in the macrophages. Eur J Pharm Sci 2019;134:102–15. [DOI] [PubMed] [Google Scholar]
- 78.Yang C, Hung Y, Tang K, et al. Discovery of 2-substituted 3-arylquinoline derivatives as potential anti-inflammatory agents through inhibition of LPS-induced inflammatory responses in macrophages. Molecules 2019;24:1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Somakala K, Tariq S, Amir M.. Synthesis, evaluation and docking of novel pyrazolo pyrimidines as potent p38α MAP kinase inhibitors with improved anti-inflammatory, ulcerogenic and TNF-α inhibitory properties. Bioorg Chem 2019;87:550–9. [DOI] [PubMed] [Google Scholar]
- 80.Abdelall EKA, Lamie PF, Ahmed AKM, et al. COX-1/COX-2 inhibition assays and histopathological study of the new designed anti-inflammatory agent with a pyrazolopyrimidine core. Bioorg Chem 2019;86:235–53. [DOI] [PubMed] [Google Scholar]
- 81.Atatreh N, Youssef AM, Ghattas MA, et al. Anti-inflammatory drug approach: synthesis and biological evaluation of novel pyrazolo[3,4-d]pyrimidine compounds. Bioorg Chem 2019;86:393–400. [DOI] [PubMed] [Google Scholar]
- 82.Tageldin GN, Ibrahim TM, Fahmy SM, et al. Synthesis, modeling and biological evaluation of some pyrazolo[3,4-d]pyrimidinones and pyrazolo[4,3-e][1,2,4]triazolo[4,3-a]pyrimidinones as anti-inflammatory agents. Bioorg Chem 2019;90:102844. [DOI] [PubMed] [Google Scholar]
- 83.Shi JB, Chen LZ, Wang BS, et al. Novel pyrazolo[4,3- d]pyrimidine as potent and orally active inducible nitric oxide synthase (iNOS) dimerization inhibitor with efficacy in rheumatoid arthritis mouse model. J Med Chem 2019;62:4013–31. [DOI] [PubMed] [Google Scholar]
- 84.Redzicka A, Czyżnikowska Ż, Wiatrak B, et al. Design and synthesis of N-substituted 3,4-pyrroledicarboximides as potential anti-inflammatory agents. Int J Mol Sci 2021;22:1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jan MS, Ahmad S, Hussain F, et al. Design, synthesis, in-vitro, in-vivo and in-silico studies of pyrrolidine-2,5-dione derivatives as multitarget anti-inflammatory agents. Eur J Med Chem 2020;186:111863. [DOI] [PubMed] [Google Scholar]
- 86.Abdel-Aziz AAM, Angeli A, El-Azab AS, et al. Synthesis and anti-inflammatory activity of sulfonamides and carboxylates incorporating trimellitimides: dual cyclooxygenase/carbonic anhydrase inhibitory actions. Bioorg Chem 2019;84:260–8. [DOI] [PubMed] [Google Scholar]
- 87.Elkamhawy A, Kim NY, Hassan AHE, et al. Thiazolidine-2,4-dione-based irreversible allosteric IKK-β kinase inhibitors: optimization into in vivo active anti-inflammatory agents. Eur J Med Chem 2020;188:111955. [DOI] [PubMed] [Google Scholar]
- 88.Ranjan Srivastava A, Bhatia R, Chawla P.. Synthesis, biological evaluation and molecular docking studies of novel 3,5-disubstituted 2,4-thiazolidinediones derivatives. Bioorg Chem 2019;89:102993. [DOI] [PubMed] [Google Scholar]
- 89.Omar YM, Abdel-Moty SG, Abdu-Allah HHM.. Further insight into the dual COX-2 and 15-LOX anti-inflammatory activity of 1,3,4-thiadiazole-thiazolidinone hybrids: the contribution of the substituents at 5th positions is size dependent. Bioorg Chem 2020;97:103657. [DOI] [PubMed] [Google Scholar]
- 90.Maghraby MTE, Abou-Ghadir OMF, Abdel-Moty SG, et al. Novel class of benzimidazole-thiazole hybrids: the privileged scaffolds of potent anti-inflammatory activity with dual inhibition of cyclooxygenase and 15-lipoxygenase enzymes. Bioorg Med Chem 2020;28:115403. [DOI] [PubMed] [Google Scholar]
- 91.Sağlık BN, Osmaniye D, Levent S, et al. Design, synthesis and biological assessment of new selective COX-2 inhibitors including methyl sulfonyl moiety. Eur J Med Chem 2021;209:112918. [DOI] [PubMed] [Google Scholar]
- 92.Li S, Tsai S, Chiang C, et al. New methyl 5-(halomethyl)-1-aryl-1H-1,2,4-triazole-3-carboxylates as selective COX-2 inhibitors and anti-inflammatory agents: design, synthesis, biological evaluation, and docking study. Bioorg Chem 2020;104:104333. [DOI] [PubMed] [Google Scholar]
- 93.Avci A, Taşci H, Kandemir Ü, et al. Synthesis, characterization, and in vivo pharmacological evaluation of novel mannich bases derived from 1,2,4-triazole containing a naproxen moiety. Bioorg Chem 2020;100:103892. [DOI] [PubMed] [Google Scholar]
- 94.Shen QK, Gong GH, Li G, et al. Discovery and evaluation of novel synthetic 5-alkyl-4-oxo-4,5-dihydro-[1,2,4]triazolo[4,3-a]quinoxaline-1-carbox-amide derivatives as anti-inflammatory agents. J Enzyme Inhib Med Chem 2020;35:85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Munir A, Khushal A, Saeed K, et al. Synthesis, in-vitro, in-vivo anti-inflammatory activities and molecular docking studies of acyl and salicylic acid hydrazide derivatives. Bioorg Chem 2020;104:104168. [DOI] [PubMed] [Google Scholar]
- 96.Phillips OA, Bosso MA, Ezeamuzie CI.. Synthesis and structure-activity relationships of novel 5-(hydroxamic acid)methyl oxazolidinone derivatives as 5-lipoxygenase inhibitors. J Enzyme Inhib Med Chem 2020;35:1471–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tang L, Luo JR, Wang XY, et al. 4-Sulfonyloxy/alkoxy benzoxazolone derivatives with high anti-inflammatory activities: synthesis, biological evaluation, and mechanims of action via p38/ERK-NF-κB/iNOS pathway. Chem Biol Drug Des 2021;97:200–9. [DOI] [PubMed] [Google Scholar]
- 98.Tang L, Gao X, Zhao B, et al. Design and synthesis of new disubstituted benzoxazolone derivatives that act as iNOS inhibitors with potent anti-inflammatory activity against LPS-induced acute lung injury (ALI). Bioorg Med Chem 2020;28:115733. [DOI] [PubMed] [Google Scholar]
- 99.Metwally NH, Mohamed MS.. New imidazolone derivatives comprising a benzoate or sulfonamide moiety as anti-inflammatory and antibacterial inhibitors: design, synthesis, selective COX-2, DHFR and molecular-modeling study. Bioorg Chem 2020;99:103438. [DOI] [PubMed] [Google Scholar]
- 100.Abdellatif KRA, Fadaly WAA, Mostafa YA, et al. Thiohydantoin derivatives incorporating a pyrazole core: design, synthesis and biological evaluation as dual inhibitors of topoisomerase-I and cycloxygenase-2 with anti-cancer and anti-inflammatory activities. Bioorg Chem 2019;91:103132. [DOI] [PubMed] [Google Scholar]
- 101.El-Sharief MAMS, Abbas SY, El-Sharief AMS, et al. 5-Thioxoimidazolidine-2-one derivatives: synthesis, anti-inflammatory activity, analgesic activity, COX inhibition assay and molecular modelling study. Bioorg Chem 2019;87:679–87. [DOI] [PubMed] [Google Scholar]
- 102.Zhu X, Chen Q, Yang Y, et al. Synthesis and anti-inflammatory effects of novel emodin derivatives bearing azole moieties. Arch Pharm (Weinheim) 2020;353:e1900264. [DOI] [PubMed] [Google Scholar]
- 103.Reale A, Brogi S, Chelini A, et al. Synthesis, biological evaluation and molecular modeling of novel selective COX-2 inhibitors: sulfide, sulfoxide, and sulfone derivatives of 1,5-diarylpyrrol-3-substituted scaffold. Bioorg Med Chem 2019;27:115045. [DOI] [PubMed] [Google Scholar]
- 104.Shawky AM, Abourehab MAS, Abdalla AN, et al. Optimization of pyrrolizine-based Schiff bases with 4-thiazolidinone motif: design, synthesis and investigation of cytotoxicity and anti-inflammatory potency. Eur J Med Chem 2020;185:111780. [DOI] [PubMed] [Google Scholar]
- 105.Attalah KM, Abdalla AN, Aslam A, et al. Ethyl benzoate bearing pyrrolizine/indolizine moieties: design, synthesis and biological evaluation of anti-inflammatory and cytotoxic activities. Bioorg Chem 2020;94:103371. [DOI] [PubMed] [Google Scholar]
- 106.Lu R, Wang Y, Liu C, et al. Design, synthesis and evaluation of 3-amide-5-aryl benzoic acid derivatives as novel P2Y14R antagonists with potential high efficiency against acute gouty arthritis. Eur J Med Chem 2021;216:113313. [DOI] [PubMed] [Google Scholar]
- 107.Wang Y, Huang W, Xin M, et al. Discovery of potent anti-inflammatory 4-(4,5,6,7-tetrahydrofuro[3,2-c]pyridin-2-yl) pyrimidin-2-amines for use as Janus kinase inhibitors. Bioorg Med Chem 2019;27:2592–7. [DOI] [PubMed] [Google Scholar]
- 108.Mugnaini C, Rabbito A, Brizzi A, et al. Synthesis of novel 2-(1-adamantanylcarboxamido)thiophene derivatives. Selective cannabinoid type 2 (CB2) receptor agonists as potential agents for the treatment of skin inflammatory disease. Eur J Med Chem 2019;161:239–51. [DOI] [PubMed] [Google Scholar]
- 109.Ghonim AE, Ligresti A, Rabbito A, et al. Structure-activity relationships of thiazole and benzothiazole derivatives as selective cannabinoid CB2 agonists with in vivo anti-inflammatory properties. Eur J Med Chem 2019;180:154–70. [DOI] [PubMed] [Google Scholar]
- 110.Liu W, Hou C, Li J, et al. Discovery of talmapimod analogues as polypharmacological anti-inflammatory agents. J Enzyme Inhib Med Chem 2020;35:187–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Talmon M, Chaudhari RD, Suryavanshi H, et al. Design, synthesis and biological evaluation of vortioxetine derivatives as new COX-1/2 inhibitors in human monocytes. Bioorg Med Chem 2020;28:115760. [DOI] [PubMed] [Google Scholar]
- 112.Roriz BC, Buccini DF, Santos BFD, et al. Synthesis and biological activities of a nitro-shiff base compound as a potential anti-inflammatory agent. Eur J Pharm Sci 2020;148:105300. [DOI] [PubMed] [Google Scholar]
- 113.Heiran R, Sepehri S, Jarrahpour A, et al. Synthesis, docking and evaluation of in vitro anti-inflammatory activity of novel morpholine capped β-lactam derivatives. Bioorg Chem 2020;102:104091. [DOI] [PubMed] [Google Scholar]
- 114.Liu Q, Mu Y, An Q, et al. Total synthesis and anti-inflammatory evaluation of violacin A and its analogues. Bioorg Chem 2020;94:103420. [DOI] [PubMed] [Google Scholar]
- 115.Chen Y, Wu B, Hao Y, et al. Structure-activity relationship studies of (E)-3,4-dihydroxystyryl alkyl sulfones as novel neuroprotective agents based on improved antioxidant, anti-inflammatory activities and BBB permeability. Eur J Med Chem 2019;171:420–33. [DOI] [PubMed] [Google Scholar]
- 116.Gu X, Chen J, Zhang Y, et al. Synthesis and assessment of phenylacrylamide derivatives as potential anti-oxidant and anti-inflammatory agents. Eur J Med Chem 2019;180:62–71. [DOI] [PubMed] [Google Scholar]
- 117.Chen LZ, Yao L, Jiao MM, et al. Novel resveratrol-based flavonol derivatives: synthesis and anti-inflammatory activity in vitro and in vivo. Eur J Med Chem 2019;175:114–28. [DOI] [PubMed] [Google Scholar]
- 118.Zhang Z, Hao K, Li H, et al. Design, synthesis and anti-inflammatory evaluation of 3-amide benzoic acid derivatives as novel P2Y14 receptor antagonists. Eur J Med Chem 2019;181:111564. [DOI] [PubMed] [Google Scholar]
- 119.Chen LZ, Wu J, Li K, et al. Novel phthalide derivatives: synthesis and anti-inflammatory activity in vitro and in vivo. Eur J Med Chem 2020;206:112722. [DOI] [PubMed] [Google Scholar]
- 120.Kumar R, Saha N, Purohit P, et al. Cyclic enaminone as new chemotype for selective cyclooxygenase-2 inhibitory, anti-inflammatory, and analgesic activities. Eur J Med Chem 2019;182:111601. [DOI] [PubMed] [Google Scholar]
- 121.Elhenawy AA,A, Harbi LM, El-Gazzar MA, et al. Naproxenylamino acid derivatives: design, synthesis, docking, QSAR and anti-inflammatory and analgesic activity. Biomed Pharmacother 2019;116:109024. [DOI] [PubMed] [Google Scholar]
- 122.Chávez-Riveros A, Hernández-Vázquez E, Ramírez-Trinidad Á, et al. Multicomponent synthesis and preliminary anti-inflammatory activity of lipophilic diphenylamines. Bioorg Med Chem Lett 2021;38:127860. [DOI] [PubMed] [Google Scholar]
- 123.Li J, Fan X, Deng J, et al. Design and synthesis of 1,3-benzothiazinone derivatives as potential anti-inflammatory agents. Bioorg Med Chem 2020;28:115526. [DOI] [PubMed] [Google Scholar]
- 124.Sakr A, Kothayer H, Ibrahim SM, et al. 1,4-Dihydroquinazolin-3(2H)-yl benzamide derivatives as anti-inflammatory and analgesic agents with an improved gastric profile: design, synthesis, COX-1/2 inhibitory activity and molecular docking study. Bioorg Chem 2019;84:76–86. [DOI] [PubMed] [Google Scholar]
- 125.Abolhasani H, Zarghi A, Komeili Movahhed T, et al. Design, synthesis and biological evaluation of novel indanone containing spiroisoxazoline derivatives with selective COX-2 inhibition as anticancer agents. Bioorg Med Chem 2021;32:115960. [DOI] [PubMed] [Google Scholar]