Abstract
Facial imaging and facial recognition technologies, now common in our daily lives, also are increasingly incorporated into health care processes, enabling touch-free appointment check-in, matching patients accurately, and assisting with the diagnosis of certain medical conditions. The use, sharing, and storage of facial data is expected to expand in coming years, yet little is documented about the perspectives of patients and participants regarding these uses. We developed a pair of surveys to gather public perspectives on uses of facial images and facial recognition technologies in healthcare and in health-related research in the United States. We used Qualtrics Panels to collect responses from general public respondents using two complementary and overlapping survey instruments; one focused on six types of biometrics (including facial images and DNA) and their uses in a wide range of societal contexts (including healthcare and research) and the other focused on facial imaging, facial recognition technology, and related data practices in health and research contexts specifically. We collected responses from a diverse group of 4,048 adults in the United States (2,038 and 2,010, from each survey respectively). A majority of respondents (55.5%) indicated they were equally worried about the privacy of medical records, DNA, and facial images collected for precision health research. A vignette was used to gauge willingness to participate in a hypothetical precision health study, with respondents split as willing to (39.6%), unwilling to (30.1%), and unsure about (30.3%) participating. Nearly one-quarter of respondents (24.8%) reported they would prefer to opt out of the DNA component of a study, and 22.0% reported they would prefer to opt out of both the DNA and facial imaging component of the study. Few indicated willingness to pay a fee to opt-out of the collection of their research data. Finally, respondents were offered options for ideal governance design of their data, as “open science”; “gated science”; and “closed science.” No option elicited a majority response. Our findings indicate that while a majority of research participants might be comfortable with facial images and facial recognition technologies in healthcare and health-related research, a significant fraction expressed concern for the privacy of their own face-based data, similar to the privacy concerns of DNA data and medical records. A nuanced approach to uses of face-based data in healthcare and health-related research is needed, taking into consideration storage protection plans and the contexts of use.
Introduction
Millions of CT (computerized tomography), MRI (magnetic resonance imaging), and PET (positron emission tomography) scans are performed in the United States each year [1]. Such medical imaging has an established standard for interoperability (i.e., the Digital Imaging and Communication in Medicine or DICOM) [2]. Perhaps not surprisingly, it has been widely hoped that the inclusion of imaging would “strengthen” precision health initiatives [1]—such as the National Institutes of Health (NIH) All of UsSM Research Program [3] and the National Cancer Institute (NCI) Cancer MoonshotSM with its Imaging Data Commons [4–5]—by linking both images and image-derived data to biomedical data abstracted from electronic health records (EHRs), genetic and genomic data, patient/participant-provided information (e.g., self-reported phenotypic data), and even consumer data (i.e., data drawn from diverse online and offline sources that might yield insights into social determinants of health). Yet traditional medical imaging is the tip of the digital imaging iceberg for precision health research. Smartphones loaded with mHealth apps (enabled by Apple ResearchKit and Android ResearchStack) are now prevalent in the United States [6–9], and we can reasonably expect a surge in selfies (i.e., photos of an individual taken by that individual), posies (i.e., photos of an individual taken by another individual), and other non-DICOM (i.e., non-clinical) images—many of which would involve human faces—becoming available for precision health research [e.g., 10].
With the increased convenience of and interest in imaging for research purposes, numerous practical, ethical, legal, and social issues must be addressed. The human face is inherently identifiable—perhaps our most public personal feature. Recent work has demonstrated that under certain conditions individuals can be reidentified from seemingly anonymous MRI scan images [11]. Patients’ medical images have inadvertently appeared in Google image searches [12], and analysis of human faces can enable inferences about one’s health status [e.g., 13–20] and even one’s genomic information [21]. Understandably, many in and out of the precision health research community wonder whether the measures taken to ensure responsible stewardship of facial imaging and imaging-derived data are appropriate and adequate. For example, scholars have examined journal editorial policies and publication of medical photographs, raising concerns about privacy and confidentiality [22, 23]. Others have focused on privacy and security concerns regarding imaging without particular regard to the uniqueness of the human head and face [e.g., 24–26]. Identifiability is just one challenging ELSI (i.e., ethical, legal, and social implication) aspect of facial imaging and precision health research, however [e.g., 21 (at Suppl. Note 5), 27]. Other issues abound. Inferring health from facial appearance, for example, suffers from well-documented biases [28–30] that emerging artificial intelligence and machine learning methods have the potential both to alleviate and exacerbate.
The emergence of new facial recognition technologies (FRT) intensifies the availability of and interest in facial imaging for precision health research purposes. Several healthcare applications of FRT have been identified, including assisting in the diagnosis of certain medical conditions (such as melanoma and certain craniofacial anomalies), detecting pain and pain relief, and accurately matching patients to their medical records [16–17, 31, 32]. Other applications focus more squarely on FRT’s uses for identification and authentication, including securing access to physical spaces or computer workstations, enabling touch-free appointment check-in for patients, and even detecting or deterring healthcare fraud [33–35].
While some have warned of FRT signaling the “end of anonymity” if left unchecked [36–38], applications of FRT in healthcare settings have received limited scholarly attention. Legal scholar Seema Mohapatra has noted that current regulatory frameworks are “not well suited” to handle medical applications of FRT [39]. Bioethicist Nicole Martinez-Martin has warned that FRT applied in a healthcare setting could erode patient trust in healthcare providers and has called for research examining patient perspectives regarding use of facial recognition in healthcare settings [40]. While the perspectives of relevant professionals on ethical issues with FRT have come into clearer view following a recent international survey [41], U.S. public perspectives on FRT and their applications in healthcare and research contexts remain poorly understood. While some empirical data about public perspectives on biometrics (including facial imaging) have been published [42–44], these have not directly examined healthcare use cases or precision health research-specific aspects and also have focused on non-U.S. perspectives. One example is the public opinion survey on use of FRT in the U.K. by the Ada Lovelace Institute [45]. Moreover, other studies of data sharing in precision health research [e.g., 46] have not focused on issues specifically related to facial imaging and derived data. Therefore, to enable a better understanding of U.S. adult perspectives on facial imaging and FRT—and how these perspectives might influence trust and participation in precision health research that involves use and sharing of facial imaging, DNA, and EHR data—we conducted two online surveys: one survey focused on six types of biometrics (including facial imaging) and a wide range of societal applications and a second survey focused on facial imaging and FRT for healthcare and research applications. Our findings regarding U.S. adult public perspectives on facial imaging and FRT in non-medical contexts will be reported elsewhere. Here we report the findings of U.S. public perspectives on facial imaging and FRT specifically in healthcare and research contexts.
Methods
We used Qualtrics Panels, a panel aggregation service provided by Qualtrics LLC (Provo, Utah, USA), to collect responses to two complementary and overlapping survey instruments. This study protocol was determined to meet exemption criteria of 45 CFR 46.104(d)(2) by the University of Pittsburgh IRB (STUDY20070193), and the study activities to be performed at Geisinger by its researchers and consultants (IRB #2020–0926) were subsequently determined not to involve “human subjects” as defined in 45 CFR 46.102.
Survey instruments were designed to incorporate new questions as well as ones adapted or inspired from published surveys [42–44]. Instruments were topically distinct, with one focused on six types of biometrics (including facial images and DNA) and their use in a wide range of societal contexts (including healthcare and research) and the other focused on facial imaging, facial recognition technology, and related data practices in health and research contexts specifically. The set of questions used in the health care and research contexts survey are provided as S1 File. An informational page explained the study, and individuals provided implicit consent to participation by proceeding beyond that informational page and responding to any questions.
Through quota sampling based on four dimensions (i.e., age, gender, race and ethnicity, and geographic region) and using eligibility criteria open to any adult residing in the U.S., we ensured our response sample would resemble the broader U.S. adult population. Anonymous survey responses were collected between November 24 and December 14, 2020. We had determined that, for simple comparisons, sample sizes for subgroups of interest as small as n = 50 would provide 80% statistical power at alpha = 0.05 to detect effect sizes of 0.23.
Statistical analyses were performed using R [47]. Survey questions eliciting nominal responses were analyzed using Pearson’s Chi-squared test. Wilcoxon sign rank tests were performed on questions eliciting ordinal responses to detect significant deviations from the sample median response. Effects of sociodemographic variables on response outcomes were tested using Kruskal-Wallis tests and post-hoc Dunn tests with Bonferroni adjustment for multiple comparisons. Associations between ordered categorical data were investigated using polychoric correlations. A statistical significance threshold of 0.05 was used. Results from the statistical tests are reported as S2 File.
Results
A total of 4,048 adults residing in the United States responded to our two surveys, with 2,038 respondents completing the first survey focused on six different biometrics and their use in societal contexts generally and 2,010 respondents completing the survey focused on FRT in healthcare settings and matters relevant to precision health research initiatives. As shown in Table 1, the responses were elicited from a diverse group of adults in terms of age, geographic region, gender, racial and ethnic background, educational attainment, household income, and political views. Demographic factors were not found to have meaningful effects on survey responses (See S2 File).
Table 1. Demographics of survey respondents.
| Demographic | All Respondents | Respondents to Health Research Contexts Survey Only | |||
|---|---|---|---|---|---|
| N = 4048 | % | N = 2010 | % | ||
| Age | 18–25 | 524 | 12.9 | 261 | 13.0 |
| 26–35 | 728 | 18.0 | 364 | 18.1 | |
| 36–45 | 678 | 16.7 | 339 | 16.9 | |
| 46–55 | 671 | 16.6 | 319 | 15.9 | |
| 56–65 | 675 | 16.7 | 338 | 16.8 | |
| 66–75 | 640 | 15.8 | 314 | 15.6 | |
| 76+ | 132 | 3.3 | 75 | 3.73 | |
| Geographic Region | South | 1491 | 36.8 | 762 | 37.9 |
| West | 999 | 24.7 | 479 | 23.8 | |
| Midwest | 840 | 20.8 | 399 | 19.9 | |
| Northeast | 713 | 17.6 | 368 | 18.3 | |
| Gender | Woman | 2029 | 50.1 | 990 | 49.3 |
| Man | 1943 | 48.0 | 978 | 48.7 | |
| Non-binary | 21 | 0.52 | 12 | 0.60 | |
| Transgender (including Man, Transgender; Woman, Transgender; or Transgender) | 13 | 0.32 | 8 | 0.40 | |
| Racial and Ethnic Background | American Indian or Alaska Native | 56 | 1.38 | 39 | 0.02 |
| Asian | 172 | 4.2 | 80 | 3.98 | |
| Black, African American, or African | 473 | 11.7 | 235 | 11.7 | |
| Hispanic, Latino, or Spanish | 470 | 11.6 | 235 | 11.7 | |
| Native Hawaiian or other Pacific Islander | 21 | 0.52 | 18 | 0.90 | |
| White | 2466 | 60.9 | 1184 | 58.9 | |
| Black, African American, or African & Hispanic, Latino, or Spanish | 9 | 0.22 | 4 | 0.20 | |
| Black, African American, or African & White | 52 | 1.28 | 30 | 1.49 | |
| Hispanic, Latino, or Spanish & White | 123 | 3.04 | 61 | 3.03 | |
| Other combination of two or more categories | 129 | 3.19 | 74 | 3.68 | |
| Educational Attainment | Grade 11 or below | 218 | 5.4 | 105 | 5.22 |
| Grade 12 or GED | 912 | 22.5 | 440 | 21.9 | |
| 1 to 3 years after high school | 1221 | 30.2 | 581 | 28.9 | |
| College 4 years or more | 967 | 23.9 | 499 | 24.8 | |
| Advanced degree | 688 | 17.0 | 360 | 17.9 | |
| Household Income | Less than $25,000 | 823 | 20.3 | 400 | 19.9 |
| $25,000 - $49,999 | 1058 | 26.1 | 481 | 23.9 | |
| $50,000 - $74,999 | 680 | 16.8 | 333 | 16.6 | |
| $75,000 - $99,999 | 496 | 12.3 | 268 | 13.3 | |
| $100,000 - $149,999 | 453 | 11.2 | 231 | 11.5 | |
| $150,000 or more | 368 | 9.1 | 208 | 10.4 | |
| Political Views | Conservative | 1099 | 27.1 | 557 | 27.7 |
| Moderate | 1568 | 38.7 | 750 | 37.3 | |
| Liberal | 1017 | 25.1 | 511 | 25.4 | |
Totals for each demographic item do not necessarily sum to N = 4048 for all respondents or N = 2010 for health research survey respondents due to item nonresponse or selections (such as “I prefer not to answer.”) that are not displayed.
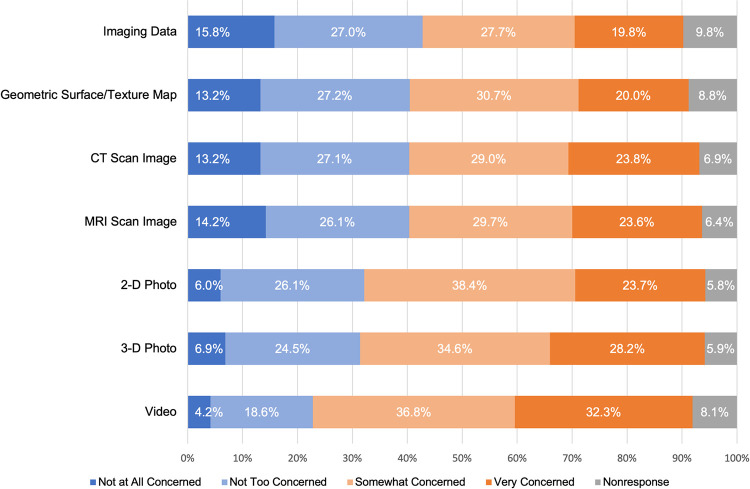
Respondents expressed varying levels of privacy concern for a health study using and sharing facial images and facial imaging data. As shown in Fig 1, reported privacy concerns were highest for video images (71.1%, 1389/2010 noting they were “very” or “somewhat” concerned) and lowest for imaging-derived data (47.4%, 953/2010) noting they were “very” or “somewhat” concerned). Demographic factors had only small or not statistically significant effects on these privacy concerns.
Fig 1. Privacy concerns regarding the use and sharing of facial images and facial imaging data in health research.
Respondents were asked the following question: “There are many different types of facial imaging, and the ability to identify an individual from these different types varies. In health research, names and other identifying information are removed from facial imaging to preserve the privacy of the individual. However, reidentification is sometimes possible. Imagine that you are participating in health research involving facial images and facial imaging data. Based on what you know, how concerned are you about your privacy if the following are used and shared as part of that health research?” Proportion of respondents indicating they were very concerned (dark orange), somewhat concerned (light orange), not too concerned (light blue), and not at all concerned (dark blue) are displayed along with item nonresponses (gray).
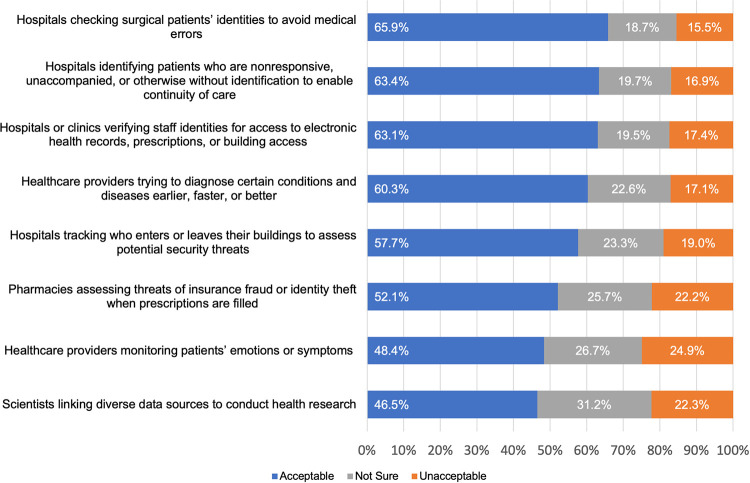
Facial recognition technologies were considered acceptable by a majority of respondents in our survey in six of the eight scenarios (as shown in Fig 2). In fact, 19.8% (800/4048) of respondents considered FRT to be acceptable in all eight of the scenarios we posed, and 53.2% (2154/4048) considered it acceptable in five of the eight scenarios. The two scenarios that failed to elicit a majority acceptance were, notably, healthcare providers monitoring patients’ emotions or symptoms (48.4%, 1879/3883 reported as acceptable) and scientists linking diverse data sources to conduct health research (46.5%, 1793/3855 reported as acceptable). Demographic factors had small or no effects on acceptability of FRT in the eight scenarios.
Fig 2. Acceptability of facial recognition technologies in eight healthcare scenarios.
For each scenario, respondents reported whether the use case was acceptable (blue) or unacceptable (orange) or reported being unsure (gray) about the (un)acceptability of FRT.
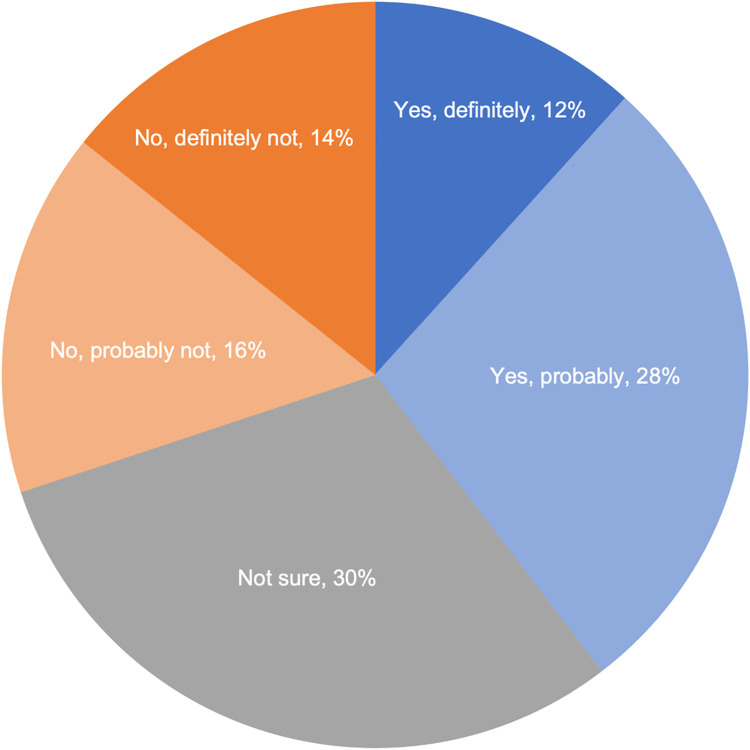
A vignette (see S1) was used to gauge respondents’ hypothetical willingness to participate in a precision health study. The vignette explained the study seeks to understand a wide range of diseases and conditions; that the study would involve use of medical records, DNA, facial imaging, and related data; and that the researchers would take steps to prevent a participant from being easily identified (e.g., use of a study ID number). Thirty percent (30.3%, 609/2010) of respondents were not sure about participating in such as study, 30.1% (605/2010) of respondents indicated they were probably or definitely unwilling to participate, and 39.6% (796/2010) of respondents indicated they were probably or definitely willing to participate (Fig 3). Demographic factors had small or no effects on willingness to participate.
Fig 3. Willingness to participate in a hypothetical precision health study.
Respondents were presented with this vignette: “For the next set of questions, imagine that you are asked to participate in a health study that seeks to understand a wide range of human diseases and conditions. The researchers will study your medical records (such test results and information about diseases and conditions); will collect a DNA sample to study your DNA information; and will collect images of your face to study along with any medical images and related information in your medical records. The researchers will not share study resources that could identify someone easily. Each participant will be given a unique study ID number.” Shades of blue denote those definitely (dark) and probably (light) willing to participate. Shades of orange denote those definitely (dark) and probably (light) not willing to participate. Gray shading denotes those not sure about participation.
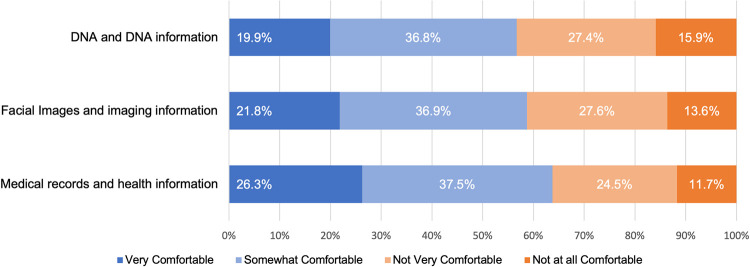
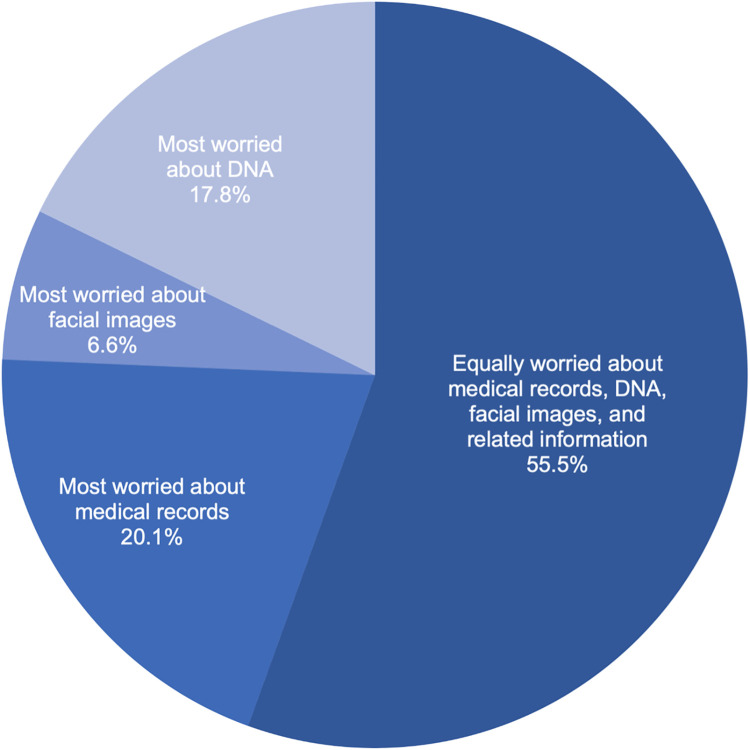
Respondents were asked to think about their relative comfort with a precision health study using three separate types of research resources (medical records, DNA, facial images, and related information) regardless of their willingness to participate in such a study. Across our response sample (Fig 4), comfort was highest for medical records and related information followed by facial imaging and related information and then, lastly, DNA and related information (with 63.7%, 58.8%, and 56.7% reporting being very or somewhat comfortable with the research item, respectively). Because comfort could encompass respondents’ consideration of multiple unspecified factors, respondents were also asked directly about their relative privacy concerns for each research resource (Fig 5). A majority of respondents (55.5%, 1116/2010) indicated they were equally worried about the privacy of medical records, DNA, and facial images collected for precision health research. One-fifth of respondents (20.1%, 405/2010) indicated that they were more concerned about their medical records than either facial imaging or DNA. Only 6.6% (132/2010) of respondents indicated they were more concerned about facial imaging and corresponding data than other types of research resources. Prior experience with medical imaging of the head or face also was also correlated with respondents’ comfort with a health study using facial imaging and imaging data (r = 0.205, p-value = 2.01E-20). Moreover, respondents’ comfort with a health study using facial images was positively correlated with the respondents’ trust in researchers (r = 0.488, p-value = 1.24E-116) and clinicians (r = 0.429, p-value = 11.38E-87) to use facial imaging and imaging data responsibly. Similar positive correlations were found between comfort with a health study using DNA and trust in researchers (r = 0.487, p-value = 1.23E-116) and clinicians (r = 0.444, p-value = 2.61E-95) using DNA and DNA data responsibly.
Fig 4. Comfort with a precision health study using medical records, facial images, DNA, and related information.
Blue shading denotes the proportion of respondents who expressed they were very (dark blue) and somewhat (light blue) comfortable with a study’s use of each of the three types of research resources. Orange shading denotes the proportion of respondents who expressed they were very (dark orange) and somewhat (light orange) uncomfortable with a study’s use of each of the three types of research resources.
Fig 5. Relative privacy concerns when information is collected for research purposes.
The darkest shade of blue denotes equal concern for the three types of research resources (medical records, DNA, and facial images). The second darkest shade of blue denotes those whose concerns for medical records were stronger (more) than their concerns about either facial images or DNA. The second lightest shade of blue denotes those whose concerns for facial images were stronger (more) than their concerns about either medical records or DNA. The lightest shade of blue denotes those whose concerns for DNA were stronger (more) than their concerns about either medical records or facial images.
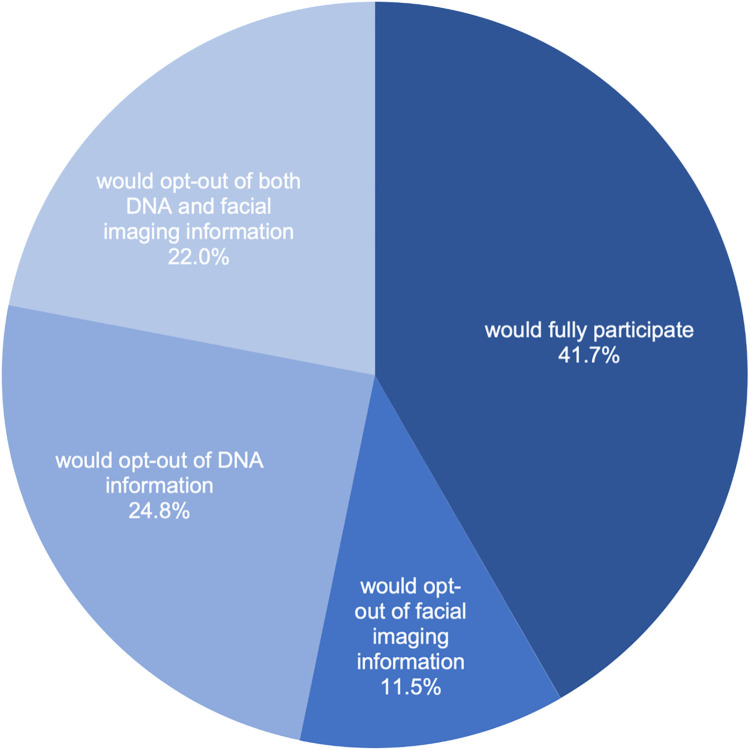
When presented with information that a hypothetical study would enable participants to opt-out of the collection and use of certain research resources even though opting out could hinder the research and reduce the health discoveries that might be possible, 41.7% (838/2010) of survey respondents indicated they would participate fully. As shown in Fig 6, despite the recognition that opting out would limit the value of an individual’s data for the research, nearly one-quarter of respondents (24.8%, 498/2010) reported they would opt out of the DNA component of the study and 22.0% (442/2010) reported they would opt out of both the DNA and facial imaging component of the study. Of those respondents who expressed to opt-out of DNA, facial imaging, or both, only 29.4% (345/1172) indicated they would be willing to pay a fee to the healthcare organization conducting the study in order to process their request to opt-out of each research resource item. Demographic factors had small or no effects on opt-out preferences.
Fig 6. Hypothetical willingness to participate and opt-out preferences for a precision health study.
The darkest shade of blue denotes willingness to participate fully including with the three types of research information (medical record, DNA, and facial imaging information). The second darkest shade of blue denotes those who would opt out of the facial imaging component. The second lightest shade of blue denotes those who would opt out of the DNA component. The lightest shade of blue denotes those who would opt out of both the DNA and facial imaging component.
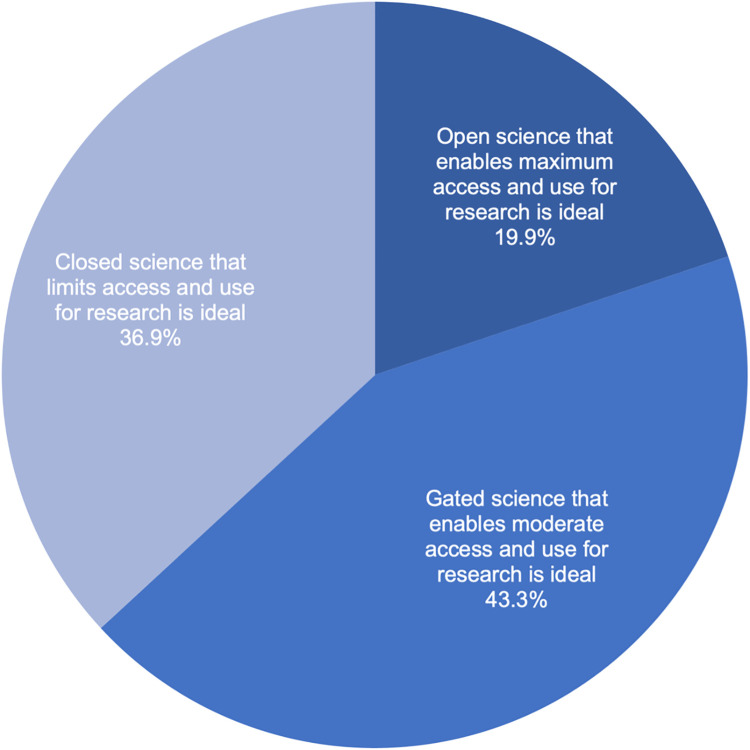
Respondents were asked to consider three possible scenarios for managing precision health research resources and to select their ideal governance design: (1) one in which research resources are unrestricted and made available to as many researchers as possible and to answer as many research questions as possible to advance science even if that increases privacy risks to participants (in other words, an “open science” that enables maximum access and use); (2) one in which research resources are controlled and made available to qualified researchers and to answer research questions reasonably related to human health (in other words, a “gated science” that enables moderate access and use); and (3) one in which research resources are restricted and made available only to a few researchers and to answer only those research questions stated at the beginning of a study to reduce privacy risks to participants (in other words, a “closed science” that strictly limits access and use). No option elicited response from a majority, as shown in Fig 7. The most popular preference among this survey sample was the middle option of “gated science” (43.3%, 870/2010), and the least popular preference was the most relaxed or “open science” option (19.9%, 399/2010). Demographic factors had only small effects on these preferences. There was a statistically significant association between preference for how research resources should be managed and willingness to participate in a hypothetical precision health study (r = 0.334, p-value = 1.28E-53).
Fig 7. Preferred management of precision health research resources.
The darkest blue shading denotes the most open of the three options, that resources should be unrestricted and made available to as many researchers as possible to answer as many research questions as possible even if that increases privacy risks to participants. The moderate blue shading denotes that research resources should be controlled and made available to qualified researchers to answer any research questions reasonably related to human health. The lightest blue shading denotes that research resources should be restricted and made available only to a few researchers and only to answer research questions stated at the beginning of the study to reduce privacy risks to participants.
Discussion
A better understanding of U.S. adult public perspectives on uses of facial imaging technologies and facial imaging data practices in health contexts is needed to guide healthcare industry and biomedical research decisions so that unintended consequences can be avoided. Here, we report survey findings of U.S. adult perspectives on the acceptability of eight specific health-related uses of facial recognition technologies as well as perspectives regarding relative privacy concerns regarding EHRs, facial imaging, DNA, and related data; willingness to participate in a hypothetical study involving such resources; hypothetical opt-out preferences and willingness to pay for those opt-out preferences; and general views regarding the ideal governance of research resources for precision health study purposes. While facial imaging has been an important part of specialized care (particularly pediatric genetics) for decades, biomedicine is changing–becoming much more anticipatory, occurring in digital spaces, involving larger and larger datasets containing data assets repurposed from diverse sources, and introducing new risks. While the U.S. public seems to have fairly high levels of trust in healthcare providers and researchers with use of facial imaging and FRT, we found that people are not necessarily fully supportive of expanded uses in healthcare settings and might even be uneasy about them. How healthcare professionals choose to proceed could have substantial impacts on levels of public trust in the profession generally [48].
Our findings have implications for the implementation of FRT in healthcare settings. Six of the eight use cases were considered acceptable uses of facial recognition technology by a majority of respondents. However, even the use case that elicited the most favorable response (using facial recognition for patient identification to avoid medical errors) was only considered acceptable by 65.9%. Notably, the two scenarios that did not elicit favorable views by a majority were (1) monitoring patients’ emotions or symptoms (considered acceptable by 48.4%) and (2) linking diverse data sources to conduct health research (considered acceptable by 46.5%). Additional research would be useful to understand what factors are contributing to the perspectives of those who expressed they were unsure about these two uses (26.7% and 31.2%, respectively) or expressed these two uses were unacceptable (24.9% and 22.3%, respectively). Our constructed scenarios notably did not include potentially relevant information to assess alternatives to FRT, relative financial costs involved, or comparative effectiveness. Before healthcare systems advance plans to implement facial recognition technologies, it would be useful to conduct an in-depth examination of these and other factors, including (1) aspects related to distrust of technology partners that might be involved; (2) mitigation strategies deployed to minimize risks of data breaches or leaks; and (3) widely varying local ordinance and state law restrictions on biometrics generally and facial recognition specifically.
Our findings also have implications for data management and sharing policies, a matter of critical importance with the anticipated implementation of the updated NIH Policy for Data Management and Sharing [49] and the ongoing development of responsible data practices for the All of UsSM Research Program [3], NCI Imaging Data Commons [4, 5], and imaging science that might be pursued via other precision health initiatives (such as the Geisinger MyCode® Community Health Initiative) [50]. Here, we observed levels of comfort with a study using facial imaging and related imaging data that fell between those levels for a study using EHR data and genomic data. When asked about relative privacy concerns specifically (as opposed to other factors that could affect perceived comfort), only 6.6% of respondents indicated they were most worried about facial images. Interestingly, of those who indicated they did not worry equally about medical records, DNA, facial imaging, and related data, the most common perspective was that they were most concerned about their medical records (not their DNA or facial images). A limitation of our study, however, is that we did not explore the possibility that individuals might prefer to opt-out of an EHR component of a hypothetical precision health study while still being willing to participate in DNA and facial imaging components. These findings suggest that, at least from U.S. public perspectives, responsible stewardship of facial imaging and imaging-derived data need not be heightened compared to the approaches taken with EHR or genomic data. Notably, however, our findings regarding willingness to participate and attitudes toward biometrics and data sharing suggest that the actual participants of precision health studies might be biased toward those individuals who are more accepting of biometrics than non-participants. Such sampling biases need to be recognized and addressed by designers of precision health initiatives upstream of proposed data use and upstream of recruitment, as individuals skeptical or critical of biometrics and broad data sharing nevertheless comprise a sizable portion of users served by health services. Additional research is needed to understand the needs, interests, and concerns of those critical of FRT and other technological advancements (i.e., “biodefectors” engaged in “informed refusals” [51])—particularly if equitable precision health practices and data justice are to be prioritized and realized. Moreover, that we did not find demographic factors (notably gender or racial and ethnic background) to have meaningful effects on perspectives on facial imaging technologies and imaging data practices in health-related contexts is worthy of further research given the known biases of FRT and documented controversies regarding FRT in social contexts outside of the health-related applications we explored here [e.g., 52–55]. Qualitative research is needed to interpret these preliminary findings.
It is convenient to distinguish the use of facial imaging directly for medical or precision health research purposes on the one hand from use of facial imaging and FRT for non-medical purposes on the other. The appropriateness of such a conceptual distinction should not be assumed without critical examination, however. Policy and regulatory frameworks often focus on the actor (as opposed to intentions or acts and omissions). HIPAA is an example of this, where the statutory requirements apply to certain actors (i.e., covered entities and, by contractual extension, business associates). Multiple use cases for FRT exist in healthcare settings, and the boundaries might not be clearly drawn regarding how a healthcare organization—not to mention individual clinicians, researchers, IRBs, security officers, administrators, executives, and others employed there—accesses and uses the diverse facial imaging resources in the organization’s possession or within its reach. Careful deliberation, interdisciplinary decision-making, and continuous evaluation are critical to ensure actors manage facial images and imaging data responsibly and deploy FRT cautiously with the ethical, legal, and social implications of doing so in mind. Purpose creep and dataveillance risks within healthcare settings warrant additional empirical and normative research.
The recent picture archiving and communication system (PACS) guidance for the healthcare industry issued by the National Institute of Standards and Technology (NIST) [56] contains nearly 400 pages of important technical information, describing PACS as the “authoritative repository of medical image information” and recognizing that it “cannot operate in isolation” (p. 1). Nevertheless, this guidance offers no mention of how its intended audience (biomedical and cybersecurity engineers, healthcare technology managers, and support staff) would or should effectively collaborate with Institutional Review Boards or others to ensure proper safeguards when trying to use images in and out of PACS for research and non-medical purposes. ELSI research is needed to understand how facial images and imaging data are being managed and used in practice and how familiar research professionals are with the privacy and cybersecurity aspects of their work. Additional ELSI research is also needed to examine whether and how a “HIPAA knowledge gap” (i.e., the gap between what individuals think HIPAA allows or disallows and what HIPAA actually allows with their data) or limited data literacy (e.g., comprehension of concepts such as “de-identified data”) influences trust in healthcare organizations and professionals and support of or opposition to precision health initiatives, healthcare applications of FRT, and policy approaches.
Supporting information
The full set of questions for the health care and research contexts survey that were programmed into Qualtrics are provided.
(DOCX)
The statistical test results performed on the survey responses are displayed.
(XLSX)
Acknowledgments
The authors appreciate the assistance of Brian M. Bot with making the data available via Synapse, the Qualtrics support provided for this project by David Trombetta, Zach Lee, and Jason Matson, and the project coordination assistance provided by Jill Ciciarelli.
Abbreviations
- CT
computerized tomography
- EHR
electronic health record
- ELSI
ethical, legal, and social implications
- FRT
facial recognition technology/ies
- MRI
magnetic resonance imaging
- NCI
National Cancer Institute
- NIH
National Institutes of Health
- NIST
National Institute of Standards and Technology
- PACS
picture archiving and communication system
- PET
positron emission tomography
Data Availability
The full dataset containing individual survey response data is available via Synapse (DOI: 10.7303/syn25618565).
Funding Statement
This study was funded by the NIH Office of the Director (OD) and National Institute of Dental and Craniofacial Research (NIDCR) through Grant No. 3R01DE027023-04S1. Contributions by SHK were also supported in part by Grant No. 5R01HG009923-03 from the National Human Genome Research Institute (NHGRI). Contributions by RCD were also supported by Grant Nos. R01CA237118 and U01CA242954 from the National Cancer Institute (NCI). Contributions by JKW were also supported in part by Geisinger. The content of this article is the authors’ responsibility and might not represent the views of the authors’ funding sources, employers, or any other person or entity. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Interagency Working Group on Medical Imaging, Committee on Science. National Science and Technology Council. Roadmap for Medical Imaging Research and Development. 2017 Dec [cited 2021 May 14]: [4-5p.]. Available from: https://imaging.cancer.gov/news_events/Roadmap-for-Medical-Imaging-Research-and-Development-2017.pdf
- 2.Digital Imaging and Communications in Medicine [cited 2021 May 14]. Available from: https://www.dicomstandard.org
- 3.National Institutes of Health (NIH). All of Us Research Program [cited 2021 May 14]. Available from: https://allofus.nih.gov
- 4.National Cancer Institute (NCI). Imaging Data Commons (IDC) [cited 2021 May 14]. Available from: https://datacommons.cancer.gov/repository/imaging-data-commons
- 5.National Cancer Institute (NCI). Award of the Imaging Data Commons: Bringing Multi-Modal Imaging Data to the Cancer Research Community. Cancer Data Science Plus Blog. 2019 Aug 14 [cited 2021 May 14]. Available from: https://datascience.cancer.gov/news-events/blog/award-imaging-data-commons-bringing-multi-modal-imaging-data-cancer-research
- 6.PEW Research Center. Mobile Fact Sheet. 2021 Apr 7 [cited 2021 May 14]. Available from: https://www.pewresearch.org/internet/fact-sheet/mobile/
- 7.Apple. Apple Introduces ResearchKit, Giving Medical Researchers the Tools to Revolutionize Medical Studies. Press release, 2016 Mar 9 [cited 2021 May 14]. Available from: https://www.apple.com/newsroom/2015/03/09Apple-Introduces-ResearchKit-Giving-Medical-Researchers-the-Tools-to-Revolutionize-Medical-Studies/
- 8.Wicklund E. ResearchStack Goes Live, Opening mHealth Studies to the Android Ecosystem. mHealth Intelligence, 2016 Oct 7 [cited 2021 May 14]. Available from: https://mhealthintelligence.com/news/researchstack-goes-live-opening-mhealth-studies-to-the-android-ecosystem
- 9.Pohl M. 325,000 Mobile Health Apps Available in 2017—Android Now the Leading mHealth Platform. Research 2 Guidance. 2017 [cited 2021 May 14]. Available from: https://research2guidance.com/325000-mobile-health-apps-available-in-2017/
- 10.Webster DE, Suver C, Doerr M, Mounts E, Domenico L, Petrie T, et al. The Mole Mapper Study, mobile phone skin imaging and melanoma risk data collected using ResearchKit, Sci Data. 2017;4: 170005. doi: 10.1038/sdata.2017.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwarz CG, Kremers WK, Therneau TM, Sharp RR, Gunter JL, Vemuri P, et al. Identification of anonymous MRI research participants with face-recognition software. NEJM. 2019;381(17): 1684–86. doi: 10.1056/NEJMc1908881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marshall Z, Brunger F, Welch V, Asghari S, Kaposy C. Open availability of patient medical photographs in Google images search results: cross-sectional study of transgender research. J Med Internet Res. 2018;20(2): e70. doi: 10.2196/jmir.8787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cole JB, Manyama M, Larson JR, Liberton DK, Ferrara TM, Riccardi SL, et al. Human facial shape and size heritability and genetic correlations. Genetics. 2017;205(2): 967–78. doi: 10.1534/genetics.116.193185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brons S, Meulstee JW, Nada RM, Kuijpers M, Bronkhorst EM, Bergé SJ. Uniform 3D meshes to establish normative facial averages of healthy infants during the first year of life. PLoS One. 2019;14(5): e0217267. doi: 10.1371/journal.pone.0217267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hallgrimsson B, Aponte JD, Katz DC, Bannister JJ, Riccardi SL, Mahasuwan N, et al. Automated syndrome diagnosis by three-dimensional facial imaging. Genet Med. 2020;22(10): 1682–1693. doi: 10.1038/s41436-020-0845-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pantel JT, Hajjir N, Danyel M, Elsner J, Abad-Perez AT, Hansen P, et al. Efficiency of computer-aided facial phenotyping (DeepGestalt) in Individuals with and without a genetic syndrome: Diagnostic accuracy study. J Med Internet Res. 2020;22(10): e19263. doi: 10.2196/19263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Javitt MJ, Vanner EA, Grajewski AL, Chang TC. Evaluation of a computer-based facial dysmorphology analysis algorithm (Face2Gene) using standardized textbook photos. Eye. 2021. doi: 10.1038/s41433-021-01563-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stephen ID, Hiew V, Coetzee V, Tiddeman BP, Perrett DI. Facial shape analysis identifies valid cues to aspects of physiological health in Caucasian, Asian, and African populations. Front Psychol. 2017;8: 1883. doi: 10.3389/fpsyg.2017.01883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jager SD, Coetzee N, Coetzee V. Facial adiposity, attractiveness, and health: a review. Front Psychol. 2018;9: 2562. doi: 10.3389/fpsyg.2018.02562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang B, Yang N, He G, Huang P, Yang Y. Identification of the facial features of patients with cancer: a deep learning-based pilot study. J Med Internet Res. 2020;22(4): e17234. doi: 10.2196/17234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sero D, Zaidi A, Li J, White J, González Zarzar T, Marazita M, et al. Facial Recognition from DNA using face-to-DNA classifiers. Nat. Commun. 2019;10: 2557. doi: 10.1038/s41467-019-10617-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roguljić M, Buljan I, Veček N, Dragun R, Marušić M, Wager E, et al. Deidentification of facial photographs: a survey of editorial policies and practices. J Med Ethics Published. 2020. Apr 6. doi: 10.1136/medethics-2019-105823 [DOI] [PubMed] [Google Scholar]
- 23.Bennett KG, Bonawitz SC, Vercler CJ. Guidelines for the ethical publication of facial photographs and review of the literature. Cleft Palate Craniofac J. 2019;56(1): 7–14. doi: 10.1177/1055665618774026 [DOI] [PubMed] [Google Scholar]
- 24.Freymann JB, Kirby JS, Perry JH, Clunie DA, Jaffe CC. Image data sharing for biomedical research—meeting HIPAA requirements for de-identification. J Digit Imaging. 2012;25(1): 14–24. doi: 10.1007/s10278-011-9422-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moore SM, Maffitt DR, Smith KE, Kirby JS, Clark KW, Freymann JB, et al. De-identification of Medical Images with Retention of Scientific Research Value. RadioGraphics. 2015;35(3): 727–35. doi: 10.1148/rg.2015140244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Desjardins B, Mirsky Y, Ortiz MP, Glozman Z, Tarbox L, Horn R, et al. DICOM Images Have Been Hacked! Now What?. Am J Roentgenol. 2020;214(4): 727–35. doi: 10.2214/AJR.19.21958 [DOI] [PubMed] [Google Scholar]
- 27.Venkatesaramani R, Malin BA, Vorobeychik Y. Re-identification of individuals in genomic datasets using public face images. 2021. Feb 17, arXiv:2102.08557v1 [cs.LG] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stephen ID, Law Smith MJ, Stirrat MR, Perrett DI. Facial skin coloration affects perceived health of human faces. Int J Primatol. 2009;30(6): 84–857. doi: 10.1007/s10764-009-9380-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Russell R, Porcheron A, Sweda JR, Jones AL, Mauger E, Morizot R. Facial contrast is a cue for perceiving health from the face. J Exp Psychol Hum Percept Perform. 2016;42(9): 1354–62. doi: 10.1037/xhp0000219 [DOI] [PubMed] [Google Scholar]
- 30.Lumaka A, Cosemans N, Mampasi AL, Mubungu G, Mvuama N, Lubala T, et al. Facial dysmorphism is influenced by ethnic background of the patient and of the evaluator. Clin Genet. 2017;92(2): 166–71. doi: 10.1111/cge.12948 [DOI] [PubMed] [Google Scholar]
- 31.Grifantini K. Detecting faces, saving lives: how facial recognition software is changing health care. IEEE Pulse. 2020. May 13. [cited 2021 May 14]. Available from: https://www.embs.org/pulse/articles/detecting-faces-saving-lives/ [DOI] [PubMed] [Google Scholar]
- 32.PEW Research Center, Health care can learn from global use of biometrics. 2020 Nov 19 [cited 2021 May 14]. Available from: https://www.pewtrusts.org/en/research-and-analysis/reports/2020/11/health-care-can-learn-from-global-use-of-biometrics
- 33.Cidon D. Making IT better: how biometrics can cure healthcare. Biometric Technol Today. 2018;7: 5–8. [Google Scholar]
- 34.Siwicki B. Biometrics entering a new era in healthcare. Healthcare IT News. 2018. Jul 30 [cited 2021 May 14]. Available from: https://www.healthcareitnews.com/news/biometrics-entering-new-era-healthcare [Google Scholar]
- 35.Yaroslav Kuflinski, How facial recognition software is revolutionizing hospital security, care, human resources. Healthcare IT Today, 2019 Aug 29 [cited 2021 May 14]. Available from: https://www.healthcareittoday.com/2019/08/29/how-facial-recognition-software-is-revolutionizing-hospital-security-care-human-resources/
- 36.Wehle KL, Anonymity, Faceprints, and the Constitution, Geo Mason L Rev. 2013;21: 409–66, at 434–5. [Google Scholar]
- 37.Nakar S, Greenbaum D, Now you see me. Now you still do: facial recognition technology and the growing lack of privacy, B U J Sci & Tech L. 2017;23: 88–123. [Google Scholar]
- 38.Simonite T. How face recognition can destroy anonymity. Wired. 2021. Apr 20 [cited 2021 May 14]. Available from: https://www.wired.com/story/how-face-recognition-destroy-anonymity/ [Google Scholar]
- 39.Mohapatra S. Use of facial recognition technology for medical purposes: balancing privacy with innovation, 43 Pepp L Rev. 2016;43: 1017–64. [Google Scholar]
- 40.Martinez-Martin N. What are important ethical implications of using facial recognition technology in health care? AMA J Ethics. 2019;21(2): E180–E187. doi: 10.1001/amajethics.2019.180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Noorden R. The Ethical Questions That Haunt Facial Recognition Research. Nature. 2020;587: 354–58. doi: 10.1038/d41586-020-03187-3 [DOI] [PubMed] [Google Scholar]
- 42.Smith A, et al. More than half of U.S. adults trust law enforcement to use facial recognition responsibly. PEW Research Center. 2019. Sept 5 [cited 2021 May 14]. Available from: https://www.pewresearch.org/internet/2019/09/05/more-than-half-of-u-s-adults-trust-law-enforcement-to-use-facial-recognition-responsibly/ [Google Scholar]
- 43.Garman RL. Barber KS. Consumer attitudes about biometric authentication. University Texas. UT CID Report #18–03, 2018. May [cited 2021 May 14]. Available from: https://perma.cc/SX9Q-KY4Q [Google Scholar]
- 44.Kugler MB. From identification to identity theft: public perceptions of biometric privacy harms. UC Irvine L Rev. 2019;10: 107–50. [Google Scholar]
- 45.Sanderson SC, Brothers KB, Mercaldo ND, Clayton EW, Antommaria AHM, Aufox SA, et al. Public attitudes toward consent and data sharing in biobank research: a large multi-site experimental survey in the US. Am J Hum Genet. 2017;100: 414–27. doi: 10.1016/j.ajhg.2017.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ada Lovelace Institute (2019). Beyond face value: public attitudes to facial recognition technology. [cited 2021 Aug 19]. Available from: www.adalovelaceinstitute.org/report/beyond-face-value-public-attitudes-to-facial-recognition-technology
- 47.R Core Team (2020). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/ [Google Scholar]
- 48.Funk C, Kennedy B. Public confidence in scientists has remained stable for decades, Pew Research Center, 2020 Aug 27 [cited 2021 May 14]. Available from: https://www.pewresearch.org/fact-tank/2020/08/27/public-confidence-in-scientists-has-remained-stable-for-decades/
- 49.Office of the Director, National Institutes of Health. Final NIH Policy for Data Management and Sharing. NOT-OD-21-013, 2020 Oct 29 [cited 2021 May 14]. Available from: https://grants.nih.gov/grants/guide/notice-files/NOT-OD-21-013.html
- 50.Carey DJ, Fetterolf SN, Davis FD, Faucett WA, Kirchner HL, Mirshahi U, et al. The Geisinger MyCode community health initiative: an electronic health record–linked biobank for precision medicine research. Genet Med. 2016;18(9): 906–13. doi: 10.1038/gim.2015.187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Benjamin R. Informed refusal: toward a justice-based bioethics. Sci, Tech, & Human Values. 2016;41(6): 967–90. [Google Scholar]
- 52.Wiggers K. NIST benchmarks show facial recognition technology still struggles to identify Black faces, VentureBeat, 2020 Sep 9. [cited 2021 Aug 19] Available from: https://venturebeat.com/2020/09/09/nist-benchmarks-show-facial-recognition-technology-still-struggles-to-identify-black-faces/
- 53.Porter, J. Federal study of top facial recognition algorithms finds ‘empirical evidence’ of bias,” The Verge, 2019 Dec 20 [cited 2021 Aug 19] Available: https://www.theverge.com/2019/12/20/21031255/facial-recognition-algorithm-bias-gender-race-age-federal-nest-investigation-analysis-amazon
- 54.K. Hill, “Wrongfully accused by an algorithm,” N. Y. Times, 2020 Jun 24 [cited 2021 Aug 19]. Available from: https://www.nytimes.com/2020/06/24/technology/facial-recognition-arrest.html
- 55.Djanegara NDT, The great misunderstanding at the core of facial recognition, Fast Company, 2021 Aug 17. [cited 2021 Aug 19]. Available from: https://www.fastcompany.com/90666477/facial-recognition-misunderstanding?partner=rss&utm_source=rss&utm_medium=feed&utm_campaign=rss+fastcompany&utm_content=rss
- 56.NIST. Securing Picture Archiving and Communication System (PACS): Cybersecurity for the healthcare sector. NIST Special Publication 1800–24. 2020 Dec [cited 2021 May 14]. Available from: https://nvlpubs.nist.gov/nistpubs/SpecialPublications/NIST.SP.1800-24.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The full set of questions for the health care and research contexts survey that were programmed into Qualtrics are provided.
(DOCX)
The statistical test results performed on the survey responses are displayed.
(XLSX)
Data Availability Statement
The full dataset containing individual survey response data is available via Synapse (DOI: 10.7303/syn25618565).