Abstract
Objective:
To assess heritable contributions to bronchopulmonary dysplasia (BPD) risk in a twin cohort restricted to gestational age (GA) at birth <29 weeks.
Study design:
250 twin pairs [192 dichorionic (DC), 58 monochorionic (MC)] born <29 weeks GA with known BPD status were identified. Three statistical methods applicable to twin cohorts (Chi-squared tests (χ2), intra-class correlations (ICC) and ACE modeling (additive genetic (A), common environmental (C) and unique environmental (E) components)) were applied. Heritability was estimated as percent variability from A. Identical methods were applied to a subcohort defined by zygosity and to an independent validation cohort.
Results:
χ2 analyses comparing whether neither, one, or both of MC (23, 19, 16) and DC (88, 56, 48) twin pairs developed BPD revealed no difference. Although there was similarity in BPD outcome within both MC and DC twin pairs by intra-class correlation (ICC) [MC ICC = 0.34, 95% CI (0.08, 0.55); DC ICC = 0.39, 95% CI (0.25, 0.51)], MC twins were not more likely than DC twins to have the same outcome (p=0.70). ACE modeling revealed no contribution of heritability to BPD risk [%A = 0.0%, 95% CI (0.0%, 43.1%)]. Validation and zygosity based cohort results were similar.
Conclusions:
Our analysis suggests that heritability is not a major contributor to BPD risk in preterm infants <29 weeks GA.
Keywords: Bronchopulmonary Dysplasia, Genetics, Disease risk, Heritability, Twin study, ACE modeling, Extremely Low Gestational Age Newborns
Bronchopulmonary dysplasia (BPD), defined as receiving supplemental oxygen (O2)1 or by a severity-stratified NIH consensus definition2 at 36 weeks postmenstrual age (PMA) remains a major morbidity among preterm infants. A BPD diagnosis acknowledges both current respiratory status and risk of chronic respiratory morbidity that is associated with higher BPD severity and lower gestational age (GA)3,4,5. BPD etiology is considered multifactorial, with hyperoxia, barotrauma, volutrauma and inflammation-based injury and impaired development and repair of the lung.6
Because only a subset of infants with similar degrees of prematurity and early illness severity develop BPD, it is reasonable to consider the role of genetic predisposition. The contributions of DNA sequence variants to BPD pathogenesis, particularly in genes involved with lung development, inflammation and repair of injury, are proposed to contribute to BPD pathogenesis7 result from associated protein dysfunction7,8.
Twins are logical subjects for the epidemiologic study of heritable disposition to disease. Conventional statistical methods that assume independent observations (as would apply with singletons) are subject to error in twin cohort analyses. Although simple pair concordance may be used, advanced statistical modeling approaches can quantify the proportions of heritable vs environmental influences responsible for disease risk and provide for statistical tests and confidence intervals.
Prior analyses of preterm twin cohorts, using a variety of statistical methods, suggest that heritable factors significantly contribute to BPD risk9,10,11. BPD incidence varies widely in these cohorts. Strong associations exist between BPD risk and both GA and birth weight 12. When BPD was first described, the mean GA at birth of diagnosed infants was 33 weeks13. With advancements in perinatal and neonatal care practices, BPD risk among infants born at GA > 29 weeks has decreased. Recent Vermont Oxford Network data suggest that < 10% of infants born at GA between 29 – 32 weeks develop BPD14 and infants at highest BPD risk are born below 29 weeks GA 5,14.
This study questioned whether, with the evolution of BPD definition and cohort characteristics over time, prior conclusions derived from twin studies regarding BPD heritability held firm. We evaluated BPD heritability within a preterm infant cohort (<29 weeks GA) whose GA distribution reflects those currently at highest BPD risk.
Methods
We collected clinical data including pulmonary and other outcomes on all infants born prior to 29 weeks gestation from two Boston high-risk perinatal centers (from 1997 through 2015 at Brigham and Women’s Hospital (BWH) and from 2004 through 2015 at Beth Israel Deaconess Medical Center (BIDMC)). We received IRB approval for medical record review from both hospitals to study morbidities and outcomes. Data collected included demographics, course of respiratory support and development of common major co-morbidities of extreme prematurity, such as patent ductus arteriosus, early onset sepsis (diagnosed at ≤ third postnatal day), late onset sepsis (diagnosed at > third postnatal day) and necrotizing enterocolitis.
Each infant was assigned a diagnosis of BPD by three definitions: 1) need for supplemental oxygen at 36 weeks PMA, 2) need for supplemental oxygen, CPAP, high flow nasal cannula (with or without oxygen), or positive pressure ventilation (with or without oxygen) at 36 weeks PMA or 3) criteria set by an NIH consensus panel for either moderate or severe BPD2 (for infants born at <32 weeks GA, Moderate BPD was defined as a need for supplemental oxygen for >28 days plus treatment with <30% O2 at 36 weeks PMA, and severe BPD as oxygen for >28 days plus >30% O2 and/or positive pressure at 36 weeks PMA). Definition #1 was considered our primary definition and definitions #2 and #3 were considered alternative definitions for secondary analyses.
Twins were characterized as monochorionic (MC) or dichorionic (DC) based on placental pathology or, when not available, prenatal sonography reports. A pilot study was performed comparing the accuracy of sonographic-based to placental pathology-based determination of chorionicity for an estimate of the magnitude of sonographic chorionicity misclassification. MC twins were considered monozygous (MZ). Dichorionic twins that were discordant for sex or blood type were considered dizygous (DZ). Dichorionic twins that were same sex and same blood type were not assigned a zygosity and were excluded from zygosity-based analyses. Analyses were conducted comparing MC to DC twins as well as MZ to DZ twins. We thus describe the univariate profile of the chorionicity determined primary cohort that yields a larger sample size with avoidance of confounding selection biases and acknowledges a presumed ~9% DZ misclassification.
The twin pairs defined by chorionicity comprised the primary study population. For confirmation, we applied an identical analytic approach to a validation cohort of similar twin pairs enrolled in the multicenter ELGAN study15. Although the ELGAN study investigated contributors to injury in the developing brain, respiratory outcome data were available. After exclusion of ELGAN subjects who had been born at our institutions, 105 twin pairs (29 MC, 76 DC) were available for analysis in the validation cohort. Chorionicity was determined by placental pathology.
Statistical Analyses
Demographic and clinical characteristics were compared between infants who did and did not develop BPD as defined by oxygen support at 36 weeks PMA (primary definition), and between MC and DC infants. We used a mixed logistic model with a random twin pair effect to account for the clustered nature of the data (twin pairs representing clusters). These analyses were performed using the GLIMMIX procedure in SAS/STAT software16. For comparisons where the variables analyzed were completely concordant (same value in both members of every twin pair) the pair was considered the unit of analysis and the Fisher exact test was used.
Our analysis of heritability was designed to consider how the BPD outcome varies among and between twin pairs. When twin siblings tend to have the same outcome, this suggests the influence of common environmental and/or genetic effects. Using chorionicity as a proxy for zygosity, a genetic effect is suggested if MC twins are more concordant for the outcome than DC twins. This analysis was also performed on a subcohort for which zygosity was confirmed.
Twin pairs were cross-classified by chorionicity (MC or DC) and by the number of individuals affected with BPD (0, 1, or 2). From this 2×3 table, we calculated the Pearson chi-squared test to test whether the within-family BPD distribution is associated with chorionicity. We measured twin similarity with the intra-class correlation (ICC) of the BPD phenotype separately within MC and DC pairs17. We also report confidence intervals for the ICCs using a goodness-of-fit approach and tested whether the two ICCs differ17. A high ICC could be due to shared environmental effects and/or due to genetic effects, but one would expect the ICC in MC pairs to be larger if there is a genetic contribution. To quantify the heritability of BPD, we used the ACE model which decomposes the total variation in “liability” of disease into additive genetic (A), common or shared environmental (C) and unique or unshared environmental (E) effects18. The model was fit with SAS/STAT software using nonlinear mixed models (NLMIXED procedure) 16,19. Heritability was estimated as the percent of the total variation in liability from the genetic component. We used the likelihood ratio test with appropriate mixture of chi-squared distributions (½ χ20 + ½ χ21) as the reference distribution to evaluate statistical significance and profile likelihood based confidence intervals (CI)20. ACE modeling was performed without adjusting for covariates but rather allowing any variation due to subject characteristics to contribute to the components of variation being modeled.
Results
Placental pathology was available for establishment of chorionicity for 92% of twin pairs (n=230). The remaining 8% (n=20) were determined by sonography. A pilot study in 121 twin pairs evaluating accuracy of chorionicity determination by sonography, as compared with placental pathology, revealed a < 3% discrepancy. Thus, use of sonography rather than pathology in 8% of our twin pairs would yield, at most, 0.3% chorionicity misclassification.
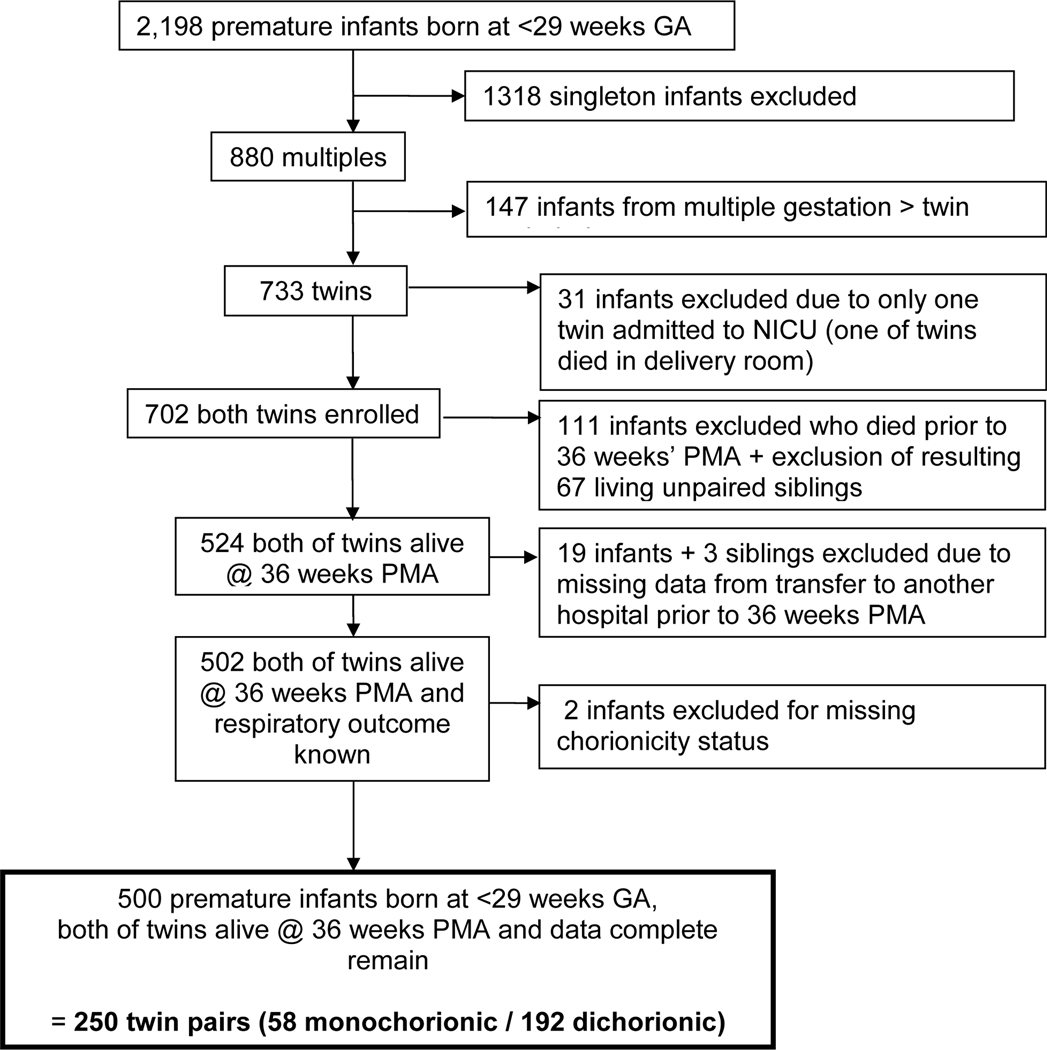
Of 2,198 total infants followed in our database, 733 were born of twin gestations. Death rates were the same whether an infant was part of a MC or DC pair (15.6% vs. 16.6%, p = 0.81). Sixty-eight percent (500 infants, 250 twin pairs (58 MC and 192 DC)) met the study inclusion criteria of documented chorionicity, survival of both twins to 36 weeks PMA and known supplemental O2 and positive pressure support status (Figure; available at www.jpeds.com). The mean (± SD) GA was 26.7 (± 1.3) (range 23–28) weeks and mean birth weight was 950 (± 208) grams. Two hundred five (41%) were diagnosed with BPD. GA and birth weight were significantly lower in the BPD group. Of the 250 twin pairs, 58 (23%) were MC. There was no difference in BPD incidence between MC and DC twins, nor was there a difference in birth weight or GA (Table I). MC twins were presumed to be monozygotic (MZ). Of the 97 same sex DC twins, 67 could be not be confirmed as DZ based on discordance of sex or blood type. Discarding these twin pairs from analysis reduced the size of the zygosity-based cohort to 183 twin pairs.
Figure 1(online).
Primary study population
Table 1.
Primary study population infant characteristics by respiratory status at 36 weeks PMA and by chorionicity. BPD is defined as need for supplemental oxygen at 36 weeks PMA. MC = monochorionic, DC = dichorionic
| Respiratory Status 36 weeks PMA | Chorionicity | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Characteristic | Total (N = 500) |
BPD (N = 203) |
No BPD (N = 297) |
p-value | MC (N = 116) |
DC (N = 384) |
p-value | |
|
| ||||||||
| Respiratory Status at 36w PMA | ||||||||
| BPD | 203 (41%) | 51 (44%) | 152 (40%) | .47 | ||||
| No BPD | 297 (59%) | 65 (56%) | 232 (60%) | |||||
|
| ||||||||
| Chorionicity | ||||||||
| Monochorionic | 116 (23%) | 51 (25%) | 65 (22%) | .47 | ||||
| Dichorionic | 384 (77%) | 152 (75%) | 232 (78%) | |||||
|
| ||||||||
| Gestational Age (w) | ||||||||
| 23–24 | 44 (9%) | 31 (15%) | 13 (4%) | <.001 | 10 (9%) | 34 (9%) | .94 | |
| 25–26 | 146 (29%) | 83 (41%) | 63 (21%) | 36 (31%) | 110 (29%) | |||
| 27–28 | 310 (62%) | 89 (43%) | 221 (74%) | 70 (60%) | 240 (63%) | |||
| mean(±SD) | 26.7 (±1.3) | 26.2 (±1.4) | 27.0 (±1.2) | 26.7 (±1.4) | 26.6 (±1.3) | |||
|
| ||||||||
| Birth weight (g) | ||||||||
| mean(±SD) | 950(±208) | 855(±185) | 1014(±198) | <.001 | 910(±194) | 962(±211) | .13 | |
|
| ||||||||
| Growth restriction (z score < −1) | .18 | |||||||
| No | 415 (83%) | 151 (74%) | 264 (89%) | <.001 | 88 (76%) | 327 (85%) | .18 | |
| Yes | 85 (17%) | 52 (26%) | 33 (11%) | 28 (24%) | 57 (15%) | |||
|
| ||||||||
| Gender | ||||||||
| Male | 261 (52%) | 113 (56%) | (50%) | .26 | 52 (45%) | 209 (54%) | .25 | |
| Female | 239 (48%) | 90 (44%) | (50%) | 64 (55%) | 175 (46%) | |||
|
| ||||||||
| Race/Ethnicity a | ||||||||
| Caucasian | 344 (70%) | 138 (69%) | 206 (70%) | .57 | 84 (76%) | 260 (68%) | .20 | |
| African-American | 60 (12%) | 26 (13%) | 34 (12%) | 8 (7%) | 52 (14%) | |||
| Hispanic | 38 (8%) | 14 (7%) | 24 (8%) | 4 (4%) | 34 (9%) | |||
| Asian | 22 (4%) | 13 (6%) | 9 (3%) | 10 (9%) | 12 (3%) | |||
| Other | 30 (6%) | 10 (5%) | 20 (7%) | 4 (4%) | 26 (7%) | |||
|
| ||||||||
| Hospital | ||||||||
| A | 354 (71%) | 131 (65%) | 223 (75%) | .03 | 84 (72%) | 270 (70%) | .87 | |
| B | 146 (29%) | 72 (35%) | 74 (25%) | 32 (28%) | 114 (30%) | |||
|
| ||||||||
| Antenatal Steroids | ||||||||
| None | 28 (6%) | 10 (5%) | 18 (6%) | .49 | 8 (7%) | 20 (5%) | .53 | |
| Partial | 140 (28%) | 51 (25%) | 89 (30%) | 26 (22%) | 114 (30%) | |||
| Complete | 322 (66%) | 142 (70%) | 190 (64%) | 82 (71%) | 250 (65%) | |||
|
| ||||||||
| Chorioamnionitis | ||||||||
| No clinical signs | 454 (91%) | 191 (94%) | 263 (89%) | .07 | 110 (95%) | 344 (90%) | .22 | |
| Clinical signs | 46 (9%) | 12 (6%) | 34 (11%) | 6 (5%) | 40 (10%) | |||
|
| ||||||||
| Mode of Ventilation at 6–18 h | ||||||||
| None or CPAP | 46 (9%) | 8 (4%) | 38 (13%) | .003 | 9 (8%) | 37 (10%) | .93 | |
| Conventional ventilation | 424 (85%) | 175 (86%) | 249 (84%) | 100 (86%) | 324 (84%) | |||
| High-frequency ventilation | 30 (6%) | 20 (10%) | 10 (3%) | 7 (6%) | 23 (6%) | |||
|
| ||||||||
| Patent Ductus Arteriosis | ||||||||
| None or untreated | 163 (33%) | 36 (18%) | 127 (43%) | <.001 | 32 (28%) | 131 (34%) | .70 | |
| Medical treatment only | 255 (51%) | 117 (58%) | 138 (46%) | 60 (52%) | 195 (51%) | |||
| Surgical ligation only | 9 (2%) | 3 (1%) | 6 (2%) | 4 (3%) | 5 (1%) | |||
| Medical and surgical ligation | 73 (15%) | 47 (23%) | 26 (9%) | 20 (17%) | 53 (14%) | |||
|
| ||||||||
| Early-onset sepsis | ||||||||
| None | 345 (69%) | 128 (63%) | 217 (73%) | .04 | 86 (74%) | 259 (67%) | .44 | |
| Clinically suspected | 145 (29%) | 73 (36%) | 72 (24%) | 26 (22%) | 119 (31%) | |||
| Culture proven | 10 (2%) | 2 (1%) | 8 (3%) | 4 (3%) | 6 (2%) | |||
|
| ||||||||
| Late-onset sepsis | ||||||||
| None | 281 (56%) | 80 (39%) | 201 (68%) | <.001 | 70 (60%) | 211 (55%) | .74 | |
| Clinically suspected | 107 (21%) | 61 (30%) | 46 (15%) | 20(17%) | 87 (23%) | |||
| Culture proven | 112 (22%) | 62 (31%) | 50 (17%) | 26 (22%) | 86 (22%) | |||
|
| ||||||||
| Necrotizing enterocolitis | ||||||||
| None | 453 (91%) | 179 (88%) | 274 (92%) | .11 | 108 (93%) | 345 (90%) | .80 | |
| Medical | 26 (5%) | 16 (8%) | 10 (3%) | 4 (3%) | 22 (6%) | |||
| Surgical | 21 (4%) | 8 (4%) | 13 (4%) | 4 (3%) | 17 (4%) | |||
N=494
BPD was more likely to occur in infants of lower GA, lower birth weight, or with growth restriction. There was no difference in sex, race/ethnicity, or antenatal steroid use between infants with or without BPD. Infants with BPD were more likely to have had a patent ductus arteriosus and/or late-onset sepsis than infants without BPD (P < .05). Patient characteristics were similar in MC and DC twins. There was no difference between hospitals in the percent of MC twins. The percentage of infants developing BPD was significantly different between the two birth hospitals.
A comparison of the GA distributions between the study and validation cohorts is presented in Table 2. GA distributions of the study and validation cohorts differed in that only the study cohort included infants born between 28 0/7 and 28 6/7 weeks. Thus, the mean GA of the validation cohort was skewed slightly toward infants born at lower GA.
Table 2.
Gestational age (GA) distribution for primary study and validation cohorts. There was no significant difference in GA distribution between Primary Study and Validation cohorts for births below 28 weeks gestation (shaded area) by Fisher’s exact test (p = .51).
| Number of Twin Pairs | |||
|---|---|---|---|
| GA (weeks) | Study | Validation | Combined |
| 23 | 3 | 1 | 4 |
| 24 | 19 | 15 | 34 |
| 25 | 25 | 22 | 47 |
| 26 | 48 | 26 | 74 |
| 27 | 70.5a | 41 | 111.5 |
| 28 | 84.5a | N/A | 84.5 |
| Total | 250 | 105 | 355 |
| Mean(±SD) | 26.7(±1.3) | 25.9(±1.1) | 26.4(±1.3) |
| Range | 23–28 | 23–27 | 23–28 |
The infants in one twin pair were born separately, at gestational ages 27 and 28 weeks.
χ2 analysis comparing whether neither, one, or both of MC and DC twin pairs developed BPD revealed no difference (p=0.71), suggesting heritability is not a major factor. This finding was confirmed in the validation population (p=0.32, Table 3).
Table 3.
Statistical analyses of for BPD heritability by X2, intra-class correlation and ACE Modeling.BPD heritability by X2, intra-class correlation (ICC) and ACE modeling in twin pairs of primary study, validation and combined cohorts by chorionicity.
| Cohort | Chorionicity | Number in Twin Pairs Affected | ACE modeling Percent of Variability (95% CI) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neither | One | Both | Total | χ2 | p | ICC (95% CI) | p | %A (Heritability) |
p | %C (Common Environmental) |
p | ||
| Study | MC | 23 | 19 | 16 | 58 | 0.69 | .71 | 0.34 (0.08,0.55) |
.70 | 0.0% (0.0%, 43.1%) |
1.0 | 56.4% (25.0%, 68.0%) |
.004 |
| DC | 88 | 56 | 48 | 192 | 0.39 (0.25,0.51) |
||||||||
| Total | 111 | 75 | 64 | 250 | |||||||||
| Validation | MC | 9 | 7 | 13 | 29 | 2.27 | .32 | 0.51 (0.15,0.75) |
.92 | 6.5% (0.0%, 67.4%) |
.44 | 66.9% (17.2%, 83.7%) |
.016 |
| DC | 34 | 19 | 23 | 76 | .49 (0.27,0.66) |
||||||||
| Total | 43 | 26 | 36 | 105 | |||||||||
| Combined | MC | 32 | 26 | 29 | 87 | 2.33 | .31 | 0.40 (0.20,0.57) |
.88 | 0.0% (0.0%, 38.6%) |
1.0 | 61.2% (33.0%, 70.4%) |
<.001 |
| DC | 122 | 75 | 71 | 268 | 0.42 (0.30,0.52) |
||||||||
| Total | 154 | 101 | 100 | 355 | |||||||||
Assessment of similarity in the BPD outcome by intraclass correlations (ICC) within twin pairs demonstrated significant familial aggregation within both MC and DC pairs: MC ICC = 0.34, 95% CI (0.08, 0.55); DC ICC = 0.39, 95% CI (0.25, 0.51). However, the difference between these correlations in MC vs. DC twins was not significant (p=0.70). This finding was replicated in the validation population: MC ICC = 0.51, DC ICC = 0.49, p=0.92 (Table 3).
The ACE model yielded a non-significant estimate of heritability. The estimated percentage of variance from additive genetic effects (%A) was 0.0% (95% CI: 0.0%, 43.1%; p=1.0). This finding was similar in the validation population, with %A 6.5% (95% CI: 0.0%, 67.4%; p=0.44). With combined cohorts, (355 twin pairs) %A = 0.0% (95% CI: 0.0%, 38.6%; p=1.0) (Table 3). The common environmental (%C) impact on BPD development was significant in both the study cohort (56.4%; 95% CI: 25.0, 68.0%; p=0.004) and the validation cohort (66.9%; 95% CI:17.2%, 83.7%; p=0.016) (Table 3). The %C in the combined cohort (n=355 pairs) was 61.2% (CI 33.0% – 70.4%, p<.001).
To address the concern that heritability might only be demonstrated in a comparison of twins selected by zygosity, we repeated the three statistical tests comparing MZ and DZ twins, excluding those DC twins with indeterminate zygosity status. Chi square, ICC and ACE modeling (%A) methods did not suggest BPD heritability (Table 4; available at www.jpeds.com). Given these results, all remaining comparisons were performed comparing twin pairs based on chorionicity to avoid bias introduced by discarding same sex twin pairs of indeterminate zygosity and to take advantage of the larger sample size, acknowledging the possibility of up to 10% misclassification of DC twins who are actually MZ.
Table 4 (online).
BPD heritability by X2, intra-class correlation (ICC) and ACE modeling in twin pairs of known zygosity (DC pairs with same gender and same blood type were excluded from this analysis). MZ = monozygotic, DZ = dizygotic
| Zygosity | Number in Twin Pairs Affected | ACE modeling Percent of Variability (95% CI) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neither | One | Both | Total | χ2 | p | ICC (95% CI) | p | %A (Heritability) | p | %C (Common Environmental) | p | |
| MZ | 23 | 19 | 16 | 58 | 1.14 | .57 | 0.34 (0.08,0.55) |
.36 | 0.0% (0.0%, 32.6%) |
1.0 | 62.5% (35.8%, 74.6%) |
.001 |
| DZ | 58 | 32 | 35 | 125 | 0.47 (0.30,0.61) |
|||||||
| Total | 81 | 51 | 51 | 183 | ||||||||
We also assessed BPD heritability using multiple definitions 2–6. In addition to categorizing infants as having BPD based on oxygen use at 36 weeks PMA (Table 3), we reclassified infants by three additional BPD definitions: 1) Oxygen or positive pressure support (CPAP, high flow nasal cannula or ventilator) with room air at 36 weeks PMA, and 2) NIH consensus definition moderate or severe BPD categories2 and 3) “death or BPD (oxygen use at 36 weeks PMA). None yielded evidence for BPD heritability using the described statistical methods (Table 5; available at www.jpeds.com).
Table 5 (online).
BPD Heritability in Primary study population using alternate definitions of BPD. MC = monochorionic, DC = dichorionic.
| Definition | Chorionicity | Number in Twin Pairs Affected | ACE modeling Percent of Variability (95% CI) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neither | One | Both | Total | χ2 | p | ICC (95% CI) | p | %A (Heritability) |
p | %C (Common Environmental) |
p | ||
| O2, CPAP or high flow nasal cannula (with or without O2) or positive pressure ventilation (with or without O2) at 36 weeks PMA | MC | 23 | 18 | 17 | 58 | .50 | .78 | 0.37 (0.12,0.58) |
.87 | 0.0% (0.0%, 47.9%) |
1.0 | 57.9% (23.3%, 69.3%) |
.004 |
| DC | 86 | 56 | 50 | 192 | 0.40 (0.26,0.52) |
||||||||
| Total | 109 | 74 | 67 | 250 | |||||||||
| Moderate or Severe BPD by NIH criteria2 | MC | 24 | 17 | 17 | 58 | .72 | .70 | 0.41 (0.15,0.61) |
.78 | 9.3% (0.0%, 62.2%) |
.40 | 50.4% (11.5%, 67.7%) |
.017 |
| DC | 88 | 58 | 46 | 192 | 0.37 (0.23,0.49) |
||||||||
| Total | 112 | 75 | 63 | 250 | |||||||||
| Composite BPD (O2 at 36 weeks) or Death | MC | 27 | 23 | 33 | 83 | .16 | .92 | 0.44 (0.23,0.61) |
.79 | 7.5% (0.0%, 49.0%) |
.39 | 56.7% (26.6%, 70.3%) |
.001 |
| DC | 91 | 80 | 102 | 273 | 0.41 (0.30,0.51) |
||||||||
| Total | 118 | 103 | 135 | 356* | |||||||||
for composite definition “BPD or Death”, total number of pairs is increased due to inclusion of twin pairs previously excluded based on death of one or both twins died prior to 36 weeks PMA
To test the hypothesis that a genetic influence might not be dominant in babies of lowest gestational age, we combined the primary and validation cohorts, divided them into two GA strata (23 – 26 weeks and 27 – 28 weeks) and repeated the three statistical tests in each of the two strata (Table 6; available at www.jpeds.com). Chi square, ICC and ACE modeling (%A) analyses did not suggest BPD heritability by any method in either GA group.
Table 6 (online).
BPD heritability by X2, intra-class correlation (ICC) and ACE modeling in MC vs. DC twin pairs in combined study and validation cohorts stratified by gestational age (23–26 weeks, 27–28 weeks). MC = monochorionic, DC = dichorionic
| Gestational Age | Chorionicity | Number in Twin Pairs Affected | ACE modeling Percent of Variability (95% CI) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neither | One | Both | Total | χ2 | p | ICC (95% CI) | p | %A (Heritability) | p | %C (Common Environmental) | p | ||
| 23–26 wks | MC | 7 | 15 | 17 | 39 | 1.01 | .60 | 0.18 (−0.13,0.46) |
.55 | 0.0% (0.0%, 58.5%) |
1.0 | 40.5% (0.0%, 57.4%) |
.06 |
| DC | 31 | 42 | 47 | 120 | 0.29 (0.11,0.45) |
||||||||
| Total | 38 | 57 | 64 | 159 | |||||||||
| 27–28 wks | MC | 25 | 11 | 12 | 48 | 2.07 | .36 | 0.51 (0.22,0.71) |
.67 | 10.6% (0.0%, 61.5%) |
.38 | 61.4% (20.9%, 79.2%) |
.008 |
| DC | 91 | 33 | 24 | 148 | .44 (0.27,0.58) |
||||||||
| Total | 116 | 44 | 36 | 196 | |||||||||
To test the hypothesis that race may influence whether BPD is heritable, we divided the primary cohort into Caucasian vs. non-Caucasian strata and repeated the three statistical tests independently in each of the two strata (Table 7; available at www.jpeds.com). Chi square, ICC and ACE modeling (%A) analyses did not suggest BPD heritability by any method in either Caucasian or Non-Caucasian twins.
Table 7 (online).
Heritability estimates of BPD stratifying the primary study population by race: Caucasian vs. Non-Caucasian. MC = monochorionic, DC = dichorionic
| Race | Chorionicity | Number in Twin Pairs Affected | ACE modeling Percent of Variability (95% CI) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neither | One | Both | Total | χ2 | p | ICC (95% CI) | p | %A (Heritability) |
p | %C (Common Environmental) |
p | ||
| Caucasian | MC | 18 | 13 | 11 | 42 | 0.15 | .93 | 0.36 (0.06,0.61) |
.80 | 0.0% (0.0%, 53.6%) |
1.0 | 58.6% (18.6%, 71.9%) |
.011 |
| DC | 60 | 37 | 33 | 130 | 0.41 (0.24,0.55) |
||||||||
| Total | 78 | 50 | 44 | 172 | |||||||||
| Non-Caucasian | MC | 3 | 6 | 4 | 13 | 2.23 | .33 | 0.07 (−0.42,0.53) |
.34 | 0.0% (0.0%, 68.6%) |
1.0 | 47.9% (0.0%, 69.7%) |
.06 |
| DC | 28 | 19 | 15 | 62 | .36 (0.11,0.57) |
||||||||
| Total | 31 | 25 | 19 | 75 | |||||||||
There are no standard power or sample size formulas for ACE modeling. We performed an approximate post hoc power calculation by using a confidence interval (CI) from our data analyses to estimate how statistical precision changes with sample size. Because our profile likelihood CIs are not symmetric, we used the half-width based on the estimate and the lower bound, as it is the lower bound that corresponds to a test of the null hypothesis of zero heritability. We used the CI for %C in the combined cohorts (Table 3) because we needed a CI whose lower bound was non-zero. Using the fact that CI widths in more standard analyses are proportional to 1/N½, we estimated the proportionality constant and calculated that if the heritability effect was 51.9% or higher, we would have had ≥80% power with our sample size of 355 twin pairs.
Discussion
Three independent twin cohort studies have suggested genetic factors contribute significantly to BPD risk9,10,11 (Table 8). These findings launched genetics-focused research efforts including single gene, GWAS and whole exome sequencing (WES) searches for candidates or pathways responsible for BPD21–25. Although differences in single gene allele frequencies between large BPD and non-BPD cohorts have been reported, major genetic determinants have not been confirmed6. Although a large GWAS study was unsuccessful in identifying specific BPD associated loci, subsequent analysis suggested association of pathways with severe BPD22, 23. Most recently, WES of severely affected infants suggested new BPD gene candidates that may reveal further understanding of pathophysiology24,25. Expression profiling studies revealed differences in gene expression between BPD and non-BPD infants, but identification of associated genetic variants remains a challenge26.
Table VIII.
Comparison of studies estimating the heritability of BPD
| Parker et al9 | Bhandari et al10 | Lavoie et al11 | Current study | ELGAN validation15 | ||
|---|---|---|---|---|---|---|
| Years subjects accrued | 1976–1990 | 1994–2004 | 1993–2006 | 1997–2015 | 2002–2004 | |
| Population size | 1872 | 450 twin pairs | 478 | 2198 | 1506 | |
| Number ot twin pairs analyzed | 108 | 252 | 159 | 250 | 105 | |
| Restrictions | <1500 g birth weight survival >28 d | ≤32 wk gestational age survrval ≥36 wk PMA | ≤30 wk gestational age | ≤28 wk gestational age survival ≥36 wk PMA | <28 wk gestational age survival ≥36 wk PMA | |
| Chonomcity known | No | Yes | Yes | Yes | Yes | |
| Zygosity known | No | Yes | Yes | Yes | No | |
| BPD definition | O2 at 28 d + abnormal CXR + symptoms | O2 at 36 wk PMA + abnormal CXR | O2 at 36 wk PMA or discharge NIH consensus criteria | O2 at 36 wk PMA O2 CPAP/RA at 36 wk PMA NIH consensus critena | O2 at 36 wk | |
| Sex (male) | 57% of BPD 46.3% of cohort | 54% of cohort | 55.7% of cohort | 56% of BPO 52% of cohort | ||
| Gestational age (wk) | Overall | 29.7 ± 2.5 | 27.9 ± 1.8 | 26.7 (±1.3) | 25.9(±1.1) | |
| mean ± SD | No BPD | 30.4 ± 2.3 | 27.0 (±1.2) | 26.2 (±1.0) | ||
| BPD | 27.7 ± 2.0 | 26.2 (±1.4) | 25.5 (±1.2) | |||
| Birth weight (g) | Overall | 1397 ± 443 | 1116 ± 314 | 950 (±208) | 873 (±189) | |
| mean ± SD | No BPD | 1211 ± 209 | 1014 (±198) | 925 (±169) | ||
| BPD | 929 ± 225 | 855 (±185) | 813 (±193) | |||
| % cohort with BPD | 17.5% | 19.8% moderate, 7.5% severe | 41% | |||
| Analysis methods | OR of BPD in twin A and twin B | ACE model* | ACE model* assuming %C = 0 (AE model) | ACE model* | ACE model* | |
| Heritability estimate | aOR 12.3 (P < .01) | unadjusted %A = 63.6% adjusted %A= 53% 95% CI (16%–89%) |
unadjusted %A = 82% 95% CI (70%–97%) |
unadjusted %A = 0.0% 95% CI (0%–43%) |
unadjusted %A = 6.5% 95% CI (0%–67%) |
CXR, chest radiograph; RA, room air.
ACE model: decomposes the total varation in “liability” of disease into additive genetic (A), common or shared environmental (C), and unique or unshared environmental (E) effects.
Over the past five decades, the BPD definition has evolved due to adoption of antenatal glucocorticoid therapy, improvements in NICU care, and resulting shifts in cohort characteristics 1–6, 27. Currently, infants born above 1,250 grams birth weight or 29 weeks GA are at relatively low BPD risk and their risk factors differ from lower GA and birth weight infants. Therefore, we focused on the highest risk population born before 29 weeks GA and applied appropriate statistical methodologies for twin pair assessment to tease out contributions of genetic and/or environmental factors to BPD development.
Differing approaches to statistical analysis, BPD definition, cohort inclusion criteria, and cohort size between this and prior studies may explain observed differences in results and conclusions (Table 8). Parker’s9 pre-surfactant era cohort was assembled when survival of infants below 28 weeks GA was low. Their inclusion criteria (<1,500g, survival beyond 28 days of life) were biased towards growth-restricted infants at more advanced gestational ages. Recent Vermont Oxford Network data suggest birth at 29 – 32 weeks GA carries a <10% risk of BPD14. Although Bhandari10 and Lavoie11 enrolled babies in the post-surfactant era and utilized contemporary BPD definitions, both of their cohorts include higher proportions of infants at low BPD risk due to higher mean GA (Table 8). The mean GA at birth of Bhandari’s cohort was older than the GA of all infants in our primary and validation cohorts.
In our combined cohort ACE model of BPD, the estimate of heritability is small (%A = 0%). This finding is consistent with the results from the χ2 and ICC analyses in our primary and validation populations, i.e. that MC twins do not have a higher concordance of outcome than DC twins, as would be expected if there is a genetic influence on development of BPD.
Thirty percent of all twins are MZ and 25–33% of these are DC29,30. Analyses focused on zygosity found no substantial differences compared with a cohort defined by chorionicity. Even though categorizing by zygosity is considered the gold standard, in the absence of universal zygosity determination (not performed in this or the prior studies9,10,11) a bias may be introduced when discarding large numbers of same sex DC twin pairs of unknown zygosity (69% of same sex DC twins in our primary cohort), as was done by Lavoie11. Our chorionicity based analysis accepted that ~9% of DC twins (used as a surrogate for DZ) are MZ. Analysis by chorionicity retains a larger cohort size; our cohort would have shrunk from 250 to 183 twin pairs if restricted by zygosity.
Biological evidence suggests that female infants have lower BPD risk.5,28 Excluding same-sex pairs reduces same-risk pairs, which in turn reduces same-outcome (concordant) pairs and creates a bias towards including discordant sex pairs in the DZ group. Higher concordance of outcomes in “mono-“ (MZ or MC) than in “di-“ (DZ or DC) pairs is evidence of hereditability, so increasing the concordance of outcomes in “mono-“ pairs or reducing concordance of outcomes in “di-“ pairs creates a bias toward finding hereditability. Even with this potential bias toward finding hereditability, results in our chorionicity-based and zygosity-based cohorts were not different (Table 4). We used chorionicity as a proxy for zygosity given that the small zygosity misclassification may be less likely to introduce bias than eliminating same-sex DC twins whose zygosity can’t be distinguished by blood type.
Our findings do not support the conclusions of prior twin studies (heritability estimates 53% – 82%, Table 8). Heritability should have been evident if the true magnitude of effect was of the size previously reported. Lavoie11 reported a hereditability estimate from a model assuming no common environmental contribution to BPD (%C = 0), because the C component was not significant in their data. The impact of environmental effects on BPD (including clinical practice differences) may vary between populations. By assuming %C = 0, their model reallocates any contribution from C to the A and E components. We believe this is an inappropriate application of the method unless there is strong evidence for no common environmental contribution. Bhandari10 reported significant hereditability estimates from the full ACE model, both unadjusted and adjusted for predictors of BPD. In our analytic approach, we let patient characteristics contribute to the shared and unshared environmental components of the ACE model rather than removing their contributions before the ACE modeling. Nevertheless, Bhandari’s10 adjusted and unadjusted heritability estimates were similar, suggesting adjustment for patient characteristics doesn’t affect conclusions. The wide confidence intervals we identified yielded limits approaching values reported in other studies with modest population size.
Lavoie11 utilized multiple BPD definitions: 1) O2 at ≥28 days, 2) O2 at ≥36 weeks PMA or, if earlier, at discharge, and 3) NIH consensus statement on BPD definition (mild/mod/severe)2. Although a model with BPD defined as O2 at ≥ 28 days of age did not yield a substantial heritability estimate, the other two definitions generated %A of 82% and 79%, suggesting a significant contribution of heritability. In our cohort, the inclusion of babies on CPAP in RA or the use of NIH consensus definitions did not alter our results, nor could we demonstrate race or gestational age strata influenced heritability.
We studied a population of lower GA infants to reflect current epidemiologic patterns in BPD. Information on care practices, such as SpO2 limits used to justify supplemental oxygen use, is missing from all studies making it difficult to quantify the possible contribution of differences in care practices. Even though stratification by GA did not identify obvious differences in heritability estimates at 23 – 26 weeks vs. 27 – 28 weeks, it is possible that a heritability factor could play a role in infants born at GA ≥ 29 weeks.
Based on findings contrary to those of Parker9, Bhandari10 and Lavoie11, we sought a validation cohort. The ELGAN cohort was younger in GA than our primary study cohort. Because findings were similar in study and validation populations, we presented data both separately and combined. The combined sample size approximated that of the largest10 prior study with a higher absolute number of affected infants.
A negative ACE model result doesn’t preclude genetic variation contributing to BPD development. Genomic studies have also yielded mixed results21–26, neither confirming nor disproving heritable factors. Genomic analyses on preterm infants are challenged by unavailable allele frequencies for unaffected premature survivors for comparison.
In conclusion, we found no evidence for a significant contribution of heritable factors to BPD risk in infants born <29 weeks gestation. We speculate that, for extremely preterm infants, the roles of immature lung structure and biochemical status trump the potential role of genetic factors in BPD pathogenesis, the latter of which may play a more significant role in the rarer instances in which BPD develops in infants born at or over 29 weeks GA. Possible factors contributing to differing results include our gestational-age restricted population, specifics of model construction and analysis, and population characteristics. The greater gestational maturity of the populations of babies included in other studies suggest that if genetic predisposition to BPD exists, its role is more prominent among preterm infants born beyond 29 weeks GA.
Acknowledgements
We thank the ELGAN Study, which was supported by the National Institute of Neurological Disorders and Stroke (5U01NS040069-05) and the National Institute of Child Health and Human Development (5P30HD018655-28) for sharing data we used for validation. We also gratefully acknowledge the important contributions of our meticulous data abstractors, Amy Zolit, Lea Horwitz, and Michele Phillips.
Supported by the National Heart Lung and Blood Institute Specialized Center of Research (HL-67699), the Brigham and Women’s Hospital Center for Clinical Investigation/Harvard Clinical and Translational Science Center (UL1 RR025758-01) from the National Center for Research Resources, and the National Human Genome Research Institute/ Eunice Kennedy Shriver National Institute of Child Health and Human Development (U19 Grant HD077671).
Abbreviations:
- ACE
Additive genetic, Common environmental, unique Environmental
- AGA
Appropriate for Gestational Age
- BIDMC
Beth Israel Deaconess Medical Center
- BPD
Bronchopulmonary Dysplasia
- BWH
Brigham and Women’s Hospital
- ELGAN
Extremely low gestational age newborn
- GA
Gestational age
- GWAS
Genome Wide Association Study
- ICC
Intraclass correlations
- O2
Oxygen
- PDA
Patent Ductus Arteriosus
- PMA
Postmenstrual age
- NICU
Newborn Intensive Care Unit
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- (1).Shennan AT, Dunn MS, Ohlsson A, Lennox K, Hoskins EM.Abnormal pulmonary outcomes in premature infants: prediction from oxygen requirement in the neonatal period. Pediatrics. 1988; 82:527–32 [PubMed] [Google Scholar]
- (2).Ehrenkranz RA, Walsh MC, Vohr BR, Jobe AH, Wright LL, Fanaroff AA, Wrage LA, Poole K; National Institutes of Child Health and Human Development Neonatal Research Network. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics. 2005; 116:1353–60 [DOI] [PubMed] [Google Scholar]
- (3).Parad RB, Davis JM, Lo J, Thomas M, Marlow N, Calvert S, Peacock JL, Greenough A. Prediction of respiratory outcome in extremely low gestational age infants. Neonatology. 2015; 107:241–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Isayama T, Lee SK, Yang J, Lee D, Daspal S, Dunn M, Shah PS; Canadian Neonatal Network and Canadian Neonatal Follow-Up Network Investigators. Revisiting the Definition of Bronchopulmonary Dysplasia. JAMA Pediatr. 2017; 171:271–279 [DOI] [PubMed] [Google Scholar]
- (5).Keller RL, Feng R, DeMauro SB, Ferkol T, Hardie W, Rogers EE, Stevens TP, Voynow JA, Bellamy SL, Shaw PA and Moore PE for the Prematurity and Respiratory Outcomes Program. Bronchopulmonary Dysplasia and Perinatal Characteristics Predict 1-Year Respiratory Outcomes in Newborns Born at Extremely Low Gestational Age: A Prospective Cohort Study. J Pediatr. 2017; 187:89–97.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Jobe AH, Steinhorn R. Can We Define Bronchopulmonary Dysplasia? J Pediatr. 2017; 188:19–23 [DOI] [PubMed] [Google Scholar]
- (7).Bhandari V, Gruen J. The Genetics of Bronchopulmonary Dysplasia. Semin Perinat. 2006; 30:185–191 [DOI] [PubMed] [Google Scholar]
- (8).Huusko JM, Karjalainen MK, Mahlman M, Haataja R, Kari MA, Andersson S, Toldi G, Tammela O, Rämet M, Lavoie PM, Hallman M; Gen-BPD Study Group. A study of genes encoding cytokines (IL6, IL10, TNF), cytokine receptors (IL6R, IL6ST), and glucocorticoid receptor (NR3C1) and susceptibility to bronchopulmonary dysplasia. BMC Medical Genetics. 2014; 15:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Parker RA, Lindstrom P, Cotton RB. Evidence from twin study implies possible genetic susceptibility to bronchopulmonary dysplasia. Semin Perinat. 1996; 20:206–209 [DOI] [PubMed] [Google Scholar]
- (10).Bhandari V, Bizzarro MJ, Shetty A, Zhong X, Page GP, Zhang H, Ment LR, Gruen JR; Neonatal Genetics Study Group.. Familial and genetic susceptibility to major neonatal morbidities in preterm twins. Pediatrics. 2006; 117:1901–1906 [DOI] [PubMed] [Google Scholar]
- (11).Lavoie PM, Pham C, Jang KL. Heritability of bronchopulmonary dysplasia, defined according to the consensus statement of the National Institutes of Health. Pediatrics. 2008; 122:479–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Jobe AH. The new bronchopulmonary dysplasia. Current Opinion in Pediatrics. 2011; 23: 167–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Northway WH Jr, Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N Engl J Med. 1967; 276:357–68 [DOI] [PubMed] [Google Scholar]
- (14).https://nightingale.vtoxford.org/home.aspx, derived from 2012. Network Data
- (15).O’Shea TM, Allred EN, Dammann O, Hirtz D, Kuban KC, Paneth N, Leviton A; ELGAN study Investigators. The ELGAN study of the brain and related disorders in extremely low gestational age newborns. Early Hum Dev. 2009; 85:719–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).SAS Institute Inc. SAS/STAT® 12.1 User’s Guide. 2012; Cary, NC: SAS Institute Inc. [Google Scholar]
- (17).Donner A, Klar N, Eliasziw M. Statistical methodology for estimating twin similarity with respect to a dichotomous trait. Genet Epidemiol. 1995; 12:267–77 [DOI] [PubMed] [Google Scholar]
- (18).Neale MC, Cardon LR. Methodology for Genetic Studies of Twins and Families. 1992; Dordrecht, The Netherlands: Kluwer Academic. [Google Scholar]
- (19).Feng R, Zhou G, Zhang M, Zhang H. Analysis of twin data using SAS. Biometrics 2009; 65:584–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Dominicus A, Skrondal A, Gjessing HK, Pedersen NL, Palmgren J. Likelihood ratio tests in behavioral genetics: problems and solutions. Behavior Genetics 2006; 36:331–340 [DOI] [PubMed] [Google Scholar]
- (21).Shaw GM, O’Brodovich HM. Progress in understanding the genetics of bronchopulmonary dysplasia. Semin Perinat. 2013; 37:85–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Wang H, St Julien KR, Stevenson DK, Hoffmann TJ, Witte JS, Lazzeroni LC, Krasnow MA, Quaintance CC, Oehlert JW, Jelliffe-Pawlowski LL, Gould JB, Shaw GM, O’Brodovich HM . A genome-wide association study (GWAS) for bronchopulmonary dysplasia. Pediatrics. 2013; 132:290–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Ambalavanan N, Cotten CM, Page GP, Carlo WA, Murray JC, Bhattacharya S, Mariani TJ, Cuna AC, Faye-Petersen OM, Kelly D, Higgins RD; Genomics and Cytokine Subcommittees of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network.. Integrated genomic analyses in bronchopulmonary dysplasia. J Pediatr. 2015; 166:531–7.e13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Carrera P, Di Resta C, Volonteri C, Castiglioni E, Bonfiglio S, Lazarevic D, Cittaro D, Stupka E, Ferrari M, Somaschini M; BPD and Genetics Study Group. Exome sequencing and pathway analysis for identification of genetic variability relevant for bronchopulmonary dysplasia (BPD) in preterm newborns: A pilot study. Clinica Chimica Acta. 2015; 451:39–45 [DOI] [PubMed] [Google Scholar]
- (25).Li J, Yu KH, Oehlert J, Jeliffe-Pawlowski LL, Gould JB, Stevenson DK, Snyder M,Shaw GM, O’Brodovich HM. Exome sequencing of neonatal blood spots identifies genes implicated in bronchopulmonary dysplasia. American Journal of Respiratory and Critical Care Medicine. 2015; 192:589–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Pietrzyk JJ, Kwinta P, Wollen EJ, Bik-Multanowski M, Madetko-Talowska A, Günther CC, Jagła M, Tomasik T, Saugstad OD. Gene expression profiling in preterm infants: New aspects of bronchopulmonary dysplasia development. PloS One. 2013; 8:e78585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Vendettuoli V, Bellu R, Zanini R, Mosca F, Gagliardi L & Italian Neonatal Network. Changes in ventilator strategies and outcomes in preterm infants. Archives of Disease in Childhood Fetal and Neonatal Edition. 2014; 99:F321–4 [DOI] [PubMed] [Google Scholar]
- (28).Peacock JL, Marston L, Marlow N, Calvert SA, Greenough A. Neonatal and infant outcome in boys and girls born very prematurely. Pediatr Res. 2012; 71:305–10 [DOI] [PubMed] [Google Scholar]
- (29).Nikkels PGJ, Hack KEA, van Gemert MJC. Pathology of twin placentas with special attenition to monochorionic twin placentas. J Clin Pathol. 2008; 61:1247–1253 [DOI] [PubMed] [Google Scholar]
- (30).Bajoria R, Kingdom J. The case for routine determination of chorionicity and zygosity in multiple pregnancy. Prenatal Diagnosis. 1997; 13:1207–1225 [PubMed] [Google Scholar]