Abstract
We present the computed tomography, ultrasonographic findings of a case of contralateral supraclavicular lymphadenopathy that occurred after AstraZeneca COVID-19 vaccination. Contralateral supraclavicular lymphadenopathy is very rare, but may be expected as an adverse reaction after COVID-19 vaccination. Radiologists as well as referring clinicians should be aware of this self-limiting process and its imaging features.
Keywords: COVID-19, Vaccines, Lymphadenopathy, Ultrasonography, Computed tomography
Competing interests
None of the authors have any conflict of interest to disclose.
Since December 2020, many vaccinations for COVID-19 have been performed worldwide. Despite the benefits of all vaccines, mild side effects including local pain, fatigue, headache, fever, chills as well as severe adverse effects including lymphadenopathy have observed after the vaccination. Recognizing the possible adverse reactions associated with vaccination requires clinicians to distinguish the expected post-vaccination transient response from actual pathologic processes. Several recent articles have reported on ipsilateral lymphadenopathy after COVID-19 vaccination. Most of those cases were axillary lymphadenopathy, supraclavicular lymphadenopathy being less common [1,2]. In fact, reports of contralateral supraclavicular lymphadenopathy are rare [3,4].
This report presents a rare case of contralateral supraclavicular lymphadenopathy following a COVID-19 vaccination along with the neck computed tomography (CT) and ultrasonography results.
Case description
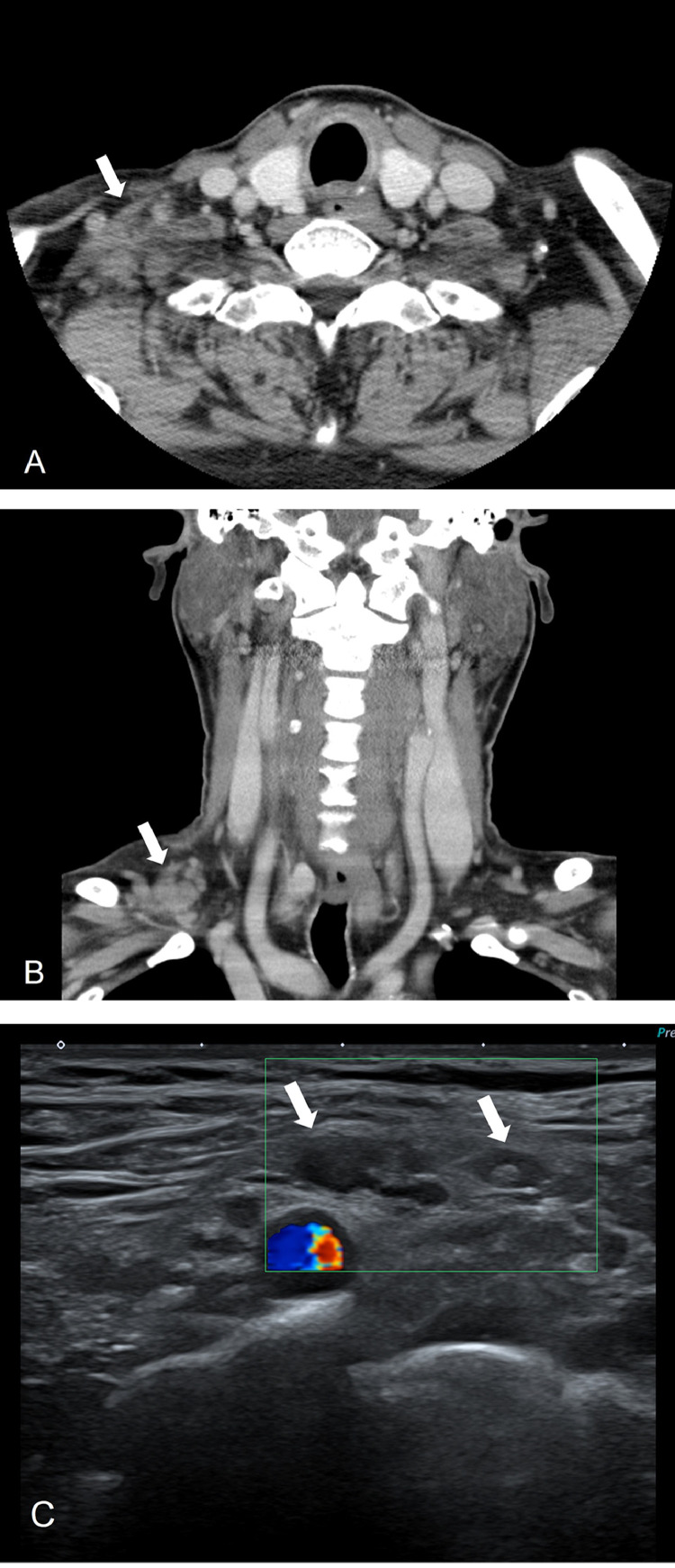
A 61-year-old male patient visited our hospital with a palpable lesion in the right supraclavicular area that had endured for 9 days. He received an intramuscular injection of a first dose COVID-19 vaccine (Oxford-AstraZeneca in his left upper arm, 14 days prior to visiting our hospital. He complained of a fever up to 39.2°C, chills and muscle pain lasting six days. He did not have any past medical history, including malignancy. Due to his fever, he was tested for COVID and the result was negative. He had no history of contact with any COVID-19 patients. On physical examination, the clinician found a soft, immovable mass of about 4 × 3.5 cm in the right supraclavicular area. His white blood cell count was elevated, at 11.62 × 1000/uL. The patient underwent neck CT for evaluation of right supraclavicular lesion, 21 days after receiving the COVID-19 vaccine. Neck CT revealed enlarged, clustered and conglomerated lymph nodes accompanied by perinodal infiltration in the right supraclavicular area. No necrosis in the lymph nodes was evident. The diameter of lesion was about 3 cm. There was no abnormal lymph node in the left supraclavicular area (Fig 1A and B). The possibility of lymphadenitis including Kikuchi disease was suggested when considering unilateral involvement with perinodal infiltration. However, the clinician had to consider the possibility of malignancy, due to the patient's old age and contralateral supraclavicular lymphadenopathy. The clinician decided to perform the ultrasonography-guided core needle biopsy of the supraclavicular lymph node. The lesion had shrunk to 1.5 cm on physical examination four weeks after the vaccination of COVID-19. Ultrasonography showed several enlarged oval lymph nodes of up to 1 cm, with asymmetric cortical thickening and preserved fatty hilum. Associated mild perinodal infiltration was evident as well. The extent of lymphadenopathy had decreased relative to the initial neck CT (Fig 1C). Ultrasonography-guided core biopsy was performed, the pathologic results revealed reactive hyperplasia with capsular and trabecular fibrosis and negative for malignancy. Therefore, we considered the development of right supraclavicular lymphadenopathy after vaccination to have been an adverse reaction to immunization. On clinical follow-up, the patient's supraclavicular lesion had improved.
Fig. 1.
61-y-old male patient with right supraclavicular lymphadenopathy that developed 5 d after first dose of AstraZeneca COVID-19 vaccine in left arm. A, B. Axial and coronal enhanced neck CT performed 21 d after vaccination demonstrated multiple enlarged, clustered lymph nodes with perinodal infiltration (arrows) in the right supraclavicular area. C. Ultrasonography performed 4 wk after vaccination showed several enlarged oval lymph nodes (arrows) with asymmetric cortical thickening, preserved fatty hilum and mild perinodal infiltration. The extent of lymphadenopathy had decreased, relative to the initial neck CT.
Discussion
We report a very rare case of contralateral supraclavicular lymphadenopathy with imaging findings including computed tomography and ultrasonography following administration of the AstraZeneca vaccine for COVID-19.
COVID-19 vaccination-related lymphadenopathy can occur in the ipsilateral axillary or supraclavicular areas associated with activation of local immune response [1,2,5]. In the Pfizer-BioNTech COVID-19 and Moderna vaccine trials, axillary swelling or lymphadenopathy were reported in 0.3% and 16% of vaccine recipients, respectively [6], [7], [8], [9]. In case of the AstraZeneca vaccine, lymphadenopathy is known to be a rare event that may affect up to one in 100 people [10].
Regional lymphadenopathy has been frequently identified by different imaging methods, due to an increase in the vaccination rate of COVID-19 in the general population [11]. There were a few reports of supraclavicular lymphadenopathy after COVID-19 vaccination [5,12,13]. However, most reports presented the ipsilateral supraclavicular lymphadenopathy after COVID-19 vaccination. Only one case series included a case with both axillary and supraclavicular lymphadenopathy [3], and just one other report included a case of contralateral retroclavicular lymphadenopathy detected on ultrasonography [4]. The instances of contralateral supraclavicular lymphadenopathy in those case series had all occurred after the administration of Pfizer-BioNTech COVID-19 vaccine [3,4]. To our knowledge, ours is the first report of the contralateral right supraclavicular lymphadenopathy demonstrated by neck CT, ultrasonography and biopsy, after AstraZeneca vaccination to the left arm.
Following COVID-19 vaccination, lymphadenopathy can prove tricky for physician, given differential diagnosis including inflammation, infection, and malignancy such as a metastasis or lymphoma. Axillary and supraclavicular lymphadenopathy resulting from COVID-19 vaccination usually affects women and typically appears 1-26 days after vaccine administration and usually improves within 4-6 weeks [2,14]. Imaging should be performed prior vaccination or postponed for at least 4-6 weeks after COVID-19 vaccination unless there are urgent clinical signs. If imaging is required, the vaccination information such as vaccination date, injection site (left or right, arm or thigh), and vaccine type should be checked and made readily available to radiologists [14].
Ultrasonographic findings in cases of supraclavicular and axillary lymphadenopathy after the COVID-19 vaccination vary, but most show oval, enlarged lymph nodes, asymmetric cortical thickening with or without loss of normal fatty hilum, central and peripheral vascular signals, and elastography patterns similar to the surrounding tissue [1,13,15]. CT findings of COVID-19 vaccine-related supraclavicular lymphadenopathy had not been reported. In our case, sonographic finding of lymphadenopathy was oval lymph nodes with asymmetric cortical thickening, preserved fatty hilum and hyperechogenicity of the adjacent fat layer, while CT showed clustered and conglomerated lymph nodes with perinodal infiltration. Biopsy was performed after considering the patient's old age and contralateral lymphadenopathy. However, recognition of supraclavicular lymphadenopathy as a self-limiting immune response in recent COVID-19 situations can avoid unnecessary aspiration or biopsy of lymph nodes. Conservative care, such as at least 6 weeks’ observation is recommended over immediate biopsy in cases of axillary and supraclavicular lymphadenopathy after recent ipsilateral or contralateral COVID-19 vaccination [14]. Radiologists should be familiar with these imaging findings to avoid misinterpretation of lymph nodes as pathologic conditions when a patient has a recent history of COVID-19 vaccination [13].
Patient consent
Written informed consent was not necessary because no patient data has been included in the manuscript.
References
- 1.Mehta N, Sales RM, Babagbemi K, Levy A.D, McGrath A.L, Drotman Unilateral axillary adenopathy in the setting of COVID-19 vaccine. Clin Imaging. 2021;75:12–15. doi: 10.1016/j.clinimag.2021.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keshavarz P, Yazdanpanah F, Rafiee F, Mizandari M. Lymphadenopathy following COVID-19 vaccination: Imaging findings review. Acad Radiol. 2021;28(8):1058–1071. doi: 10.1016/j.acra.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D'Auria D, Fulgione L, Romeo V, Stanzione A, Maurea S, Brunetti A. Ultrasound and shear-wave elastography patterns of COVID-19 mRNA vaccine-related axillary, supra and subclavicular lymphadenopathy. Clin and Transl Imaging. 2021 doi: 10.1007/s40336-021-00441-0. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hagen C, Nowack M, Messerli M, Saro F, Mangold F, Bode PK. Fine needle aspiration in COVID-19 vaccine-associated lymphadenopathy. Swiss Med Wkly. 2021;151:w20557. doi: 10.4414/smw.2021.20557. [DOI] [PubMed] [Google Scholar]
- 5.Fernández-Prada M, Rivero-Calle I, Calvache-González A, Martinón-Torres F. Acute onset supraclavicular lymphadenopathy coinciding with intramuscular mRNA vaccination against COVID-19 may be related to vaccine injection technique, Spain, January, and February 2021. Euro Surveil. 2021;26(10) doi: 10.2807/1560-7917.ES.2021.26.2100193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meo SA, Bukhari IA, Akram J, Meo AS, Klonoff DC. COVID-19 vaccines: comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna Vaccines. Eur Rev Med Pharmacol Sci. 2021;25(3):1663–1669. doi: 10.26355/eurrev_202102_24877. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Local Reactions, Systemic Reactions, Adverse Events, and Serious Adverse Events: Pfizer-BioNTech COVID-19 vaccine [Accessed 22 August 2021]; Available from: https://www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html.
- 9.Centers for Disease Control and Prevention. Local Reactions, Systemic Reactions, Adverse Events, and Serious Adverse Events: Moderna COVID-19 Vaccine. [Accessed 22 August 2021]; Available from: https://www.cdc.gov/vaccines/covid-19/info-by-product/moderna/reactogenicity.html.
- 10.Information for UK recipients on COVID 19 vaccine AstraZeneca-regulation 174 information for UK recipients. [Accessed 19 July 2021]; Available from: https://www.cntw.nhs.uk/resource-library/information-for-uk-recipients-on-covid-19-vaccine-astrazeneca-regulation-174-information-for-uk-recipients/
- 11.Lehman CD, Lamb LR, D'Alessandro HA. Mitigating the impact of coronavirus disease (COVID-19) vaccinations on patients undergoing breast imaging examinations: a pragmatic approach. Am J Roentgenol. 2021;217:584–586. doi: 10.2214/AJR.21.25688. [DOI] [PubMed] [Google Scholar]
- 12.Washington T, Bryan R, Clemow C. Adenopathy following COVID-19 vaccination. Radiology. 2021;299(3):E280–E281. doi: 10.1148/radiol.2021210236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim B, Park Y, Kim EK, Lee SH. Supraclavicular lymphadenopathy after COVID-19 vaccination in Korea: serial follow-up using ultrasonography. Clin Imaging. 2021;79:201–203. doi: 10.1016/j.clinimag.2021.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Becker AS, Perez-Johnston R, Chikarmane SA, Chen MM, Homsi ME, Feigin KN. Multidisciplinary recommendations regarding post-vaccine adenopathy and radiologic imaging: radiology scientific expert panel. Radiology. 2021;300(2):E323–E327. doi: 10.1148/radiol.2021210436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cocco G, Delli Pizzi A, Fabiani S, Cocco N, Boccatonda A, Frisone A. Lymphadenopathy after the anti-COVID-19 Vaccine: Multiparametric ultrasound findings. Biology. 2021;10(7):652. doi: 10.3390/biology10070652. [DOI] [PMC free article] [PubMed] [Google Scholar]