Abstract
Introduction:
Critically ill COVID-19 patients are at increased risk of thrombosis with an enhanced risk of bleeding. We aimed to explore the role of anti-factor Xa levels in optimizing the high-intensity anticoagulation’s safety and efficacy and finding possible associations between D-dimer levels, cytokine storm markers, and COVID-19-induced coagulopathy or thrombophilia.
Methods:
Retrospective cohort study conducted on 69 critically ill COVID-19 patients who received three regimens of higher intensity anticoagulation.
Results:
Seventeen patients (24.6%) received high-dose enoxaparin prophylaxis, 29 patients (42%) received therapeutic doses of enoxaparin, and 23 patients (33.3%) were on therapeutic unfractionated heparin infusion. Fewer than one-third of the whole cohort (n = 22; 31.8%) achieved the target range of anti-factor Xa. The patients were divided into three subgroups based on anti-factor Xa target status within each anticoagulation regimen; when compared, the only association observed among them was for interleukin-6 levels, which were significantly higher in both the “above the expected range” and “below the expected range” groups compared with the “within the expected range” group (p = 0.009). Major bleeding episodes occurred in 14 (20.3%) patients and were non-significantly more frequent in the “below the expected anti-factor Xa range group” (p = 0.415). Seven patients (10.1%) developed thrombosis. The majority of patients had anti-factor Xa levels below the expected ranges (four patients, 57.1%).
Conclusion:
Conventional anti-factor Xa ranges may not be appropriate as a predictive surrogate for bleeding in critically ill COVID-19. The clinical decision to initiate therapeutic anticoagulation preemptively may be individualized according to thrombosis and bleeding risks. Cytokine storm markers, namely, interleukin-6, may play a role in COVID-19-induced coagulopathy or thrombophilia.
Keywords: Anti-factor Xa, anticoagulation, bleeding, COVID-19, intensive care unit, thrombosis
Introduction
Hematological complications of coronavirus disease 2019 (COVID-19), caused by the novel coronavirus (SARS-CoV-2), emerged recently and evolved in real-time. Complications ranging from simple hematological changes to arterial and venous thrombosis contribute to the disease’s morbidity and mortality. 1 Histopathologic studies revealed diffuse alveolar damage with severe inflammation, thrombosis, and thrombotic microangiopathy of the lung’s small vessels and capillaries. In an autopsy case series, thrombosis of small and mid-sized pulmonary arteries was demonstrated in all patients. 2 Endotheliitis and thrombotic microangiopathy have also been reported in extrapulmonary organs, which might be due to the intense proinflammatory response that signals cytokine storm initiation. 3 D-dimer has emerged as the most widely recognized indicator of a probable hypercoagulable state in COVID-19 patients, and elevated levels are positively correlated with mortality. 4
Despite the current prophylactic dose of anticoagulation (AC) in patients with COVID-19, several case series from intensive care units (ICUs) have reported high rates of venous thromboembolism (VTE), pulmonary embolism (PE), extracorporeal circuits thrombosis, and arterial events leading to limb ischemia and ischemic strokes. 5 Thus, clinicians worldwide implemented a variety of more intense AC approaches. Consequently, many hospitals’ and clinical societies’ protocols advocated initiating higher prophylactic or even therapeutic doses of anticoagulants in critically ill COVID-19 patients according to their D-dimer levels.6,7
It may be prudent to monitor AC in those patients to avoid aggravating bleeding risks. Activated partial thromboplastin time (aPTT) correlates poorly with the activity of unfractionated heparin (UFH) in plasma, and predicting dose optimization of enoxaparin is complicated; accordingly, anti-factor Xa (anti-FXa) has been suggested as a surrogate marker of the extent of AC. 8 Furthermore, the studies have found that anti-FXa measurement is more accurate than aPTT for monitoring therapy, particularly at the lower end of its therapeutic target. 9 In addition, because of the variable activity of aPTT in the presence of acute-phase proteins, lupus anticoagulants, or liver failure, the anti-FXa assay is preferable for monitoring the therapeutic activity of UFH.10,11
To date, two studies in a small number of patients with COVID-19 that assessed utilizing the anti-FXa assay for monitoring therapy did not have conclusive results. No studies have assessed the role of anti-FXa levels for monitoring the efficacy of higher than usual prophylactic doses of AC therapy in critically ill COVID-19 patients.12,13 Therefore, the aims of this study were: 1 to determine if AC regimens using higher than usual doses are effective in preventing thrombosis; as indicated by anti-FXa levels 2 to examine a possible association between D-dimer levels, inflammatory markers, and COVID-19-induced coagulopathy or thrombophilia; and 3 to monitor the incidence of bleeding as complication.
Methods
Study population
This retrospective cohort study was conducted in the ICUs of King Khalid University Hospital (KKUH), Riyadh, Saudi Arabia. Patients were recruited from 27 April 2020 until 15 August 2020. The KKUH Institutional Review Board approved the study; the need for patient consent was waived because of the study’s retrospective design. Patients >18 years of age who received higher intensity AC within the initial 24–48 h after hospital admission, with anti-FXa level measured during their ICU stay, and with a confirmed positive COVID-19 determined by real-time polymerase chain reaction (RT-PCR) were included. Pregnant women and patients with lupus anticoagulants, a known underlying hypercoagulable state, chronic liver disease, platelet count <50 × 103/μL, those who were post-cardiac arrest, died within 48 h of admission, had VTE on admission, or had inappropriate sample timing were excluded.
Data collection
Electronic health records were accessed to retrieve basic demographic characteristics, medical history, and comorbidities. Platelet count, prothrombin time (PT), aPTT, international normalized ratio (INR), fibrinogen, D-dimer, and anti-FXa were measured on the same day. Markers of inflammation—including peak D-dimer, fibrinogen, interleukin-6 (IL-6), procalcitonin, C-reactive protein (CRP), and ferritin levels—were also obtained from patients’ records. Complications including acute kidney injury, the need for renal replacement therapy, respiratory failure necessitating mechanical ventilation, and the development of new-onset VTE or arterial thrombosis were recorded. Routine VTE screening by performing lower limb Doppler was not a part of our institution protocol.
Major bleeding, as defined by The International Society on Thrombosis and Haemostasis (ISTH), was recorded. Symptomatic bleeding, including intracranial, intraspinal, intraocular, retroperitoneal, intraarticular, pericardial, intramuscular bleed with compartment syndrome, or bleeding causing a ⩾2 g/dL decrease in hemoglobin level or leading to transfusion of ⩾2 units of packed red blood cell (RBC), was recorded. 14 Other data obtained included the use of vasopressors or antiplatelet agents, mechanical ventilator days, ICU length of stay, ICU mortality, hospital length of stay, and all-cause 28-day mortality.
AC regimens
The patients received the following three AC regimens in the ICU:
“High-dose prophylaxis”: enoxaparin 40, 50, or 60 mg subcutaneously (SC) according to body weight and D-dimer level every 12 h according to the Saudi Ministry of Health protocol. 7
“Therapeutic enoxaparin”: 1 mg/kg SC every 12 h.
“Therapeutic unfractionated heparin (UFH) infusion.”
Therapeutic enoxaparin and UFH infusion were typically used if there was any evidence of thrombosis (including superficial veins), worsening hypoxemia despite improving X-ray and proper lung compliance, D-dimer ⩾2 μg/mL, or rapidly increasing D-dimer. The AC regimen was initiated at the discretion of the treating team. It was guided by the patient’s estimated glomerular filtration rate (eGFR) using the Cockcroft–Gault equation. Therapeutic UFH infusion was started if eGFR was <30 mL/min/1.73 m2.
Anti-FXa activity
Following our institutional clinical pharmacy protocol, plasma anti-FXa activity was measured after reaching steady-state of the anticoagulant (after ⩾3 doses of enoxaparin, or 4–6 h after starting intravenous UFH infusion). Blood sampling in patients receiving enoxaparin was timed to measure “the peak level” 4–6 h after the last AC dose. Our usual practice was to sample for aPTT and anti-FXa simultaneously if the patient received UFH infusion to ensure result concordance.
The reference ranges we used were based on previous studies investigating the efficacy of anti-FXa in non-COVID-19 patients and the drug reference index, and they were as follows:
Enoxaparin for VTE prophylaxis: 0.2–0.4 unit/mL.
Enoxaparin for VTE treatment (twice daily dosing): 0.6–1 units/mL.
UFH for VTE treatment: 0.3–0.7 unit/mL.
Whole blood was sampled in 3.2% sodium citrate tubes (BD Vacutainer) for coagulation testing at the hospital clinical chemistry and hematology laboratory. Parameters measured included PT, aPTT, D-dimer, fibrinogen, antithrombin (AT) activity, and platelet count. Blood sampling for anti-FXa activity (peak anti-FXa) was performed using STA®-Liquid Anti-Xa (Stago, Asnières-sur-Seine, France); as per our standard lab procedure, without adding any exogenous antithrombin.
Anti-FXa groups
We divided the entire cohort according to anti-FXa levels, with subgroups based on anti-FXa target status within each AC regimen:
“Within range” included patients whose anti-FXa level was within the expected range, whether prophylactic or therapeutic.
“Above the expected range” included patients with supraprophylactic or supratherapeutic values.
“Below the expected range” included patients with subprophylactic or subtherapeutic values.
Statistical analysis
Data were analyzed using SPSS version 20.0. (IBM Corp., Armonk, NY, USA). The Kolmogorov–Smirnov test was used to verify distribution normality. Comparisons between groups for categorical variables were assessed using the chi-square test (Monte Carlo). For continuous variables, analysis of variance (ANOVA) was used to compare >2 groups; the Kruskal–Wallis test was used for data not normally distributed, and Dunn’s post hoc multiple comparisons test was used for pairwise comparisons. The significance level was established at 5%.
Results
Patient characteristics
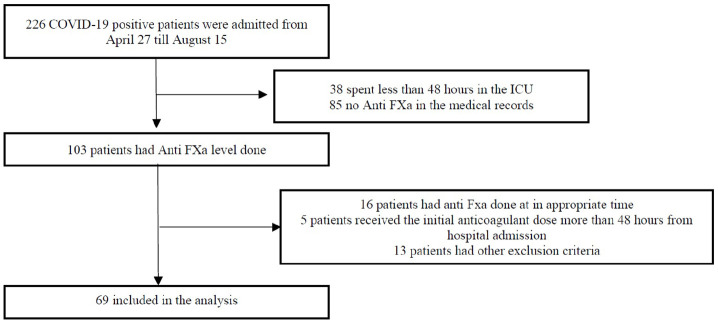
Sixty-nine critically ill patients with RT-PCR confirmed COVID-19 who had anti-FXa levels measured were included in the study (Figure 1). Most patients were male (68.1%), with a median age of 58.4 years (interquartile range (IQR) = 51–65) and approximately half had diabetes mellitus and/or hypertension. Only two (2.9%) patients had a history of VTE (one deep vein thrombosis (DVT) and one PE), and both cases were provoked. Three patients had a history of cerebrovascular accident (CVA); all were ischemic. None of the patients had chronic liver disease or a history of past or current malignancy (Supplemental Table 1).
Figure 1.
Study flow chart.
Seventeen patients (24.6%) received high-dose enoxaparin prophylaxis, 29 patients (42%) received therapeutic doses of enoxaparin, and 23 patients (33.3%) were on UFH infusion. More patients receiving UFH infusion had multiple comorbidities at baseline compared with the other two regimens (Table 1).
Table 1.
Comparison of patients’ clinical and laboratory parameters according to the three anticoagulation regimens.
| High-dose prophylaxis enoxaparin (n = 17) | Therapeutic enoxaparin (n = 29) | Therapeutic UFH infusion (n = 23) | p-value | |
|---|---|---|---|---|
| Characteristics, number (%) | ||||
| Female | 6 (35.3%) | 9 (31%) | 7 (30.4%) | 0.940 |
| Male | 11 (64.7%) | 20 (69%) | 16 (69.6%) | |
| Age, median (IQR) (years) | 59 (46–61) | 59 (51–65) | 62 (56.5–69) | 0.277 |
| BMI, median (IQR) (kg/m2) | 32.1 (28.4–40) | 28.3 (24.8–32.4) | 31.2 (28.1–33.6) | 0.239 |
| Comorbidities, number (%) | ||||
| Previous VTE | 0 (0%) | 1 (3.4%) | 1 (4.3%) | 1.000 |
| Diabetes mellitus | 5 (29.4%) | 14 (48.3%) | 18 (78.3%) | 0.007 |
| Hypertension | 8 (47.1%) | 12 (41.4%) | 14 (60.9%) | 0.369 |
| Previous CVA | 0 (0%) | 0 (0%) | 3 (13%) | 0.046 |
| Ischemic heart disease | 0 (0%) | 1 (3.4%) | 5 (21.7%) | 0.030 |
| Heart failure | 0 (0%) | 1 (3.4%) | 5 (21.7%) | 0.030 |
| Atrial fibrillation | 0 (0%) | 0 (0%) | 1 (4.3%) | 0.579 |
| CKD | 0 (0%) | 0 (0%) | 11 (47.8%) | 0.001 |
| Coagulation parameters, median (IQR) | ||||
| INR (0.8–1.3) | 1.1 (1–1.2) | 1.1 (1.1–1.2) | 1.2 (1.1–1.2) | 0.290 |
| aPTT (25–39 s) | 37.7 (34–44.9) | 37.1 (33.4–48) | 56.9 (50.7–78) | <0.001 |
| Current fibrinogen (2–4 g/L) | 7.1 (5–8.3) | 6.6 (5–7.6) | 5.9 (4.5–6.5) | 0.614 |
| Current D-dimer (0.22–0.45 mcg/mL FEU) | 2.3 (1.4–4.7) | 1.9 (1.3–3.4) | 3.8 (2.8–5.6) | 0.019 |
| Peak D-dimer (0.22–0.45 mcg/mL FEU) | 3.8 (3.3–12.7) | 5.4 (3–15.8) | 13.8 (6.5–19.7) | 0.107 |
| Platelets (×10³/μL) | 320 (184–368) | 385 (308–466) | 208 (182.5–279) | <0.001 |
| Medications, number (%) | ||||
| Aspirin | 1 (5.9%) | 7 (24.1%) | 6 (26.1%) | 0.295 |
| Plavix | 0 (0%) | 2 (6.9%) | 3 (13%) | 0.355 |
| Pressors | 3 (17.6%) | 10 (34.5%) | 19 (82.6%) | <0.001 |
| Complications, number (%) | ||||
| Bleeding | 2 (11.8%) | 6 (20.7%) | 6 (26.1%) | 0.599 |
| Arterial thrombosis | 0 (0%) | 0 (0%) | 1 (4.3%) | 0.579 |
| VTE | 0 (0%) | 2 (6.9%) | 4 (17.4%) | 0.184 |
| AKI | 5 (29.4%) | 11 (37.9%) | 18 (78.3%) | 0.003 |
| CRRT | 1 (5.9%) | 1 (3.4%) | 17 (73.9%) | <0.001 |
| Invasive MV | 7 (41.2%) | 14 (48.3%) | 23 (100%) | <0.001 |
| Cytokine storm markers, median (IQR) | ||||
| LDH (84–246 unit/L) | 701 (608–880) | 717 (599–978) | 814 (473–1040) | 0.946 |
| Ferritin (13–150 mcg/L) | 1828 (1367–3801) | 1580 (783–4500) | 2896 (1065–8542) | 0.240 |
| CK (26–192 unit/L) | 177.5 (97–379) | 266 (97–452) | 580 (252–1702) | 0.017 |
| Procalcitonin (0.0.2–0.1 ng/mL) | 0.2 (0.1–2) | 0.4 (0.2–1.6) | 4 (0.5–9.3) | 0.011 |
| IL-6 (1.5–7 pg/mL) | 174.5 (57.4–1552) | 172 (104.2–266) | 151 (113.5–224.4) | 0.707 |
| CRP (<10 mg/L) | 191 (171–226) | 231 (176–318) | 207.5 (107–291) | 0.217 |
| Peak fibrinogen (2–4 g/L) | 7.5 (6.3–8.9) | 7.6 (6.4–7.9) | 7.8 (6.1–8.5) | 0.997 |
| Outcomes | ||||
| ICU length of stay, median (IQR), days | 15 (9–23) | 16 (11–26) | 26 (19–31.5) | 0.012 |
| ICU mortality, number (%) | 6 (35.3%) | 11 (37.9%) | 19 (82.6%) | 0.002 |
AKI: acute kidney injury; aPTT: activated partial thromboplastin time; BMI: body mass index; CK: creatine kinase; CKD: chronic kidney disease; CRP: C-reactive protein; CRRT: continuous renal replacement therapies; CVA: cerebrovascular accident; eGFR: estimated glomerular filtration rate; INR: international normalized ratio; IQR: interquartile range; LDH: lactate dehydrogenase; IL-6: interleukin-6; MV: mechanical ventilation; VTE: venous thromboembolism.
Comparison of patients among the three AC regimens
Patients in the UFH group had significantly higher D-dimer levels, and significantly lower platelet counts at the time of anti-FXa sampling compared with the other two groups. All patients in this group were on invasive mechanical ventilation, 19 (82.6%) were on pressors, and 18 (78.3%) developed acute kidney injury (AKI), with 17 (73.9%) requiring continuous renal replacement therapy (CRRT). These patients had higher procalcitonin levels compared with the other two regimens. Moreover, they had significantly longer ICU length of stay and ICU mortality (Table 1).
Anti-FXa level targets
Fewer than one-third of the patients (n = 22; 31.8%) achieved the target range level of anti-FXa. The majority of patients on each AC regimen had anti-FXa levels, either below or above the expected range (Table 2). Patients who received UFH infusion were more likely to have subtherapeutic anti-FXa levels (78.2%), despite having a therapeutic (56.5%) or supratherapeutic (21.7%) aPTT level. None of the patients on UFH infusion had anti-FXa levels above the expected range. Patients receiving the two enoxaparin regimens had varied anti-FXa levels and did not show a predominant distribution in any of the three anti-FXa range groups.
Table 2.
Comparison of the three anti-FXa groups according to the anticoagulation regimen and bleeding and thrombotic complications.
| Anti-FXa within the expected range (n = 22) | Out of expected range (n = 47) | p-value | ||
|---|---|---|---|---|
| Anti-FXa above the expected range (n = 10) | Anti-FXa below the expected range (n = 37) | |||
| Anticoagulant regimen, number (%) | ||||
| High-dose enoxaparin prophylaxis | 5 (29.4%) | 5 (29.4%) | 7 (41.1%) | 0.125 |
| Therapeutic enoxaparin | 12 (41.3%) | 5 (17.2%) | 12 (41.3%) | 0.215 |
| Therapeutic UFH infusion | 5 (21.7%) | 0 | 18 (78.2%) | 0.007 |
| Complications, number (%) | ||||
| Bleeding | 3 (13.6%) | 1 (10%) | 10 (27%) | 0.415 |
| Arterial thrombosis | 0 (4.5%) | 0 (0%) | 1 (2.7%) | 0.463 |
| VTE | 3 (13.6%) | 0 (0%) | 3 (8.1%) | 0.602 |
UFH: unfractionated heparin; VTE: venous thromboembolism.
Biological factors and anti-FXa levels
To elucidate the possible influence of various biological factors, we compared the three anti-FXa groups according to baseline demographics, comorbidities, coagulation parameters, inflammatory and cytokine storm markers, and pharmacological interventions (Supplemental Table 2). The only association observed among the three groups was for IL-6 levels, which were significantly higher in both the “above the expected range” and “below the expected range” groups compared with the “within the expected range” group (Figure 2). None of the patients had hyperbilirubinemia or hypertriglyceridemia sufficient to affect anti-FXa levels.
Figure 2.
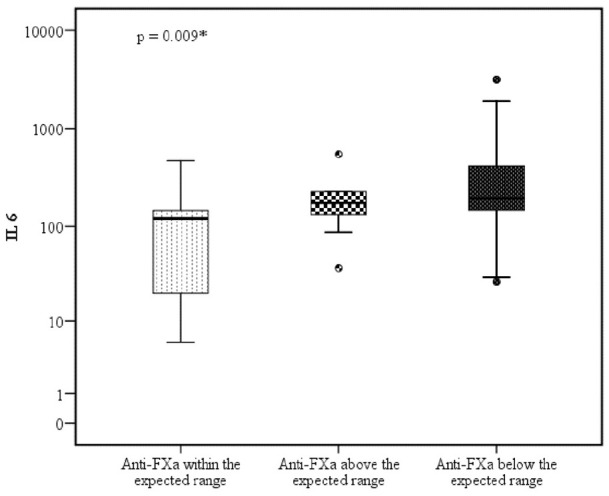
Interleukin-6 level according to anti-FXa levels.
Bleeding complications
Major bleeding episodes occurred in 14 (20.3%) patients, 6 in each therapeutic AC regimen, and 2 in the high-dose prophylactic enoxaparin regimen. The major bleeding incidents were more frequent in the “below the expected anti-FXa range group” (Table 1), although the difference did not reach statistical significance.
Six (43%) patients with bleeding events were receiving UFH (Table 2 and Supplemental Table 3). Despite having therapeutic aPTT levels, anti-FXa levels were below target. Among all patients with bleeding, eight had gastrointestinal (GI) bleeding; two had intramuscular hematomas, one had a subdural hematoma, and one had massive epistaxis. One patient in the “above the expected range” group had a 4-g/dL drop in hemoglobin. Although strongly suspected, the source of bleeding could not be identified (relevant investigations excluded intra- and extravascular hemolysis). The three patients in the “within the expected range” group had upper GI bleeding. None of the 14 cases had either a fatal bleed or required an invasive intervention.
Thrombotic complications
Seven patients (10.1%) developed thrombosis (Table 2 and Supplemental Table 4). The majority of these patients were receiving UFH (n = 5; 71.4%). One patient developed arterial thrombosis in the form of acute lower limb ischemia. The majority of patients had anti-FXa levels below the expected ranges (n = 4; 57.1%). No thrombotic episodes were observed in the enoxaparin high-dose prophylactic regimen.
ICU length of stay and mortality
Thirty-six (52%) patients died. ICU length of stay was longer, and ICU mortality was higher in the UFH group. There were no significant mortality differences in the three subgroups based on anti-FXa target levels (Table 2, Figure 3, and Supplemental Table 2).
Figure 3.
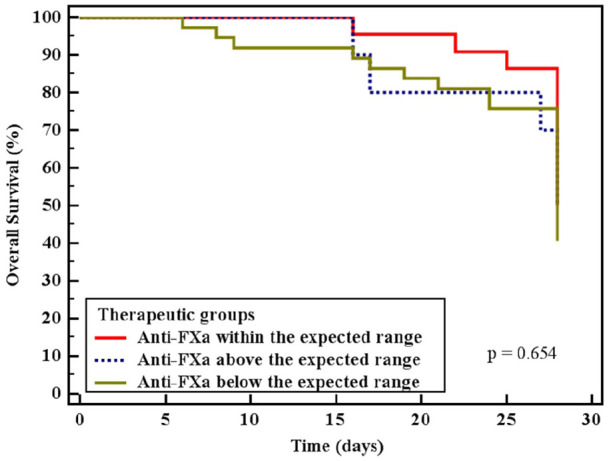
All cause 28 days mortality according to anti-FXa levels.
Discussion
Variable responses and possible resistance to anticoagulants contribute to the high thrombosis incidence among critically ill COVID-19 patients who are admitted to the ICU,5,15 and there is a paucity of data that can inform the optimum AC dose for COVID-19 patients. 16 Clinicians need a practical test to guide AC optimization, especially if higher doses are used than those included in standard regimens. Monitoring anti-FXa activity has been suggested to allow improved titration of low molecular weight heparin (LMWH) and UFH doses in non-COVID-19 patients. 9
To our knowledge, this is the first study to evaluate anti-FXa in critically ill COVID-19 patients receiving three different AC regimens and correlate biological factors and cytokine storm markers with anti-FXa levels. Fewer than one-third of the patients achieved anti-FXa levels within the typically acceptable target levels, while most values were in subprophylactic and subtherapeutic ranges.
We also observed that patients receiving therapeutic UFH infusion were less likely to achieve anti-FXa target levels despite having concomitant therapeutic aPTT levels. Spontaneously and falsely elevated aPTT levels have been observed in severe COVID-19 cases. For example, in a case series reported by Bowles et al., 17 20% of their patients had elevated aPTT levels, of which 91% had lupus anticoagulant. Consequently, clinicians responding to aPTT levels might withhold or reduce the intensity of AC due to perceived over-AC and fear of bleeding. 17
We are unaware of a universally accepted anti-FXa reference range for the high prophylactic dose of enoxaparin. We believe that the reference range we used during our analysis (0.2–0.4 IU/mL), which we compiled from multiple trials that used a maximum enoxaparin dose of 30 mg twice daily, might be suboptimal. Nevertheless, despite higher than usual doses of enoxaparin for VTE prophylaxis, 41% of patients in the high-dose enoxaparin group had anti-FXa levels <0.2 IU/mL. Likewise, 41% of the patients on therapeutic enoxaparin AC had subtherapeutic anti-FXa levels (<0.6 U/mL).
Our data support two small recent reports of anti-FXa guided AC therapy in COVID-19 patients. Vlot et al. 12 studied 16 COVID-19 patients admitted to the ICU, who received 5700 IU nadroparin twice daily, which is almost four times the regular thromboprophylactic dose. They concluded that anti-FXa was within the target range of pharmacodynamic endpoints, while viscoelastic tests demonstrated a procoagulant pattern. 12 Similarly, in a letter to the editor, Dutt et al. 13 reported that 95% of 20 COVID-19 patients requiring intensive care had subprophylactic anti-FXa levels while receiving a prophylactic dose of enoxaparin compared with 27% of 22 COVID-19 patients admitted to the ward.
We examined additional possible associations with anti-FXa levels. In the absence of discrepancies in preanalytical and analytical factors due to environmental conditions and technique, biological and inflammatory factors are the main elements to affect anticoagulant pharmacokinetics. Common biological factors known to affect anti-FXa levels include increased acute-phase reactants, inflammatory markers, impaired renal function, severe hyperbilirubinemia, and extremes of age, and weight. 18 In addition, there is concern that the use of vasopressors may reduce the effectiveness of LMWH. Critically ill patients receiving vasopressor support were found to have significantly lower anti-FXa levels than patients who were not. 19 The likely mechanism is decreased absorption of LMWH from the subcutaneous tissues due to reduced perfusion. This could have contributed to low anti-FXa levels in some of our patients.
Due to the myriad factors that can contribute to subprophylactic and subtherapeutic anti-FXa levels in critically ill patients, we further divided the cohort into three groups according to anti-FXa levels to better understand the role of biological factors and inflammatory markers. We observed a significantly elevated IL-6 level among patients who failed to achieve a target anti-FXa level. Cytokine storm is recognized as a hallmark of severe COVID-19 infection; therefore, IL-6 became strongly implicated in the pathophysiology of COVID-19, and several clinical trials are examining the potential use of IL-6 receptor antagonists to divert the course of the disease.20–22 A systemic review that compared IL-6 levels in severe and non-severe COVID-19 cases found that mean IL-6 levels were more than three times higher in patients with complicated COVID-19 than in those with a non-complicated disease and that IL-6 levels were associated with an increased risk of mortality. 23 The elevated IL-6 levels in our patients suggest an excessive inflammatory response and more severe disease, which likely contributed to the non-optimal levels of anti-FXa.
Our 10% incidence of arterial and venous thrombosis was lower than that of earlier studies, which reported a thrombosis incidence of 20%–30%.24,25 The higher doses of prophylactic or therapeutic AC administered to our patients may explain our decreased incidence of thrombosis, which is in keeping with the more recently reported radiographically confirmed VTE rate of 4.8%, and overall thrombosis rate of 9.5% in COVID-19 patients. 26 We acknowledge that the absence of evidence is not evidence of absence; the lack of routine VTE screening in our ICUs while relying on clinical suspicion to look for possible VTE might have contributed to the underdiagnosis of possible thrombotic events.
Twenty percent of our patients suffered bleeding complications. Although we could not establish an explicit time-based correlation between the anti-FXa levels and the bleeding in many of the patients, bleeding was far more common among patients who received therapeutic compared with prophylactic AC. A previous meta-analysis for the non-COVID population reported that the risk of bleeding with therapeutic doses of enoxaparin was four times higher than with prophylactic doses, especially in patients with renal impairment. 27 Some recent studies in critically ill COVID-19 patients also reported significant bleeding complications, particularly in those receiving therapeutic doses of AC.28,29
Interestingly, we observed only one bleeding episode among the patients with supraprophylactic or supratherapeutic anti-FXa levels. Instead, bleeding incidence was the highest among patients whose anti-FXa levels were below target, although the difference among anti-FXa target groups did not reach statistical significance. Apart from the therapeutic doses of AC, other confounding factors might have contributed to the bleeding incidents, including gram-negative sepsis in four patients, thrombocytopenia in two patients, and concomitant antiplatelet therapy in three patients. Although those patients had a major bleeding episode based on the International Society of Haemostasis and Thrombosis (ISHT) criteria, none of the 14 cases had fatal bleeding or required an invasive intervention.
Our findings indicate that the conventional target ranges for anti-FXa levels may not be appropriate for COVID-19 patients, particularly for those receiving therapeutic doses of AC. In addition, although higher intensity AC might be advantageous for reducing thrombotic events, it appears to enhance bleeding risk even in patients with subprophylactic and subtherapeutic anti-FXa levels, and underscores the need for a thorough evaluation of bleeding risk in these patients.
Reported ICU mortality for patients with COVID-19 varied among studies and ranged from 34.0% to 49.7% in a recent meta-analysis. 30 Our ICU mortality was similar among groups based on anti-FXa status; however, the mortality rate was higher for patients who received therapeutic UFH compared with the other two AC groups. This difference is likely multifactorial, with UFH patients being sicker, having more comorbidities, with a higher incidence of AKI, pressors utilization, and invasive mechanical ventilation in addition to higher procalcitonin levels, which may indicate coexisting bacterial infection.
This study has some limitations. Apart from being retrospective, our groups are small, and we could not perform sample size calculation, which precludes reaching firm conclusions. With the small numbers, this study was underpowered to test for all relevant covariate interactions reliably. Furthermore, the variation in anti-FXa activity among groups did not lead to differences in ICU length of stay or ICU mortality, which may be more clinically meaningful endpoints. These results should be validated in larger prospective studies.
Conclusion
Patients with COVID-19 are at increased risk of thrombosis as well as at enhanced risk of bleeding. There is an urgent need to define effective pharmacological thromboprophylaxis regimens for the management of COVID-19 patients admitted to ICU to reduce the risk of thrombosis without significantly increasing the risk of bleeding. Conventional anti-FXa ranges may not be appropriate as a predictive surrogate for bleeding in COVID-19 patients, particularly those receiving therapeutic AC. Given the bleeding risk associated with anticoagulants, the preemptive clinical decision to initiate therapeutic AC should be individualized and tailored for each patient according to thrombosis and bleeding risk.
Supplemental Material
Supplemental material, sj-docx-1-smo-10.1177_20503121211049931 for Assessment of anti-factor Xa activity in critically ill COVID-19 patients receiving three different anticoagulation regimens by Mohammed A Hamad, Shereen A Dasuqi, Aamer Aleem, Rasha A Omran, Rakan M AlQahtani, Fahad A Alhammad and Abdulaziz H Alzeer in SAGE Open Medicine
Acknowledgments
This study was supported by the College of Medicine Research Centre, Deanship of Scientific Research, King Saud University, Riyadh, Saudi Arabia. The authors also thank Mr Amgad Hamza for his help during statistical analysis of the data.
Footnotes
Author contributions: M.A.H. contributed to the conceptualization, methodology, investigation, data curation, writing—original draft, and writing—review and editing. S.A.D. contributed to the investigation data curation, project administration, methodology, writing—original draft, and writing—review and editing. A.A. contributed to the methodology, validation, and writing—review and editing. R.A.O. contributed to writing—original draft and validation. R.M.A.Q. contributed to the writing—review and editing. F.A.A. contributed to the writing review and editing. A.H.A. contributed to the supervision, writing—original draft, and writing—review and editing. All authors read and approved the final manuscript.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Ethical approval for this study was obtained from The KKUH Institutional Review Board no. E-20-5174.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the College of Medicine Research Centre, Deanship of Scientific Research, King Saud University, Riyadh, Saudi Arabia.
Informed consent: The need for patient consent was waived because of the study’s retrospective design.
ORCID iD: Shereen A Dasuqi  https://orcid.org/0000-0002-7631-5573
https://orcid.org/0000-0002-7631-5573
Supplemental material: Supplemental material for this article is available online.
References
- 1. Mason RJ. Pathogenesis of COVID-19 from a cell biology perspective. Eur Respir J 2020; 55(4): 2000607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sigurd F, Kristijan L, Peter S, et al. Pulmonary arterial thrombosis in COVID-19 with fatal outcome: results from a prospective, single-center, clinicopathologic case series. Ann Intern Med 2020; 173(5): 350–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhang T, Sun LX, Feng RE. Comparison of clinical and pathological features between severe acute respiratory syndrome and coronavirus disease 2019. Zhonghua Jie He He Hu Xi Za Zhi 2020; 43(6): 496–502 (in Chinese). [DOI] [PubMed] [Google Scholar]
- 4. Zhang L, Yan X, Fan Q, et al. D-dimer levels on admission to predict in-hospital mortality in patients with COVID-19. J Thromb Haemost 2020; 18(6): 1324–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Klok FA, Kruip van der Meer NJM, Arbous MS, et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res 2020; 191: 148–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Paranjpe I, Fuster V, Lala A, et al. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol 2020; 76(1): 122–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ministry of Health. Coronavirus diseases 19 (COVID-19) guidelines, July 2020, version 2.1, https://www.moh.gov.sa/Ministry/MediaCenter/Publications/Documents/MOH-therapeutic-protocol-for-COVID-19.pdf
- 8. Barnes GD, Burnett A, Allen A, et al. Thromboembolism and anticoagulant therapy during the COVID-19 pandemic: interim clinical guidance from the anticoagulation forum. J Thromb Thrombolysis 2020; 50(1): 72–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hutt Centeno E, Militello M, Gomes MP. Anti-Xa assays: what is their role today in antithrombotic therapy? Cleve Clin J Med 2019; 86(6): 417–425. [DOI] [PubMed] [Google Scholar]
- 10. Wool GD, Lu CM. Pathology consultation on anticoagulation monitoring: factor X-related assays. Am J Clin Pathol 2013; 140(5): 623–634. [DOI] [PubMed] [Google Scholar]
- 11. Tang N, Li D, Wang X, et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. JTH 2020; 18: 844–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vlot EA, Van den Dool EJ, Hackeng CM, et al. Anti Xa activity after high dose LMWH thrombosis prophylaxis in covid 19 patients at the intensive care unit. Thromb Res 2020; 196: 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dutt T, Simcox D, Downey C, et al. Thromboprophylaxis in COVID-19: anti-FXa-the missing factor? Am J Respir Crit Care Med 2020; 202(3): 455–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 2005; 3(4): 692–694. [DOI] [PubMed] [Google Scholar]
- 15. McLaughlin K, Rimsans J, Sylvester KW, et al. Evaluation of antifactor-Xa heparin assay and activated partial thromboplastin time values in patients on therapeutic continuous infusion unfractionated heparin therapy. Clin Appl Thromb Hemost 2019; 25: 876030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Spyropoulos AC, Levy JH, Ageno W, et al. Scientific and Standardization Committee communication: clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost 2020; 18(8): 1859–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bowles L, Platton S, Yartey N, et al. Lupus Anticoagulant and abnormal coagulation tests in patients with COVID-19. N Engl J Med 2020; 383(3): 288–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Smythe MA, Priziola J, Dobesh PP, et al. For the practical management of the heparin anticoagulants in the treatment of venous thromboembolism. J Thromb Thrombolysis 2016; 41(1): 165–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jochberger S, Mayr V, Luckner G, et al. Antifactor Xa activity in critically ill patients receiving antithrombotic prophylaxis with standard dosages of certoparin: a prospective, clinical study. Crit Care 2005; 9: R541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395(10223): 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Favalli EG, Ingegnoli F, De Lucia O, et al. COVID-19 infection and rheumatoid arthritis: faraway, so close! Autoimmun Rev 2020; 19(5): 102523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gritti G, Raimondi F, Ripamonti D. IL-6 signalling pathway inactivation with siltuximab in patients with COVID-19 respiratory failure: an observational cohort study. medRxiv 2020, https://www.medrxiv.org/content/10.1101/2020.04.01.20048561v4
- 23. Aziz M, Fatima R, Assaly R. Elevated interleukin-6 and severe COVID-19: a meta-analysis. J Med Virol 2020; 92: 2283–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hang C, Shen L, Le KJ, et al. Incidence of venous thromboembolism in hospitalized coronavirus disease 2019 patients: a systematic review and meta-analysis. Front Cardiovasc Med 2020; 7: 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Porfidia A, Valeriani E, Pola R, et al. Thromboembolism in patients with COVID-19: systematic review and meta-analysis. Thromb Res 2020; 196: 67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Al-Samkari H, Karp Leaf RS, Dzik WH, et al. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood 2020; 136(4): 489–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lim W, Dentali F, Eikelboom JW, et al. Meta-analysis: low-molecular-weight heparin and bleeding in patients with severe renal insufficiency. Annals of Internal Medicine 2006; 144(9): 673–684. [DOI] [PubMed] [Google Scholar]
- 28. Megan F, Elsa L, Olivier P, et al. Thrombotic and hemorrhagic events in critically ill COVID-19 patients: a French monocenter retrospective study. Crit Care 2020; 24: 275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Desborough MJR, Doyle AJ, Griffiths A, et al. Image-proven thromboembolism in patients with severe COVID-19 in a tertiary critical care unit in the United Kingdom. Thromb Res 2020; 193: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Armstrong RA, Kane AD, Cook TM. Outcomes from intensive care in patients with COVID-19: a systematic review and meta-analysis of observational studies. Anaesthesia 2020; 75(10): 1340–1349. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-smo-10.1177_20503121211049931 for Assessment of anti-factor Xa activity in critically ill COVID-19 patients receiving three different anticoagulation regimens by Mohammed A Hamad, Shereen A Dasuqi, Aamer Aleem, Rasha A Omran, Rakan M AlQahtani, Fahad A Alhammad and Abdulaziz H Alzeer in SAGE Open Medicine